Abstract
Background
The recent emergence of the Coronavirus Disease (COVID-19) disease had been associated with reports of fungal infections such as aspergillosis and mucormycosis especially among critically ill patients treated with steroids. The recent surge in cases of COVID-19 in India during the second wave of the pandemic had been associated with increased reporting of invasive mucormycosis post COVID-19. There are multiple case reports and case series describing mucormycosis in COVID-19.
Purpose
In this review, we included most recent reported case reports and case-series of mucormycosis among patients with COVID-19 and describe the clinical features and outcome.
Results
Many of the mucormycosis reports were eported from India, especially in COVID-19 patients who were treated and recovered patients. The most commonly reported infection sites were rhino-orbital/rhino-cerebral mucormycosis. Those patients were diabetic and had corticosteroids therapy for controlling the severity of COVID-19, leading to a higher fatality in such cases and complicating the pandemic scenario. The triad of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), corticosteroid use and uncontrolled diabetes mellitus have been evident for significant increase in the incidence of angioinvasive maxillofacial mucormycosis. In addition, the presence of spores and other factors might play a role as well.
Conclusion
With the ongoing COVID-19 pandemic and increasing number of critically ill patients infected with SARS-CoV-2, it is important to develop a risk-based approach for patients at risk of mucormycosis based on the epidemiological burden of mucormycosis, prevalence of diabetes mellitus, COVID-19 disease severity and use of immune modulating agents including the combined use of corticosteroids and immunosuppressive agents in patients with cancer and transplants.
Keywords: SARS-CoV-2, COVID-19, Mucormycosis
Introduction
The current Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection is associated with a wide clinical spectrum of Coronavirus Disease 2019 (COVID-19) that ranges from being asymptomatic to severe disease requiring intensive care unit (ICU) admission [1–7]. The rate of admission to ICU is about 5% of all COVID-19 patients [8, 9]. Severe COVID-19 pneumonia is associated with immune dysregulation and cytokine syndrome leading to the increased use of immunomodulators [10, 11]. Emerging fungal infections such as aspergillosis were described in critically ill patients treated with steroids [12]. The mortality rate of SARS-CoV-2 infection in critically ill patients co-infected with aspergillosis was high [13].
Since the emergence of the COVID-19 pandemic, it has been suspected that mucormycosis might cause significant morbidity to infected patients. This was based on a retrospective analysis of SARS and influenza cases as suggested by Song et al. [14]. The more vulnerable individuals are those requiring hospitalization and intensive care, which represent advanced stage of their disease [15]. The recent surge in cases of COVID-19 in India during the second wave of the pandemic had been associated with increased reporting of invasive mucormycosis post COVID-19, of up to 9000 cases and are continuously being reported to be rising, popularly known as black fungal infection [16–18]. In this review, we describe the important risk factors, clinical presentation and outcome of mucormycosis in patients infected with SARS-CoV-2.
Incidence and prevalence
The occurrence of mucormycosis, a rare disease, in the general population was previously cited as 0.005 to 1.7 per million population [19]. However, the incidence of mucormycosis in India was reported to be 0.14/1000 diabetic patients which is 80 times higher than that reported in other parts of the world[20] and more than that in the general population based on computational-modeling [21]. Given the large number of diabetic patients in India of almost 62 million, mucormycosis has caused large public health burden in India [20]. In one study, diabetes mellitus was the underlying disease in 54–76% of mucormycosis cases with 8–22% presenting with diabetic ketoacidosis [22]. In addition, there had been geographic difference in the rate of diabetes mellitus among patients with mucormycosis in India. Even prior to COVID-19, the prevalence of diabetes mellitus was a major risk factor with regional differences ranging from 67% in North India to 22% among patients from the South of India [23]. The true incidence of rhino-orbital mucormycosis in COVID-19 patients is not known. However, there are multiple case reports describing mucormycosis in COVID-19 and most of these case reports are presently from India, especially in COVID-19 treated and recovered patients those were diabetic and corticosteroids were administered injudiciously for controlling severity of COVID-19, leading to a higher fatality in such cases and complicating the pandemic scenario [17, 18, 24–37].
Risk factors
There are multiple possible contributing factors for the development of mucormycosis among patients with COVID-19 and these include diabetes mellitus, obesity, use of corticosteroid, and the development of cytokine storms (Fig. 1). The triad of SARS-CoV-2, steroid and uncontrolled diabetes mellitus have contributed towards a significant increase in the incidence of angioinvasive maxillofacial mucormycosis [30]. However, the presence of spores and other factors might play a role as well [38]. The contribution of diabetes mellitus per se to the development of rhino-orbital-cerebral mucormycosis was the most common underlying comorbidity in 340 of 851 (40%) patients who were included in a meta-analysis, with an odds ratio (OR) of 2.49 (95% CI 1.77–3.54) compared to the next possible factor of having hematological malignancies with an OR of 0.76 (0.44–1.26) [19]. The role of Interleukin 6 blockers as a risk factor for mucormycosis is not clear [39]. Whether the combined use of steroids and interleukin 6 blockers will increase the risk of mucormycosis compared to the use of steroids alone needs more studies.
Fig. 1.
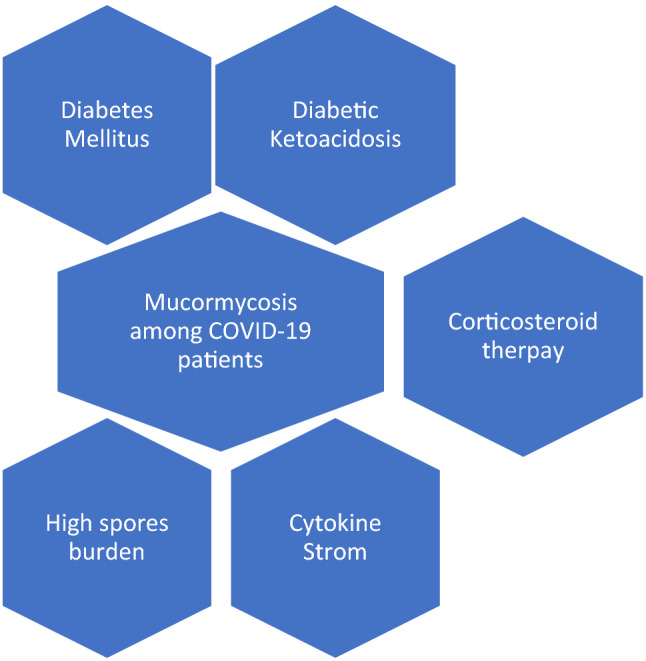
Possible contributing Factors for the development of Mucormycosis among COVID-19 patients
Clinical features and management
Literature review identified 30 publications of case reports and case series of mucormycosis among COVID-19 patients [24–26, 30, 31, 33–37, 40–55]. Of all the reports, 11 publications were from India [24–26, 30–37]. The most commonly reported infection sites were rhino-orbital/rhino-cerebral mucormycosis[24–26, 30, 32–37, 40, 42, 45, 47, 52–54]. Other presentations included pulmonary [31, 41, 43, 44, 49, 51, 55], cutaneous [46], disseminated [56] and gastrointestinal [48] diseases. The reported organisms were Rhizopus spp. [24, 31, 36, 41–44, 47, 49, 51, 55] and the others were reported as unspecified Mucorale [25, 26, 30, 33–35, 37, 40, 45, 48, 50, 52, 54]. The management of mucormycosis is usually difficult and requires urgent medical and surgical debridement while the choice of drug to treat mucormycosis is Amphotericin B [23, 57] and Amphotericin was used in 23 of the included studies [24–26, 30–37, 40–44, 46, 47, 49–54] and surgical debridement was reported in 20 of the included studies [24–26, 30, 32–37, 40, 44–47, 50–54]. The majority of the included patients in this review underwent surgical resection/debridement [24–26, 30, 32–37, 40, 44–47, 50–54].
Outcomes and prognosis
Before the COVID-19 era, mucormycosis is known for its poor prognosis, especially with delayed management may lead to a high mortality rate. There was no difference in the mortality between solid organ transplants and diabetes mellitus with a mortality of about 28%, (2/7 (28.57%) vs 5/18 (27.78%); p = 0.66 in patients with solid organ transplant and diabetes mellitus, respectively) [58]. However, another study showed higher mortality of 49% among diabetes mellitus patients compared to 30% among non-diabetic patients[58]. Morbidity and mortality were linked to the invasive nature of the underlying disease[59]. However, even with COVID-19, early intravenous anti-fungal treatment and surgical debridement were associated with favorable outcomes[26].
Discussion
The etiologic agent of mucormycosis are ubiquitous in nature and thus may easily be acquired, and its global epidemiology has been studied by several investigators, and may pose a threat during ongoing pandemic as has been observed in India [17, 23, 27, 57, 60, 61]. Due to the steep rise in cases of mucormycosis (black fungus infection) amid the second COVID-19 pandemic wave and its association with severe complications and associated higher fatality rate in post COVID-19 patients, this rare disease is now a notifiable disease in India. It is postulated that the use of non-sterile medical supplies might be associated with spore contamination and higher exposure of patients to mucormycosis [62, 63]. As summarized in Tables 1 and 2, most patients had severe COVID-19 pneumonia requiring intensive care, intubation and ventilation. In addition, most patients had underlying diabetes mellitus and received steroids [28, 64, 65]. The presence of diabetes mellitus is a major predisposing factor for mucormycosis as described in a meta-analysis among 600 (70%) of 851 patients with rhino-orbital–cerebral mucormycosis [19]. The presence of diabetes mellitus among patients with COVID-19 was estimated to be 17% in one study [66] and 9% in another study [67]. However, the presence of diabetes mellitus might be higher in other populations and may be more than 50% [4–6]. One meta-analysis showed that diabetes mellitus was associated with an odds ratio (OR) of 2.40 (95% CI 1.98–2.91) for severe disease [68], OR of 1.64 (95% CI 2.30–1.08) in a second meta-analysis [69], and an OR of 2.04, 95% CI 1.67–2.50 in a third meta-analysis [66]. Corticosteroid are currently the only medication that had shown conclusively to be effective in the treatment of COVID-19 in clinical trials therapy [70–72]. The RECOVERY trial utilized dexamethasone at a dose of 6 mg intravenous or oral once a day for treatment of COVID-19 [73]. Systemic steroids could further exaggerate the underlying glycemic control as well as impede the body’s immune system. The use of high dose corticosteroid had been used in patients with COVID-19 disease [74]and the use of such medications required assessment [75]. One study showed that adherence to the use of low dose corticosteroid and good glycemic control were important in having no mucormycosis among 1027 ICU patients despite the use of corticosteroids in 89% and that 40% had diabetes mellitus [76]. The presence of these pre-disposing factors in association with high fungal spore burden in certain localities and communities may set the perfect storm for the development of mucormycosis in patients with COVID-19 patients.
Table 1.
Summary of clinical characteristics of the included studies of SARS-CoV-2 and mucormycosis co-infections, 2020–2021
| Author, year, study location | Study design, setting | Age (years) | Male, n (%) | Underlying diseases | Mechanical ventilation, n (%) | Use of systemic corticosteroid therapy | Risk factors for mucormycosis | Histopathologic identification of an organism with a structure typical of Mucorales | Mucormycosis classificationa | Clinical symptoms and signs of mucormycosis | Description of mucormycosis and etiologic agent |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alekseyev et al. (2021), United States [40] | Retrospective, case report, single centre | 41 | 1 (100) | Diabetes | No | Yes | Uncontrolled diabetes, diabetic ketoacidosis | NA | Putative | Peripheral bilateral lung infiltrates with extension into the sinuses and intracranial abscess in the infratemporal fossa with cavernous sinus enhancement | Rhino-cerebral mucormycosis/Mucorale(unspecified) |
| Bellanger et al. (2021), France [41] | Retrospective, case report, single centre | 55 | 1 (100) | Lymphoma | Yes | Yes | Hematopoietic cell transplantation, steroid for SARS-CoV-2 | NA | Putative | Non-specific bilateral ground glass opacities with development of pulmonary fibrosis | Pulmonary mucormycosis/Rhizopus microsporus |
| Dallalzadeh et al. (2021), United States [42] | Retrospective, case reports, single centre | 48 | 2 (100) | Diabetes (n = 2) | NA | Yes (n = 2) | Uncontrolled diabetes, diabetic ketoacidosis | No | Definite | Right sino-nasal cavity and anterior skull base extending to bilateral frontal lobes | Rhino-orbital mucormycosis/Rhizopus spp. |
| Garg et al. (2021), India [31] | Retrospective, case report, single centre | 55 | 1 (100) | Diabetes, hypertension, coronary artery disease, cardiomyopathy, end-stage renal disease | Yes | Yes | Uncontrolled diabetes, steroid for SARS-CoV-2 | No | Putative | Cough, expectoration, and burning micturition. A thick-walled cavity in the right upper lobe was confirmed | Pulmonary mucormycosis/Rhizopus microsporus |
| Hanley et al. (2020), United Kingdom [56] | Retrospective, case series, multi-centre | 22 | 7 (70) | Pancreatitis | Yes | Yes | Steroid for SARS-CoV-2 | Yes | Definite (post-mortem) | NA | Disseminated (involving the hilar lymph nodes, heart, brain, and kidney)/Mucorale (unspecified) |
| Johnson et al. (2021), United States [43] | Retrospective, case report, single centre | 79 | 1 (100) | Diabetes, hypertension | Yes | Yes | Diabetes, steroid for SARS-CoV-2 | Yes | Probable | Bilateral ground-glass opacities and infiltrates; then extensive bilateral pneumonia and new development of bilateral upper lobe cavitations were revealed | Pulmonary mucormycosis/Rhizopus arrhizus |
| Kanwar et al. (2021), United States [44] | Retrospective, case report, single centre | 56 | 1 (100) | End-stage renal disease (hemodialysis) | Yes | Yes | NA | Yes | Definite | Patchy ground glass infiltrates with pleural effusion with an increased area of density concerning for blood | Pulmonary mucormycosis/Rhizopus azygosporus |
| Karimi‐Galougahi et al. (2021), Iran [45] | Retrospective, case report, single centre | 61 | 0 (0) | Diabetes | 0 (0) | Yes | Uncontrolled diabetes, steroid for SARS-CoV-2 | Yes | Definite | Right hemifacial pain and numbness, decreased visual acuity, chemosis, proptosis, frozen eye, complete loss of vision, and fixed mydriasis | Rhino-orbital mucormycosis/Mucorale (unspecified) |
| Khatri et al. (2021), United States [46] | Retrospective, case report, single centre | 68 | 1 (100) | Diabetes, hypertension, coronary artery disease, OSA, renal failure | Yes | Yes | Diabetes, hypertension, solid organ transplantation | Yes | Definite | Purplish skin discoloration with fluctuant swelling was noted in the right axilla, at the prior IABP catheter insertion site | Cutaneous mucormycosis/Rhizopus microsporus |
| Maini et al. (2021), India [32] | Retrospective, case report, single centre | 38 | 1 (100) | None | No | Yes | Steroid for SARS-CoV-2 | Yes | Definite | Patient developed chemosis and pain in the left eye | Sino-orbital mucormycosis/Rhizopusoryzae |
| Mehta et al. (2020), India [33] | Retrospective, case report, single centre | 60 | 1 (100) | Diabetes | 1 (100) | Yes | Uncontrolled diabetes, steroid for SARS-CoV-2 | Yes | Definite | Unilateral facial swelling, unilateral periorbital facial pain, eyelid oedema, ptosis, proptosis, right orbital cellulitis, acute vision loss | Rhino-orbital-cerebral mucormycosis/Mucorale (unspecified) |
| Mekonnen et al. (2021), United States [47] | Retrospective, case report, single centre | 60 | 1 (100) | Diabetes, asthma, hypertension, hyperlipidaemia | Yes | Yes | Uncontrolled diabetes, steroid for SARS-CoV-2 | Yes | Definite | Right globe proptosis, oedema of the eyelids and conjunctival chemosis. extensive opacification of right maxillary, ethmoid, and frontal sinuses | Rhino-orbital mucormycosis/Rhizopus spp. |
| Monte Junior et al. (2020), Brazil [48] | Retrospective, case report, single centre | 86 | 1 (100) | Hypertension | Yes | Yes | Steroid for SARS-CoV-2 | Yes | Definite | Gastric ulcers, acute diarrhea, melena, severe anemia, and fever | Gastrointestinal mucormycosis/Mucorale (unspecified) |
| Moorthy et al. (2021), India [30] | Retrospective, case series, multi-centre | Median (IQR), 55.5 (48–63) | 15 (83.3) | Diabetes (n = 16) | NA | Yes (n = 16) | Uncontrolled diabetes (n = 6), steroid for SARS-CoV-2 (n = 16) | Yes | Definite (n = 17) | Patients presented with one or more of the following symptoms: facial cellulitis, maxillary sinusitis, headache, necrosis of palatal bone/mucosa or acute loss of vision | Sinusitis alone (n = 3), Rhino-orbital (n = 6), Rhino-orbital-cerebral (n = 5), Rhino-cerebral (n = 3)/Mucorale (unspecified) |
| Pasero et al. (2020), Italy [49] | Retrospective, case report, single centre | 66 | 1 (100) | Hypertension | Yes | No | Lymphopenia | Yes | Putative | Pulmonary infiltrates with an increase of parenchymal thickening of the whole left lung, cavitary lesions in left lung and pleural effusion, opacification of the left maxillary sinus | Pulmonary mucormycosis/Rhizopus spp. |
| Pauli et al. (2021), Brazil [50] | Retrospective, case report, single centre | 50 | 0 (0) | Diabetes | NA | No | Uncontrolled diabetes | Yes | Definite | Ulcerated lesion with coagulative necrosis, hemorrhage, and abundant neutrophils | Palatal ulcer/Mucorale (unspecified) |
| Placik et al. (2020), United States [51] | Retrospective, case report, single centre | 49 | 1 (100) | None | Yes | Yes | Steroid for SARS-CoV-2 | Yes | Definite | Right pneumothorax, bronchopulmonary fistula, necrotic empyema | Pulmonary mucormycosis/Rhizopus spp. |
| Rao et al. (2021), India [34] | Retrospective, case report, single centre | 66 | 1 (100) | Diabetes | No | Yes | Uncontrolled diabetes, steroid for SARS-CoV-2 | Yes | Definite | Periorbital pain followed by sudden onset of vision loss in the left eye | Rhino‑orbital mucormycosis/Mucorale (unspecified) |
| Ravani et al. (2021), India [35] | Retrospective, case series, single centre | Mean, 56.3 | NA | Diabetes (n = 19); plus, other comorbidities (hypertension/ischemic heart disease/kidney disease) | NA | Yes | Uncontrolled diabetes, steroid for SARS-CoV-2 | NA | NA | The most common presentation was diminution of vision (< 6/60 in 80.64% patients) and ophthalmoplegia (77.4%). The most common imaging findings were orbital cellulitis (61.29%) and pansinusitis (77.4%) | Rhino‑orbital mucormycosis/Mucorale (unspecified) |
| Revannavar et al. (2021), India [36] | Retrospective, case report, single centre | NA | 0 (0) | Diabetes | No | No | Uncontrolled diabetes | Yes | Definite | Patient presented with left-sided facial pain, complete ptosis | Rhino‑orbital mucormycosis/Rhizopus spp. |
| Saldanha et al. (2021), India [37] | Retrospective, case report, single centre | 32 | 0 (0) | Diabetes | No | No | Uncontrolled diabetes | Yes | Definite | Patient presented with left eye complete ptosis and left facial pain | Sino-orbital mucormycosis/Mucorale (unspecified) |
| Sarkar et al. (2021), India [24] | Retrospective, case series, multi-centre | Median (IQR), 46.5 (30.7–59.7) | 8 (80) | Diabetes (n = 10) | Yes (n = 9) | Yes (n = 10) | Diabetic ketoacidosis (n = 9) | NA | Definite (n = 4), probable (n = 2) | NA | Rhino-orbital (n = 5), Rhino-orbital-cerebral (n = 1)/Rhizopus (n = 4), Mucor (n = 2) |
| Sen et al. (2021), India [25] | Retrospective, case series, multi-centre | Median (IQR), 61.4 (46.8–73.1) | 6 (100) | Diabetes (n = 5), hypertension (n = 1), coronary artery disease (n = 1) | NA | All patients received systemic corticosteroids for SARS-CoV-2 except for one patient | Uncontrolled diabetes (n = 3), steroid for SARS-CoV-2 (n = 5), diabetic ketoacidosis (n = 2) | Yes | Definite (n = 5), probable (n = 1) | All patients complained of pain, redness, and periocular swelling as initial symptoms. This was followed by acute, progressive, drooping of eyelids, limitation of ocular movements, and painful loss of vision | Rhino-orbital-cerebral mucormycosis/Mucorale (unspecified) |
| Sharma et al. (2021), India [26] | Prospective, case series, single centre | NA | 15 (65.2) | Diabetes (n = 21), hypertension (n = 14), renal failure (n = 1) | NA | Yes (n = 23) | Uncontrolled diabetes (n = 12) | No | NA | Intra-orbital extension was seen in 43.47% of cases, while intracranial extension was only seen in 8.69% | Intra-orbital (n = 10), intra-cranial (n = 2) and palatal (n = 1) |
| Veisi et al. (2021), Iran [52] | Retrospective, case reports, single centre | 40 (Case 1) and 54 (Case 2) | 1 (50) |
None (Case 1) Diabetes (Case 2) |
No | Yes (n = 2) | Diabetes (n = 1), steroid for SARS-CoV-2 (n = 2) | Yes (n = 2) | Definite |
Bilateral visual loss and periorbital pain with complete blepharoptosis and ophthalmoplegia together with mild proptosis (Case 1) Left orbital pain and periorbital swelling together with progressive vision loss (Case 2) |
Rhino-orbital (n = 1) and/or rhino-orbito-cerebral (n = 1) mucormycosis/Mucorale (unspecified) |
| Waizel-Haiat et al. (2021), Mexico [53] | Retrospective, case report, single centre | 24 | 0 (0) | Diabetes | Yes | NA | Uncontrolled diabetes, diabetic ketoacidosis | No | Probable | Severe left lid edema with extension to the upper lip and malar region, left proptosis with a hyperemic conjunctiva, and an opaque cornea | Rhino-orbital mucormycosis/Lichteimia (Absidia) spp. |
| Werthman-Ehrenreich et al. (2021), United States [54] | Retrospective, case report, single centre | 33 | 0 (0) | Diabetes, asthma, hypertension | NA | No | Diabetic ketoacidosis | NA | Definite | Necrotic palate, necrotic nasal, left eye ptosis, altered mental status, ophthalmoplegia proptosis | Rhino-orbital-cerebral mucormycosis/Mucorale(unspecified) |
| Zurl et al. (2021), Austria [55] | Retrospective, case report, single centre | 53 | 1 (100) | Myelodysplastic syndrome, acute myeloid leukemia | Yes | Yes | Intensive chemotherapy (neutropenia), steroid for SARS-CoV-2 (n = 5) | Yes | Definite (post-mortem) | Increase of bilateral infiltrates and the patient developed severe ARDS | Pulmonary mucormycosis/Rhizopus microspores |
| Pakdel et al.; (2021), [78] | Cross-sectional descriptive multicenter study | Median 52 years (range 14–71) | 15 and 9 (66%) male | 86% diabetes mellitus | NA | 7 (46.6%) | Diabetes and Steroid | Yes | Definite | Variable | Rhino-orbital |
| Singh et al. (2021); India [79] | Case report | 48 | 1 M | None | No | No | NA | Yes | Definite | Abdominal pain, nausea, vomiting | Gastrointestinal mucormycosis |
| Arjun et al. (2021); India [80] | Case series | 53.0 ± 12.1 years | 10 cases (80%) | 30% had coronary artery disease | NA | Yes in 80% | Corticosteroid | Yes | Definite | Headache and facial pain | Rhino-orbital |
| Saidha et al. (2021); India [81] | Case series | 47 | 6 cases (66%) | Diabetes Mellitus | NA | In 1 patient | Diabetes Mellitus | Yes | Definite | Headache and facial pain | Paranasal sinusitis |
| Jain et al. (2021); India [82] | Case report | 57 | Female | Diabetes Mellitus | No | Yes | Diabetes Mellitus | Yes | Definite | Abdominal pain, nausea, vomiting | Abdominal |
| Baskar et al. (2021); India [83] | Case report | 28 | Male | None | No | No | None | Yes | Definite | Acute loss of vision | Rhino-orbital |
| Joshi et al. (2021), India [84] | Case series | 55.2 ± 13 years | 16 men, 9 women | 22 had DM; 2 HIV | 20 (80%) | Yes | 6 (27%) | Yes (n = 10) | Radiographic and histopathology in selected patients | Variable | Rhino-orbito-cerebral |
| Sen et al. (2021); India [85] | Case series | Mean age 51.9 years | 2826 patients; male 71% | Diabetes mellitus 78% | NA | 87% | Diabetes and Steroid | NA | Definite | Variables | NA; rhino-orbital-cerebral mucormycosis |
ARDS acute respiratory distress syndrome, IABP intra-aortic balloon pump, NA not available, spp. species, SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, OSA obstructive sleep apnea
aDefinite—if histopathologic, cytopathologic or direct microscopic examination of a specimen obtained by needle aspiration or biopsy in which hyphae or melanized yeast-like forms were seen accompanied by evidence of associated tissue damage OR Recovery of a hyaline or pigmented mold by culture of a specimen obtained by a sterile procedure from a normally sterile and clinically or radiologically abnormal site consistent with an infectious disease process, excluding BAL fluid, a paranasal or mastoid sinus cavity specimen, and urine OR Blood culture that yielded a mold (e.g., Fusarium species) in the context of a compatible infectious disease process OR Amplification of fungal DNA by PCR combined with DNA sequencing when molds were seen in formalin-fixed paraffin-embedded tissue. Probable—concluded as the presence of combined host factors and clinical criterion with mycological evidence and if only the criteria for a host factor and a clinical criterion were met but mycological criteria were absent, possible mucormycosis was diagnosed. Putative—if none of the criteria were met but Mucor is attributed as a pathogen and patient was treated for it
Table 2.
Summary of therapy and outcome of mucormycosis among SARS-CoV-2 infected patients
| Author, year, study location | Time between diagnosis of SARS-CoV-2 and mucormycosis (days) | Surgical debridement made | Antifungal treatment | Treatment outcome |
|---|---|---|---|---|
| Alekseyev et al. (2021), United States [40] | NA | Yes | Amphotericin B | Survived |
| Bellanger et al. (2021), France [41] | 15 | NA | Amphotericin B | Died |
| Dallalzadeh et al. (2021), United States [42] | 6 | No | Amphotericin B, isavuconazole | Died (n = 2) |
| Garg et al. (2021), India [31] | 17 | Scheduled for right upper lobectomy | Amphotericin B | Survived |
| Hanley et al. (2020), United Kingdom [56] | NA | No | No | Died |
| Johnson et al. (2021), United States [43] | NA | NA | Amphotericin B, voriconazole | Discharged |
| Kanwar et al. (2021), United States [44] | 16 | Yes | Amphotericin B | Died |
| Karimi‐Galougahi et al. (2021), Iran [45] | 21 | Yes | Systemic antifungals (Unspecified) | Survived |
| Khatri et al. (2021), United States [46] | 90 | Yes | Amphotericin B, posaconazole | Died |
| Maini et al. (2021), India [32] | 18 | Yes | Amphotericin B, fluconazole | Survived |
| Mehta et al. (2020), India [33] | 10 | Yes | Amphotericin B | Died |
| Mekonnen et al. (2021), United States [47] | 7 | Yes | Amphotericin B, caspofungin, posaconazole; | Died |
| Monte Junior et al. (2020), Brazil [48] | 5 | No | No | Died |
| Moorthy et al. (2021), India [30] | NA | Yes (n = 7) | Amphotericin B | Survived (n = 11),died (n = 6) and lost to follow-up (n = 1) |
| Pasero et al. (2020), Italy [49] | 17 | No | Amphotericin B, isavuconazole | Died |
| Pauli et al. (2021), Brazil [50] | 8 | Yes | Amphotericin B | Survived |
| Placik et al. (2020), United States [51] | 14 | Yes | Amphotericin B | Died |
| Rao et al. (2021), India [34] | NA | Yes | Amphotericin B | Survived |
| Ravani et al. (2021), India [35] | NA | Yes (n = 19) | Amphotericin B (n = 19) | Survived (n = 18), died (n = 1) |
| Revannavar et al. (2021), India [36] | NA | Yes | Amphotericin B | Survived |
| Saldanha et al. (2021), India [37] | NA | Yes | Amphotericin B | Survived |
| Sarkar et al. (2021), India [24] | NA | Yes | Amphotericin B | Improved (n = 1), died (n = 4), unchanged (n = 4), exenteration (n = 1) |
| Sen et al. (2021), India [25] | Mean ± SD (minimum–maximum), 15.6 ± 9.6 (3–42) | Yes | Amphotericin B, voriconazole/posaconazole (n = 5) | Survived (n = 5) |
| Sharma et al. (2021), India [26] | NA | Yes | Amphotericin B | Survived (n = 23) |
| Veisi et al. (2021), Iran [52] | 8 (Case 1) and 7 (Case 2) | Yes (n = 2) | Amphotericin B (n = 2) | Died (Case 1) and discharged (Case 2) |
| Waizel-Haiat et al. (2021), Mexico [53] | 6 | Yes | Amphotericin B | Died |
| Werthman-Ehrenreich et al. (2021), United States [54] | 2 | Yes | Amphotericin B | Died |
| Zurl et al. (2021), Austria [55] | NA | No | None | Died |
| Pakdel et al.; (2021), Iran [78] | 1–37 | 33% | 6 (40%) combined antifungal | 7 (47%) died |
| Singh et al. (2021); India [79] | 19 | Yes | Liposomal amphotericin B | Recovered |
| Arjun et al. (2021); India [80] | 17.0 ± 3.6 | Yes | Amphotericin B deoxycholate and isavuconazole | 10% died |
| Saidha et al. (2021); India [81] | NA | Yes | Amphotericin | Recovered |
| Jain et al. (2021); India [82] | 15 | Yes | NA | Recovered |
| Baskar et al. (2021); India [83] | On diagnosis | Yes | Amphotericin | Recovered |
| Joshi et al. (2021), India [84] | Not indicated | yes in 10 (45%) | Amphotericin | 14 (63%) died |
| Sen et al. (2021); India [85] | 10–15 | 56% had functional endoscopic sinus surgery (FESS)/paranasal sinus (PNS) debridement, 15% orbital exenteration in 15%, 17% both FESS/PNS debridement and orbital exenteration | Amphotericin B in 73% | Mortality 14% |
The outcome was favorable for patients who had surgical debridement in three case series [25, 26, 35]. With the ongoing COVID-19 pandemic and increasing number of critically ill patients infected with SARS-CoV-2, it is important to develop a risk-based approach for patients at risk of mucormycosis based on the epidemiological burden of mucormycosis, prevalence of diabetes mellitus, COVID-19 disease severity and use of immune modulating agents including the combined use of steroids and immunosuppressive agents in patients with cancer and transplants. A suggested approach for aspergillosis in COVID-19 was developed [77] and a similar approach is needed for mucormycosis in SARS-CoV-2 infected patients. Whether a mold prophylaxis is required in high-risk patients need further studies.
Early diagnosis of cases of mucormycosis, timely treatment with prescribed drugs and surgical operations, checking glycemic levels and judicious use of corticosteroids in patients with COVID-19 along with adopting appropriate hygienic and sanitization measures would aid in limiting the rising cases of this fungal infection. In-depth studies are required to investigate how COVID-19 is triggering mucormycosis infections in patients and why mainly most cases are being reported from India as compared to other countries amidst second wave of ongoing pandemic.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest.
References
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;6736:1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicastri E, D’Abramo A, Faggioni G, De Santis R, Mariano A, Lepore L, et al. Coronavirus disease (COVID-19) in a paucisymptomatic patient: Epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Eurosurveillance. 2020 doi: 10.2807/1560-7917.ES.2020.25.11.2000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Omari A, Alhuqbani WN, Zaidi ARZ, Al-Subaie MF, AlHindi AM, Abogosh AK, et al. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: a descriptive cross-sectional study. J Infect Public Health. 2020;13:1639–1644. doi: 10.1016/j.jiph.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Mutair A, Alhumaid S, Alhuqbani WN, Zaidi ARZ, Alkoraisi S, Al-Subaie MF, et al. Clinical, epidemiological, and laboratory characteristics of mild-to-moderate COVID-19 patients in Saudi Arabia: an observational cohort study. Eur J Med Res. 2020;25:61. doi: 10.1186/s40001-020-00462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AlJishi JM, Alhajjaj AH, Alkhabbaz FL, AlAbduljabar TH, Alsaif A, Alsaif H, et al. Clinical characteristics of asymptomatic and symptomatic COVID-19 patients in the Eastern Province of Saudi Arabia. J Infect Public Health. 2021;14:6–11. doi: 10.1016/j.jiph.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, et al. Coronavirus disease 2019–COVID-19. Clin Microbiol Rev. 2020;33:1–48. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirupathi R, Muradova V, Shekhar R, Salim SA, Al-Tawfiq JA, Palabindala V. COVID-19 disparity among racial and ethnic minorities in the US: a cross sectional analysis. Travel Med Infect Dis. 2020;38:101904. doi: 10.1016/j.tmaid.2020.101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Tawfiq JA, Leonardi R, Fasoli G, Rigamonti D. Prevalence and fatality rates of COVID-19: what are the reasons for the wide variations worldwide? Travel Med Infect Dis. 2020;35:101711. doi: 10.1016/j.tmaid.2020.101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, et al. COVID-19 associated pulmonary aspergillosis (CAPA)—from immunology to treatment. J Fungi. 2020;6:1–17. doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahmer T, Kriescher S, Herner A, Rothe K, Spinner CD, Schneider J, et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective AspCOVID-19 study. PLoS ONE. 2021 doi: 10.1371/journal.pone.0238825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185:599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangneux JP, Bougnoux ME, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med. 2020 doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BBC. Black fungus: India reports nearly 9,000 cases of rare infection—BBC News 2021. 2021. https://www.bbc.com/news/world-asia-india-57217246. Accessed 28 May 2021.
- 17.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021 doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas S. Mucormycosis: The “black fungus” maiming Covid patients in India—BBC News 2021. 2021. https://www.bbc.com/news/world-asia-india-57027829. Accessed 29 May 2021.
- 19.Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Chander J, Kaur M, Singla N, Punia RPS, Singhal SK, Attri AK, et al. Mucormycosis: battle with the deadly enemy over a five-year period in India. J Fungi. 2018 doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A. Chakrabarti, P. Sood DWD. Estimating fungal infection burden in India using computational models: mucormycosis burden as a case study. ESCMID. 2021.
- 22.Prakash H, Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9:1–12. doi: 10.3390/microorganisms9030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakash H, Ghosh AK, Rudramurthy SM, Singh P, Xess I, Savio J, et al. A prospective multicenter study on mucormycosis in India: epidemiology, diagnosis, and treatment. Med Mycol. 2019;57:395–402. doi: 10.1093/mmy/myy060. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar S, Gokhale T, Choudhury S, Deb A. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002–1004. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69:244–252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021 doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chegini Z, Didehdar M, Khoshbayan A, Rajaeih S, Salehi M, Shariati A. Epidemiology, clinical features, diagnosis and treatment of cerebral mucormycosis in diabetic patients: a systematic review of case reports and case series. Mycoses. 2020;63:1264–1282. doi: 10.1111/myc.13187. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt K, Agolli A, Patel MH, Garimella R, Devi M, Garcia E, et al. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries. 2021;9:e126. doi: 10.15190/d.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyer O. COVID-19: India sees record deaths as “black fungus” spreads fear. BMJ. 2021;373:n1238. doi: 10.1136/bmj.n1238. [DOI] [PubMed] [Google Scholar]
- 30.Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG, et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids—an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021 doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186:289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V. Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep. 2021 doi: 10.1016/j.ijscr.2021.105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020 doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao R, Shetty AP, Nagesh CP. Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of COVID-19: clinico-radiological features. Indian J Ophthalmol. 2021;69:1627–1630. doi: 10.4103/ijo.IJO_1053_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69:1563–1568. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revannavar SM, Supriya P, Samaga L, Vineeth K. COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021 doi: 10.1136/bcr-2021-241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saldanha M, Reddy R, Vincent MJ. Title of the article: paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020;6:1–20. doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Candel FJ, Peñuelas M, Tabares C, Garcia-Vidal C, Matesanz M, Salavert M, et al. Fungal infections following treatment with monoclonal antibodies and other immunomodulatory therapies. Rev Iberoam Micol. 2020;37:5–16. doi: 10.1016/j.riam.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases. 2021;12:85–89. doi: 10.14740/jmc3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellanger A-P, Navellou J-C, Lepiller Q, Brion A, Brunel A-S, Millon L, et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect Dis Now. 2021 doi: 10.1016/j.idnow.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dallalzadeh LO, Ozzello DJ, Liu CY, Kikkawa DO, Korn BS. Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit (London) 2021 doi: 10.1080/01676830.2021.1903044. [DOI] [PubMed] [Google Scholar]
- 43.Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep. 2021;32:64–67. doi: 10.1016/j.mmcr.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanwar A, Jordan A, Olewiler S, Wehberg K, Cortes M, Jackson BR. A fatal case of rhizopus azygosporus pneumonia following covid-19. J Fungi. 2021;7:1–6. doi: 10.3390/jof7030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karimi-Galougahi M, Arastou S, Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19) Int Forum Allergy Rhinol. 2021 doi: 10.1002/alr.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khatri A, Chang KM, Berlinrut I, Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient—case report and review of literature. J Med Mycol. 2021 doi: 10.1016/j.mycmed.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC, et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthal Plast Reconstr Surg. 2021;37:E40–E42. doi: 10.1097/IOP.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Do Monte ES, Dos Santos MEL, Ribeiro IB, De Oliveira LG, Baba ER, Hirsch BS, et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin Endosc. 2020;53:746–749. doi: 10.5946/CE.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasero D, Sanna S, Liperi C, Piredda D, Pietro BG, Casadio L, et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020. 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed]
- 50.Pauli MA, Pereira LM, Monteiro ML, de Camargo AR, Rabelo GD. Painful palatal lesion in a patient with COVID-19. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021. 10.1016/j.oooo.2021.03.010. [DOI] [PMC free article] [PubMed]
- 51.Placik DA, Taylor WL, Wnuk NM. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol Case Rep. 2020;15:2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: A case report. Eur J Ophthalmol. 2021 doi: 10.1177/11206721211009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waizel-Haiat S, Guerrero-Paz JA, Sanchez-Hurtado L, Calleja-Alarcon S, Romero-Gutierrez L. A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus. 2021 doi: 10.7759/cureus.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264.e5–264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zurl C, Hoenigl M, Schulz E, Hatzl S, Gorkiewicz G, Krause R, et al. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically Ill COVID-19 patient with underlying hematological malignancy. J Fungi. 2021;7:1–4. doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Obaidi M, Youssefi B, Bardwell J, Bouzigard R, Le CH, Zangeneh TT. A comparative analysis of mucormycosis in immunosuppressed hosts including patients with uncontrolled diabetes in the Southwest United States. Am J Med. 2021 doi: 10.1016/j.amjmed.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 59.Bellazreg F, Hattab Z, Meksi S, Mansouri S, Hachfi W, Kaabia N, et al. Outcome of mucormycosis after treatment: report of five cases. New Microbes New Infect. 2015;6:49–52. doi: 10.1016/j.nmni.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camara-Lemarroy CR, González-Moreno EI, Rodríguez-Gutiérrez R, Rendón-Ramírez EJ, Ayala-Cortés AS, Fraga-Hernández ML, et al. Clinical features and outcome of mucormycosis. Interdiscip Perspect Infect Dis. 2014 doi: 10.1155/2014/562610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szarpak L. Mucormycosis - a serious threat in the COVID-19 pandemic? J Infect. 2021 doi: 10.1016/j.jinf.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hartnett KP, Jackson BR, Perkins KM, Glowicz J, Kerins JL, Black SR, et al. A guide to investigating suspected outbreaks of mucormycosis in healthcare. J Fungi. 2019;5:69. doi: 10.3390/jof5030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alsuwaida K. Primary cutaneous mucormycosis complicating the use of adhesive tape to secure the endotracheal tube. Can J Anesth. 2002;49:880–882. doi: 10.1007/BF03017426. [DOI] [PubMed] [Google Scholar]
- 64.John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe covid-19 converge: the perfect storm for mucormycosis. J Fungi. 2021 doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verma DK, Bali RK. COVID-19 and mucormycosis of the craniofacial skeleton: causal, contributory or coincidental? J Maxillofac Oral Surg. 2021;20:165–166. doi: 10.1007/s12663-021-01547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giri M, Puri A, Wang T, Guo S. Comparison of clinical manifestations, pre-existing comorbidities, complications and treatment modalities in severe and non-severe COVID-19 patients: a systemic review and meta-analysis. Sci Prog. 2021 doi: 10.1177/00368504211000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin T, Li Y, Ying Y, Luo Z. Prevalence of comorbidity in Chinese patients with COVID-19: systematic review and meta-analysis of risk factors. BMC Infect Dis. 2021 doi: 10.1186/s12879-021-05915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Zhong X, Wang Y, Zeng X, Luo T, Liu Q. Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS ONE. 2021 doi: 10.1371/journal.pone.0250602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng S, Zhao Y, Wang F, Chen Y, Kaminga AC, Xu H. Comorbidities’ potential impacts on severe and non-severe patients with COVID-19: A systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e24971. doi: 10.1097/MD.0000000000024971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol. 2020 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCreary EK, Coronavirus PJM, Disease, Treatment: a review of early and emerging options. Open Forum Infect Dis. 2019;2020:7. doi: 10.1093/ofid/ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.AlBahrani S, Al-Tawfiq JA, Jebakumar AZ, Alghamdi M, Zakary N, Seria M, et al. Clinical features and outcome of low and high corticosteroids in admitted COVID-19 patients. J Epidemiol Glob Health. 2021 doi: 10.2991/jegh.k.210521.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez-Morales AJ, Sah R, Millan-Oñate J, Gonzalez A, Montenegro-Idrogo JJ, Scherger S, et al. COVID-19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs. Ther Adv Infect Dis. 2021 doi: 10.1177/20499361211027065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mulakavalupil B, Vaity C, Joshi S, Misra A, Pandit RA. Absence of Case of Mucormycosis (March 2020–May 2021) under strict protocol driven management care in a COVID-19 specific tertiary care intensive care unit. Diabetes Metab Syndr Clin Res Rev. 2021;15:102169. doi: 10.1016/j.dsx.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pakdel F, Ahmadikia K, Salehi M, Tabari A, Jafari R, Mehrparvar G, et al. Mucormycosis in patients with COVID-19: a cross-sectional descriptive multicenter study from Iran. Mycoses. 2021 doi: 10.1111/myc.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh RP, Gupta N, Kaur T, Gupta A. Rare case of gastrointestinal mucormycosis with colonic perforation in an immunocompetent patient with COVID-19. BMJ Case Rep. 2021;14:e244096. doi: 10.1136/bcr-2021-244096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arjun R, Felix V, Niyas VKM, Kumar MAS, Krishnan RB, Mohan V, et al. COVID-19 associated rhino-orbital mucormycosis: a single centre experience of ten cases. QJM An Int J Med. 2021 doi: 10.1093/qjmed/hcab176. [DOI] [PubMed] [Google Scholar]
- 81.Saidha PK, Kapoor S, Das P, Gupta A, Kakkar V, Kumar A, et al. Mucormycosis of paranasal sinuses of odontogenic origin post COVID19 infection: a case series. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jain M, Tyagi R, Tyagi R, Jain G. Post-COVID-19 gastrointestinal invasive mucormycosis. Indian J Surg. 2021 doi: 10.1007/s12262-021-03007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baskar HC, Chandran A, Reddy CS, Singh S. Rhino-orbital mucormycosis in a COVID-19 patient. BMJ Case Rep. 2021;14:e244232. doi: 10.1136/bcr-2021-244232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patankar SH, Joshi AR, Muthe MM, Athawale A, Achhapalia Y. CT and MRI findings of invasive mucormycosis in the setting of COVID-19: experience from a single center in India. Am J Roentgenol. 2021 doi: 10.2214/AJR.21.26205. [DOI] [PubMed] [Google Scholar]
- 85.Sen M, Honavar SG, Bansal R, Sengupta S, Rao R, Kim U, et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69:1670–1692. doi: 10.4103/ijo.IJO_1565_21. [DOI] [PMC free article] [PubMed] [Google Scholar]


