Abstract
Orbital infarction syndrome is an uncommon pathology with devastating consequences. It is frequently secondary to atherothrombotic phenomena in the internal carotid artery. We report a case of a 66-year-old male with uncontrolled diabetes and use of systemic steroids for COVID-19, who presented with a sudden loss of vision in the left eye, with total ophthalmoplegia and diffuse opacification of the retina. On imaging, he was found to have features of rhino-orbital cellulitis with ischemia of the orbital tissue secondary to isolated ophthalmic artery obstruction (OAO) with a patent internal carotid artery. KOH mount of deep nasal swab was confirmatory of mucor. This is the first reported case of orbital infarction syndrome in the setting of COVID-19.
Keywords: COVID-19 pandemic, delayed sequential bilateral cataracts surgery, immediate sequential bilateral cataract surgery
Orbital infarction syndrome (OIS) is an extremely rare entity defined as ischemia of the intraorbital structures including the intraocular tissue, optic nerve, extraocular muscles and orbital fat.[1,2,3,4,5,6] Clinically, it is characterised by a sudden and profound vision loss, periorbital ache, blepharoptosis, total ophthalmoplegia, and chorioretinal ischemia.[1,2,3,4,5,6,7,8,9,10,11,12] Frequently, the site of arterial occlusion is the common or internal carotid artery (ICA), with very few cases reported due to isolated occlusion of the ophthalmic artery (OA).[1,2,3,4,5,6,7,8,9,10,11,12] Vascular occlusions are a known association of the novel SARS-CoV2 virus due its ability to induce a thrombogenic state.[13] One of the mainstays of treatment of COVID-19 involves the use of steroids. An unfortunate consequence of immunosuppression in these patients is an increased incidence of rhino-orbito-cerebral mucormycosis.[14] In this context, we report a rare case of OIS secondary to mucormycosis in a patient with COVID-19.
Case Report
A 66-year-old male presented with a five-day history of headache associated with periorbital pain followed by sudden onset of vision loss in the left eye for 3 days. He had been diagnosed with COVID-19 and had been receiving systemic steroids for the past 12 days. He was a poorly controlled diabetic with a history of laser treatment of proliferative diabetic retinopathy in both the eyes a few years prior.
On examination, his best corrected visual acuity in the right eye was 20/40 with no light perception in the left eye. Intraocular pressure in both eyes was 15 mmHg. The right eye showed an unremarkable anterior segment with a briskly reactive pupil and lasered diabetic retinopathy. In the left eye, there was periorbital edema, a 3 mm axial proptosis, complete ptosis and chemosis [Fig. 1a]. The pupil was mid-dilated and fixed [Fig. 1b], non-reactive to both direct and consensual light. There was complete absence of ocular movements in all directions of gaze [Fig. 1c-f]. The left fundus showed optic disc edema with Drantz hemorrhage at the temporal disc margin [Fig. 2]. There was box-car appearance of retinal veins with attenuation of arteries, suggestive of a recent partial recanalization with diffuse opacification of the retina including the fovea, extending to the periphery with no cherry red spot. Neurological examination revealed anaesthesia of the territory of the ophthalmic branch of trigeminal nerve with absence of corneal reflex on the left side. The patient also had an inability to raise the left eyebrow with ipsilateral facial nerve function otherwise intact.
Figure 1.
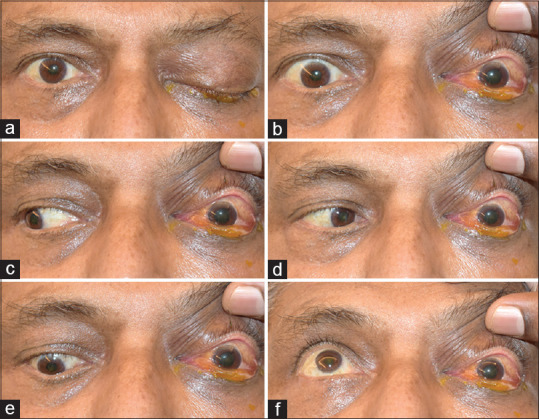
External photographs reveals complete left ptosis and proptosis (a), chemosis and fixed dilated left pupil (b), and absence of left ocular movements in all directions of gaze (c-f)
Figure 2.
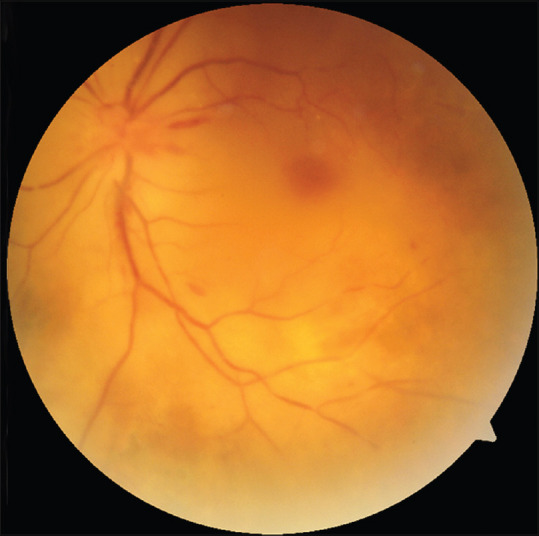
Left fundus image shows disc edema with Drantz hemorrhage at the temporal disc margin, along with boxcarring of veins with attenuation of arteries, suggestive of a recent partial recanalization. There is diffuse opacification of the retina including the fovea, extending to the periphery with no cherry red spot
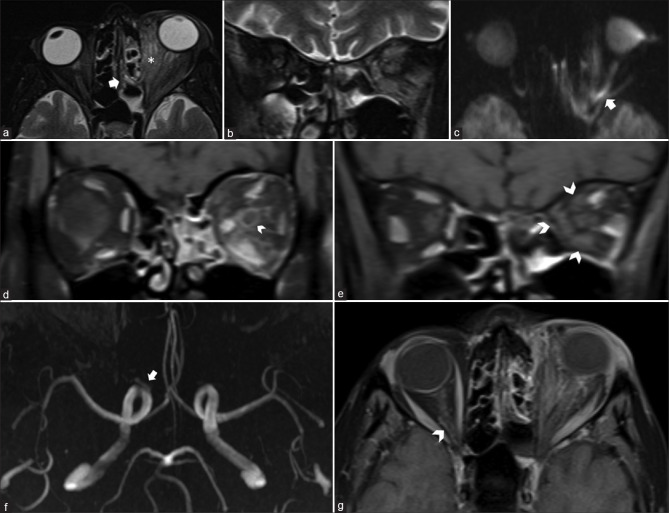
A contrast enhanced magnetic resonance imaging (MRI) of the orbit and brain [Fig. 3] showed features of ethmoid sinusitis with left orbit cellulitis and intervening osteomyelitis. In addition to inflammatory changes in the orbit, the optic nerve showed features of ischemia and perineuritis. The extraocular muscles appeared enlarged and edematous. On post-contrast scans, they showed the expected enhancement in their anterior aspect but absent enhancement in the posterior segments of their bellies consistent with infarction. MR angiography revealed isolated occlusion of the left ophthalmic artery with maintained patency of the rest of the intracranial vasculature.
Figure 3.
MRI axial (a) and coronal (b) T2 fat saturated [fs] images reveal features of ethmoid sinusitis [arrow] with orbital inflammatory changes manifested by orbital fat stranding (* in a), EOM enlargement and T2 hyperintensity. The left optic nerve also shows T2 hyperintensity in (b) with hyperintense diffusion restriction on axial diffusion weighted image (arrow in c) suggestive of optic nerve infarction. Post-contrast coronal T1fs in retro-ocular section shows abnormal left perioptic nerve sheath enhancement (arrowhead) consistent with optic perineuritis, abnormal orbital fat enhancement and maintained expected enhancement of the EOMs. More posterior sections (e) near the orbital apex show absent enhancement of EOMs consistent with infarction, with relative sparing of the lateral rectus. MR Angiography (f) shows non-visualization of the left ophthalmic artery, in comparison to the origin of the right OA which is seen clearly (arrowhead). Post-contrast axial T1fs (g) shows the orbital segment of the OA on the right side (arrowhead) but not on the left side
Blood investigations revealed elevated inflammatory markers and D-dimer levels. KOH mount of the endoscopic-guided deep nasal swab confirmed the presence of fungal hyphae consistent with mucor. In view of extensive involvement, the patient underwent functional endoscopic sinus surgery with orbital exenteration and intravenous liposomal amphotericin.
Discussion
The orbit is primarily supplied by the ophthalmic artery which is the first major branch of the ICA soon after its emergence from the cavernous sinus.[15] In its course through the orbit, the OA gives rise to several branches including the central retinal and posterior ciliary arteries, muscular arteries to the extraocular muscles and levator palpebrae superioris (LPS), the supraorbital, arteries to the lacrimal gland, areolar tissue and eyelids, to terminate as the dorsal nasal and supratrochlear arteries.[15] The external carotid artery (ECA) normally contributes only to a small percentage of the orbital blood supply via the maxillary and superficial temporal arteries, wherein the orbital branches of the infraorbital artery and middle meningeal artery anastomose with the branches of OA.[15]
Orbital infarctions are exceptionally rare by virtue of an extensive collateral network in the orbit that serve as anastomoses between the ICA and ECA territories.[7] Indeed, the OA itself is a major anastomotic channel with flow often reversed in the event of ICA occlusion or stenosis.[16] OIS is most often the result of extensive ICA or common carotid artery atherothrombotic occlusions.[1,2,3,4,5,6,7,8,9,10,11,12] Other causes include craniotomies or endovascular procedures,[3,4,8,10,12] mucomycosis,[1,3] giant cell arteritis,[1] and sclerotherapy for vascular malformations.[11] For patients undergoing craniotomies, the mechanism of OIS is thought to be prolonged extensive pressure onto orbital tissues from improper positioning or direct orbital compression, resulting in inadequate tissue perfusion.[2]
Isolated OA occlusion usually presents with symptoms that are limited to chorioretinal, and less commonly optic nerve ischemia by virtue of these being end arteries. The orbital tissues however usually continue to remain perfused via ECA collaterals.[7] The presence of orbital ischemia in this patient with isolated OA occlusion can be explained by multiple synergistic factors. In addition to predisposing anatomical variations that may have limited ECA supply to the orbit, the presence of a COVID-induced prothrombotic state (as evidenced by elevated inflammatory markers and D-dimer levels) could have compromised ECA collateralization. Lastly, the sudden nature of the occlusion with minimal time for collateral development could have further exacerbated the ischemia.
The use of systemic steroids as part of the management of COVID-19 infection in the setting of poorly controlled diabetes mellitus in this patient possibly led to the development of rhino-orbital mucormycosis, resulting in proptosis and periorbital pain. Angioinvasion by the mucor led to OA occlusion resulting in orbital ischemia that further compounded the periorbital pain. Total ophthalmoplegia and complete ptosis along with sudden loss of vision mimicked an orbital apex syndrome which is usually caused by inflammation or compression at the orbital apex.
However in our case, the MRI showed a conspicuous lack of abnormal soft tissue or enhancement around the orbital apex. Instead, it is the ischemia of the ocular motor nerves combined with direct ischemic damage of the extraocular muscles and LPS that led to these symptoms. Ischemia of the ophthalmic division of trigeminal nerve explains the loss of corneal reflex and periorbital anaesthesia. The loss of both direct and consensual light reflex was due to ischaemia of both the afferent and efferent pathways of the pupillary reflex. Interestingly, the patient had all other facial movements intact apart from an inability to raise the ipsilateral eyebrow. This was likely due to ischemia of the frontalis muscle which is supplied by the branches of the OA rather than the involvement of facial nerve.
In conclusion, OIS is a devastating condition resulting from hypoperfusion of the orbital tissues from a variety of causes. Clinically, it can be easily mistaken for an orbital apex syndrome; however, the presentation of OIS is far more extensive with more profound deficits. In addition to demonstrating the fundoscopic and radiological aspects of this disease, the case also highlights the need for a meticulous neuro-ophthalmic evaluation in all patients with orbital infection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Borruat FX, Bogousslavsky J, Uffer S, Klainguti G, Schatz NJ. Orbital infarction syndrome. Ophthalmology. 1993;100:562–8. doi: 10.1016/s0161-6420(93)31606-4. [DOI] [PubMed] [Google Scholar]
- 2.Brinjikji W, Nicholson PJ, Hilditch CA, Tsang COA, Krings T. Orbital infarction syndrome following mechanical thrombectomy secondary to embolization in new territory. World Neurosurg. 2018;117:326–9. doi: 10.1016/j.wneu.2018.06.082. [DOI] [PubMed] [Google Scholar]
- 3.Yang SW, Kim SY, Chung J, Kim KB. Two cases of orbital infarction syndrome. Korean J Ophthalmol. 2000;14:107–11. doi: 10.3341/kjo.2000.14.2.107. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman CF, Van Patten PD, Golnik KC, Kopitnik TA, Jr, Anand R. Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology. 1995;102:594–8. doi: 10.1016/s0161-6420(95)30979-7. [DOI] [PubMed] [Google Scholar]
- 5.Maier P, Feltgen N, Lagrèze WA. Bilateral orbital infarction syndrome after bifrontal craniotomy. Arch Ophthalmol. 2007;125:422–3. doi: 10.1001/archopht.125.3.422. [DOI] [PubMed] [Google Scholar]
- 6.Larrosa Campo D, Fuentes Castañón D, Calleja Puerta S. Global orbital infarction syndrome after a carotid artery dissection. JAMA Neurol. 2019;76:111–2. doi: 10.1001/jamaneurol.2018.2880. [DOI] [PubMed] [Google Scholar]
- 7.Bogousslavsky J, Pedrazzi PL, Borruat FX, Regli F. Isolated complete orbital infarction: A common carotid artery occlusion syndrome. Eur Neurol. 1991;31:72–6. doi: 10.1159/000116650. [DOI] [PubMed] [Google Scholar]
- 8.Valls Carbó A, Gutiérrez Sánchez de la Fuente M, Pérez García C, Gómez Ruiz MN. Orbital infarction syndrome after mechanical thrombectomy in acute ischemic stroke. BMJ Case Rep. 2020;13:e234158. doi: 10.1136/bcr-2019-234158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fargeot G, Lecler A, Lamirel C, Savatovsky J, Obadia M. MRI findings in orbital infarction syndrome. Rev Neurol (Paris) 2018;174:571–3. doi: 10.1016/j.neurol.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Morofuji Y, Tsutsumi K, Takahata H, Kawahara I, Hirao T, Toda K, et al. Radiological findings of orbital infarction syndrome following intracranial aneurysm surgery. Clin Neurol Neurosurg. 2013;115:1546–8. doi: 10.1016/j.clineuro.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Sio WS, Lee SH, Liang IC. Orbital infarction syndrome after multiple percutaneous sclerotherapy sessions for facial low-flow vascular malformation: A case report and literature review. Indian J Ophthalmol. 2016;64:595–7. doi: 10.4103/0301-4738.191508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SW, Kang KT, Jun JH, Jang JH, Kim YC. Orbital infarction syndrome after cerebral aneurysm surgery: A case series and literature review. Medicine (Baltimore) 2020;99:e21277. doi: 10.1097/MD.0000000000021277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and eye: A review of ophthalmic manifestations of COVID-19. Indian J Ophthalmol. 2021;69:488–509. doi: 10.4103/ijo.IJO_297_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: A tale of two pathogens. Indian J Ophthalmol. 2021;69:244–52. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayreh SS. Orbital vascular anatomy. Eye (Lond) 2006;20:1130–44. doi: 10.1038/sj.eye.6702377. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds PS, Greenberg JP, Lien LM, Meads DC, Myers LG, Tegeler CH. Ophthalmic artery flow direction on color flow duplex imaging is highly specific for severe carotid stenosis. J Neuroimaging. 2002;12:5–8. doi: 10.1111/j.1552-6569.2002.tb00082.x. [DOI] [PubMed] [Google Scholar]