Abstract
Regulation of IP3 receptors (IP3Rs) by IP3 and Ca2+ allows regenerative Ca2+ signals, the smallest being Ca2+ puffs, which arise from coordinated openings of a few clustered IP3Rs. Cells express thousands of mostly mobile IP3Rs, yet Ca2+ puffs occur at a few immobile IP3R clusters. By imaging cells with endogenous IP3Rs tagged with EGFP, we show that KRas-induced actin-interacting protein (KRAP) tethers IP3Rs to actin beneath the plasma membrane. Loss of KRAP abolishes Ca2+ puffs and the global increases in cytosolic Ca2+ concentration evoked by more intense stimulation. Over-expressing KRAP immobilizes additional IP3R clusters and results in more Ca2+ puffs and larger global Ca2+ signals. Endogenous KRAP determines which IP3Rs will respond: it tethers IP3R clusters to actin alongside sites where store-operated Ca2+ entry occurs, licenses IP3Rs to evoke Ca2+ puffs and global cytosolic Ca2+ signals, implicates the actin cytoskeleton in IP3R regulation and may allow local activation of Ca2+ entry.
Subject terms: Cytoskeletal proteins, Calcium signalling, Super-resolution microscopy, Actin, Endoplasmic reticulum
Calcium signals initiated by IP3 receptors in ER membranes regulate most cellular activities. Here, the authors show that KRas-induced actininteracting protein (KRAP) tethers a small subset of IP3 receptors to actin and licenses them to evoke cytosolic calcium signals.
Introduction
Cytosolic Ca2+ signals regulate diverse activities in all eukaryotic cells1. Most of these signals are due to the opening of Ca2+-permeable channels, which then allow Ca2+ to flow into the cytosol across either the plasma membrane (PM) or the membranes of intracellular organelles, primarily the endoplasmic reticulum (ER). Inositol 1,4,5-trisphosphate receptors (IP3Rs), the most widely expressed of these Ca2+ channels, reside in the membranes of the ER from which they release Ca2+ when they bind IP32,3. This Ca2+ flux delivers Ca2+ to the cytosol and to the cytosolic surface of other organelles, notably mitochondria4 and lysosomes5. By depleting the ER of Ca2+, IP3-evoked Ca2+ release also stimulates store-operated Ca2+ entry (SOCE) across the PM. Stromal interaction molecule 1 (STIM1), which straddles the ER membrane, detects the loss of ER Ca2+ through its luminal Ca2+-binding sites causing it to unfurl cytosolic domains. These domains reach across narrow membrane contact sites (MCS) between the ER and PM to contact Orai Ca2+ channels, causing them to open and allow Ca2+ to flow into the cell through the SOCE pathway6,7. IP3Rs are therefore Ca2+-signalling hubs: in all animal cells, they link the extracellular stimuli that evoke the formation of IP3 to delivery of Ca2+ from the ER to the cytosol or other organelles, and to the regulation of Ca2+ entry across the PM through SOCE.
IP3 is not alone sufficient to stimulate the opening of an IP3R. Instead, IP3 binding to all four subunits of a tetrameric IP3R8 primes it to respond to Ca2+, which then evokes channel opening3. In the presence of IP3, IP3Rs can thereby evoke regenerative signals through Ca2+-induced Ca2+ release (CICR). The smallest of these regenerative events are Ca2+ puffs, which are local cytosolic Ca2+ signals that arise from coordinated openings of a few clustered IP3Rs as Ca2+ released by an open IP3R stimulates the opening of its neighbours9 (Supplementary Fig. 1a). Ca2+ puffs, which can be evoked by all three IP3R subtypes10,11, allow local Ca2+ signalling and they have been thought to be the building blocks for larger Ca2+ signals, although that view has recently been challenged12. Cells typically express several thousand IP3Rs, most of which are mobile13–15, yet Ca2+ puffs, whether evoked by endogenous signalling pathways or by the uniform release of cytosolic IP3 from a caged precursor, occur repeatedly at rather few immobile sites within a cell16–21. In seeking to address this long-standing conundrum we recently reported that Ca2+ puffs occur at only a few immobile IP3R clusters alongside the PM and adjacent to the sites where STIM1 accumulates when SOCE is activated20. We described these responsive IP3R clusters as licensed IP3Rs to suggest an additional level of regulation that precedes gating by IP3 and Ca2+.
Here, we identify this additional form of regulation and demonstrate that IP3Rs are licensed to respond when they are tethered to actin by KRas-induced actin-interacting protein (KRAP). We show that all Ca2+ signals, whether local or global, require KRAP-mediated licensing of IP3Rs and that endogenous KRAP limits the capacity of IP3Rs to respond. This additional, and obligatory, level of IP3R regulation reveals an important role for the actin cytoskeleton in regulating IP3-evoked Ca2+ signals and suggests mechanisms whereby local depletion of the ER might control SOCE.
Results
Licensed IP3Rs associate with actin
We used HeLa cells with endogenous type 1 IP3Rs tagged with enhanced green fluorescent protein (EGFP) to address the mechanisms that license immobile IP3Rs adjacent to the PM to evoke Ca2+ puffs20. IP3R1 is the major subtype in HeLa cells22, but we showed previously that an antibody to EGFP immunoprecipitated all three IP3R subtypes from EGFP-IP3R1 HeLa cells in the same ratio as their overall expression, confirming that EGFP-IP3R1 subunits assemble with the other IP3R subtypes20. Thus, EGFP-IP3R1 puncta probably report the presence of all three IP3R subtypes in EGFP-IP3R1 HeLa cells.
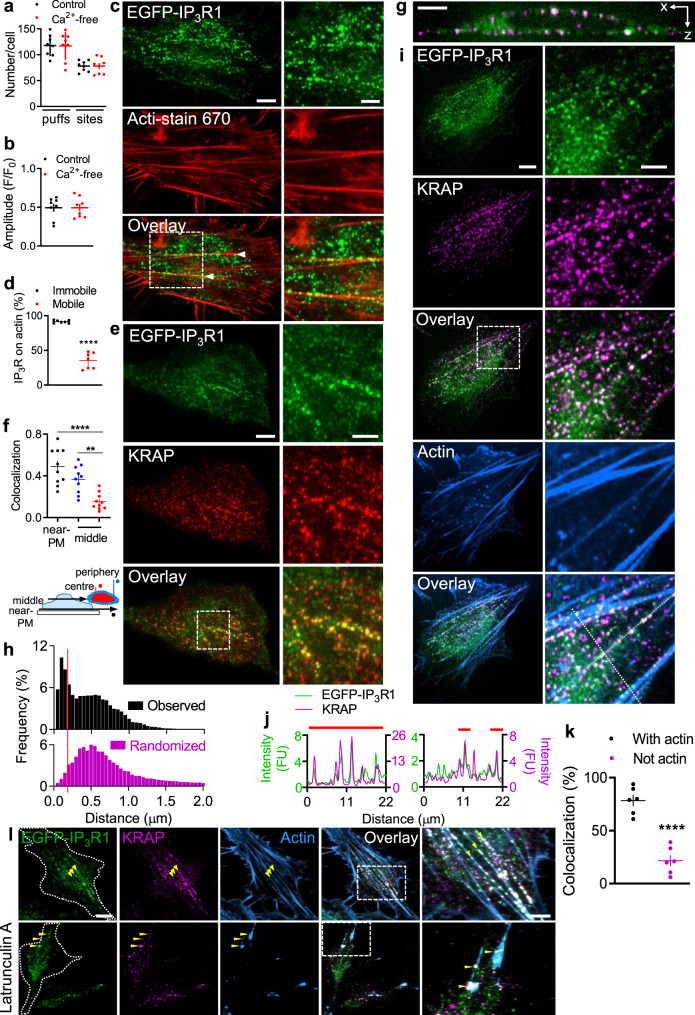
We first considered whether Ca2+ leaking across the PM might provide a local increase in cytosolic free Ca2+ concentration ([Ca2+]c) that then selectively stimulates IP3Rs immediately beneath the PM. Previous work with SH-SY5Y neuroblastoma cells9 and human embryonic kidney (HEK) cells11 established that Ca2+ puffs do not require extracellular Ca2+. We confirmed these findings in HeLa cells by demonstrating that the frequency and amplitude of Ca2+ puffs evoked by photolysis of caged IP3 (ci-IP3) were unaffected by removing extracellular Ca2+ (Fig. 1a, b). We conclude that licensing cannot be due to sensitization of IP3Rs near the PM by Ca2+ leaking across the PM.
Fig. 1. Immobile IP3Rs associate with actin and KRAP.
a Numbers of Ca2+ puffs and Ca2+-release sites detected in EGFP-IP3R1 HeLa cells in the 20 s after photolysis of ci-IP3 with or without extracellular Ca2+ (2.5 mM BAPTA added immediately before UV flash). Mean ± s.d., 8 cells. b Mean puff amplitudes for individual cells (mean ± s.e.m., n = 8 cells). The results establish that Ca2+ leaking across the PM does not account for Ca2+ puffs occurring near the PM. c TIRF images of EGFP-IP3R1 HeLa cells show some IP3R puncta colocalized with actin filaments (arrows). Manders split coefficient for IP3Rs: 0.27 ± 0.07 (mean ± s.d., 11 cells from 5 independent dishes). Scale bars (c,e,i,l), 10 µm (5 µm in enlargements). d IP3R puncta categorized as mobile or immobile and whether they colocalize with actin filaments (mean ± s.d., 7 cells). ****P < 0.0001, Student’s t test. e TIRF images of cells immunostained for KRAP. Typical of 22 cells from 8 independent dishes. f Manders split coefficient for IP3R colocalizing with KRAP in different cell regions. Mean ± s.d., n = 10 cells for each analysis, **P < 0.01, ****P < 0.0001, one-way ANOVA with Bonferroni’s test. g z-projection showing colocalization (white) of KRAP and IP3Rs near PM. Scale bar, 5 μm. Typical of 10 cells from 5 independent dishes. h, Frequency distribution of centre-to-centre distances between each IP3R punctum (14886 puncta from 22 cells) and nearest KRAP punctum. Observed values and values after randomization of positions of the KRAP puncta (100 iterations) are shown. 28 ± 7% (mean ± s.d., n = 22 cells) of IP3R puncta are within 160 nm (red line) of a KRAP punctum (4.2 ± 0.9% after randomization; P < 0.0001, Student’s t test) (Supplementary Fig. 3e). Scale truncated at 2 μm, beyond which 0.13% of observed values occur. i, Confocal section (close to coverslip) of cell stained for actin and KRAP. Typical of 6 cells from 6 independent dishes. j Fluorescence intensity profiles (from i) along actin fibres (left) or perpendicular to them (right). Red lines show regions with actin. FU, fluorescence unit. k Summary (mean ± s.e.m., 6 cells; ~1500 puncta analysed for each) show distributions of colocalized IP3R-KRAP puncta (centre-centre distances < 160 nm) relative to actin. ****P < 0.0001, Student’s t test. i TIRF images show effects of latrunculin A (5 μM, 30 min) on colocalization of IP3R and KRAP with actin filaments. Colocalized IP3R and KRAP puncta associate with actin, even as it depolymerizes to leave residual filaments. Typical of 5 cells from 5 independent dishes.
The immobility of licensed IP3Rs alongside the PM20, the presence of a cortical actin cytoskeleton in most animal cells23, evidence that IP3Rs interact with actin24–26, and identification of actin-binding proteins as partners of IP3Rs in HeLa cells27 led us to consider whether actin might anchor licensed IP3Rs. Total internal reflection fluorescence microscopy (TIRFM) revealed that 27 ± 7% of EGFP-IP3R1 clusters colocalize with actin filaments (Fig. 1c, Supplementary Movie 1); this fraction is similar to the immobile fraction of peripheral IP3Rs (~25%) reported previously20. Live-cell imaging confirmed that immobile IP3R puncta selectively associate with actin (Fig. 1d, Supplementary Fig. 1b–e). Cytochalasin D and latrunculin A caused actin depolymerization, and as actin filaments retracted many immobile IP3R puncta retreated with them and remained associated with residual filaments (Supplementary Fig. 1f–i, Supplementary Movies 2, 3)28. We detected no association of IP3Rs with intermediate filaments29–32 (Supplementary Fig. 2, Supplementary Movie 4). These results suggest that IP3R puncta are immobilized alongside the PM by association with actin.
KRas-induced actin-interacting protein ties IP3Rs to actin
KRas-induced actin-interacting protein (KRAP), now designated IP3 receptor-interacting domain-containing protein 2 (ITPRID 2), was originally identified as a large actin-binding protein that is over-expressed in a colon cancer cell line expressing activated KRas33. KRAP is now known to be widely expressed33,34 and to associate with all three IP3R subtypes29,35,36. Results from co-immunoprecipitation and immunocytochemical analyses suggest that the N-terminal region of KRAP may interact with IP3Rs, while the C-terminal may interact with actin (Supplementary Fig. 3a); it remains unclear whether these interactions are direct or via intermediary proteins33,35,37,38. The functions of KRAP are poorly understood, but it affects both the subcellular distribution of IP3Rs29 and IP3-evoked Ca2+ release35,39. Proteins related to KRAP are also implicated in Ca2+ signalling33,40–43 (Supplementary Fig. 3a). These observations, alongside evidence from the HeLa cell interactome suggesting that KRAP and IP3Rs share partners, including several that interact with actin27 (Supplementary Fig. 3b), prompted us to consider whether KRAP might license IP3Rs.
HeLa cells express KRAP35 (Supplementary Fig. 3c), and TIRFM and spinning-disc confocal microscopy revealed that endogenous KRAP form puncta, some of which colocalize with a subset of EGFP-IP3Rs (Fig. 1e). The colocalization was restricted to regions close to the PM (Fig. 1f, g, Supplementary Fig. 3d). We used an object-based colocalization method44 to study nearest-neighbour distances between IP3R and KRAP puncta. This confirmed that 28 ± 7% of IP3R puncta colocalized with KRAP, significantly more than expected from randomly distributed puncta (Fig. 1h, Supplementary Fig. 3e). Most IP3R puncta that colocalized with KRAP were associated with actin filaments (Fig. 1i–k), but only with filaments close to the PM (Supplementary Fig. 3f, Supplementary Movies 5, 6). Furthermore, colocalized IP3R-KRAP puncta retreated with residual actin as it depolymerized after the addition of latrunculin A (Fig. 1l).
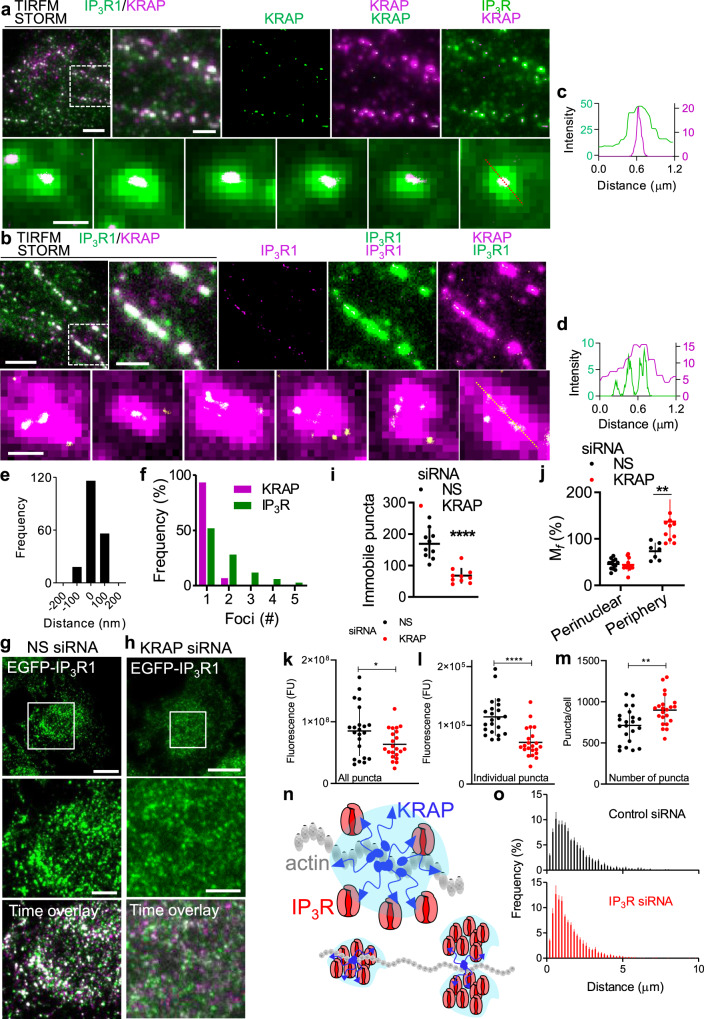
Immobile IP3R puncta are stable, but loose, confederations of about eight IP3Rs, some of which are too far apart to interact directly (Fig. 2a–d)20. Using super-resolution microscopy (stochastic optical reconstruction microscopy, STORM) and TIRFM, we showed that IP3Rs and KRAP cluster around the same centroid in each punctum. However, IP3Rs were often quite widely distributed within a punctum, whereas KRAP was more tightly clustered (Fig. 2a–f), suggesting that KRAP tethers a loose cluster of IP3Rs to actin. Loss of KRAP reduced the number of immobile IP3R puncta in the TIRF field, without affecting IP3R expression or the distribution of actin (Fig. 2g–i, Supplementary Fig. 4a–h). Fluorescence recovery after photobleaching (FRAP) analyses confirmed that loss of KRAP reduced the IP3R immobile fraction at the cell periphery, but the immobility of perinuclear IP3Rs was unaffected (Fig. 2j, Supplementary Fig. 4i, j). We conclude that different mechanisms immobilize central and near-PM IP3Rs; only the latter requires KRAP and only these near-PM IP3Rs tethered to actin are licensed to evoke Ca2+ puffs20. The overall decrease in fluorescence of IP3R puncta in the TIRF field after the loss of KRAP (26 ± 9.7%, Fig. 2k, Supplementary Fig. 4k) and the properties of the remaining puncta, which are more abundant and less bright than in control cells, are consistent with loss of KRAP causing loss of bright puncta tethered beneath the PM, rather than disaggregation of individual IP3R puncta (Fig. 2l, m, Supplementary Fig. 4k–m). We suggest that KRAP, by interacting directly with IP3Rs or with molecules that scaffold the IP3R cluster, tethers one or two pre-assembled IP3R clusters to actin (Fig. 2n).
Fig. 2. KRAP tethers immobile IP3Rs to actin near the PM.
a, b, STORM images of KRAP overlying TIRF images of IP3R (a), and STORM images of IP3R overlying TIRF images of KRAP (b). Scale bars, 5 µm in first image, 2 µm in enlargements of boxed areas, 500 nm in images of individual puncta. Typical of at least 3 experiments. The resolution of our STORM images (FWHM 20–25 nm) is too low to confidently distinguish single IP3R tetramers (~20 nm across) from tightly packed small clusters. Pixels evident in the enlarged overlays are from the TIRF, rather than STORM, images (pixel sizes 100 nm and 10 nm, respectively). c, d, Fluorescence intensity profiles across puncta (dashed lines in (a) and (b)). e, f, Summary shows distances between centroids of KRAP and IP3R within each punctum (e, 190 puncta, 3 cells) and distribution of numbers of resolved foci within each punctum (f, 118 IP3R puncta from 4 cells, 150 KRAP puncta from 3 cells). g, h, TIRF images of EGFP-IP3R1 HeLa cells transfected with non-silencing (NS) (g) or KRAP siRNA (h) and time overlays (30-s interval) showing mobile and immobile IP3R puncta. Scale bars, 10 μm (5 µm for enlargements). i, Summary (mean ± s.e.m., 10 cells from 5 independent dishes, with 41–253 puncta analysed in each) shows numbers of immobile IP3R puncta per cell. ****P < 0.0001, Student’s t test. j Effects of siRNA on mobile fractions (Mf) determined by FRAP for peripheral and perinuclear IP3R puncta (Supplementary Fig. 4i, j). Mean ± s.d., n = 7 cells (peripheral, NS siRNA), 12 cells (peripheral and perinuclear, KRAP siRNA), and 13 cells (perinuclear, NS siRNA). **P < 0.01 Student’s t test. k–m, Effects of siRNA on the sum of the fluorescence intensities of all IP3R puncta in the TIRF field (k), the average intensities of individual puncta (l), and numbers of puncta (m). Mean ± s.d., n = 22 cells for each, ****P < 0.0001, **P < 0.01, *P < 0.05, Student’s t test (k–m). n KRAP, via its interaction with IP3Rs or scaffold molecules (pale blue), tethers pre-assembled clusters of sparsely distributed IP3Rs to actin. o Effects of siRNA against the three IP3R subtypes (10 cells) or NS siRNA (9 cells) on distribution of distances between the centroids of each STIM1 punctum and the nearest actin-associated KRAP punctum in cells treated with thapsigargin (P < 0.05, for both distributions relative to distances after randomization of KRAP distribution; Costes randomization test for each cell).
KRAP directs IP3Rs to the sites where they are licensed
We next considered whether KRAP guides IP3Rs to actin immediately beneath the PM or vice versa. There was no difference in the colocalization of near-PM KRAP with actin in cells with and without IP3Rs, suggesting that KRAP can be appropriately targeted without help from IP3Rs (Supplementary Fig. 5a, b). Licensed IP3Rs park immediately beneath the PM and alongside the sites where SOCE occurs, but they are excluded from the narrow MCS where STIM1 and Orai1 interact20. The association of immobile IP3Rs with actin through KRAP explains the exclusion of IP3Rs from the MCS because most actin filaments terminate at least 100 nm from SOCE MCS45. It does not, however, explain the preferential positioning of licensed IP3Rs alongside SOCE MCS20. We used cells with endogenous STIM1 tagged with EGFP (STIM1-EGFP HeLa cells)46 together with siRNA to reduce expression of all IP3R subtypes to examine any effects of IP3Rs on the subcellular distribution of KRAP and STIM1. Loss of Ca2+ from the ER caused STIM1 puncta to accumulate beneath the PM, and these puncta accumulated alongside, but not coincident with, KRAP (Supplementary Fig. 5c–i). The distribution of distances between each STIM1 punctum and the nearest actin-associated KRAP punctum revealed that their preferential affiliation was unaffected by loss of IP3Rs (Fig. 2o). These observations show that it is the association of KRAP with actin that determines the location of immobile IP3Rs near the PM and alongside the ER-PM junctions where SOCE occurs. We conclude that KRAP guides IP3Rs to the sites where they are licensed.
KRAP is required for Ca2+ puffs and global Ca2+ signals
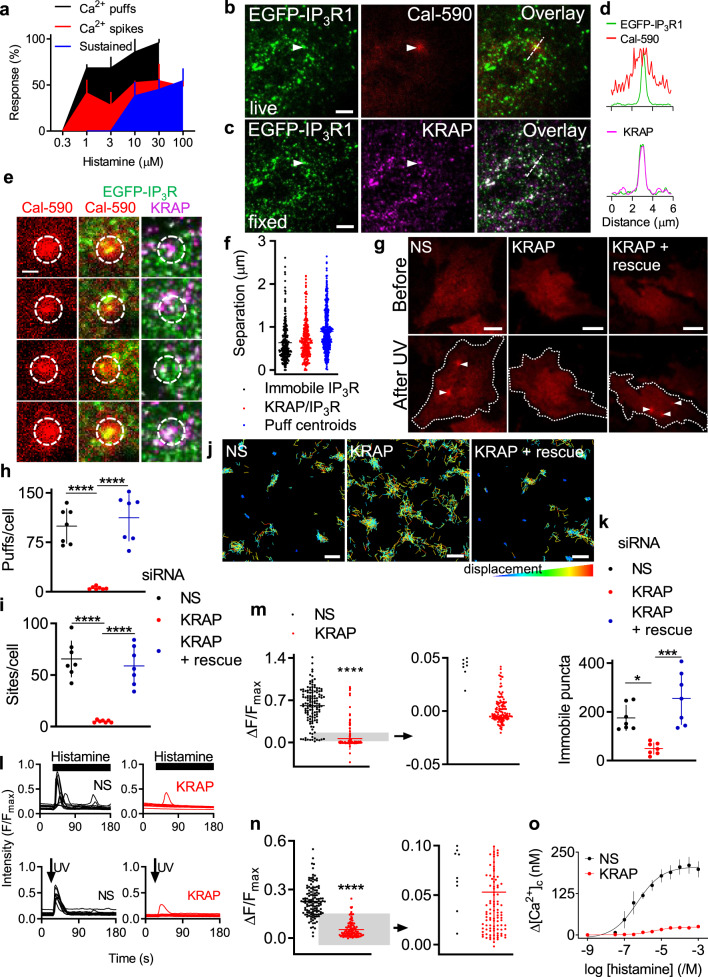
Ca2+ puffs provide local Ca2+ signals at low stimulus intensities. As stimulus intensities increase and more IP3Rs are primed with IP3, Ca2+ signals invade the entire cell as Ca2+ waves, the frequency of which increases with stimulus intensity47. We confirmed this hierarchy of Ca2+ signals in HeLa cells stimulated with histamine to evoke IP3 formation through endogenous pathways (Fig. 3a, Supplementary Fig. 6a). Since all three IP3R subtypes can generate Ca2+ puffs10,11, it has been widely assumed that Ca2+ puffs are the building blocks for all IP3-evoked Ca2+ signals. However, it is not known whether, during signal propagation, Ca2+ puffs recruit only further Ca2+ puffs (licensed IP3Rs) or additional IP3Rs (e.g., mobile IP3Rs). Indeed, recent evidence suggests that global Ca2+ signals may not be entirely associated with underlying Ca2+ puffs12.
Fig. 3. KRAP is required for IP3-evoked Ca2+ signals.
a Hierarchical recruitment of Ca2+ signals by histamine (Supplementary Fig. 6a). b TIRF images of part of an EGFP-IP3R1 HeLa cell showing IP3Rs and a Ca2+ puff (arrow) evoked by photolysis of ci-IP3, and their overlay. c Same cell after KRAP immunostaining shows IP3Rs, KRAP and overlay. Arrow highlights location of Ca2+ puff. Scale bars (b, c), 5 µm. Images typical of 3 cells from 3 independent experiments (b, c). d Fluorescence intensity profiles along dashed lines in (b) and (c). e Examples of colocalized KRAP/IP3R puncta and Ca2+ puffs evoked by photolysis of ci-IP3. Scale bar, 2 μm. Typical of 3 cells from 3 independent experiments. f Centre-centre distances measured from Ca2+ puffs to immobile IP3Rs (black, n = 3 cells), IP3R/KRAP puncta (red, n = 3 cells), or between centres of successive Ca2+ puffs at the same site (blue, n = 19 cells) (Supplementary Fig. 6b, c). Individual values with mean ± s.d for the indicated number of cells. g TIRF images of Ca2+ puffs evoked by photolysis (150 ms) of ci-IP3 after the indicated treatments. Scale bars, 10 µm. h, i, Summary (mean ± s.d, 7 cells, with 4–152 puffs detected per cell). ****P < 0.0001, one-way ANOVA with Bonferroni’s test. j Single-particle trajectories (coloured according to frame-to-frame displacement) of EGFP-IP3R1 puncta in cells treated with NS or KRAP siRNA alone or with expression of siRNA-resistant KRAP (rescue). Scale bars, 500 nm. k Summary (mean ± s.d., 7 cells with 16–407 puncta per cell analysed) shows numbers of immobile IP3R puncta per cell. *P < 0.05, ***P < 0.001, one-way ANOVA with Bonferroni’s test. l Randomly selected traces (10 cells for each) show responses from EGFP-IP3R1 HeLa cells treated with NS or KRAP siRNA and stimulated with a maximally effective concentration of histamine (100 μM) or intense photolysis of ci-IP3 (600 ms). m, n Summary (mean ± s.d.) shows increases in [Ca2+]c (reported as ΔF/Fmax) evoked by histamine (m, n = 157 and 125 cells from 7 and 6 independent siRNA treatments for KRAP and NS siRNA, respectively) or photolysis of ci-IP3 (n, n = 109 and 127 cells from 6 and 7 independent siRNA treatments for KRAP and NS siRNA). Enlargements around ΔF/Fmax = 0 highlight many unresponsive KRAP siRNA-treated cells. ****P < 0.0001, Student’s t test. Different Ca2+ indicators were used with histamine (Cal-590, Kd = 561 nM) and ci-IP3 (Calbryte 590, Kd = 1400 nM). o Effect of NS and KRAP siRNA on peak increases in [Ca2+]c (Δ[Ca2+]c) evoked by the indicated concentrations of histamine in populations of EGFP-IP3R1 HeLa cells stimulated in HBS. Mean ± s.e.m., n = 4, each with 3 replicates.
By using TIRFM to record IP3R mobility and Ca2+ signals after photolysis of ci-IP3, and then fixing cells to visualize KRAP, we established that Ca2+ puffs occur at sites populated by KRAP and immobile IP3R puncta (Fig. 3b–f). Because the centroids of successive Ca2+ puffs wander within a site48–50, reflecting recruitment of sparsely distributed IP3Rs within a punctum (Fig. 2a–d, n, Supplementary Fig. 6b, c), there is some variability in the separation of Ca2+ puff centroids and KRAP/IP3R puncta. However, the distances between successive Ca2+ puffs at a site, between Ca2+ puffs and immobile IP3R, and between Ca2+ puffs and KRAP/IP3R puncta are similar (Fig. 3f). This indicates that Ca2+ puffs occur at KRAP-associated immobile IP3R puncta (Fig. 3b–f).
In cells with KRAP expression reduced by siRNA, there were very few immobile IP3R puncta, IP3-evoked Ca2+ puffs were almost abolished, and the number of sites where Ca2+ puffs occurred was massively reduced (Fig. 3g–k, Supplementary Fig. 6d–g, Supplementary Movies 7–9). By adjusting detection thresholds, we confirmed that the lack of Ca2+ puffs in KRAP-depleted cells was not a result of failing to detect small Ca2+ puffs (Supplementary Fig. 6h, i). We conclude that KRAP is required to both immobilize IP3R clusters on actin and to allow them to evoke Ca2+ puffs.
We next considered whether the global Ca2+ signals evoked by more intense stimulation also required KRAP. A maximally effective histamine concentration, which evoked large global increases in [Ca2+]c in normal cells, caused a negligible increase in [Ca2+]c in cells without KRAP (Fig. 3l–n). Nor was there any greater response when the histamine concentration was increased to one-hundred times that required to evoke a maximal response in normal cells (Fig. 3o). Global Ca2+ signals evoked by intense photolysis of ci-IP3 were also abolished in cells without KRAP (Fig. 3l–n). Neither IP3R expression nor ER Ca2+ content was reduced by KRAP siRNA (Supplementary Fig. 4c, d) indeed the ER Ca2+ content was slightly greater in cells without KRAP (Supplementary Fig. 4n, o), perhaps a consequence of reduced basal IP3R activity. The effects of siRNA-mediated knockdown of KRAP expression on IP3R mobility and Ca2+ puffs were reversed by expression of a siRNA-resistant KRAP (Fig. 3g–k). We conclude that all cytosolic Ca2+ signals, local and global, require IP3Rs licensed by their association with KRAP.
Endogenous KRAP determines the sensitivity of cells to IP3
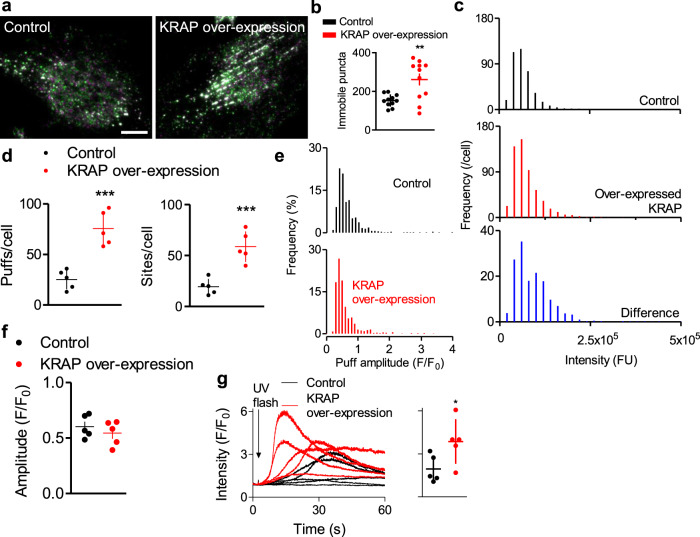
Only a small fraction of IP3Rs is licensed to respond. We therefore considered whether KRAP might determine the number of licensed IP3Rs. Over-expressing KRAP in EGFP-IP3R1 HeLa cells (to 9.5-times endogenous levels, Supplementary Fig. 6j) increased the number of immobile IP3R puncta without increasing the number of IP3Rs within a punctum (Fig. 4a–c). We conclude that even though not all KRAP is associated with IP3Rs (Fig. 1e), the availability of endogenous KRAP controls the number, rather than the size, of immobile IP3R puncta. This aligns with evidence that KRAP captures pre-existing IP3R clusters and tethers them to actin (Fig. 2k–m, Supplementary Fig. 4k–m). Consistent with that interpretation, over-expressed KRAP increased the frequency of Ca2+ puffs evoked by photolysis of ci-IP3 and the number of sites where they occurred, but not the amplitude of individual Ca2+ puffs (Fig. 4d–f, Supplementary Movie 10). We confirmed that our identification of more Ca2+-release sites was not due to improved detection efficiency arising from more frequent Ca2+ puffs (Supplementary Fig. 6k). The increased activity caused Ca2+ signals to propagate globally after much shorter intervals and to evoke larger increases in [Ca2+]c (Fig. 4g). Even in cells stimulated with a maximal histamine concentration, KRAP over-expression caused the peak Ca2+ signal to increase by 23 ± 8%. We conclude that endogenous KRAP limits the number of licensed IP3Rs and thereby the ability of a cell to evoke cytosolic Ca2+ signals.
Fig. 4. Endogenous KRAP determines the number of licensed IP3Rs.
a TIRFM images of EGFP-IP3R1 at 30-s intervals (green and magenta) show more immobile IP3R puncta (white) in cells over-expressing KRAP. Scale bar, 10 μm. b Summary (mean ± s.d., 11 cells) show numbers of immobile IP3R puncta per cell. **P < 0.01, Student’s t test. c Fluorescence intensity distributions for EGFP-IP3R in control and cells over-expressing KRAP (4762–6559 puncta from 12 cells). The difference plot confirms that KRAP over-expression does not affect the size of IP3R puncta. d Numbers of Ca2+ puffs and sites detected during the 5.5-s interval after photolysis of ci-IP3 (individual values, mean ± s.d., 5 cells). ***P < 0.001, Student’s t test. e, Distribution of Ca2+ puff amplitudes (61–142 Ca2+ puffs in each of 5 cells). f Summary results (mean ± s.e.m., 5 cells) show mean puff amplitudes. P > 0.05, Student’s t test. g Examples of whole-cell Cal-590 fluorescence changes evoked by photolysis (150 ms) of ci-IP3 in control and KRAP-over-expressing cells. Histogram shows peak amplitudes (mean ± s.d. n = 5 cells). *P < 0.05, Student’s t test.
Discussion
Ca2+ puffs are elementary regenerative events evoked by IP3 in mammalian cells; yet while most IP3Rs are mobile, Ca2+ puffs occur repeatedly at the same sites within a cell16–18. We have now resolved this long-standing conundrum by identifying an additional level of IP3R regulation that precedes IP3R gating by IP3 and Ca2+. We have shown that KRAP licenses IP3Rs to respond by tethering loose confederations of IP3Rs to actin filaments immediately beneath the PM and alongside the sites where SOCE occurs (Fig. 5a, b). All IP3-evoked cytosolic Ca2+ signals, whether local (Ca2+ puffs) or global, require licensing of IP3Rs by KRAP (Fig. 5a, b). Licensing by KRAP is probably a feature of all IP3Rs since all three IP3R subtypes interact with KRAP29,35,36, and loss of KRAP abolishes IP3-evoked Ca2+ signals in HeLa cells (Fig. 3l–o), which express all three IP3R subtypes20.
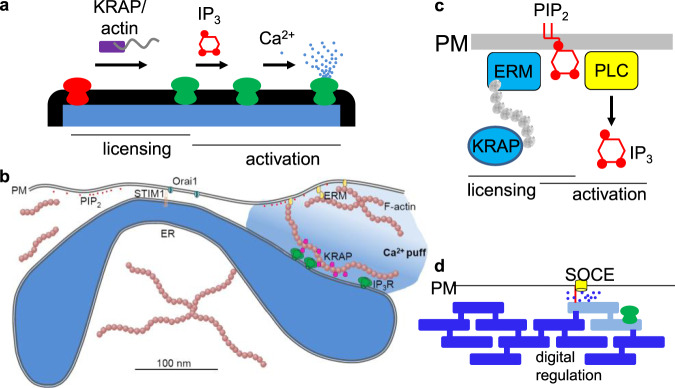
Fig. 5. KRAP licenses IP3Rs to evoke Ca2+ signals.
a IP3-evoked Ca2+ release requires licensing by the association of IP3Rs with KRAP and actin. IP3R activation is then triggered by sequential binding of IP3 and Ca2+. b Organization of IP3Rs near SOCE junctions, drawn to approximate scale. c PIP2 may regulate both IP3R licensing by controlling assembly of actin filaments (through ezrin, radixin and moesin (ERM), for example) and as the substrate from which IP3 is produced by PLC. d, Local release of Ca2+ from ER by licensed IP3Rs immediately beneath the PM may allow digital regulation of SOCE.
How can an obligatory need for KRAP in intact cells be reconciled with evidence that IP3Rs can open under experimental conditions where their association with KRAP or actin is unlikely? Such interactions are impossible, for example, after functional reconstitution of purified IP3R protein51 or in patch-clamp recordings52, and they are unlikely in permeabilized cells;3 yet in each case, IP3 evokes opening of IP3Rs. We suggest that IP3Rs in intact cells are probably constitutively repressed, and that licensing by KRAP relieves the inhibition. We have not yet explored the molecular basis of the repression. IRBIT (IP3R-binding protein released by IP3)53, Bcl2 (B-cell lymphoma 2)54, annexin A155 and many other signals inhibit IP3Rs56, and they may be lost when cells are disrupted, perhaps thereby alleviating the need for KRAP in analyses of broken cells.
A second issue arises from observations that not all IP3-evoked Ca2+ signals in all cells arise immediately beneath the PM. We consider two possible explanations. It may be that in other cells or in response to appropriate regulatory signals, IP3Rs may be licensed by association with KRAP in different subcellular locations, allowing them to initiate Ca2+ signals away from the PM. Alternatively, and consistent with recent analyses of local and global Ca2+ signals in HEK cells12, a flurry of Ca2+ puffs mediated by licensed IP3Rs may provide a signal (perhaps mediated by a decrease in ER luminal [Ca2+])12 that ignites the activity of unlicensed IP3Rs. Hence, and consistent with our observations (Fig. 3), all IP3-evoked Ca2+ signals would require licensing of IP3Rs by KRAP: either directly to evoke Ca2+ puffs, or indirectly (through the prior occurrence of Ca2+ puffs) to evoke global Ca2+ signals. The second scheme also aligns with evidence from pancreatic acinar cells. In these cells, KRAP is located at the apical pole of each cell57, where IP3-evoked Ca2+ signals initiate before propagating via IP3Rs and ryanodine receptors to the basal pole;58 disruption of the actin cytoskeleton perturbs these local Ca2+ signals and the apical distribution of IP3Rs26.
Licensing of IP3Rs by KRAP is likely to have further implications. By positioning responsive IP3R clusters alongside the MCS where SOCE occurs, KRAP may ensure that activation of IP3Rs selectively depletes the ER that is best placed to regulate SOCE. We speculate that this may allow substantial local depletion of small ER compartments close to the PM and thereby allow local digital regulation of SOCE (Fig. 5d)20,59. In this way, the substantial ER depletion required to effectively activate STIM160 may be achieved without massive loss of Ca2+ from the entire ER, which might otherwise compromise other ER functions like protein folding. KRAP takes its name from the discovery that it is up-regulated in many KRas-transformed cells33. Licensing of IP3Rs by KRAP may, therefore, provide another site of interaction between endogenous Ras and Ca2+ signalling61. Finally, the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2), is both the source of IP3 and a key regulator of actin filaments62, suggesting that PIP2 may control both licensing of IP3Rs and their activation by IP3 (Fig. 5c).
We conclude that licensing of IP3Rs by KRAP allows cells to respond to IP3 and determines the spatial organization of cytosolic Ca2+ signals. Licensing may allow dynamic actin filaments to regulate Ca2+ signalling, and IP3Rs to regulate SOCE through local depletion of ER Ca2+ stores (Fig. 5)20,59.
Methods
Materials
Cal-590 AM, Calbryte 590 AM and Fluo-8 AM were from AAT Bioquest (Sunnyvale, CA, USA). A membrane-permeant form of caged-IP3 (ci-IP3/PM: d-2,3-O-isopropylidene-6-O-(2-nitro-4,5-dimethoxy)benzyl-myo-inositol 1,4,5-trisphosphate hexakis(propionoxymethyl) ester) was from SiChem (Bremen, Germany). Acti-stain 670 phalloidin (# PHDN1-A) was from Cytoskeleton (Denver, CO, USA). BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) was from Molekula (Darlington, UK). Histamine was from Sigma-Aldrich. Bovine serum albumin (BSA) was from Europa Bio-Products (Ely, UK). Withaferin A was from AdipoGen Life Sciences (Liestal, Switzerland). AlexaFluor-568 phalloidin and restriction enzymes were from ThermoFisher. Human fibronectin was from Merck Millipore (Watford, UK). Plasmids encoding the following proteins were from: LifeAct-mCherry-N1 (Addgene #40908)63, LifAct-7-iRFP670 (Addgene #103032)64, human KRAP with an N-terminal Myc-DDK tag (OriGene, Rockville, MD, USA, #RC205550), mCherry-keratin (Addgene #55066) and mCherry-vimentin-N-18 (Addgene #55158, deposited by Michael Davidson, Florida State University, FL, USA). Cytochalasin D and latrunculin-A were from Tocris (Abingdon, UK). Antibodies (for Western blotting, WB; or immunocytochemistry, IC) were from: β-actin (mouse monoclonal; WB 1:10000; Cell Signaling Technology, Leiden, Netherlands, #8H10D10, undefined clone #); KRAP (rabbit polyclonal; WB 1:1000; IC 1:400; ProteinTech, Manchester, UK, #14157-1-AP), the same KRAP primary antibody was custom-conjugated to YF-594 for TIRF imaging during STORM analyses of IP3Rs (1:400, ProteinTech); GFP Tag-AlexaFluor-647 (STORM 1:400, ThermoFisher, #31852); IP3R1 (rabbit, raised against a C-terminal peptide 2732–2750 of rat IP3R1; WB 1:1000, Merck Millipore, #AB5882); IP3R2 (rabbit, custom-made to a C-terminal peptide GFLGSNTPHENHHMPPH; WB 1:500, IC 1:200, Pocono Rabbit Farm and Laboratory);65 IP3R3 (mouse monoclonal; WB 1:1000, IC, 1:200, BD Transduction Laboratories, Wokingham, UK, #610313, clone 2); STIM1 (rabbit monoclonal; WB 1:800, Cell Signaling Technology, #5668, undefined clone #); vimentin (chicken polyclonal; IC 1:1000, Novus Biologicals, Centennial, CO, USA, #NB300-223); GFP-Booster Atto488 (IC 1:500, Chromotek, Planegg-Martinsried, Germany #gba488); donkey anti-rabbit IgG-HRP (WB 1:5000, SantaCruz, Heidelberg, Germany, SC-2313); donkey anti-mouse IgG-HRP (WB 1:2000, Santa Cruz, SC-2314); goat anti-rat IgG-HRP (WB 1:5000, Santa Cruz, SC-2020); goat anti-rabbit AlexaFluor-594 (IC 1:1000, ThermoFisher, #A11012); goat anti-rabbit AlexaFluor-647 (IC and STORM 1:500, ThermoFisher, #A21244); goat anti-mouse AlexaFluor-568 (IC 1:1000, ThermoFisher, #A11004); and goat anti-chicken AlexaFluor-568 (IC 1:1000, ThermoFisher, #A11041). Additional sources of materials are provided in relevant methods.
Cell culture and transfection
HEK cells, HEK cells devoid of all IP3Rs (HEK-3KO, Kerafast, Boston, MA, USA)8, HeLa cells, STIM1-EGFP HeLa cells66 and EGFP-IP3R1 HeLa cells20 were cultured in Dulbecco’s modified Eagle’s medium/F-12 with Gluta-MAX (ThermoFisher, Waltham, MA, USA) supplemented with foetal bovine serum (FBS, 10%, Sigma-Aldrich, Gillingham, Dorset, UK). The cells were maintained at 37 °C in humidified air with 5% CO2 and passaged every 3-4 days using Gibco TrypLE Express (ThermoFisher). We have reported an extensive characterization of the EGFP-IP3R1 HeLa cell line, in which monomeric EGFP was attached to the N-terminal of all copies of the endogenous IP3R1 gene (ITPR1) using TALENs (transcription activator-like effector nucleases)20. The characterization included evidence that EGFP-IP3R1 is functional. In the STIM1-EGFP HeLa cell line, CRISPR/Cas9 was used to add monomeric EGFP to the C-terminal of one of the two STIM1 genes66. For STIM1-EGFP HeLa cells, we have confirmed that the only fluorescent protein in the cells is STIM1-EGFP and that histamine-evoked Ca2+ signals and SOCE are unperturbed by the gene-editing46. KRAP is up-regulated in some cells with mutant KRas33, but we confirmed by pyrosequencing (Source Bioscience, Nottingham, UK) that there were no mutations in codons 12, 13 (exon 2), 59, 61 (exon 3), 117 or 146 (exon 4) of the KRAS gene in the EGFP-IP3R1 HeLa cells.
For imaging, cells were grown on 35-mm glass-bottomed dishes (Cellvis, IBL Baustoff+Labor GmbH, Gerasdorf bei Wein, Austria) coated with human fibronectin (10 μg ml-1). Regular screening confirmed that all cells were free of mycoplasma. The authenticity of the EGFP-IP3R1 HeLa cells (Eurofins, London, UK) and HEK-3KO cells (DNA Diagnostic Center, Fisher Scientific) was verified by short-tandem repeat profiling.
For transient transfections, EGFP-IP3R1 HeLa cells grown on Cellvis imaging dishes were transfected with the appropriate plasmid (1–2 µg DNA per dish) using ViaFect (Promega, Madison, WI, USA) transfection reagent (3 µl per 1 µg DNA) according to the manufacturer’s instructions. Cells were used after 24 h.
Western blotting
Cells grown on 75-cm2 culture flasks were harvested using either enzyme-free cell dissociation buffer (ThermoFisher) or, for siRNA experiments, by scraping cells into lysis medium (LM, 150 mM NaCl, 0.5 mM EDTA, 1% Triton X-100, 10 mM Tris/HCl, pH 7.5) containing a protease inhibitor mini-tablet with EDTA (Pierce, 1 tablet per 10 ml). Cells were then lysed by incubation in LM at 4 °C for 1 h, sonicated (Transonic ultrasonic bath, 3 × 10 s), and the supernatant was collected (20,000 × g, 30 min). Since actin-associated proteins are more resistant to detergent-extraction, and KRAP facilitates IP3R association with actin (Fig. 1), the Triton X-100 in LM was supplemented with 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulphate (SDS)29 for western blot (WB) analyses of IP3Rs before and after KRAP knock-down (Supplementary Fig. 4c, d).
For WB, proteins were separated using NuPAGE 3–8% Tris-acetate gels (ThermoFisher) and transferred onto an iBlot PVDF membrane using an iBlot gel-transfer device (ThermoFisher). The PVDF membrane was blocked by incubation (1 h, 20 °C) in TBST, which comprised Tris-buffered saline (TBS: 137 mM NaCl, 20 mM Tris, pH 7.6) supplemented with bovine serum albumin (BSA, 5%) and Tween-20 (0.1%). The blocked membrane was incubated (16 h, 4 °C) with primary antibody (in TBST with 1% BSA), washed (3 × 5 min) in TBST, and incubated (1 h, 20 °C) with horseradish peroxidase (HRP)-conjugated secondary antibody in TBST with 1% BSA. The antibodies used and their dilutions are listed in the Materials section. After further washes with TBST (3 × 5 min), HRP was detected using ECL Prime Western blotting detection reagents (GE Healthcare Life Sciences, Little Chalfont. UK) and a PXi chemiluminescence detection system (Syngene, Cambridge, UK). Where main figures show only part of a WB, the complete WB is shown in Supplementary Fig. 7.
Treatment with siRNA
Cells grown in either glass-bottomed imaging dishes or multi-well plates (6 or 96 wells) were transfected with siRNA directed against KRAP (50 nM, #AM16708 or #4392420, ThermoFisher) or a non-silencing (NS) control siRNA (50 nM, #AM4611, ThermoFisher) using either siPORT NeoFX transfection reagent (ThermoFisher, 220 ng siRNA per μl reagent) or a Neon transfection system (ThermoFisher) according to the manufacturer’s instructions. The two KRAP siRNAs were used interchangeably and with indistinguishable knockdown efficiencies (Supplementary Fig. 4a, b). Cells were used after 72 h. We confirmed by immunostaining that after treatment with KRAP siRNA, KRAP was undetectable in 90 ± 0.1% of cells (55 cells from 3 independent experiments) and 0 ± 0 % for cells treated with NS siRNA (53 cells from 3 experiments). The very small residual histamine-evoked Ca2+ signals (~11%, Fig. 3o) in cell populations treated with KRAP siRNA are likely attributable to the 10% of cells with residual KRAP. Images of cells treated with either siRNA were randomly selected for single-cell analyses.
For siRNA-mediated knockdown of the three IP3R subtypes, cells were simultaneously transfected with siRNA to each IP3R subtype (40 nM of each FlexiTube siRNA, Qiagen, Hilden, Germany: IP3R1, #S100034545; IP3R2, #S100034552; IP3R3, #S100034580) or NS siRNA (120 nM, Qiagen #1027281). Cells were used after 72 h.
A siRNA-resistant version of the KRAP plasmid was prepared by digesting the plasmid encoding human KRAP (Origene, #RC205550) at Bst1107I and ScaI restriction sites. This was followed by the introduction of a DNA string of the excised region containing silent mutations at the siRNA target site with 30 bp flanking regions of the two restriction sites (ThermoFisher), into the plasmid using Gibson assembly (Gibson Assembly Master Mix, New England Biolabs, Ipswich, MA, USA). The sequence of the DNA from which the siRNA-targeted mRNA sequence derives (bp 2011–2029 of the coding sequence) was: GCTAAATGCAGTGATATGA in the native DNA, and GCGAAGTGTTCAGACATGA in the mutated form (mutated residues are underlined). The modified and native DNAs encode the same protein sequence.
Immunocytochemistry
Cells grown on fibronectin-coated Cellvis glass-bottomed dishes were washed three times in phosphate-buffered saline (PBS, 20 °C), fixed with paraformaldehyde (4%) in PBS (30 min), washed three-times in PBS, and permeabilized by incubation (5 min) in PBS containing Triton X-100 (0.25%). After three washes with PBS, cells were incubated (1 h) in PBS containing BSA (5%), washed briefly, and then incubated (1 h) with primary antibody (details in Materials section) in PBS containing BSA (3%). After three washes with PBS, cells were incubated (1 h) with an appropriate AlexaFluor-conjugated secondary antibody, fluorescent phalloidin or GFP-Booster (details in Materials section and legends) in PBS containing BSA (3%). The cells were then washed three times with PBS before imaging. For matched analyses of live and immunostained cells (Fig. 3b–f), fixation, permeabilization and immunostaining were performed without moving the sample from the microscope stage.
Fluorescence microscopy
Fluorescence microscopy used an inverted Olympus IX83 microscope equipped with a 100× oil-immersion TIRF objective (numerical aperture, NA 1.49), a multi-line laser bank (488 nm, 561 nm, 638 nm and 647 nm) and an iLas2 targeted laser illumination system (Cairn, Faversham, UK). In some experiments that required larger fields of view, we used a ×60 (NA 1.45) oil-immersion TIRF objective (Fig. 3l–n). Excitation light was transmitted through a quad dichroic beam splitter (TRF89902-QUAD, Chroma). Emitted light was passed through appropriate filters (Cairn Optospin; peak/bandwidth: 525/50, 630/75 and 700/75 nm) and detected with either an iXon Ultra 897 electron multiplying charge-coupled device (EMCCD) camera (512 × 512 pixels, Andor) or a Prime 95B scientific complementary metal oxide semiconductor (sCMOS) camera (1200 × 1200 pixels, Photometrics). Spinning-disc confocal microscopy (SDCM) used a spinning disc with a 70-μm pinhole (X-Light, CrestOptics, Rome, Italy). For total internal reflection fluorescence microscopy (TIRFM), the penetration depth was 90–120 nm. The iLas2 illumination system was used for TIRFM and FRAP.
Super-resolution confocal microscopy used an inverted Nikon Eclipse Ti2 microscope equipped with a ×100 oil-immersion TIRF objective (NA 1.49), an X-Light V3 spinning-disc confocal unit with 50-µm pinholes (CrestOptics), and a Live-SR super-resolution module (Gataca Systems, Massy, France). Excitation light (470, 555 and 640 nm) from a laser diode illuminator (89 North, Williston, VT, USA) was transmitted through a quad-band filter set (405/470/555/640 nm). Emitted light was passed through an appropriate emission filter (GFP, TRITC or Cy5) and detected with a Prime 95B camera. Super-resolution was achieved by multifocal structured illumination67 using the Live-SR module and optical reassignment processing to give a lateral resolution of approximately 120 nm.
Before analysis, all fluorescence images were corrected for background by subtraction of fluorescence detected from a region outside the cell. Image capture and processing used MetaMorph Microscopy Automation and Image Analysis Software (version 7.10.1.161, Molecular Devices, San Jose, CA, USA) and Fiji (https://fiji.sc)68, respectively. Confocal images were deconvolved using the Microvolution deconvolution algorithm (version 2015.05)69. All images are presented in RGB colour (16-bit) format.
Stochastic optical reconstruction microscopy (STORM)
The methods used to generate STORM images were exactly as described20. Cells were fixed in PBS containing paraformaldehyde (4%, 30 s), permeabilized and immunostained. For identification of KRAP by STORM, we used a rabbit polyclonal primary antibody (1:400) and goat anti-rabbit AlexaFluor-647 secondary antibody (1:500); IP3Rs were identified in TIRF from their EGFP fluorescence. To identify IP3Rs using STORM, EGFP-IP3R1 was visualized using GFP Tag-AlexaFluor-647 (1:400); KRAP was identified in TIRF using the same primary antibody used for STORM analyses, but conjugated directly to YF-594 (custom-made by ProteinTech, Manchester, UK). The medium used for STORM comprised: Tris-HCl (50 mM), pH 8.0, NaCl (10 mM), glucose (555 mM), catalase (Sigma-Aldrich, 34 µg ml−1), glucose oxidase (Sigma-Aldrich, 560 µg ml−1), 2-mercaptoethanol (Sigma-Aldrich, 1%) and cyclooctatetraene (Sigma-Aldrich, 1%). For STORM imaging, stochastic blinking of individual fluorophores was achieved by first bleaching cells in semi-TIRF mode using full laser power (647 nm). Images (256 × 256 pixels, 20,000 frames, 50 frames per s) were then acquired in TIRF at a lower laser power (647 nm) using a TIRF objective (×100, NA = 1.49, with an intermediate magnification of × 1.6) and an Andor iXon Ultra EMCCD camera. Image acquisition and fitting used WaveTracer (MetaMorph) to detect genuine blinking events followed by fitting a centroid to each blinking event within each 10 nm × 10 nm pixel. Point localizations of blinking events from each of the 20,000 frames were collated to construct a super-resolution STORM image20. STORM images generated using four-colour TetraSpeck microspheres (diameter 0.1 µm, ThermoFisher) confirmed that there was no chromatic aberration or drift during image acquisition. The lateral resolution for STORM (full-width at half-maximal amplitude, FWHM) determined using TetraSpeck microspheres was of 21.8 ± 4.0 nm, and 24.2 ± 5.6 nm when determined using STORM images generated with AlexFluor 647.
Fluorescence recovery after photobleaching (FRAP)
The methods used for wide-field FRAP measurements, after photobleaching a circular region of interest (ROI), were exactly as described previously20. Briefly, time-lapse images (~1 frame per second) were acquired in epifluorescence mode using a 488-nm laser. A circular ROI (diameter = 2.3 μm) was rapidly photobleached by raster scanning at either a perinuclear or peripheral region of a cell, using a 395-nm laser and an iLas2 laser illumination system. Recovery of fluorescence was then recorded. Images were background-corrected by subtracting the fluorescence recorded from a region outside the cell. The mobile fraction was calculated using the ImageJ plugin, FRAP profiler (http://worms.zoology.wisc.edu/ImageJ/FRAP_Profiler.java).
Colocalization analyses
These analyses required different methods to accommodate our need to quantify relationships between fluorophores where only subsets of particles were colocalized (e.g., KRAP and IP3R); the need to compare two or three colocalized fluorophores; our use of TIRF and spinning-disc confocal microscopy, where the latter contributes more out-of-focus fluorescence; and our need to compare both punctate (e.g., KRAP and IP3R) and elongated (e.g., actin) structures, where only the punctate structures are amenable to object-based analyses.
Since only subpopulations of IP3R and KRAP puncta colocalize, we used an object-based method (DiAna, version 1.1)44 to quantify their colocalization by measuring centre-to-centre distances between IP3R and KRAP puncta (Fig. 1h, k, Supplementary Fig. 2a–d, 3e). The same methods were used to determine separations of KRAP and STIM1 puncta (Fig. 2o). The separations between the centroids of Ca2+ puffs and either immobile IP3R, KRAP/IP3R or the centroids of successive Ca2+ puffs were manually determined (Fig. 3f). Background-corrected TIRF images of fluorescence from EGFP-IP3R and immunostained KRAP were segmented using a spot segmentation method, which identifies all local intensity maxima in a frame and then uses a threshold to select the local maximum for each object. The algorithm then computes the radial distribution of pixel intensities around each local maximum to define a border intensity threshold for the objects. It then progresses radially from each local maximum and includes pixels if their intensities are both above the border intensity threshold and below the intensity of the pixel that was last accepted; acceptance also requires that a pixel is adjacent to other accepted pixels. Centre-to-centre distances between the segmented spots (IP3R and KRAP puncta, which need not be circular) are then calculated. Since these measurements provide sub-pixel localization accuracy, we considered IP3R and KRAP puncta to be colocalized if their centre-to-centre distance was <160 nm (the width of a single pixel in all analyses, except for super-resolution confocal microscopy where the pixel size was 65 nm and criterion for colocalization was a separation of <130 nm). Our aim was to use similar criteria for colocalization for each analysis, while ensuring that the number of pixels used to define colocalization for each imaging method came closest to the theoretical lateral resolution of the method. To assess the statistical significance of the colocalization, the algorithm randomizes (100 iterations) the segmented image of all KRAP puncta before re-calculating the centre-to-centre distances44. This analysis was used to provide the cumulative distribution of centre-to-centre distances for unperturbed IP3R puncta and randomly distributed KRAP puncta with its 95% confidence interval (CI) (Supplementary Fig. 3e). The same approach was applied to all object-based colocalization analyses to establish their statistical significance.
Colocalization analyses of Ca2+ puffs with EGFP-IP3Rs again compared distances between centroids, but using coordinates for Ca2+ puffs derived from FLIKA (see the section on Analysis of Ca2+ puffs) and coordinates for EGFP-IP3R puncta derived from Fiji.
For colocalization analyses of actin (which are not amenable to object-based methods) with IP3R or KRAP, actin images were first Gaussian-filtered (σ = 0.5) to remove uneven background fluorescence. We then used the Fiji JACoP plugin70 to calculate the Mander’s split coefficient, which reports the fraction of IP3Rs or KRAP colocalized with actin (Fig. 1d, Supplementary Fig. 5b).
For confocal sections away from the PM, object-based analyses were impracticable because the increased out-of-focus fluorescence prevented reliable identification of local fluorescence maxima with DiAna. To quantify colocalization of IP3R and KRAP puncta in confocal sections, deconvolved images were Gaussian-filtered (σ = 0.5) to remove noise. For confocal sections remote from the coverslip, a peripheral region of interest (ROI) (i.e. close to the PM) was defined by an annulus extending 15 pixels (i.e. 2.4 μm) inward from the cell boundary, while the remaining core formed the central ROI (Fig. 1f). For the confocal section closest to the coverslip (i.e. near-PM; similar to the TIRF field), the ROI was defined by the entire region enclosed by the cell boundary. Colocalization between IP3R and KRAP puncta was measured (Mander’s split coefficient for IP3R) using the JACoP plugin70 for each ROI (Fig. 1f).
Since methods for quantifying colocalization of more than two fluorophores by object-based colocalization methods are not well developed, we measured the colocalization of IP3R, KRAP and actin using a mask to define regions of the cell containing actin filaments, and compared them with regions without actin (Fig. 1k). For each cell, an area of 576–768 µm2 (~30% of the cell area) was used for analysis. Similar methods were used for analyses of vimentin filaments. Deconvolved confocal images were Gaussian-filtered for actin staining (σ = 0.5). Centre-to-centre distances between IP3R and KRAP puncta were then measured using the DiAna plugin44 for ROI within and outside the actin mask. We considered IP3R and KRAP puncta to be colocalized if their centre-to-centre distance was <160 nm (< 130 nm for super-resolution confocal microscopy).
Analysis of Ca2+ signals in single cells
For analysis of Ca2+ puffs, EGFP-IP3R1 HeLa cells were grown on fibronectin-coated Cellvis dishes and transfected with siRNA (see the section on Treatment with siRNA) or plasmid encoding KRAP (see the section on Cell culture and transfection). Before use, cells were washed twice with HBS and incubated (1 h) in HBS containing Cal-590-AM (2 μM, 37 °C) or Calbryte 590-AM (2 μM, 20 °C) and ci-IP3/PM (1 μM), washed twice with HBS, and then incubated (30 min, 20 °C) in HBS containing EGTA-AM (5 μM). EGTA is a slow Ca2+-buffer that limits regenerative propagation of global Ca2+ signals without perturbing Ca2+ puffs71. After two further washes, cells were incubated in HBS (30 min 20 °C), washed and imaged in HBS at 20 °C. Fluorescence was recorded for 32.5 s by TIRFM (561-nm excitation, 630/75 nm emission, 20 frames per s) with illumination for 25 ms in every 50-ms capture interval to minimize photobleaching. A flash of ultraviolet (UV) light (150–600 ms, 395/20 nm, SPECTRA X-light engine, Lumencor, Beaverton, OR, USA) was delivered after 2.5 s to photolyse ci-IP3. Images were collected using MetaMorph, corrected for background fluorescence, and Ca2+ puffs were detected and analysed using the FLIKA algorithm (version 1)72. Similar methods (using Cal-590, but without ci-IP3/PM loading) were used for analyses of the effects of histamine on Ca2+ puffs and global increases in [Ca2+]c (Fig. 3a, Supplementary Fig. 6a). Similar methods, but without EGTA-loading, were used for analyses of global Ca2+ signals evoked by histamine (Cal-590) or photolysis of ci-IP3 (Calbryte 590) (Fig. 3l–n); the responses are reported as ΔF/Fmax, where ΔF is the peak increase in fluorescence and Fmax is fluorescence from the Ca2+-saturated indicator.
Measurements of [Ca2+]c in cell populations
EGFP-IP3R1 HeLa cells were grown in clear-bottomed 96-well plates (Greiner Bio-One, Stonehouse, Gloucester, UK) coated with fibronectin (10 μg ml−1) (see the section on Cell culture and transfection). Cells were then washed and loaded with Fluo-8 by incubation in HBS with Fluo-8 AM (2 μM, 60 min, 20 °C), washed and incubated in HBS (60 min, 20 °C) before experiments. A FlexStation 3 plate-reader (Molecular Devices) was used to measure Fluo-8 fluorescence at 1.4-s intervals at 20 °C (excitation 490 nm, emission at 525 nm). Responses to histamine were determined in HBS, and responses to ionomycin were determined after the addition of BAPTA in Ca2+-free HBS to chelate extracellular Ca2+ (final BAPTA concentration = 2.5 mM, free [Ca2+] < 40 nM). Fluorescence (F) was recorded (SoftMax Pro, version 5.4, Molecular Devices) and calibrated to [Ca2+]c from [Ca2+]c = KD(F − Fmin)/(Fmax − F), where the KD of Fluo-8 for Ca2+ is 389 nM, and Fmin and Fmax are the minimal and maximal fluorescence signals determined after addition of Triton X-100 (1%) with either BAPTA (2.5 mM) for Fmin or CaCl2 (10 mM) for Fmax.
Quantification of immobile IP3Rs
For convenience, we often show immobile IP3R puncta by overlaying pseudocoloured images collected at intervals of 30 s, such that the two colours (green and magenta) overlay for immobile IP3Rs (white; e.g., Figs. 2g, h, 4a)20. But for all quantitative analyses, we used single-particle tracking (Fiji TrackMate plugin, version 3.8, https://git.io/v6uz2)73 and trajectory classification (TraJCassifier, version 0.83)74 to identify immobile IP3R puncta. We confirmed the congruence of the two approaches previously20. TrackMate requires selection of unique thresholds for detection of puncta in each image73, but we confirmed the validity of the thresholds by both manual inspection of images and by demonstrating that the fraction of whole-cell fluorescence attributed to puncta was consistent. For example, in TIRF analyses of cells treated with NS or KRAP siRNA (Fig. 2g, h), the total fluorescence attributed to IP3R puncta relative to whole-cell fluorescence was 56 ± 2% and 53 ± 1%, respectively (mean ± s.e.m., n = 22 cells, P > 0.05, Student’s t test). Using TrackMate, the sub-pixel localization of each IP3R punctum in time-lapse TIRF images (usually 10 frames per s for 30 s) was obtained before linking the particles to generate track segments, followed by gap-closing to link the track segments. A displacement threshold of 1.5 μm s−1 was employed to track and link particles since the maximal velocity of the particles did not exceed this limit. TraJClassifier, a validated plugin for trajectory classification74, was used to classify single-particle trajectories from TrackMate. This algorithm uses a machine-learning approach to classify single-particle trajectories into diffusive, sub-diffusive, confined and directed trajectories. For inclusion in the classification analysis, the minimum trajectory length was 45 frames. This algorithm does not specifically identify immobile particles, which fall within the category of sub-diffusive particles. From each sub-diffusive trajectory, we used the exponent (α) from the plot of mean squared displacement (MSD, γ2) versus time (t) for anomalous sub-diffusion (γ2 = 4Dtα, where D is the diffusion coefficient)74,75 and classified particles with α < 0.1 as immobile. The value (α < 0.1) was chosen from the analysis of simulated trajectories performed by Thorsten Wagner (University of Dortmund).
Statistics
Most results are presented as mean ± s.d. or s.e.m. from n independent analyses. Statistical comparisons used paired (where indicated) or unpaired, two-tailed Student’s t tests, or analysis of variance (ANOVA) with the Bonferroni’s correction for multiple comparisons (PRISM version 8, GraphPad, San Diego, CA, USA). Significance levels are shown as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Variances of the product of two variables (for Supplementary Fig. 4k) were computed according to ref. 76 (details in Supplementary Table 1). Methods used to assess the statistical significance of colocalization analyses are described in the appropriate methods section. Further statistical details are provided in figure legends and in the summary of all statistical analyses (Supplementary Table 1).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of additional supplementary files
Acknowledgements
The authors thank Martyn Reynolds and Stephen Tovey (Cairn, UK) for help with super-resolution confocal microscopy. Supported by a Wellcome Senior Investigator Award (grant no. 101844), and by a grant (grant no. BB/T012986/1) and studentship (to H.A.S) from the Biotechnology and Biological Sciences Research Council UK. P.A.-A. is a research fellow of Emmanuel College, Cambridge.
Source data
Author contributions
N.B.T. performed most experiments. H.A.S. performed the super-resolution confocal microscopy analyses. P.A.-A. completed analyses of Ca2+ signals in cell populations after KRAP knockdown. C.W.T. and N.B.T. conceived the project. C.W.T., N.B.T. P.A.-A. and H.A.S. analysed and interpreted results, and wrote the manuscript.
Data availability
All data required to support the conclusions of this paper are provided in Figs. 1–5, Supplementary Figs 1–7, Supplementary Table 1, Supplementary Movies 1–10 and the Source Data File. All software and algorithms used are freely available from sources listed in Methods. Materials and primary data are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nagendra Babu Thillaiappan, Email: nagendrababu@qu.edu.qa.
Colin W. Taylor, Email: cwt1000@cam.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24739-9.
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi AM, Taylor CW. IP3 receptors—lessons from analyses ex cellula. J. Cell Sci. 2018;132:jcs222463. doi: 10.1242/jcs.222463. [DOI] [PubMed] [Google Scholar]
- 4.Bartok A, et al. IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nat. Commun. 2019;10:3726. doi: 10.1038/s41467-019-11646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atakpa P, Thillaiappan NB, Mataragka S, Prole DL, Taylor CW. IP3 receptors preferentially associate with ER-lysosome contact sites and selectively deliver Ca2+ to lysosomes. Cell Rep. 2018;25:3180–3193. doi: 10.1016/j.celrep.2018.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan PG. The STIM1-ORAI1 microdomain. Cell Calcium. 2015;58:357–367. doi: 10.1016/j.ceca.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis RS. Store-operated calcium channels: from function to structure and back again. Cold Spring Harb. Persp. Biol. 2019;10:a035055. doi: 10.1101/cshperspect.a035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzayady KJ, et al. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci. Signal. 2016;9:ra35. doi: 10.1126/scisignal.aad6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith IF, Parker I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl Acad. Sci. USA. 2009;106:6404–6409. doi: 10.1073/pnas.0810799106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mataragka S, Taylor CW. All three IP3 receptor subtypes generate Ca2+ puffs, the universal building blocks of IP3-evoked Ca2+ signals. J. Cell Sci. 2018;131:jcs220848. doi: 10.1242/jcs.220848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lock JT, Alzayady KJ, Yule DI, Parker I. All three IP3 receptor isoforms generate Ca2+ puffs that display similar characteristics. Sci. Signal. 2018;11:eaau0344. doi: 10.1126/scisignal.aau0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lock JT, Parker I. IP3-mediated global Ca2+ signals arise through two temporally and spatially distinct modes of Ca2+ release. eLife. 2020;9:e55008. doi: 10.7554/eLife.55008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreri-Jacobia M, Mak D-OD, Foskett JK. Translational mobility of the type 3 inositol 1,4,5-trisphosphate receptor Ca2+ release channel in endoplasmic reticulum membrane. J. Biol. Chem. 2005;280:3824–3831. doi: 10.1074/jbc.M409462200. [DOI] [PubMed] [Google Scholar]
- 14.Pantazaka E, Taylor CW. Differential distribution, clustering and lateral diffusion of subtypes of inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2011;286:23378–23387. doi: 10.1074/jbc.M111.236372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith IF, Swaminathan D, Dickinson GD, Parker I. Single-molecule tracking of inositol trisphosphate receptors reveals different motilities and distributions. Biophys. J. 2014;107:834–845. doi: 10.1016/j.bpj.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keebler MV, Taylor CW. Endogenous signalling pathways and caged-IP3 evoke Ca2+ puffs at the same abundant immobile intracellular sites. J. Cell Sci. 2017;130:3728–3739. doi: 10.1242/jcs.208520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lock JT, Smith IF, Parker I. Comparison of Ca2+ puffs evoked by extracellular agonists and photoreleased IP3. Cell Calcium. 2017;63:43–47. doi: 10.1016/j.ceca.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lock JT, Smith IF, Parker I. Spatial-temporal patterning of Ca2+ signals by the subcellular distribution of IP3 and IP3 receptors. Sem. Cell Dev. Biol. 2019;94:3–10. doi: 10.1016/j.semcdb.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith IF, Wiltgen SM, Shuai J, Parker I. Ca2+ puffs originate from preestablished stable clusters of inositol trisphosphate receptors. Sci. Signal. 2009;2:ra77. doi: 10.1126/scisignal.2000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thillaiappan NB, Chavda AP, Tovey SC, Prole DL, Taylor CW. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat. Commun. 2017;8:1505. doi: 10.1038/s41467-017-01644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellefsen KL, Parker I. Dynamic Ca2+ imaging with a simplified lattice light-sheet microscope: a sideways view of subcellular Ca2+ puffs. Cell Calcium. 2018;71:34–44. doi: 10.1016/j.ceca.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itzhak DN, Tyanova S, Cox J, Borner GH. Global, quantitative and dynamic mapping of protein subcellular localization. eLife. 2016;5:e16950. doi: 10.7554/eLife.16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chugh P, Paluch EK. The actin cortex at a glance. J. Cell Sci. 2018;131:jcs186254. doi: 10.1242/jcs.186254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukatsu K, et al. Lateral diffusion of inositol 1,4,5-trisphosphate receptor type 1 is regulated by actin filaments and 4.1N in neuronal dendrites. J. Biol. Chem. 2004;279:48976–48982. doi: 10.1074/jbc.M408364200. [DOI] [PubMed] [Google Scholar]
- 25.Fukatsu K, Bannai H, Inoue T, Mikoshiba K. Lateral diffusion of inositol 1,4,5-trisphosphate receptor type 1 in Purkinje cells is regulated by calcium and actin filaments. J. Neurochem. 2010;114:1720–1733. doi: 10.1111/j.1471-4159.2010.06885.x. [DOI] [PubMed] [Google Scholar]
- 26.Turvey MR, Fogarty KE, Thorn P. Inositol (1,4,5)-trisphosphate receptor links to filamentous actin are important for generating local Ca2+ signals in pancreatic acinar cells. J. Cell Sci. 2005;118:971–980. doi: 10.1242/jcs.01693. [DOI] [PubMed] [Google Scholar]
- 27.Hein MY, et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell. 2015;163:712–723. doi: 10.1016/j.cell.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 28.Fujimoto T, Miyawaki A, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae is linked to actin filaments. J. Cell Sci. 1995;108:7–15. doi: 10.1242/jcs.108.1.7. [DOI] [PubMed] [Google Scholar]
- 29.Dingli F, Parys JB, Loew D, Saule S, Mery L. Vimentin and the K-Ras-induced actin-binding protein control inositol-(1,4,5)-trisphosphate receptor redistribution during MDCK cell differentiation. J. Cell Sci. 2012;125:5428–5240. doi: 10.1242/jcs.108738. [DOI] [PubMed] [Google Scholar]
- 30.Bargagna-Mohan P, et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 2007;14:623–634. doi: 10.1016/j.chembiol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. Intermediate filaments play a pivotal role in regulating cell architecture and function. J. Biol. Chem. 2015;290:17145–17153. doi: 10.1074/jbc.R115.640359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiu Y, et al. Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J. Cell Sci. 2017;130:892–902. doi: 10.1242/jcs.196881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inokuchi J, et al. Deregulated expression of KRAP, a novel gene encoding actin-interacting protein, in human colon cancer cells. J. Hum. Genet. 2004;49:46–52. doi: 10.1007/s10038-003-0106-3. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto T, et al. Altered energy homeostasis and resistance to diet-induced obesity in KRAP-deficient mice. PLoS ONE. 2009;4:e4240. doi: 10.1371/journal.pone.0004240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto T, et al. KRAS-induced actin-interacting protein is required for the proper localization of inositol 1,4,5-trisphosphate receptor in the epithelial cells. Biochem. Biophys. Res. Commun. 2011;407:438–443. doi: 10.1016/j.bbrc.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto T, Shirasawa S. Identification of KRAP-expressing cells and the functional relevance of KRAP to the subcellular localization of IP3R in the stomach and kidney. Int. J. Mol. Med. 2012;30:1287–1293. doi: 10.3892/ijmm.2012.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimoto T, et al. Determination of the critical region of KRAS-induced actin-interacting protein for the interaction with inositol 1,4,5-trisphosphate receptor. Biochem. Biophys. Res. Commun. 2011;408:282–286. doi: 10.1016/j.bbrc.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Fujimoto T, Shirasawa S. KRAS-induced actin-interacting protein: a potent target for obesity, diabetes and cancer. Anticancer Res. 2011;31:2413–2417. [PubMed] [Google Scholar]
- 39.Fujimoto T, et al. KRAS-induced actin-interacting protein regulates inositol 1,4,5-trisphosphate-receptor-mediated calcium release. Biochem. Biophys. Res. Commun. 2011;408:214–217. doi: 10.1016/j.bbrc.2011.03.112. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki H, Fujimoto T, Tanaka M, Shirasawa S. Tespa1 is a novel component of mitochondria-associated endoplasmic reticulum membranes and affects mitochondrial calcium flux. Biochem. Biophys. Res. Commun. 2013;433:322–326. doi: 10.1016/j.bbrc.2013.02.099. [DOI] [PubMed] [Google Scholar]
- 41.Javed AA, Naz RK. Human cleavage signal-1 protein; cDNA cloning, transcription and immunological analysis. Gene. 1992;112:205–211. doi: 10.1016/0378-1119(92)90377-2. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, et al. Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling. Nat. Immunol. 2012;13:560–568. doi: 10.1038/ni.2301. [DOI] [PubMed] [Google Scholar]
- 43.Liang J, et al. Tespa1 regulates T cell receptor-induced calcium signals by recruiting inositol 1,4,5-trisphosphate receptors. Nat. Commun. 2017;8:15732. doi: 10.1038/ncomms15732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilles JF, Dos Santos M, Boudier T, Bolte S, Heck N. DiAna, an ImageJ tool for object-based 3D co-localization and distance analysis. Methods. 2017;115:55–64. doi: 10.1016/j.ymeth.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh TS, Chen YJ, Chang CL, Lee WR, Liou J. Cortical actin contributes to spatial organization of ER-PM junctions. Mol. Biol. Cell. 2017;28:3171–3180. doi: 10.1091/mbc.e17-06-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Y, Thillaiappan NB, Taylor CW. The store-operated Ca2+ entry complex comprises a small cluster of STIM1 associated with one Orai1 channel. Proc. Natl Acad. Sci. USA. 2021;118:e2010789118. doi: 10.1073/pnas.2010789118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thurley K, et al. Reliable encoding of stimulus intensities within random sequences of intracellular Ca2+ spikes. Sci. Signal. 2014;7:ra59. doi: 10.1126/scisignal.2005237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura H, et al. Cooperative and stochastic calcium releases from multiple calcium puff sites generate calcium microdomains in intact Hela cells. J. Biol. Chem. 2012;287:24563–24572. doi: 10.1074/jbc.M111.311399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demuro A, Parker I. Multi-dimensional resolution of elementary Ca2+ signals by simultaneous multi-focal imaging. Cell Calcium. 2008;43:367–374. doi: 10.1016/j.ceca.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiltgen SM, Smith IF, Parker I. Superresolution localization of single functional IP3R channels utilizing Ca2+ flux as a readout. Biophys. J. 2010;99:437–446. doi: 10.1016/j.bpj.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferris CD, Huganir RL, Supattapone S, Snyder SH. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989;342:87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- 52.Mak DO, Vais H, Cheung KH, Foskett JK. Patch-clamp electrophysiology of intracellular Ca2+ channels. Cold Spring Harb. Protoc. 2013;2013:787–797. doi: 10.1101/pdb.top066217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiefer H, et al. Inositol 1,4,5-trisphosphate receptor-binding protein released with inositol 1,4,5-trisphosphate (IRBIT) associates with components of the mRNA 3’ processing machinery in a phosphorylation-dependent manner and inhibits polyadenylation. J. Biol. Chem. 2009;284:10694–10705. doi: 10.1074/jbc.M807136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rong YP, et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc. Natl Acad. Sci. USA. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vais H, et al. ER-luminal [Ca2+] regulation of InsP3 receptor gating mediated by an ER-luminal peripheral Ca2+-binding protein. eLife. 2020;9:e53531. doi: 10.7554/eLife.53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prole DL, Taylor CW. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J. Physiol. 2016;594:2849–2866. doi: 10.1113/JP271139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujimoto T, et al. Analysis of KRAP expression and localization, and genes regulated by KRAP in a human colon cancer cell line. J. Hum. Genet. 2007;52:978–984. doi: 10.1007/s10038-007-0204-8. [DOI] [PubMed] [Google Scholar]
- 58.Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993;74:669–677. doi: 10.1016/0092-8674(93)90514-Q. [DOI] [PubMed] [Google Scholar]
- 59.Taylor CW, Machaca K. IP3 receptors and store-operated Ca2+ entry: a license to fill. Curr. Opin. Cell Biol. 2019;57:1–7. doi: 10.1016/j.ceb.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Bird GS, et al. STIM1 is a calcium sensor specialized for digital signaling. Curr. Biol. 2009;19:1–6. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cullen PJ. Decoding complex Ca2+ signals through the modulation of Ras signaling. Curr. Opin. Cell Biol. 2006;18:157–161. doi: 10.1016/j.ceb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Janmey PA, Bucki R, Radhakrishnan R. Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms. Biochem. Biophys. Res. Commun. 2018;506:307–314. doi: 10.1016/j.bbrc.2018.07.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smyth JW, et al. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ. Res. 2012;110:978–989. doi: 10.1161/CIRCRESAHA.111.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padilla-Rodriguez M, et al. The actin cytoskeletal architecture of estrogen receptor positive breast cancer cells suppresses invasion. Nat. Commun. 2018;9:2980. doi: 10.1038/s41467-018-05367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nerou EP, Riley AM, Potter BVL, Taylor CW. Selective recognition of inositol phosphates by subtypes of inositol trisphosphate receptor. Biochem. J. 2001;355:59–69. doi: 10.1042/bj3550059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu F, et al. Remodeling of ER–plasma membrane contact sites but not STIM1 phosphorylation inhibits Ca2+ influx in mitosis. Proc. Natl Acad. Sci. USA. 2019;116:10392–10401. doi: 10.1073/pnas.1821399116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.York AG, et al. Resolution doubling in live, multicellular organisms via multifocal structured illumination microscopy. Nat. Methods. 2012;9:749–754. doi: 10.1038/nmeth.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruce MA, Butte MJ. Real-time GPU-based 3D deconvolution. Opt. Express. 2013;21:4766–4773. doi: 10.1364/OE.21.004766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 71.Parker I, Smith IF. Recording single-channel activity of inositol trisphosphate receptors in intact cells with a microscope, not a patch clamp. J. Gen. Physiol. 2010;136:119–127. doi: 10.1085/jgp.200910390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ellefsen KL, Settle B, Parker I, Smith IF. An algorithm for automated detection, localization and measurement of local calcium signals from camera-based imaging. Cell Calcium. 2014;56:147–156. doi: 10.1016/j.ceca.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaqaman K, et al. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner T, Kroll A, Haramagatti CR, Lipinski HG, Wiemann M. Classification and segmentation of nanoparticle diffusion trajectories in cellular micro environments. PLoS ONE. 2017;12:e0170165. doi: 10.1371/journal.pone.0170165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saxton MJ, Jacobson K. Single-particle tracking: applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 1997;26:373–399. doi: 10.1146/annurev.biophys.26.1.373. [DOI] [PubMed] [Google Scholar]
- 76.Colquhoun D. Lectures in Biostatistics. Clarendon Press; 1971. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of additional supplementary files
Data Availability Statement
All data required to support the conclusions of this paper are provided in Figs. 1–5, Supplementary Figs 1–7, Supplementary Table 1, Supplementary Movies 1–10 and the Source Data File. All software and algorithms used are freely available from sources listed in Methods. Materials and primary data are available from the corresponding authors upon reasonable request. Source data are provided with this paper.