Abstract
Optimal condition was determined to prepare horse oil-in-water (O/W) emulsion stabilized by different HLB system. Span 60 and Tween 60 were used to achieve the predetermined HLB values ranging from 10 to 14 and the surfactant concentrations were adjusted to 10–20%. Fifteen formulated O/W emulsions were characterized by mean particle diameter, zeta-potential (ZP), polydispersity index, and encapsulation efficiency (EE, %). Mean particle diameter decreased with increasing HLB value and surfactant concentration. Particles of the emulsion with HLB 12 and surfactant concentration at 15% were distributed in the size of below 500 nm. The particle diameter and EE (%) of the emulsion with HLB 11 or 12 and surfactant concentration at 15 or 20% were not significantly changed during storage at 40 °C for 15 days. These results suggest the characteristics of horse oil O/W emulsion are dependent on HLB values and surfactant concentration so that affect to emulsion properties during storage.
Keywords: Horse oil, Oil-in-water emulsion, Emulsion formulation, Emulsion stability, HLB system
Introduction
Horse oil extracted from horse fatty meats has been widely used to cure skin as a folk medicine in Asia such as Korea, Mongolia, Japan, and China (Jang et al., 2014). Along with this benefit, horse oil has been reported to show anti-inflammation, anti-bactericidal, and skin moisturizing effects (Choi et al., 2014; Kim et al., 2020). As these benefits of horse oil are known, cosmetic products containing horse oil have become popular (Kim et al., 2016; Lee and Park, 2013). However, horse oil has some limitation to utilize because it can be liquid or solid depending on the ambient temperature (Park et al., 2019). This makes consumers uncomfortable to use horse oil as skin care products. To overcome this limitation, the O/W emulsion made of horse oil can be a promising candidate.
Emulsion has been applied for various cosmetic products, such as body and face wash, essence, cream, and lotion depending on their viscosity or appearances (An et al., 2004; Lim, 2004). The O/W emulsion is the type of emulsion that oil phase is dispersed in aqueous phase. This emulsion contains particles with a mean particle diameter of more than 1 μm and is thermodynamically unstable (Mun et al., 2006). On the other hand, nano-sized emulsion is the oil-in-water colloidal dispersion, usually in 20–500 nm particle size distributed in the system with good physical stability (De Oca-Ávalos et al., 2017; Porras et al., 2004). Generally, nano-sized emulsion is prepared by high energy emulsification method using a high-speed and high-pressure homogenizer to produce nano-scale particles (Mulia et al., 2018; Wang et al., 2018). The stable nano-emulsions are formed when oil phase, aqueous phase, and hydrophilic-lipophilic balance (HLB) and concentration of surfactant are well matched in the right sequences (Llinares et al., 2018). Additionally, nano-emulsions possibly enhance therapeutic efficacy by maximizing the penetration of useful component (Jaiswal et al., 2015; Kale and Deore, 2017).
The O/W emulsion with less than 100 nm of particle sizes was formed by formulation of nonionic surfactant mixtures, hydrophobic (Span 20, 40, 60, and 80) and hydrophilic (Tween 80) surfactants (Cho et al., 2008). According to Griffin (1949), a stable emulsion can be formulated with surfactants or blends of surfactants, which were compatible with the required HLB value of the oil phase used. The use of surfactant mixtures increases the repulsive interactions of particles in the emulsion and furthermore improves their flocculation stability (McClements and Jafari, 2018). The required HLB value is an essential parameter related to the emulsion formulation because it allows the selection of suitable surfactants or even a surfactant mixture required to produce a stable emulsified system (Egito et al., 2018). The HLB method is useful as a rough guide for surfactant selection and the calculated HLB values can be used to determine hydrophilic or lipophilic of the emulsifiers in O/W or W/O emulsion. Non-ionic surfactants are generally recognized as being safe and biocompatible (Sanjeewani and Sakeena, 2013). Currently, there are not many studies or data on formulating O/W emulsion with horse oil (Choi et al., 2014; Cho and Kim, 2020). The objectives of this study were to prepare O/W emulsions made of horse oil using non-ionic surfactants by adjusting HLB system and to determine the characteristics and emulsion stability of the prepared O/W emulsions during storage.
Materials and methods
Materials and chemicals
Horse fatty meats were purchased from Sansaemi Agricultural Co. (Jeju, Korea). Sorbitan monostearate (Span 60) and polyethylene glycol sorbitan monostearate (Tween 60) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals used in the current study were analytical grade.
Preparation of O/W emulsion with horse oil
Prior to the preparation of O/W emulsion, horse oil was extracted from horse fatty meats by the method of Cho and Kim (2020). Acid value of the final refined horse oil was below 0.2 mg KOH/g and horse oil was stored in a freezer until use.
The O/W emulsions made of horse oil were prepared based on the HLB system, which binary nonionic surfactants with low and high HLB values were combined. As binary surfactants, Span 60 (HLB = 4.7) and Tween 60 (HLB = 14.9) were used to achieve the predetermined HLB values of 10, 11, 12, 13, and 14 using the following equation (Griffin, 1949).
X = the target HLB value of 10, 11, 12, 13, and 14.
The surfactant blends were directly added to horse oil at the concentration of 10, 15, or 20%, which were determined by preliminary test to have a stable form of emulsion. The mixture of horse oil (30%, w/w) and surfactants was pre-heated at 60 °C and homogenized at high speed using a homogenizer (T25D, Ika, Staufen, Germany) at 10,000 rpm for 1 min. Ultrapure water (50, 55, or 60%) at 60 °C was poured to the homogenized mixture for 5 min and the final mixture was stirred at room temperature for 20 min. Fifteen formulations of the O/W emulsion (E1 to E15) were prepared and the composition of them are shown in Table 1. After emulsifying, the formulated emulsions were poured into 60 mL-wide neck glass bottles and then stored at 40 °C in the dark for 30 days. After 15 days of storage, the formulated emulsions were not stable so that the data obtained during the storage of 0, 5, 10, and 15 days were reported in the results.
Table 1.
The composition of O/W emulsions composed of 30% horse oil (w/w) with various HLB values and surfactant concentrations
| O/W emulsion of horse oil | HLB value | Surfactant (%, w/w) | Distilled water (%, w/w) |
|---|---|---|---|
| E1 | 10 | 10 | 60 |
| E2 | 15 | 55 | |
| E3 | 20 | 50 | |
| E4 | 11 | 10 | 60 |
| E5 | 15 | 55 | |
| E6 | 20 | 50 | |
| E7 | 12 | 10 | 60 |
| E8 | 15 | 55 | |
| E9 | 20 | 50 | |
| E10 | 13 | 10 | 60 |
| E11 | 15 | 55 | |
| E12 | 20 | 50 | |
| E13 | 14 | 10 | 60 |
| E14 | 15 | 55 | |
| E15 | 20 | 50 |
Characterization of horse oil O/W emulsion
The mean particle diameter, ZP, polydispersity index (PDI), and particle size distribution of the O/W emulsions were determined using a light scattering analyzer (Delsamax pro, Beckman Coulter, Brea, CA, USA). For the measurement, the O/W emulsions were diluted to 500-fold with distilled water to prevent multiple scattering. The ZP was indicated as absolute value in the results. The pH values were measured at 25 °C by a pH meter (SevenExcellence, Mettler-Toledo, Schwerzenbach, Switzerland).
The EE (%) was measured by the method of Davidov-Pardo and McClements (2015) as following. The O/W emulsions were diluted in hexane (1:3), vortexed for 2 min, and centrifuged at 2,700 × g for 15 min (1248R, Labogene Co., Daejeon, Korea). The supernatant of the solution was collected and the absorbance at 272 nm was measured using a spectrophotometer (OPTIZEN 2120UV, Mecasys, Daejeon, Korea). The absorbance of the horse oil concentrations ranging from 0 to 10% was obtained at 272 nm for the calibration curve. The EE (%) of the O/W emulsion was calculated by the following equation:
where A: amount of horse oil initially added to the emulsion and B: amount of horse oil escaped from the emulsion.
Statistical analysis
All experiments in this study were performed in triplicate. The results were given as means ± standard deviation. Statistical comparisons were performed by analysis of variance (ANOVA) followed by Duncan’s multiple range test using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Significant difference were considered at p < 0.05.
Results and discussion
Characteristics of O/W emulsion prepared with horse oil
Fifteen O/W emulsions were formulated with 30% horse oil depending on various HLB values and surfactant concentrations (Table 1). These formulations were prepared based on the assumption that the particles of the O/W emulsions were nano-sized and the emulsions were stable at the optimum HLB value and surfactant concentration of nonionic surfactant blends.
The characteristics of fifteen O/W emulsions prepared with different HLB values and surfactant concentrations are shown in Table 2. Briefly, the mean particle diameter, ZP, PDI, and pH values of fifteen formulations were different depending on the HLB values and surfactant concentrations. The mean particle diameters of O/W emulsions were in the range of 220.28 to 1033.28 nm and the E1, E2, E3, E4, E7, and E10 formulations were not nano-sized emulsions (diameter < 500 nm). As the surfactant concentration increased from 10 to 15 and 20%, the mean particle diameters were decreased (Table 2). Ramisetty et al. (2015) reported that the decrease in particle size of coconut oil in water emulsion was observed when the surfactant concentration increased. Small-sized particles in the emulsion improved the emulsion stability and reduced the sedimentation or creaming, a gravitational separation (Pathak, 2017). The large particle sizes of the O/W emulsions formulated with 10% surfactants might be attributed to insufficient surfactant amounts to cover all interfaces of oil and water. Additionally, the particle sizes lowered with increasing HLB values ranging from 10 to 12 (Table 2). These were similar to the results of O/W emulsions made with lippia and peppermint oil as reported by Orafidiya and Oladimeiji (2002), which results showed that increasing the hydrophilicity of the surfactant blends led to the decrease of the particle size.
Table 2.
Characteristics of O/W emulsion formulated with various HLB values and surfactant concentrations
| O/W emulsion of horse oil | Mean particle diameter (nm) | Zeta-potential (mV) | Polydispersity index | pH |
|---|---|---|---|---|
| E1 | 1033.28 ± 41.27a | 19.96 ± 2.85c | 0.19 ± 0.04c | 7.63 ± 0.01c |
| E2 | 886.15 ± 46.67b | 16.35 ± 2.84b | 0.16 ± 0.06c | 7.66 ± 0.02b |
| E3 | 676.02 ± 44.74c | 21.50 ± 3.54c | 0.20 ± 0.08c | 7.74 ± 0.00a |
| E4 | 882.93 ± 58.54b | 26.60 ± 1.00e | 0.14 ± 0.10c | 7.45 ± 0.02gh |
| E5 | 386.83 ± 4.70ef | 32.30 ± 0.46g | 0.23 ± 0.01c | 7.49 ± 0.01e |
| E6 | 353.52 ± 4.49fg | 32.03 ± 0.92g | 0.48 ± 0.14ab | 7.56 ± 0.01d |
| E7 | 577.65 ± 11.73d | 24.05 ± 1.18d | 0.44 ± 0.00b | 7.41 ± 0.00i |
| E8 | 255.93 ± 4.06h | 29.38 ± 0.24f | 0.21 ± 0.00c | 7.47 ± 0.02ef |
| E9 | 240.52 ± 3.29h | 29.76 ± 0.42f | 0.20 ± 0.02c | 7.46 ± 0.01fg |
| E10 | 610.65 ± 28.38d | 8.94 ± 1.35a | 0.56 ± 0.01a | 7.21 ± 0.02m |
| E11 | 334.62 ± 9.27g | 21.13 ± 0.50c | 0.52 ± 0.12ab | 7.34 ± 0.01j |
| E12 | 222.17 ± 4.74h | 23.82 ± 0.68d | 0.20 ± 0.02c | 7.43 ± 0.02hi |
| E13 | 396.77 ± 11.79ef | 19.59 ± 0.83c | 0.57 ± 0.00a | 7.24 ± 0.02l |
| E14 | 246.70 ± 4.89h | 21.37 ± 0.32c | 0.57 ± 0.00a | 7.26 ± 0.01l |
| E15 | 220.28 ± 3.72h | 20.72 ± 0.51c | 0.55 ± 0.05a | 7.28 ± 0.01k |
Each value is expressed as mean ± standard deviation of triplicate determination. Values with different letters in a column are significantly different by Duncan’s multiple range test (p < 0.05). The O/W emulsions were made with 30% horse oil, 10–20% surfactant, and 10–14 HLB values. The ZP value was shown in absolute value
The particles of the O/W emulsions with horse oil were found to be negatively charged thus the ZP values were present in absolute values (Table 2). The ZP values of E5 and E6 formulations were the highest, 32.03 and 32.30 mV, and those of E8 and E9 formulations were followed as 29.38 and 29.76 mV, respectively. When compared to 15 and 20% surfactant concentrations within the emulsions with the same HLB value, there were no significant differences in the ZP values except E2 and E3 or E11 and E12 formulations. The ZP values of the O/W emulsions increased as increasing the HLB values of 10 to 11 or 12, which the ratio of Tween 60 (HLB 14.9) increased. However, the ZP values decreased when the HLB values increased to 13 and 14. The ZP value is a useful parameter to predict the emulsion stability because it reflects the electrostatic interaction of moving particles (Gutierrez et al., 2008). High ZP value indicated the flocculation or coalescence of particles were prevented so that increasing the emulsion stability (McClements, 2005). The formulations of E5, E6, E8, and E9 could be remained with high stability compared to other formulations of the O/W emulsions.
The PDI of all O/W emulsion formulations were shown from 0.16 to 0.57 which varied with HLB values and surfactant concentration (Table 2). Mostly, the PDI above 0.3 indicates a polydispersed system which requires high energy at the emulsification process (Izquierdo et al., 2002). The formulations of E5, E8, and E9 were possibly stable nano-sized emulsions with low PDI. The pH values of all O/W emulsion formulations were in the range of 7.21 to 7.74 and they slightly decreased with increasing HLB values. The study of Macedo et al. (2006) showed the similar trend that pH was changed depending on the HLB values. According to the results of mean particle diameter, ZP, PDI, and pH values, the O/W emulsions with HLB value of 11 or 12 and surfactant concentration of 15 or 20% (E5, E8, and E9 formulations) could be expected as a stable emulsion.
Particle size distribution of O/W emulsion prepared with horse oil
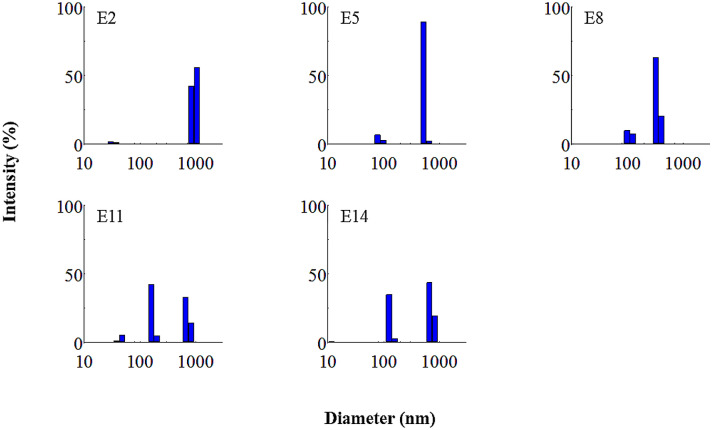
Figure 1 shows the particle size distribution of the O/W emulsions formulated by HLB values ranging from 10 to 14 at 15% of surfactant concentration (E2, E5, E8, E11, and E14 formulation). The average value of main particle diameters of E2, E5, and E8 emulsion was 961, 522, and 343 nm, respectively. The particle diameters of E11 and E14 emulsions were relatively wide ranges from 123 to 712 nm and 163 to 712 nm. As shown in Fig. 1, the distributions of particle size in the E2, E5, and E8 emulsions become narrow as its HLB value increased from 10 to 11 and 12. The mean particle diameters of those emulsions declined from 886 to 256 nm as the HLB value increased from 10 to 12 (Table 2). Nano-emulsions are characterized by particle size and its distribution in the dispersed phase of the emulsion (De Oca-Ávalos et al., 2017). The narrow range and high intensity of the particle size distribution mean high protection from Ostwald ripening of particles in emulsions (Klaus et al., 2012). Because the relatively small particles in O/W emulsion made of horse oil were more hydrophilic than large particles, they were possibly merged to aqueous phase and coagulated with large particles. The particles of this type of emulsion could be increased during storage and finally separated by creaming. Llinares et al. (2018) reported that the particle size of O/W emulsion prepared with rosemary oil tended to decrease as the HLB value increased, which results were similar to those in the current study. As discussed above, the E8 formulation with HLB 12 and surfactant concentration at 15% was nano-sized emulsion showing the particle size below 500 nm and narrow range of particle size distributions.
Fig. 1.
Particle size distribution of O/W emulsions made of horse oil and formulated with various HLB values. The O/W emulsions of E2, E5, E8, E11, and E14 were made with 30% horse oil, 15% surfactant, and HLB values of 10, 11, 12, 13, and 14, respectively
Emulsion stability of O/W emulsion prepared with horse oil during storage
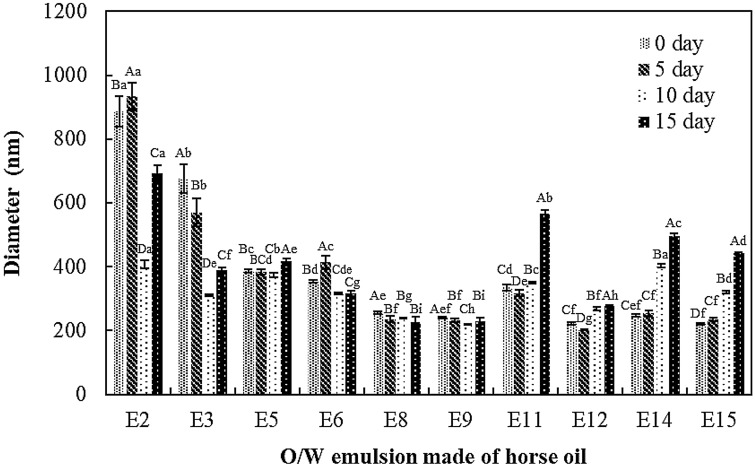
Changes in particle sizes of O/W emulsions formulated with HLB 10 to 14 and surfactant concentration at 15 and 20% during storage at 40 °C for 15 days were shown in Fig. 2. Prior to mention the changes in particle sizes of the emulsion during storage, the temperature at 40 °C was chosen for storage test. When compared the particle sizes of E8 formulation stored at 25 and 40 °C for 30 days, the particle sizes of emulsions stored at both temperature were not significantly changed (data not shown). The E2 and E3 emulsions (HLB 10) had large particle sizes at 0 day and the particles became small after 15 days. Possibly, the sedimentation occurred to the E2 and E3 O/W emulsions and small particles remained in the emulsions after 15 days of storage. The formulations of E5 and E6 kept the particle sizes around 400 nm during storage. Only the mean particle sizes of E8 and E9 formulations were not significantly changed during storage. The particle sizes of E11, E12, E14, and E15 formulations (HLB 13 and 14) were increased after 15 days at 40 °C. These differences were attributed to different HLB values. When the HLB value increased, the O/W emulsion could be destabilized because the dispersed particles were highly hydrophilic. Additionally, the wide particle size distribution of those formulations were observed (data not shown). As the concentration of Tween 60 in surfactants blends increased, hydrophile of dispersed phase increased which possibly shifted to continuous phase and coagulated other particles (Yamashita et al., 2017). Large particles easily grow through the interchange with small particles (Djerdjev and Beattie, 2008; Zhou et al., 2018).
Fig. 2.
Changes in particle sizes of O/W emulsions made of horse oil and formulated with HLB 10 to 14 and surfactant concentration at 15 and 20% during the storage of 0, 5, 10, and 15 days at 40 °C in the dark. Each value is expressed as mean ± standard deviation of triplicate determination. Values with the different lowercase letters (a–i) within the storage day and values with the different uppercase letters (A–D) within the O/W emulsion formulation are significantly different by Duncan’s multiple range test (p < 0.05)
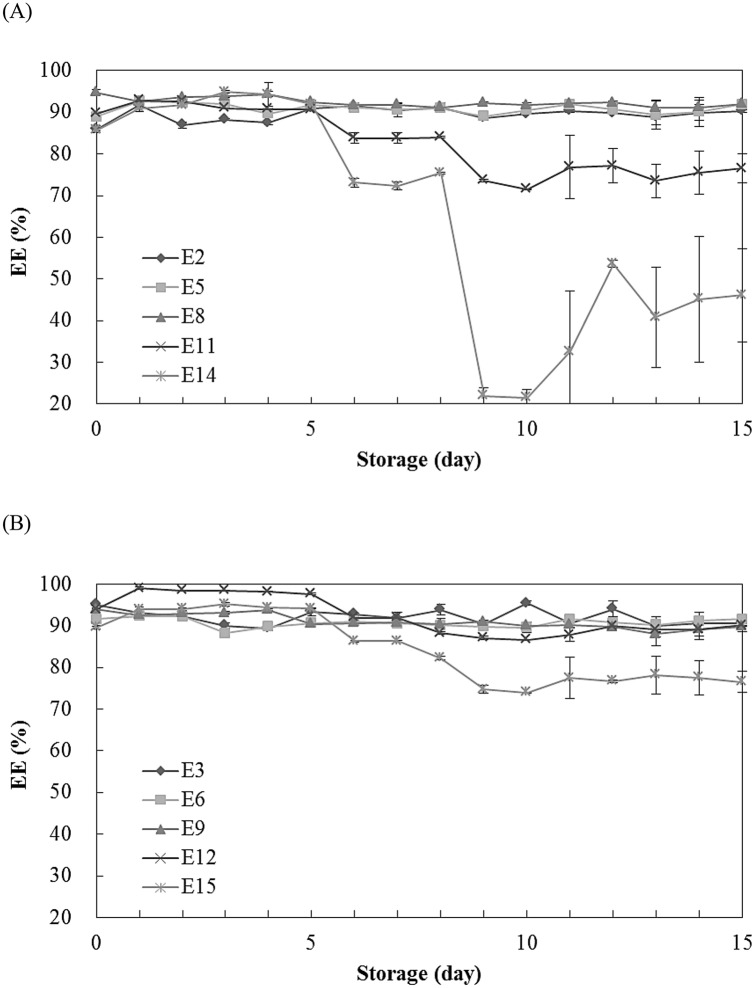
Changes in encapsulation efficiency (EE) of O/W emulsions formulated with HLB 10 to 14 and surfactant concentration at 15 and 20% during storage were shown in Fig. 3(A), (B), respectively. The EE (%) values of the O/W emulsions with 10% surfactant concentration (E1, E4, E7, E10, and E13 formulation) were not shown since the phase separation of the emulsion was observed during storage of 15 days at 40 °C. The EE (%) of E2, E5, E11, and E14 formulation was 85.9, 88.9, 89.7, and 85.6% at 0 day, respectively, and after 1 day they were increased to 91.7, 92.6, 92.8, and 90.9%. However, the EE (%) of E8 formulation was not significantly changed from 94.8 to 92.5% (0 to 1 day). The EE (%) of E2, E5, and E8 formulation were not significantly changed during storage; however, the EE of E11 and E14 formulation decreased. Especially, the EE values of E14 formulation were greatly decreased for 9 and 10 days along with the observation of the great increase of particle diameter (Fig. 2). This result confirmed that the HLB value of 14 and surfactant concentration of 15% were not suitable to formulate O/W emulsion made of horse oil. Figure 3(B) showed the EE (%) of E3, E6, E9, and E12 formulations were not significantly changed during storage. The EE (%) of E3, E6, and E9 formulations were maintained at the high value (EE > 90%) for 15 days because those emulsions contained enough concentration of surfactant blends. Capan et al. (1999) reported that the EE value was reduced when the stability of emulsion was low. These results showed that the O/W emulsion with 30% horse oil could be encapsulated well when used the surfactant concentration of 15 or 20% at HLB value below 15.
Fig. 3.
Changes in encapsulation efficiency (EE, %) of O/W emulsions made of horse oil and formulated with various HLB values and 15% (A) and 20% (B) surfactant concentration during the storage of 15 days at 40 °C in the dark. The O/W emulsions of E2, E5, E8, E11, and E14 were made with 30% horse oil, 15% surfactant, and HLB values of 10, 11, 12, 13, and 14, respectively (A). The O/W emulsions of E3, E6, E9, E12, and E15 were made with 30% horse oil, 20% surfactant, and HLB values of 10, 11, 12, 13, and 14, respectively (B)
In conclusion, the O/W emulsion formulations with different HLB values and concentrations of surfactant blends were prepared with 30% horse oil and 50, 55, and 60% ultrapure water using binary nonionic surfactants (Span 60 and Tween 60). When the O/W emulsion was formulated at the HLB value of 12 and surfactant concentration of 15% (E8 formulation in the current study), the emulsion was a stable nano-particle sized emulsion. Additionally, the characteristics of emulsion were not significantly changed during storage at 40 °C for 15 days. These results suggested that the characteristics of horse oil in water emulsions were depending on HLB values and surfactant concentrations so that affected to emulsion stability during storage.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2019R1F1A1048400).
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Youn Hyung Park, Email: rotifer3@naver.com.
Hyun Jung Kim, Email: hyunjkim@jejunu.ac.kr.
References
- An BJ, Lee JT, Lee IC, Kwak JH, Park JM, Park CI. Preparation and stabilization of an O/W emulsion using liquid crystalline phases. Journal of Oil and Applied Science. 2004;21:31–36. [Google Scholar]
- Capan Y, Woo BH, Gebrekidan S, Ahmed S, DeLuca PP. Influence of formulation parameters on the characteristics of poly (D, L-lactide-co-glycolide) microspheres containing poly (L-lysine) complexed plasmid DNA. Journal of Controlled Release. 1999;60:279–286. doi: 10.1016/S0168-3659(99)00076-0. [DOI] [PubMed] [Google Scholar]
- Cho MJ, Kim HJ. Effects of rendering and α-tocopherol addition on the oxidative stability of horse fat. Food Science and Biotechnology. 2020;29:169–177. doi: 10.1007/s10068-019-00653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Kim S, Bae EK, Mok CK, Park J. Formulation of a cosurfactant-free o/w microemulsion using nonionic surfactant mixtures. J of Food Science. 2008;73:E115–E121. doi: 10.1111/j.1750-3841.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Choi KH, Lee YS, Yoon JH, Yoo WK, Kim MR, Lee KS, Cho JW. Effect of horse oil on anti-bacterial, inflammatory cytokines, and type I collagen expressions in human HaCaT keratinocytes and fibroblasts. Korean Journal of Dermatology. 2014;52:1–6. [Google Scholar]
- Davidov-Pardo G, McClements DJ. Nutraceutical delivery systems: resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chemistry. 2015;167:205–212. doi: 10.1016/j.foodchem.2014.06.082. [DOI] [PubMed] [Google Scholar]
- De Oca-Ávalos JMM, Candal RJ, Herrera ML. Nanoemulsions: stability and physical properties. Current Opinion in Food Science. 2017;16:1–6. doi: 10.1016/j.cofs.2017.06.003. [DOI] [Google Scholar]
- Djerdjev AM, Beattie JK. Enhancement of Ostwald ripening by depletion flocculation. Langmuir. 2008;24:7711–7717. doi: 10.1021/la800140s. [DOI] [PubMed] [Google Scholar]
- Egito EST, Machado LA, Farias IEG, Silva KGH, Oliveir G. HLB Concept: a way to never forget it. Biomedical Journal of Scientific & Technical Research. 2018;10:1–2. [Google Scholar]
- Griffin WC. Classification of surface-active agents by HLB. Journal of Cosmetic Science. 1949;1:311–326. [Google Scholar]
- Gutiérrez G, Lobo A, Allende D, Cambiella A, Pazos C, Coca J, Benito JM. Influence of coagulant salt addition on the treatment of oil-in-water emulsions by centrifugation, ultrafiltration, and vacuum evaporation. Separation Science and Technology. 2008;43:1884–1895. doi: 10.1080/01496390801973953. [DOI] [Google Scholar]
- Izquierdo P, Esquena J, Tadros TF, Dederen C, Garcia MJ, Azemar N, Solans C. Formation and stability of nano-emulsions prepared using the phase inversion temperature method. Langmuir. 2002;18:26–30. doi: 10.1021/la010808c. [DOI] [Google Scholar]
- Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SK, An S, Jeon SH. The moisturizing effect and formulation test of the cosmetics composed by horse oil liposomes. Korean Journal of Aesthetics and Cosmetology. 2014;12:813–820. [Google Scholar]
- Kale SN, Deore SL. Emulsion micro emulsion and nano emulsion: a review. Systematic Reviews in Pharmacy. 2017;8:39–47. doi: 10.5530/srp.2017.1.8. [DOI] [Google Scholar]
- Kim HJ, Kim D, Kim NY, Kim JS, Jang A. Anti-wrinkle anti-inflammatory effects of a combination of topically applied horse oil and dietary enzyme hydrolysates from horse bone. Process Biochemistry. 2020;90:257–267. doi: 10.1016/j.procbio.2019.11.010. [DOI] [Google Scholar]
- Kim KB, Kang KP, Lee YK, Park, KH. A method for refining horse oil, the refined horse oil and a cosmetic composition comprising the refined horse oil. Korea Patent. No. 10-10-1632601 (2016)
- Klaus A, Tiddy GJ, Solans C, Harrar A, Touraud D, Kunz W. Effect of salts on the phase behavior and the stability of nano-emulsions with rapeseed oil and an extended surfactant. Langmuir. 2012;28:8318–8328. doi: 10.1021/la300435t. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Park CI. Application of rheological theory to assess emulsion stability and physical skincare barrier. Journal of Skin Barrier Research. 2013;15:65–69. [Google Scholar]
- Lim KH. Emulsion inversion and emulsion transition. Journal of the Korean Oil Chemists Society. 2004;21:267–273. [Google Scholar]
- Llinares R, Santos J, Trujillo-Cayado LA, Ramírez P, Muñoz J. Enhancing rosemary oil-in-water microfluidized nanoemulsion properties through formulation optimization by response surface methodology. LWT-Food Science and Technology. 2018;97:370–375. doi: 10.1016/j.lwt.2018.07.033. [DOI] [Google Scholar]
- Macedo JP, Fernandes LL, Formiga FR, Reis MF, Júnior TN, Soares LA, Egito EST. Micro-emultocrit technique: a valuable tool for determination of critical HLB value of emulsions. Pharmaceutical Science and Technology. 2006;7:E146–E152. doi: 10.1208/pt070121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements DJ. Food emulsions: principles, practices, and techniques. 3. Boca Raton, LA, USA: CRC Press; 2005. [Google Scholar]
- McClements DJ, Jafari SM. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Advances in Colloid and Interface Science. 2018;251:55–79. doi: 10.1016/j.cis.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Mulia K, Putri GA, Krisanti E. Encapsulation of mangosteen extract in virgin coconut oil based nanoemulsions: Preparation and characterization for topical formulation. Materials Science Forum. 2018;929:234–242. doi: 10.4028/www.scientific.net/MSF.929.234. [DOI] [Google Scholar]
- Mun S, Decker EA, McClements DJ. Effect of molecular weight and degree of deacetylation of chitosan on the formation of oil-in-water emulsions stabilized by surfactant–chitosan membranes. Journal of Colloid and Interface Science. 2006;296:581–590. doi: 10.1016/j.jcis.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Orafidiya LO, Oladimeji FA. Determination of the required HLB values of some essential oils. International Journal of Pharmaceutics. 2002;237:241–249. doi: 10.1016/S0378-5173(02)00051-0. [DOI] [PubMed] [Google Scholar]
- Park YH, Cho MJ, Kim HJ. Comparison of physicochemical characteristics of horse fat, lard, and beef-tallow. Korean Journal of Food Science and Technology. 2019;51:1–6. [Google Scholar]
- Pathak M. Nanoemulsions and their stability for enhancing functional properties of food ingredients. In: Oprea AE, Grumezescu AM, editors. Nanotechnology Applications in Food. Cambridge, MA, USA: Academic Press; 2017. pp. 87–106. [Google Scholar]
- Porras M, Solans C, González C, Martínez A, Guinart A, Gutiérrez JM. Studies of formation of W/O nano-emulsions. Colloids and Surfaces A. 2004;249:115–118. doi: 10.1016/j.colsurfa.2004.08.060. [DOI] [Google Scholar]
- Ramisetty KA, Pandit AB, Gogate PR. Ultrasound assisted preparation of emulsion of coconut oil in water: Understanding the effect of operating parameters and comparison of reactor designs. Chemical Engineering and Processing. 2015;88:70–77. doi: 10.1016/j.cep.2014.12.006. [DOI] [Google Scholar]
- Sanjeewani NA, Sakeena MHF. Formulation and characterization of virgin coconut oil (VCO) based emulsion. International Journal of Scientific and Research Publications. 2013;3:1–6. [Google Scholar]
- Wang X, Zhu C, Peng T, Zhang W, Zhang J, Liu H, Wu C. Enhanced stability of an emulsion enriched in unsaturated fatty acids by dual natural antioxidants fortified in both the aqueous and oil phases. Food Hydrocolloids. 2018;82:322–328. doi: 10.1016/j.foodhyd.2018.02.012. [DOI] [Google Scholar]
- Zhou H, Xu S, Sun Z. Real-time in situ observation of shear modulus evolution during Ostwald ripening of colloidal crystallization. Journal of Crystal Growth. 2018;502:35–40. doi: 10.1016/j.jcrysgro.2018.06.027. [DOI] [Google Scholar]