Abstract
Objectives
To determine the clinical features and course of thyroid-associated ophthalmopathy (TAO) in a large sample of Chinese patients.
Design and methods
We retrospectively identified a cohort of consecutive patients diagnosed with TAO at the West China Hospital from October 1, 2009 to October 1, 2019. We analysed clinical data from 3620 patients, including demographic data, clinical manifestations, ophthalmology examinations, and prognosis.
Results
TAO most frequently occurred with hyperthyroidism, with most patients developing TAO after thyroid disease (TD). The TAO phenotype was asymmetric in 375 (50.7%) euthyroid patients, 25 (27.8%) hypothyroid patients, and 314 (12.1%) hyperthyroid patients (p < 0.0001). The most frequent symptom was lid lag and the most commonly involved extraocular muscle was the inferior rectus. Severity assessment (NOSPECS score) and clinical activity assessment (Clinical Activity Scores, CAS) differed significantly between male and female patients (P < 0.000). The majority (88.8%) of patients had clinically inactive TAO, and only 3.2% of cases were sight-threatening. Regarding the clinical process, 75.5% of patients had an active phase time less than 12 months and 2.1% showed complete remission.
Conclusions
TAO most commonly develops in females and is closely related to hyperthyroidism. Euthyroid TAO often has an asymmetric clinical phenotype. CAS combined with magnetic resonance imaging can improve the detection of TAO. NOSPECS scores should be slightly refined regarding the criteria for corneal involvement. Clinical management of TAO should be individualized according to CAS or NOSPECS assessments and a multidisciplinary approach is paramount. A minority of patients showed complete remission.
Subject terms: Eye manifestations, Epidemiology
Introduction
Thyroid-associated ophthalmopathy (TAO), also known as Graves’ orbitopathy (GO) and thyroid eye disease [1], is closely associated with autoimmune thyroid disease (TD), although the precise aetiology remains unclear. Patients typically present with lid swelling, lid retraction, proptosis, and diplopia. In some severe TAO cases, exposure keratitis and dysthyroid optic neuropathy (DON) may occur [2].
TAO most commonly occurs with hyperthyroidism, but can be seen in euthyroid and hypothyroid patients as well [3]. There is a close temporal relationship between TAO and hyperthyroidism [4]. Radioactive iodine (RAI) therapy is one of the most common treatments for hyperthyroidism, but may be a risk factor for TAO [5]. The clinical course of TAO involves two stages: the active phase and quiescence, as described by Rundle’s curve [6]. Studies on the clinical course of TAO are scarce, and the existing studies involved small samples with limited data. To better understand the symptoms and clinical course of TAO, studies with larger samples are needed.
In this study, we retrospectively evaluated a large cohort of Chinese patients to obtain more reliable epidemiological and clinical data of TAO. We hope that this work will provide further insight into the management and prognosis of TAO.
Patients and methods
This is a retrospective analysis study carried out at a single centre.
Patients
All patients diagnosed with TAO who attended the ophthalmic clinic at West China Hospital over the 10 years period from October 1, 2009 to October 1, 2019 were included in this study. The diagnosis of TAO was based on Bartley’s criteria [7]. This study was approved by the hospital’s internal ethics review board and all patients provided written informed consent.
Data collection
The medical records of all patients were retrospectively reviewed for the following: thyroid status, active phase duration, visual acuity, eyelid status, conjunctival status, exophthalmos and intraocular pressure (IOP), Clinical Activity Scores (CAS), severity assessment (NOSPECS score), ocular motility and diplopia, corneal status, complications, treatment modality, and last-known status. On each follow-up visit, photographs of the face were taken to help evaluate disease progression.
Definitions used in this study
1. Upper eyelid retraction was defined as an upper eyelid at or above the superior corneoscleral limbus in the primary position without frontalis muscle contraction [7].
Lower eyelid retraction was defined as a lower eyelid below the inferior corneoscleral limbus in the primary position [8].
2. Exophthalmos and IOP
For this study, we considered exophthalmos as a Hertel exophthalmometry measurement higher than 14 mm [9].
IOP was measured using a non-contact tonometer in the primary position. IOP above 21 mmHg was considered as ocular hypertension.
3. Activity and severity were assessed using the CAS [10] and NOSPECS score [11], respectively. A CAS score ≥3 indicates an active period, whereas a CAS score <3 indicates a clinically inactive disease [12].
Data analysis
Statistical analysis was performed using SPSS 19.0 software. Demographic data are reported using descriptive statistics, including percentages, mean, and standard deviation. The clinical features of male and female patients were compared using the Chi-square test. P < 0.05 indicated statistical significance.
Results
Demographic data
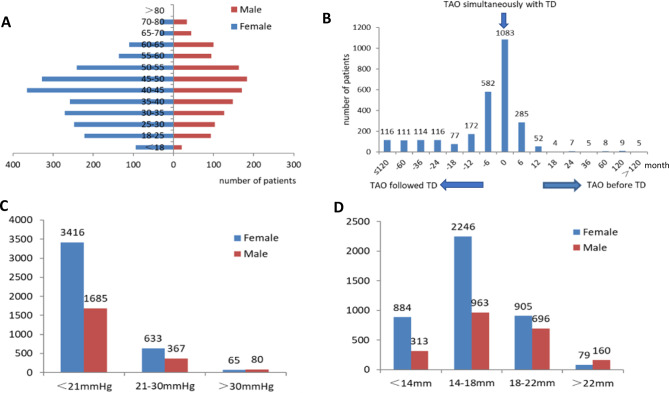
A total of 3620 patients were included in this study, comprising 772 cases of monocular TAO and 2848 cases of binocular TAO. The cohort included 2341 females aged 6–84 years (mean age 42.0 ± 13.09) and 1279 males aged 4–80 years (mean age 45.5 ± 13.9). The female-male ratio was 1.83. On average, male patients were older than female patients, with the peak age of TAO incidence being 45–50 years for males and 40–45 years for females. Figure 1a shows the demographic characteristics of the patients.
Fig. 1. Demographic characteristic and clinical characteristic of TAO.
a Age of onset distribution for patients with TAO. b Temporal relationship between the diagnosis of TAO and thyroid disease (Hyperthyroidism, hypothyroidism, hashimoto’s thyroiditis, thyroid cancer). c IOP in TAO patients. d Exophthalmos in TAO patients.
Thyroid function and TAO
TAO is most common in patients with hyperthyroidism, but also occurred in euthyroid or hypothyroid individuals. Table 1 shows the specific thyroid functional status of all patients.
Table 1.
Thyroid disease and therapeutic approach of hyperthyroidism and TAO.
| Thyroid disease | Total (%) | Monocular (%) | Binocular (%) |
|---|---|---|---|
| Hyperthyroidism* | 2585 (71.4%) | 314 (12.1%) | 2271 (87.9%) |
| Euthyroidism* | 739 (20.4%) | 375 (50.7%) | 364 (49.3%) |
| Subclinical hyperthyroidism | 122 (3.4%) | 43 (35.2%) | 79 (64.8%) |
| Hypothyroidism* | 90 (2.5%) | 25 (27.8%) | 65 (72.2%) |
| Hashimoto thyroiditis | 38 (1.0%) | 7 (18.4%) | 31 (81.6%) |
| Thyroid cancer | 33 (0.9%) | 6 (18.2%) | 27 (81.8%) |
| Subclinical hypothyroidism | 13 (0.4%) | 2 (15.4%) | 11 (84.6%) |
| Treatment of hyperthyroidism | Number (%) | ||
|---|---|---|---|
| Anti-thyroid drugs | 1808 (69.9%) | ||
| Radioiodine | 751 (29.1%) | ||
| Thyroidectomy | 13 (0.5%) | ||
| Untreated | 11 (0.4%) | ||
| Radiofrequency ablation | 2 (0.1%) |
| Treatment in our hospital | Number (%) | Prior treatment in other hospital | Number |
|---|---|---|---|
| Wait-and-see/local | 861 (29.5%) | TA | 146 |
| TA | 1342 (45.9%) | TA + RT | 5 |
| TA + RT | 463 (15.8%) | TA + C | 32 |
| TA + C | 35 (1.2%) | C + D | 5 |
| RT + C | 4 (0.1%) | C | 177 |
| C + D | 5 (0.2%) | RT | 134 |
| multiple | 212 (7.3%) | RT + C | 22 |
| 99 Tc-MDP | 43 | ||
| multiple | 16 |
*There was a significant difference regarding asymmetric phenotype in these three groups (P < 0.0001).
C systemic corticosteroids, RT radiotherapy of the orbits, D surgical orbital decompression, TA periorbitally triamcinolone acetonide injections, Multiple two or more therapeutic approach.
As shown in Table 1, hyperthyroidism was most commonly treated with anti-thyroid drugs (1808; 69.9%), although 29.1% of patients received RAI. Among patients who received RAI therapy, the majority (454, 60.5%) developed TAO, 53 patients experienced aggravation of pre-existing TAO, and four patients had TAO relapse.
Figure 1b presents the relationship between the duration of TD and TAO. As shown, TAO most commonly developed after (1288; 46.9%) TD, but may develop simultaneously (39.4%) or before (13.7%) TD.
Ophthalmic features
Among the 3260 patients with TAO, the most frequent symptom was lid lag, followed by upper eyelid retraction and lid swelling. Proptosis and restrictive dyskinesia were also common. The clinical features that were significantly different (P < 0.05) between male and female patients are shown in Table 2. Typical clinical manifestations of TAO are shown in Fig. 2.
Table 2.
Clinical features of patients with TAO.
| Symptoms | Female (%) | Male (%) | P value | Total (%) |
|---|---|---|---|---|
| Eye pain | 588 (25.1%) | 308 (24.1%) | 0.49 | 896 (24.8%) |
| epiphora | 546 (23.3%) | 307 (24.0%) | 0.645 | 853 (23.6%) |
| diplopia | 581 (24.8%) | 536 (41.9%) | *** | 1117 (30.9%) |
| dry eye | 111 (4.7%) | 45 (3.5%) | 0.083 | 156 (4.3%) |
| photophobia | 259 (11.1%) | 123 (9.6%) | 0.176 | 382 (10.6%) |
| foreign body sensation | 297 (12.7%) | 123 (9.6%) | 0.006 | 420 (11.6%) |
| proptosis | 1576 (67.3%) | 898 (70.2%) | 0.074 | 2474 (68.3%) |
| blurring of vision | 201 (8.6%) | 133 (10.4%) | 0.072 | 334 (9.2%) |
| Physical sign | ||||
| lid lag | 2016 (86.1%) | 1040 (81.3%) | *** | 3056 (84.4%) |
| upper eyelid retraction | 1706 (72.9%) | 831 (65.0%) | *** | 2537 (70.1%) |
| lid swelling | 1612 (68.9%) | 836 (65.4%) | 0.032 | 2448 (67.6%) |
| restrictive dyskinesia | 1297 (55.4%) | 973 (76.1%) | *** | 2270 (62.7%) |
| lagophthalmos | 416 (17.8%) | 248 (19.4%) | 0.229 | 664 (18.3%) |
| fat pockets | 384 (16.4%) | 351 (27.4%) | *** | 735 (20.3%) |
| lower eyelid retraction | 255 (10.9%) | 206 (16.1%) | *** | 461 (12.7%) |
| conjunctival congestion | 249 (10.6%) | 292 (22.8%) | *** | 541 (14.9%) |
| chemosis | 237 (10.1%) | 201 (15.7%) | *** | 438 (12.1%) |
| redness of eyelids | 14 (0.6%) | 6 (0.5%) | 0.617 | 20 (0.6%) |
| redness of caruncle | 20 (0.9%) | 45 (3.5%) | *** | 65 (1.8%) |
| corneal involvement | 351 (15.0%) | 184 (14.4%) | 0.623 | 535 (14.8%) |
| strabismus | 179 (7.6%) | 225 (17.6%) | *** | 404 (11.2%) |
| trichiasis | 128 (5.5%) | 65 (5.1%) | 0.622 | 193 (5.3%) |
| compensatory head posture | 52 (2.2%) | 38 (3.0%) | 0.166 | 90 (2.5%) |
| TAO activity | *** | |||
| active | 192 (8.2%) | 215 (16.8%) | 407 (11.2%) | |
| TAO Severity | *** | |||
| mild (NOSPECS I, II) | 275 (11.7%) | 80 (6.3%) | 355 (9.8%) | |
| moderate (NOSPECS III, IV) | 1690 (72.2%) | 997 (78.0%) | 2687 (74.2%) | |
| severe (NOSPECS V, IV) | 376 (16.1%) | 202 (15.8%) | 578 (16.0%) | |
| Complication and Comorbidities | ||||
| Dysthyroid Optic Neuropathy | 25 | 18 | - | 43 (1.2%) |
| corneal ulcer | 41 | 30 | - | 71 (2.0%) |
| myasthenia gravis | 15 | 12 | - | 27 (0.7%) |
| pregnant | 25 | 0 | - | 25 (0.7%) |
| Mitochondrial myopathy | 0 | 1 | - | 1 |
| multiple sclerosis | 1 | 0 | - | 1 |
| intraorbital tumour | 2 | 2 | - | 4 |
| Sjogren syndrome | 1 | 0 | - | 1 |
***P < 0.0001.
-The data were not to perform statistical analysis.
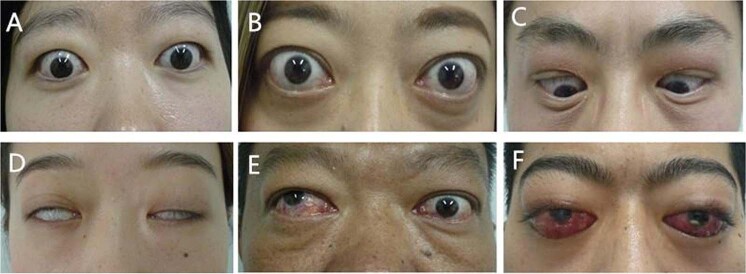
Fig. 2. Representative clinical features of TAO patients.
a Bilateral upper eyelid swelling and retraction. b Bilateral proptosis and upper and lower eyelid retraction. c Bilateral upper eyelid swelling and lag. d Lagophthalmos (incomplete eye closure) in binoculus. e Strabismus in right eye. f Severe conjunctival congestion and chemosis in binoculus and ulcer of cornea in left eye.
According to CAS scores, most patients (3213; 88.8%) had clinically inactive TAO; active TAO only accounted for 11.2% of patients. There were more men than women in the active phase, with 215 and 192 cases, respectively. Regarding NOSPECS severity scores, 355 (9.8%) patients presented as mild, 74.2% as moderate, and 16.0% as severe. Only 3.2% of cases were sight-threatening, including 71 (2.0%) cases of corneal ulceration and 43 (1.2%) cases of DON. The mean age of DON patients was 55 ± 12.06 years, and the median age was 54. The NOSPECS scores and CAS differed significantly between male and female patients.
Exophthalmos and IOP
The same trained technician performed the IOP and exophthalmos examinations for all patients included in the analysis. We excluded patients presenting with strabismus (404 cases), severe conjunctival chemosis (22 cases), and corneal ulcer (71 cases) to enable more accurate measurement of IOP and exophthalmos. After applying these exclusion criteria, a total of 3123 patients (6246 eyes) were assessed regarding IOP and exophthalmos. Exophthalmos was present in 80.8% of total eyes (Fig. 1d). Notably, there were 51 patients for which both eyes showed normal exophthalmos but the difference between the two eyes was greater than 2 mm. By contrast, the majority of eyes (5101; 81.7%) had normal IOP; only 2.3% of eyes had IOP >30 mmHg (Fig. 1c).
Imaging findings
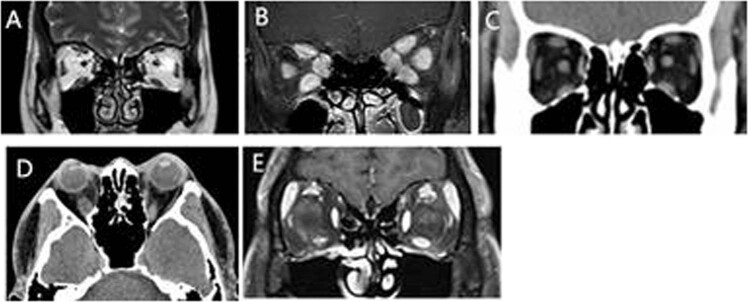
Imaging examinations were not performed for patients without exophthalmos and restrictive dyskinesia. As a result, orbit imaging was available for 2736 patients, including computed tomography (CT) for 1725 cases and magnetic resonance imaging (MRI) for 1011 cases. Four subgroups were distinguished according to increases in extraocular muscles volume (MV) and fat volume (FV): (i) 452(16.5%) orbits showed no increase in fat or MV (Fig. 3a), (ii) 69 (2.5%) orbits showed only a FV increase (Fig. 3c), (iii) 1717 (62.8%) orbits showed only a MV increase (Fig. 3b), (iv) and 498 (18.2%) orbits showed both FV and MV increases (Fig. 3d).
Fig. 3. Image of the orbits of TAO patient.
a Coronal MRI shows patients with normal MV and FV. b Coronal fat-suppressed T1-weighted image presents the enlargement of MV of a patient. c Coronal CT scan presents only FV increased with normal MV. d Axial CT scan displays both MV and FV enlarged. e Coronal fat-suppressed T1-weighted image reveals the enlargement of lacrimal gland.
Of the 2215 patients (4430 eyes) presenting with enlarged extraocular muscles, the most commonly involved muscle was the inferior rectus (3539 eyes; 79.9%), followed by the superior rectus (55.6%), medial rectus (49.5%), and lateral rectus (28.2%). Furthermore, 1,212 (27.4%) eyes had one extraocular muscle enlarged, 20.1% had two enlarged, 20.3% had three enlarged, and 21.0% had all four extraocular muscles enlarged. Lacrimal gland enlargement is also a clinical manifestation of TAO and was observed in 320 of 2736 (11.7%) patients (Fig. 3e).
Treatment and follow-up
Follow‐up data for each patient ranged from 3 to 120 months. A total of 2922 patients whose last-known status was the quiescent phase were included in this follow-up. We defined the quiescent phase as CAS <3 and a stable condition for 6 months or longer, which was assessed by reviewing the medical records and patients’ observations.
After a complete evaluation of the severity and activity of the disease, clinicians developed individualized treatment regimens for these 2922 patients, as reported in Table 1. Since some of these patients were referrals, they were treated at the early stage of TAO at a primary care hospital. The prior therapeutic approaches were diversified in dosage and modality, generally resulting in poor therapeutic effects. Therefore, clinical evaluation and treatment of TAO requires further strengthening.
To gain a better understanding of the clinical course of TAO, we evaluated the duration of the active phase in these 2922 patients. The mean duration was 11.35 (±11.09) months and the median duration was 9 months. The active phase duration was <12 months for the majority of patients (2206; 75.5%), followed by 18.7% with a duration of 12–24 months, 3.6% with a duration of 24–36 months, and 2.2% with a duration of å 60 months. Regarding recovery over time, 60 (2.1%) patients showed complete remission of TAO.
Discussion
TAO is an inflammatory disease of the orbit. Many case studies have focused on the clinical features of TAO. However, the incidence and clinical manifestations of TAO vary with ethnicity [13], and there are limited data available for Chinese patients. To fill this research gap, the present study presents the clinical characteristics of TAO in a large sample of Chinese patients.
Our results showed that TAO was more prevalent in women, with a female-to-male ratio of 1.83:1. These data are roughly in agreement with a study conducted in Singapore which found a ratio of 1.76:1 [14]. In previous studies of Asian populations, the prevalence of TAO in females relative to males was found to be lower than that in in Caucasian populations: a female-to-male ratio of 6:1 was found in the United Kingdom [15] and 6.06:1 in the USA [16]. In the present study, the peak age of incidence was 45–50 years for males and 40–45 years for females. This finding is consistent with the literature, where the peak age of onset is higher in males than females [8, 9, 16].
Previous studies have demonstrated that thyroid function is closely related to TAO. TAO commonly occurs with hyperthyroidism, but may also present in euthyroid or hypothyroid patients [17]. However, there is a lack of studies reporting subclinical thyroid disorders, which were included in our study. The rate of euthyroid patients in our study (20.4%) is quite similar to that reported in an epidemiological study conducted in India by Reddy et al. [18]. Based on the available literature, the clinical phenotype of euthyroid patients with TAO is significantly less severe and presents more often as unilateral [19]. We confirmed that TAO in euthyroid patients was more likely to present as unilateral ophthalmopathy (P < 0.0001). However, we observed unilateral TAO in 21.3% of our patients, which was higher than the rate reported in previous studies (9–15%) [20].
It is well-known that there is a strong temporal relationship between TD and eye manifestations [3]. However, some studies have shown that TAO occurring after TD is the most common condition [9], whereas other studies report simultaneous onset as more common [4]. In our study, the diagnosis of TAO tended to follow the diagnosis of TD (46.9%). Furthermore, the time interval between TD and TAO was usually 12 months (79.2%). Currently, it is widely accepted that, in most patients, ocular symptoms and hyperthyroidism occur within an 18 month interval of each other [21]. Development or worsening of TAO after RAI therapy for hyperthyroidism has been reported in 15–39% of patients, particularly in smokers [22]. It is not clear why RAI exacerbates or induces TAO. A prospective study of 114 patients revealed that the development of hypothyroidism and elevated TSH after RAI might be important adverse factors [23]. In our study, the majority of patients (60.5%) developed new onset TAO, with only 7.1% of cases being aggravation of pre-existing TAO. Alternatively, it may be that patients with TAO are more likely to seek medical advice at an ophthalmology clinic after RAI treatment.
Unlike previous studies where eyelid retraction was the most common sign of TAO [24], we found eyelid lag to be the most common sign. There are several possible reasons for this. First, this may be due to differences in geographical region, as our previous clinical study conducted in China found a similar result [25]. Second, it may be due to the sample size. Confirmation of the reason for these differences would require larger studies. Proptosis is a common symptom of TAO [26]. Our study found that the incidence of proptosis was 80.8% (total eyes). This number is higher than that reported in previous studies, probably due to different normal reference values. In China the normal reference value is 14 mm [9], but the upper limit of normal exophthalmos values in Caucasians is 20 mm [16]. In addition, the presence of a high incidence of myopia in TAO patients could lead to exacerbation of proptosis.
Since its publication in 1977, the modified NOSPECS by Werner has been widely used [11]. We found that 535 (14.8%) patients had corneal involvement, which is considered a severity grade of V if they do not have DON. However, only 71 patients had severe corneal involvement, such as corneal ulcer, with part of the rest corneal involvement usually disappearing with local measures. Therefore, this does not necessarily indicate more severe eye disease. These findings suggest that NOSPECS criteria may require some modification when evaluating grade V.
CAS is used to identify active disease, as described by Mourits et al. [10]. According to CAS, most of our patients (88.8%) were inactive. However, in routine clinical practice, we found inflammation in many patients with CAS <3 when we evaluated the extraocular muscle using enhanced MRI (data not shown). Therefore, our results support that CAS score alone cannot detect all active stage patients. Thus, the combination of CAS with enhanced MRI could improve the sensitivity of disease activity detection [27].
The sight-threatening complications of TAO were corneal ulcer and DON, which were present in 3.2% of our patients. This is similar to previous research, where 3–5% of cases posed a threat to eyesight [28]. There appears to be a consensus in the literature that myasthenia gravis is associated with TAO [29], which was confirmed in our large cohort study.
Additional imaging examinations indicated that MV enlargement was the most common manifestation, with some patients showing both MV and FV increases. However, only FV augmentation without extraocular muscle involvement is rare. Our results are similar to those of a 2009 study by Regensburg [30]. It is possible that this phenomenon is related to the pathogenesis of TAO, such that increased orbital MV is an early phenomenon in TAO but increased FV occurs relatively later. Notably, increased MV is associated with more severe TAO [31]. Regarding extraocular muscle involvement, the present result is in line with previous findings that the inferior rectus is the most commonly involved extraocular muscle, followed by the superior rectus, medial rectus, and lateral rectus. These findings are in accordance with another Chinese study [9]. Lacrimal gland changes in TAO have been noted by previous researchers [32]. In the present study, 11.7% of patients had patent lacrimal gland enlargement.
According to EUGOGO guidelines [12], mild GO should be treated with local treatment and control of risk factors. It should be noted that the patient population in the current study was recruited from a tertiary referral clinic, and so tended to have moderate to severe disease requiring more interventions. In our clinical practice, periorbital triamcinolone acetonide injections are administered frequently due to their powerful effects on upper-lid retraction, which have been confirmed in previous research [33]. Of course, therapies need to be individualized according to CAS or NOSPECS assessments, with systemic glucocorticoids and radiotherapy being the first line of treatment for active moderate to severe TAO [34]. Treatment plans should be designed by multidisciplinary teams, including endocrinologists, ophthalmologists, and radiotherapists.
Rundle documented that the clinical course of TAO involves two stages, an initial dynamic phase with inflammation followed by a static phase [35]. Previous studies have demonstrated that the active phase lasts 6–18 months in non-smokers and 2–3 years in smokers [36]. In our study, the mean duration of the active phase was 11.35 months. Consistent with reports for various diseases with dynamic phases ranging from few months to 5 years before disease stabilization [37], our study found a range of duration from 3 to 168 months. Regarding prognosis, we found that 2.1% of patients showed complete remission of ocular involvement. By contrast, in a retrospective study by Tanda et al. 58.1% of patients experienced complete remission [38]. However, the sample in Tanda et al. comprised patients with mild and inactive TAO.
Our study has several limitations. First, it was a retrospective study. Eye photographs and medical records for all patients were systematically assessed to maintain the authenticity of the data. Second, it was a single-centre study. However, because our hospital is considered the best hospital in western China and many patients in neighbouring provinces choose our hospital for medical treatment, our patient sample may be representative of the region. A notable strength of our study is the large dataset. For these reasons, we believe that our findings will be of great clinical value for the treatment of TAO in China.
Summary
What was known before
Previous studies were mainly concern demographic and clinical features in TAO, but less in clinical course of disease and prognosis.
Large sample reports are very limited.
What this study adds
This is the first study with such large sample size and extensive follow-up time evaluating Chinese TAO patients.
The work analysed clinical characteristics and clinical course of Chinese patients with TAO and can make you understand the performance and development of TAO more comprehensively.
We observed 75.5% patients had an active phase time lesser than 12 months
Supplementary information
Acknowledgments
Funding
This work was supported by the 1·3·5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (grant numbers 2018HXFH024), and the science and technology program of Sichuan province (grant numbers 2018SZ0128).
Author contributions
BX: conception, design, data collection, literature search, and preparation and editing of the manuscript. MY: collection and interpretation of data. YJ and WM: critically revising the manuscript for important intellectual content. All authors reviewed and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Ethics approval was approved by the medical ethics committee of West China Hospital, Sichuan University.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41433-020-01246-7) contains supplementary material, which is available to authorized users.
References
- 1.Fang S, Huang Y, Wang N, Zhang S, Zhong S, Li Y, et al. Insights into local orbital immunity: evidence for the involvement of the Th17 cell pathway in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2019;104:1697–711. doi: 10.1210/jc.2018-01626. [DOI] [PubMed] [Google Scholar]
- 2.Tramunt B, Imbert P, Grunenwald S, Boutault F, Caron P. Sight-threatening Graves’ orbitopathy: twenty years’ experience of a multidisciplinary thyroid-eye outpatient clinic. Clin Endocrinol. 2019;90:208–13. doi: 10.1111/cen.13880. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus JH. Epidemiology of Graves’ orbitopathy (GO) and relationship with thyroid disease. Best Pract Res Clin Endocrinol Metab. 2012;26:273–9. doi: 10.1016/j.beem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Wiersinga WM, Smit T, van der Gaag R, Koornneef L. Temporal relationship between onset of Graves’ ophthalmopathy and onset of thyroidal Graves’ disease. J Endocrinol Invest. 1988;11:615–9. doi: 10.1007/BF03350193. [DOI] [PubMed] [Google Scholar]
- 5.Ponto KA, Zang S, Kahaly GJ. The tale of radioiodine and Graves’ orbitopathy. THYROID. 2010;20:785–93. doi: 10.1089/thy.2010.1640. [DOI] [PubMed] [Google Scholar]
- 6.GB B. Rundle and his curve. Arch Ophthalmol. 2011;129:356–8. doi: 10.1001/archophthalmol.2011.29. [DOI] [PubMed] [Google Scholar]
- 7.Bartley GB, GC Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol. 1995;119:792–5. doi: 10.1016/S0002-9394(14)72787-4. [DOI] [PubMed] [Google Scholar]
- 8.Lim SL, Lim AK, Mumtaz M, Hussein E, Wan BW, Khir AS. Prevalence, risk factors, and clinical features of thyroid-associated ophthalmopathy in multiethnic Malaysian patients with Graves’ disease. Thyroid. 2008;18:1297–301. doi: 10.1089/thy.2008.0044. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Ye H, Ding Y, Chen G, Liu Z, Xu J, et al. Clinical characteristics of moderate-to-severe thyroid associated ophthalmopathy in 354 Chinese cases. PLoS ONE. 2017;12:e176064. doi: 10.1371/journal.pone.0176064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol. 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 11.Werner SC. Modification of the classification of the eye changes of Graves’ disease. Am J Ophthalmol. 1977;83:725. doi: 10.1016/0002-9394(77)90140-4. [DOI] [PubMed] [Google Scholar]
- 12.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly G, Marcocci C, et al. The 2016 European thyroid association/European Group on Graves’ orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 2016;5:9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chng C, Seah LL, Khoo DHC. Ethnic differences in the clinical presentation of Graves’ ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26:249–58. doi: 10.1016/j.beem.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Lim NC, Sundar G, Amrith S, Lee KO. Thyroid eye disease: a Southeast Asian experience. Br J Ophthalmol. 2015;99:512–8. doi: 10.1136/bjophthalmol-2014-305649. [DOI] [PubMed] [Google Scholar]
- 15.Mellington FE, Dayan CM, Dickinson AJ, Hickey AJ, MacEwen CJ, McLaren J, et al. Management of thyroid eye disease in the United Kingdom: a multi-centre thyroid eye disease audit. Orbit. 2017;36:159–69. doi: 10.1080/01676830.2017.1280057. [DOI] [PubMed] [Google Scholar]
- 16.GB B. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477–588. [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Cestari DM, Fortin E. Thyroid eye disease. Curr Opin Ophthalmol. 2018;29:528–34. doi: 10.1097/ICU.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 18.Reddy SV, Jain A, Yadav S, Sharma K, Bhatia E. Prevalence of Graves’ ophthalmopathy in patients with Graves’ disease presenting to a referral centre in north India. Indian J Med Res. 2014;139:99–104. [PMC free article] [PubMed] [Google Scholar]
- 19.Eckstein AK, Losch C, Glowacka D, Schott M, Mann K, Esser J, et al. Euthyroid and primarily hypothyroid patients develop milder and significantly more asymmetrical Graves ophthalmopathy. Br J Ophthalmol. 2009;93:1052–6. doi: 10.1136/bjo.2007.137265. [DOI] [PubMed] [Google Scholar]
- 20.Kashkouli MB, Kaghazkanani R, Heidari I, Ketabi N, Jam S, Azarnia S, et al. Bilateral versus unilateral thyroid eye disease. Indian J Ophthalmol. 2011;59:363–6. doi: 10.4103/0301-4738.83612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahn RS. Graves’ ophthalmopathy. New Engl J Med. 2010;362:726–38. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stan MN, Bahn RS. Risk factors for development or deterioration of Graves’ ophthalmopathy. Thyroid. 2010;20:777–83. doi: 10.1089/thy.2010.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kung AW, Yau CC, Cheng A. The incidence of ophthalmopathy after radioiodine therapy for Graves’ disease: prognostic factors and the role of methimazole. J Clin Endocrinol Metab. 1994;79:542–6. doi: 10.1210/jcem.79.2.7913934. [DOI] [PubMed] [Google Scholar]
- 24.Smith TJ, HL Graves’ disease. N Engl J Med. 2016;375:1552–65. doi: 10.1056/NEJMra1510030. [DOI] [PubMed] [Google Scholar]
- 25.Wang YJ, He WM. Clinical analysis of 403 cases of thyroid associated ophthalmopathy. Chin J Ophthalmol. 2013;49:685–90. [PubMed] [Google Scholar]
- 26.Prummel MF, Bakker A, Wiersinga WM, Baldeschi L, Mourits MP, Taylor PK, et al. Multi-center study on the characteristics and treatment strategies of patients with Graves’ orbitopathy: the first European Group on Graves’ Orbitopathy experience. Eur J Endocrinol. 2003;148:491–5. doi: 10.1530/eje.0.1480491. [DOI] [PubMed] [Google Scholar]
- 27.Das T, Roos J, Patterson AJ, Graves MJ, Murthy R. T2-relaxation mapping and fat fraction assessment to objectively quantify clinical activity in thyroid eye disease: an initial feasibility study. Eye. 2019;33:235–43. doi: 10.1038/s41433-018-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Taylor PK, Marcocci C, et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158:273–85. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 29.Bojikian KD, Francis CE. Thyroid eye disease and myasthenia gravis. Int Ophthalmol Clin. 2019;59:113–24. doi: 10.1097/IIO.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 30.Regensburg NI, Wiersinga WM, Berendschot TTJM, Potgieser P, Mourits MP. Do subtypes of Graves’ orbitopathy exist? Ophthalmology. 2011;118:191–6. doi: 10.1016/j.ophtha.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Potgieser PW, de Win MMML, Wiersinga WM, Mourits MP. Natural course of mild Graves orbitopathy: increase of orbital fat but decrease of muscle volume with increased muscle fatty degeneration during a 4-year follow-up. Ophthal Plast Recons. 2019;35:456–60. doi: 10.1097/IOP.0000000000001319. [DOI] [PubMed] [Google Scholar]
- 32.Bingham CM, Harris MA, Realini T, Nguyen J, Hogg JP, Sivak-Callcott JA. Calculated computed tomography volumes of lacrimal glands and comparison to clinical findings in patients with thyroid eye disease. Ophthal Plast Recons. 2014;30:116–8. doi: 10.1097/IOP.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 33.Xu D, Chen Y, Xu H, Li H, Zhang Z, Liu Y. Long-term effect of triamcinolone acetonide in the treatment of upper lid retraction with thyroid associated ophthalmopathy. Int J Ophthalmol. 2018;11:1290–5. doi: 10.18240/ijo.2018.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcocci C, Marinò M. Treatment of mild, moderate-to-severe and very severe Graves’ orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012;26:325–37. doi: 10.1016/j.beem.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Menconi F, Profilo MA, Leo M, Sisti E, Altea MA, Rocchi R, et al. Spontaneous improvement of untreated mild Graves’ ophthalmopathy: rundle’s curve revisited. Thyroid. 2014;24:60–66. doi: 10.1089/thy.2013.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel P, Khandji J, Kazim M. Recurrent thyroid eye disease. Ophthal Plast Recons. 2015;31:445–8. doi: 10.1097/IOP.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 37.Selva D, Chen C, King G. Late reactivation of thyroid orbitopathy. Clin Exp Ophthalmol. 2004;32:46–50. doi: 10.1046/j.1442-9071.2004.00756.x. [DOI] [PubMed] [Google Scholar]
- 38.Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, et al. Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed Graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98:1443–9. doi: 10.1210/jc.2012-3873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.