Abstract
Although hormones such as glucocorticoids have been broadly accepted in recent decades as general neuromodulators of memory processes, sex steroid hormones such as the potent oestrogen 17β-oestradiol have been less well recognized by the scientific community in this capacity. The predominance of female subjects in studies of oestradiol and memory, and the general (but erroneous) perception that oestrogens are ‘female’ hormones, have probably prevented oestradiol from being more widely considered as a key memory modulator in both sexes. Indeed, although considerable evidence supports a crucial role for oestradiol in regulating learning and memory in females, a growing body of literature indicates a similar role in males. This Review discusses the mechanisms of oestradiol signalling and provides an overview of oestradiol’s effects on spatial, object-recognition, social and fear memories. Although the primary focus is on data collected in females, effects of oestradiol on memory in males will be discussed, as will sex differences in the molecular mechanisms that regulate oestrogenic modulation of memory, which may have important implications for the development of future cognitive therapeutics.
ToC blurb
Sex steroid hormones such as the potent oestrogen 17β-oestradiol (E2) have only recently started to be acknowledged as important neuromodulators. Taxier, Gross and Frick review E2 signalling in the brain and its effects on different types of memory.
Introduction
Oestrogens, androgens and progestogens are most commonly associated with their roles in reproduction, despite their involvement in a broad range of physiological and neural functions. One particular realm in which these sex-steroid hormones exert wide-ranging effects is that of learning and memory. In particular, considerable effort has been devoted to understanding how 17β-oestradiol (E2), the most potent and prevalent circulating oestrogen, regulates the function of the hippocampus, a bilateral medial temporal lobe structure with extensive connections to numerous cortical and subcortical brain regions. Although the hippocampus is not the only brain region important for memory, damage or dysfunction to this structure leads to deficits in the formation and retention of numerous types of memories, including those used for spatial navigation, recognizing objects and conspecifics, and recalling fear-associated contexts. However, roles for sex-steroids in regulating the function of mnemonic brain regions like the hippocampus have rarely been considered by scientists other than neuroendocrinologists.
In the early 1990s, a series of papers describing the effects of oestrogens on neuronal morphology and plasticity in the hippocampus was paradigm shifting for the field of neuroendocrinology1–5. These publications demonstrated that dendritic spine density on CA1 pyramidal neurons in the hippocampus fluctuates across the reproductive (oestrous) cycle of female rats, and is increased in bilaterally ovariectomized [G] female rats by exogenous E2 treatment3. Other contemporaneous work showed that E2 potentiates hippocampal excitatory synaptic plasticity5–7 and protects hippocampal neurons from drug-induced excitoxicity8. Collectively, this body of work unlocked a new research frontier that has broadened understanding of how E2 regulates hippocampal function to modulate memory. Since their publication, these findings have inspired much research examining actions of E2 and other sex-steroid hormones in non-reproductive, pro-cognitive brain regions including the hippocampus and prefrontal cortex (PFC). A subset of this newer research has unveiled very rapid actions of E2 on molecular and cellular mechanisms in the hippocampus, PFC, amygdala and other regions that facilitate memory-consolidation processes.
Although the myriad effects of oestrogens such as E2 on hippocampal function have been intensely studied by neuroendocrinologists for nearly three decades, oestrogens have yet to be as widely accepted in neuroscience as general neuromodulators of cognitive processes. As oestrogens exert numerous effects in multiple cognitive brain areas in both sexes9,10,11–13, elucidating how these hormones regulate memory could promote better mental health outcomes in people of all genders by identifying potential therapeutic targets for neurodegenerative diseases, mood disorders and other conditions in which memory dysfunction features prominently. Effective treatments for memory loss are sorely lacking, highlighting an urgent need to characterize the neural mechanisms underlying memory formation, including those mediated by oestrogens. Moreover, such information will greatly inform our general understanding of how memories are formed. Thus, more broadly considering oestrogens and related sex-steroid hormones as important neuromodulators of memory formation will provide fundamental insights into the neurobiology of memory and new avenues for drug development.
The goal of this Review is to highlight the importance of E2 as a critical modulator of synaptic plasticity and memory circuitry. Although other sex-steroid hormones can regulate memory, the influence of E2 on learning and memory is the most extensively documented and thus E2 is the focus of this Review. The discussion will centre upon the effects of E2 in rats and mice because their size and short lifespans afford convenient mammalian systems in which to explore molecular mechanisms of E2 action in the brain, and thus the preponderance of data are from these species. However, non-human primate studies (reviewed in11–13) have also provided insights about oestrogenic regulation of memory and neuronal morphology that corroborate findings from rodent studies, highlighting the translational potential of this work. Here, we first outline molecular mechanisms underlying E2 signalling in the rodent brain, including both classical and non-classical signalling. Next, we describe several forms of learning and memory modulated by E2 in rodents, and the mechanisms thus far identified that underlie this mediation. Although findings from female rodents are the primary focus, data from males are also discussed, as are sex differences in the neural mechanisms through which E2 influences memory. Last, we conclude by discussing the broad health implications of considering E2 as a neuromodulator, and speculate about next steps necessary to further advance knowledge about oestrogenic regulation of memory. We hope readers will be convinced of the importance of E2 as a general neuromodulator that, like stress hormones and growth factors, should be considered in mainstream models of the neurobiological mechanisms underlying memory.
Oestradiol signalling
Unlike neurotransmitters, which are stored in vesicles after synthesis for later release, steroid hormones are generated in response to a stimulus and released immediately. Sex-steroid biosynthesis begins with the catabolism of cholesterol to progesterone, which is then broken down into the androgens testosterone and androstenedione, which are subsequently converted to the oestrogens E2 and oestrone, respectively14,15. In this synthesis pathway, E2 is generated from testosterone via the enzyme aromatase. The primary sources of sex steroids in both sexes are the gonads, which synthesize progestogens, androgens and oestrogens in varying sex-dependent amounts (with more androgens in males, and more progestogens and oestrogens in females). Hormones synthesized in the gonads can exert paracrine effects on adjacent cells in the gonads, or endocrine effects on distant tissues via the bloodstream16. However, other tissues synthesize sex-steroid hormones14, including adipose tissue, the adrenal glands and numerous brain areas, including the hippocampus16–19 (Box 1). In the brain, steroids including E2 exert rapid paracrine or synaptocrine effects on neighbouring cells20,21.
Box 1 |. A role for hippocampally synthesized oestrogens in memory.
Although the gonads are a primary source of oestrogens in both sexes, oestrogens are synthesized in numerous tissues, including the brain. Of most relevance to learning and memory, the enzyme aromatase, which converts testosterone to 17β-oestradiol (E2), is widely expressed in the brain and has been shown to produce E2 locally in regions such as the hypothalamus and hippocampus17,18,200,237,238. In the hippocampus, neuron-derived de novo E2 supports multiple aspects of synaptic plasticity, including synaptogenesis and long-term potentiation (LTP)19,204,239,240. In hippocampal cultures from female rats, pharmacologically blocking de novo E2 synthesis with the aromatase inhibitor letrozole results in reduced spine density, decreased expression of synaptic proteins, and impaired LTP19,204,239.
Interestingly, neural E2 synthesis seems to be regulated by neuronal activity. For example, activation of NMDA receptors in cultured hippocampal neurons or in hippocampal slices increased E2 synthesis200,203, and exposure to a learning stimulus in ovariectomized mice increased de novo E2 synthesis in the hippocampus, an effect blocked by letrozole226. In vivo, systemic letrozole treatment decreases CA1 dendritic spine density and levels of hippocampal synaptic proteins in both ovary-intact and ovariectomized females240, demonstrating that neuron-derived E2 contributes to hippocampal synaptic plasticity regardless of other sources of the hormone.
The central importance of de novo hippocampal E2 synthesis in both sexes is illustrated by studies in which letrozole infusion into the dorsal hippocampus of gonadectomized male or female mice impaired consolidation of spatial and object recognition memory215,226. Similarly, hippocampal implants of the aromatase inhibitor ATD impaired spatial memory in male zebra finches241, suggesting that the importance of hippocampal E2 synthesis is conserved across species. Supporting this conclusion are recent data showing oral letrozole treatment impaired spatial working memory and reduced hippocampal intrinsic excitability in male and female marmosets228.
Aromatase outside of the hippocampus also seems to be important for memory consolidation, as infusion of letrozole into the perirhinal cortex of gonad-intact male mice impaired short- and long-term memory in an object placement memory task224. In humans, letrozole is an authority-approved treatment for oestrogen receptor (ER)-positive breast cancer; however, women taking letrozole may experience a number of adverse side effects that affect memory242. For example, women taking letrozole for ER-positive breast cancer exhibited episodic memory deficits, supporting the idea that brain-synthesized oestrogens are crucial in memory processes227.
In female mammals, circulating E2 is synthesized by the ovaries, which release fluctuating levels into the bloodstream as part of the reproductive cycle. During the human menstrual cycle and rodent oestrous cycle, oestrogen levels rise to promote follicle maturation in advance of ovulation, peak to stimulate ovulation and then return to baseline as the degenerating follicle secretes progesterone in preparation for implantation of a fertilized egg. In mice and rats, the oestrous cycle consists of four approximately 12–24-hour long stages: proestrus, oestrus, metoestrus and dioestrus22,23. E2 levels surge during proestrus and remain high through early oestrus, after which they plunge in late oestrus and remain low throughout metoestrus, rising again during late dioestrus. These fluctuations are reflected in the hippocampus, where E2 levels are substantially higher during proestrus than during other phases24, as is CA1 dendritic spine density2 and neurogenesis25. Interestingly, hippocampal E2 levels in ovariectomized rats are similar to those of ovary-intact rats in dioestrus and metoestrus24, reflecting hippocampal E2 synthesis in the absence of the ovaries. As discussed in Box 1, de novo hippocampal E2 synthesis is activity-dependent and crucial for rapid synaptic plasticity and memory formation. Oestrogen receptor (ER) expression is also influenced by the oestrous cycle26.
Classical and non-classical signalling.
Early research demonstrated that sex-steroid receptors act as transcription factors, dimerizing and translocating to the nucleus upon ligand activation to bind hormone response elements [G] and regulate gene transcription27. E2 binds two ER subtypes, ERα and ERβ, to accomplish this ‘classical’ mechanism of steroid hormone action, resulting in slow transcriptional changes that become evident within hours to days28. However, data from the 1960s revealed that E2 could increase cellular concentrations of cyclic AMP (cAMP) in uterine tissue within seconds29, a time course too rapid for classical genomic signalling. Similarly rapid effects on the order of seconds to minutes were later found in the brain, where E2 altered neuronal activity in the preoptic area of the hypothalamus30. More recent studies have characterized myriad ‘non-classical’, membrane-initiated mechanisms through which oestrogen rapidly triggers cell signalling and epigenetic processes to produce downstream alterations in gene expression, local protein synthesis, actin polymerization, synaptic physiology and dendritic spine morphology31–35.
Non-classical oestrogen signalling is initiated at the cell membrane and influences cellular processes through the activation of downstream second messenger pathways. The identities of the ERs responsible for this signalling have been controversial, as the early characterization of ERα and ERβ as purely nuclear receptors seemed to exclude a role in membrane-initiated signalling. However, technological advances have since permitted observation of these receptors at the cell membrane36–40, and studies using overexpression or deletion of ERα and ERβ demonstrated that these classical receptors are indeed responsible for many of the defining effects of rapid E2 action, including increased cAMP response-element binding protein (CREB) phosphorylation and activation of extracellular signal-regulated kinase–mitogen activated protein kinase (ERK–MAPK) signalling38,41,42. Evidence of the classical ERs (that is, ERα and ERβ) in dendritic spines and axon terminals of neurons43–45 suggests that these receptors could be directly involved in synaptic signalling.
How classical ERs initiate non-classical oestrogen signaling is still not thoroughly understood. The field continues to struggle with technical limitations, such as insufficient antibody validation46, in studying the subcellular localization of ERs, and many questions remain about the nature of the interaction between classical ERs and the cell membrane. The ERα and ERβ structures do not contain prototypical transmembrane domains47,48, indicating that these receptors are probably not fully inserted into the membrane. However, ERs undergo post-translational lipidation49–51 and associate with caveolins [G]52,53. Both of these factors contribute to the ability of ERs to localize to and associate with the cell membrane50,53–55.
In addition, how ERα and ERβ, which do not have inherent kinase activity, can interact with and activate downstream second messengers is unclear. One explanation is that ERs localized to the membrane co-opt the signalling machinery of other membrane receptors. At the membrane, ERα and ERβ can physically interact with and activate metabotropic glutamate receptors (mGluRs) independent of glutamate release, resulting in rapid ERK and CREB phosphorylation56,57. Functional coupling of ERs to mGluRs is observed throughout the nervous system and seems to contribute to the effects of E2 on memory consolidation, motivated behaviour and sexual receptivity [G]57–59. ERs also interact with receptor tyrosine kinases at the membrane, activating signalling at the insulin-like growth factor receptor 1 and the neurotrophin receptor TRKB through both rapid and gene-expression-dependent mechanisms60–63. Activation of these receptors subsequently engages neuroprotective and plasticity-related signalling cascades62–66. The integration of ERs into a signalling complex with other receptors, G proteins and kinases at the cell membrane is facilitated by multiple scaffold and adaptor proteins. These include: caveolins, which facilitate ER interaction with mGluRs as well as membrane association50,53,55; p130CAS, which couples ERs to kinases such as SRC and phosphoinositide 3-kinase (PI3K)67; and striatin, which binds caveolins and G proteins at the membrane68.
Non-classical E2 signalling also occurs through recently identified membrane-localized ERs. Although multiple putative ERs exist, the most well defined and studied is G protein-coupled oestrogen receptor (GPER; also known as GPR30). Characterized in the early 2000s, GPER is a 7-transmembrane-domain G-protein-coupled receptor activated by oestrogens69,70. It most frequently interacts with Gα/s subunits, leading to increased levels of cAMP and activation of the ERK, protein kinase A (PKA) and PI3K signalling pathways69–72. GPER is highly expressed in the brain, including in forebrain regions such as cortex, hippocampus and hypothalamus73,74, and pharmacological targeting of GPER has implicated it in mediating oestrogen effects on cognitive75–78, social79 and reproductive behaviours80,81. Given the considerable overlap of GPER and classical ER expression in regions like the hippocampus and PFC, as well as similarities in the outcomes of their activation, an intriguing question is whether these ER subtypes use parallel or distinct mechanisms to modulate neuronal function and behaviour. Current evidence suggests that the answer to this question is complex, with classical ERs and GPER often producing similar or complementary outcomes but through distinct molecular mechanisms78,82.
Intracellular cascades.
E2 activation of membrane-localized receptors triggers numerous intracellular signalling events that ultimately influence synaptic plasticity in cognitive brain regions. These downstream cellular and molecular mechanisms have been best elucidated in the hippocampus (Fig. 1). Activation of membrane-localized ERs in cultured hippocampal neurons leads to rapid calcium influx83,84 and activation of phospholipase C and adenylyl cyclase signalling via G protein activation56,85. In turn, these events activate small GTPases like RHOA35,82 and a host of kinase pathways, including the ERK–MAPK56,84,86–89, PI3K–AKT62,89–92, PKA93–95, protein kinase C (PKC)56,96 and JUN N-terminal kinase (JNK)78 pathways. The activation of these second messenger cascades influences processes that regulate synaptic structure and function, such as local protein synthesis, actin polymerization, ion channel dynamics and gene expression.
Fig. 1 |. Membrane-initiated oestrogen signalling and downstream intracellular events.
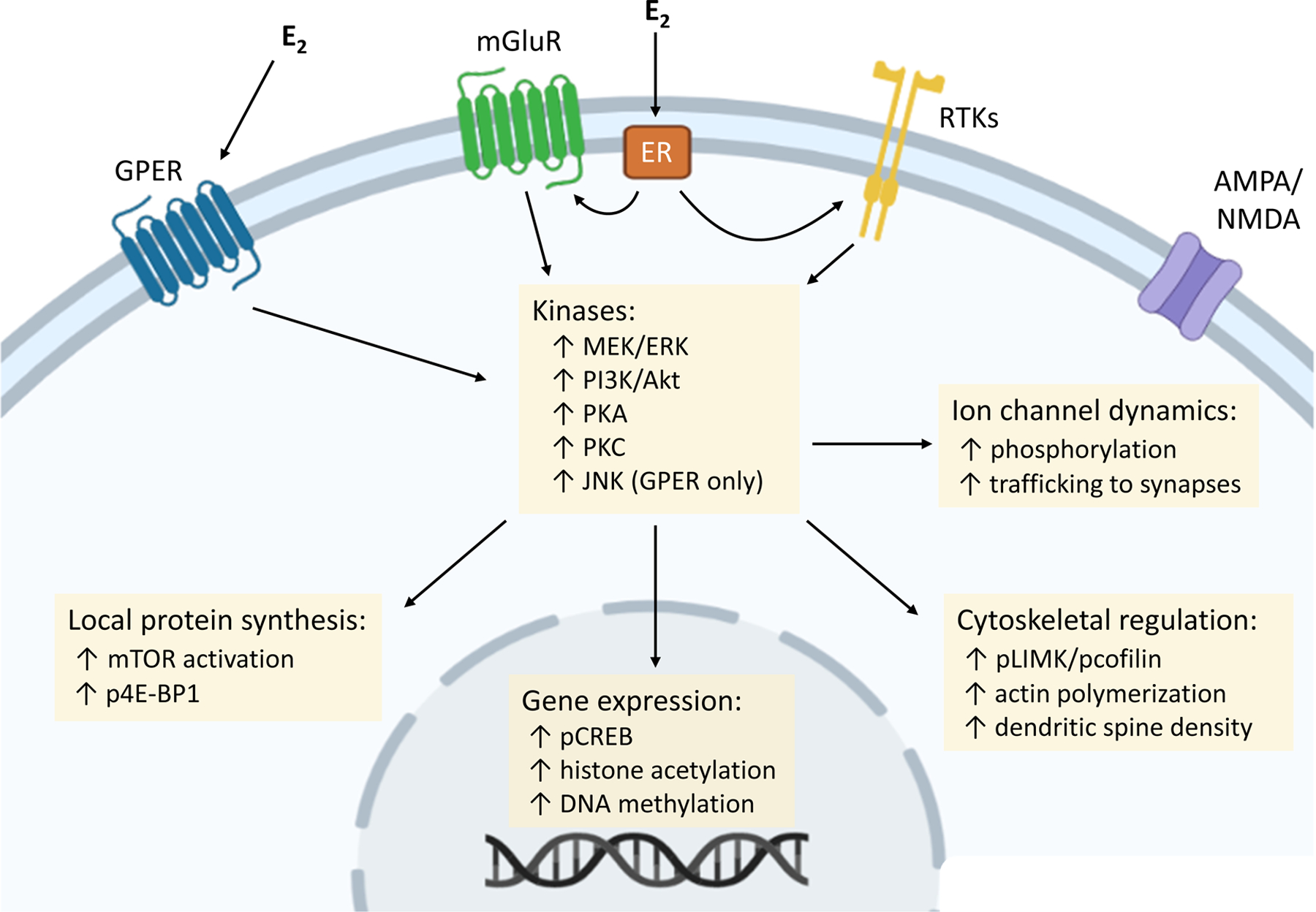
Intracellular processes are initiated by 17β-oestradiol (E2) binding to the G protein-coupled oestrogen receptor (GPER), or functional interaction between the canonical oestrogen receptors (ERα and ERβ) and other receptors located at the membrane (such as metabotropic glutamate receptors (mGluRs)). Several kinase cascades, key for the memory-enhancing effects of E2, rapidly increase activity in response to membrane-initiated signalling events. ERα and ERβ seem to trigger similar kinases, whereas GPER activates distinct signalling pathways such as JUN N-terminal kinase (JNK). In turn, kinase activity facilitates additional regulatory processes, including protein synthesis, ion channel phosphorylation and trafficking, gene expression, and cytoskeletal regulation. AMPAR, AMPA receptor; ERK, extracellular signal-regulated kinase; MEK, mitogen-activated protein kinase kinase; mTOR, mechanistic target of rapamycin; NMDAR, NMDA receptor; p4E-BP, phosphorylated 4E-binding protein 1; pCREB, phosphorylated cAMP response-element binding protein; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; PKC, protein kinase C; pLIMK, phosphorylated LIM kinase; RTK, receptor tyrosine kinase
E2 activation of ERK and AKT stimulates local protein translation by activating mechanistic target of rapamycin (mTOR) signalling and promoting the phosphorylation of translational initiation factors such as 4E-binding protein 1 (4E-BP1)32,82,97,98, and this new protein synthesis is required for E2-induced spinogenesis in the hippocampus99. Recruitment of actin-remodelling pathways also contributes to E2-induced structural plasticity: E2 activation of RHOA elicits phosphorylation of LIM kinase (LIMK) and its substrate, the actin-binding protein cofilin, to promote actin polymerization35,100–103. LIMK–cofilin activation and enhanced actin polymerization contribute to E2 effects on spine structure and synaptic transmission35,100,103. Moreover, although membrane-initiated E2 signalling is often referred to as ‘non-genomic’ to differentiate from the classical effects of E2, the intracellular signalling cascades initiated by surface ERs ultimately influence gene expression. E2 activation of ERK signalling rapidly induces phosphorylation of the transcription factor CREB56,83,88,104 and produces epigenetic modifications such as histone acetylation31,105,106, both of which increase expression of genes with neurotrophic effects, such as Bdnf106.
Synaptic function.
E2-induced changes in intracellular signalling ultimately alter synaptic function. In the hippocampus, the general result of E2 exposure is a rapid increase in excitatory neurotransmission. Applying E2 to hippocampal slices increases intrinsic excitability of CA1 pyramidal neurons107,108, enhances baseline excitatory neurotransmission5,6,35,109–111 and enhances long-term potentiation (LTP) at CA3–CA1 synapses35,109,111–114.
The effects of E2 on synaptic function correlate with increases in dendritic spine density5,110,113,114 and result primarily from regulation of ionotropic glutamate receptors. In hippocampal neurons, E2 influences the phosphorylation and subcellular trafficking of both AMPA receptors (AMPARs) and NMDA receptors (NMDARs)112,115–120. AMPAR-mediated excitatory postsynaptic potentials can be positively modulated by E2 6,35; however, many of the effects of E2 on synaptic plasticity rely on NMDAR-dependent mechanisms5,109,110,120,121. In particular, NMDARs containing the NR2B subunit are required for E2 enhancement of LTP120,121. E2 can also increase hippocampal excitability by suppressing inhibitory GABAergic signalling; however, this mechanism varies by sex122,123.
In sum, considerable data supports that the rapid cell-signalling mechanisms resulting from activation of surface-localized ERs enhance the multiple forms of synaptic plasticity thought to be cellular substrates for learning and memory. As we discuss below, the activation of intracellular signaling, and the resulting effects on neuronal structure and function, are necessary for the beneficial effects of E2 on memory in female rodents.
E2 modulation of memory in females
The numerous classical and non-classical effects of E2 on brain function provide abundant opportunities for this hormone to influence learning and memory. Copious data supporting a modulatory influence of E2 on hippocampal plasticity has led most rodent researchers to focus on forms of learning and memory mediated by the hippocampus, such as spatial- and object-based memories. However, oestrogens also regulate other types of learning and memory, including social learning, social discrimination, and fear memory mediated by the amygdala, perirhinal cortex, prefrontal cortex, and other regions. Thus, the sections below will focus not only on the hippocampus, but also on these brain regions as well.
Studies of ER-null mice114,124–126 or viral vectors to modulate ERα expression127 have suggested key roles for ERα and ERβ in various forms of memory. However, pharmacological manipulations are the most common method of interrogating roles for E2 and ERs in memory processes (Table 1). Although the compounds used afford better temporal specificity than genetic manipulations, they lack absolute specificity for one ER over the other. However, most can be used at doses that promote preferential binding to a single ER. Ongoing synthesis of more potent and selective ER compounds will undoubtedly help pinpoint discrete functions of specific ERs128.
Table 1.
Compounds used to demonstrate a role for specific estrogen receptors in memory
| Target | Action | Drug | Chemical Name | References |
|---|---|---|---|---|
| ERα | Agonist |
|
|
29, 125, 150, 177, 197 |
| Antagonist |
|
|
209, 210 | |
| ERβ | Agonist |
|
|
29, 99, 125, 150, 177, 197 |
| Antagonist |
|
|
125, 209, 210 | |
| ERα & ERβ | Antagonist |
|
|
62, 175, 197 |
| GPER | Agonist |
|
|
51, 53, 79, 148, 150, |
| Antagonist |
|
|
50, 53, 197 | |
| Aromatase | Enzymatic inhibitor |
|
|
194–200 |
Most studies examining the neuromodulatory role of E2 have been conducted in bilaterally ovariectomized female rodents, although some reports have examined memory in naturally cycling females or in males (with or without gonadectomy [G]). Although the widespread use of ovariectomized females has limitations that require cautious considerations about extrapolating effects to the natural cycle, this model has permitted more systematic investigations of oestrogen actions than is possible in the presence of daily ovarian hormone fluctuations. Much of this work has used young rodents (2–3 months old), but a rich literature also exists on the mnemonic effects of E2 in middle-aged rodents (for example, 14–19 months old) and aged rodents (older than 20 months)129,130 (Box 1). Besides animal age, there are several other experimental variables that influence behavioural and mechanistic outcomes. Timing of administration relative to testing, length of administration (for example, chronic (that is, over several days) or acute (that is, a single dose)) and dose can vary across studies, making direct comparisons across studies challenging. Moreover, when E2 is administered systemically, the site of E2 action is often unknown. Indeed, given that E2 is also synthesized in the brain (Box 2), differentiating the effects of brain-derived E2 from systemically administered E2, or from gonadal E2 in ovary-intact rodents, poses a unique challenge. In addition, age at ovariectomy and the duration between ovariectomy and first E2 treatment have also been demonstrated to affect oestrogenic regulation of memory129,130. The age at testing and duration of oestrogen deprivation are particularly important, as ER expression in the rodent hippocampus declines not only with age, but also several months after bilateral ovariectomy131,132. Combined, these data highlight the importance of experimental parameters when designing studies or interpreting data. The sections below mirror the preponderance of literature using young ovariectomized rodents (but see Box 2 and reviews129,133–135 for additional information relevant to ageing female and male rodents). As a comprehensive synthesis of all types of memory modulated by E2 is beyond the scope of this Review, the text below focuses on selected forms of memory and behavioural tasks that have proved especially useful to pinpoint the neural mechanisms underlying E2-facilitated memory.
Box 2 |. The effects of oestradiol in ageing rodents.
Ageing female rodents are frequently used to examine the effects of oestrogen deprivation and replacement during the transition to reproductive senescence (see refs 133,134,243 for recent reviews). In humans, menopause onset coincides with follicular depletion, whereas oestropause, the menopausal parallel in rodents, is driven by alterations in the function of the hypothalamus–pituitary–gonad axis244,245. Given this difference, researchers should be cautious when interpreting studies using rodent models of reproductive ageing. Nevertheless, the ageing rodent model has proved useful in understanding the effects of oestrogen deprivation, timing of hormone therapy administration and age-associated alterations in responsivity to 17β-oestradiol (E2).
Broadly, E2 deprivation as a consequence of ageing or artificial long-term ovarian hormone depletion lowers sensitivity to the memory-enhancing effects of E2. In spatial memory tasks such as the Morris water maze (MWM), systemic E2 administration typically enhances memory in middle-aged (14–19-month-old), but not aged (20-month-old or older), ovariectomized female rodents 246–249. However, E2 can still facilitate memory in aged females under certain experimental conditions. Chronic, high doses of E2 can elicit better memory in the MWM in aged ovariectomized female rats250 and in aged female mice251, and chronic E2 administration over 3 weeks enhanced object memory in aged ovariectomized female mice252.
Age and duration of oestrogen deprivation also modulate the effects of systemic E2 treatment on spatial reference and working memory. For example, chronic systemic E2 treatment enhanced spatial working memory in middle-aged ovariectomized rats in a water-motivated radial arm maze (RAM) task253. However, daily systemic injections of E2 given to ageing female mice ovariectomized at 17.5 months did not affect spatial reference or working memory in a water-motivated RAM task, whereas intermittent E2 treatment mimicking normal hormonal cycling impaired spatial reference and working memory254. As with the MWM, chronic E2 treatment enhanced spatial reference memory in aged ovariectomized rats, but only when rats were primed with additional acute injections of E2255. These data suggest not only an age-related loss of responsivity to the memory-enhancing effects of E2, but also a detrimental effect of E2 on memory in aged rodents. Supporting the idea that the duration of hormone loss affects responsivity to E2, systemic E2 implants administered to 12- and 17-month-old mice immediately after ovariectomy enhanced spatial working memory in a RAM256, whereas such E2 treatment given to 17-month-olds 5 months after ovariectomy no longer enhanced spatial working memory. These data support the well-accepted notion that the ageing brain is less responsive to E2 after long periods of oestrogen deprivation.
As in the maze tasks, ageing female rodents or rodents subjected to delayed oestrogen replacement post-ovariectomy exhibit diminished sensitivity to the beneficial effects of E2 in object memory consolidation. For example, rats treated with systemic E2 9 or 15 months post-ovariectomy exhibited intact memory for a previously seen object91. However, increasing the treatment delay to 19 months post-ovariectomy eliminated the memory-facilitating effects of E2162, consistent with other findings that E2 typically enhances object recognition and object placement memory in middle-aged, but not aged, female rodents248,257. Combined, these data suggest that E2 is less efficacious when given to aged rodents, especially when post-ovariectomy E2 treatment is delayed.
Several potential mechanisms may underlie decreased sensitivity to E2 in older rodents. For example, E2-induced hippocampal transcription is reduced in aged female rodents258, and several studies suggest that the expression of oestrogen receptors ERα and ERβ diminishes with age131,259,260. The precise mechanisms underlying decreased responsivity to E2 in advanced age remain to be determined.
Spatial and object memory
The tasks most commonly used to assess hippocampal-dependent spatial and object memory include the Morris water maze (MWM), the radial arm maze (RAM), and object recognition (OR) and object placement (OP; also known as object location) tasks (Fig. 2). In the MWM and RAM, animals use extra-maze cues to traverse a round or wheel-shaped maze to escape from water or locate a food or water reward. Memory in these tasks is typically measured across multiple days, enabling within- and between-subject performance to be assessed over time. Whereas both the MWM and RAM involve explicit motivational stimuli to compel performance, the OR and OP tasks rely on the animal’s inherent proclivity for novelty. That is, mice and rats will spend more time investigating a novel object, or a familiar object in a novel location, than a previously investigated object in a familiar location. One advantage of these single-trial learning tasks is that they permit learning assessment while minimizing appetitive or aversive confounds. Moreover, the rapidity with which object tasks are learned allows effects of acute hormone administration to be associated with specific neural events and discrete phases of memory formation (including acquisition [G], consolidation [G] and retention [G]).
Fig. 2 |. Behavioural approaches to studying oestrogenic effects on memory.
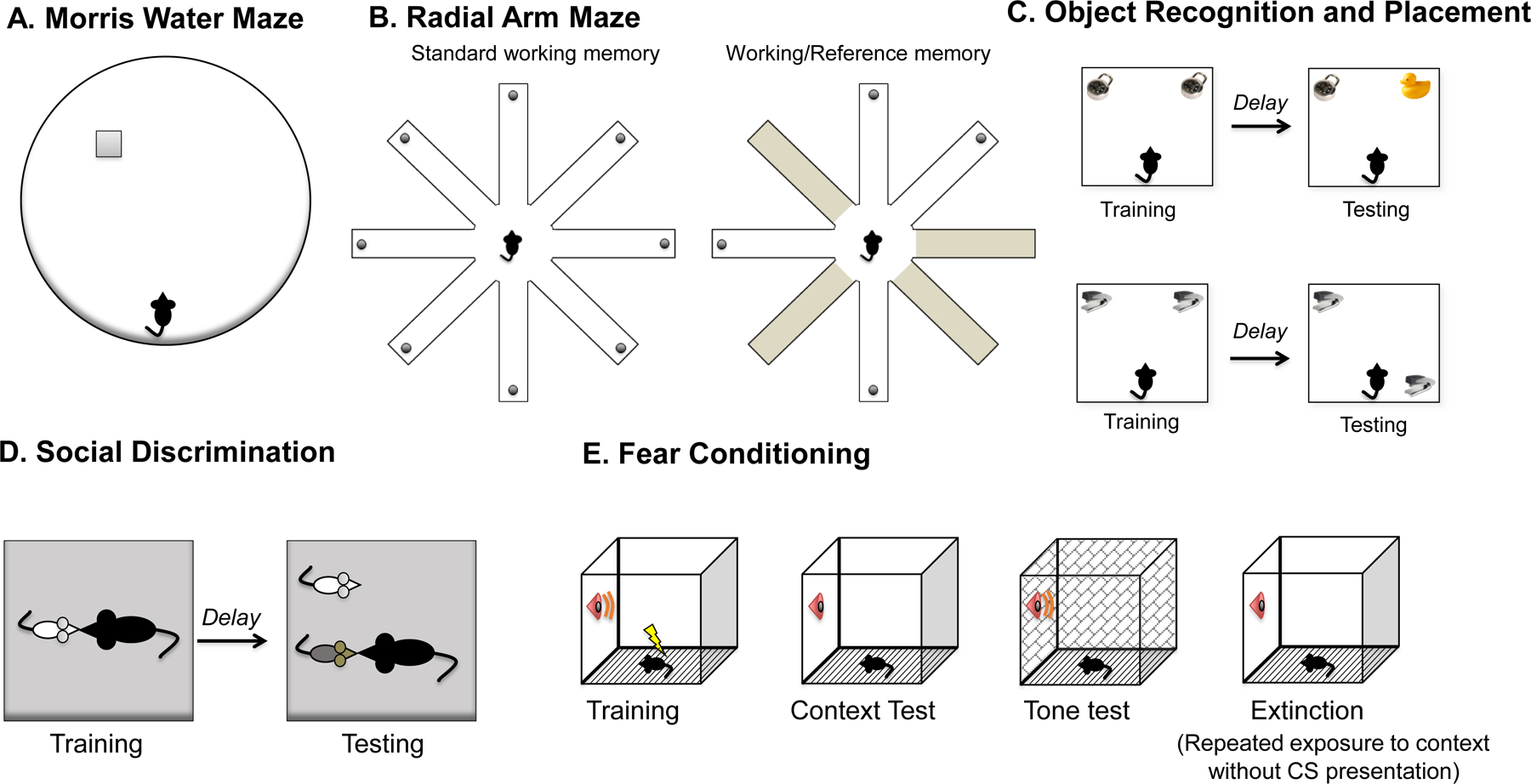
a | The Morris water maze tests a rodent’s ability to navigate in space and remember the location of spatial cues in the environment229,230. Rodents are placed in a large round pool of water made opaque with nontoxic paint or powdered milk, and must use extramaze spatial cues to navigate to a platform hidden just below the surface of the water229,230. Measures of performance include time to reach the platform, distance swum and swim speed. During probe trials in which the hidden platform is inaccessible to subjects for a portion of, or throughout, the trial, memory for the platform location can be assessed by measuring the number of times that animals cross the platform location and/or the time spent in close proximity to the location of the platform. b | The radial arm maze also tests spatial navigation abilities. Food- or water-restricted subjects traverse a wheel-shaped maze in which they must retrieve food or water rewards from the ends of 8 or 12 long arms that radiate from a round central platform231,232. Rewards are not replaced, so animals should visit each arm only once. In the standard version, all arms are baited to measure working memory, which is memory for information that changes from trial to trial. In a common variation, half of the arms are baited to measure working memory and half are never baited to measure reference memory, or memory for information that does not change from trial to trial. c | Object recognition and object placement tasks typically consist of a training trial, during which animals explore objects in an open field for 5–20 minutes, followed by a delay (minutes to days) and a single testing phase, during which a new object is introduced (object recognition) or a training object is moved (object placement)233,234. Because rodents are drawn to novelty, animals that remember the identity and location of the training objects will spend more time than chance with a novel of moved object during testing. d | Social recognition can be tested using a habituation–dishabituation task or a social discrimination task. In habituation–dishabituation, subjects are repeatedly exposed to the same stimulus conspecific, leading to a decrease in investigative behavior (habituation) as the stimulus animal becomes known. Animals are then exposed to a novel conspecific, and, if social recognition memory is intact, investigative behaviour returns (dishabituation). In social discrimination, animals undergo a test trial where they are simultaneously exposed to a familiar conspecific, seen previously in habituation trials, and a novel conspecific. Increased investigative behaviour of the novel conspecific is indicative of intact social recognition memory. e | Fear memory is most commonly studied using classical conditioning paradigms that pair a stimulus (a cue or context) with a foot shock, generating a learned association that leads to fear responses (such as freezing) upon subsequent presentations of the conditioned stimulus (CS). Cued and contextual fear conditioning differ in their underlying neurocircuitry, with cued fear being independent of hippocampal function, and contextual fear requiring hippocampal input235. Extinction can be tested with repeated presentation of the context in the absence of CS presentation236. Figure adapted with permission from Frick and Fortress (2015)236.
Morris water maze.
Studies using the MWM to test oestrogenic regulation of spatial reference memory present conflicting data on the role of E2 in this form of memory. For example, chronic systemic E2 given to ovariectomized rats or high E2 levels during the rat oestrous cycle can impair spatial learning and memory136–138, whereas high E2 levels in cycling mice were associated with enhanced spatial learning139, and ovariectomized rats given systemic E2 72 and 48 hours before testing exhibited better memory for a hidden platform140. Moreover, high plasma E2 in ovary-intact female meadow voles correlated with longer latency to locate the escape platform during acquisition trials, whereas lower plasma E2 was associated with better learning141. Given the varied reported effects of E2 level on spatial MWM learning in different rodent studies, E2 dose and species may determine how E2 affects spatial memory in this task.
Although few studies have examined the acute effects of intracranially administered E2 on MWM performance, existing research suggests beneficial effects. For example, E2 infusion into the dorsal hippocampus of young ovariectomized rats immediately, but not 3 hours, after spatial MWM training facilitates retention 24 hours later, indicating that E2 specifically enhances spatial memory consolidation142.
Radial arm maze.
The RAM allows researchers to disambiguate effects of E2 on spatial reference memory [G] and working memory (Fig. 2). Broadly, chronic systemic E2 injections in ovariectomized rats enhance spatial working memory in the RAM, an effect that manifests most prominently after several days of E2 administration143–145. Consistent with this, chronic systemic E2 treatment in rats rescues ovariectomy-induced deficits in working memory, but not reference memory146. Similarly, intracerebroventricular E2 infusion reduces working memory errors in ovariectomized rats147. As such, E2 appears to facilitate spatial working memory, but not spatial reference memory, in the RAM among young ovariectomized rodents.
Many brain regions, including the PFC, are required to coordinate the effects of E2 in the RAM, although the effects of E2 in these brain regions differ by dose. For example, spatial working memory in ovariectomized rats was facilitated by infusion of a high, but not a low, dose of E2 into the PFC, and infusion of a low, but not high, dose into the dorsal hippocampus148. Thus, effects of E2 on spatial working memory in the RAM may depend on both dose and brain region148.
Single-trial learning.
It is difficult to disaggregate the rapid non-classical effects of E2 from its long-term classical effects in tasks such as the MWM and RAM, because learning in these tasks requires multiple trials across several days. Moreover, both tasks involve motivational components (for example, escape stress and thirst or hunger) to compel performance, which may alter how the brain responds to E2. By contrast, single-trial learning tasks such as OR and OP afford more precise assessment of the neural mechanisms through which E2 facilitates memory formation. As illustrated below, findings from studies using these tasks to assess the effects of E2 on spatial and recognition memory are considerably more consistent than those from maze studies.
As in the MWM and RAM, memory in the OR and OP tasks is negatively affected by ovariectomy149,150. However, unlike in the MWM and RAM, exogenous E2 consistently improves spatial and object recognition memory in the object tasks. For example, systemic E2 given immediately post-training enhances memory consolidation in the OR and OP tasks among young ovariectomized rats and mice.151–153. However, systemic E2 does not enhance memory when administered 2 hours after training152, suggesting that the window in which E2 exerts pro-cognitive effects on OR and OP memory consolidation is time-limited and probably dependent on non-classical mechanisms.
E2 infused immediately, but not 3 hours, post-training into the dorsal hippocampus similarly enhances memory consolidation in the OR and OP tasks, indicating that the dorsal hippocampus has a crucial role in mediating the rapid effects of E2 on memory consolidation57,87,154,155. The perirhinal cortex also plays a part, as E2 infused immediately post-training into the perirhinal cortex of ovariectomized rats enhanced their preference for a novel object over a familiar object compared with vehicle-treated controls156. However, perirhinal E2 infusions also impaired object memory consolidation in a delayed-non-match-to-sample task [G], complicating the role of this brain region in mediating the influence of E2 on OR memory156,157. Overall, these data suggest that E2 generally enhances OR and OP memory consolidation in young ovariectomized rodents when it is administered either pre-training or immediately post-training.
Molecular mechanisms.
The memory-enhancing effects of E2 in the hippocampus involve numerous cellular and molecular events in female rodents. Several cell-signalling cascades are rapidly activated in response to E2, including the ERK, mTOR and PI3K–AKT pathways; activity in these pathways is required for E2 to enhance memory consolidation in the OR and OP tasks among ovariectomized mice57,87,91,97,158. Within the dorsal hippocampus, these signalling cascades are initiated by E2 binding to hippocampal ERα and ERβ, but interestingly, not to GPER57,78. Indeed, although GPER activation in the dorsal hippocampus enhances OR and OP memory consolidation in ovariectomized females, these effects are independent of E2 signalling78.
The ability of E2 to activate dorsal hippocampal cell signalling is closely tied to its ability to mediate epigenetic processes associated with facilitated memory. ERK activation is necessary for E2 to increase acetylation of histone H3 within the dorsal hippocampus of ovariectomized mice105,106. Dorsal hippocampal E2 infusion also decreases levels of histone deacetylases 2 and 3 in the hippocampus of ovariectomized mice, thereby providing a mechanism for increased H3 acetylation in the E2-treated hippocampus105,106. Importantly, infusion of a histone acetylation inhibitor prevents E2 from enhancing OR memory consolidation in ovariectomized mice, demonstrating an crucial role for histone acetylation in the mnemonic effects of E231,105.
In ovariectomized females, systemic E2159 and intrahippocampally administered E2 increase the dendritic spine density of CA1 pyramidal neurons, an effect dependent on ERK and mTOR signalling99. Consistent with these data, elevated E2 robustly influences hippocampal synaptic plasticity by facilitating LTP121,160,161, which correlates with enhanced memory in hippocampus-dependent tasks120,162.
Although much remains to be learned, researchers are well on their way towards characterizing the neurobiological mechanisms through which E2 modulates spatial and object memory consolidation.
Social cognition
The term ‘social cognition’ encompasses various processes, but perhaps the most well studied in animals is social recognition. Memory for conspecifics and past social interactions allows for the formation of meaningful relationships and selection of appropriate behavioural responses in future social interactions, which are crucial abilities in social species163. Social discrimination is heavily influenced by neuroendocrine mechanisms; however, much of this research has focused on the neuropeptides oxytocin and vasopressin, so only more recently have sex-steroid hormones become appreciated as modulators of social memory164.
Ovary-intact female mice trained on a habituation–dishabituation task (Fig. 2) during proestrus show better discrimination of a novel conspecific 24 hours later relative to females trained in dioestrus, suggesting that elevated E2 during training may improve long-term social recognition165. Other studies specifically testing effects of exogenous E2 on social recognition in ovariectomized rodents have found that systemic E2 given before training improves social memory in a habituation–dishabituation task166–168.
Many of these studies use chronic E2 replacement before training and then test memory at extended timepoints, suggesting that classical actions of E2 are involved. Indeed, E2 action at ERα increases transcription of the oxytocin receptor in the medial amygdala (MeA)169, a region where oxytocin signalling is crucial for social recognition memory170. However, rapid membrane-initiated E2 signalling also contributes substantially to these effects. In a modified social discrimination task where acquisition and recall occur within 40 minutes of drug administration, systemic E2 enhanced social recognition memory in ovariectomized rats33. The relatively short period between training and testing makes it unlikely that classical E2 mechanisms have a role in this effect, thereby implicating membrane-initiated signalling as the key mediator. Furthermore, rapid E2-induced CA1 plasticity correlates with this memory enhancement; within 40 minutes of systemic or intracranial administration, E2 increased CA1 dendritic spine density in ovariectomized mice, which was surprisingly associated with reduced AMPAR-mediated signalling33,119. This change in excitatory signalling may reflect the generation of new silent synapses [G].
The effects of E2 on social recognition memory are mediated by both classical receptors and GPER. ERα plays a particularly important role, with several studies reporting that ERα-null or ERα-deficient female rodents show impaired social recognition memory165,169,171,172. Systemic injection of the ERα-selective agonist PPT (propyl pyrazole triol) improved memory among ovariectomized mice in a social discrimination task within 40 minutes, suggesting that rapid action of membrane-localized ERα is important for this effect173. The GPER agonist G1 induces similarly rapid improvements in social recognition memory when systemically injected in ovariectomized mice77. However, whether ERα and GPER initiate similar cellular and molecular mechanisms to facilitate social recognition memory is unclear. Interestingly, the facilitative effects of ERα and GPER on social recognition do not seem to extend fully to ERβ. Social recognition memory in female ERβ-null mice is either unaffected or only modestly impaired165,169,171, and treatment with the ERβ-selective agonist DPN (diarylpropionitrile) in ovariectomized mice either impairs or does not improve social recognition memory173.
Similar to spatial and object recognition memory, the hippocampus is a critical locus of E2 action for social recognition memory. Infusions of E2, PPT or G1 directly into the dorsal hippocampus of ovariectomized mice induce a rapid enhancement of social recognition memory that is correlated with increased CA1 spine density and modulation of excitatory neurotransmission119,174. However, E2 also modulates the function of other brain regions crucial for social recognition memory. For example, knockdown of ERα in the MeA of ovariectomized female rats abolishes social recognition memory172, and infusion of ERα and GPER agonists into the MeA of ovariectomized mice rapidly enhances social recognition175. An integrated understanding of how E2 works across multiple brain regions, receptor subtypes and signalling mechanisms to influence social cognition and recognition behaviour remains an area ripe for future research.
Fear memory
The study of fear conditioning and its underlying circuitry and mechanisms is a cornerstone of the learning and memory field. Nevertheless, despite its prominence, researchers have until relatively recently largely ignored how sex-steroid hormones such as E2 affect fear learning. Existing work reveals an interesting, and at times contradictory, role for E2 in modulating both the acquisition and extinction [G] of fear memory.
Fear acquisition.
Studies examining how E2 modulates fear acquisition report both enhancing and impairing effects. For example, chronic systemic E2 enhanced cued and context-dependent fear conditioning in ovariectomized mice176,177, and fear-potentiated startle in ovariectomized rats178, suggesting that E2 enhances acquisition of fear learning. However, other findings reveal impaired contextual fear conditioning [G] (CFC) in proestrus rats relative to males or rats in oestrus179. Similarly, ovary-intact or ovariectomized rats given E2 for 2 days before CFC froze less than did males or untreated ovariectomized rats180, suggesting E2 may impair fear learning.
These seemingly contradictory findings probably result from differences in experimental approach, with dose and timing of E2 administration as important variables. High-dose systemic E2 days or weeks before CFC enhanced acquisition in ovariectomized mice, with lower doses of E2 having no effect181,182. By contrast, when E2 was injected into ovariectomized rats 30 minutes before CFC training, high doses impaired acquisition, whereas a low dose enhanced learning183. These results suggest important differences in how E2 dose and treatment duration interact to impact fear learning, but further research is needed to more clearly define these relationships.
Fear extinction.
Research on the role of E2 in modulating fear extinction paints a clearer picture than does the fear acquisition literature, with evidence from passive avoidance tasks184, conditioned taste aversion185 and both cued and conditioned fear conditioning186–188 supporting the conclusion that E2 improves extinction learning. In ovary-intact female rats, fear-extinction training during proestrus produces more rapid learning across trials186 and reduced freezing during extinction recall testing187 compared with extinction during metoestrus. E2 seems to be both necessary and sufficient for these effects, as systemic E2 given to metoestrus or ovariectomized rats enhances extinction learning186,187, whereas systemic blockade of ERs in proestrus rats impairs extinction recall187. Similarly, reducing circulating E2 via hormonal contraceptives impairs extinction recall in cycling female rats189.
As with fear acquisition, the influence of E2 on extinction is dose-dependent. In cycling female rats, E2 exhibits an inverted-U dose–response effect on extinction learning, such that rats with very low (untreated metoestrus) or very high (proestrus or metoestrus given high dose E2) levels of E2 exhibited poor extinction recall, and rats with moderate E2 levels (untreated proestrus or metoestrus given low dose E2) extinguished well190.
ERβ is an important mediator of the effects of E2 on fear extinction. Systemic injection of the ERβ-selective agonist DPN before extinction training improves extinction learning in ovariectomized or metoestrus rats, whereas the ERα-selective agonist PPT has no effect186,188. Infusion of DPN into the dorsal hippocampus of ovariectomized rats enhanced contextual fear extinction learning, suggesting that this brain region is an important locus for ERβ activity in this paradigm186. However, E2 has more pervasive actions in the neurocircuitry of fear extinction beyond the hippocampus. The amygdala, a central region in fear extinction, shows widespread expression of ER subtypes16 and structural and synaptic plasticity following E2 treatment191,192. The infralimbic cortex (IL) sends excitatory projections that regulate amygdala subregions during extinction193, and is also sensitive to oestrogens194. E2 seems to modulate the activity and connectivity of these regions during extinction recall; studies using FOS [G] as a marker of neuronal activity report that metoestrus rats treated with E2 displayed less amygdalar activation188,195 and greater IL activation188 following extinction recall than did untreated controls. Both the oestrous-cycle phase and exogenous E2 treatment influence the activity of IL–amygdala projections in female rats195,196, suggesting that E2 increases IL-driven activation of an inhibitory circuitry in the amygdala that reduces fear responses.
Fear generalization.
In addition to fear acquisition and extinction, E2 also influences fear generalization [G]. Ovariectomized rats treated with systemic E2 show increased generalization of fear to neutral contexts relative to untreated controls197. Similar to extinction, this effect seems to depend primarily on hippocampal ERβ signalling, as infusion of the ERβ-selective agonist DPN, but not ERα-selective PPT, into the hippocampus of ovariectomized rats increases fear generalization198.
That E2 can enhance both fear expression (via acquisition and generalization) as well as extinction may at first seem contradictory, but is not surprising given that these processes share certain underlying molecular mechanisms. Moreover, fear acquisition, generalization and extinction all require new learning. Thus, the effects of E2 on all aspects of fear learning highlight its actions as a general promoter of learning and memory.
Together with findings from the spatial, object and social memory tasks discussed above, the fear memory data suggest that E2 is uniquely positioned as a general neuromodulator that broadly facilitates new learning of many sorts.
E2 modulation of memory in males
Although oestrogens are often (incorrectly) considered ‘female’ hormones, accumulating research indicates a primary role for E2 in enhancing cognition in male rodents. Indeed, E2 levels in the male rat hippocampus are higher than in that of cycling females199,200. Emerging findings, reviewed below, imply that E2 may influence memory via distinct molecular mechanisms in males and females. Why might these differences matter if the beneficial effects on memory are similar in both sexes? From a drug discovery standpoint, these data suggest the potential need to develop sex-specific treatments for memory dysfunction in humans. Treatments that target mechanisms through which E2 modulates memory in one sex (for example, ERK in females), but not in the other, are bound for failure in half the population. Additional research to determine which oestrogenic mechanisms are sex-specific and which are common to both sexes will substantially advance the development of effective memory therapeutics for both men and women.
Sex differences in hippocampal E2 signalling.
In the hippocampus, E2 acts as a neuromodulator in both males and females, but an emerging literature has found notable sex differences in how E2 influences hippocampal neurotransmission and the molecular underpinnings of these effects. In both sexes, E2 potentiates excitatory neurotransmission in the hippocampus6,35,111, and although some molecules and pathways, such as SRC, ERK–MAPK, calcium/calmodulin-dependent protein kinase II (CAMKII) and TRKB signalling, contribute to these effects in both sexes35,201,202, others are sex-specific. For example, potentiation of excitatory post-synaptic currents and LTP in the hippocampus depend on PKA in female rats, but not in males202. The sexes also differ in the ER subtypes that mediate neural excitation, as hippocampal glutamatergic neurotransmission is regulated presynaptically by ERβ in females and ERα in males, and postsynaptically by GPER in females and ERβ in males111. Other work has similarly shown differential contributions of ERα versus ERβ in facilitating LTP between males and females35,201. Differing roles of ER subtypes in modulating excitatory signalling may arise from sex differences in subcellular ER localization, which in turn could lead to sex-specific recruitment of signalling kinases by E2167,201.
Unlike excitatory signalling, inhibitory hippocampal neurotransmission seems to be modulated by E2 only in females and through a sex-specific mechanism. In the ovariectomized female rat hippocampus, ERα activates post-synaptic mGluR1 signalling to stimulate endocannabinoid release, which inhibits presynaptic GABAergic terminals122. The functional coupling of ERs with mGluR subtypes has previously been shown to occur only in females56 and further investigation has found that although ER–mGluR complexes can exist in both sexes, E2 only activates mGluR-dependent signalling in females123.
A similar sex difference has been found with locally synthesized E2 in the hippocampus. Although male and female rodents both express aromatase and synthesize E2 in the hippocampus17,200,203, systemic aromatase inhibition reduces hippocampal synapse number and severely impairs LTP induction in female mice, but has no effect on synapse number and only modestly reduces LTP in males204. Similarly, aromatase-null female mice were shown to have reduced hippocampal spine density compared with wild-type female mice, whereas spine density was unaffected in male aromatase-null mice205. However, in a forebrain-specific aromatase-null mouse, both males and females exhibited reduced hippocampal spine density206. Understanding the cellular and molecular consequences of local oestrogen synthesis in the male hippocampus will require further investigation207.
Oestrogenic modulation of cognition in males.
Despite differences in the mechanisms of E2 signalling in cognition-related regions of the brain, E2 exerts similar mnemonic benefits in males and females. Systemic injection or dorsal hippocampal infusion of E2 given immediately post-training enhances memory consolidation in the OR and OP tasks in gonad-intact and gonadectomized male rats and mice208,209. Notably, sex differences in the mechanisms that drive the beneficial effects of E2 on these tasks have been found: in contrast to its central importance to the memory-enhancing effects of E2 in ovariectomized mice, dorsal hippocampal ERK phosphorylation is not necessary for E2 to facilitate spatial and object memory consolidation in male mice, regardless of gonadal status209. In the MWM, gonad-intact adult male rats and aged male mice treated with systemic E2 immediately post-training exhibited enhanced spatial memory compared with vehicle-treated males210,211. Gonad-intact adult males receiving chronic E2 also made fewer working memory errors in the RAM than did vehicle-treated controls212. Local synthesis of oestrogens is also important for memory processes in male rodents, as aromatase-null male mice exhibit deficits in social recognition213. Furthermore, acute oral aromatase inhibition increases errors in a working memory task in male rats214, and acute intrahippocampal aromatase inhibition prevents object and spatial memory consolidation in gonadectomized male mice215. As such, the data thus far suggest that hippocampally-synthesized E2 is important for memory formation in male rodents, as has been observed in female rodents. Although much more remains to be learned, these selected findings support a beneficial effect of E2 on memory formation in male rodents.
Conclusions and future directions
This Review has illustrated some of the myriad ways in which E2, acting in several brain regions via multiple ERs, can facilitate numerous forms of learning and memory (Fig. 3). Three decades of research have demonstrated that E2 is a potent regulator of the neural mechanisms that are critically important for memory formation, including cell signalling, gene expression, protein synthesis, extrinsic and intrinsic excitability, dendritic spine morphology and neurogenesis. Thus, researchers studying the neurobiology of learning and memory should recognize oestrogens as essential neuromodulators akin to other well-accepted modulatory factors such as glucocorticoids and growth factors.
Fig. 3 |. Summary of oestrogenic actions on memory processes.
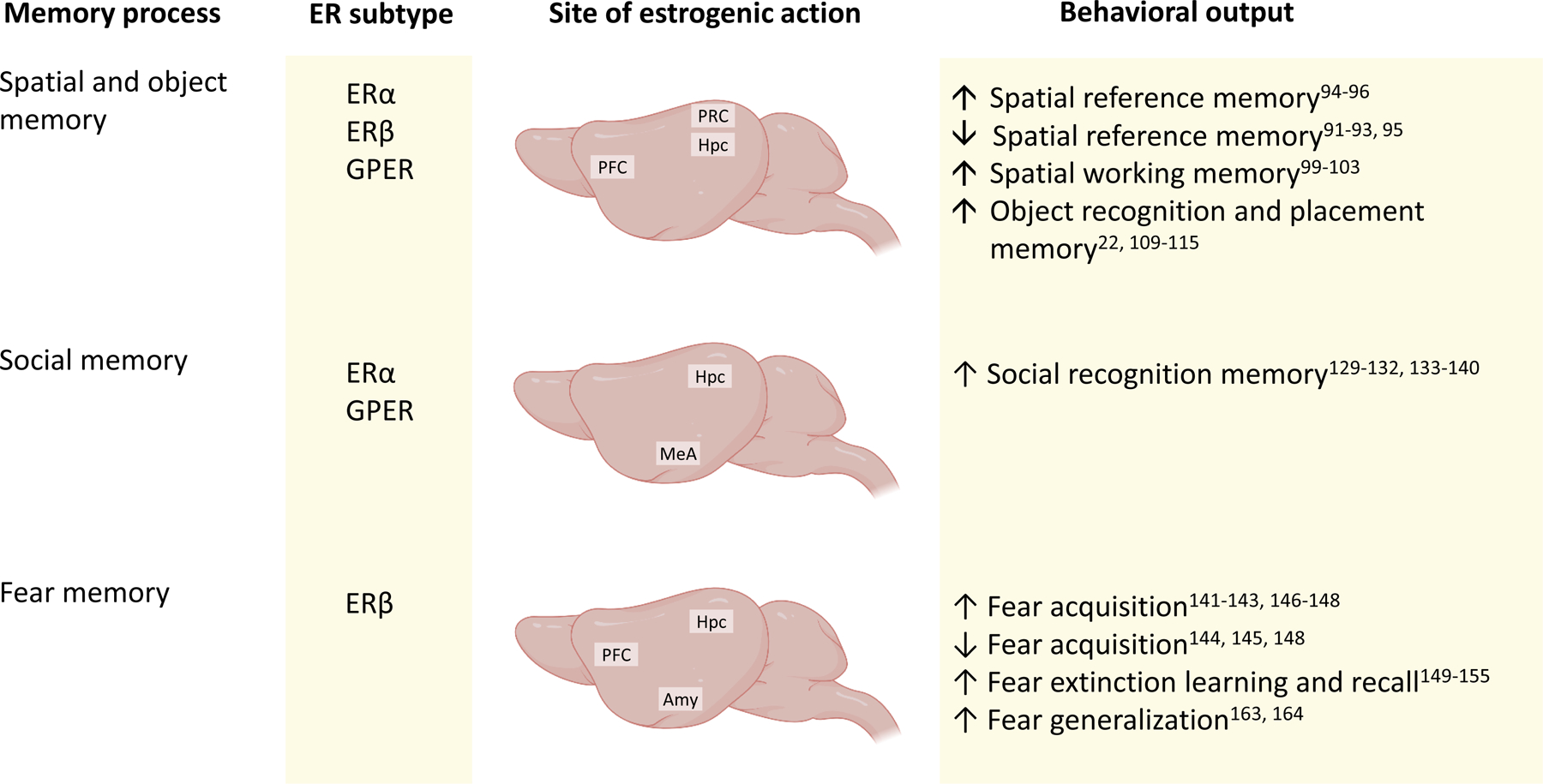
This schematic shows the receptors and brain regions involved in the effects of 17β-oestradiol (E2) on memory processes, and the behavioural output of the actions of E2 in young female rodents. Spatial reference memory142, working memory148 and object memory57,87,154,155,173 are facilitated by E2 (but see discussion in main text), and are largely dependent on the hippocampus (HPC) and prefrontal cortex (PFC). The perirhinal cortex (PRC) is also involved in E2-mediated object recognition memory156,157. Social recognition memory is facilitated by oestrogen receptor-α (ERα) and G protein-coupled oestrogen receptor (GPER) signalling in the HPC and medial amygdala (MeA)119,172–174. Fear acquisition, fear extinction learning and recall, and fear generalization are regulated by ERβ in the PFC, amygdala (Amy), and HPC186,188,195,196,198.
Widespread acceptance of oestrogenic neuromodulation may have been historically slow to take hold because of the strong association between oestrogens and female reproduction, which has led to the erroneous perceptions that oestrogens are ‘female’ hormones and that cyclic oestrogen fluctuations in females confound experimental outcomes due to increased variability. In fact, oestrogens are important modulators of memory and neural function in both sexes216,217. Depending on the behaviour being assessed, ovary-intact females do not necessarily exhibit more behavioural variability than males218, which argues for greater consideration of the modulatory influence of oestrogens in females and in males. Future progress on this issue may stem from the US National Institutes of Health’s 2016 policy requiring vertebrate animal researchers to consider sex as a biological variable219–221, as more explicit comparisons between the sexes could lead to new insights about hormonal regulation of cognition. Although some investigators were initially resistant to this policy, attitudes towards the requirement are improving222. Greater appreciation of oestrogens as neuromodulators that exert wide-ranging effects in all individuals — not just females — is crucial for the advancement of both basic and clinical science.
To further advance knowledge about oestrogenic modulation in both sexes, future studies should exploit newer technologies including single-cell sequencing, ‘omics’-level analyses, and targeted genetic manipulations to pinpoint crucial molecules and cellular processes that underlie oestrogenic facilitation of learning and memory. For example, a multiplexed CRISPR–Cas9 gene-editing approach could be used to simultaneously target multiple oestrogen-responsive genes implicated in neurodegenerative disease to understand their role in memory function223. Future research must also evolve from a focus on oestrogenic effects in individual brain regions to addressing how oestrogens concurrently influence multiple brain areas within memory circuits. Recent work using multiplexed chemogenetic silencing has shown that the memory-enhancing effects of E2 in the dorsal hippocampus requires concurrent activity of the dorsal hippocampus and PFC155. Thus, determining how brain regions interact synergistically to support oestrogen-mediated memory processes is a crucial next step for the field. As part of this approach, researchers should also consider cell-type specificity, as memory-modulating effects of E2 on cell types such as inhibitory neurons and glial cells have been overshadowed by a predominant focus on excitatory neurons.
By leveraging new technologies and asking circuit-level and cell-type-specific questions in both males and females, scientists will discover fundamental new insights into the ways in which E2-induced modulation of brain function influences memory formation. Given the dearth of effective treatments for memory dysfunction in various disorders, this information could provide valuable new avenues for therapeutic development that benefits both sexes.
Acknowledgements
The Frick lab is supported by the US National Institutes of Health (R01MH107886, 2R15GM118304-02, F31MH118822, F32MH118782), the Alzheimer’s Association (SAGA-17-419092), the University of Wisconsin System, and the University of Wisconsin-Milwaukee Research Foundation, Office of Undergraduate Research, and College of Letters and Science.
Glossary terms
- Ovariectomized
Ovariectomy involves surgical removal of the ovaries to eliminate ovarian hormone cycling. Subjects that have undergone ovariectomy are considered ovariectomized.
- Hormone response elements
Short DNA sequence within the promoter region of a gene that binds a hormone receptor complex to enable gene transcription.
- Caveolins
Integral membrane proteins that form functional microdomains of receptors and their associated signaling proteins at the plasma membrane.
- Sexual receptivity
A positive state of responsivity towards the initiation of sexual behaviour by another individual. Often indicated by a species-specific mating posture.
- Gonadectomy
Surgical removal of the gonads (ovaries or testes); because ovariectomy is the preferred term for females, gonadectomy is most commonly used for males.
- Acquisition
A process through which information is learned through physical or sensory interaction with environmental stimuli.
- Consolidation
Process through which learned information is encoded and stored to form a memory that can be recalled at a later time.
- Retention
Storage of acquired and consolidated information that enables subsequent recall or retrieval of the information.
- Spatial reference memory
Memory for locations that do not change over time — for example, the layout of buildings on a college campus — used for navigating through an environment.
- Spatial working memory
Memory for locations that change over time — for example, the locations of your keys or your car in your campus parking lot.
- Delayed non-match-to-sample task
Test of memory for items that differ from an initial stimulus array, assessed at some delay after original stimulus presentation.
- Silent synapses
Immature synapses containing few AMPA receptors, which could allow for greater synaptic potentiation and learning facilitation upon interaction with a training stimulus.
- Extinction
Process whereby a learned association between two stimuli (for example, shock occurs in context A) becomes unlearned through repetitive exposure to one stimulus (context) without the other (shock).
- Contextual fear conditioning
Model of fear learning in which repeated exposure to footshocks in one context eventually elicits fear (freezing) to the context in the absence of shock.
- FOS
An immediate early gene and transcription factor that is activated rapidly and transiently in response to neuronal activity, leading to expression of memory-related genes.
- Generalization
Process whereby a stimulus–response association learned in one context (for example, a stimulus induces fear) becomes transferred to another similar context.
Footnotes
Competing interests
The authors declare the following competing interests: K.M.F. is a co-founder and the Chief Scientific Officer of Estrigenix Therapeutics, Inc, and is listed as an inventor of a pending patent held by the University of Wisconsin-Milwaukee, Marquette University, and Concordia University Wisconsin: ‘Substituted (4’-hydroxyphenyl)cycloalkane and (4’-hydroxyphenyl)cycloalkene compounds and uses thereof as selective agonists of the estrogen receptor beta isoform for enhanced memory consolidation’, inventors W. A. Donaldson, D. S. Sem & K.M.F.; WO2018183800A1. The other authors have no competing interests to declare.
Peer review information
Nature Reviews Neuroscience thanks H. Bimonte-Nelson, who co-reviewed with V. Bernaud, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Gould E, Woolley CS, Frankfurt M & McEwen BS Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci 10, 1286–1291 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolley C & McEwen B Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci 12, 2549–2554 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolley CS & McEwen BS Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol 336, 293–306 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Woolley CS & McEwen BS Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J. Neurosci 14, 7680–7687 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolley CS, Weiland NG, McEwen BS & Schwartzkroin PA Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci 17, 1848–1859 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong M & Moss R Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J. Neurosci 12, 3217–3225 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Q & Moss RL 17β-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J. Neurosci 16, 3620–3629 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azcoitia I, Sierra A & Garcia-Segura LM Estradiol prevents kainic acid-induced neuronal loss in the rat dentate gyrus. NeuroReport 9, 3075–3079 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Frick KM, Tuscher JJ, Koss WA, Kim J & Taxier LR Estrogenic regulation of memory consolidation: a look beyond the hippocampus, ovaries, and females. Physiol. Behav 187, 57–66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossetti MF, Cambiasso MJ, Holschbach MA & Cabrera R Oestrogens and progestagens: synthesis and action in the brain. J. Neuroendocrinol 28, (2016). [DOI] [PubMed] [Google Scholar]
- 11.Hara Y, Waters EM, McEwen BS & Morrison JH Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev 95, 785–807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison JH & Baxter MG The aging cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci 13, 240–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumitriu D, Rapp PR, McEwen BS & Morrison JH Estrogen and the aging brain: an elixir for the weary cortical network. Ann. N. Y. Acad. Sci 1204, 104–112 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller WL & Auchus RJ The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev 32, 81–151 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Compagnone NA & Mellon SH Neurosteroids: biosynthesis and function of these novel neuromodulators. Front. Neuroendocrinol 21, 1–56 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Österlund M, Kuiper GJM, G., Gustafsson J-Å & Hurd YL Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain1First published on the World Wide Web on 10 December 1997.1. Mol. Brain Res 54, 175–180 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Prange‐Kiel J, Wehrenberg U, Jarry H & Rune GM Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus 13, 226–234 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Stanić D et al. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β, and androgen receptors. PLoS ONE 9, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kretz O et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci 24, 5913–5921 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balthazart J & Ball GF Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci 29, 241–249 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Remage-Healey L, Saldanha CJ & Schlinger BA Estradiol synthesis and action at the synapse: Evidence for “synaptocrine” signaling. Front. Endocrinol 2, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen Edgar. The oestrous cycle in the mouse. Am. J. Anat 30, 297–371 (1922). [Google Scholar]
- 23.Long JA & Evans HM The oestrous cycle in the rat and its associated phenomena (University of California Press, 1922). [Google Scholar]
- 24.Kato A et al. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front. Neural Circuits 7, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawluski JL, Brummelte S, Barha CK, Crozier TM & Galea LAM Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front. Neuroendocrinol 30, 343–357 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Mendoza-Garcés L et al. Differential expression of estrogen receptors in two hippocampal regions during the estrous cycle of the rat. Anat. Rec 294, 1913–1919 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Balthazart J, Choleris E & Remage-Healey L Steroid and the brain: 50 years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm. Behav 99, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasudevan N & Pfaff DW Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front. Neuroendocrinol 29, 238–257 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Szego CM & Davis JS Adenosine 3’,5’-monophosphate in rat uterus: acute elevation by estrogen. Proc. Natl. Acad. Sci. U. S. A 58, 1711–1718 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly MJ, Moss RL & Dudley CA Differential sensitivity of preoptic-septal neurons to microelectrophoressed estrogen during the estrous cycle. Brain Res 114, 152–157 (1976). [DOI] [PubMed] [Google Scholar]
- 31.Zhao Z, Fan L & Frick KM Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. U. S. A 107, 5605–5610 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akama KT & McEwen BS Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J. Neurosci 23, 2333–2339 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan A et al. Low doses of 17 β -Estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology 37, 2299–2309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woolley CS Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol 47, 657–680 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Kramár EA et al. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J. Neurosci 29, 12982–12993 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pappas TC, Gametchu B & Watson CS Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J 9, 404–410 (1995). [DOI] [PubMed] [Google Scholar]
- 37.Watsona CS, Norfleet AM, Pappas TC & Gametchu B Rapid actions of estrogens in GH3/B6 pituitary tumor cells via a plasma membrane version of estrogen receptor-α. Steroids 64, 5–13 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Razandi M, Pedram A, Greene GL & Levin ER Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in chinese hamster ovary cells. Mol. Endocrinol 13, 307–319 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Clarke CH et al. Perimembrane localization of the estrogen receptor α protein in neuronal processes of cultured hippocampal neurons. Neuroendocrinology 71, 34–42 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Gorosito SV, Lorenzo AG & Cambiasso MJ Estrogen receptor α is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience 154, 1173–1177 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Razandi M, Pedram A, Park ST & Levin ER Proximal events in signaling by plasma membrane estrogen receptors. J. Biol. Chem 278, 2701–2712 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Ábrahám IM, Todman MG, Korach KS & Herbison AE Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology 145, 3055–3061 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Blaustein JD Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology 131, 1336–1342 (1992). [DOI] [PubMed] [Google Scholar]
- 44.Milner TA et al. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol 429, 355–371 (2001). [PubMed] [Google Scholar]
- 45.Milner TA et al. Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J. Comp. Neurol 491, 81–95 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Andersson S et al. Insufficient antibody validation challenges oestrogen receptor beta research. Nat. Commun 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Kumar R, Santen RJ & Song RX-D The role of adapter protein Shc in estrogen non-genomic action. Steroids 69, 523–529 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Russell KS, Haynes MP, Sinha D, Clerisme E & Bender JR Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc. Natl. Acad. Sci. U. S. A 97, 5930–5935 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acconcia F, Ascenzi P, Fabozzi G, Visca P & Marino M S-palmitoylation modulates human estrogen receptor-α functions. Biochem. Biophys. Res. Commun 316, 878–883 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Pedram A et al. A conserved mechanism for steroid receptor translocation to the plasma membrane. J. Biol. Chem 282, 22278–22288 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Meitzen J et al. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology 154, 4293–4304 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlegel A, Wang C, Katzenellenbogen BS, Pestell RG & Lisanti MP Caveolin-1 potentiates estrogen receptor α (ERα) signaling. J. Biol. Chem 274, 33551–33556 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Razandi M, Oh P, Pedram A, Schnitzer J & Levin ER ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol. Endocrinol 16, 100–115 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Acconcia F et al. Palmitoylation-dependent estrogen receptor α membrane localization: regulation by 17β-estradiol. Mol. Biol. Cell 16, 231–237 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulware MI, Kordasiewicz H & Mermelstein PG Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci 27, 9941–9950 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boulware MI et al. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci 25, 5066–5078 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boulware MI, Heisler JD & Frick KM The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J. Neurosci 33, 15184–15194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez LA et al. Estradiol facilitation of cocaine self-administration in female rats requires activation of mGluR5. eNeuro 3, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dewing P et al. Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J. Neurosci 27, 9294–9300 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kahlert S et al. Estrogen receptor α rapidly activates the IGF-1 receptor pathway. J. Biol. Chem 275, 18447–18453 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Mendez P, Azcoitia I & Garcia-Segura LM Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Mol. Brain Res 112, 170–176 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Spencer-Segal JL et al. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience 202, 131–146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kramár EA, Babayan AH, Gall CM & Lynch G Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience 239, 3–16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quesada A & Micevych PE Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J. Neurosci. Res 75, 107–116 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Selvamani A & Sohrabji F The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J. Neurosci 30, 6852–6861 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witty CF, Gardella LP, Perez MC & Daniel JM Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: A role for insulin-like growth factor-I. Endocrinology 154, 842–852 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Cabodi S et al. p130Cas interacts with estrogen receptor α and modulates non-genomic estrogen signaling in breast cancer cells. J. Cell Sci 117, 1603–1611 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Lu Q et al. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor α. Proc. Natl. Acad. Sci. U. S. A 101, 17126–17131 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Filardo EJ, Quinn JA, Bland KI & Frackelton AR Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol 14, 1649–1660 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Thomas P, Pang Y, Filardo EJ & Dong J Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Filardo EJ, Quinn JA, Frackelton AR & Bland KI Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol 16, 70–84 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Revankar CM, Cimino DF, Sklar LA, Arterburn JB & Prossnitz ER A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Hazell GGJ et al. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol 202, 223–236 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brailoiu E et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol 193, 311–321 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Hammond R, Nelson D, Kline E & Gibbs RB Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm. Behav 62, 367–374 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hawley WR, Grissom EM, Moody NM, Dohanich GP & Vasudevan N Activation of G-protein-coupled receptor 30 is sufficient to enhance spatial recognition memory in ovariectomized rats. Behav. Brain Res 262, 68–73 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Gabor C, Lymer J, Phan A & Choleris E Rapid effects of the G-protein coupled oestrogen receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice. Physiol. Behav 149, 53–60 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Kim J, Szinte JS, Boulware MI & Frick KM 17β-Estradiol and agonism of G-protein-coupled estrogen receptor enhance hippocampal memory via different cell-signaling mechanisms. J. Neurosci 36, 3309–3321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ervin KSJ, Mulvale E, Gallagher N, Roussel V & Choleris E Activation of the G protein-coupled estrogen receptor, but not estrogen receptor α or β, rapidly enhances social learning. Psychoneuroendocrinology 58, 51–66 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Anchan D, Gafur A, Sano K, Ogawa S & Vasudevan N Activation of the GPR30 receptor promotes lordosis in female mice. Neuroendocrinology 100, 71–80 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Long N, Serey C & Sinchak K 17β-estradiol rapidly facilitates lordosis through G protein-coupled estrogen receptor 1 (GPER) via deactivation of medial preoptic nucleus μ-opioid receptors in estradiol primed female rats. Horm. Behav 66, 663–666 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Briz V & Baudry M Estrogen regulates protein synthesis and actin polymerization in hippocampal neurons through different molecular mechanisms. Neuroendocr. Sci 5, 22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao L, Chen S, Ming Wang J & Brinton RD 17β-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience 132, 299–311 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Wu T-W, Chen S & Brinton RD Membrane estrogen receptors mediate calcium signaling and MAP kinase activation in individual hippocampal neurons. Brain Res 1379, 34–43 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moss RL & Gu Q Estrogen: mechanisms for a rapid action in CA1 hippocampal neurons. Steroids 64, 14–21 (1999). [DOI] [PubMed] [Google Scholar]
- 86.Kuroki Y, Fukushima K, Kanda Y, Mizuno K & Watanabe Y Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur. J. Pharmacol 400, 205–209 (2000). [DOI] [PubMed] [Google Scholar]
- 87.Fernandez SM et al. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci 28, 8660–8667 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee SJ et al. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience 124, 549–560 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Yokomaku D et al. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol. Endocrinol 17, 831–844 (2003). [DOI] [PubMed] [Google Scholar]
- 90.Spencer JL, Waters EM, Milner TA & McEwen BS Estrous cycle regulates activation of hippocampal Akt, LIMK, and neurotrophin receptors in C57BL6 mice. Neuroscience 155, 1106–1119 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan L et al. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J. Neurosci 30, 4390–4400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruiz-Palmero I, Hernando M, Garcia-Segura LM & Arevalo M-A G protein-coupled estrogen receptor is required for the neuritogenic mechanism of 17β-estradiol in developing hippocampal neurons. Mol. Cell. Endocrinol 372, 105–115 (2013). [DOI] [PubMed] [Google Scholar]
- 93.Lewis MC, Kerr KM, Orr PT & Frick KM Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav. Neurosci 122, 716–721 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato K, Akaishi T, Matsuki N, Ohno Y & Nakazawa K β-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res 1150, 108–120 (2007). [DOI] [PubMed] [Google Scholar]
- 95.Gu Q, Korach KS & Moss RL Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology 140, 660–666 (1999). [DOI] [PubMed] [Google Scholar]
- 96.Hasegawa Y et al. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res 1621, 147–161 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Fortress AM, Fan L, Orr PT, Zhao Z & Frick KM Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn. Mem 20, 147–155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sarkar SN, Smith LT, Logan SM & Simpkins JW Estrogen-induced activation of extracellular signal-regulated kinase signaling triggers dendritic resident mRNA translation. Neuroscience 170, 1080–1085 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tuscher JJ, Luine V, Frankfurt M & Frick KM Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J. Neurosci 36, 1483–1489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuen GS, McEwen BS & Akama KT LIM kinase mediates estrogen action on the actin depolymerization factor Cofilin. Brain Res 1379, 44–52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao Y et al. Estrogen receptor alpha and beta regulate actin polymerization and spatial memory through an SRC-1/mTORC2-dependent pathway in the hippocampus of female mice. J. Steroid Biochem. Mol. Biol 174, 96–113 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Yildirim M et al. Estrogen and aging affect synaptic distribution of phosphorylated LIM Kinase (LIMK) in CA1 region of female rat hippocampus. Neuroscience 152, 360–370 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim J et al. Dorsal hippocampal actin polymerization is necessary for activation of G-protein-coupled estrogen receptor (GPER) to increase CA1 dendritic spine density and enhance memory consolidation. J. Neurosci 39, 9598–9610 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou Y, Watters JJ & Dorsa DM Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology 137, 2163–2166 (1996). [DOI] [PubMed] [Google Scholar]
- 105.Zhao Z, Fan L, Fortress AM, Boulware MI & Frick KM Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J. Neurosci 32, 2344–2351 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fortress AM, Kim J, Poole RL, Gould TJ & Frick KM 17β-Estradiol regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Learn. Mem 21, 457–467 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carrer HF, Araque A & Buño W Estradiol regulates the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. J. Neurosci 23, 6338–6344 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumar A & Foster TC 17beta-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J. Neurophysiol 88, 621–626 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Foy MR et al. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J. Neurophysiol 81, 925–929 (1999). [DOI] [PubMed] [Google Scholar]
- 110.Pozzo-Miller LD, Inoue T & Murphy DD Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. J. Neurophysiol 81, 1404–1411 (1999). [DOI] [PubMed] [Google Scholar]
- 111.Oberlander JG & Woolley CS 17β-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J. Neurosci 37, 12314–12327 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bi R, Broutman G, Foy MR, Thompson RF & Baudry M The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc. Natl. Acad. Sci. U. S. A 97, 3602–3607 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith CC & McMahon LL Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J. Neurosci 25, 7780–7791 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu F et al. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci 11, 334–343 (2008). [DOI] [PubMed] [Google Scholar]
- 115.Xu X et al. Bisphenol-A rapidly promotes dynamic changes in hippocampal dendritic morphology through estrogen receptor-mediated pathway by concomitant phosphorylation of NMDA receptor subunit NR2B. Toxicol. Appl. Pharmacol 249, 188–196 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Avila JA et al. Estradiol rapidly increases GluA2-mushroom spines and decreases GluA2-filopodia spines in hippocampus CA1. Hippocampus 27, 1224–1229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Waters EM et al. Effects of estrogen and aging on synaptic morphology and distribution of phosphorylated Tyr1472 NR2B in the female rat hippocampus. Neurobiol. Aging 73, 200–210 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Potier M et al. Temporal memory and its enhancement by estradiol requires surface dynamics of hippocampal CA1 N-Methyl-D-Aspartate receptors. Biol. Psychiatry 79, 735–745 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Phan A et al. Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc. Natl. Acad. Sci. U. S. A 112, 16018–16023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vedder LC, Smith CC, Flannigan AE & McMahon LL Estradiol-induced increase in novel object recognition requires hippocampal NR2B-containing NMDA receptors. Hippocampus 23, 108–115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Smith CC & McMahon LL Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J. Neurosci 26, 8517–8522 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang GZ & Woolley CS Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74, 801–808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tabatadze N, Huang G, May RM, Jain A & Woolley CS Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J. Neurosci 35, 11252–11265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fugger HN, Foster TC, Gustafsson J & Rissman EF Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res 883, 258–264 (2000). [DOI] [PubMed] [Google Scholar]
- 125.Walf AA, Koonce CJ & Frye CA Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol. Learn. Mem 89, 513–521 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rissman EF, Heck AL, Leonard JE, Shupnik MA & Gustafsson J-A Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc. Natl. Acad. Sci. U. S. A 99, 3996–4001 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Witty CF, Foster TC, Semple-Rowland SL & Daniel JM Increasing hippocampal estrogen receptor alpha levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogens. PloS One 7, e51385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hanson AM et al. A-C estrogens as potent and selective estrogen receptor-beta agonists (SERBAs) to enhance memory consolidation under low-estrogen conditions. J. Med. Chem 61, 4720–4738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frick KM Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm. Behav 55, 2–23 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boulware MI, Kent BA & Frick KM The impact of age-related ovarian hormone loss on cognitive and neural function. Curr. Top. Behav. Neurosci 10, 165–184 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Mehra RD, Sharma K, Nyakas C & Vij U Estrogen receptor α and β immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res 1056, 22–35 (2005). [DOI] [PubMed] [Google Scholar]
- 132.Zhang Q-G et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. J. Neurosci 29, 13823–13836 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daniel JM Estrogens, estrogen receptors, and female cognitive aging: the impact of timing. Horm. Behav 63, 231–237 (2013). [DOI] [PubMed] [Google Scholar]
- 134.Luine V & Frankfurt M Estrogenic regulation of memory: the first 50 years. Horm. Behav 121, 104711 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Foster TC Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus 22, 656–669 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Warren SG & Juraska JM Spatial and nonspatial learning across the rat estrous cycle. Behav. Neurosci 111, 259–266 (1997). [DOI] [PubMed] [Google Scholar]
- 137.Daniel JM, Roberts SL & Dohanich GP Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol. Behav 66, 11–20 (1999). [DOI] [PubMed] [Google Scholar]
- 138.Chesler EJ & Juraska JM Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Horm. Behav 38, 234–242 (2000). [DOI] [PubMed] [Google Scholar]
- 139.Frick KM & Berger-Sweeney J Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav. Neurosci 115, 229–237 (2001). [DOI] [PubMed] [Google Scholar]
- 140.Sandstrom NJ & Williams CL Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci 115, 384–393 (2001). [PubMed] [Google Scholar]
- 141.Galea LA, Kavaliers M, Ossenkopp KP & Hampson E Gonadal hormone levels and spatial learning performance in the Morris water maze in male and female meadow voles, Microtus pennsylvanicus. Horm. Behav 29, 106–125 (1995). [DOI] [PubMed] [Google Scholar]
- 142.Packard MG & Teather LA Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport 8, 3009–3013 (1997). [DOI] [PubMed] [Google Scholar]
- 143.Daniel JM, Fader AJ, Spencer AL & Dohanich GP Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm. Behav 32, 217–225 (1997). [DOI] [PubMed] [Google Scholar]
- 144.Bimonte HA & Denenberg VH Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology 24, 161–173 (1999). [DOI] [PubMed] [Google Scholar]
- 145.Luine VN, Richards ST, Wu VY & Beck KD Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm. Behav 34, 149–162 (1998). [DOI] [PubMed] [Google Scholar]
- 146.Gibbs RB & Johnson DA Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 149, 3176–3183 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nelson BS, Springer RC & Daniel JM Antagonism of brain insulin-like growth factor-1 receptors blocks estradiol effects on memory and levels of hippocampal synaptic proteins in ovariectomized rats. Psychopharmacology (Berl.) 231, 899–907 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sinopoli KJ, Floresco SB & Galea LAM Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol. Learn. Mem 86, 293–304 (2006). [DOI] [PubMed] [Google Scholar]
- 149.Wallace M, Luine V, Arellanos A & Frankfurt M Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126, 176–182 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Fonseca CS et al. Object recognition memory and temporal lobe activation after delayed estrogen replacement therapy. Neurobiol. Learn. Mem 101, 19–25 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Inagaki T, Gautreaux C & Luine V Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm. Behav 58, 415–426 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Luine VN, Jacome LF & MacLusky NJ Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 144, 2836–2844 (2003). [DOI] [PubMed] [Google Scholar]
- 153.Gresack JE & Frick KM Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol. Biochem. Behav 84, 112–119 (2006). [DOI] [PubMed] [Google Scholar]
- 154.Pereira LM, Bastos CP, de Souza JM, Ribeiro FM & Pereira GS Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor α. Neurobiol. Learn. Mem 114, 1–9 (2014). [DOI] [PubMed] [Google Scholar]
- 155.Tuscher JJ, Taxier LR, Schalk JC, Haertel JM & Frick KM Chemogenetic suppression of medial prefrontal-dorsal hippocampal interactions prevents estrogenic enhancement of memory consolidation in female mice. eNeuro 6, ENEURO.0451-18.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gervais NJ, Jacob S, Brake WG & Mumby DG Systemic and intra-rhinal-cortical 17-β estradiol administration modulate object-recognition memory in ovariectomized female rats. Horm. Behav 64, 642–652 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Gervais NJ, Hamel LM, Brake WG & Mumby DG Intra-perirhinal cortex administration of estradiol, but not an ERβ agonist, modulates object-recognition memory in ovariectomized rats. Neurobiol. Learn. Mem 133, 89–99 (2016). [DOI] [PubMed] [Google Scholar]
- 158.Taxier LR, Philippi SM, Fortress AM & Frick KM Dickkopf-1 blocks 17β-estradiol-enhanced object memory consolidation in ovariectomized female mice. Horm. Behav 114, 104545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luine V & Frankfurt M Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience 239, 34–45 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Warren SG, Humphreys AG, Juraska JM & Greenough WT LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res 703, 26–30 (1995). [DOI] [PubMed] [Google Scholar]
- 161.Good M, Day M & Muir JL Cyclical changes in endogenous levels of oestrogen modulate the induction of LTD and LTP in the hippocampal CA1 region. Eur. J. Neurosci 11, 4476–4480 (1999). [DOI] [PubMed] [Google Scholar]
- 162.Vedder LC, Bredemann TM & McMahon LL Estradiol replacement extends the window of opportunity for hippocampal function. Neurobiol. Aging 35, 2183–2192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferguson JN, Young LJ & Insel TR The neuroendocrine basis of social recognition. Front. Neuroendocrinol 23, 200–224 (2002). [DOI] [PubMed] [Google Scholar]
- 164.Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M & Choleris E Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci 126, 97–109 (2012). [DOI] [PubMed] [Google Scholar]
- 165.Sánchez-Andrade G & Kendrick KM Roles of α- and β-estrogen receptors in mouse social recognition memory: Effects of gender and the estrous cycle. Horm. Behav 59, 114–122 (2011). [DOI] [PubMed] [Google Scholar]
- 166.Hliňáck Z Social recognition in ovariectomized and estradiol-treated female rats. Horm. Behav 27, 159–166 (1993). [DOI] [PubMed] [Google Scholar]
- 167.Tang AC et al. Effects of long-term estrogen replacement on social investigation and social memory in ovariectomized C57BL/6 mice. Horm. Behav 47, 350–357 (2005). [DOI] [PubMed] [Google Scholar]
- 168.Spiteri T & Ågmo A Ovarian hormones modulate social recognition in female rats. Physiol. Behav 98, 247–250 (2009). [DOI] [PubMed] [Google Scholar]
- 169.Choleris E et al. An estrogen-dependent four-gene micronet regulating social recognition: A study with oxytocin and estrogen receptor-α and -β knockout mice. Proc. Natl. Acad. Sci 100, 6192–6197 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ferguson JN, Aldag JM, Insel TR & Young LJ Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci 21, 8278–8285 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Choleris E et al. Involvement of estrogen receptor α, β and oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Genes Brain Behav 5, 528–539 (2006). [DOI] [PubMed] [Google Scholar]
- 172.Spiteri T et al. The role of the estrogen receptor α in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav. Brain Res 210, 211–220 (2010). [DOI] [PubMed] [Google Scholar]
- 173.Phan A, Lancaster KE, Armstrong JN, MacLusky NJ & Choleris E Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology 152, 1492–1502 (2011). [DOI] [PubMed] [Google Scholar]
- 174.Lymer J, Robinson A, Winters BD & Choleris E Rapid effects of dorsal hippocampal G-protein coupled estrogen receptor on learning in female mice. Psychoneuroendocrinology 77, 131–140 (2017). [DOI] [PubMed] [Google Scholar]
- 175.Lymer JM et al. Estrogens and their receptors in the medial amygdala rapidly facilitate social recognition in female mice. Psychoneuroendocrinology 89, 30–38 (2018). [DOI] [PubMed] [Google Scholar]
- 176.Morgan MA & Pfaff DW Effects of estrogen on activity and fear-related behaviors in mice. Horm. Behav 40, 472–482 (2001). [DOI] [PubMed] [Google Scholar]
- 177.Jasnow AM, Schulkin J & Pfaff DW Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm. Behav 49, 197–205 (2006). [DOI] [PubMed] [Google Scholar]
- 178.Hiroi R & Neumaier JF Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behav. Brain Res 166, 93–100 (2006). [DOI] [PubMed] [Google Scholar]
- 179.Markus EJ & Zecevic M Sex differences and estrous cycle changes in hippocampus-dependent fear conditioning. Psychobiology 25, 246–252 (1997). [Google Scholar]
- 180.Gupta RR, Sen S, Diepenhorst LL, Rudick CN & Maren S Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats11Published on the World Wide Web on 1 December 2000. Brain Res 888, 356–365 (2001). [DOI] [PubMed] [Google Scholar]
- 181.McDermott CM, Liu D, Ade C & Schrader LA Estradiol replacement enhances fear memory formation, impairs extinction and reduces COMT expression levels in the hippocampus of ovariectomized female mice. Neurobiol. Learn. Mem 118, 167–177 (2015). [DOI] [PubMed] [Google Scholar]
- 182.Matsumoto YK, Kasai M & Tomihara K The enhancement effect of estradiol on contextual fear conditioning in female mice. PLOS ONE 13, e0197441 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barha CK, Dalton GL & Galea LA Low doses of 17α -estradiol and 17β-estradiol facilitate, whereas higher doses of estrone and 17α- and 17β-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology 35, 547–559 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rivas-Arancibia S & Vazquez-Pereyra F Hormonal modulation of extinction responses induced by sexual steroid hormones in rats. Life Sci 54, PL363–PL367 (1994). [DOI] [PubMed] [Google Scholar]
- 185.Yuan DL & Chambers KC Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Horm. Behav 36, 1–16 (1999). [DOI] [PubMed] [Google Scholar]
- 186.Chang Y-J et al. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus 19, 1142–1150 (2009). [DOI] [PubMed] [Google Scholar]
- 187.Milad MR, Igoe SA, Lebron-Milad K & Novales JE Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164, 887–895 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zeidan MA et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol. Psychiatry 70, 920–927 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Graham BM & Milad MR Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol. Psychiatry 73, 371–378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Graham BM & Scott E Effects of systemic estradiol on fear extinction in female rats are dependent on interactions between dose, estrous phase, and endogenous estradiol levels. Horm. Behav 97, 67–74 (2018). [DOI] [PubMed] [Google Scholar]
- 191.de Castilhos J, Forti CD, Achaval M & Rasia-Filho AA Dendritic spine density of posterodorsal medial amygdala neurons can be affected by gonadectomy and sex steroid manipulations in adult rats: a Golgi study. Brain Res 1240, 73–81 (2008). [DOI] [PubMed] [Google Scholar]
- 192.Ferri SL, Hildebrand PF, Way SE & Flanagan-Cato LM Estradiol regulates markers of synaptic plasticity in the hypothalamic ventromedial nucleus and amygdala of female rats. Horm. Behav 66, 409–420 (2014). [DOI] [PubMed] [Google Scholar]
- 193.Amano T, Unal CT & Paré D Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci 13, 489–494 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shansky RM et al. Estrogen promotes stress sensitivity in a prefrontal cortex–amygdala pathway. Cereb. Cortex 20, 2560–2567 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maeng LY et al. Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J. Neurosci. Res 95, 163–175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rey CD, Lipps J & Shansky RM Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal–amygdala circuits. Neuropsychopharmacology 39, 1282–1289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lynch J, Cullen PK, Jasnow AM & Riccio DC Sex differences in the generalization of fear as a function of retention intervals. Learn. Mem 20, 628–632 (2013). [DOI] [PubMed] [Google Scholar]
- 198.Lynch JF, Winiecki P, Vanderhoof T, Riccio DC & Jasnow AM Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiol. Learn. Mem 130, 83–92 (2016). [DOI] [PubMed] [Google Scholar]
- 199.Ooishi Y et al. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. J. Steroid Biochem. Mol. Biol 131, 37–51 (2012). [DOI] [PubMed] [Google Scholar]
- 200.Hojo Y et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. U. S. A 101, 865–870 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang W et al. Memory-related synaptic plasticity is sexually dimorphic in rodent hippocampus. J. Neurosci 38, 7935–7951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jain A, Huang GZ & Woolley CS Latent sex differences in molecular signaling that underlies excitatory synaptic potentiation in the hippocampus. J. Neurosci 39, 1552–1565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fester L et al. Control of aromatase in hippocampal neurons. J. Steroid Biochem. Mol. Biol 160, 9–14 (2016). [DOI] [PubMed] [Google Scholar]
- 204.Vierk R et al. Aromatase inhibition abolishes LTP generation in female but not in male mice. J. Neurosci 32, 8116–8126 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhou L et al. Oestradiol-induced synapse formation in the female hippocampus: roles of oestrogen receptor subtypes. J. Neuroendocrinol 26, 439–447 (2014). [DOI] [PubMed] [Google Scholar]
- 206.Lu Y et al. Neuron-derived estrogen regulates synaptic plasticity and memory. J. Neurosci 39, 2792–2809 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brandt N & Rune GM Sex-dependency of oestrogen-induced structural synaptic plasticity: Inhibition of aromatase versus application of estradiol in rodents. Eur. J. Neurosci 1–12 (2019) doi: 10.1111/ejn.14541. [DOI] [PubMed] [Google Scholar]
- 208.Jacome LF et al. Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology 157, 1357–1362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Koss WA, Haertel JM, Philippi SM & Frick KM Sex differences in the rapid cell signaling mechanisms underlying the memory-enhancing effects of 17β-estradiol. eNeuro 5, ENEURO.0267-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Frye CA, Rhodes ME & Dudek B Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res 1036, 101–108 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Packard MG Posttraining estrogen and memory modulation. Horm. Behav 34, 126–139 (1998). [DOI] [PubMed] [Google Scholar]
- 212.Heikkinen T, Puoliväli J, Liu L, Rissanen A & Tanila H Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm. Behav 41, 22–32 (2002). [DOI] [PubMed] [Google Scholar]
- 213.Pierman S et al. Activational effects of estradiol and dihydrotestosterone on social recognition and the arginine-vasopressin immunoreactive system in male mice lacking a functional aromatase gene. Horm. Behav 54, 98–106 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Alejandre-Gomez Misael, Garcia-Segura Luis Miguel & Gonzalez-Burgos Ignacio. Administration of an inhibitor of estrogen biosynthesis facilitates working memory acquisition in male rats. Neurosci. Res 58, 272–277 (2007). [DOI] [PubMed] [Google Scholar]
- 215.Koss WA & Frick KM Activation of androgen receptors protects intact male mice from memory impairments caused by aromatase inhibition. Horm. Behav 111, 96–104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Koss WA & Frick KM Sex differences in hippocampal function. J. Neurosci. Res 95, 539–562 (2017). [DOI] [PubMed] [Google Scholar]
- 217.Frick KM, Kim J, Tuscher JJ & Fortress AM Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn. Mem 22, 472–493 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Prendergast BJ, Onishi KG & Zucker I Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev 40, 1–5 (2014). [DOI] [PubMed] [Google Scholar]
- 219.Clayton JA Studying both sexes: a guiding principle for biomedicine. FASEB J 30, 519–524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Clayton JA Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol. Behav 187, 2–5 (2018). [DOI] [PubMed] [Google Scholar]
- 221.Brooks CE & Clayton JA Sex/Gender influences on the nervous system: Basic steps toward clinical progress. J. Neurosci. Res 95, 14–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Woitowich NC & Woodruff TK Implementation of the NIH sex-inclusion policy: Attitudes and opinions of study section members. J. Womens Health 28, 9–16 (2018). [DOI] [PubMed] [Google Scholar]
- 223.Sandoval A, Elahi H & Ploski JE Genetically engineering the nervous system with CRISPR-Cas. eNeuro 7, ENEURO.0419-19.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mitchnick KA et al. Dissociable involvement of estrogen receptors in perirhinal cortex-mediated object-place memory in male rats. Psychoneuroendocrinology 107, 98–108 (2019). [DOI] [PubMed] [Google Scholar]
- 225.Kim J & Frick KM Distinct effects of estrogen receptor antagonism on object recognition and spatial memory consolidation in ovariectomized mice. Psychoneuroendocrinology 85, 110–114 (2017). [DOI] [PubMed] [Google Scholar]
- 226.Tuscher JJ et al. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm. Behav 83, 60–67 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bayer J et al. The effect of estrogen synthesis inhibition on hippocampal memory. Psychoneuroendocrinology 56, 213–225 (2015). [DOI] [PubMed] [Google Scholar]
- 228.Gervais NJ et al. Adverse effects of aromatase inhibition on the brain and behavior in a nonhuman primate. J. Neurosci 39, 918–928 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Morris R Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 (1984). [DOI] [PubMed] [Google Scholar]
- 230.Vorhees CV & Williams MT Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc 1, 848–858 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Olton DS The radial arm maze as a tool in behavioral pharmacology. Physiol. Behav 40, 793–797 (1987). [DOI] [PubMed] [Google Scholar]
- 232.Olton DS & Papas BC Spatial memory and hippocampal function. Neuropsychologia 17, 669–682 (1979). [DOI] [PubMed] [Google Scholar]
- 233.Ennaceur A & Delacour J A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res 31, 47–59 (1988). [DOI] [PubMed] [Google Scholar]
- 234.Ennaceur A & Aggleton JP Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp. Brain Res 100, 85–92 (1994). [DOI] [PubMed] [Google Scholar]
- 235.Phillips RG & LeDoux JE Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci 106, 274–285 (1992). [DOI] [PubMed] [Google Scholar]
- 236.Frick KM, Fortress AM Pharmacological manipulation of learning and memory. In: The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition, Bimonte-Nelson H (Ed). Heidelberg, Germany: Springer. pp. 165–210 (2015). [Google Scholar]
- 237.Naftolin F, Ryan KJ & Petro Z Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology 90, 295–298 (1972). [DOI] [PubMed] [Google Scholar]
- 238.Sato SM & Woolley CS Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. eLife 5, e12917(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Prange‐Kiel J et al. Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus 16, 464–471 (2006). [DOI] [PubMed] [Google Scholar]
- 240.Zhou L et al. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology 151, 1153–1160 (2010). [DOI] [PubMed] [Google Scholar]
- 241.Bailey DJ, Ma C, Soma KK & Saldanha CJ Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology 154, 4707–4714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Blaustein JD Treatments for breast cancer that affect cognitive function in postmenopausal women. Policy Insights Behav. Brain Sci 4, 170–177 (2017). [Google Scholar]
- 243.Bimonte-Nelson HA, Acosta JI & Talboom JS Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules 15, 6050–6105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wise PM Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol and progesterone concentrations in middle-aged rats. Life Sci 31, 165–173 (1982). [DOI] [PubMed] [Google Scholar]
- 245.Richardson SJ & Nelson JF Follicular depletion during the menopausal transition. Ann. N. Y. Acad. Sci 592, 13–20 (1990). [DOI] [PubMed] [Google Scholar]
- 246.Talboom JS, Williams BJ, Baxley ER, West SG & Bimonte-Nelson HA Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol. Learn. Mem 90, 155–163 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Markham JA, Pych JC & Juraska JM Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm. Behav 42, 284–293 (2002). [DOI] [PubMed] [Google Scholar]
- 248.Gresack JE, Kerr KM & Frick KM Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol. Learn. Mem 88, 393–408 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Singh M, Meyer EM, Millard WJ & Simpkins JW Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res 644, 305–312 (1994). [DOI] [PubMed] [Google Scholar]
- 250.Foster TC, Sharrow KM, Kumar A & Masse J Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol. Aging 24, 839–852 (2003). [DOI] [PubMed] [Google Scholar]
- 251.Frick KM, Fernandez SM & Bulinski SC Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience 115, 547–558 (2002). [DOI] [PubMed] [Google Scholar]
- 252.Vaucher E et al. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol. Aging 23, 87–95 (2002). [DOI] [PubMed] [Google Scholar]
- 253.Prakapenka AV et al. Contrasting effects of individual versus combined estrogen and progestogen regimens as working memory load increases in middle-aged ovariectomized rats: one plus one does not equal two. Neurobiol. Aging 64, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gresack JE & Frick KM Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res 1115, 135–147 (2006). [DOI] [PubMed] [Google Scholar]
- 255.Markowska AL & Savonenko AV Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J. Neurosci 22, 10985–10995 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Daniel JM, Hulst JL & Berbling JL Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 147, 607–614 (2006). [DOI] [PubMed] [Google Scholar]
- 257.Gresack JE, Kerr KM & Frick KM Short-term environmental enrichment decreases the mnemonic response to estrogen in young, but not aged, female mice. Brain Res 1160, 91–101 (2007). [DOI] [PubMed] [Google Scholar]
- 258.Aenlle KK & Foster TC Aging alters the expression of genes for neuroprotection and synaptic function following acute estradiol treatment. Hippocampus 20, 1047–1060 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Adams MM et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-α in the hippocampus of female rats. J. Neurosci 22, 3608–3614 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Waters EM et al. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res 1379, 86–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


