Abstract
Purpose:
The aim of this study was to explore whether prolonged and consistent face mask use might be associated with worsening of dry eye symptoms in patients with dry eye disease (DED).
Methods:
Subjects with a previous diagnosis of DED. Their OSDI scores were compared with those recorded in Fall 2019 using the Wilcoxon paired test. Participants were stratified by face mask use: heavy (Group A) or standard (Group B) face mask users. Heavy use was defined as wearing any type of face mask for at least 6 hours a day, at least 5 days per week in the last 2 months.
Results:
67 subjects (mean age: 45.27 ± 10.06 SD years, 40% males and 60% females). Median OSDI score in Fall 2019: 18.75. Median OSDI in Spring 2020: 20.83. The Hodges-Lehmann median difference was 2.09 (95% CI [1.05, 4.17]) (P < 0.0001). The population was then stratified into heavy and standard face mask users: Group A included heavy users (31 subjects; mean age: 42.81 ± 10.48 SD years; 35% males and 65% females), Group B included standard users (36 subjects; mean age: 47.39 ± 9.31 SD years; 44% males and 56% females). The Hodges-Lehmann median difference was 5.21 (95% CI [3.13, 7.29]) in Group A (P < 0.0001), and 1.04 (95% [0, 2.08]) in Group B (P = 0.0177).
Conclusion:
Prolonged and consistent face mask use is associated with an increase in OSDI scores. Whether face mask use is responsible for the worsening of symptoms of DED remains to be elucidated.
Keywords: COVID-19, DED, dry eye disease, face mask, keratoconjunctivitis sicca, SARS-CoV-19
Dry eye disease (DED) presents with a constellation of symptoms caused by tear film instability. It is estimated that as many as 16.4 million people in the United States are affected.[1] The most common symptoms include grittiness, foreign body sensation, conjunctival hyperemia, and itching. DED results from a defective or deficient tear film.[2] When tears are insufficient or evaporate easily, the cornea remains exposed to external irritative agents for a variable amount of time. In general, the longer the time, the worse the symptoms. Risk factors for DED include aging, local inflammatory conditions such as blepharitis or atopy, topical or systemic medications such as antihistamines or contraceptive pills, lifestyle factors such as prolonged video terminal work, alcohol, smoking, and environmental factors, such as wind, temperature, and humidity.[3,4,5,6]
In recent months, the SARS-CoV-2 outbreak has forced millions of people worldwide to change their lifestyles. In some countries, including Italy, face mask use has become mandatory in all indoor spaces open to the public.[7] Examples include hospitals, supermarkets, shops, cafés, or shared means of transportation, such as buses or trains. Therefore, those who work in these places are always required to wear a face mask. Following a recent surge in the number of patients presenting to our clinic with complaints of DED, chalazia, and styes, we speculated on whether consistent and prolonged face mask use might aggravate the symptoms of DED. For this purpose, we conducted a cross-sectional study in patients with pre-existing DED to explore the potential association between prolonged face mask use and worsening of DED symptoms.
Methods
Subjects with a pre-existing diagnosis of DED were recruited between April and June 2020 (referred as Spring 2020). All participants signed a written consent form upon enrollment. The study was approved by the Institutional Review Board of the University of Bologna and adhered to the tenets of the Helsinki Declaration.
Inclusion criteria included: DED of mild or moderate severity (OSDI 13–32 points), first diagnosis of DED in 2018 or earlier, age between 18 and 60, TBUT of less than 10 seconds in either eye, consistent respirator or surgical face mask use in the 60 days prior. Exclusion criteria included: Severe DED (OSDI >32 points), active ocular surface conditions other than DED (e.g. episcleritis or conjunctivitis) in the four weeks prior, regular face mask use in 2019, history of atopy, prescription of anti-histamine or hypotensive eye drops, prior intraocular or refractive surgeries in either eye, autoimmune (e.g. Rheumatoid arthritis or Sjogren) or neurologic diseases (e.g. Parkinson's disease or Bell's palsy).
Participants received a comprehensive ocular examination. The type and average number of daily instillations of eye lubricants were noted. TBUT was calculated non-invasively for each eye using the Tomey RT-7000. TBUT score was averaged across three measurements. Each patient completed the “Ocular Surface Disease Index© (OSDI©) 2” questionnaire,[8] which is a scientifically validated 12-item questionnaire that evaluates severity of DED symptoms during the week prior. Results were compared with values obtained from the same subjects at the end of the previous year, from September to December 2019 (referred as Fall 2019), when face mask use outside the surgical setting was rare. If more than one OSDI score had been recorded for the same patient during this period, the most recent was used. This score is referred in the text as the baseline score. Participants were later stratified in two groups based on face mask use. Group A included subjects who reported wearing a face mask for at least six hours a day, for at least five days a week. These were labeled as heavy users. Group B included the remaining subjects, labeled as standard users.
OSDI scores were compared using the non-parametric Wilcoxon paired test. Normality of data was tested using the D’Agostino–Pearson test. Statistical significance was set at P < 0.05. The software used for conducting the statistical analysis was MedCalc (MedCalc Software v. 19.3 for Microsoft Windows).
Results
A total of 67 subjects were recruited in this study. Mean age: 45.27 ± 10.06 SD years. 40% males and 60% females. Table 1 shows a statistically-significant increase in the median OSDI score between Fall 2019 and Spring 2020.
Table 1.
Results of the Wilcoxon test for paired samples on the whole study population
| Fall 2019 | Spring 2020 | |
|---|---|---|
| Sample size | 67 | 67 |
| Lowest OSDI value | 14.58 | 12.50 |
| Highest OSDI value | 22.92 | 35.42 |
| Median | 18.75 | 20.83 |
| 95% CI for the median | 16.67 to 20.83 | 18.75 to 22.92 |
| Interquartile range | 16.67 to 20.83 | 18.75 to 25.00 |
| Hodges-Lehmann median difference | 2.09 | |
| 95% Confidence interval | 1.05 to 4.17 | |
| Two-tailed probability | P<0.0001 |
The same statistical analysis was repeated after stratifying subjects by face mask use. Subjects wearing a face mask for at least 6 hours a day, for at least 5 days a week during the 60 days prior were included in Group A (31 subjects; Mean age: 42.81 ± 10.48 SD years; 35% males and 65% females). Group B included the remaining subjects (36 subjects; Mean age: 47.39 ± 9.31 SD years; 44% males and 56% females).
Results are shown in Tables 2 and 3. Table 2 shows a statistically-significant increase in the median OSDI score of Group A between Fall 2019 and Spring 2020. The highest absolute increase in Group A was: +12.50. Group A mostly included healthcare workers such as physicians, nurses, and dentists, as well as pharmacists, shop assistants, and bus drivers. Table 3 shows no statistically significant change in the median OSDI score of Group B between Fall 2019 and Spring 2020. The highest absolute increase in Group B was: +4.17. Data are also depicted in Figs. 1 and 2.
Table 2.
Results of the Wilcoxon test for paired samples on Group A subjects. Group A subjects reported wearing a facemask for at least 6 hours a day, for at least 5 days a week during the 60 days prior
| Fall 2019 | Spring 2020 | |
|---|---|---|
| Sample size | 31 | 31 |
| Lowest OSDI value | 14.58 | 12.50 |
| Highest OSDI value | 22.92 | 35.42 |
| Median OSDI value | 16.67 | 22.92 |
| 95% CI for the median | 16.67 to 20.83 | 19.97 to 25.86 |
| Interquartile range | 16.67 to 20.83 | 18.75 to 27.08 |
| Hodges-Lehmann median difference | 5.21 | |
| 95% Confidence interval | 3.13 to 7.29 | |
| Two-tailed probability | P<0.0001 |
Table 3.
Results of the Wilcoxon test for paired samples on Group B subjects. Group B includes the remaining subjects
| Fall 2019 | Spring 2020 | |
|---|---|---|
| Sample size | 36 | 36 |
| Lowest OSDI value | 14.58 | 12.50 |
| Highest OSDI value | 22.92 | 27.08 |
| Median OSDI value | 18.75 | 18.75 |
| 95% CI for the median | 16.67 to 20.83 | 18.75 to 20.83 |
| Interquartile range | 16.67 to 20.83 | 16.67 to 20.83 |
| Hodges-Lehmann median difference | 1.04 | |
| 95% Confidence interval | 0.00 to 2.08 | |
| Two-tailed probability | P=0.0177 |
Figure 1.
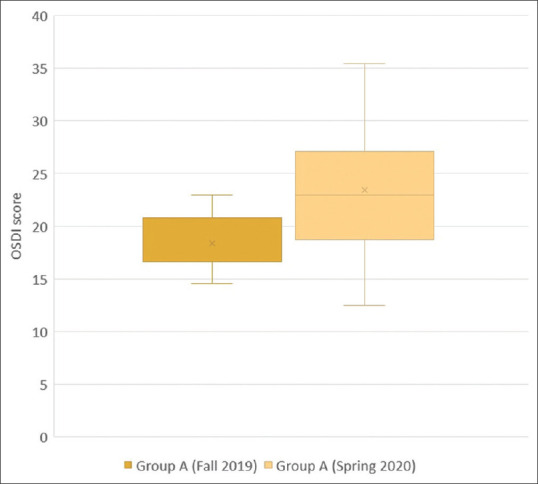
Comparison of OSDI scores of heavy face mask users (Group A) between Fall 2019 and Spring 2020
Figure 2.
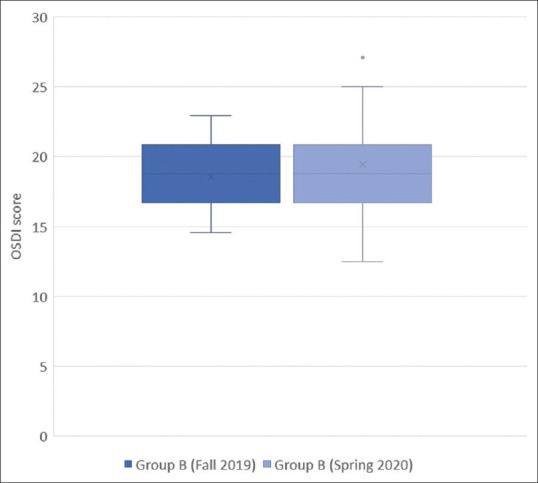
Comparison of OSDI scores of standard face mask users (Group B) between Fall 2019 and Spring 2020
Discussion
This cross-sectional study shows an association between prolonged face mask use and worsening of DED symptoms. After stratifying the study population into heavy (Group A) or standard (Group B) face mask users, those who reported wearing a face mask for at least 6 hours a day, at least 5 days a week during the 60 days prior showed an higher increase in their OSDI scores (P < 0.001), as compared to standard users (Group B). Group A mostly included subjects who work in indoor places open to the public. Examples include healthcare workers such as physicians, nurses, and dentists, as well as bus drivers, shop clerks, pharmacists, and others.
Should prolonged face mask use be found to be an aggravating factor for DED symptoms, the pathophysiological explanation remains to be elucidated. In general, respirators and surgical masks should not alter blood gas composition, unless the subject is exercising.[9] Furthermore, blood gas composition is unlikely to influence the severity of DED symptoms. One possible explanation for the worsening of DED symptoms might be related to loose fitting of masks around the nose. Without a proper seal, respiratory gases might be diverted upwards towards the eyes. As these masks do not readily dissipate air, the microclimate inside face masks – hence the type of gases that are diverted upwards - can be quite different from that of ambient air. This is especially true when using respirators not equipped with an exhaust valve. Previous studies have shown that temperature and humidity of gases inside a face mask can be higher.[10,11] The poor ventilation caused by wearing a face mask in turn decreases heat loss from the respiratory tract, which generally accounts for 11% of the total heat loss from the body. This has been found to influence the heart rate, thermal stress, and subjective perception of discomfort.[10,12] The proposed mechanism of air escape leads to fogging of spectacles and possibly worsening of DED symptoms. This study shows that the effect of face masks on DED would only become apparent after prolonged and consistent daily application, rather than sporadic use.
Despite the bothersome effects that prolonged face mask use might have on DED symptoms, authors of this study would never recommend against the use of personal protective equipment (PPE). Instead, authors promote consistent face mask wear for personal and community protection. We also recommend users with worsening DED symptoms to increase either the number of installations or density of eye lubricants. In addition, placing tape around the nose area might help. In fact, tape helps form an airtight seal that prevents respiratory gases to reach the ocular surface.
Limitations of this study include the subjective nature of the OSDI score, the risk for recall bias, and the presence of confounders other than age and gender. Additional studies with larger populations are encouraged.
Conclusion
The results of our study show that prolonged and consistent face mask use is associated with an increase in OSDI scores. Whether face mask use is solely responsible for the worsening of symptoms of DED remains to be elucidated.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–8. doi: 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Lollett IV, Galor A. Dry eye syndrome: Developments and lifitegrast in perspective. Clin Ophthalmol. 2018;12:125–39. doi: 10.2147/OPTH.S126668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villatoro AJ, Fernández V, Claros S, Alcoholado C, Cifuentes M, Merayo-Lloves J, et al. Regenerative rherapies in dry eye disease: From growth factors to cell therapy. Int J Mol Sci. 2017;18:2264. doi: 10.3390/ijms18112264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziemanski JF, Wolters LR, Jones-Jordan L, Nichols JJ, Nichols KK. Relation between dietary essential fatty acid intake and dry eye disease and meibomian gland dysfunction in postmenopausal women. Am J Ophthalmol. 2018;189:29–40. doi: 10.1016/j.ajo.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–8. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 6.Librando A, Carlesimo SC, Albanese G, Albanese GM, Migliorini R, Pacella E. Effectiveness of 0.1% topical salicylic acid on blepharoconjunctivitis affecting glaucoma patients treated with topical prostaglandin analogues: A prospective randomized trial. Int J Ophthalmol. 2018;11:1936–40. doi: 10.18240/ijo.2018.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loffredo L, Pacella F, Pacella E, Tiscione G, Oliva A, Violi F. Conjunctivitis and COVID-19: A meta-analysis. 2020;92:1413–4. doi: 10.1002/jmv.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekaran B, Fernandes S. “Exercise with face mask; Are we handling a devil's sword?”-A physiological hypothesis. Med Hypotheses. 2020;144:110002. doi: 10.1016/j.mehy.2020.110002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Tokura H, Guo YP, Wong AS, Wong T, Chung J, et al. Effects of wearing N95 and surgical face masks on heart rate, thermal stress and subjective sensations. Int Arch Occup Environ Health. 2005;78:501–9. doi: 10.1007/s00420-004-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi C, Tokura H. The effects of two kinds of mask (with or without exhaust valve) on clothing microclimates inside the mask in participants wearing protective clothing for spraying pesticides. Int Arch Occup Environ Health. 2004;77:73–8. doi: 10.1007/s00420-003-0472-3. [DOI] [PubMed] [Google Scholar]
- 12.Guo YP, Yi L, Tokura H, Wong TK, Chung JW, Gohel MD, et al. Evaluation on masks with exhaust valves and with exhaust holes from physiological and subjective responses. J Physiol Anthropol. 2008;27:93–102. doi: 10.2114/jpa2.27.93. [DOI] [PubMed] [Google Scholar]


