Atrial fibrillation (AF) is the most prevalent sustained cardiac arrhythmia worldwide1 and it is associated with high morbidity and mortality, ultimately making it a major public health burden.
Omega-3 fatty acids (O3FA) supplementation are being utilized in clinical practice to reduce cardiovascular disease (CVD) risk in patients with elevated plasma triglycerides. Safety has been, however, questioned as several cardiovascular (CV) outcomes trials of O3FA supplementation showed a potential increase of AF, when compared with placebo.2
In this meta-analysis of randomized controlled trials (RCTs), we investigated whether O3FA supplementation is associated with an increased risk for AF compared to placebo. We conducted a systematic search of RCTs of O3FA supplementation on CV outcomes, which also included data on the incidence of AF up to November 2020.
The main endpoint of the study was the onset of AF. For inferential purposes, a frequentist pairwise meta-analysis was conducted by using Poisson regression model with random study effects. Incidence rate ratio (IRR) and 95% confidence interval (CI) were chosen over relative risk as outcome measure because of the different follow-up of the selected studies.
The pairwise meta-analysis was performed with the R statistical software (4.0.0 version) using R package ‘meta’. Heterogeneity across studies was assessed with Cochran’s Q method and I2 testing. A threshold of P < 0.10 was used to define the presence of heterogeneity for the Q test. I2 was considered substantial when it was >50%. The presence of publication bias for small study effect appraisal was assessed by visual examination of funnel plots and was quantified by Egger’s test and Begg’s test.
Detailed characteristics of the five included studies are presented in Supplementary material online, Table S1.3–7
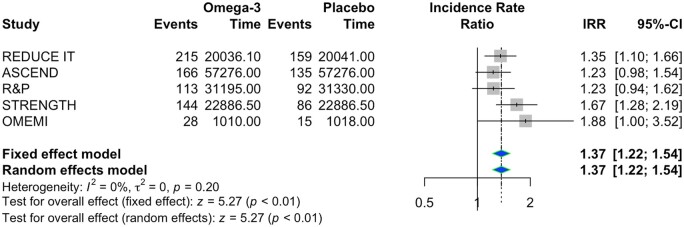
In the random effect model, O3FA supplementation was associated with an increased risk of incident AF as compared with placebo [IRR 1.37, 95% CI (1.22–1.54), P < 0.001] (Figure 1). There were no significant statistical heterogeneity between studies and no publication bias, even if the funnel plot suggested some asymmetry. As a sensitivity analysis, we included the VITAL rhythm trial. Results confirmed a higher risk of AF in the group receiving O3FA supplementation as compared with placebo [IRR 1.29, 95% CI (1.13–1.48), P = 0.0002] (Supplementary material online, Figure S1).
Figure 1.
Forest plot for atrial fibrillation events. IRR, incidence rate ratio.
The results of this meta-analysis show that individuals at high risk for, or with established CVD and elevated plasma triglycerides treated with O3FA supplementation have a significantly higher incidence of AF events, compared to placebo. We also found no heterogeneity and no small study publication bias.
O3FA supplementation has been utilized to reduce CVD and CVD-related mortality in patients at high CV risk and elevated plasma triglycerides. The CV benefits, however, have been inconsistent; specifically, in the REDUCE-IT trial, a high dose of O3FA supplementation with highly purified icosapent ethyl was associated with a significantly lower risk for the primary composite CV endpoint3. Contrarily, the recent STRENGTH trial, which utilized the same dose of O3FA supplementation, but containing a formulation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), was neutral on the composite primary endpoint.6 Moreover, lower doses of O3FA supplementation have not improved CV outcomes.2
Although the positive effects of O3FA supplementation on the composite CV endpoints have been inconsistent, most trials have been associated with an increased risk for AF occurrence. Patients receiving icosapent ethyl in the REDUCE-IT3 trial presented a significantly greater risk for incidence AF or atrial flutter, which was further confirmed in STRENGTH. Even lower doses of O3FA supplementation has shown a potential signal for increased risk for AF,2,4,5 proposing that regardless of the dose of O3FA supplementation implemented in the trials and potential-related benefits on CV outcomes, O3FA supplementation may increase the risk for AF. The results of our meta-analysis confirmed this finding, which is concerning given the large proportion of patients eligible for treatment with O3FA supplementation.
The conflicting results of the beneficial effects of O3FA supplementation on CV outcomes along with their potential risk for harm, highlight the need for future studies to ultimately confirm the potential beneficial effects of this class of drugs.
Moreover, the mechanisms through which O3FA supplementation may increase the risk for AF remain largely unknown, clearly highlighting the need for more mechanistic clinical trials to investigate such effects. In fact, O3FA have been previously shown to stabilize the cardiac membrane resulting in protective effects against arrhythmias, including ventricular arrhythmias.2,8 Yet, some previous studies reported a higher post-operative AF in patients with elevated O3FA levels. Of note, dedicated studies investigating the effects of O3FA supplementation on ventricular arrhythmias, and in targeted high-risk populations (e.g. post-myocardial infarction patients) remain to be determined and further study encouraged.
This study has some limitations such as the lack of systematic haemorrhagic risk of the patients, the lack of a systematic search for AF events in the individual studies, and the fact that some of the studies did not include AF as a prespecified outcome, potentially resulting in under-reporting of AF-related events. We conducted a study-level meta-analysis, with individual aspects of the participants not being accounted for. Moreover, although placebo arms were often different among the trials, we did not find heterogeneity in the results. Finally, we only included a sensitivity analysis on the VITAL rhythm results presented at the American Heart Association 2020, as the fully data have not been disclosed yet.
In conclusion, our study suggests that O3FA supplementation is associated with an increased risk of AF in patients with elevated plasma triglyceride and at elevated CV risk. This proposes that the risk of AF should be considered when prescribing O3FA supplementation in this population.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Funding
S.C. was supported by a Career Development Award 19CDA34660318 from the American Heart Association and by the Clinical and Translational Science Awards Program UL1TR002649 from National Institutes of Health to Virginia Commonwealth University.
Conflict of interest: G.B.-Z. has consulted for Cardionovum, Innovheart, Meditrial, and Replycare. The remaining authors have nothing to disclose.
Supplementary Material
References
- 1. Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles-Gonzalez JF, Paydak H.. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation 2014;129:2371–2379. [DOI] [PubMed] [Google Scholar]
- 2. Lombardi M, Chiabrando JG, Vescovo GM, Bressi E, Del Buono MG, Carbone S, Koenig RA, Van Tassell BW, Abbate A, Biondi-Zoccai G, Dixon DL.. Impact of different doses of omega-3 fatty acids on cardiovascular outcomes: a pairwise and network meta-analysis. Curr Atheroscler Rep 2020;22:45. [DOI] [PubMed] [Google Scholar]
- 3. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, Tardif J-C, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 4. Kromhout D, Giltay EJ, Geleijnse JM; For the AOTG. N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–2026. [DOI] [PubMed] [Google Scholar]
- 5. Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R; Risk and Prevention Study Collaborative Group. N-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 2013;368:1800–1808. [DOI] [PubMed] [Google Scholar]
- 6. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundström T, Agrawal R, Menon V, Wolski K, Nissen SE.. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020;324:2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, Nilsen DWT, Tveit A, Fagerland MW, Solheim S, Seljeflot I, Arnesen H; OMEMI Investigators. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized controlled trial. Circulation 2020. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.052209. [DOI] [PubMed] [Google Scholar]
- 8.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–455. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.