Abstract
Cancer and associated medical treatments affect patients' health-related quality of life (HRQoL) by decreasing functional dimensions of physical, social, cognitive, and emotional well-being, while increasing short and late-term symptoms. Exercise, however, is demonstrated to be a useful therapy to improve cancer patients' and survivors’ HRQoL, yet the effectiveness of high-intensity training (HIT) exercise is uncertain. This systematic review and meta-analysis aimed to analyse the effects of HIT on HRQoL dimensions in cancer patients and survivors as well as evaluate the optimal prescription of HIT. The search followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) and examined Web of Science and PubMed (Medline) databases. Data were analysed utilizing Review Manager Software. Twenty-two articles were included in the systematic review and 17 in the meta-analysis. Results showed HIT improved global quality of life, physical functioning, role functioning, social functioning, cognitive functioning, fatigue, pain, dyspnea, and insomnia, compared to an inactive control group, yet no differences were found between HIT and low to moderate-intensity exercise interventions. Particular improvements in HRQoL were observed during cancer treatment and with a training duration of more than eight weeks, a frequency of 2 days/week, and a volume of at least 120 min/week, including 15 min or more of HIT. Our findings whilst encouraging, highlight the infancy of the extant evidence base for the role of HIT in the HRQoL of cancer patients and survivors.
Subject terms: Cancer, Health care
Introduction
Cancer survivorship continues to increase, with the latest data indicating an estimated 16.9 million people have survived cancer in the United States. This figure is projected to reach more than 26 million by 20401. Moreover, by 2040, 73% of cancer survivors will be at least 65 years old, suggesting a higher comorbidity burden1. Cancer and associated therapies can have severe consequences, including treatment-related side effects that decrease health-related quality of life (HRQoL). HRQoL represents the perception of an individual’s current physical, social, emotional, and cognitive health (functional dimensions), together with individual wellbeing and the cancer symptoms suffered2. HRQoL is an important variable to consider when making clinical decisions2, and HRQoL correlates with patients' cardiorespiratory fitness3 and cancer-specific mortality4 in different types of cancer such as breast5–7, lung8, colon9, prostate10.
Short- and long-term11 effects of cancer treatments have been shown to compromise patients’ HRQoL. Short-term effects include symptoms of fatigue12, weight loss13, weight gain14, sarcopenia and cachexia15, nausea/vomiting16, pain17, hair loss18, dyspnea19, insomnia (sleep disturbance)20, constipation21, and drowsiness22. Symptoms such as diarrhea, appetite loss, sore mouth, and sweating are also reported23. Late effects of chemotherapy and radiation therapy most commonly include secondary cancers24 and cardiovascular disease25. Short-term and late effects vary depending on a patients’ medical history and treatment exposures11, and can directly impact a survivors physical and mental health, which can worsen with the increased comorbidities that likely occur with aging26. Thus, cancer patients’ HRQoL functional capacities, which include physical, emotional, cognitive, social, and mental components, may be negatively affected during and after treatment, and this negative experience may last throughout survivorship27.
In addition to pharmacological therapies, numerous interventions (e.g. psychological therapies, meditation, alternative medicines) are available that aim to reduce the effects of cancer, including treatment-related side effects of cancer drugs28. The role of exercise as a cancer therapy appears promising given its potential impact on variables such as physical and mental health, cancer symptoms, and clinical components29. Exercise programs are known to help improve the management of treatment side-effects, improve functional outcomes30, enhance global quality of life, and help manage fatigue31. Several meta-analyses and systematic reviews have investigated the role of exercise in the HRQoL of cancer survivors, trying to approximate the best dose–response and the critical exercise loading characteristics of frequency, intensity, type (FITT). Sweegers et al. (2018) and Buffat et al. (2017) concluded that supervised exercise programmes can improve HRQoL and physical function32,33 and are more beneficial than unsupervised interventions. Furthermore, in Hong et al. (2019) meta-analysis, positive social functioning effects were found as a result of exercise, with the largest benefits seen when exercise sessions were 45 to 90 min in duration34. Moreover, physical cancer symptoms of fatigue, pain, insomnia, and dyspnoea also showed a decrease with exercise programmes35.
Low-intensity exercise has been shown to improve depression, anxiety, and overall physical functioning36. Moderate-to-vigorous exercise has demonstrated improvements in physical function and reductions in cancer-related consequences30. High Intensity Training (HIT) has yielded positive effects37 on cardiorespiratory fitness38, strength39, and body composition40, as well as reduced tumor growth41. Although the use of HIT as part of cancer-related therapy is increasing, its benefits on HRQoL are unclear. Toohey et al. (2017) systematically reported a higher effect on HRQoL in patients using HIT. Whereas Mugele et al. (2019) stated that HIT did not improve global health status, pain, fatigue, or insomnia. Adams’ et al. (2018) found improvements in cancer-related fatigue and self-esteem when assessed by the Functional Assessment of Cancer Therapy-Fatigue questionnaire. These reviews point out the need for further investigation to clarify the possible beneficial effects of HIT in cancer patients and survivors. Therefore, the primary aim of this study was to explore the effect of HIT on HRQoL dimensions in cancer patients and survivors. Second, we aimed to evaluate the characteristics of HIT for each HRQoL dimension with regard to the intervention timing related to the cancer treatment, mode of exercise, and dose (i.e. duration and frequency).
Methods
The methodology of the current systematic review was carried out according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines42. The systematic review was registered with the International Prospective Register of systematic reviews (PROSPERO), identification number CRD42020167203. In this manuscript, we reported the effects on each HRQoL dimension, which includes more than 100 meta-analyses that have been summarized in tables and in the supplementary data. For readers interested in the effects of HIT on cardiorespiratory fitness outcomes in cancer, these have been reported elsewhere43.
Data sources and searches
PubMed (MEDLINE) and Web of Sciences (which includes articles indexed in the KCI-Korean Journal Database, MEDLINE, Russian Science Citation Index, and SciELO Citation Index) databases were used for article searches. The boolean operators employed were (cancer or “neoplasm”) and (HIIT or “high intensity”) and (“quality of life” or “hrqol” or “qol”), limiting the results to articles published in the last 10 years and written in English or Spanish. The search was done from November 2019 to March 2020. The search for published studies was independently performed by two authors (A.M.L-P and D.C-M.), and disagreements were resolved through discussion.
The inclusion criteria established to select the articles were: (a) studies involving any kind of cancer patients, (b) interventions with any kind of high-intensity exercise, (c) articles with any HRQoL outcome registered, and (d) investigations including at least one other group to compare the effects of HIT. Additionally, interventions were excluded in cases of being a letter to the editor, a consensus or guideline, a study protocol or study design, a case report, a follow-up study, meta-analysis, or systematic review. The current review considered high-intensity training as any program (cardiovascular and/or resistance exercise) whose authors classified it as “high-intensity”, including both high intensity interval training and high intensity training.
Risk of bias assessment
The analysis of the risk of bias was done using the PEDro scale. The scale is known as a valid and reliable instrument to assess eligibility, allocation to groups, blinding of allocation, and comparison between groups at baseline and its outcomes44. The leading reason for its selection is due to it being the most used in the Sport Sciences for Health scientific area45.
Data extraction
The main data of participants, intervention, comparisons, results, and study design (PICOS) of each group included in the articles were reported according to the PRISMA methodology42. Regarding participants, studies sample size, patients age (mean and standard deviation) and body mass index (BMI), type of cancer, stage, cancer treatment, and exercise intervention timing concerning the therapy phase were reported. The intervention characteristics registered were: program length (in weeks), duration of sessions, weekly frequency, a description of the exercise, its corresponding intensity (control and progression), and adherence data. HRQoL was the outcome reported in this review. The questionnaires used in the different studies were the European Organisation for Research and Treatment of Cancer quality of life Questionnaire C30 (EORTC QLQ-C30), the Functional Assessment of Cancer Therapy (FACT), and the Short-form 36 (SF-36). Results regarding the questionnaires used are in the meta-analysis figures or the supplementary data tables. Of all the surveys, the EORTC QLQ-C30 questionnaire was the most commonly reported and the one with more specific variables analyzed46. To make a representative analysis, the EORTC QLQ-C30 dimensions were used to group FACT and SF-36 results. We examined all the questionnaire’s dimensions and related items to establish similarities between the categories. We did this even if there were named differences, but their items evaluated the same topic. Item categorization was analyzed by two of the researchers (AMLP and DCM) who discussed similarities and differences to classify them in useful variables for the meta-analysis. The variables were divided into categories consistent with EORTC QLQ.C30 dimensions, distinguishing global health, functional scale, and symptoms scale. The data from those items that did not correspond to any variable group created, or was registered by less than three articles, was not included in the literature part.
Statistical analysis
Post-intervention means and standard deviations were extracted from the articles and analyzed using Review Manager Software (RevMan, 5.3)47 based on; High-intensity exercise group (HIEG), low-to-moderate exercise group (LMEG), and inactive control group (CG). When outcomes were evaluated on scales with opposite directions, (e.g. pain or fatigue), one of the results directions was multiplied by − 148. The results were reported using standardized mean differences (SMDs) and interpreted according to the Cochrane Handbook48 i.e. small effects with scores < 0.4, moderate effects from 0.4 to 0.7, and large effects with > 0.7. The statistical method employed was inverse variance with random effects49 and the interval confidence (CI) utilized was 95%.
Different analyses were computed for each dimension (Global health, Physical functioning, Role functioning/physical role, Emotional functioning/wellbeing and mental health, Cognitive functioning, Social functioning, Fatigue/vitality, Nausea, Body pain, Dyspnoea, Constipation, Insomnia, Diarrhoea and Appetite loss). The described procedure was carried out, first, to analyze the difference according to the type of intervention group: LMEG or CG. Secondary calculations were performed contrasting HIEG and CG outcomes with more detail making the following subgroups analysis: (1) interventions conducted before, during, or after cancer treatment, (2) interventions of ≤ 8 weeks or > 8 weeks, (3) only aerobic exercise programs or work-outs with any resistance component, (4) studies where participants exercised ≤ 2 times per week or those > 3 times per week (5) interventions of ≤ 120 min or > 120 min per week, (6) training designs with a high-intensity aerobic session part of 15 min or less and separately those with greater than 15 min duration.
Results
Study selection
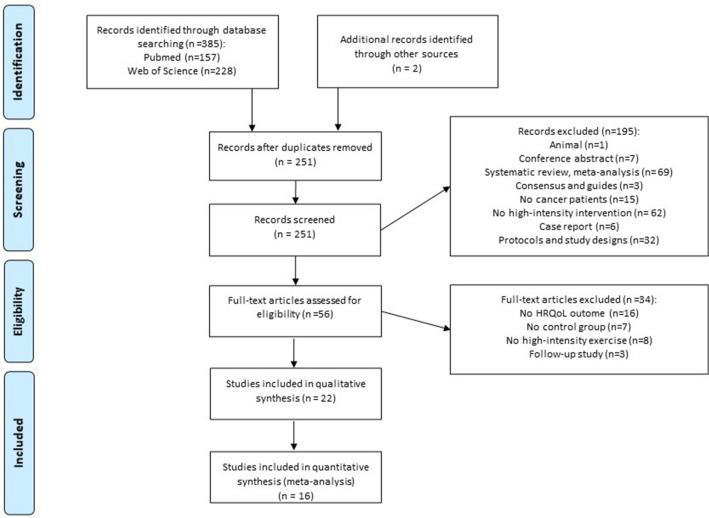
Figure 1 sets out the data from the study selection process. The search obtained 385 articles, 157 in the PubMed database and 228 in Web of Science. Two more papers were identified in the references of articles and were therefore included50,51. One hundred thirty-five of the found studies were duplicated, so 251 were screened by examining the title and abstract. Following the exclusion criteria, one animal intervention, seven conference abstracts, 69 reviews, three consensus or guideline writings, 15 studies not focused on cancers, 62 not involving a high-intensity intervention, six case reports, and 32 study designs were removed. Fifty-six articles were full-text analyzed. From those, 16 were excluded because they did not have HRQoL as a variable, seven did not include a CG, eight did not carry out a HIT programme (most of them were respiratory exercises), and three were follow-up studies. In total, 22 studies were eligible for the systematic review, and from those sixteen had data to be included in the meta-analysis process.
Figure 1.
Study flow diagram.
Risk of bias
The risk of bias was evaluated using the PEDro scale and ranged from 3 to 8, see Table 1 (being 10 the best score of the scale). The mean of the scores was 6.3. All the articles fulfilled Items 1 (“the election criteria were specified”) and 10 (“the results of between-group statistical comparisons are reported for at least one key outcome”). Item 5 “there was blinding of all subjects” and 6 “there was blinding of all therapists who administered the therapy” were only reaches by two of the includes studies52.
Table 1.
Risk of bias using PEDro scale.
| Validity | External item | Internal items | Statistic items | Total score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Pereira et al. (2020) | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 6 |
| Mijwel et al. (2018) | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Christensen et al. (2018) | Y | Y | N | Y | N | N | N | Y | Y | Y | N | 5 |
| Adams et al. (2018) | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Persoon et al. (2017) | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Brunet et al. (2017) | Y | N | N | N | N | N | N | Y | N | Y | Y | 3 |
| Van Waart et al. (2017) | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 6 |
| Dunne et al. (2016) | Y | Y | Y | Y | N | Y | Y | Y | N | Y | Y | 8 |
| Toohey et al. (2016) | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Waked,et al. (2016) | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 6 |
| Schmitt et al. (2016) | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 6 |
| Edvardsen et al. (2015) | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Martin et al. (2015) | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | 7 |
| Moller et al. (2015) | Y | Y | Y | Y | N | N | Y | N | N | Y | Y | 6 |
| Kampshoff et al. (2015) | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | 7 |
| Van Wart et al. (2015) | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Midtgaard et al. (2013) | Y | Y | Y | Y | N | N | N | N | Y | Y | Y | 6 |
| Andersen et al. (2013) | Y | Y | Y | Y | N | N | N | N | Y | Y | Y | 6 |
| Cormie et al. (2013) | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Hwang et al. (2012) | Y | Y | Y | Y | N | N | Y | N | N | Y | Y | 6 |
| Adamsen et al. (2009) | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
Y: Yes, the item was satisfied in the experimental protocol; N: No, the item was not satisfied in the experimental protocol Items: (1) Eligibility criteria; (2) Random allocation; (3) Concealed allocation; (4) Similarity of the groups at baseline; (4, 7–11) Key outcomes; (5–7) Blinding process; (8) Final measure with 85% of the initial sample size; (9) intent-to-treat analysis; (10) Between-group comparisons report; (11) Point and variability measures.
Characteristics of the participants
Table 2 shows the meta-analysis participants’ baseline characteristics. The information from the articles included in the systematic review but not in the meta-analysis are presented in Supplementary Table S1 and Table S2. The global sample size of the systematic review was 2457, composed of 1080 participants of HIEG, 385 which participated in LMEG, and 992 from inactive CG and. Participants aged ranged from 27.8 to 72 with a mean of 51.56 in HIEG, 53.35 years in LMEG, and 51.6 in CG Patients' mean BMI ranged from 22.6 to 31 kg/m2 and often were not regularly physically active.
Table 2.
Baseline characteristics of the participants included in the meta-analysis.
| Study | Design | Group | Sample size (% of females) | Age (SD) | Cancer type (%) | Treatment | Timing | BMI |
|---|---|---|---|---|---|---|---|---|
| Egegaard et al. (2019) | Feasibility Study | CG | n = 7 (71.4%) | 65 (4.7) | Non-smallcell lung Cancer (NSCLC) | Chemoradiotherapy | During | 24.2 (1.9) |
| HIEG | n = 8 (62.5%) | 64 (5.8) | During | 24.1 (4.4) | ||||
| Mijwel et al. (2018) | Randomized Clinical Trial | CG | n = 60 (100%) | 52.6 (10.2) | Breast cancer | Chemotherapy | During | NR |
| HIEG-R | n = 74 (100%) | 52.7 (10.3) | NR | |||||
| HIEG-A | n = 72 (100%) | 54.4 (10.3) | NR | |||||
| Adams et al. (2018) | Phase 2 Randomized Controlled Trial | CG | n = 28 (0%) | 43.3 (9.9) | Testicular cancer |
Surgery (96.4%) Radiotherapy (17.9%) Chemotherapy (28.6%) |
After | 27.9 (4.2) |
| HIEG | n = 35 (0%) | 44.0 (11.6) |
Surgery (88.6%) Radiotherapy (17.1%) Chemotherapy (42.9%) |
After | 27.2 (5.0) | |||
| Van Waart et al. (2017) | Pilot trial | CG | n = 8 (10.6%) | 56.7 (10.6) | Colon cancer |
Surgery (25%) Radiotherapy (13%) Prescribed chemotherapy (100%) |
During | 23.5 (3.1) |
| HIEG | n = 7 (71%) | 57.7(13.2) | Surgery (57%) Prescribed chemotherapy (100%) | During | 25.1(4.2) | |||
| LIEG-H | n = 8 (38%) | 60.1 (7.3) | Surgery (6%) Prescribed chemotherapy (100%) | During | 23.6 (2.1) | |||
| Persoon et al. (2017) | Randomized controlled trial | CG | n = 55 (33%) | 56 | Multiple myeloma (53%) (Non-)Hodgkin lymphoma (47%) | NR |
After transplanta-tion |
NR |
| HIEG | n = 54 (46%) | 53.5 | Multiple myeloma (54%) (Non-)Hodgkin lymphoma (46%) | NR |
After Transplanta-tion |
NR | ||
| Toohey et al. (2016) | Pilot study | HIEG | n = 8 (100%) | 47.25(13.49) | Colon (6.25%) Cervical (6.25%) Melanoma (6.25%) Ovarian (12.5%) Breast (56.25%) Breast and uterine (6.25%) Breast and liver (6.25%) |
Surgery (18.75%) Surgery + chemotherapy (12.5%) Surgery + radiation (6.25%) Surgery + chemotherapy + endocrine (12.5%) Surgery + chemotherapy + radiation + endocrine (50%) |
After | NR |
| MIEG | n = 8 (100%) | 55.88 (11.81) | After | NR | ||||
| Schmitt et al. (2016) | Single arm, non- randomized | HIEG | n = 13 (100%) | 53 (8) |
Breast (85%) Ovarian (8%) Non-invasive urotelial (8%) Metastases (15%) |
Surgery (100%) Chemotherapy (54%) Radiation (69%) Antihormonal (69%) |
After | 27.0 (5.3) |
| LIEG | n = 13 (100%) | 54 (9) |
Breast (77%) colon (8%) vaginal (8%) Non-Hodgkin0s lymphoma (8%) Metastases (8%) |
Surgery (100%) Chemotherapy (69%) Radiation (69%) Antihormonal (54%) |
After | 26.2 (4.3) | ||
| Dunne et al. (2016) | Randomized clinical trial | CG | n = 17 (23.5%) | 62 | Colorectal liver metastasis | Chemotherapy (60%) | Before | 29.7 (4.2) |
| HIEG | n = 20 (35%) | 61 | Chemotherapy (58.82%) | Before | 29.7 (4.2) | |||
| Kampshoff et al. (2015) | Randomized controlled trial | CG | n = 92 (78%) | 54 (10.9) |
Breast (63%) Colon (17%) Ovarian (6%) Lymphoma (9%) Cervix (2%) Testicles (4%) |
Surgery (88%) Radiation (53%) Surgery + radiation (51%) Immunotherapy (20%) Homonal therapy (47%) | After | NR |
| HIEG | n = 91 (80%) | 54 (11.0) | Breast (68%) Colon (17%) Ovarian (4%) Lymphoma (10%) Testicles (1%) | Surgery (91%) Radiation (51%) Surgery + radiation (45%) Immunotherapy (18%) Homonal therapy (50%) | After | NR | ||
| LMIEG | n = 95 (82%) | 53 (11.3) |
Breast (65%) Colon (20%) Ovarian (3%) Lymphoma (9%) Cervix (2%) |
Surgery (92%) Radiation (43%) Surgery + radiation (41%) Immunotherapy (26%) Homonal therapy (42%) |
After | NR | ||
| Martin et al. (2015) c) | Randomised controlled trial | CG | n = 35 (0%) | 66.9 (6.6) | Prostate cancer | Surgery (77.14%) Radiation (28.57%) Brachytherapy (11.43%) ADT (20%) | After | 28 (3.7) |
| HIEG | n = 27 (0%) | 65.3 (7) | Surgery (81.48%) Radiation (18.52%) Brachytherapy (11.11%) ADT (11.11%) | After | 27.6 (4.1) | |||
| LIEG | n = 25 (0%) | 65 (6.3) |
Surgery (92%) Radiation (8%) ADT (12%) |
After | 26.4 (2.8) | |||
| Martin et al. (2015) a) | Randomised controlled trial | CG | n = 40(100%) | 57.2 (9.8) | Breast cancer | Surgery (100%) Chemotherapy (67%) Radiation (71%) Hormone (98%) | After | 26.3 (5.2) |
| HIEG | n = 13 (100%) | 53.5 (9) |
Surgery (100%) Chemotherapy (77%) Radiation (54%) Hormone (85%) |
After | 27.9 (5.3) | |||
| LIEG | n = 19(100%) | 58.2 (9.6) |
Surgery (100%) Chemotherapy (63%) Radiation (90%) Hormone (82%) |
After | 26.6 (4.8) | |||
| Van Waart et al. (2015) | Randomized Clinical Trial | CG | n = 77 (100%) | 51.6 (8.8) | Breast cancer | Surgery (78%) Radiation (78%) | During | NR |
| HIEG | n = 76 (97%) | 49.9 (8.4) | Surgery (74%) Radiation (79%) | During | NR | |||
| LIEG-H | n = 77(100%) | 50.5 (10.1) | Surgery (81%) Radiation (78%) | During | NR | |||
| Møller et al. (2015) | Randomised feasibility study | CG | n = 16 (12.5%) | 46.95 (9.19) | Colon and breast cancer | Chemotherapy | During | 25.54 (4.9) |
| HIEG | n = 15 (7.14%) | 57.17 (10.51) | Colon and breast cancer | Chemotherapy | During | 24.39 (5.27) | ||
| LIEG | n = 77(100%) | 48.49 (8.41) | Colon and breast cancer | Chemotherapy | During | 23.8 (2.59) | ||
| Edvardsen et al. (2015) | Randomised controlled trial | CG | n = 31 (52%) | 65.9 (8.5) | Lung cancer | Surgery (100%) Chemotherapy (29%) Radiation (13%) | After surgey | 25.1 (5.2) |
| HIEG | n = 30 (57%) | 64.4 (9.3) |
Surgery (100%) Chemotherpy (30%) Radiation (10%) |
After surgey | 25.4 (5.1) | |||
| Cormie et al. (2013) | Randomised controlled trial | CG | n = 19 (100%) | 58.6 (6.7) | Breast cancer |
Surgery (89.5%) Chemotherapy (63.2%) Radiotherapy (89.5%) Hormonotherapy (57.9%) |
After | 28.2(6.0) |
| HIEG | n = 22 (100%) | 56.1 (8.1) |
Surgery (90.9%) Chemotherapy (90.9%) Radiotherapy (77.3%) Hormonotherapy (63.6%) |
After | 30.8 (6.5) | |||
| LIEG | n = 21 (100%) | 57.0 (10.0) |
Surgery (100%) Chemotherapy (90.5%) Radiotherapy (81.0%) Hormonotherapy (66.7%) |
After | 30.4(5.7) | |||
| Andersen et al. (2013) | Randomised controlled trial | CG | n = 107 (72%) | 47.8 (10.4) |
Breast (47.66%) Bowel (14.02%) Ovaries (8.41%) Testicles (6.54%) Oesophagus (0.93%) Brain (1.87%) Cervix (1.87%) Pharynx (0.93%) Pancreas (1.87%) Stomach (0.93%) Hematological (9.43%) |
NR | During | NR |
| HIEG | n = 106 (79.2%) | 47.1 (10.8) |
Breast (49.05%) Bowel (13.21%) Ovaries (10.38%) Testicles (6.6%) Oesopagus (0.94%) Brain (0.94%) Cervix (1.88%) Pharynx (1.88%) Pancreas (0.94%) Stomach (0.94%) Hematological (10.38%) |
NR | During | NR | ||
| Hwang et al. (2012) | Randomised controlled trial | CG | n = 11 (36.4%) | 58.5 (8.2) | Lung cancer |
Surgery (36.4%) Chemotherapy (45.6%) Radiotherapy (45.5%) |
During | 23.1 (2.6) |
| HIEG | n = 13 (61.5%) | 61.0 (6.3) |
Surgery (69.3%) Chemotherapy (76.9%) Radiotherapy (61.5%) |
During | 22.6 (2.4) | |||
| Adamsen et al. (2009) |
Randomized control trial |
CG | n = 134 (70.9%) | 47.2 (10.6) | Breast (44.03%) Bowel (12.68%) Ovaries (8.2%) Testicular (6.7%) Oesophagus (2.23%) Brain (2.98%) Cervix (1.5%) Pharynx (0.74%) Pancreas (1.5%) Stomach (1.5%) Hematological malignancies (11.2%) | Chemotherapy | During | NR |
| HIEG | n = 135 (74.8%) | 47.2 (10.7) |
Breast (44.44%) Bowel (13.33%) Ovaries (11.85%) Testicular (5.18%) Oesophagus (1.48%) Brain (0.74%) Cervix (2.96%) Pharynx (1.48%) Pancreas (0.74%) Stomach (0.74%) Hematological malignancies (9.63%) |
Chemotherapy | During | NR |
CG: control group, HIEG: high-intensity exercise group, HIEG-R: high-intensity resistance exercise group, HIEG-E: high-intensity endurance exercise group, LIEG: low-intensity exercise group, LMIEG: low to moderate exercise group, NR: not reported.
The selected articles involved different types of cancer. Some papers specified the intervention in one type of cancer, such as breast cancer39,51,53–57 (being the most common in the studies included), colon cancer51,58,59, lung cancer60–62 prostate cancer54, testicles cancer63 or rectal cancer50. Other authors designed programmes mixing participants with different kinds of cancer37,57,64–69. Moreover, the exercise interventions found could be distinguished by the timing within the cancer pathway: before59, during50,51,53,56–58,60,61,68–71 or after treatment54,62,65,66,72,73.
Characteristics of the exercise programs
The intervention descriptions are reported in Table 3 (meta-analysis articles) and in Supplementary Table S3. The mean duration of interventions was 12 weeks and the median was 10 weeks. Interventions were three weeks66, six weeks50,51,68,69, seven weeks71, eight weeks54,56,61 twelve weeks39,72–75, sixteen weeks53, eighteen weeks65, 36 weeks55 and 12 months62. HIEG participants trained with a mean frequency of 2.8 times/week, so most of the interventions programs were delivered 3 times/week37,50,51,54,55,60–63,68,69,75,76, although in some were 2 days/week39,53,57,58,65,67,70 and 5 days/week71. All high-intensity interventions were supervised and conducted indoors, except Schmitt et al. (2016), who evaluated the effects of a program performed outside on a paved uphill road66. Mean duration was 62.5 min but included sessions of 20 min71,75, 20 to 30 min74, 30 to 40 min61,72, 40 min50, 50 min57,58, 60 min39,53,54,60,65, 70 min55, 75 min66,70 and 90 min51,56,68,69.
Table 3.
Description of the high-intensity exercise interventions included in the meta-analysis.
| Study | Group | Duration | Sessions duration | Weekly frequency | Setting | Exercise description | Intensity progression and control | Attendance |
|---|---|---|---|---|---|---|---|---|
| Egegaard et al. (2019) | CG | 7 weeks | Daily life | Activity tracker (Garmin®vívosmart®) | ||||
| HIEG | 7 weeks | 20 min | 5 times per week |
5 min warm-up HIIT: 1st and 3rd cycle ergometer intervals: 5 × 30 s with 30 s rest2nd cycle ergometer interval: continuous cycling |
Moderate-to-high intensity Warm-up: 50–60% (W Peak Power) 1st, 3rd interval 80–95% (W Peak Power) 2nd interval: 80% (W Power Peak) Additional control: HR |
Sessions: 90.0% and adherence Simple size: 100% | ||
| Mijwel et al. (2018) | CG | Written American College of Sports Medicine exercise recommendations | ||||||
| HIEG-R | 16 weeks | 60 min | 2 times/week | Exercise clinic |
5 min aerobic warm-up HIIT cycle exercise: 3 × 3 min intervals with 1 min recovery Resistance: 8–12 high-load repetitions of the major muscle groups |
Warm-up: 10–12 RPE Resistance: 70%-80% (RM) Aerobic: moderate 13–15 RPE HIIT: intervals at 16–18 RPE |
Sessions: 68% Simple size: 88% |
|
| HIEG-A | 16 weeks | 60 min | 2 times/week | Exercise clinic |
5 min aerobic warm-up HIIT cycle exercise: 3 × 3 min intervals with 1 min recovery Aerobic: 20 min of cycle ergometer, elliptical ergometer, or treadmill moderate continuous exercise |
Warm-up: 10–12 RPE HIIT: intervals at 16–18 RPE From 70% RM to 80% RM |
Sessions: 63% | |
| Adams et al. (2018) | CG | 12 weeks | ||||||
| HIEG | 12 weeks | 35 min | 3 times/week | Supervised |
5 min warm-up and cool-down HIIT:4 × 4 min intervals with 3 min active recovery |
Warm-up: at ± 5% of the ventilatory threshold Intervals: from 75 to 95% VO2máx Recovery: 5%-10% of the ventilatory threshold |
Sessions: 99% Simple size: 100% |
|
| Van Waart et al. (2017) | CG | Moderate intensity leisure-time sports | ||||||
| HIEG |
From the first cycle of chemotherapy to 3 weeks after the last cycle |
50 min | 2 times/week | Supervised |
Resistance: 20 min 6 large muscle groups 2 series of 8 repetitions Cardiovascular MHIT: 30 min + 30 min physically active 5 days/Week |
Moderate to high Resistance: 80%RM Aerobic: 80% (predicted maximal workload) Adjustment: 1RM testing was repeated every 3 weeks Additional control: RPE |
Sessions: 61% | |
| LIEG-H | 30 min | 5 times/week | Home-based | Written individual information |
Low intensity: 12–14 RPE Additional control:Activity Diary |
NR | ||
| Persoon et al. (2017) | CG | 18 weeks | ||||||
| HIEG | 18 weeks | 60 min |
1st–12th week 2 times/week 13th week 1 time/week |
Physiotherapy center |
High-intensity resistance: 6 standardized exercise muscles. Week 1–12: 2 series of 10 repetitions HIIT: 2 series of8 min cycling Week 1–8 30 s blocks with 60 s blocks Week 9–12 30 s blocks |
Resistance: 65–80% RM Aerobic: 30 s blocks at 65% (maximal short exercise capacity) 60 s blocks at 30% (maximal short exercise capacity) Load adjustment every 4 weeks by a performing of the indirect 1-RM measurements and the steep ramp test |
Sessions: 86% Simple size: 92.6% |
|
| Toohey et al. (2016) | HIEG | 12 weeks | 20 min | 3 times/week | Supervised |
5 min warm-up HIIT: 7 × 30 s intervals with 1 min rest 5 min cool-down |
Intervals ≥ 85% (HRmax) From 3 intervals in the first session to 7 intervals in week 5 Additional control: RPE and blood pressure |
Sessions: 93.75% Simple size: 100% |
| MIEG | 12 weeks | 30 min | 3 times/week | Supervised |
5 min warm-up 20 min cycle continuos Aerobic 5 min cool-down |
≤ 55% predicted maximal heart rate | NR | |
| Schmitt et al. (2016) | HIEG |
3 weeks 8 sessions |
3 times/week | Outside (paved up-hill road) |
5 min warm-up HIIT: 8 × 1 min intervals walking 2 min active recovery |
Warm-up: 70% (HRpeak) Intervals: > 95% (HRpeak) |
93% partipants all sessions | |
| LMIEG | 3 weeks | 75 min | 6 sessions | Outside (paved up-hill road) and Inside |
60 min walking 15 min indoor cycling |
Cycling: 60% (HRpeak) | ||
| Dunne et al. (2016) | CG | 4 weeks | ||||||
| HIEG | 4 weeks | warm-up + 30 min + cool-down | 12 sessions | Clinic |
Cycle ergometer exercise Warm-up HIIT: Intervals of high and moderate intensity |
High intensity > 90% (VO2 peak) Moderate intensity > 60% (VO2peak) |
Sessions: 99% Simple size: 95% | |
| Kampshoff et al. (2015) | CG | 12 weeks | ||||||
| HIEG | 12 weeks | Depending on de week | 2 times/week | Supervised |
Resistance: six exercise large groups 2 series of 10 repetitions HIIT: 1st–4th week: 2 × 8 min cycling intervals 30 s + 60 s blocks 4th–end:2 × 8 min cycling intervals 30 s + 30 s blocks 5th week-end additional HIIT session: 8 min of cycling intervals 30 s + 30 s blocks and 8 min 3 × 5 min continuous ergometer with 1 min rest |
Resistance: 70%-85% (RM) Aerobic: 30 s Interval 65% (MSEC) 60 s Interval:30% (MSEC) Continuous ergometer: 80% (HRR) Every four weeks, the training progress was evaluated utilizing the steep ramp test and RM test and the workload is adjusted accordingly |
Sessions: 74% and more than 80% of the sessions Simple size: 92% |
|
| LMIEG | 12 weeks | 2 times/week | Supervised |
Resistance: six exercise large groups 2 series of 10 repetitions Interval aerobic: 1st–4th week: 2 × 8 min cycling intervals 30 s + 60 s blocks 4th–end: 2 × 8 min cycling intervals 30 s + 30 s blocks 5th week-end additional Aerobic session: 8 min of cycling intervals 30 s + 30 s blocks and 8 min 3 × 5 min continuos ergometer with 1 min rest |
Resistance: 70%-85% (RM) Aerobic: 30 s Interval 45% maximum short exercise capacity (MSEC) 60 s Interval:30% (MSEC) Continuos ergometer: 40%-50% (HRR) |
Sessions: 70% | ||
| Martin et al. (2015) c) | CG | 8 weeks | ||||||
| HIEG | 8 weeks | 60 min | 3 times/week | University clinic |
25 min HIT 25 min resistance 10 min static stretching |
Aerobic: 75%-80% (VO2 max) Resistance: 65–80% RM Increase 5% VO2 middle of the programme Additional control: HR |
Sessions: 90% Simple size: 96% | |
| LIEG | 8 weeks | 60 min | 3 times/week | University clinic |
25 min Aerobic 25 min resistance 10 min static stretching |
Aerobic: 60%-65% (VO2 max) Resistance: 50–65% RM Increase 5% VO2 middle of the programme |
||
| Martin et al. (2015) a) | CG | 8 weeks | ||||||
| HIEG | 8 weeks | 60 min | 3 times/week | University clinic |
25 min HIT 25 min resistance 10 min static stretching |
Aerobic: Week 1- 4 75% (VO2 max) Week 5–8 80% (VO2max) Resistance: 65–80% RM Increase 5% VO2 middle of the programme Additional control: HR |
Sessions: 90% Simple size: 96% | |
| LIEG | 8 weeks | 60 min | 3 times/week | University clinic |
25 min Aerobic 25 min resistance 10 min static stretching |
Aerobic: Week 1- 4 60% (VO2 max) Week 5–8 65% (VO2max) Resistance:50–65% RM Increase 5% VO2 middle of the programme |
||
| Van Waart et al. (2015) | CG | Moderate intensity leisure-time sports | ||||||
| HIEG |
From the first cycle of chemotherapy to 3 weeks after the last cycle |
50 min | 2 times/week | Supervised |
Resistance: 20 min 6 large muscle groups 2 series of 8 repetitions Cardiovascular MHIT: 30 min + 30 min physically active 5 days/Week |
Moderate to high Resistance: 80%RM Aerobic: 50%-80% (predicted maximal workload) Adjustment: Resistance: 1 RM testing every 3 weeks Aerobic: Borg Scale, with a threshold of less than 12 for the increase and more than 16 for decrease of intensity Additional control: RPE |
NR | |
| LIEG-H | 30 min | 5 times/week | Home-based | Written individual information |
Low intensity: 12–14 RPE Additional control: Activity Diary |
NR | ||
| Møller et al. (2015) | CG | 12 weeks | ||||||
| HIEG | 12 weeks | 90 min (hiit sessions) |
9 h/ week (HIIT and Low-intensity sessions) |
Copenhagen University Hospital |
High-intensity sessions: 30 min warm-up HIT resistance: 45 min, 3series of 5–8 repetitions HIIT:15 min cardiovascular cool-down (stretching and coordination training) Low- intensity sessions: 30–90 min of body awareness, relaxation or massage |
Resistance:70–100% RM- 5.5 METSs Aerobic: 70–250 W, 85–95% (HRmax) 15 METs |
Sessions: 74% Simple size: 82% | |
| LIEG-H | 12 weeks | At home | Low/moderate recreational physical activity level of 30 min/day and 10 000 steps/day, five times/week | Podometer data | NR | |||
| Edvardsen et al. (2015) | CG | 20 weeks | ||||||
| HIEG | 20 weeks | 60 min | 3 times/week | Fitness centers |
Warm-up HIIT: Interval uphill treadmill walking Resistance 3 series of leg press, leg extension, back extension, seat row, bicep curls, and chest-and-shoulder press |
Intervals 80–95% (HRpeak) Resistance: 6–12 RM Increase of Interval intensity and duration based on the patient’s improvement, ability to cope with dyspnoea and feelings of well-being or fatigue on each exercise day Additional control: RPE |
Sessions: 88 ± 29% Simple size: 83% |
|
| Cormie et al. (2013) | CG | 3 months | ||||||
| HIEG | 3 months | 60 min | 2 times/week | Supervised |
10 min warm-up HIT resistance: 1–4 sets of 6 exercise upper body and 2 lower body 5 min cool-down |
Resistance: 75%-85% RM using 10–6 RM Resistance increased 5–10% for the next set and/or training session if participants were able to perform more repetitions than the RM’s Additional control: RPE |
NR | |
| LIEG | 3 months | 60 min | 2 times /week | Supervised |
10 min warm-up Resistance: 1–4 sets of 6 exercise upper body and 2 lower body 5 min cool-down |
Resistance: 55%-65% RM using 20–15 RM Additional control: RPE |
NR | |
| Andersen et al. (2013) | CG | |||||||
| HIEG | 6 weeks | 90 min (hiit sessions) |
9 h/ week (HIIT and Low-intensity sessions) |
Copenhagen University Hospital |
High-intensity sessions: 30 min warm-up HIIT:10 min cycling interval Cool-down (stretching and coordination training) Resistance Low- intensity sessions: 30–90 min of body awareness, relaxation or massage |
Intervals: 85–95% (HRpeak) | NR | |
| Hwang et al. (2012) | CG | General exercise instructions and Theraband Elastic Band | ||||||
| HIEG | 8 weeks | 30–40 min | 3 times/week | Clinic |
Treadmill o cycling ergometer sessions 10 min warm-up HIIT:2–5 min intervals with an active recovery 5 min cool-down |
Intervals: 80% (VO2peak) 15–17 RPE Recovery: 60% (V02peak) 11–13 RPE Intensity and duration were adjusted every 1–2 weeks based on the individual’s exercise response Additional control: HR, blood pressure and oxygen saturation |
Sessions: 71.2% Simple size: 85% |
|
| Adamsen et al. (2009) | CG | |||||||
| HIEG | 6 weeks | 90 min (HIT sessions) |
9 h/ week (HIT and Low intensity sessions) |
Copenhagen University Hospital |
High-intensity sessions: 30 min warm-up HIT resistance 45 min: 3 series of 5–8 repetitions HIIT: 15 min cardiovascular interval training: cool-down (stretching and coordination training) Low- intensity sessions: 30–90 min of body awareness, relaxation or massage |
Resistance:70–100% RM- 5.5 METSs Aerobic: 70–250 W, 85–95% (HRmax) 15 METs |
Sessions: 70.8% Simple size: 87,4% |
CG: control group, HIEG: high-intensity exercise group, LIEG: low-intensity exercise group, LMIEG: low to moderate exercise group, HIT: high-intensity training, HIIT: high-intensity interval training, MHIT: moderate to high intensity training, METs: Metabolic equivalent of task, RM: maximum repetition, HR: heart rate, RPE: the rating of perceived exertion, MSEC: maximum short exercise capacity, VO2: oxygen consumption, NR: not reported.
All HIT components included a cardiovascular exercise component, except Cormie et al. (2013), which included resistance training only39. In some interventions, HIT was conducted in interval bouts of 30 s65,71,73, 1 min66 3 min53, 4 min63, 5 to 8 min61. Others incorporated HIT utilizing continuous aerobic training protocols51,54,57,68,69. Across the studies, there were a variety of methodologies used to set high intensity depending on VO2: 95% VO2 max.63, > 90% VO2 peak, 80% VO2peak61, 80% VO2max54; based on heart rate: > 85% HRmax75, 95% HRpeak51,60,66,68,69; based on power: 95% Wpeak power71; based on Borg’s Rating of perceived exertion scale: 18 of the Borg’s Rating of perceived exertion scale53; and based on the maximum short exercise capacity (MSEC)65,67, 80% of predicted maximal workload57,58. Of equal importance, the prescribed rest during HIT varied from 30 s71 to 1 min53,73,75, 2 min66, or 3 min of active recovery63. Most of the interventions supplemented the HIT component either with resistance training51,53,54,57,58,60,62,65,68–70,73 Other studies complemented HIT with low-intensity sessions like body awareness, relaxation, or massage51,68,69. Data regarding participants' adherence are presented in Table 3 and Supplementary data (Table S3). The mean percentage rate of sessions completed for participants in each group was HIEG 76.7%; LMEG 72.9%; aerobic exercise 82.3%; and resistance exercise 74.0%.
Health-related quality of life outcomes
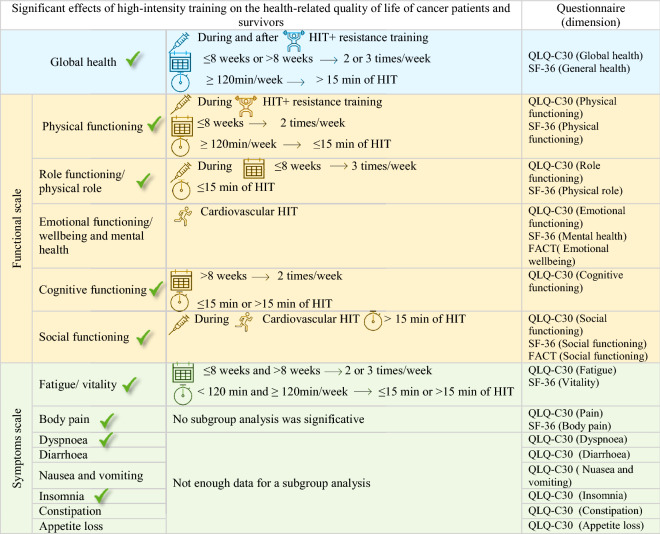
According to the EORTC QLQ-C30 scoring manual, the results, including SF-36 and FACT-G questionnaires, were divided into three categories shown in Table 4: Global health status, Functional scales, and Symptom scales77. Below are the results of the exercise programmes characteristics showing the interventions needed to achieve higher HRQoL benefits. Tthe supplementary data included explain the description of each HRQoL dimension results with their corresponding figure resume (from Figure S1 to Figure S10), and all the meta-analyses performed are reported in the supplementary data (from Supplementary Figure S11 to Supplementary Figure S61).
Table 4.
Sum of HRQoL results obtained and the corresponding questionnaires used.
 : Significant differences between the control group and the high-intensity exercise group.
: Significant differences between the control group and the high-intensity exercise group.
No comparison between the high-intensity exercise group and the low-moderate group was significant.
QLQ-C 30: European Organisation for Research and Treatment of Cancer quality of life Questionnaire, SF-36: Short-form 36, FACT-G: Functional Assessment of Cancer Therapy (General).
Physical exercise and treatment timing
When the HIEG exercise programs were implemented during cancer treatments, physical functioning (p = 0.0005, with SMD of 0.42 and a 95% CI from 0.18 to 0.66) role functioning (p = 0.0003, with SMD of 0.35 and a 95% CI from 0.16 to 0.54) and social functioning (p = 0.03, with SMD of 0.12 and a 95% CI from 0.01 to 0.23) seemed to improve more than the CG. In contrast, those variables were not significantly improved from after-treatment interventions. Moreover, outcomes of global health dimensions showed similar between-group differences in exercise programs conducted during (p = 0.02, with SMD of 0.22 and a 95% CI from 0.03 to 0.40) and after (p = 0.003, with SMD of 0.30 and a 95% CI from 0.10 to 0.50) cancer treatments.
Intervention length
Results showed higher between-group differences (HIEG vs CG) when performing HIT in exercise programs of ≤ 8 weeks duration, including physical function (p = 0.04, with SMD of 0.04 and a 95% CI from 0.01 to 0.45) and role functioning (p = 0.02, with SMD of 0.26 and a 95% CI from 0.04 to 0.49). For HIT programs lasting more than 8 weeks there was no significant between-group differences (physical function p = 0.05, role functioning p = 0.07). However, cognitive functioning reached higher significant differences between the CG and the EG in interventions longer than 8 weeks (p = 0.04, with SMD of 0.20 and a 95% CI from 0.01 to 0.40). All HIT durations showed significant differences between CG and HIEG in the global health dimensions (≤ 8 weeks: p = 0.04; > 8 weeks: p = 0.002) and fatigue (≤ 8 weeks: p = 0.008; > 8 weeks: p = 0.001).
Exercising frequency
The physical (p = 0.005, with SMD of 0.37 and a 95%CI from 0.11 to 0.62) and cognitive functioning (p = 0.003, with SMD of 0.25 and a 95% CI from 0.08 to 0.42) dimensions showed significant between-group differences with higher improvements in HIEG vs CG in interventions conducted 2 times/week. In comparison, 3 times/week programs did not show significant between-group differences (physical p = 0.09; cognitive p = 0.18). However, patients in the HIEG scored higher than the CG in role functioning with a frequency of 3 times/week (p = 0.04, with SMD of 0.21 and a 95% CI from 0.01 to 0.42), while no significant results were observed in interventions with lower frequency (p = 0.05). All of the reported exercise frequencies showed significant differences between CG and HIEG in global health (2 times/week: p = 0.002; 3 times/week: p = 0.03) and fatigue (2 times/week: p = 0.005; 3 times/week: p = 0.001) dimensions.
Minutes of exercise per week
Results showed significant improvements in HIEG compared to CG in global health (p = 0.03, with SMD of 0.18 and a 95% CI from 0.02, to 0.42) and physical functioning (p = 0.006, with SMD of 0.24 and a 95% CI from 0.07 to 0.40) only when patients exercised at least 120 min/week. The fatigue dimension was significantly improved in both shorter (< 120 weekly minutes: [p = 0.01]) and longer bouts per week (≥ 120 weekly minutes [p = 0.0005]).
Type of exercise programme
Interventions that combined resistance training and HIT showed better improvements compared to the CG in global health (p = 0.0008, with an SMD of 0.25 and a 95% CI from 0.10 to 0.39) and physical functioning (p = 0.0006, with an SMD of 0.34 and a 95% CI from 0. 15 to 0.53). Whereas programs involving only cardiovascular/aerobic HIT achieved significant between-group differences in emotional (p = 0.007, with SMD of 0.36 and a 95% CI from 0.10 to 0.63) and social functioning (p = 0.03, with SMD of 0.29 and a 95% CI from 0.03 to 0.55).
High-intensity training part duration
Patients who participated in programs with components of HIT totalling ≤ 15 min increased their physical (p = 0.003, with an SMD of 0.29 and a 95% CI from 0.10 to 0.48) and role function (p = 0.0004, with an SMD of 0.34 and a 95% CI from 0.15 to 0.54) in contrast to the CG. However, in HIT lasting longer than 15 min, no significant between-group differences were seen in those variables (p = 0.05 and p = 0.27, respectively). Moreover, when the HIT portion lasted more than 15 min, global health (p = 0.001, with an SMD of 0.32 and a 95% CI from 0.13 to 0.51) and social functioning (p = 0.03, with an SMD of 0.17 and a 95% CI from 0.01 to 0.33) seemed to improve more in the HIEG than in the CG. For cognitive (≤ 15 min: p = 0.04; > 15 min: p = 0.02) and fatigue (≤ 15 min: p = 0.0005; > 15 min: p = 0.01) improvements, both longer and shorter HIT durations showed significant between-group differences.
Furthermore, HIEG reported significant improvements compared to a CG in the overall comparison of bodily pain, dyspnea and insomnia (p = 0.02, with an SMD of − 0.18 and a 95% CI from − 0.21 to − 0.02 in pain analysis; p = 0.002 with an SMD of − 0.34 and a 95% CI from − 0.55 to − 0.13 in the dyspnoea results and p = 0.003, with an SMD of − 0.29 and a 95% CI from − 0.47 to − 0.10 in insomnia). There were no significant between-group differences in diarrhea, nausea, constipation, and appetite loss dimensions.
The meta-analysis did not include the global calculation of HRQoL because of the data heterogeneity from the different questionnaires’ measures, despite this there were significant improvements in most of the articles analyzed54–56,59,62,69,74,75 as the Supplementary Tables S4 and S5 report.
Discussion
This study aimed to explore the effect HIT on HRQoL dimensions in cancer patients and survivors. We also aimed to evaluate the optimal characteristics of HIT for dimensions of HRQoL with respect to intervention timing and cancer treatment, mode of exercise, and exercise dose. We found that HIT improves global quality of life, physical functioning, role functioning, social functioning, cognitive functioning, fatigue, pain, dyspnoea, and insomnia, compared to an inactive control group. The inclusion of resistance training seemed critical to improvements in global health and physical functioning. No significant differences were found when the effects of HIT were compared to low to moderate-intensity exercise. Improvements in HRQoL were observed during cancer treatment when training occured for more than eight weeks, with a frequency of 2 days/week, and a volume of at least 120 min/week with the HIT component duration in each session of at least 15 min.
Global health and physical function were the most commonly reported variables studied in exercise and cancer reviews, and findings here suggest that HIT consistently shows improvements in these outcomes compared to an inactive control group32,33. Data support positive global health changes with intense exercise37, but are contrary to Mugele et al. (2019), who focused solely on High Intensity Interval Training (HIIT) in their systematic review38. The broader definition of HIT might explain the data we observed here, but it is clear further studies are required to understand the role of HIT, including HIIT, specifically on HRQoL outcomes in cancer.
The subgroup analysis made regarding an intervention’s timing showed statistically positive effects in the global health dimension, physical functioning, role functioning, and social functioning during cancer treatments. Only the global health dimension showed a positive increase in after-treatment HIT. In line with our findings, the functional variables of HRQoL decrease progressively across chemotherapy78. Our data suggest that this decline might be moderated with HIT, particularly regarding depression and anxiety, function79 and activities of daily living80. Most of the negative side effects of cancer and its treatments are related to reduced physical functioning, reduced mobility due to surgery or chemotherapy81, lymphedema82, negative body composition changes as sarcopenia83, or osteoporosis84. Providing opportunities to mitigate these deleterious effects through HIT is highly important since more than half of all cancer patients develop a mobility disability because of the disease and its treatments’ adverse side-effects85.
Exercise interventions can and should be an important therapeutic modality prior to the onset of medical treatment86. Exercise has been shown to increase baseline physical functioning, reduce treatment-related impediments30, and help a patient maintain overall strength during treatment87. Post-treatment exercise can help the patient return to baseline and reduce subsequent side-effects88. The meta-analysis underlined the important role of resistance training in improving global health and physical function. Incorporating strength training in HIT programs is likely to increase muscle function, reduce the risk of sarcopenia, and reduce the risk of mortality89 and treatment toxicity90. This has been shown independent of age, cancer stage, or BMI91, and is partly explained through an anti-inflammatory response92. Further, resistance training may regulate deficiencies in skeletal muscle and adipose tissue known as cachexia93,94. However, it should be noted that interventions which included resistance training had lower adherence rates compared to aerobic exercise, which has been reported in other chronic disease patients95. Poor adherence might also explain why social and emotional functioning only significantly increased in the aerobic component programs, not in the resistance exercise modalities. To improve adherence, researchers and exercise specialists might wish to adopt a co-production approach, seeking to co-create the specific training strategies with people who have a cancer diagnosis, taking into account what matters most to them95.
Cancer and its associated treatments can cause severe side-effects during drug therapy, with pain and fatigue the most common96. Fatigue-related to cancer is reported by 70% of cancer patients97. The complaint of cancer-related fatigue is associated with immune response dysregulation, inflammation, metabolic and mitochondrial function impairment, neuroendocrine function impairment, and genetic biomarkers98; however, with exercise, these parameters can be improved99. To decrease fatigue, HIT, as well as other exercise modes, seems to be effective30, possibly more so than pharmacological or psychological therapies100. Other symptoms like pain, insomnia, and dyspnea also appear to improve via exercise35and without aggravating cancer symptoms, although this requires further investigation101.
Interventions lasting more than 8 weeks reported greater increases in HRQoL compared to shorter duration programs, which is consistent with a previous review of HIT interventions37. Greater improvements across a range of cancer-related outcomes were observed with exercising 3 times/week compared to training 2 times/week, except for role functioning (3 times/week). The American College of Sports Medicine recommends exercising two to three times/week101, which agrees with the findings of our study and a previous meta-analysis37. Three exercise sessions per week will also make it easier for individual cancer patients to achieve 120 min of weekly exercise, which seems to be important for increasing HRQoL, particularly when each session includes at 15 min of HIT. Some programs have included family members with hospitalized patients102.
This article presents valuable information about the role of high-intensity exercise as part of treatment and recovery in cancer, specifically in terms of HRQoL. The data from the systematic review and meta-analysis should be viewed in the light of the following limitations. Only articles written in English or Spanish were included, so not all the available information was analyzed. The intervention description, as well as the subgroup meta-analysis, was undertaken with the published available details. Where a study had incomplete data (e.g. sessions’ duration, HIT minutes, after intervention mean and standard deviation, etc.), data were omitted to the corresponding subgroup calculation. For the meta-analysis procedure, data from at least three articles were needed to make a subgroup analysis. Thus, assessments concerning the cancer type and all the subgroups analysis considering each intervention characteristic were not possible. Therefore, more information could be added with further studies. It must be considered that three of the included articles combined HIT with body awareness, relaxation, or massage interventions, each of which could influence HRQoL.
Conclusion
This is the first meta-analysis exploring the effects of HIT on the HRQoL of cancer patients and survivors. Data from this systematic review and meta-analysis suggests that HIT as part of exercise therapy for people with a cancer diagnosis can improve global health and provide physical, cognitive, and social functioning benefits compared to controls. In addition, fatigue, bodily pain, dyspnea, and insomnia decreases can be achieved with HIT, all with similar outcomes observed using low-moderate intensity exercise. Dimensions of HRQoL showed the largest positive effects when the programs were delivered as part of cancer treatment and included resistance training. Ultimately, exercise programs may need to be longer than 8 weeks, with a HIT frequency of 2 times/week, and a total duration of at least 120 min/week, including a HIT component of more at least 15 min, to achieve the highest return in HRQoL. However, as it is the first meta-analysis about the effects of HIT in the HRQoL of cancer patients and survivors, further research is required to support our findings.
Supplementary Information
Author contributions
Conceptualization A.M.L.-P., D.C.-M. and X.M.; Methodology, A.M.L.-P., D.C.-M. and A.J.; Software, A.M.L.-P. and D.C.-M.; Validation, D.C.-M., A.M.L.-P, X.M.; Formal Analysis, A.M.L.-P., D.C.-M.; Investigation, G.L, L.H., R.J.C and A.J; Resources, A.M.L.-P. X.M and D.C.-M; Data Curation, A.M.L.-P, and D.C.-M.; Writing—Original Draft Preparation, D.C.-M., A.M.L.-P and X.M.; Writing—Review & Editing, A.J, D.C.-M. and A.M.L.-P.; Supervision, G.L, L.H., R.J.C and X.M; Project Administration, D.C.-M. and A.J. All authors have read and approved the published version of the manuscript.
Funding
The author A.M.L.-P. is supported by the Industrial Doctorate Spanish National grant program, part of the Strategic Plan on Science and Innovation Support of the Spanish Ministry of Science, Innovation and Universities. The predoctoral industry grant identification number is DIN2018-010129.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94476-y.
References
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the "Silver Tsunami": Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016;25:1029–1036. doi: 10.1158/1055-9965.epi-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cella DF, Tulsky DS. Quality of life in cancer: Definition, purpose, and method of measurement. Cancer Investig. 1993;11:327–336. doi: 10.3109/07357909309024860. [DOI] [PubMed] [Google Scholar]
- 3.Herrero F, et al. Is cardiorespiratory fitness related to quality of life in survivors of breast cancer? J. Strength Cond. Res. 2006;20:535–540. doi: 10.1519/r-18215.1. [DOI] [PubMed] [Google Scholar]
- 4.Sitlinger A, Zafar SY. Health-related quality of life: The impact on morbidity and mortality. Surg. Oncol. Clin. N. Am. 2018;27:675–684. doi: 10.1016/j.soc.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Vulpen JK, Peeters PH, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: A meta-analysis. Maturitas. 2016;85:104–111. doi: 10.1016/j.maturitas.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Konieczny M, Cipora E, Sygit K, Fal A. Quality of life of women with breast cancer and socio-demographic factors. Asian Pac. J. Cancer Prev. 2020;21:185–193. doi: 10.31557/apjcp.2020.21.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yfantis A, et al. Health-related quality of life of young women with breast cancer. Review of the literature. J. B.U.O.N. 2018;23:1–6. [PubMed] [Google Scholar]
- 8.Yoo JS, et al. The association of physical function and quality of life on physical activity for non-small cell lung cancer survivors. Support Care Cancer. 2020 doi: 10.1007/s00520-020-05302-6. [DOI] [PubMed] [Google Scholar]
- 9.Balhareth A, Aldossary MY. Impact of physical activity and diet on colorectal cancer survivors' quality of life: A systematic review. World J Surg. 2019;17:153. doi: 10.1186/s12957-019-1697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers SK, et al. Trajectories of quality of life, life satisfaction, and psychological adjustment after prostate cancer. Psychooncology. 2017;26:1576–1585. doi: 10.1002/pon.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro CL. Cancer survivorship. N. Engl. J. Med. 2018;379:2438–2450. doi: 10.1056/NEJMra1712502. [DOI] [PubMed] [Google Scholar]
- 12.Yang S, Chu S, Gao Y, Ai Q, Liu Y. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells. 2019 doi: 10.3390/cells8070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Fabbro E, Orr TA, Stella SM. Practical approaches to managing cancer patients with weight loss. Curr. Opin. Support. Palliat. Care. 2017;11:272–277. doi: 10.1097/spc.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg MM, et al. Weight change during chemotherapy in breast cancer patients: A meta-analysis. BMC Cancer. 2017;17:259. doi: 10.1186/s12885-017-3242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr. Clin. Pract. 2017;32:30–39. doi: 10.1177/0884533616680354. [DOI] [PubMed] [Google Scholar]
- 16.Navari RM. Managing nausea and vomiting in patients with cancer: What works. Oncology. 2018;32:121–125, 131, 136. [PubMed] [Google Scholar]
- 17.Feller L, et al. Pain: Persistent postsurgery and bone cancer-related pain. J. Int. Med. Res. 2019;47:528–543. doi: 10.1177/0300060518818296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freites-Martinez A, et al. Hair disorders in patients with cancer. J. Am. Acad. Dermatol. 2019;80:1179–1196. doi: 10.1016/j.jaad.2018.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meriggi F. Dyspnea in cancer patients: A well-known and neglected symptom. Rev. Recent Clin. Trials. 2018;13:84–88. doi: 10.2174/1574887113666180326112116. [DOI] [PubMed] [Google Scholar]
- 20.Fleming L, et al. Insomnia in breast cancer: A prospective observational study. Sleep. 2019 doi: 10.1093/sleep/zsy245. [DOI] [PubMed] [Google Scholar]
- 21.Larkin PJ, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018;29(Suppl 4):iv111–iv125. doi: 10.1093/annonc/mdy148. [DOI] [PubMed] [Google Scholar]
- 22.Yennurajalingam S, Barla SR, Arthur J, Chisholm GB, Bruera E. Frequency and characteristics of drowsiness, somnolence, or daytime sleepiness in patients with advanced cancer. Palliat. Support. Care. 2019;17:459–463. doi: 10.1017/s1478951518000779. [DOI] [PubMed] [Google Scholar]
- 23.Erickson JM, et al. Symptoms and symptom clusters in adolescents receiving cancer treatment: A review of the literature. Int. J. Nurs. Stud. 2013;50:847–869. doi: 10.1016/j.ijnurstu.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Singh GK, Yadav V, Singh P, Bhowmik KT. Radiation-induced malignancies making radiotherapy a “two-edged sword”: A review of literature. World J. Oncol. 2017;8:1–6. doi: 10.14740/wjon996w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellegrini L, et al. MicroRNAs in cancer treatment-induced cardiotoxicity. Cancers. 2020 doi: 10.3390/cancers12030704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng HS, Roder D, Koczwara B, Vitry A. Comorbidity, physical and mental health among cancer patients and survivors: An Australian population-based study. Asia Pac. J. Clin. Oncol. 2018;14:e181–e192. doi: 10.1111/ajco.12677. [DOI] [PubMed] [Google Scholar]
- 27.Byar, K. L., Berger, A. M., Bakken, S. L. & Cetak, M. A. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncology nursing forum (2006). [DOI] [PubMed]
- 28.Kalter J, et al. Effects and moderators of psychosocial interventions on quality of life, and emotional and social function in patients with cancer: An individual patient data meta-analysis of 22 RCTs. Psychooncology. 2018;27:1150–1161. doi: 10.1002/pon.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Courneya KS, et al. The group psychotherapy and home-based physical exercise (group-hope) trial in cancer survivors: Physical fitness and quality of life outcomes. Psychooncology. 2003;12:357–374. doi: 10.1002/pon.658. [DOI] [PubMed] [Google Scholar]
- 30.Stout NL, Baima J, Swisher AK, Winters-Stone KM, Welsh J. A systematic review of exercise systematic reviews in the cancer literature (2005–2017) PM & R. 2017;9:S347–s384. doi: 10.1016/j.pmrj.2017.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott K, Posmontier B. Exercise interventions to reduce cancer-related fatigue and improve health-related quality of life in cancer patients. Holist. Nurs. Pract. 2017;31:66–79. doi: 10.1097/hnp.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 32.Sweegers MG, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2018;52:505–513. doi: 10.1136/bjsports-2017-097891. [DOI] [PubMed] [Google Scholar]
- 33.Buffart LM, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Hong F, Ye W. Exercise intervention improves clinical outcomes, but the "time of session" is crucial for better quality of life in breast cancer survivors: A systematic review and meta-analysis. Cancers. 2019 doi: 10.3390/cancers11050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakano J, et al. Effects of aerobic and resistance exercises on physical symptoms in cancer patients: A meta-analysis. Integr. Cancer Ther. 2018;17:1048–1058. doi: 10.1177/1534735418807555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cramer H, Lange S, Klose P, Paul A, Dobos G. Yoga for breast cancer patients and survivors: A systematic review and meta-analysis. BMC Cancer. 2012;12:412. doi: 10.1186/1471-2407-12-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toohey K, Pumpa K, McKune A, Cooke J, Semple S. High-intensity exercise interventions in cancer survivors: A systematic review exploring the impact on health outcomes. J. Cancer Res. Clin. Oncol. 2018;144:1–12. doi: 10.1007/s00432-017-2552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mugele H, et al. High-intensity interval training in the therapy and aftercare of cancer patients: A systematic review with meta-analysis. J. Cancer Surviv. Res. Pract. 2019;13:205–223. doi: 10.1007/s11764-019-00743-3. [DOI] [PubMed] [Google Scholar]
- 39.Cormie P, et al. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: A randomised controlled trial. J. Cancer Surviv. 2013;7:413–424. doi: 10.1007/s11764-013-0284-8. [DOI] [PubMed] [Google Scholar]
- 40.Devin JL, et al. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: A randomised controlled trial. J. Cancer Surviv. Res. Pract. 2016;10:467–479. doi: 10.1007/s11764-015-0490-7. [DOI] [PubMed] [Google Scholar]
- 41.Devin JL, et al. Acute high intensity interval exercise reduces colon cancer cell growth. J. Physiol. 2019;597:2177–2184. doi: 10.1113/JP277648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Page M J, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ372, 71 10.1136/bmj.n71 (2021). [DOI] [PMC free article] [PubMed]
- 43.Lavín‐Pérez, A. M. et al. High‐intensity exercise to improve cardiorespiratory fitness in cancer patients and survivors: A systematic review and meta‐analysis. Scand. J. Med. Sci. Sports31(2), 265-294 (2020). [DOI] [PubMed]
- 44.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003;83:713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- 45.Moseley AM, Elkins MR, Van der Wees PJ, Pinheiro MB. Using research to guide practice: The Physiotherapy Evidence Database (PEDro) Braz. J. Phys. Ther. 2019 doi: 10.1016/j.bjpt.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fayers, P. et al. The EORTC QLQ-C30 scoring manual. European Organisation for Research and Treatment of Cancer, 3 (2001)
- 47.RevMan, R. The Nordic Cochrane centre, the Cochrane collaboration. Book [computer program] (2014).
- 48.Higgins, J. P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., & Welch, V. A. (Eds.). Cochrane handbook for systematic reviews of interventions. John Wiley & Sons (2011).
- 49.Schmidt FL, Oh IS, Hayes TL. Fixed-versus random-effects models in meta-analysis: Model properties and an empirical comparison of differences in results. Br. J. Math. Stat. Psychol. 2009;62:97–128. doi: 10.1348/000711007X255327. [DOI] [PubMed] [Google Scholar]
- 50.Brunet J, Burke S, Grocott MP, West MA, Jack S. The effects of exercise on pain, fatigue, insomnia, and health perceptions in patients with operable advanced stage rectal cancer prior to surgery: a pilot trial. BMC Cancer. 2017;17:153. doi: 10.1186/s12885-017-3130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Møller T, et al. The challenge of preserving cardiorespiratory fitness in physically inactive patients with colon or breast cancer during adjuvant chemotherapy: A randomised feasibility study. BMJ Open Sport. Exerc. Med. 2015;1:e000021–e000021. doi: 10.1136/bmjsem-2015-000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro) Aust. J. Physiother. 2002;48:43–50. doi: 10.1016/S0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 53.Mijwel S, et al. Adding high-intensity interval training to conventional training modalities: Optimizing health-related outcomes during chemotherapy for breast cancer: The OptiTrain randomized controlled trial. Breast Cancer Res. Treat. 2018;168:79–93. doi: 10.1007/s10549-017-4571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin E, Battaglini C, Hands B, Naumann FL. Higher-intensity exercise helps cancer survivors remain motivated. J. Cancer Surviv. Res. Pract. 2016;10:524–533. doi: 10.1007/s11764-015-0498-z. [DOI] [PubMed] [Google Scholar]
- 55.Pereira-Rodríguez JE, et al. Fatiga asociada al cáncer de mama luego de un programa de entrenamiento. Acta Médica Costarricense. 2020;62:18–25. doi: 10.51481/amc.v62i1.1056. [DOI] [Google Scholar]
- 56.Waked IS, Attalla AF, Deghidi AHN. High intensity physical training exercise program in improving breast cancer related fatigue. Int. J. Physiother. 2016;3:29–34. doi: 10.15621/ijphy/2016/v3i1/88905. [DOI] [Google Scholar]
- 57.van Waart H, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. J. Clin. Oncol. 2015;33:1918–1927. doi: 10.1200/jco.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 58.van Waart H, et al. Recruitment to and pilot results of the PACES randomized trial of physical exercise during adjuvant chemotherapy for colon cancer. Int. J. Colorectal. Dis. 2018;33:29–40. doi: 10.1007/s00384-017-2921-6. [DOI] [PubMed] [Google Scholar]
- 59.Dunne DF, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br. J. Surg. 2016;103:504–512. doi: 10.1002/bjs.10096. [DOI] [PubMed] [Google Scholar]
- 60.Edvardsen E, et al. High-intensity training following lung cancer surgery: A randomised controlled trial. Thorax. 2015;70:244–250. doi: 10.1136/thoraxjnl-2014-205944. [DOI] [PubMed] [Google Scholar]
- 61.Hwang C-L, Yu C-J, Shih J-Y, Yang P-C, Wu Y-T. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support. Care Cancer. 2012;20:3169–3177. doi: 10.1007/s00520-012-1452-5. [DOI] [PubMed] [Google Scholar]
- 62.Midtgaard J, et al. Efficacy of multimodal exercise-based rehabilitation on physical activity, cardiorespiratory fitness, and patient-reported outcomes in cancer survivors: A randomized, controlled trial. Ann. Oncol. 2013;24:2267–2273. doi: 10.1093/annonc/mdt185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams SC, et al. Effects of high-intensity interval training on fatigue and quality of life in testicular cancer survivors. Br. J. Cancer. 2018;118:1313–1321. doi: 10.1038/s41416-018-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toohey KL. Effects of high vs. moderate intensity exercise on functional fitness and quality of life in cancer survivors: A pilot study. Med. Sci. Sports Exerc. 2015;47:464–464. doi: 10.1249/01.mss.0000477705.08629.eb. [DOI] [Google Scholar]
- 65.Persoon S, et al. Randomized controlled trial on the effects of a supervised high intensity exercise program in patients with a hematologic malignancy treated with autologous stem cell transplantation: Results from the EXIST study. PLoS ONE. 2017 doi: 10.1371/journal.pone.0181313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmitt J, Lindner N, Reuss-Borst M, Holmberg HC, Sperlich B. A 3-week multimodal intervention involving high-intensity interval training in female cancer survivors: A randomized controlled trial. Physiol. Rep. 2016 doi: 10.14814/phy2.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kampshoff CS, et al. Participation in and adherence to physical exercise after completion of primary cancer treatment. Int. J. Behav. Nutr. Phys. Act. 2016 doi: 10.1186/s12966-016-0425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adamsen L, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ Br. Med. J. 2009 doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andersen C, et al. The effects of a six-week supervised multimodal exercise intervention during chemotherapy on cancer-related fatigue. Eur. J. Oncol. Nurs. 2013;17:331–339. doi: 10.1016/j.ejon.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Christensen JF, et al. Safety and feasibility of preoperative exercise training during neoadjuvant treatment before surgery for adenocarcinoma of the gastro-oesophageal junction. BJS Open. 2019;3:74–84. doi: 10.1002/bjs5.50110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egegaard T, Rohold J, Lillelund C, Persson G, Quist M. Pre-radiotherapy daily exercise training in non-small cell lung cancer: A feasibility study. Rep. Pract. Oncol. Radiother. 2019;24:375–382. doi: 10.1016/j.rpor.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adams S, et al. A randomized controlled trial of the effects of high-intensity aerobic interval training on fatigue, psychosocial function, and health-related quality of life in testicular cancer survivors. Psychooncology. 2018;27:68–68. [Google Scholar]
- 73.Kampshoff CS, et al. Randomized controlled trial of the effects of high intensity and low-to-moderate intensity exercise on physical fitness and fatigue in cancer survivors: Results of the Resistance and Endurance exercise After ChemoTherapy (REACT) study. BMC Med. 2015;13:275. doi: 10.1186/s12916-015-0513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toohey K, et al. Does low volume high-intensity interval training elicit superior benefits to continuous low to moderate-intensity training in cancer survivors? World J. Clin. Oncol. 2018;9:1–12. doi: 10.5306/wjco.v9.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toohey K, et al. A pilot study examining the effects of low-volume high-intensity interval training and continuous low to moderate intensity training on quality of life, functional capacity and cardiovascular risk factors in cancer survivors. PeerJ. 2016 doi: 10.7717/peerj.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Venkatesan K, Zacharakis E, Andrich DE, Mundy AR. Conservative management of urorectal fistulae. Urology. 2013;81:1352–1356. doi: 10.1016/j.urology.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 77.Fayers, P., Aaronson, N. K., Bjordal, K. & Sullivan, M. EORTC QLQ–C30 scoring manual. European Organisation for Research and Treatment of Cancer (1995).
- 78.Mayrbäurl B, et al. Quality of life across chemotherapy lines in patients with advanced colorectal cancer: A prospective single-center observational study. Support Care Cancer. 2016;24:667–674. doi: 10.1007/s00520-015-2828-0. [DOI] [PubMed] [Google Scholar]
- 79.Pergolotti M, et al. Activities, function, and health-related quality of life (HRQOL) of older adults with cancer. J. Geriatr. Oncol. 2017;8:249–254. doi: 10.1016/j.jgo.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brekke MF, La Cour K, Brandt Å, Peoples H, Wæhrens EE. The association between ADL ability and quality of life among people with advanced cancer. Occup. Ther. Int. 2019;1–10:2019. doi: 10.1155/2019/2629673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Groef A, et al. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: A systematic review. Arch. Phys. Med. Rehabil. 2015;96:1140–1153. doi: 10.1016/j.apmr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 82.Allam O, et al. The impact of radiation on lymphedema: a review of the literature. Gland Surg. 2020;9:596–602. doi: 10.21037/gs.2020.03.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baracos VE, Arribas L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018;29:ii1–ii9. doi: 10.1093/annonc/mdx810. [DOI] [PubMed] [Google Scholar]
- 84.Handforth C, D’Oronzo S, Coleman R, Brown J. Cancer Treatment and Bone Health. Calcif. Tissue Int. 2018;102:251–264. doi: 10.1007/s00223-017-0369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campbell G, Hagan T, Gilbertson-White S, Houze M, Donovan H. Cancer and treatment-related symptoms are associated with mobility disability in women with ovarian cancer: A cross-sectional study. Gynecol. Oncol. 2016;143:578–583. doi: 10.1016/j.ygyno.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simcock, R. Principles and guidance for prehabilitation within the management and support of people with cancer in partnership with acknowledgements.Prehabilitation in Cancer Treatment (2019).
- 87.Padilha CS, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: A meta-analysis. J. Cancer Surviv. 2017;11:339–349. doi: 10.1007/s11764-016-0592-x. [DOI] [PubMed] [Google Scholar]
- 88.Fuller JT, Hartland MC, Maloney LT, Davison K. Therapeutic effects of aerobic and resistance exercises for cancer survivors: A systematic review of meta-analyses of clinical trials. Br. J. Sports Med. 2018;52:1311. doi: 10.1136/bjsports-2017-098285. [DOI] [PubMed] [Google Scholar]
- 89.Villaseñor A, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: The HEAL study. J. Cancer Surviv. Res. Pract. 2012;6:398–406. doi: 10.1007/s11764-012-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prado CM, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.ccr-08-2242. [DOI] [PubMed] [Google Scholar]
- 91.Caan BJ, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malietzis G, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann. Surg. 2016;263:320–325. doi: 10.1097/sla.0000000000001113. [DOI] [PubMed] [Google Scholar]
- 93.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat. Rev. Dis. Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 94.Hardee JP, Counts BR, Carson JA. Understanding the role of exercise in cancer cachexia therapy. Am. J. Lifestyle Med. 2017;13:46–60. doi: 10.1177/1559827617725283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plotnikoff RC, Courneya KS, Trinh L, Karunamuni N, Sigal RJ. Aerobic physical activity and resistance training: An application of the theory of planned behavior among adults with type 2 diabetes in a random, national sample of Canadians. Int. J. Behav. Nutr. Phys. Act. 2008;5:61–61. doi: 10.1186/1479-5868-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niklasson A, Paty J, Rydén A. Talking about breast cancer: Which symptoms and treatment side effects are important to patients with advanced disease? The Patient. 2017;10:719–727. doi: 10.1007/s40271-017-0242-z. [DOI] [PubMed] [Google Scholar]
- 97.Tavio M, Milan I, Tirelli U. Cancer-related fatigue (review) Int. J. Oncol. 2002;21:1093–1099. [PubMed] [Google Scholar]
- 98.Saligan LN, et al. The biology of cancer-related fatigue: A review of the literature. Support. Care Cancer. 2015;23:2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27:10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 100.Mustian KM, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue. JAMA Oncol. 2017;3:961. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Campbell KL, et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019;51:2375–2390. doi: 10.1249/mss.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martínez-Velilla N, et al. An exercise program with patient’s involvement and family support can modify the cognitive and affective trajectory of acutely hospitalized older medical patients: A pilot study. Aging Clin. Exp. Res. 2015 doi: 10.1007/s40520-015-0434-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.