Abstract
Objective
To determine how many patients with chronic osteoarthritis pain respond to various non-surgical treatments.
Data sources
PubMed and the Cochrane Library.
Study selection
Published systematic reviews of randomized controlled trials (RCTs) that included meta-analysis of responder outcomes for at least 1 of the following interventions were included: acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), topical NSAIDs, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, cannabinoids, counseling, exercise, platelet-rich plasma, viscosupplementation, glucosamine, chondroitin, intra-articular corticosteroids, rubefacients, or opioids.
Synthesis
In total, 235 systematic reviews were included. Owing to limited reporting of responder meta-analyses, a post hoc decision was made to evaluate individual RCTs with responder analysis within the included systematic reviews. New meta-analyses were performed where possible. A total of 155 RCTs were included. Interventions that led to more patients attaining meaningful pain relief compared with control included exercise (risk ratio [RR] of 2.36; 95% CI 1.79 to 3.12), intra-articular corticosteroids (RR = 1.74; 95% CI 1.15 to 2.62), SNRIs (RR = 1.53; 95% CI 1.25 to 1.87), oral NSAIDs (RR = 1.44; 95% CI 1.36 to 1.52), glucosamine (RR = 1.33; 95% CI 1.02 to 1.74), topical NSAIDs (RR = 1.27; 95% CI 1.16 to 1.38), chondroitin (RR = 1.26; 95% CI 1.13 to 1.41), viscosupplementation (RR = 1.22; 95% CI 1.12 to 1.33), and opioids (RR = 1.16; 95% CI 1.02 to 1.32). Preplanned subgroup analysis demonstrated no effect with glucosamine, chondroitin, or viscosupplementation in studies that were only publicly funded. When trials longer than 4 weeks were analyzed, the benefits of opioids were not statistically significant.
Conclusion
Interventions that provide meaningful relief for chronic osteoarthritis pain might include exercise, intra-articular corticosteroids, SNRIs, oral and topical NSAIDs, glucosamine, chondroitin, viscosupplementation, and opioids. However, funding of studies and length of treatment are important considerations in interpreting these data.
Résumé
Objectif
Déterminer le nombre de patients souffrant de douleur due à l’arthrose chronique qui répondent à divers traitements non chirurgicaux.
Qualité des données
PubMed et la bibliothèque Cochrane.
Sélection des études
Nous avons inclus les revues systématiques publiées d’études randomisées contrôlées (ERC) qui incluaient une méta-analyse des résultats chez les sujets répondants pour au moins 1 des interventions suivantes : l’acétaminophène, les anti-inflammatoires non stéroïdiens (AINS) par voie orale, les AINS topiques, les inhibiteurs de la recapture de la sérotoninenorépinéphrine (IRSN), les antidépresseurs tricycliques, les cannabinoïdes, le counseling, l’exercice, le plasma riche en plaquettes, la viscosupplémentation, la glucosamine, la chondroïtine, les corticostéroïdes intra-articulaires, les rubéfiants ou les opioïdes.
Synthèse
Au total, 235 revues systématiques ont été incluses. En raison des rapports limités des méta-analyses sur les sujets répondants, une décision a été prise a posteriori d’évaluer les ERC individuelles qui comportaient une analyse des sujets répondants parmi les revues systématiques incluses. De nouvelles méta-analyses ont été effectuées, dans la mesure du possible. Un total de 155 ERC ont été retenues. Parmi les interventions qui ont entraîné un soulagement plus significatif de la douleur chez les patients par rapport aux groupes témoins figuraient l’exercice (risque relatif [RR] = 2,36; IC à 95 % de 1,79 à 3,12), les corticostéroïdes intra-articulaires (RR = 1,74; IC à 95 % de 1,15 à 2,62), les IRSN (RR = 1,53; IC à 95 % de 1,25 à 1,87), les AINS par voie orale (RR = 1,44; IC à 95 % de 1,36 à 1,52), la glucosamine (RR = 1,33; IC à 95 % de 1,02 à 1,74), les AINS topiques (RR = 1,27; IC à 95 % de 1,16 à 1,38), la chondroïtine (RR = 1,26; IC à 95 % de 1,13 à 1,41), la viscosupplémentation (RR = 1,22; IC à 95 % de 1,12 à 1,33), et les opioïdes (RR = 1,16; IC à 95 % de 1,02 à 1,32). Une analyse planifiée de sousgroupes n’a démontré aucun effet avec la glucosamine, la chondroïtine ou la viscosupplémentation dans les études qui étaient financées seulement par le secteur public. Dans l’analyse des études d’une durée de plus de 4 semaines, les bienfaits des opioïdes n’étaient pas statistiquement significatifs.
Conclusion
Parmi les interventions qui pourraient procurer un soulagement significatif de la douleur causée par l’arthrose chronique, on peut mentionner l’exercice, les corticostéroïdes intra-articulaires, les IRSN, les AINS par voie orale et topiques, la glucosamine, la chondroïtine, la viscosupplémentation et les opioïdes. Par ailleurs, le financement des études et la durée du traitement sont des facteurs importants à considérer dans l’interprétation de ces données.
Osteoarthritis is one of the most common chronic medical conditions experienced by older persons and a frequent reason patients visit their primary care providers.1,2 The hallmark of diagnosis of osteoarthritis is pain (most commonly in the knees, hips, shoulders, and hands), and many patients experience impairment in function and quality of life.3
Various interventions have been evaluated for the management of osteoarthritis in primary care. These interventions are frequently evaluated in isolation, making it difficult to determine the relative efficacy of individual treatments.4–7 Guideline groups have performed systematic reviews of multiple interventions; however, they generally report standard mean differences or the direction of effect—outcomes that are not readily translatable to clinical practice.8,9 The reporting of average improvement in pain scores is not always clinically useful, as patients might not get an average response.10 For this reason, some have advocated that analysis of responders—the proportion of patients achieving outcomes that patients consider meaningful—provides a clearer picture of how many patients will receive clinical benefit from a specific therapeutic intervention.10
We therefore performed a systematic review of systematic reviews with a focus on randomized controlled trials (RCTs) that included a responder analysis in the treatment of osteoarthritis. This review will provide practical information to assist with shared informed decision making in the management of osteoarthritis pain.
METHODS
We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and systematic review of systematic reviews protocols.11,12
Data sources
Two authors (J.T., D.P.), in consultation with a medical librarian, performed a search for systematic reviews of RCTs in the PubMed and Cochrane databases. For both databases, all articles published up to and including in April 2019 were searched using the key words osteoarthritis and systematic review.
Systematic review selection
Inclusion criteria included systematic reviews of RCTs in adults with osteoarthritis. The RCTs had to be placebo controlled for all interventions except for exercise, for which placebo control is not possible. Reviews had to include one of the following interventions: acetaminophen, oral nonsteroidal anti-inflammatory drugs (NSAIDs), topical NSAIDs, serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, cannabinoids, counseling, exercise, platelet-rich plasma, viscosupplementation, glucosamine, chondroitin, intraarticular corticosteroids, rubefacients, or opioids. Systematic reviews that included more than 1 unique treatment for osteoarthritis pain were also included. We excluded non-English systematic reviews, studies of back osteoarthritis, and pediatric and nonhuman studies. Dual title, abstract, and full-text reviews were completed to determine eligibility based on the above criteria (completed by D.P., J.T., B.T., C.S.K., A.J.L., G.M.A., S.M., S.G., J.M., M.R.K., N.D., K.C., R.C., R.T., C.R.F.). Disagreements over inclusion were resolved by third-party consensus.
Systematic reviews that focused on a single intervention and met criteria for inclusion were ranked based on publication year, inclusion of a responder meta-analysis (assessment of proportion of patients who achieved a clinically meaningful improvement in pain), and a modified AMSTAR (A Measurement Tool to Assess Systematic Reviews) quality assessment.13 For each intervention, the top 5 systematic reviews were used where available. In addition, 5 systematic reviews that focused on multiple interventions for osteoarthritis were chosen based on the publication date. This was done to ensure recent clinical trials were not missed.
As responder meta-analyses were infrequently reported and not consistently available for each intervention, we made a post hoc decision to use individual RCTs from the identified systematic reviews for the responder analysis.
Data extraction
A Microsoft Excel extraction template was developed and used to record RCT data via dual extraction. This included author, publication year, report of responder analysis, responder outcome, number of patients, osteoarthritis location, intervention, comparator, duration of study, duration of treatment at which the outcome was reported, and intervention and placebo outcome rates.
Data synthesis
We created a hierarchy of responder outcomes, prioritizing the Osteoarthritis Research Society International responder criteria and scales assessing pain rather than pain and function.14 Pain was prioritized over function, as pain predominates the lived experience of osteoarthritis and is often the presenting issue in primary care offices.15 The hierarchy of responder outcomes can be found in the appendix, available from CFPlus* (Table A1). The highest ranked outcome at the longest reported followup was included in the meta-analysis for each intervention. We made an a priori decision to perform subgroup analyses that included the size of the trial (< 150 patients and ≥ 150 patients), funding (industry or clearly publicly funded), and duration (≤ 4 weeks, > 4 to < 12 weeks, and ≥ 12 weeks). We hypothesized that larger, longer, and publicly funded trials would be at lower risk of bias and less likely to overexaggerate benefit.
Quality assessment
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool to report the certainty of the evidence.16 Confidence of each outcome was evaluated based on risk of bias, inconsistency, indirectness, imprecision, and publication bias.
Adverse events
Meta-analyzed adverse event data were extracted from included systematic reviews for each intervention. In cases where the 5 most recent systematic reviews failed to provide quantifiable adverse event data, we reviewed up to 20 of the most recent reviews previously identified in our initial search.
SYNTHESIS
Figure 1 provides details of the study flow (PRISMA) for 1757 unique articles. After exclusion by title and abstract, full-text review was performed on 353 articles, and 118 were excluded for various reasons, leaving a total of 235 systematic reviews.
Figure 1.
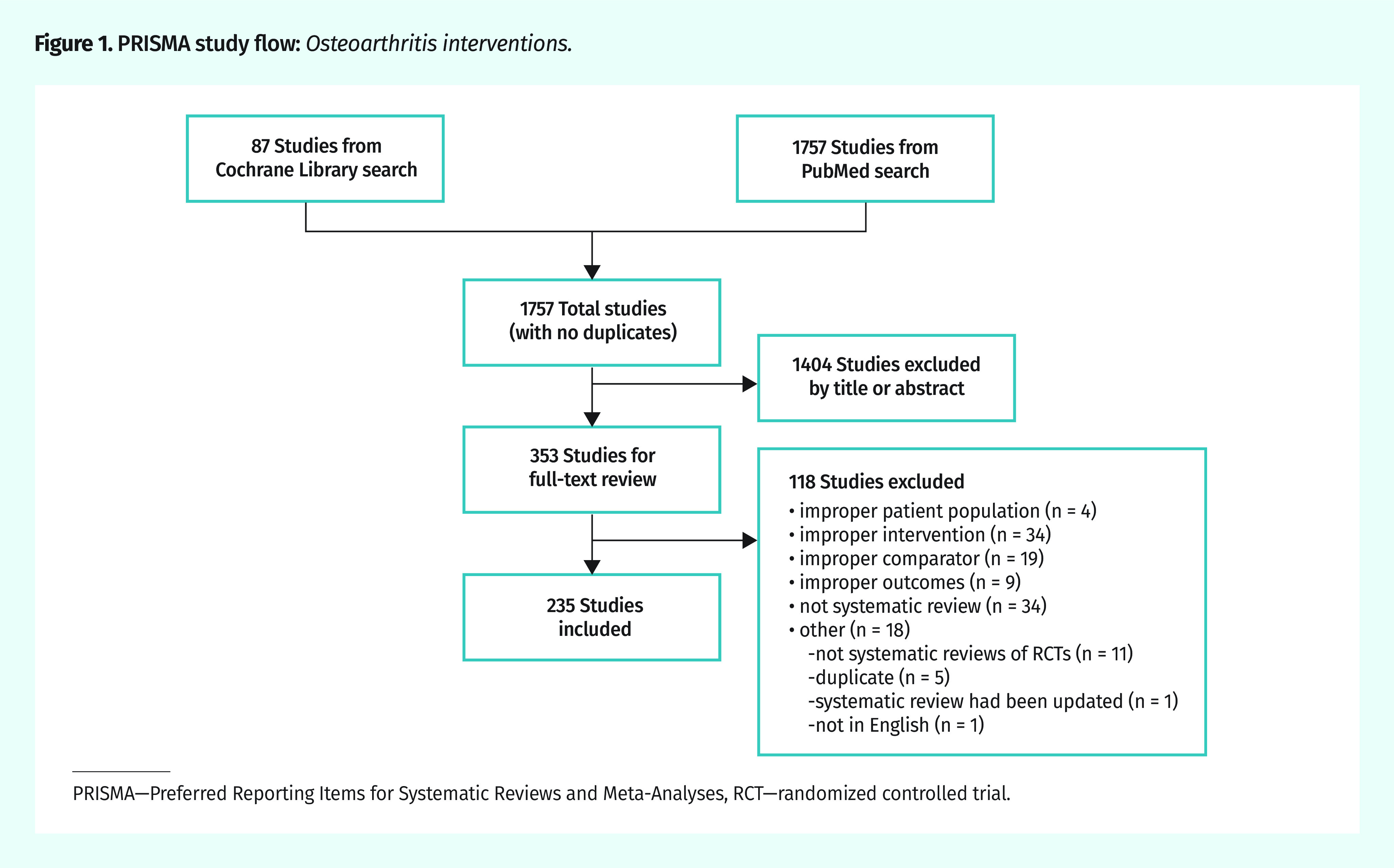
PRISMA study flow: Osteoarthritis interventions.
PRISMA—Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT—randomized controlled trial.
Full details of the primary efficacy results are available in Table 1, with interventions arranged in order of decreasing efficacy (based on risk ratios [RRs]). Full details of the subgroup analysis on funding source are available in Table 2. Individual metagraphs for responder outcomes for the longest reported outcomes, as well as subgroup analyses of size, funding, and duration are available from CFPlus (Figures A1 to A10).* Adverse events that could be retrieved from meta-analyses in systematic reviews are available from CFPlus (Table A2).*
Table 1.
Proportion of patients attaining meaningful pain relief for each intervention compared with control: Ordered by descending efficacy (based on risk ratio).
| TREATMENT TYPE | RCTS | INTERVENTION EVENT RATE, % (N/N) | CONTROL EVENT RATE, % (N/N) | NNT | TIME FRAME | CERTAINTY OF THE EVIDENCE (GRADE)* | RISK RATIO (95% CI) |
|---|---|---|---|---|---|---|---|
| Exercise | 11 | 47 (341/723) | 21 (138/644) | 4 | 6 to 104 wk |
0000 Low |
2.36 (1.79–3.12) |
| Intra-articular corticosteroids | 7 | 50 (203/410) | 31 (91/296) | 6 | 4 to 24 wk |
0000 Very low |
1.74 (1.15–2.62) |
| SNRIs (duloxetine only) | 6 | 64 (655/1030) | 43 (443/1030) | 5 | 10 to 18 wk |
0000 Moderate |
1.53 (1.25–1.87) |
| NSAIDs (oral) | 43 | 57 (12 201/21 495) | 39 (2838/7204) | 6 | 4 to 104 wk |
0000 Moderate |
1.44 (1.36–1.52) |
| Glucosamine | 9 | 47 (384/824) | 37 (306/819) | 11 | 4 to 156 wk |
0000 Very low |
1.33 (1.02–1.74) |
| NSAIDs (topical) | 22 | 61 (2357/3892) | 47 (1602/3373) | 8 | 1 to 12 wk |
0000 Low |
1.27 (1.16–1.38) |
| Chondroitin | 9 | 57 (707/1250) | 45 (553/1227) | 9 | 12 to 48 wk |
0000 Moderate |
1.26 (1.13–1.41) |
| Viscosupplementation | 31 | 53 (1748/3291) | 44 (1300/2963) | 11 | 2 to 160 wk |
0000 Very low |
1.22 (1.12–1.33) |
| Opioids (oral) | 15 | 47 (1795/3854) | 43 (1048/2412) | 32 | 10 d to 24 wk |
0000 Very low |
1.16 (1.02–1.32) |
| Acetaminophen | 2 | 47 (240/513) | 43 (204/478) | NSS | 6 to 24 wk |
0000 Low |
1.17 (0.83–1.64) |
GRADE—Grading of Recommendations Assessment, Development and Evaluation; NNT—number needed to treat; NSAID—nonsteroidal anti-inflammatory drug; NSS—not statistically significant; RCT—randomized controlled trial; SNRI—serotonin-norepinephrine reuptake inhibitor.
Details of GRADE Assessment are available in the appendix from CFPlus (Table A8).
Table 2.
Proportion of patients with a clinically meaningful response, based on funding source: Only studies with clear public or industry funding were analyzed. Those with unclear funding sources were not included in the analysis.
| TREATMENT TYPE | RCTS | INDUSTRY FUNDING | INTERVENTION EVENT RATE, % (N/N) | CONTROL EVENT RATE, % (N/N) | RISK RATIO (95% CI) | NNT | P VALUE* |
|---|---|---|---|---|---|---|---|
| Exercise | 0 | Industry funding | NA† | NA† | NA† | NA† | NA† |
| 10 | Clearly publicly funded | 47 (332/703) | 22 (136/634) | 2.38 (1.78–3.18) | 4 | ||
| Intra-articular corticosteroids | 3 | Industry funding | 50 (129/257) | 44 (68/155) | 1.15 (0.85–1.55) | NSS | .05 |
| 3 | Clearly publicly funded | 49 (46/94) | 17 (14/82) | 2.66 (1.22–5.77) | 4 | ||
| SNRIs (duloxetine only) | 6 | Industry funding | 64 (655/1030) | 43 (443/1030) | 1.53 (1.25–1.87) | 5 | NA† |
| 0 | Clearly publicly funded | NA† | NA† | NA† | NA† | ||
| NSAIDs (oral) | 39 | Industry funding | 57 (11 785/20 810) | 39 (2560/6518) | 1.42 (1.34–1.50) | 6 | .008 |
| 1 | Clearly publicly funded | 67 (214/318) | 57 (178/313) | 1.18 (1.05–1.34) | 10 | ||
| Glucosamine | 6 | Industry funding | 41 (176/425) | 25 (105/423) | 1.62 (1.28–2.05) | 7 | .006 |
| 3 | Clearly publicly funded | 52 (208/399) | 51 (201/396) | 0.99 (0.76–1.28) | NSS | ||
| NSAIDs (topical) | 14 | Industry funding | 56 (1594/2847) | 48 (1354/2808) | 1.19 (1.09–1.31) | 13 | NA† |
| 0 | Clearly publicly funded | NA† | NA† | NA† | NA† | ||
| Chondroitin | 8 | Industry funding | 54 (505/932) | 41 (375/914) | 1.30 (1.14–1.49) | 8 | .10 |
| 1 | Clearly publicly funded | 64 (202/318) | 57 (178/313) | 1.12 (0.98–1.27) | NSS | ||
| Viscosupplementation | 23 | Industry funding | 54 (1502/2805) | 45 (1137/2555) | 1.20 (1.10–1.32) | 12 | .72 |
| 1 | Clearly publicly funded | 36 (30/84) | 32 (27/84) | 1.11 (0.73–1.70) | NSS | ||
| Opioids (oral) | 14 | Industry funding | 46 (1740/3743) | 43 (992/2293) | 1.17 (1.02–1.34) | 32 | NA† |
| 0 | Clearly publicly funded | NA† | NA† | NA† | NA† | ||
| Acetaminophen | 2 | Industry funding | 47 (240/513) | 43 (204/478) | 1.17 (0.83–1.64) | NSS | NA† |
| 0 | Clearly publicly funded | NA† | NA† | NA† | NA† |
NA—not applicable, NNT—number needed to treat, NSAID—nonsteroidal anti-inflammatory drug, NSS—not statistically significant, RCT—randomized controlled trial, SNRI—serotonin-norepinephrine reuptake inhibitor.
P value for testing between subgroup differences.
Not applicable, as one subgroup was not present.
Treatment options
Exercise.
Eleven RCTs with 1367 patients followed for 6 to 104 weeks were suitable for meta-analysis. The most common type of exercise included was physiotherapy-guided exercise programs. Meta-analysis revealed 47% of patients receiving exercise therapy and 21% in the control group attained meaningful pain relief (RR = 2.36; 95% CI 1.79 to 3.12), for a number needed to treat (NNT) of 4 (Figure 2).17–22 No industry-funded trials were identified. Primary and subgroup meta-analyses are available from CFPlus (Figures A1a–d).*
Figure 2.
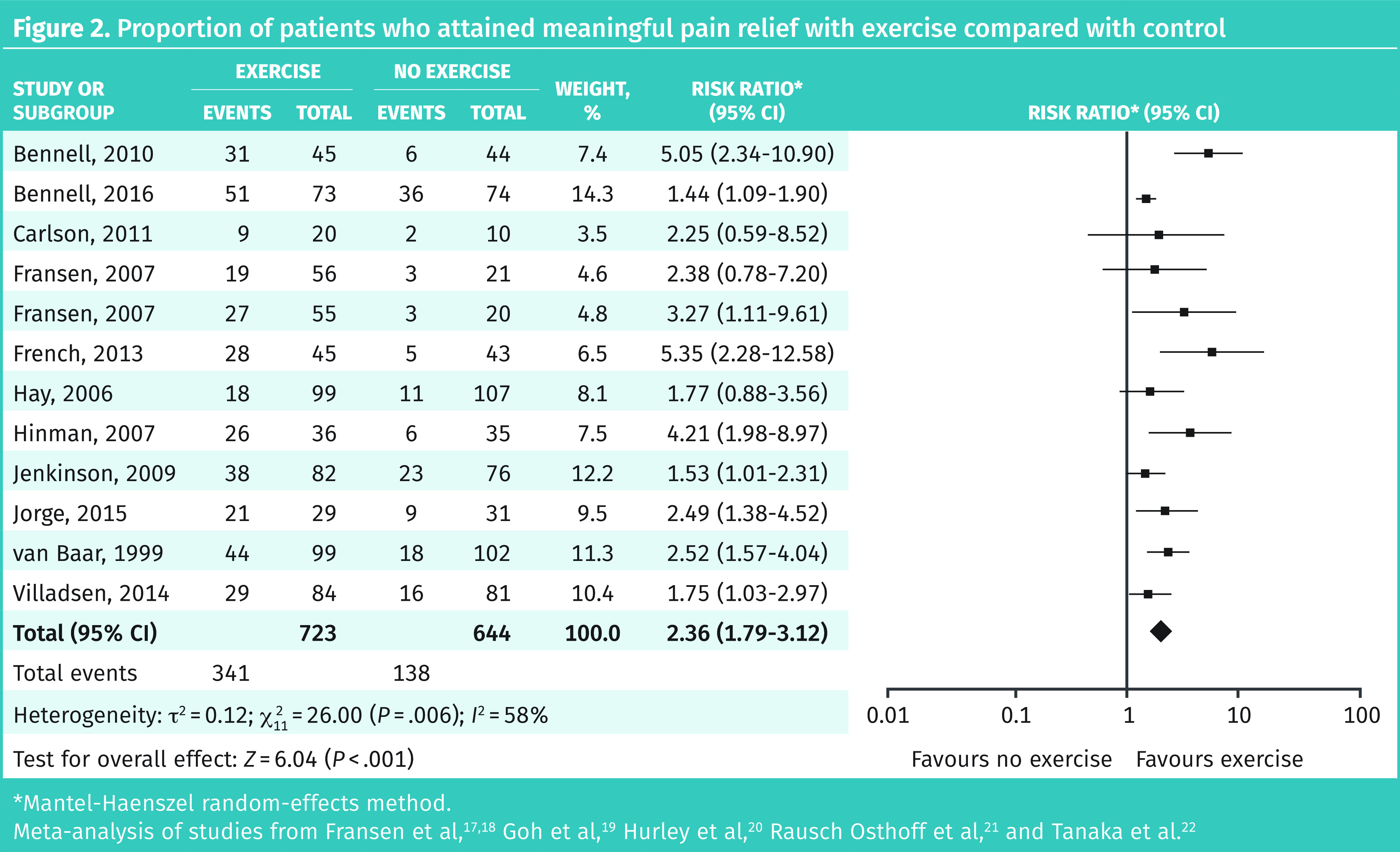
Proportion of patients who attained meaningful pain relief with exercise compared with control
*Mantel-Haenszel random-effects method.
Meta-analysis of studies from Fransen et al,17,18 Goh et al,19 Hurley et al,20 Rausch Osthoff et al,21 and Tanaka et al.22
Intra-articular corticosteroids.
Seven RCTs with 706 patients followed for 4 to 24 weeks were suitable for meta-analysis. Meta-analysis revealed 50% of patients receiving steroid injections and 31% receiving placebo attained meaningful pain relief (RR = 1.74; 95% CI 1.15 to 2.62), for an NNT of 6. Studies 12 weeks or longer showed no difference from placebo. Primary and subgroup meta-analyses are available from CFPlus (Figures A2a–e).*
SNRIs (duloxetine only).
Six RCTs with 2060 patients followed for 10 to 18 weeks were suitable for meta-analysis (all examining duloxetine). Meta-analysis revealed 64% of patients receiving duloxetine and 43% receiving placebo attained meaningful pain relief (RR = 1.53; 95% CI 1.25 to 1.87), for an NNT of 5. No publicly funded trials were identified. Primary and subgroup meta-analyses are available from CFPlus (Figures A3a–d).*
Oral NSAIDs.
Forty-three RCTs with 28 699 patients followed for 4 to 104 weeks were suitable for meta-analysis. Meta-analysis revealed 57% of patients receiving oral NSAIDs and 39% receiving placebo attained meaningful pain relief (RR = 1.44; 95% CI 1.36 to 1.52), for an NNT of 6. In a subgroup analysis of trial funding, the publicly funded trial demonstrated smaller benefit (RR = 1.18; 95% CI 1.05 to 1.34). Primary and subgroup meta-analyses are available from CFPlus (Figures A4a–e).*
Glucosamine.
Nine RCTs with 1643 patients followed for 4 to 156 weeks were suitable for meta-analysis. Meta-analysis revealed 47% of patients receiving glucosamine and 37% receiving placebo attained meaningful pain relief (RR = 1.33; 95% CI 1.02 to 1.74), for an NNT of 11. In a subgroup analysis of trial funding, publicly funded trials found no significant benefit with glucosamine versus placebo (RR = 0.99; 95% CI 0.76 to 1.28). Primary and subgroup meta-analyses are available from CFPlus (Figures A5a–e).*
Topical NSAIDs.
Twenty-two RCTs with 7265 patients followed for 1 to 12 weeks were suitable for meta-analysis. Meta-analysis revealed 61% of patients receiving topical NSAIDs and 47% receiving placebo attained meaningful pain relief (RR = 1.27; 95% CI 1.16 to 1.38), for an NNT of 8. No publicly funded trials were identified. Primary and subgroup meta-analyses are available from CFPlus (Figures A6a–e).*
Chondroitin.
Nine RCTs with 2477 patients followed for 12 to 48 weeks were suitable for meta-analysis. Meta-analysis revealed 57% of patients receiving chondroitin and 45% receiving placebo attained meaningful pain relief (RR = 1.26; 95% CI 1.13 to 1.41), for an NNT of 9. In a subgroup analysis of trial funding, the publicly funded trial did not demonstrate a statistically significant benefit (RR = 1.12; 95% CI 0.98 to 1.27). Primary and subgroup meta-analyses are available from CFPlus (Figure A7a–e).*
Viscosupplementation.
Thirty-one RCTs with 6254 patients followed for 2 to 160 weeks were suitable for meta-analysis. Meta-analysis revealed 53% of patients receiving viscosupplementation and 44% receiving placebo attained meaningful pain relief (RR = 1.22; 95% CI 1.12 to 1.33), for an NNT of 11. In a subgroup analysis of trial funding, the publicly funded trial did not demonstrate a statistically significant benefit (RR = 1.11; 95% CI 0.73 to 1.70). Primary and subgroup meta-analyses are available from CFPlus (Figures A8a–e).*
Opioids.
Fifteen RCTs with 6266 patients followed for 10 days to 24 weeks were suitable for meta-analysis. Meta-analysis revealed 47% of patients receiving opioids and 43% receiving placebo attained meaningful pain relief (RR = 1.16; 95% CI 1.02 to 1.32), for an NNT of 32. No publicly funded trials were identified. In a subgroup analysis of efficacy based on duration of treatment, opioids did not show statistically significantly more responders than placebo after 4 weeks (Figure 3).23–28 Primary and subgroup meta-analyses are available from CFPlus (Figures A9a–e).*
Figure 3.
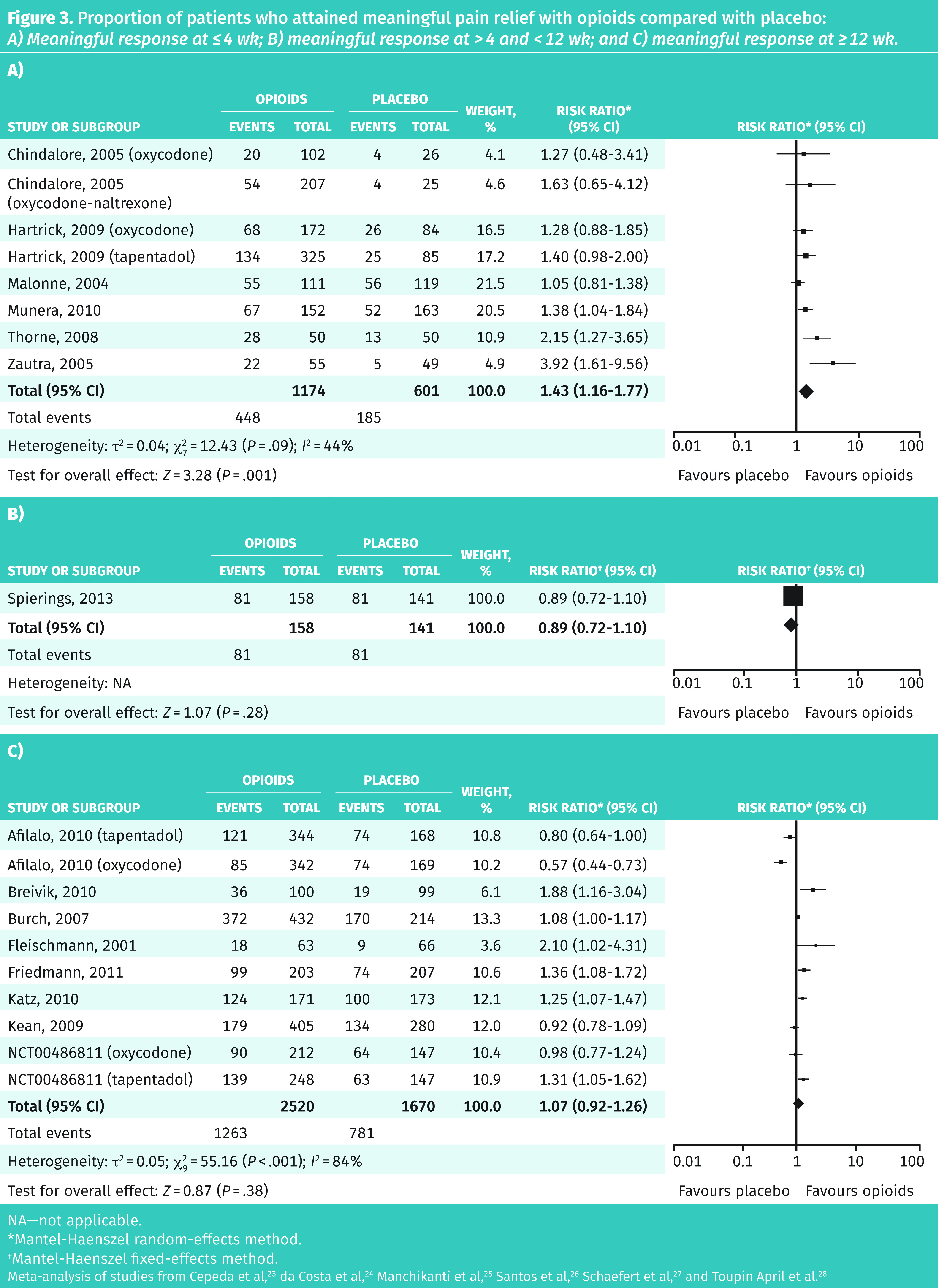
Proportion of patients who attained meaningful pain relief with opioids compared with placebo: A) Meaningful response at ≤ 4 wk; B) meaningful response at > 4 and < 12 wk; and C) meaningful response at ≥ 12 wk.
NA—not applicable.
*Mantel-Haenszel random-effects method.
†Mantel-Haenszel fixed-effects method.
Meta-analysis of studies from Cepeda et al,23 da Costa et al,24 Manchikanti et al,25 Santos et al,26 Schaefert et al,27 and Toupin April et al.28
Acetaminophen.
Two RCTs with 991 patients followed for 6 to 24 weeks were suitable for meta-analysis. Meta-analysis revealed no statistically significant difference between acetaminophen and placebo (RR = 1.17; 95% CI 0.83 to 1.64). No publicly funded trials were identified. Primary and subgroup meta-analyses are available from CFPlus (Figures A10a–b).*
Rubefacients.
One RCT of 113 patients compared 0.025% capsaicin to vehicle placebo and found no statistical difference at 4, 8, or 12 weeks.29
Interventions lacking responder analysis.
We did not identify any systematic reviews that included a responder analysis for platelet-rich plasma injections, counseling, cannabinoids, or tricyclic antidepressants.
Quality assessment
The results of our modified AMSTAR assessment for each of the included systematic reviews is presented in Table A3, available from CFPlus.* Of 649 RCTs identified from systematic reviews across all interventions, 155 (24%) provided a responder analysis. Of those 155 RCTs, only 11 had at least 150 participants, were longer than 8 weeks, and were clearly publicly funded (Table 3). Heterogeneity of the primary outcome ranged from 48% to 82% (I2 statistic).
Table 3.
Number of RCTs with responder analysis and quality characteristics
| INTERVENTION | NO. OF RCTS FOUND* | NO. OF RCTS IDENTIFIED WITH RESPONDER ANALYSIS (%)† | OF THOSE WITH A RESPONDER ANALYSIS SAMPLE SIZE ≥ 150 | DURATION > 8 WK | CLEARLY PUBLICLY OR NOT INDUSTRY FUNDED | NO. MEETING ALL CRITERIA (%)‡ |
|---|---|---|---|---|---|---|
| Exercise | 237 | 11 (5) | 5 | 10 | 11 | 5 (2) |
| Intra-articular corticosteroids | 32 | 7 (22) | 1 | 7 | 3 | 0 (0) |
| SNRIs (duloxetine only) | 6 | 6 (100) | 6 | 6 | 0 | 0 (0) |
| NSAIDs (oral) | 115 | 43 (37) | 42 | 23 | 1 | 1 (1) |
| Glucosamine | 31 | 9 (29) | 4 | 7 | 3 | 3 (10) |
| NSAIDs (topical) | 30 | 22 (73) | 15 | 8 | 0 | 0 (0) |
| Chondroitin | 19 | 9 (47) | 4 | 9 | 1 | 1 (5) |
| Viscosupplementation | 166 | 31 (19) | 17 | 26 | 1 | 1 (1) |
| Opioids (oral) | 32 | 15 (47) | 12 | 9 | 0 | 0 (0) |
| Acetaminophen | 10 | 2 (20) | 2 | 1 | 0 | 0 (0) |
| Total | 649 | 155 (24) | 108 | 106 | 20 | 11 (2) |
AMSTAR—A Measurement Tool to Assess Systematic Reviews, NSAID—nonsteroidal anti-inflammatory drug, RCT—randomized controlled trial, SNRI—serotonin-norepinephrine reuptake inhibitor.
Retrieved from the top 5 systematic reviews (ranked by most recent year published and AMSTAR rating) from the review.
See methods section for responder criteria.
Responder analysis in addition to sample size ≥ 150, duration > 8 wk, and clearly publicly funded.
Adverse events
In general, adverse events were poorly and inconsistently reported in the systematic reviews (Table A2, available from CFPlus*). One outcome consistently reported across interventions is withdrawal due to adverse events. Withdrawals due to adverse events were not significantly greater for most interventions, except for opioids (number needed to harm [NNH] of 8 to 10), SNRIs (NNH = 15), topical NSAIDs (NNH = 50), and viscosupplementation (statistically significant, but absolute numbers not reported).
DISCUSSION
To our knowledge, this is the first meta-analysis to report responder data—a key patient-oriented outcome for osteoarthritis pain management—for a variety of interventions.
Our analysis suggests that exercise is an effective intervention for chronic osteoarthritis pain regardless of study size or time frame studied. Exercise has consistently been recommended by international guideline groups as the first-line treatment in osteoarthritis management. The type of exercise is likely not important.8,9,30–33
Pharmacotherapies such as NSAIDs and duloxetine demonstrate smaller but statistically significant benefit that continues beyond 12 weeks. Opioids appear to have short-term benefits that attenuate after 4 weeks, and intra-articular steroids after 12 weeks. Limited data (based on 2 RCTs) suggest that acetaminophen is not helpful. These findings are consistent with recent Osteoarthritis Research Society International guideline recommendations that no longer recommend acetaminophen for osteoarthritis pain management and strongly recommend against the use of opioids.8
Limited benefit was observed with other interventions including glucosamine, chondroitin, and viscosupplementation. When only publicly funded trials were examined for these interventions, the results were no longer statistically significant.
We found that adverse events were inconsistently reported in the systematic reviews, which is consistent with data suggesting that published studies under-report adverse events compared with unpublished studies.34 Overall, withdrawal due to adverse events was consistently reported and found to be greater in patients using opioids, SNRIs, topical NSAIDs, and viscosupplementation.
Strengths and limitations
Strengths of this review include a focus on responder analyses and the completion of a priori subgroup analyses for funding, trial size, and length. When data were available to compare public to industry-funded trials, we found that in most cases efficacy decreased in publicly funded trials. Limitations include the chosen design of this review. It is possible we missed RCTs that had not been identified in the included systematic reviews. In prioritizing RCTs with responder analysis, we might have inadvertently overestimated the treatment effect of some interventions, as RCTs that did not achieve a clinically important response might have been less likely to report these data. Heterogeneity was high for most outcomes, which might reflect differences in study populations (eg, affected joint), dosing, responder outcome reported, or other unidentified study differences.
The adverse events reported are likely missing potential serious and rare adverse events because these are not reported well in RCTs and are generally only captured comprehensively in observational studies, which follow large numbers of patients for long periods of time (eg, dependence potential of opioids, or NSAIDs and gastrointestinal bleeds).35,36
In 2000, a European League Against Rheumatism systematic review of 680 studies of knee osteoarthritis found that only 5 of them reported responder outcomes (20% reduction in pain).37 In our search, 155 (24%) RCTs included a responder analysis. Additional limitations include the lack of moderately sized publicly funded trials that followed patients for more than 8 weeks. Only 2% of trials met all these criteria in addition to the inclusion of responder analyses. This suggests that 98% of research being conducted is not meeting critical quality criteria to allow for accurate interpretation of potential treatment benefits of the multitude of available interventions for osteoarthritis. In an effort to facilitate shared informed decision making with our patients, future studies evaluating osteoarthritis interventions should be at least 12 weeks long, of reasonable size, and publicly funded, and report the proportion of patients with 30% or 50% improvement in pain.
Conclusion
Evidence suggests that exercise is an effective intervention for achieving meaningful pain relief in osteoarthritis. This is followed by intra-articular corticosteroids, SNRIs, oral and topical NSAIDs, glucosamine, chondroitin, viscosupplementation injections, and opioids. Both acetaminophen and rubefacients were similar to placebo. No efficacy responder data were identified for platelet-rich plasma, counseling, cannabinoids, or tricyclic antidepressants as treatment options for osteoarthritis. Adverse event data for interventions in osteoarthritis are limited and further research is required.
Findings of this systematic review were used to develop a clinical decision aid (page 191)38 and will be combined with similar systematic reviews and tools on other types of pain to inform future guideline development.
Acknowledgments
We thank Janice Kung, MLIS, for her assistance with creating the search strategy, our peer reviewers for their valuable feedback, and the Alberta College of Family Physicians and the College of Family Physicians of Canada for continued support. This project was funded by Alberta Health through the Primary Health Care Opioid Response Initiative.
Editor’s key points
▸ Osteoarthritis is one of the most common chronic medical conditions experienced by older persons and a frequent reason patients visit their primary care providers. While various interventions have been evaluated, they are frequently evaluated in isolation, making it difficult to determine the relative efficacy of treatments. Systematic reviews of multiple interventions have generally reported standard mean differences or the direction of effect—outcomes not readily translatable to clinical practice.
▸ Analysis of responders—the proportion of patients achieving outcomes patients consider meaningful—might provide a clearer picture of how many patients will receive clinical benefit from a therapeutic intervention. This systematic review of systematic reviews focused on randomized controlled trials that included meta-analysis of responder outcomes for non-surgical interventions.
▸ Findings of this systematic review were used to develop a clinical decision aid (page 191) and will be combined with similar systematic reviews and tools on other types of pain to inform future guideline development.
Points de repère du rédacteur
▸ L’arthrose est l’un des problèmes de santé chroniques les plus courants dont souffrent les personnes plus âgées et une fréquente motivation de leurs consultations auprès de leurs professionnels des soins primaires. Quoique diverses interventions aient été évaluées, elles le sont souvent isolément, ce qui complique la détermination de l’efficacité relative des traitements. Des revues systématiques sur de multiples interventions ont généralement signalé des différences de moyennes standard ou la direction des effets, mais ce sont des résultats qui ne sont pas aisément transposables à la pratique clinique.
▸ L’analyse des sujets répondants, notamment la proportion de patients qui obtiennent des résultats qu’ils considèrent significatifs, pourrait dégager un portait plus clair du nombre de patients qui tireraient des bienfaits cliniques d’une intervention. Cette revue systématique de revues systématiques se concentre sur les études contrôlées randomisées qui incluent une méta-analyse des résultats chez les sujets répondants que procurent des interventions non chirurgicales.
▸ Les constatations de cette revue systématique ont été utilisées pour élaborer une aide à la décision clinique (page e86), et elles seront combinées à des revues systématiques et à des outils semblables sur d’autres types de douleurs pour servir de fondement à la production de lignes directrices futures.
Footnotes
The hierarchy of responder outcomes; individual metagraphs for responder outcomes; subgroup analyses of size, funding, and duration; primary and subgroup meta-analyses for the various interventions; details of the GRADE analysis; the modified AMSTAR assessment; and the reported adverse events are available at www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
Contributors
All authors were part of the Evidence Review Team and contributed to preparing the manuscript for submission.
Competing interests
None declared
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.Finley CR, Chan DS, Garrison S, Korownyk C, Kolber MR, Campbell S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician. 2018;64:832–40. [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. Erratum in: Lancet 2013;381(9867):628. [DOI] [PubMed] [Google Scholar]
- 4.JÜni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, et al. Intraarticular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328. doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leopoldino AO, Machado GC, Ferreira PH, Pinheiro MB, Day R, McLachlan AJ, et al. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database Syst Rev. 2019;(2):CD013273. doi: 10.1002/14651858.CD013273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regnaux JP, Lefevre-Colau MM, Trinquart L, Nguyen C, Boutron I, Brosseau L, et al. High-intensity versus low-intensity physical activity or exercise in people with hip or knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD010203. doi: 10.1002/14651858.CD010203.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614. doi: 10.1002/14651858.CD005614.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–89. doi: 10.1016/j.joca.2019.06.011. Epub 2019 Jul 3. [DOI] [PubMed] [Google Scholar]
- 9.Geenen R, Overman CL, Christensen R, Àsenlöf P, Capela S, Huisinga KL, et al. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77(6):797–807. doi: 10.1136/annrheumdis-2017-212662. Epub 2018 May 3. [DOI] [PubMed] [Google Scholar]
- 10.Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ. 2013;346:f2690. doi: 10.1136/bmj.f2690. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. Epub 2009 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–20. doi: 10.1016/j.jclinepi.2008.10.009. Epub 2009 Feb 20. [DOI] [PubMed] [Google Scholar]
- 14.Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12(5):389–99. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Wallis JA, Taylor NF, Bunzli S, Shields N. Experience of living with knee osteoarthritis: a systematic review of qualitative studies. BMJ Open. 2019;9(9):e030060. doi: 10.1136/bmjopen-2019-030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015. Epub 2011 Jan 5. [DOI] [PubMed] [Google Scholar]
- 17.Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;(1):CD004376. doi: 10.1002/14651858.CD004376.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. 2014;(4):CD007912. doi: 10.1002/14651858.CD007912.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh SL, Persson MSM, Stocks J, Hou Y, Welton NJ, Lin J, et al. Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: systematic review and network meta-analysis. Sports Med. 2019;49(5):743–61. doi: 10.1007/s40279-019-01082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurley M, Dickson K, Hallett R, Grant R, Hauari H, Walsh N. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst Rev. 2018;(4):CD010842. doi: 10.1002/14651858.CD010842.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rausch Osthoff AK, Juhl CB, Knittle K, Dagfinrud H, Hurkmans E, Braun J, et al. Effects of exercise and physical activity promotion: meta-analysis informing the 2018 EULAR recommendations for physical activity in people with rheumatoid arthritis, spondyloarthritis and hip/knee osteoarthritis. RMD Open. 2018;4(2):e000713. doi: 10.1136/rmdopen-2018-000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka R, Ozawa J, Kito N, Moriyama H. Effects of exercise therapy on walking ability in individuals with knee osteoarthritis: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. 2016;30(1):36–52. doi: 10.1177/0269215515570098. [DOI] [PubMed] [Google Scholar]
- 23.Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2006;(3):CD005522. doi: 10.1002/14651858.CD005522.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Da Costa BR, Nüesch E, Kasteler R, Husni E, Welch V, Rutjes AW, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2014;(9):CD003115. doi: 10.1002/14651858.CD003115.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manchikanti L, Ailnani H, Koyyalagunta D, Datta S, Singh V, Eriator I, et al. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician. 2011;14(2):91–121. [PubMed] [Google Scholar]
- 26.Santos J, Alarcão J, Fareleira F, Vaz-Carneiro A, Costa J. Tapentadol for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2015;(5):CD009923. doi: 10.1002/14651858.CD009923.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaefert R, Welsch P, Klose P, Sommer C, Petzke F, Häuser W. Opioids in chronic osteoarthritis pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Schmerz. 2015;29(1):47–59. doi: 10.1007/s00482-014-1451-1. [DOI] [PubMed] [Google Scholar]
- 28.Toupin April K, Bisaillon J, Welch V, Maxwell LJ, Jüni P, Rutjes AW, et al. Tramadol for osteoarthritis. Cochrane Database Syst Rev. 2019;(5):CD005522. doi: 10.1002/14651858.CD005522.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman RD, Aven A, Holmburg E, Pfeifer LM. Capsaicin cream 0.025% as monotherapy for osteoarthritis: a double-blind study. Semin Arthritis Rheum. 1994;23(Suppl 3):25–32. 3. [Google Scholar]
- 30.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 31.National Clinical Guideline Centre . Osteoarthritis: care and management in adults. London, UK: National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 32.Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66(3):622–36. doi: 10.1002/art.38290. [DOI] [PubMed] [Google Scholar]
- 33.Batterham SI, Heywood S, Keating JL. Systematic review and meta-analysis comparing land and aquatic exercise for people with hip or knee arthritis on function, mobility and other health outcomes. BMC Musculoskelet Disord. 2011;(12):123. doi: 10.1186/1471-2474-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golder S, Loke YK, Wright K, Norman G. Reporting of adverse events in published and unpublished studies of health care interventions: a systematic review. PLoS Med. 2016;13(9):e1002127. doi: 10.1371/journal.pmed.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moe S, Kirkwood J, Allan GM. Incidence of iatrogenic opioid use disorder. Can Fam Physician. 2019;65:724. (Eng), e431–2 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 36.Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Reglat A, Nicotra F, et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project) Drug Saf. 2012;35(12):1127–46. doi: 10.1007/BF03261999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2000;59(12):936–44. doi: 10.1136/ard.59.12.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindblad AJ, McCormack J, Korownyk CS, Kolber MR, Ton J, Perry D, et al. PEER simplified decision aid: osteoarthritis treatment options in primary care. Can Fam Physician. 2020;66:191–3. (Eng), e86–8 (Fr). [PMC free article] [PubMed] [Google Scholar]


