Abstract
Aims
None of the existing studies on adherence have directly measured levels of all medications (or their metabolites) in patients with heart failure (HF).
Methods and results
We used liquid chromatography–tandem mass spectrometry to measure the presence of prescribed drugs (diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and mineralocorticoid receptor antagonists) in the urine of patients reviewed 4–6 weeks after hospitalization with HF. Patients were unaware that adherence was being assessed. Of the 341 patients studied, 281 (82.4%) were adherent, i.e. had all prescribed drugs of interest detectable in their urine. Conversely, 60 patients (17.6%) were partially or completely non-adherent. Notably, 24 of the 60 were non-adherent to only diuretic therapy and only seven out of all 341 patients studied (2.1%) were completely non-adherent to all prescribed HF drugs. There were no major differences in baseline characteristics between adherent and non-adherent patients.
Conclusion
Non-adherence, assessed using a single spot urine measurement of drug levels, was confirmed in one of five patients evaluated 4–6 weeks after hospitalization with HF.
Keywords: Adherence, Heart failure, Mass spectrometry, Urine
Introduction
Adherence is a foundation of successful pharmacological therapy. In heart failure (HF), higher adherence is associated with lower mortality and less frequent HF hospitalization and better adherence may reduce the need for costly advanced device therapies and even cardiac transplantation.1 Conversely, non-adherence is an independent predictor of a higher risk of hospitalization for HF and poorer survival.1,2 It is suggested that non-adherence rates range from 5% to 60% in some cohorts with HF, but the true prevalence is not known.3 Many patient factors associated with poor adherence such as higher number of comorbid conditions, complexity of medical regimens, and depression, are all prevalent amongst patients with HF.4 However, the rates of non-adherence reported in patients with HF vary widely in existing studies. This may in part reflect the different ways in which adherence was measured in these studies, including by pill counting, questionnaires, electronic-monitoring devices, and review of prescription claim databases.5–9 None of these methods are considered entirely reliable as they depend, variously, on patient reporting or return of pill boxes, accuracy of electronic records and patients taking the treatment which they have been dispensed, among others. Indeed, each of these methods is thought to overestimate true adherence.10 Direct measurement of drug levels provides objective evidence of adherence. Assays for most drugs prescribed in HF are available and have been used to examine adherence in other health conditions.11 This has seldom been performed in contemporary cohorts of patients with HF, although adherence to digoxin therapy using serum concentrations in patients with HF is well described.12 We have used liquid chromatography–tandem mass spectrometry assays to directly measure the presence of prescribed drugs in the urine of patients with HF.
Methods
Patients studied
In this post hoc exploratory analysis, we used a previously reported cohort of near consecutive patients hospitalized with HF who were enrolled in a prospective observational study of the association between microvolt T-wave alternans and mortality.13,14 Patients were recruited from three hospitals in Glasgow from December 2006 to January 2009. Heart failure was defined according to European Society of Cardiology guidelines and brain natriuretic peptide >100 pg/mL was required for enrolment in the study.15 Urine samples were obtained at the study visit, 4–6 weeks after hospital discharge and stored at −70°C.
Adherence measure
Urine samples were batched analysed after purification by solvent extraction and dilution. The analysis took place on an Agilent Technologies 1290 series High Pressure Liquid Chromatograph interfaced with an Agilent Technologies 6460 Triple Quad Mass Spectrometer fitted with a Jetstream electrospray source. The techniques used have been described previously.11 Each urine sample was analysed twice, in positive and negative ion mode. A potential of 40 medications was measured as listed in our previous work.11 The assay is a qualitative yes/no assay. Compounds were identified by using 2 or 3 unique m/z ratios and most of the compounds have a detection limit of 5–10 nanogram per mL. Non-detection implies that the medication of interest was not ingested for at least four half-lives prior to sample collection; this is generally more than 24 h except for loop diuretics such as furosemide where it is between 4 and 12 h.16 Further details of the technique used are described in the Supplementary material online, Appendix. As these analyses were conducted retrospectively, patients were not aware that their adherence to prescribed therapies was being assessed. Patients provided written consent for storage of samples of urine for future biomarker analyses.
Definition of adherence
Full (complete) adherence was defined as detection of all prescribed HF medications in a patient’s urine. Two categories of non-adherence were considered; non-adherence to diuretic therapy alone, and non-adherence to disease-modifying drugs. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and mineralocorticoid receptor antagonists were classed as ‘disease-modifying therapy’. The term partial non-adherence is used to refer to patients who were non-adherent to one or more of their prescribed HF medications, irrespective of class of medication. We looked specifically at non-adherence to diuretic therapy, as patients may choose to (or be advised to) withhold diuretics when travelling to attend hospital appointments or when undertaking other journeys or social outings.
Statistical analyses
Categorical data are presented as numbers and percentages. For continuous data, normally distributed data are summarized as the mean and standard deviation. Medians and interquartile ranges are used where data were not normally distributed.
When comparing parameters between patients who were adherent and those who were not, a P-value of <0.05 was considered statistically significant. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.1.2.
The study was approved by the Local Ethical Committee and complied with the Declaration of Helsinki. As collection of urine was only commenced after a protocol amendment, consent for storing and further testing of urine samples was provided by a subset of patients in the original study. The data underlying this article are available in the article and in its online supplementary material.
Results
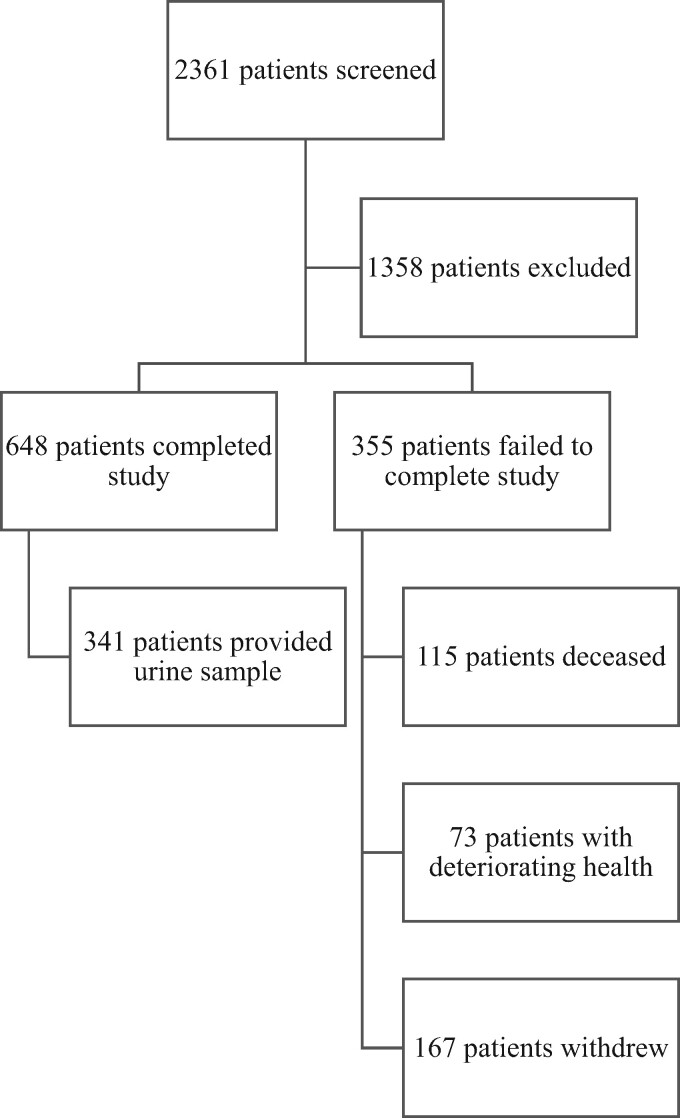
A total of 1003 patients were enrolled during a hospital admission. Of these, 648 patients returned for the 4- to 6-week study visit. A CONSORT diagram of original study recruitment is shown in Figure 1. Once ethical approval for obtaining a urine sample was in place, 341 of 342 consecutive patients attending the study visit provided a sample. All 341 samples were analysed. The mean left ventricular ejection fraction (LVEF) was 40% (SD 11.6%). A total of 77% of patients had reduced ejection fraction, defined at time of recruitment as an ejection fraction <50%. One patient in the cohort had an implantable cardioverter-defibrillator.
Figure 1.
CONSORT diagram of original study recruitment.
Considering assays of all drugs prescribed for HF, 281 of these 341 patients (82.4%) were fully adherent, i.e. had all the prescribed drugs of interest for HF detectable in their urine sample. Conversely, 60 of the 341 patients (17.6%) were partially or completely non-adherent with prescribed HF treatments. Of note, 24 of the 60 (7% of the study population, 40.0% of non-adherent patients), were non-adherent to only diuretic therapy. Of all 341 patients, 7 (2.1%) were completely non-adherent to their prescribed HF medications Of the 60 patients with partial or complete non-adherence, non-adherence was evident for 22 of 47 prescribed an angiotensin converting enzyme inhibitor (ACE-I) [9.1% of all patients prescribed ACE-I (n = 242)]. The equivalent numbers for other drugs were: angiotensin receptor blocker (ARB) 7/12 (18.9% of 37), beta-blocker 9/36 (3.9% of 232), mineralocorticoid receptor antagonist 2/7 (3.9% of 51), and diuretic 33/60 (9.7% of 339) (categories are not mutually exclusive). The clinical characteristics of the patients according to adherence subgroup are shown in Table 1.
Table 1.
Baseline characteristics according to adherence category
| Adherent to all medication (A) | Non-adherent to at least one medication (B) | P-value A/B | Non-adherent to one or more disease-modifying therapy (C) | Non-adherent to diuretic only (D) | P-value C/D | |
|---|---|---|---|---|---|---|
| 281a | 60a | 36a | 24a | |||
| Demographics | ||||||
| Age (years) | 72.1 (10.3) | 69.7 (12.1) | 0.42 | 71.6 (12.6) | 67.0 (11.0) | 0.14 |
| Male | 170 (60.5) | 40 (66.7) | 0.47 | 26 (72.2) | 14 (58.3) | 0.28 |
| SIMD quintile | 0.93 | 0.20 | ||||
| Total | 267 | 59 | 35 | 24 | ||
| 1 | 133 (49.8) | 29 (49.2) | 16 (45.7) | 13 (54.2) | ||
| 2 | 46 (17.2) | 13 (22.0) | 8 (22.9) | 5 (20.8) | ||
| 3 | 32 (12.0) | 7 (11.9) | 6 (17.1) | 1 (4.2) | ||
| 4 | 27 (10.1) | 5 (8.5) | 4 (11.4) | 1 (4.2) | ||
| 5 | 29 (10.9) | 5 (8.5) | 1 (2.9) | 4 (16.7) | ||
| Heart failure status | ||||||
| LVEF (%) | 40.7 (11.7) | 37.7 (10.7) | 0.07 | 36.8 (10.6) | 39.0 (10.8) | 0.45 |
| NYHA classification | 0.62 | 0.26 | ||||
| I | 4 (1.4) | 2 (3.3) | 0 (0.0) | 2 (8.3) | ||
| II | 181 (64.4) | 37 (61.7) | 23 (63.9) | 14 (58.3) | ||
| III | 94 (33.5) | 21 (35.0) | 13 (36.1) | 8 (33.3) | ||
| IV | 2 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Duration of diagnosis >2 years | 89 (31.7) | 20 (33.3) | 0.94 | 11 (30.6) | 9 (37.5) | 0.20 |
| Ischaemic aetiology | 250 (89.0) | 50 (83.3) | 0.27 | 30 (83.3) | 20 (83.3) | 1.00 |
| Clinical signs and symptoms | ||||||
| Heart rate (b.p.m.) | 74.6 (14.9) | 78.3 (16.9) | 0.14 | 78.0 (19.2) | 78.8 (13.0) | 0.86 |
| Systolic blood pressure (mmHg) | 129.8 (22.3) | 137.3 (24.1) | 0.05 | 135.5 (22.0) | 140.1 (27.2) | 0.49 |
| Diastolic blood pressure (mmHg) | 67.0 (13.1) | 71.8 (12.1) | 0.006 | 70.0 (12.7) | 74.5 (10.9) | 0.16 |
| Orthopnoea | 90 (32.0) | 14 (23.3) | 0.22 | 8 (22.2) | 6 (25.0) | 1.00 |
| PND | 44 (15.7) | 6 (10.0) | 0.32 | 4 (11.1) | 2 (8.3) | 1.00 |
| Ankle swelling | 85 (30.2) | 15 (25.0) | 0.53 | 7 (19.4) | 8 (33.3) | 0.24 |
| Laboratory results | ||||||
| Estimated GFR (mL/min) | 58.0 (42.0–61.0) | 61.0 (49.5–61.0) | 0.10 | 61.0 (47.3–61.0) | 61.0 (58.0–61.0) | 0.47 |
| Potassium (mmol/L) | 4.0 (3.7–4.3) | 4.2 (3.8–4.4) | 0.09 | 4.1 (3.7–4.3) | 4.3 (4.1–4.7) | 0.08 |
| BNP (pg/mL) | 391.0 (202.0–870.0) | 430.5 (221.8–744.3) | 0.77 | 470.5 (261.8–799.8) | 393.0 (212.5–613.0) | 0.36 |
| Medical history | ||||||
| Hypertension | 188 (66.9) | 34 (56.7) | 0.14 | 19 (52.8) | 15 (62.5) | 0.60 |
| Valvular heart disease | 130 (46.3) | 28 (46.7) | 1.0 | 16 (44.4) | 12 (50.0) | 0.79 |
| Atrial fibrillation | 150 (53.4) | 33 (55.0) | 0.9 | 21 (58.3) | 12 (50.0) | 0.60 |
| Diabetes mellitus | 98 (34.9) | 17 (28.3) | 0.37 | 11 (30.6) | 6 (25.0) | 0.77 |
| COPD | 82 (29.2) | 15 (25.0) | 0.64 | 10 (27.8) | 2 (20.8) | 0.76 |
| Anaemia | 114 (40.7) | 34 (56.7) | 0.03 | 23 (63.9) | 11 (45.8) | 0.19 |
| Urinary incontinence | 24 (8.5) | 10 (16.7) | 0.09 | 4 (11.1) | 6 (25.0) | 0.18 |
| Previous MI | 116 (41.3) | 30 (50.0) | 0.25 | 18 (50.0) | 12 (50.0) | 1.00 |
| Previous PCI | 37 (13.2) | 11 (18.3) | 0.31 | 4 (11.1) | 7 (29.2) | 0.10 |
| Previous CABG | 50 (17.8) | 10 (16.7) | 1.0 | 5 (13.9) | 5 (20.8) | 0.50 |
| Depression | 68 (24.2) | 12 (20.0) | 0.62 | 5 (13.9) | 7 (29.2) | 0.19 |
| Smoking | 198 (70.5) | 42 (70.0) | 1.0 | 26 (72.2) | 16 (66.7) | 0.78 |
| Alcohol | 199 (70.8) | 46 (76.7) | 0.43 | 28 (77.8) | 18 (75.0) | 1.00 |
| Number of comorbidities (max 18) | 5 (0–9) | 5 (0–10) | 0.69 | 4 (1–10) | 5 (0–9) | 0.61 |
| Medications | ||||||
| ACE-I | 194 (69.0) | 48 (80.0) | 0.116 | 29 (80.6) | 19 (79.2) | 1.00 |
| ARB | 25 (8.9) | 12 (20.0) | 0.020 | 10 (27.8) | 2 (8.3) | 0.10 |
| ACE-I or ARB | 212 (75.4) | 56 (93.3) | 0.002 | 36 (100.0) | 20 (83.3) | 0.02 |
| Beta-blocker | 196 (69.9) | 36 (60.0) | 0.170 | 23 (63.9) | 13 (54.2) | 0.59 |
| Spironolactone | 44 (15.7) | 7 (11.7) | 0.551 | 4 (11.1) | 3 (12.5) | 1.00 |
| Digoxin | 47 (16.7) | 13 (21.7) | 0.355 | 9 (25.0) | 4 (16.7) | 0.53 |
| Loop diuretic | 272 (96.8) | 60 (100.0) | 0.370 | 36 (100.0) | 24 (100.0) | — |
| Thiazide diuretic | 5 (1.8) | 2 (1.7) | 1.000 | 0 (0.0) | 1 (4.2) | 0.40 |
| Number of cardiovascular medications prescribed | 4 (1–8) | 4 (1–7) | 0.359 | 3.5 (1–6) | 4 (1–7) | 0.48 |
| Number of non-cardiovascular medications prescribed | 3 (1–10) | 3 (1–6) | 0.339 | 3 (1–6) | 2 (1–5) | 0.09 |
Values are represented as mean ± standard deviation, n (%), or median (interquartile range).
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CABG, coronary artery bypass grafting; COPD, chronic obstructive airways disease; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PND, paroxysmal nocturnal dyspnoea; SIMD, Scottish Index of Multiple Deprivation.
The total number of patients studied was 341; of these, 333 were prescribed a diuretic and 311 disease-modifying drugs.
Comparison of adherent and non-adherent patients
There were few statistically significant differences between patients adherent and those non-adherent to their prescribed medications. In terms of medical history, non-adherent patients were more likely to be anaemic (P = 0.03) and there were higher proportions of non-adherent patients with urinary incontinence (16.7% vs. 8.5%; P = 0.09) although this difference was not statistically significant. Systolic and diastolic blood pressures (BPs) were higher (systolic BP: 137 mmHg vs. 130 mmHg; P = 0.05 and diastolic BP: 72 mmHg vs. 67 mmHg; P = 0.006) and LVEF tended to be lower (37.7 vs. 40.7%; P = 0.07) in non-adherent patients. Potassium levels tended to be higher in patients found to be non-adherent to any medication and highest in those non-adherent to disease-modifying therapy (although this difference was not statistically significant). A higher proportion of patients in lower deprivation categories were non-adherent to prescribed HF medications when compared with those in higher deprivation categories (49.2% vs. 8.5%) when categorized according to the Scottish Index of Multiple Deprivation. The number of prescribed cardiac and non-cardiac medications was the same for patients adherent and those non-adherent to their prescribed medications.
Patients non-adherent to diuretics only
There were few statistically significant differences between patients non-adherent to diuretics only compared with other HF treatments. In comparison to patients non-adherent to disease-modifying therapies, those non-adherent to diuretic therapy tended to have higher serum potassium levels (4.30 mmol/L vs. 4.10 mmol/L; P = 0.08) and were less likely to be prescribed an ACE-I or ARB (100.0 vs. 83.3%). A higher proportion of patients non-adherent to diuretics reported urinary incontinence compared with those non-adherent to disease-modifying therapies, and patients who were adherent (25% vs. 16.7% vs. 8.5).
Discussion
Adherence to prescribed medications can be assessed in a variety of ways. This is the largest report of the use of liquid chromatography–tandem mass spectrometry to assess the presence, or absence, of multiple prescribed medications in the urine of patients with HF. Using this objective approach, approximately 18% of patients in our cohort were observed to be non-adherent to one or more of their prescribed HF treatments. Both patients and investigators were unaware at the time of urine collection that treatment adherence would be investigated.
Comparison of our observed rate of non-adherence to those reported in previous studies in patients with HF is difficult in the context of the differing methods of assessment utilized in previous reports. Indeed, only one previous study has assessed directly the presence or absence of prescribed medications in biological samples from patients with HF.17 Pelouch et al.17 used serum drug levels to show that 25% patients with chronic HF were non-adherent to one or more prescribed drugs. In the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Programme non-adherence (defined as presumed consumption of less than 80% of prescribed study-drug as estimated by pill count over the whole duration of the trial), the non-adherence rate was 11%.1 In the Systolic Heart failure treatment with the If-inhibitor ivabradine Trial (SHIFT), non-adherence was defined very differently as premature and permanent discontinuation of study-drug (i.e. before the final study visit or death); the non-adherence rate in SHIFT was 19.8%.18 Both of these reports are from clinical trials, which included frequent monitoring and patient-prescriber contact, which might have led to higher adherence rates than in routine practice. Moreover, only adherence to study-drug was assessed. On the other hand, both of those reports considered persistence of tablet taking over time whereas we used a single spot urine sample. Another approach considered to give reliable data on adherence is the electronic medication event monitoring system (MEMS). Viana et al.19 used MEMS to measure adherence to 3 different drugs over a median of 96 (range 49–180) days in patients attending an HF clinic in Portugal. Patients were categorized as adherent when they took ≥88% of doses prescribed. Of the 63 patients studied, 22% were classified as non-adherent to ACE-Is, 30% to beta-blockers, and 30% to loop diuretics. These reported rates of non-adherence are higher than those found in our study where of 341 patients studied, 9.1% were non-adherent to ACE-Is, 3.9% to beta-blockers, and 9.7% to loop diuretics. The influence of the ‘spot-check’ nature of our study rather than a cumulative percentage measure of adherence on different visits may partly explain the observed differences. However, the MEMS method of measuring adherence relies on patient participation and patients are aware their medications are being monitored, which may improve medication adherence rates. Despite our study patients being unaware that their pill taking was being monitored, the measured adherence rates were higher than previously observed. Other observational studies in HF have reported non-adherence rates which varied from 17% to 44%, with one meta-analysis estimating a mean non-adherence rate of 27%.20
Perhaps the most appropriate and direct comparison of non-adherence rates is with a prior study, using the same analytical technique applied to a spot urine sample, in 208 patients with hypertension attending a tertiary care clinic.11 In that study, 10% of patients were totally non-adherent (defined as the absence of all prescribed anti-hypertensive medications in the urine) and a further 15% partially non-adherent (the absence of one or more, but not all, prescribed medications). The highest rates of any non-adherence were observed in patients with inadequate BP control (28.8%) and in patients referred for consideration of renal denervation (23.5%). In a larger study of 676 patients with hypertension, partial non-adherence was observed in 41.6%.21 In a further study of patients with resistant hypertension referred for renal denervation, median non-adherence was 26.2% and adherence patterns of individuals fluctuated over a 17-month period.22
High non-adherence rates have also been reported in other studies in hypertension and in studies of primary and secondary prevention, assessed using a variety of methods.23,24
It was also notable in these analyses patients who were non-adherent to diuretic therapy were more likely to report urinary incontinence. In clinical practice, patients are often advised to adjust their diuretic dose according to need and told it is permissible to delay or omit dosing if they must make a journey, for example to a hospital clinic. High rates of urinary incontinence have previously been reported in patients with HF. Hwang et al.25 surveyed a group of 89 patients with chronic HF and found 49% of patients described urinary incontinence. Higher doses of diuretics were noted in patients who described themselves as incontinent and, when compared to continent patients, reported missing or altering a diuretic dose more frequently.
Adherence in HF might be better than in these other conditions because HF is a highly symptomatic condition and HF therapies improve symptoms and quality of life and reduce the risk of HF hospitalization and premature death. These may be powerful motivational determinants of adherence, especially in patients recently hospitalized with worsening HF. Use of liquid chromatography–tandem mass spectrometry as a direct and objective measure of prescription medications has several potential applications in clinical practice. Most obviously, non-adherent patients might be targeted for intensive education and counselling in relation to the importance of taking prescribed therapy. Patients hospitalized with worsening HF, especially those with frequent admissions, might be particularly appropriate candidates for this investigation and intervention. Further work using this technique is required to examine the effects of non-adherence on long term outcomes.
Limitations
This was a post hoc analysis of a prospective observational study and is subject to limitations inherent to this type of analysis. The patients we studied had recently been hospitalized with HF and were participating in a clinical research study. These features may have resulted in better adherence when compared to ‘real world’ ambulatory patients with HF in the community. Although our initial cohort consisted of consecutive, unselected, patients recruited in hospital, only 648 of 1003 (64.6%) of patients returned for a follow-up visit. However, 341 of 342 (99.7%) consecutive patients invited to provide a urine sample were able to.
All prescription medications are supplied free of charge to patients in the Scottish National Health Service and payment or co-payment might reduce adherence in other healthcare systems. Marital status, a factor well-recognized to be associated with medication adherence, was not collected for this cohort of patients.
Liquid chromatography–tandem mass spectrometry provides a snap-shot, rather than a longitudinal, assessment only of adherence to medication. While this direct measure is one of the strengths of the study, an impending clinic appointment has been shown to influence adherence behaviour although patients in this study were not aware that their adherence to medication was being assessed.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Funding
M.T., I.S. and P.G. are supported by the British Heart Foundation Clinical Study grant CS/17/3/32799.
Conflict of interest: R.S.G. reports grants from the British Heart Foundation, grants, personal fees and non-financial support from Abbott, personal fees and non-financial support from Boston Scientific, personal fees and non-financial support from Novartis, personal fees from Vifor. J.J.V.M.c.M. reports other from Roche Pharmaceuticals, during the conduct of the study; other from Novartis, other from Cardiorentis, other from Amgen, other from Oxford University/Bayer, other from GlaxoSmithKline, other from Theracos, other from Abbvie, other from DalCor, other from Pfizer, other from Merck, other from AstraZeneca, other from Bristol Myers Squibb (BMS), other from Kidney Research UK (KRUK)/Kings College Hospital, London/Vifor-Fresenius Pharma, outside the submitted work. I.S. reports grants and personal fees from Novartis. All other authors have declared no conflict of interest.
Supplementary Material
References
- 1. Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, Yusuf S, Michelson EL, Pfeffer MA; CHARM investigators. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet 2005;366:2005–2011. [DOI] [PubMed] [Google Scholar]
- 2. Ghali JK, Kadakia S, Cooper R, Ferlinz J.. Precipitating factors leading to decompensation of heart failure. Traits among urban blacks. Arch Intern Med 1988;148:2013–2016. [PubMed] [Google Scholar]
- 3. Oosterom-Calo R, van Ballegooijen AJ, Terwee CB, Te Velde SJ, Brouwer IA, Jaarsma T, Brug J.. Determinants of adherence to heart failure medication: a systematic literature review. Heart Fail Rev 2013;18:409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masoudi FA, Baillie CA, Wang Y, Bradford WD, Steiner JF, Havranek EP, Foody JM, Krumholz HM.. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998-2001. Arch Intern Med 2005;165:2069–2076. [DOI] [PubMed] [Google Scholar]
- 5. Gupta P, Patel P, Horne R, Buchanan H, Williams B, Tomaszewski M.. How to screen for non-adherence to antihypertensive therapy. Curr Hypertens Rep 2016;18:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu JR, Moser DK, Chung ML, Lennie TA.. Objectively measured, but not self-reported, medication adherence independently predicts event-free survival in patients with heart failure. J Card Fail 2008;14:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohachick P, Burke LE, Sereika S, Murali S, Dunbar-Jacob J.. Adherence to angiotensin-converting enzyme inhibitor therapy for heart failure. Prog Cardiovasc Nurs 2002;17:160–166. [DOI] [PubMed] [Google Scholar]
- 8. Chui MA, Deer M, Bennett SJ, Tu W, Oury S, Brater DC, Murray MD.. Association between adherence to diuretic therapy and health care utilization in patients with heart failure. Pharmacotherapy 2003;23:326–332. [DOI] [PubMed] [Google Scholar]
- 9. Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Buch P, Sørensen R, Folke F, Gadsbøll N, Rasmussen S, Køber L, Madsen M, Torp-Pedersen C.. Persistent use of evidence-based pharmacotherapy in heart failure is associated with improved outcomes. Circulation 2007;116:737–744. [DOI] [PubMed] [Google Scholar]
- 10. Muzzarelli S, Brunner-La Rocca H, Pfister O, Foglia P, Moschovitis G, Mombelli G, Stricker H.. Adherence to the medical regime in patients with heart failure. Eur J Heart Fail 2010;12:389–396. [DOI] [PubMed] [Google Scholar]
- 11. Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, Samani NJ, Gupta P, Madira W, Stanley A, Williams B.. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart 2014;100:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muzzarelli S, Brunner-La Rocca H, Pfister O, Foglia P, Moschovitis G, Mombelli G, Stricker H.. Adherence to the medical regime in patients with heart failure. Eur J Heart Fail 2010;12:389–396. [DOI] [PubMed] [Google Scholar]
- 13. Jackson CE, Myles RC, Tsorlalis IK, Dalzell JR, Rocchiccioli JP, Rodgers JR, Spooner RJ, Greenlaw N, Ford I, Gardner RS, Cobbe SM, Petrie MC, McMurray JJ.. Spectral microvolt T-wave alternans testing has no prognostic value in patients recently hospitalized with decompensated heart failure. Eur J Heart Fail 2013;15:1253–1261. [DOI] [PubMed] [Google Scholar]
- 14. Jackson CE, Myles RC, Tsorlalis IK, Dalzell JR, Spooner RJ, Rodgers JR, Bezlyak V, Greenlaw N, Ford I, Cobbe SM, Petrie MC, McMurray J.. Profile of microvolt T-wave alternans testing in 1003 patients hospitalized with heart failure. Eur J Heart Fail 2012;14:377–386. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P.. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 16. Moffat A, Osselton D, Widdop B, Watts J.. Clarke's Analysis of Drugs and Poisons. 4th ed. London, UK: Pharmaceutical Press; 2011 [Google Scholar]
- 17. Pelouch R, Voříšek V, Furmanová V, Solař M.. The assessment of serum drug levels to diagnose non-adherence in stable chronic heart failure patients. Acta Medica (Hradec Kralove) 2019;62:52–57. [DOI] [PubMed] [Google Scholar]
- 18. Böhm M, Lloyd SM, Ford I, Borer JS, Ewen S, Laufs U, Mahfoud F, Lopez-Sendon J, Ponikowski P, Tavazzi L, Swedberg K, Komajda M.. Non-adherence to ivabradine and placebo and outcomes in chronic heart failure: an analysis from SHIFT. Eur J Heart Fail 2016;18:672–683. [DOI] [PubMed] [Google Scholar]
- 19. Viana M, Laszczynska O, Mendes S, Friões F, Lourenço P, Bettencourt P, Lunet N, Azevedo A.. Medication adherence to specific drug classes in chronic heart failure. J Manag Care Spec Pharm 2014;20:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krueger K, Botermann L, Schorr SG, Griese-Mammen N, Laufs U, Schulz M.. Age-related medication adherence in patients with chronic heart failure: a systematic literature review. Int J Cardiol 2015;184:728–735. [DOI] [PubMed] [Google Scholar]
- 21. Gupta P, Patel P, Štrauch B, Lai FY, Akbarov A, Marešová V, White CMJ, Petrák O, Gulsin GS, Patel V, Rosa J, Cole R, Zelinka T, Holaj R, Kinnell A, Smith PR, Thompson JR, Squire I, Widimský J Jr, Samani NJ, Williams B, Tomaszewski M.. Risk factors for nonadherence to antihypertensive treatment. Hypertension 2017;69:1113–1120. [DOI] [PubMed] [Google Scholar]
- 22. Wunder C, Persu A, Lengelé JP, Mg Georges C, Renkin J, Pasquet A, Carlier M, Zhang ZY, Staessen JAEuropean Network Coordinating Research on Renal Denervation (ENCOReD)Toennes SW.. Adherence to antihypertensive drug treatment in patients with apparently treatment-resistant hypertension in the INSPiRED pilot study. Blood Press 2019;28:168–172. [DOI] [PubMed] [Google Scholar]
- 23. Jackevicius CA, Li P, Tu JV.. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation 2008;117:1028–1036. [DOI] [PubMed] [Google Scholar]
- 24. Senior V, Marteau TM, Weinman J; Genetic Risk Assessment for FH Trial (GRAFT) Study Group. Self-reported adherence to cholesterol-lowering medication in patients with familial hypercholesterolaemia: the role of illness perceptions. Cardiovasc Drugs Ther 2004;18:475–481. [DOI] [PubMed] [Google Scholar]
- 25. Hwang R, Chuan F, Peters R, Kuys S.. Frequency of urinary incontinence in people with chronic heart failure. Heart Lung 2013;42:26–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.