Abstract
The Fontan circulation is characterized as a nonpulsatile flow propagation without a pressure-generating ventricle. However, flow through the Fontan circulation still exhibits oscillatory waves as a result of pressure changes generated by the systemic single ventricle. Identification of discrete flow patterns through the Fontan circuit may be important to understand single ventricle performance. Ninety-seven patients with Fontan circulation underwent phase-contrast MRI of the right pulmonary artery, yielding subject-specific flow waveforms. Principal component (PC) analysis was performed on preprocessed flow waveforms. Principal components were then correlated with standard MRI indices of function, volume, and aortopulmonary collateral flow. The first principal component (PC) described systolic versus diastolic-dominant flow through the Fontan circulation, accounting for 31.3% of the variance in all waveforms. The first PC correlated with end-diastolic volume (R = 0.34, P = 0.001), and end-systolic volume (R = 0.30, P = 0.003), cardiac index (R = 0.51, P < 0.001), and the amount of aortopulmonary collateral flow (R = 0.25, P = 0.027)—lower ventricular volumes and a smaller volume of collateral flow—were associated with diastolic-dominant cavopulmonary flow. The second PC accounted for 19.5% of variance and described late diastolic acceleration versus deceleration and correlated with ejection fraction—diastolic deceleration was associated with higher ejection fraction. Principal components describing the diastolic flow variations in pulmonary arteries are related to the single ventricle function and volumes. Particularly, diastolic-dominant flow without late acceleration appears to be related to preserved ventricular volume and function, respectively.
NEW & NOTEWORTHY The exact physiological significance of flow oscillations of phasic and temporal flow variations in Fontan circulation is unknown. With the use of principal component analysis, we discovered that flow variations in the right pulmonary artery of Fontan patients are related to the single ventricle function and volumes. Particularly, diastolic-dominant flow without late acceleration appears to be related to more ideal ventricular volume and systolic function, respectively.
Keywords: flow, Fontan circulation, MRI
INTRODUCTION
Total cavopulmonary connection (TCPC) or the Fontan circulation is characterized by passive, nonpulsatile flow propagation through the pulmonary arteries without a pressure-generating ventricle (4, 5). Consequently, minimal pressure loss in pulmonary arteries and optimal flow hemodynamic conditions are critical for the energetically efficient Fontan system (7, 24). However, flow-through Fontan circulation still exhibits oscillatory waves as a result of pressure changes generated by the systemic single ventricle and due to intrathoracic pressure changes associated with the respiration cycle (10, 12, 19). A limited amount of MRI-based investigations have attempted to describe the in vivo flow characteristics and categorize the phasic and temporal flow variations of the Fontan circulation, but the exact physiological significance of these flow oscillations is yet to be determined.
The noninvasive flow hemodynamic evaluation of the Fontan circulation is typically performed using phase-contrast MRI (11). A typical clinical protocol interrogates all major vessels of the Fontan circulation to provide accurate net flow information and also to quantify the burden of collateral flow (8, 33). Prior research studies have additionally reported computational fluid dynamics and four-dimensional flow MRI analyses of the flow hemodynamic patterns within the TCPC with respect to the flow-mediated energy loss, anatomical variability, and surgical technique (16, 24, 30, 31). However, less attention has been paid to the oscillatory nature of the flow through the pulmonary arteries and general characteristics of flow profiles. Unfortunately, the nonpulsatile condition without a physiologically synchronized pressure waveform disables any form of the pulse wave or wave-intensity analyses in pulmonary arteries post-TCPC. Therefore, a qualitative time-domain analysis of flow profile would have to rely on the extraction of features from highly heterogenous flow profiles encountered in Fontan circulation (10, 12, 16), which could be assigned to respective events in the cardiac cycle. Principal component analysis (PCA) is a statistical method used to transform large sets of possibly correlated variables into a smaller set that contains most of the information present in the original collection. PCA has been applied in numerous biomedical applications to identify the most important underlying features within a large pool of related input variables to test associations with a dependent variable describing a physiological concept. Typical applications include dimensionality reduction of complex data sets, such as microarray analyses. PCA can also be leveraged for shape analysis, including identification of important anatomic and geometric variations in vascular morphologies (2, 3, 34).
The purpose of this study was to investigate flow profile characteristics generated by phase-contrast MRI in pulmonary arteries of patients with Fontan circulation using PCA. PCA enabled us to decompose complex flow profile curves into a smaller set of component patterns, which we then compared with relevant clinical parameters. We further sought to investigate whether identified flow profile characteristics correspond to specific events within the cardiac cycle and whether they are associated with the MRI hemodynamics pertinent to Fontan circulation. We hypothesized that different flow profile characteristics would be associated with the single ventricle size and function and that recognized features would be associated with single ventricle morphology.
METHODS
Patients who underwent single ventricle palliation, culminating in a Fontan operation, were referred to the Single Ventricle Care Program, Multidisciplinary Clinic, at Children’s Hospital Colorado between June 2015 and April 2019. In total, 97 patients underwent comprehensive, clinically indicated cardiac MRI evaluation of ventricular function and size, along with the quantitative assessment of flow hemodynamics through the systemic and cavopulmonary circulations. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB) and was part of the Fontan at Altitude Registry for Outcomes study with an approved waiver of informed consent.
MRI acquisition.
Previously described MRI protocol with prescribed sequences for the single ventricle evaluation was applied in standardized fashion. Standard, balanced, steady-state free precession stacks of short-axis images covering the ventricles from base to apex were used for volumetric and functional analysis. Ventricular volumes were then derived from the manual segmentation of the endocardial contours and indexed to the body-surface area. Phase-contrast MRI, ECG-gated sequences were used to generate tissue intensity and phase velocity maps of the thoracic aorta, applying a 1.5 or 3.0 tesla magnet (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany, or Ingenia, Philips Medical Systems, Best, The Netherlands) using a phased-array body-surface coil.
Typical free-breathing phase-contrast MRI sequence parameters included the following: repetition time, 14–28 ms/30–40 cardiac phases; echo time, 2.2–3.5 ms; matrix, 160 × 256; flip angle, 25°, with 100% k-space sampling and no further temporal interpolation; the cross-sectional pixel resolution ranged between 0.82 × 0.82 mm2 and 1.56 × 1.56 mm2, with a slice thickness of 5 mm. Phase-contrast MRI acquisition time for individual pulmonary arteries varied between 2 and 3 min depending on heart rate. Velocity-encoding values were selected to accommodate for the pulmonary arterial flow in Fontan circulation and ranged between 50 and 100 cm/s. Aortopulmonary collateral burden was evaluated as the percent ratio of the net caval flow to the net aortic flow, as described previously (33).
Flow profile characterization.
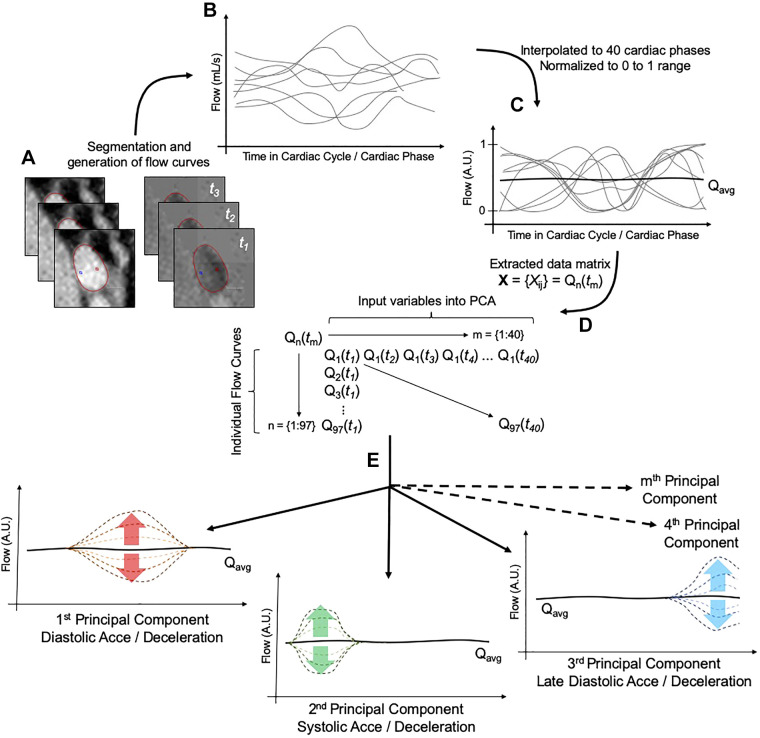
Flow through the right pulmonary artery was analyzed using Circle CVI42 (Calgary, Canada). Patient-specific flow hemodynamic curves were generated using a standard approach using parallel segmentation of both magnitude and phase images. Proximal segments of branch pulmonary arteries were, in all, considered patients free of any stenoses, noticeable by MRI. We decided to interrogate the right pulmonary artery due to a known tendency for higher pulmonary blood flow and hence, better signal for PCA analysis. Additionally, flow through the right pulmonary artery is considerably easier for evaluation because its lumen is easier for temporal segmentation and delineates from other structures compared with the left pulmonary artery. Generated flow curves were then imported into Matlab 2018a (Mathworks, Natick, MA) for further postprocessing and PCA. The overall postprocessing pipeline is graphically depicted in Fig. 1. First, all flow curves were interpolated to achieve 40 cardiac phases within the cardiac cycle using a cubic spline interpolation. Temporally standardized flow curves were then normalized to a range from 0 to 1. Following these steps, principal component analysis was performed on the postprocessed flow curves, where input variables were represented by the scaled flow magnitude at each individual cardiac phase [Q(t1) to Q(t40)].
Fig. 1.
Workflow diagram of the principal component analysis (PCA) of the pulmonary arterial flow (Q). A and B: magnitude and phase images acquired by phase-contrast MRI (A) were segmented in parallel to yield patient-specific flow profiles (B). C: generated flow profiles were then preprocessed by temporal interpolation and magnitude normalization. D: finalized matrix of data sets serving as input into PCA consisted of 40 columns representing the time step in cardiac cycle and input variables and 97 rows representing the individual flow curves and sample size. E: generated principal components (here, hypothetically depicted) would then describe a variation from the averaged flow curve (Qavg) at any phase of the cardiac cycle (t). Acce, acceleration; A.U., arbitrary units.
PCA uses an orthogonal transformation to convert a set of potentially correlated variables [flow at individual cardiac phases Q(t1), Q(t2), Q(t3), ..., Q(t40)] into a set of new, linearly uncorrelated vectors, known as principal components (PCs). The resulting PCs are ranked in the order of decreasing variance by which they are described the original data set. In other words, PCA seeks to identify underlying patterns in the collected set of flow profiles and describes them in terms of a smaller number of parameters (PCs and their corresponding variances). In this study, PCs can be envisioned as flow shape modulators, where a given PC may either increase or decrease flow within a given portion of the cardiac cycle. Such hypothetical flow modulators are described in Fig. 1. Since the sample size in this study was smaller than the number of input variables (number of cardiac phases = 40), the PCA yielded, in total, 40 different principal components. The inclusion of the PCs for the correlative analysis with MRI hemodynamics and additional analyses was based on the explained variance of each principal component (first five principal components were analyzed).
Statistical analysis.
Analyses were performed in Prism (version 7.0 or higher; GraphPad Software Inc., La Jolla, CA) and freely available Estimation-Stats (13). Variables were checked for the distributional assumption of normality using normal plots, in addition to Kolmogorov–Smirnov and Shapiro–Wilk tests. Hemodynamic characteristics between different patient groups identified by the principal component analysis were compared using Student’s t test for normally distributed continuous variables or Mann–Whitney test for non-normally distributed variables and graphically displayed using the Gardner–Altman estimation plot for two groups using Estimation-Stats (13). The relationships between the principal components and MRI hemodynamics were analyzed by simple linear regression analysis using Pearson’s R value. Analyses were considered exploratory and hypothesis generating, and adjustments for multiple comparisons were not used. Significance was based on an α value of 0.05.
RESULTS
Patient demographics and MRI hemodynamics are summarized in Table 1. From the total of 97 patients, 42 (43%) were diagnosed with hypoplastic left heart syndrome (HLHS), 30 (31%) had tricuspid atresia (TA), and 25 (26%) had a spectrum of other single ventricle diagnoses, including double-outlet right ventricle with hypoplastic left heart (n = 13), double-inlet left ventricle (n = 5), transposition of great arteries with hypoplastic right ventricle (n = 4), atrioventricular septal defect with aortic valve hypoplasia (n = 1), and heterotaxy with unbalanced right ventricular-dominant canal (n = 2). The median age at the time of total cavopulmonary connection was 2.7 yr (range: 1.4 to 4.1 yr), with 60 patients undergoing the completion with extracardiac conduit and 37 patients with lateral tunnel. At our institution, all patients also undergo the creation of the fenestration due to a high prevalence of increased pulmonary vascular resistance, otherwise leading to low cardiac output syndrome with reduced preload. Nineteen patients were on pulmonary vasodilator therapy (16 phosphodiesterase-5 inhibitors and 3 endothelin receptor antagonists). At the time of cardiovascular magnetic resonance (CMR), 17 patients had a Fontan physiology-related major clinical complication, including the heart failure (n = 9); 6 patients exhibited the Fontan circulatory failure; and 3 patients had heart failure due to reduced ventricular systolic function, protein-losing enteropathy (n = 5), plastic bronchitis (n = 2), and liver disease due to cirrhosis (n = 2). In the interim, three patients underwent heart transplantation, and one died. The mean ejection fraction was 48 ± 7%, with an end-diastolic volume index of 101 ± 33 mL/m2, an end-systolic volume of 54 ± 23 mL/m2, and a cardiac index 4.1 ± 1.6 L⋅min−1⋅m−2.
Table 1.
Demographics and MRI hemodynamics
| Age, yr | 11 (5–16) |
| BSA, m2 | 1.07 ± 0.48 |
| Female, n (%) | 40 (41) |
| HLHS, n (%) | 42 (43) |
| Tricuspid atresia, n (%) | 30 (31) |
| Others, n (%) | 25 (26) |
| EDVi, mL/m2 | 101 ± 33 |
| ESVi, mL/m2 | 54 ± 23 |
| SVi, mL/m2 | 47 ± 15 |
| EF, % | 48 ± 7 |
| CI, L⋅min−1⋅m−2 | 4.1 ± 1.6 |
| Ao-P collateral flow, % | 23 (14–41) |
Values are means ± SD or median with corresponding interquartile range. Ao-P, aortopulmonary flow as percent of the net aortic flow; BSA, body-surface area; CI, cardiac index; EDVi, end-diastolic volume index; EF, ejection fraction; ESVi, end-systolic volume index; HLHS, hypoplastic left heart syndrome; SVi, stroke volume index.
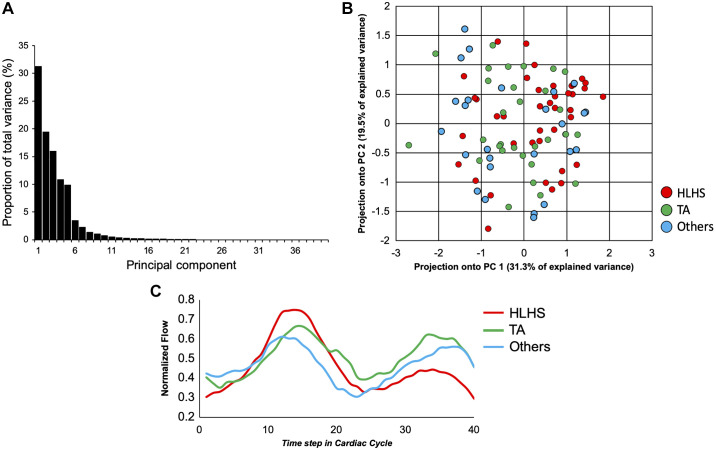
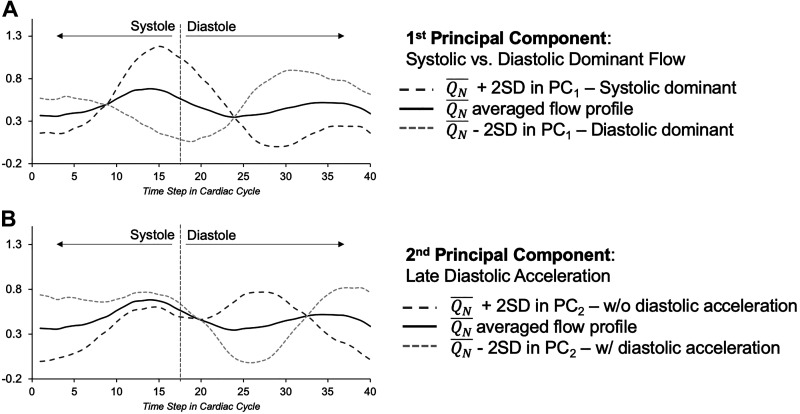
The scree plot, representing the overall proportion of the explained variance of all 40 principal components, along with the projection of the single ventricle morphology on the first two principal components, is depicted in Fig. 2. Together, the first five PCs accounted for 87.6% of the variance in all sampled flow curves (31.3, 19.5, 16.0, 10.9, and 9.9% for PCs 1–5, respectively). The first two principal components and their modulating effect on the mean pulmonary arterial flow are depicted in Fig. 3. Qualitatively, the first PC primarily reflected the relative predominance of systolic or diastolic flow. In other words, the net flow or propensity toward the higher flow rate in patients with Fontan circulation tends to be higher either during the single ventricle systole or diastole. The second PC primarily describes late diastolic flow acceleration or deceleration, which occurs during the phase of atrial contraction.
Fig. 2.
A: the scree plot of the principal components describing the proportion of total variance of each principal component. B: projection of different single ventricle subtypes onto the first and the second principal component (PC 1 and PC 2, respectively). There were no major clusters of data sets with an apparent trend along the first principal component progressing from the spectrum of complex single ventricle diagnoses (Others) to tricuspid atresia (TA) and hypoplastic left heart syndrome (HLHS). C: patient group-specific averaged flow curves for HLHS (red), TA (green), and others (blue).
Fig. 3.
The dark and light dashed curves represent ± 2 SD within given principal component from the averaged (black line) flow profile (QN). A: 1st principal component (PC1) described systolic (dark dashes)- versus diastolic (light dashes)-dominant flow. B: 2nd principal component (PC2) described either diastolic flow acceleration (light dashes) or deceleration (dark dashes).
To explore the physiological relevance of the observed principal components, we performed linear regression analysis with standard MRI hemodynamics. The summary of the correlative analysis is depicted in Table 2. The first PC correlated with the end-diastolic volume index (R = 0.34, P = 0.001), end-systolic volume index (R = 0.30, P = 0.003), cardiac index (R = 0.51, P < 0.001), and aortopulmonary collateral flow index to the aortic net flow (R = 0.25, P = 0.027). The second principal component correlated with the ejection fraction (R = 0.38, P < 0.001). Lastly, the fifth principal component correlated with the end-diastolic volume index (R = −0.22, P = 0.034).
Table 2.
Correlation among principal components and MRI hemodynamics
| PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|
| R, P Value | R, P Value | R, P Value | R, P Value | R, P Value | |
| EF | −0.10, 0.335 | 0.38, <0.001 | 0.10, 0.356 | 0.08, 0.432 | 0.07, 0.489 |
| EDVi | 0.34, 0.001 | −0.01, 0.927 | −0.02, 0.881 | 0.06, 0.591 | −0.22, 0.034 |
| ESVi | 0.30, 0.003 | −0.08, 0.459 | −0.06, 0.537 | 0.03, 0.808 | −0.19, 0.065 |
| CI | 0.51, <0.001 | 0.13, 0.24 | −0.10, 0.360 | 0.08, 0.470 | −0.13, 0.229 |
| Ao-P Coll | 0.25, 0.027 | 0.10, 0.398 | 0.02, 0.831 | 0.14, 0.211 | −0.03, 0.794 |
Values reported as Pearson R with corresponding P value. Bold represents statistically significant correlations with P < 0.05. Ao-P Coll, aortopulmonary collateral flow as percent of the net aortic flow; CI, cardiac index; EDVi, end-diastolic volume index; EF, ejection fraction; ESVi, end-systolic volume index; PC, principal component.
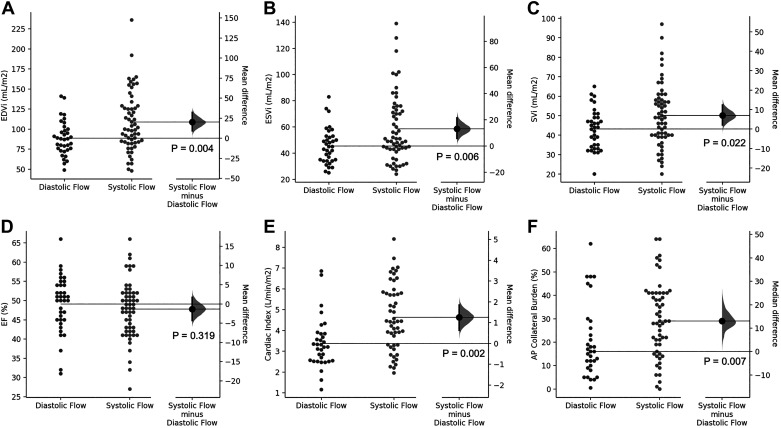
Given the observed correlations between MRI hemodynamics and the first two PCs, we performed further subgroup analyses based on the flow hemodynamic changes described by the principal components. Because the first PC primarily differentiated between the systolic- and diastolic-dominant flow patterns, we chose to divide patients by the ratio of the maximum diastolic flow to the maximum systolic flow. Patients with a ratio > 1 were classified as diastolic dominant, whereas those with a ratio < 1 were classified as systolic dominant. Differences in standard MRI hemodynamics for the considered groups are depicted in Table 3 and Fig. 4. Compared with patients with systolic-dominant flow, patients with dominant diastolic flow had a decreased end-diastolic volume index (89 vs. 109 mL/m2, P = 0.004), end-systolic volume index (45 vs. 59 mL/m2, P = 0.006), stroke volume index (43 vs. 50 mL/m2, P = 0.002), and cardiac index (3.4 vs. 4.6 L⋅min−1⋅m−2, P = 0.002). Additionally, patients with the diastolic-dominant flow had lower aortopulmonary collateral flow burden (17 vs. 29%, P = 0.023).
Table 3.
Flow differentiation by the first principal component
| Diastolic | Systolic | P Value | |
|---|---|---|---|
| n | 38 | 59 | |
| EDVi, mL/m2 | 89 ± 21 | 109 ± 37 | 0.004 |
| ESVi, mL/m2 | 45 ± 14 | 59 ± 26 | 0.006 |
| SVi, mL/m2 | 43 ± 10 | 50 ± 16 | 0.022 |
| EF, % | 50 ± 6 | 48 ± 8 | 0.319 |
| CI, L⋅min−1⋅m−2 | 3.4 ± 1.2 | 4.6 ± 1.6 | 0.002 |
| Ao-P Coll, % | 17 (11–28) | 29 (18–41) | 0.007 |
Values are means ± SD or median with corresponding interquartile range. Ao-P Coll, aortopulmonary collateral flow as percent of the net aortic flow; CI, cardiac index; EDVi, end-diastolic volume index; EF, ejection fraction; ESVi, end-systolic volume index; SVi, stroke volume index.
Fig. 4.
The summary of subgroup analyses based on the 1st principal component describing diastolic- or systolic-dominant flow. Patients with diastolic-dominant flow had reduced end-diastolic volume index (EDVi; A), end-systolic volume index (ESVi; B), and stroke volume indices (SVi; C), but there was no difference in ejection fraction (EF; D). Additionally, patients with diastolic-dominant flow had reduced cardiac index (CI; E) and lesser burden of the aortopulmonary (AP) collateral flow (F). All graphs are depicted as means ± SD, except F, which is displayed as median with corresponding interquartile ranges.
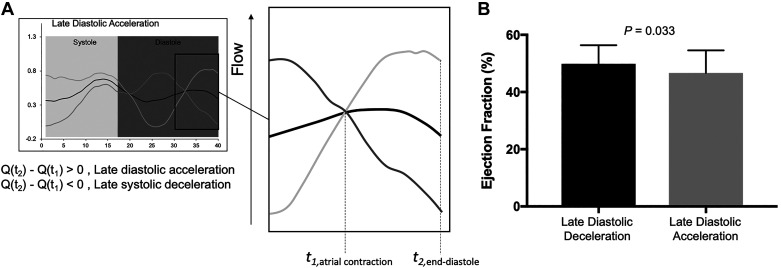
Similarly, we applied the primary differentiating feature, recognized by the second principal component—late diastolic acceleration—and categorized patients based on the following criteria: 1) the presence of late diastolic acceleration, as defined by positive difference in flow at the end of the cardiac cycle and flow at the beginning time of atrial contraction and 2) the presence of late diastolic deceleration, as defined by the negative difference in flow at the end of the cardiac cycle and flow at the beginning time of atrial contraction (Fig. 5). Patients with late diastolic deceleration had an increased ejection fraction when compared with those with late diastolic acceleration (50 ± 7 vs. 47 ± 7%, P = 0.033). No further differences in considered MRI hemodynamics were observed between patients with late diastolic acceleration and deceleration.
Fig. 5.
A: the subanalysis based on the 2nd principal component. Late diastolic acceleration was defined as a positive flow difference (Q) between the flow at end of the cardiac cycle (t2) and beginning of the atrial contraction (t1). B: late diastolic deceleration was defined as a negative flow difference.
DISCUSSION
Low pulmonary vascular resistance and minimal flow-mediated energy loss are important components of efficient flow transduction across the pulmonary circulation in the absence of a subpulmonary ventricle (5, 24). However, additional diastolic suction-driving forces generated by the single ventricle and respiratory muscles are important contributors to the overall transpulmonary pressure gradients (12, 14). Our results show that nonpulsatile flow in the pulmonary arteries of patients with the Fontan circulation exhibits oscillatory characteristics associated with single ventricle volume and systolic function, important predictors of clinical outcomes in patients with functional single ventricle (23). The two most important characteristics of cavopulmonary flow by our analysis were related to diastole (both whether flow pattern had a dominant diastolic wave and also the nature at end diastole), suggesting that single ventricular diastolic function and the trans-atrioventricular valve suction that is generated during ventricular filling play an important role in accelerating flow through the precapillary pulmonary circulation.
Systolic- versus diastolic-dominant flow.
Previous studies using phase-contrast MRI have described the biphasic flow pattern through pulmonary arteries in patients with Fontan circulation (10, 15), which was similar to our observed averaged flow pattern. However, the specific alterations in observed phasic patterns were not delineated, due to small sample size in considered studies. In our study, the first PC, accounting for 31.3% of total flow profile variation in the right pulmonary artery, delineated patients with either systolic- or diastolic-dominant flow. Furthermore, diastolic-dominant flow was associated with the smaller ventricular size and lower volume of the aortopulmonary collateral flow. During early diastole, ventricular relaxation generates an aspirating suction force that propagates through the pulmonary veins toward the capillary bed by means of a backward traveling expansion wave that assists with the generation of preload (1, 32). This expansion wave accelerates the blood flow and reduces pressure in the pulmonary arteries (22, 25, 29), in other words, relaxing single ventricle functions, such as suctioning—aspirating force assisting the blood return from pulmonary veins. Whether an expansion wave can propagate beyond the capillary network with sufficient intensity to induce the observed diastolic increase in flow rate is yet to be determined. However, with the onset of diastolic dysfunction, restrictive ventricular filling attenuates the relaxation, and the expansion wave decreases (21, 32).
Single ventricle stiffness and impaired relaxation properties have been emphasized as important predictors of exercise capacity, as well as early and long-term clinical outcomes after Fontan operation (1, 6, 9, 23). The myocardial structural changes with ventricular dilation are typical manifestations of diastolic dysfunction (20a). Indeed, increased end-systolic and end-diastolic single ventricle volumes were previously associated with death and transplantation (23). This finding was partially supported in our study by an observed inverse association between diastolic-dominant flow and elevated single ventricle volumes. We believe that the similarly observed association with the cardiac index was primarily driven by an elevated stroke volume index in patients with systolic-dominant flow. However, we previously observed an increased cardiac index in patients with Fontan circulation and pulmonary hypertension, which seemed to be more related to increased heart rate (25, 26). We also speculate that these patients were in a compensatory stage with preserved ventricular-vascular coupling, leading to normal stroke volume and consequently, elevated cardiac index.
Further studies by Border et al. (1) and Garofalo et al. (6) associated isovolumic ventricular relaxation τ and β constants, respectively, with worse, early postoperative outcomes. Given that the magnitude of expansion waves generated by ventricular relaxation is reduced by increased ventricular volume and elevated diastolic pressures (32), we speculate that reduced diastolic cavopulmonary flow might be a sign of diastolic dysfunction in patients with Fontan physiology. However, in this study, we did not directly study the effect of single ventricle compliance on expansion waves traveling through the pulmonary vasculature, and furthermore, physiological models will be needed to assess the effect of the Fontan single ventricle relaxation properties on pulmonary flow patterns. Standard echocardiographic Doppler and tissue-Doppler markers of diastolic dysfunction also have been shown to be highly abnormal in patients with Fontan circulation, yet their exact interpretation in the setting of single ventricle physiology remains uncertain (18, 20). Lastly, we did not evaluate the flow profiles with respect to the pulmonary vascular resistance and presence of pulmonary hypertension. The pulmonary hypertension is in the setting of single ventricle physiology, difficult to evaluate, and is influenced by the presence of fenestration and collateral flow. Our future studies will focus on combined catheterization and MRI studies evaluating pulmonary vascular resistance in patients with Fontan physiology and its effect on a generated pulmonary flow profile.
Late diastolic acceleration versus deceleration.
The second PC described the flow variation during the late portion of diastole corresponding to atrial contraction. Atrial function is an increasingly recognized marker of ventricular diastolic dysfunction (17). The dominant atrium in patients with Fontan circulation has been shown to undergo functional and morphological remodeling, resulting in reduced compliance and increased reliance on active atrial contraction to support ventricular filling (17a). In the scenario of adequate coupling of atrial contraction and ventricular filling, atria with preserved contractile function generate a backward compression wave that is transduced to the pulmonary circulation, manifesting as A-wave reversal on a pulsed-wave Doppler pattern. Such a compression wave temporarily increases pressure and decelerates flow. However, at the stage of severe diastolic dysfunction, pressure- and volume-overloaded atria lose effective contractile function (20a), and the magnitude of the backward traveling compression wave is consequently reduced. We speculate that patients with Fontan circulation and preserved atrial function generate a sufficient backward compression wave, which momentarily decelerates flow during late diastole. The pattern of late diastolic flow acceleration/deceleration in pulmonary arteries of patients with Fontan physiology was also noticeable in a study by Hager et al. (10). In this study, patients with late diastolic deceleration were more likely to have a higher ventricular ejection fraction, but there was no association with ventricular size. We did not measure the single atrial volume, as this measurement might be prone to considerable error. Future studies combining atrial strain analyses and MRI-based flow studies will be necessary to address the relationship between the atrial function and late diastolic flow patterns in pulmonary arteries.
Limitations.
We acknowledge several limitations related to this study. First, acquired flow hemodynamic profiles were acquired only in the right pulmonary artery. Therefore, nonuniform flow distribution between left and right pulmonary arteries or distal pulmonary arterial stenoses might have affected the generated flow profiles. We attempted to mitigate this problem by only selecting patients without known right and left pulmonary arterial obstructions. Second, in this study, we did not investigate the effect of respiration on pulmonary arterial flow. Respiration is an important contributor to the overall aspirating forces aiding with the central venous return. However, previous investigations that study an impact of respiration of pulmonary arterial flow in Fontan patients have suggested that respiration primarily modulates the overall magnitude of the flow, whereas phasic cardiac patterns remain consistent (12, 14). Third, this study did not directly study the physiological relationship between the components of the ventricular diastolic function (ventricular stiffness and myocardial relaxation) and flow hemodynamic patterns observed in pulmonary arteries. We are planning to perform a detailed, diastology-oriented study in the near future with particular attention to the preload factor. Assessment of the diastolic dysfunction in patients with single ventricle physiology is rather challenging, with a majority of standard Doppler indices considered unreliable. Our future investigations will attempt to characterize diastolic dysfunction using MRI-based tissue tracking and relate these findings to pulmonary flow patterns. We consider this study as explorative and hypothesis generating, and we encourage replication in a larger patient size and in patients with long-term clinical outcomes.
Conclusions.
Principal components describing the flow variations in the right pulmonary artery of Fontan patients are related to the single ventricle function and volumes. Particularly, diastolic-dominant flow without late acceleration appears to be related to more ideal ventricular volume and systolic function, respectively. We find a relationship between the ventricular diastolic function and pulmonary artery flow patterns in patients with Fontan circulation; further physiological models will be necessary to confirm this relationship. Our results imply that pulmonary arterial flow profile characteristics in patients with Fontan circulation may provide physiological, mechanistic, and clinical insights into overall function of the single ventricle and aid with the overall follow-up of this patient population.
GRANTS
This study was supported by a generous gift from the Rady Family Foundation and Jayden DeLuca Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S., B.S.F., K.S.H., A.Y., and M.V.D.M. conceived and designed research; M.S., K.L.C., R.J., G.J.M., A.J.B., L.P.B., and M.V.D.M. performed experiments; M.S., S.M.H., K.L.C., M.L.S., G.J.M., A.J.B., L.P.B., and M.V.D.M. analyzed data; M.S., B.S.F., S.M.H., K.S.H., R.J., M.B.M., J.J., M.L.S., G.J.M., A.J.B., D.D.I., A.Y., and M.V.D.M. interpreted results of experiments; M.S. prepared figures; M.S., B.S.F., and M.V.D.M. drafted manuscript; M.S., B.S.F., S.M.H., K.S.H., K.L.C., R.J., M.B.M., J.J., M.L.S., G.J.M., A.J.B., L.P.B., D.D.I., A.Y., and M.V.D.M. edited and revised manuscript; M.S., B.S.F., S.M.H., K.S.H., K.L.C., R.J., M.B.M., J.J., M.L.S., G.J.M., A.J.B., L.P.B., D.D.I., A.Y., and M.V.D.M. approved final version of manuscript.
REFERENCES
- 1.Border WL, Syed AU, Michelfelder EC, Khoury P, Uzark KC, Manning PB, Pearl JM. Impaired systemic ventricular relaxation affects postoperative short-term outcome in Fontan patients. J Thorac Cardiovasc Surg 126: 1760–1764, 2003. doi: 10.1016/j.jtcvs.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Bruse JL, Zuluaga MA, Khushnood A, McLeod K, Ntsinjana HN, Hsia TY, Sermesant M, Pennec X, Taylor AM, Schievano S. Detecting clinically meaningful shape clusters in medical image data: metrics analysis for hierarchical clustering applied to healthy and pathological aortic arches. IEEE Trans Biomed Eng 64: 2373–2383, 2017. doi: 10.1109/TBME.2017.2655364. [DOI] [PubMed] [Google Scholar]
- 3.Casciaro ME, Craiem D, Chironi G, Graf S, Macron L, Mousseaux E, Simon A, Armentano RL. Identifying the principal modes of variation in human thoracic aorta morphology. J Thorac Imaging 29: 224–232, 2014. doi: 10.1097/RTI.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 4.de Leval MR, Kilner P, Gewillig M, Bull C, McGoon DC. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg 96: 682–695, 1988. doi: 10.1016/S0022-5223(19)35174-8. [DOI] [PubMed] [Google Scholar]
- 5.Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart 98: 1098–1104, 2012. doi: 10.1136/heartjnl-2011-301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garofalo CA, Cabreriza SE, Quinn TA, Weinberg AD, Printz BF, Hsu DT, Quaegebeur JM, Mosca RS, Spotnitz HM. Ventricular diastolic stiffness predicts perioperative morbidity and duration of pleural effusions after the Fontan operation. Circulation 114, Suppl: I56–I61, 2006. doi: 10.1161/CIRCULATIONAHA.105.001396. [DOI] [PubMed] [Google Scholar]
- 7.Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart 102: 1081–1086, 2016. doi: 10.1136/heartjnl-2015-307467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glatz AC, Rome JJ, Small AJ, Gillespie MJ, Dori Y, Harris MA, Keller MS, Fogel MA, Whitehead KK. Systemic-topulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes. Circ Cardiovasc Imaging 5: 218–225, 2012. doi: 10.1161/CIRCIMAGING.111.966986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein BH, Connor CE, Gooding L, Rocchini AP. Relation of systemic venous return, pulmonary vascular resistance, and diastolic dysfunction to exercise capacity in patients with single ventricle receiving fontan palliation. Am J Cardiol 105: 1169–1175, 2010. doi: 10.1016/j.amjcard.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Hager A, Fratz S, Schwaiger M, Lange R, Hess J, Stern H. Pulmonary blood flow patterns in patients with Fontan circulation. Ann Thorac Surg 85: 186–191, 2008. doi: 10.1016/j.athoracsur.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Hauser JA, Taylor AM, Pandya B. How to image the adult patient with fontan circulation. Circ Cardiovasc Imaging 10: 1–7, 2017. doi: 10.1161/CIRCIMAGING.116.004273. [DOI] [PubMed] [Google Scholar]
- 12.Hjortdal VE, Emmertsen K, Stenbøg E, Fründ T, Schmidt MR, Kromann O, Sørensen K, Pedersen EM. Effects of exercise and respiration on blood flow in total cavopulmonary connection: a real-time magnetic resonance flow study. Circulation 108: 1227–1231, 2003. doi: 10.1161/01.CIR.0000087406.27922.6B. [DOI] [PubMed] [Google Scholar]
- 13.Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A. Moving beyond P values: data analysis with estimation graphics. Nat Methods 16: 565–566, 2019. doi: 10.1038/s41592-019-0470-3. [DOI] [PubMed] [Google Scholar]
- 14.Honda T, Itatani K, Takanashi M, Kitagawa A, Ando H, Kimura S, Nakahata Y, Oka N, Miyaji K, Ishii M. Contributions of respiration and heartbeat to the pulmonary blood flow in the Fontan circulation. Ann Thorac Surg 102: 1596–1606, 2016. doi: 10.1016/j.athoracsur.2016.03.101. [DOI] [PubMed] [Google Scholar]
- 15.Houlind K, Stenbøg EV, Sørensen KE, Emmertsen K, Hansen OK, Rybro L, Hjortdal VE. Pulmonary and caval flow dynamics after total cavopulmonary connection. Heart 81: 67–72, 1999. doi: 10.1136/hrt.81.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis K, Schnell S, Barker AJ, Garcia J, Lorenz R, Rose M, Chowdhary V, Carr J, Robinson JD, Rigsby CK, Markl M. Evaluation of blood flow distribution asymmetry and vascular geometry in patients with Fontan circulation using 4-D flow MRI. Pediatr Radiol 46: 1507–1519, 2016. doi: 10.1007/s00247-016-3654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jone P-N, Schäfer M, Li L, Craft M, Ivy DD, Kutty S. Right atrial deformation in predicting outcomes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging 10: e006250, 2017. doi: 10.1161/CIRCIMAGING.117.006250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Khoo NS, Smallhorn JF, Kaneko S, Kutty S, Altamirano L, Tham EB. The assessment of atrial function in single ventricle hearts from birth to Fontan: a speckle-tracking study by using strain and strain rate. J Am Soc Echocardiogr 26: 756–764, 2013. doi: 10.1016/j.echo.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Margossian R, Sleeper LA, Pearson GD, Barker PC, Mertens L, Quartermain MD, Su JT, Shirali G, Chen S, Colan SD; Pediatric Heart Network Investigators . Assessment of diastolic function in single-ventricle patients after the Fontan procedure. J Am Soc Echocardiogr 29: 1066–1073, 2016. doi: 10.1016/j.echo.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markl M, Geiger J, Kilner PJ, Föll D, Stiller B, Beyersdorf F, Arnold R, Frydrychowicz A. Time-resolved three-dimensional magnetic resonance velocity mapping of cardiovascular flow paths in volunteers and patients with Fontan circulation. Eur J Cardiothorac Surg 39: 206–212, 2011. doi: 10.1016/j.ejcts.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Mertens L, Khairy P. Right ventricular diastolic function in congenital heart disease. Can J Cardiol 29: 755–756, 2013. doi: 10.1016/j.cjca.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 20a.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh KJ, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29: 277–314, 2016. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Ntsinjana HN, Chung R, Ciliberti P, Muthurangu V, Schievano S, Marek J, Parker KH, Taylor AM, Biglino G. Utility of cardiovascular magnetic resonance-derived wave intensity analysis as a marker of ventricular function in children with heart failure and normal ejection fraction. Front Pediatr 5: 65, 2017. doi: 10.3389/fped.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker KH. An introduction to wave intensity analysis. Med Biol Eng Comput 47: 175–188, 2009. doi: 10.1007/s11517-009-0439-y. [DOI] [PubMed] [Google Scholar]
- 23.Rathod RH, Prakash A, Kim YY, Germanakis IE, Powell AJ, Gauvreau K, Geva T. Cardiac magnetic resonance parameters predict transplantation-free survival in patients with fontan circulation. Circ Cardiovasc Imaging 7: 502–509, 2014. [Erratum in Circ Cardiovasc Imaging 11: e000021, 2018]. doi: 10.1161/CIRCIMAGING.113.001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rijnberg FM, Hazekamp MG, Wentzel JJ, de Koning PJH, Westenberg JJM, Jongbloed MRM, Blom NA, Roest AAW. Energetics of blood flow in cardiovascular disease: Concept and clinical implications of adverse energetics in patients with a fontan circulation. Circulation 137: 2393–2407, 2018. doi: 10.1161/CIRCULATIONAHA.117.033359. [DOI] [PubMed] [Google Scholar]
- 25.Schäfer M, Wilson N, Ivy DD, Ing R, Abman S, Browne LP, Morgan G, Ross M, McLennan D, Barker AJ, Fonseca B, Di Maria M, Hunter KS, Truong U. Noninvasive wave intensity analysis predicts functional worsening in children with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 315: H968–H977, 2018. doi: 10.1152/ajpheart.00227.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schäfer M, Younoszai A, Truong U, Browne LP, Mitchell MB, Jaggers J, Campbell DN, Hunter KS, Ivy DD, Di Maria MV. Influence of aortic stiffness on ventricular function in patients with Fontan circulation. J Thorac Cardiovasc Surg 157: 699–707, 2019. doi: 10.1016/j.jtcvs.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 29.Su J, Hilberg O, Howard L, Simonsen U, Hughes AD. A review of wave mechanics in the pulmonary artery with an emphasis on wave intensity analysis. Acta Physiol (Oxf) 218: 239–249, 2016. doi: 10.1111/apha.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundareswaran KS, Haggerty CM, de Zélicourt D, Dasi LP, Pekkan K, Frakes DH, Powell AJ, Kanter KR, Fogel MA, Yoganathan AP. Visualization of flow structures in Fontan patients using 3-dimensional phase contrast magnetic resonance imaging. J Thorac Cardiovasc Surg 143: 1108–1116, 2012. doi: 10.1016/j.jtcvs.2011.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang E, Haggerty CM, Khiabani RH, de Zélicourt D, Kanter J, Sotiropoulos F, Fogel MA, Yoganathan AP. Numerical and experimental investigation of pulsatile hemodynamics in the total cavopulmonary connection. J Biomech 46: 373–382, 2013. doi: 10.1016/j.jbiomech.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Jalali F, Sun YH, Wang JJ, Parker KH, Tyberg JV. Assessment of left ventricular diastolic suction in dogs using wave-intensity analysis. Am J Physiol Heart Circ Physiol 288: H1641–H1651, 2005. doi: 10.1152/ajpheart.00181.2004. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead KK, Harris MA, Glatz AC, Gillespie MJ, DiMaria MV, Harrison NE, Dori Y, Keller MS, Rome JJ, Fogel MA. Status of systemic to pulmonary arterial collateral flow after the fontan procedure. Am J Cardiol 115: 1739–1745, 2015. doi: 10.1016/j.amjcard.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao F, Zhang H, Wahle A, Thomas MT, Stolpen AH, Scholz TD, Sonka M. Congenital aortic disease: 4D magnetic resonance segmentation and quantitative analysis. Med Image Anal 13: 483–493, 2009. doi: 10.1016/j.media.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]