Abstract
Approximately half of genetic disease-associated mutations cause aberrant splicing. However, a widely applicable therapeutic strategy to splicing diseases is yet to be developed. Here, we analyze the mechanism whereby IKBKAP-familial dysautonomia (FD) exon 20 inclusion is specifically promoted by a small molecule splice modulator, RECTAS, even though IKBKAP-FD exon 20 has a suboptimal 5′ splice site due to the IVS20 + 6 T > C mutation. Knockdown experiments reveal that exon 20 inclusion is suppressed in the absence of serine/arginine-rich splicing factor 6 (SRSF6) binding to an intronic splicing enhancer in intron 20. We show that RECTAS directly interacts with CDC-like kinases (CLKs) and enhances SRSF6 phosphorylation. Consistently, exon 20 splicing is bidirectionally manipulated by targeting cellular CLK activity with RECTAS versus CLK inhibitors. The therapeutic potential of RECTAS is validated in multiple FD disease models. Our study indicates that small synthetic molecules affecting phosphorylation state of SRSFs is available as a new therapeutic modality for mechanism-oriented precision medicine of splicing diseases.
Subject terms: Chemical biology, RNA splicing, Peripheral neuropathies
Familial dysautonomia is caused by splicing mutation of IKBKAP gene, which induces skipping of exon 20 and subsequent functional loss. Here, the authors report that a synthetic splice modulator RECTAS ameliorates pathogenic exon 20 skipping and shows therapeutic effects in cellular and animal models.
Introduction
Splicing mutations through the creation or disruption of splicing cis-elements induce constitutive gain or loss or the alternative splicing of target exons, which in turn prevent functional protein production by generating stop codons or frameshifts. Pre-mRNA splicing is catalyzed by five essential small nuclear RNAs (U1, U2, U4, U5, and U6 snRNA) and more than 100 proteins. In addition to these essential factors (e.g., U1 small nuclear ribonucleoprotein (snRNP) to recognize the 5′-splice site and U2 snRNP to recognize the branchpoint sequence), various trans-acting splicing factors are recruited to cis-regulatory elements to orchestrate alternative splicing. Several approaches have been taken to amend mutation-derived splicing defects by targeting either cis-regulatory elements or trans-acting splice factors. The former approach includes the antisense-oligonucleotide drug nusinersen, which rescues deficient SMN2 exon 7 inclusion for spinal muscular atrophy therapy1–3, and small-molecule compounds, such as SMN-C3 and RG7916, that also promote SMN2 exon 7 inclusion, in part by directly binding to an imperfect RNA helix formed by the exon 7 5′-splice site and U1 snRNA acting for bulge repair4–8. Examples of the latter approach include small-molecule inhibitors for CDC-like kinase (CLK) or serine/arginine-rich protein kinase (SRPK)9, which then inactivate serine/arginine-rich splicing factors (SRSFs) through serine phosphorylation in their carboxy-terminal RS domain. Previously, we synthesized CLK inhibitors10 and demonstrated their therapeutic potential in disease models of cystic fibrosis (c.3849 + 10 kb C > T mutation of cystic fibrosis transmembrane conductance regulator (CFTR))11, Duchenne muscular dystrophy (c.4303 G > T mutation of dystrophin)12, and anhidrotic ectodermal dysplasia with immunodeficiency (EDA-ID; IVS6 + 866 C > T mutation of NF-kappa-B essential modulator (NEMO))13.
In parallel, we developed a dual-color splicing reporter system combining two different fluorescent proteins to identify both cis-elements and trans-acting factors involved in targeting alternative splicing events in worms, mice, and cultured cells14–16. Recently, we applied this system to screen a chemical library and found a small-molecule RECTAS, which corrects abnormal splicing caused by the IVS20 + 6 T > C splicing mutation of the (inhibitor of κ light polypeptide gene enhancer in B-cells, kinase complex-associated protein (IKBKAP) gene17. The homozygous mutation is mainly found in the Ashkenazi Jewish population and is responsible for >99.5% of cases of familial dysautonomia (FD) (also known as Riley-Day syndrome or hereditary sensory/autonomic neuropathy type III)18–20. FD is an autosomal recessive genetic disease, and autonomic neurons and peripheral sensory neurons derived from neural crest cells are impaired in development, function, and survival18–20. Patients with FD exhibit various symptoms including cardiovascular instability, recurrent pneumonia, vomiting/dysautonomic crisis, gastrointestinal dysfunction, decreased sensitivity to pain and temperature, and defective lacrimation. The IVS20 + 6 T > C mutation causes abnormal IKBKAP exon 20 skipping, due to impaired recognition of the mutant 5′ splice site by U1 snRNP21,22, leading to a frameshift and the generation of a premature termination codon in exon 21 of IKBKAP mRNA20. This frameshift in turn results in reduced expression of IKAP (or ELP1), compromising tRNA modification and neuronal cell survival17,23,24. RECTAS directly affects mRNA splicing, and our transcriptome analyses revealed that RECTAS affects the splicing of only a limited set of genes, suggesting that it has high specificity and a direct role in correcting IKBKAP aberrant splicing17. Sequence analysis of the responsive exons suggests that RECTAS promotes exon recognition by either inhibiting splicing repressors, such as heterogeneous nuclear ribonucleoproteins (hnRNPs), or stimulating splicing activators. RBM24 functions as a tissue-dependent splice activator by binding to a cryptic intronic splicing enhancer element (IVS20 + 13 to +29) downstream of the intronic 5′ splice-site mutation of IKBKAP-FD and promotes U1 snRNP recognition of the mutant 5′ splice site25. Thus, undetectable RBM24 expression in neuronal tissues explains the greater extent of aberrant splicing of IKBKAP-FD exon 20 in neurons25. In this study, we revealed that SRSF6 is also involved in exon 20 recognition and that RECTAS specifically rectifies aberrant splicing by promoting the SRSF6 function.
Results
Binding of SRSF6 to an intronic splice enhancer (ISE) is required for IKBKAP-FD exon 20 inclusion
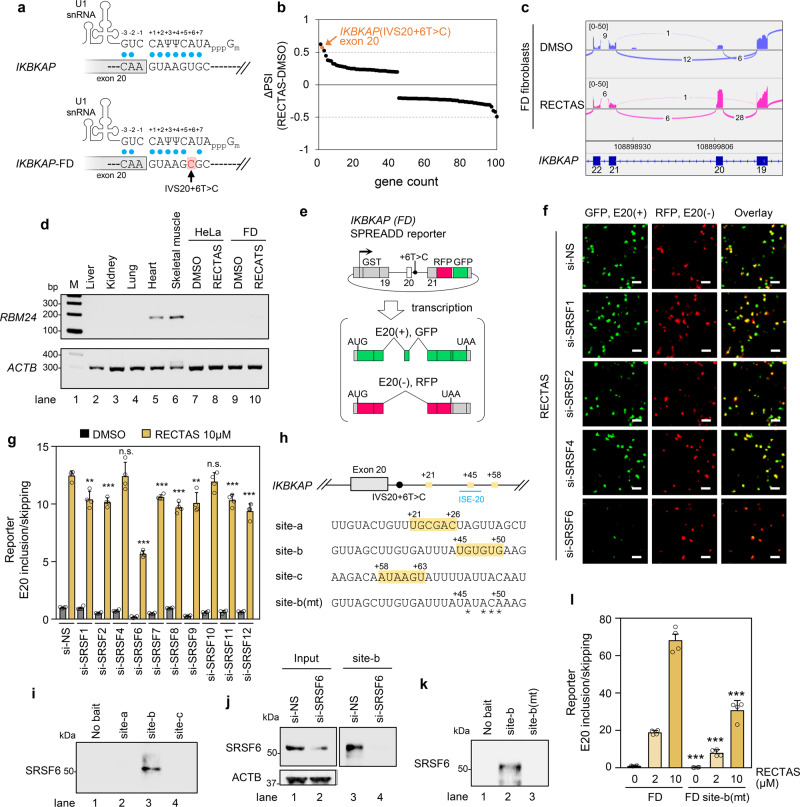
IKBKAP-FD exon 20 has a suboptimal 5′ splice site, due to the IVS20 + 6 T > C mutation. It lacks complementarity to U1 snRNA at the −1 and +6 positions (Fig. 1a), and such suboptimal splice sites are more prone to be regulated by alternative splicing factors11–13,26,27, as we reported for promotion of spliceosomal recognition of this 5′ splice site by RBM24 in skeletal muscle tissue25. The RNA sequencing (RNA-Seq) evaluation of the transcriptome of FD patient fibroblasts homozygous for the IKBKAP IVS20 + 6 T > C mutation following RECTAS treatment revealed a highly selective action of RECTAS on IKBKAP-FD exon 20, with the second-highest ΔPSI (percent spliced-in) of 618 altered splicing events with ≥0.1 of | ΔPSI | (Fig. 1b, c and Supplementary Data 1). As we supposed that RECTAS rescued exon 20 skipping as part of the overall context of splicing regulation, we checked whether RBM24 function is involved in RECTAS-induced exon 20 inclusion. Western blot for human tissues as well as HeLa and FD patient fibroblasts, responder cells to RECTAS17, indicates RBM24 expression is exclusive in heart and skeletal muscle but absent in RECTAS responder cells, irrespective of RECTAS treatment (Fig. 1d). These observations ruled out the involvement of RBM24 in RECTAS-induced splice modification. Therefore, we hypothesized that RECTAS interacts with an unidentified splicing regulator(s) that promotes the restoration of normal exon 20 inclusion. To test this hypothesis, we examined loss-of-function study by RNAi knockdown for individual SRSFs, a major family of alternative splicing activators, and compared the extent of RECTAS-induced promotion of IKBKAP-FD exon 20 inclusion, using a dual fluorescent splicing reporter system we previously developed (Fig. 1e)11,13,17. We observed a marked reduction (~60%) in exon 20 inclusion when SRSF6 was knocked down (Fig. 1f, g), whereas depletion of nine other SRSFs yielded no or only a modest effect on this splicing event (Fig. 1f, g).
Fig. 1. IKBKAP-FD exon 20 requires SRSF6 binding to an ISE.
a The IKBKAP exon 20 5′ splice site has nearly optimal pairing to U1 snRNA, whereas the IVS20 + 6 T > C creates an additional mismatch at +6 position, resulting in suboptimal recognition by U1 snRNP. Blue dots indicate base pairing. b Top 100 differential splice events by | ΔPSI (PSIRECTAS-PSIDMSO) | following RECTAS treatment (2 µM, 8 h) in FD patient fibroblasts. c Sashimi plot with junction read number of IKBKAP-FD exon 19–22 for DMSO- or RECTAS-treated FD patient fibroblasts. The displayed read count range was set to 0–50. d RT-PCR for RBM24 expression in human tissues, and cell lines treated with 10 µM RECTAS or 0.1% DMSO for 24 h. RBM24 and ACTB were detected with primers oAM669 + oAM671 and oAM13 + oAM14, respectively. e Diagram of reporter system for IKBKAP-FD exon 20 splicing13,17. GFP is translated when exon 20 is included (E20(+)), whereas RFP is translated when it is skipped (E20(−)). f, g Microscopic images (f) and quantification of IKBKAP-FD exon 20 inclusion (g), following treatment with RECTAS (10 µM) or 0.1% DMSO for 24 h in HeLa cells transfected with the indicated siRNAs for 72 h. h SRSF6-binding motifs starting at +21 nt (site-a), +45 nt (site-b), and +58 nt (site-c), predicted by ESE finder 3.028,29 are shown. RNA sequences for pull-down assays are indicated. Asterisks in site-b(mt) indicate mutated ribonucleotides. ISE-20 from Sinha et al.30 is also indicated. i–k Western blot of SRSF6 by 1H4 antibody for RNA pull-down products or no bait RNA control for HeLa cells (i), site-b pull-down products for HeLa cells treated with siRNA for 72 h (j), and site-b and site-b(mt) RNA pull-down products for HeLa cells (k). l Quantification of IKBKAP exon 20 inclusion rate, following RECTAS treatment for 24 h in HeLa cells with IKBKAP-FD or site-b(mt)-mutant IKBKAP (FD site-b(mt)) reporter. Data from four replicates are shown in (f), (g), and (l), and two replicates are shown in (i), (j), and (k). Representative data of two experiments are shown in (d). Columns, mean; bars, SE; n.s., P ≥ 0.05; **P < 0.01; ***P < 0.001 for unpaired two-tailed t test in (g) (vs. the RECTAS 10 µM of si-NS: si-SRSF1, P = 2.3 × 10−3; si-SRSF2, P = 1.7 × 10−4; si-SRSF4, P = 0.94; si-SRSF6, 1.5 × 10−7; si-SRSF7, P = 1.9 × 10−4; si-SRSF8, P = 1.0 × 10−4; si-SRSF9, 3.0 × 10−3; si-SRSF10, P = 0.28; si-SRSF11, P = 6.1 × 10−4; si-SRSF12, P = 1.8 × 10−4) and (l) (vs. the FD of corresponding RECTAS concentration: 0 µM, P = 2.0 × 10−4; 5 µM, P = 4.4 × 10−5; 10 µM, P = 1.0 × 10−4).
These observations indicate that SRSF6 is a crucial regulator of IKBKAP-FD exon 20 inclusion in the therapeutic splicing environment provided by RECTAS treatment. We further asked whether SRSF6 directly controls IKBKAP-FD exon 20 inclusion. For this purpose, we searched for potential SRSF6-recognition sites based on the systematic evolution of ligands by exponential enrichment (SELEX) motifs for SRSF6 with ESE finder 3.028,29 for a region downstream or upstream of the exon 20 5′-splice site (−100 to +100 nucleotides (nt)) of IKBKAP. We identified three candidate motifs 21, 45, and 58 nt downstream of the exon 20 5′ splice site (site-a, site-b, and site-c, respectively, in Fig. 1h). Subsequent validation of SRSF6 binding by RNA pull-down assay with biotin-conjugated RNAs revealed that only site-b has a high affinity to a cellular protein inferred to be SRSF6 (Fig. 1i). The band of expected SRSF6 disappeared in the pull-down products from SRSF6-knockdown cells, confirming its identity as SRSF6 (Fig. 1j). In addition, SRSF6 binding was lost when point mutations (site-b(mt) in Fig. 1h) were introduced in the SRSF6-binding motif (Fig. 1k). The functional significance of SRSF6 binding for IKBKAP-FD exon 20 recognition was subsequently examined by introducing a site-b(mt) mutation into the IKBKAP-FD exon 20 splicing reporter, and a reduction in RECTAS-led exon 20 inclusion was observed, comparable to that seen in SRSF6 knockdown cells (~60%) (Fig. 1l). These combined observations indicate that site-b is an ISE, and whose recognition by SRSF6 is crucial to provide a splicing context that restores normal splicing of IKBKAP-FD exon 20 in the presence of RECTAS. We note that the identified SRSF6-dependent ISE overlaps with a 20-nt region with ISE activity (dubbed ISE-20) revealed by our previous antisense-oligonucleotide walk30 (Fig. 1h). In addition, the SRSF6-binding site (+45 to +50) somewhat close to that of RBM24 (+13 to +29)25 imply RBM24 binding may interfere with SRSF6 binding, and may impair the effect of RECTAS in tissues with RBM24 expression.
Reciprocal control of IKBKAP-FD exon 20 splicing by RECTAS and CDC-like kinase (CLK) inhibitors suggests that CLKs are pivotal regulators
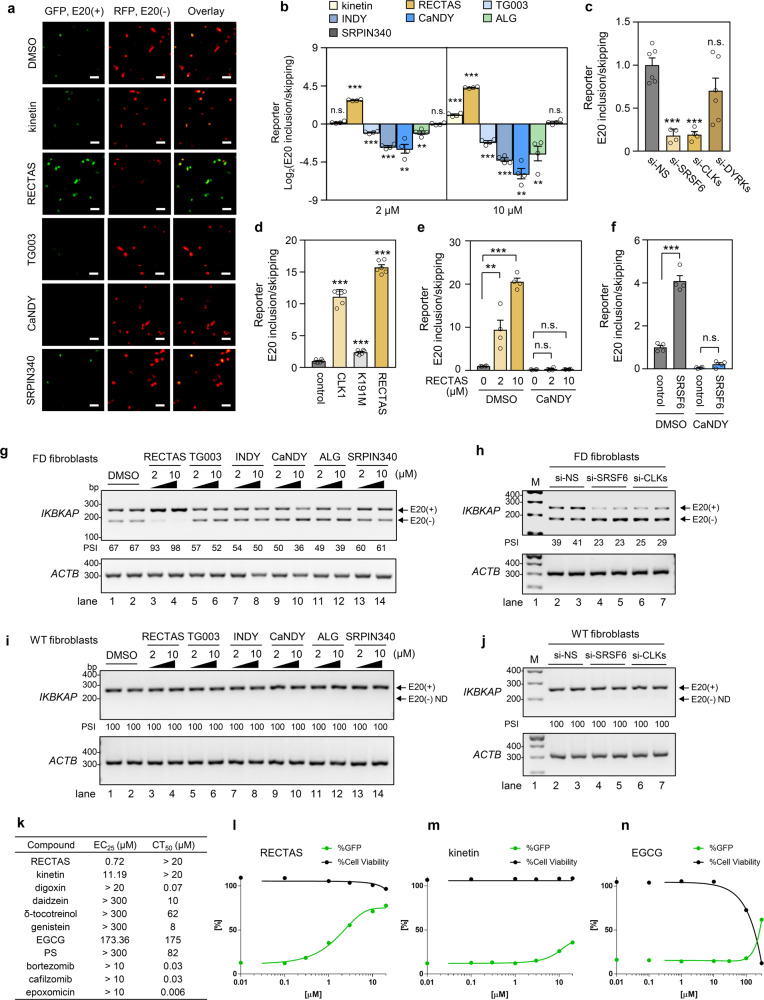
As SRSF6 is functionally activated by serine phosphorylation in its carboxyl-terminal RS domain, we next asked if kinases upstream of SRSF6 promote IKBKAP-FD exon 20 inclusion. For this purpose, we examined the effects of the following synthetic inhibitors of potential upstream SRSF kinases on IKBKAP-FD exon 20 recognition with our splicing reporter: TG003 for CLKs;10,12,13 INDY, CaNDY, and ALGERNON for dual-specificity tyrosine phosphorylation-regulated kinases (DYRKs)31–33 and CLKs11, and SRPIN340 for SRSF protein kinases (SRPKs)34,35. We used RECTAS and the plant cytokinin kinetin17,36,37 as controls with opposite effect to that expected for inhibitors of SRSF6 kinases. Treatment of cells with TG003, INDY, CaNDY, and ALGERNON at 2 and 10 µM resulted in the dose-dependent promotion of IKBKAP-FD exon 20 skipping (Fig. 2a, b), in contrast to the increased inclusion of exon 20 by RECTAS treatment (Fig. 2a, b), whereas the exon-inclusion effect of kinetin was considerably weaker, consistent with our prior observation17 (Fig. 2a, b). SRPIN340 had no effect on IKBKAP-FD exon 20 splicing (Fig. 2a, b). Some inhibitors of CLKs and DYRKs show cross-inhibition due to structural similarity at the ATP-binding pocket between these two kinase families11,25,31–33,38,39, and depletion of CLK or DYRK isoforms by RNAi was conducted to distinguish their effects. As a result, we found that the depletion of CLK isoforms inhibited IKBKAP-FD exon 20 recognition similarly to SRSF6-depleted cells (Fig. 2c), whereas depletion of DYRK isoforms had no effect (Fig. 2c). Moreover, overexpression of CLK1 mimicked the effect of RECTAS on the inclusion of IKBKAP-FD exon 20 (Fig. 2d), whereas the kinase-inactive K191M mutant of CLK1 had no effect (Fig. 2d). Dependency of RECTAS activity on CLK activities was further assessed by evaluating RECTAS response in cells under CLK inhibition. We recently reported CaNDY as a potent pan-CLK inhibitor11, and found that there is no exon 20 inclusion-promoting activity by RECTAS when CLKs were functionally inactivated (Fig. 2e). Moreover, we found SRSF6 overexpression also has exon 20 inclusion-promoting activity as seen in RECTAS treatment or CLK1 overexpression (DMSO conditions in Fig. 2f). However, this SRSF6 overexpression effect was also canceled when CLKs are inhibited (CaNDY conditions in Fig. 2f). These observations together support the idea that RECTAS depends on CLK1 activity for exon 20 inclusion-promoting activity. We further validated the effects of compound treatment (RECTAS, TG003, INDY, CaNDY, ALGERNON, and SRPIN340) and knockdown of SRSF6 or CLK in FD patient fibroblasts with the homozygous IKBKAP-FD IVS20 + 6 T > C mutation, and confirmed the increased IKBKAP-FD exon 20 skipping by CLK inhibition (Fig. 2g and Supplementary Fig. 1a) or depletion for SRSF6 or CLK (Fig. 2h and Supplementary Fig. 2a) for the endogenous transcripts. Primary fibroblasts without IVS20 + 6 T > C mutation of IKBKAP showed no exon 20 skipping following CLK inhibition (Fig. 2i and Supplementary Fig 1b) or depletion for SRSF6 or CLK (Fig. 2j and Supplementary Fig. 2b), showing the effect of RECTAS depends on the suboptimal nature of exon 20 donor sites due to IVS20 + 6 T > C (Fig. 1a). In addition, we also compared RECTAS with compounds previously reported for exon 20 inclusion promotion40, and we confirmed that RECTAS shows the highest activity (0.72 μM of EC25) over those we tested (Fig. 2k, l). Two compounds, kinetin and (−)-epigallocatechin gallate (EGCG), reached EC25 of RECTAS (Fig. 2k, m, n), but were 15.5- and 241-fold less potent and there was also cytotoxicity around the effective range for EGCG (Fig. 2n).
Fig. 2. A pivotal control of IKBKAP-FD exon 20 splicing by RECTAS and a CLK inhibitor.
a, b Microscopic images (a) and IKBKAP-FD exon 20 inclusion rates (b) following compound treatment (2 or 10 µM) or solvent only (0.1% DMSO) for 24 h in HeLa cells transfected with IKBKAP-FD reporter. ALG indicates ALGERNON in (b). c HeLa cells with IKBKAP-FD reporter were transfected with siRNA for si-SRSF6, si-CLKs, si-DYRKs, or si-NS for 72 h, and IKBKAP-FD exon 20 inclusion rates were plotted by GFP/RFP ratio. d IKBKAP-FD exon 20 inclusion was quantified by the GFP/RFP ratio of IKBKAP-FD reporter in HeLa cells, transfected with empty vector (control), CLK1 expression vector, or inactive mutant CLK1 (K191M) expression vector for 24 h, or treated with RECTAS (2 µM) for 24 h. e, f IKBKAP-FD exon 20 inclusion rates in response to RECTAS treatment for 24 h (e) or transfection with SRSF6 expression vector for 24 h (f) were quantified by GFP/RFP ratio in HeLa cells, transfected with IKBKAP-FD reporter with or without CaNDY treatment (10 µM, 24 h). control, empty p3XFLAG-CMV14 vector transfection in (f). g, h RT-PCR for IKBKAP-FD exon 19–21 for FD patient fibroblasts (P2) treated with compounds (2 or 10 µM) or solvent only (0.1 % DMSO) for 24 h (g), or transfected with si-NS, si-SRSF6, or si-CLKs for 72 h (h). i, j RT-PCR for IKBKAP-FD exon 19–21 for fibroblasts from a healthy individual (C1) treated with compounds (2 or 10 µM) or solvent only (0.1% DMSO) for 24 h (i), or transfected with si-NS, si-SRSF6, or si-CLKs for 72 h (j). k–n 25% effective concentration (EC25) and 50% cytotoxic concentration (CT50 for 72 h treatment) were shown for indicated compounds. EC25 was determined with %GFP of 20 µM RECTAS set to 100%. Individual plots for RECTAS (l), kinetin (m), and (−)-epigallocatechin gallate (EGCG) (n) are shown separately. Data from four replicates are shown in (a), (b), (c), (e), and (f), six replicates in (d), four replicates for %GFP, and three replicates for %Cell viability in (k–n). Representative data from two replicates are shown in (g–j). Columns, mean; bars, SE; n.s., P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001 for unpaired two-tailed t test in (b) (vs. the 0.1% DMSO control) (kinetin: 2 µM, P = 9.2 × 10−2; 10 µM, P = 8.8 × 10−5; RECTAS: 2 µM, P = 3.2 × 10−8; 10 µM, 3.2 × 10−9; TG003: 2 µM, 2.3 × 10−5; 10 µM, 1.3 × 10−5; INDY: 2 µM, 1.5 × 10−6; 10 µM, 1.6 × 10−6; ALG: 2 µM, P = 4.1 × 10−3; 10 µM, P = 7.1 × 10−3; SRPIN340: 2 µM, P = 0.90; 10 µM, P = 0.27), (c) (vs. si-NS: si-SRSF6, P = 6.7 × 10−5; si-CLKs, P = 7.1 × 10−5; si-DYRKs, P = 0.11), (d) (vs. control vector: CLK1, P = 1.1 × 10−9; K191M, P = 1.0 × 10−5; RECTAS, P = 6.1 × 10−12), (e) (P = 9.0 × 10−3 and P = 3.9 × 10−7 for RECTAS 2 µM and 10 µM without CaNDY, and P = 0.53 and 0.13 for RECTAS 2 µM and 10 µM with CaNDY), and (f) (DMSO, P = 3.1 × 10−5; CaNDY, P = 8.0 × 10−2). E20 (+), exon 20 inclusion product; E20 (−), exon 20 skipping product in (a), (g), (h), (i), and (j); ND, not detectable in (i) and (j); PSI, percent spliced-in in (g–j). IKBKAP and ACTB were detected with primers oAM138 + oAM139 and oAM13 + oAM14, respectively in (g–j).
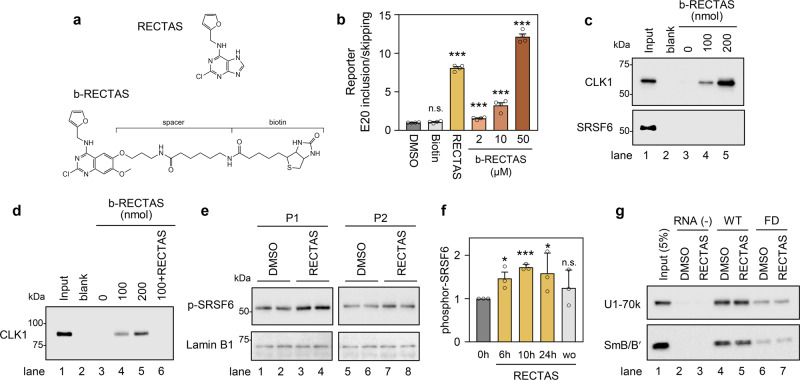
Having confirmed that CLK-dependent SRSF6 activation is required for restoration of normal splicing of IKBKAP-FD exon 20 by RECTAS, we then asked whether RECTAS affects the functions of CLKs and SRSF6. For this purpose, we synthesized a biotin-linked RECTAS analog (b-RECTAS) (Fig. 3a), which retained the IKBKAP-FD exon 20 inclusion-promoting activity, whereas biotin alone at 50 μM had no effect (Fig. 3b). We used b-RECTAS in a compound pull-down assay from HeLa cell lysates by neutravidin to evaluate its binding affinity to CLK1 and SRSF6. We found that b-RECTAS binds to CLK1 in a dose-dependent manner but not to SRSF6 (Fig. 3c). Recombinant CLK1 protein also bound to b-RECTAS in a dose-dependent manner, indicating their direct interaction (Fig. 3d). The interaction between b-RECTAS and CLK1 was effectively competed out by incubating with 500 nmol of free RECTAS (lane 6 in Fig. 3d). Western blotting for RECTAS-treated FD patient fibroblasts revealed that the SRSF6 phosphorylation was enhanced following RECTAS treatment (Fig. 3e), and this effect was detected by 6 h and disappeared by 10 h after washout of RECTAS (Fig. 3f). Since it was recently reported that several small molecules stabilize U1 snRNA-donor paring for SMN exon 7 through 5′-splice-site bulge repair8, we tested whether RECTAS has such activity on IKBKAP exon 20 donor site. RNA pull-down assay by IKBKAP exon 20 donor sequences with or without IVS20 + 6 T > C reproduced weakened affinity to U1 snRNP (detected by U1-70k and SmB/B′ subunits) by IVS20 + 6 T > C mutation (lanes 4 and 6 in Fig. 3g), whereas RECTAS had no effect on their affinity, denying its role in donor-U1 snRNA paring (lanes 5 and 7 in Fig. 3g).
Fig. 3. RECTAS directly interacts with CDC-like kinase 1 (CLK1), and it enhances cellular SRSF6 activity.
a Structures of RECTAS and b-RECTAS. b Extent of IKBKAP-FD exon 20 inclusion represented by GFP/RFP ratio, following treatment with biotin (50 µM), RECTAS (2 µM), b-RECTAS (2, 10, and 50 µM), or solvent only (0.1% DMSO) for 24 h in reporter-transfected HeLa cells. c, d Western blotting of pull-down products by b-RECTAS (0, 100, or 200 nmol/reaction) for HeLa cells transfected with a flag-tagged CLK1 expression vector (c) or GST-tagged recombinant CLK1 (d). RECTAS (500 nmol) was incubated with 100 nmol b-RECTAS for the competition assay in (d). e, f Western blot (e) for phosphor SRSF6 in FD patient primary fibroblasts (P1 and P2), treated with RECTAS (10 µM) or solvent only (0.1% DMSO) for 24 h. f Phosphor SRSF6 in FD fibroblasts (P1) was quantified at 0, 6, 10, 24 h after RECTAS (10 µM) treatment (columns, 0 h, 6 h, 10 h, and 24 h), as well as 10 h after washout of compound following RECTAS treatment (10 µM) for 24 h (column, wo). Lamin B1 served as a loading control in (e). g Western blot for U1-70k and SmB/B’ in pull-down products with biotin-conjugated RNA surrounding IKBKAP exon 20 donor site with (oAM154) or without (oAM153) IVS20 + 6 T > C mutation (labeled as FD and WT, respectively). Pull-down was conducted in the presence of 10 µM RECTAS with 1% DMSO, or 1% DMSO only. RNA (−), control sample without RNA bait. Input, HeLa nuclear extract of 5% input amount. Representative data from four replicates were shown in (b). Representative data from two replicates were shown in (c), (d), and (g). Data from two experiments are shown in (e, f) and three experiments are shown in (f). Columns, mean; bars, SE; n.d. ≥0.05; *P < 0.05; ***P < 0.001 for unpaired two-tailed t test in (b) (biotin, P = 0.26; RECTAS, P = 2.5 × 10−8; b-RECTAS 2 µM, 5.5 × 10−4; b-RECTAS 10 µM, 7.5 × 10−4; b-RECTAS 50 µM, 9.6 × 10−8) and one-tailed t test in (f) (vs. the 0 h control: 6 h, P = 1.6 × 10−2; 10 h, P = 2.8 × 10−5; 24 h, P = 4.8 × 10−2; wo, P = 0.18). No sample was loaded in blank lanes in (c, d).
The therapeutic effect of RECTAS in FD disease models
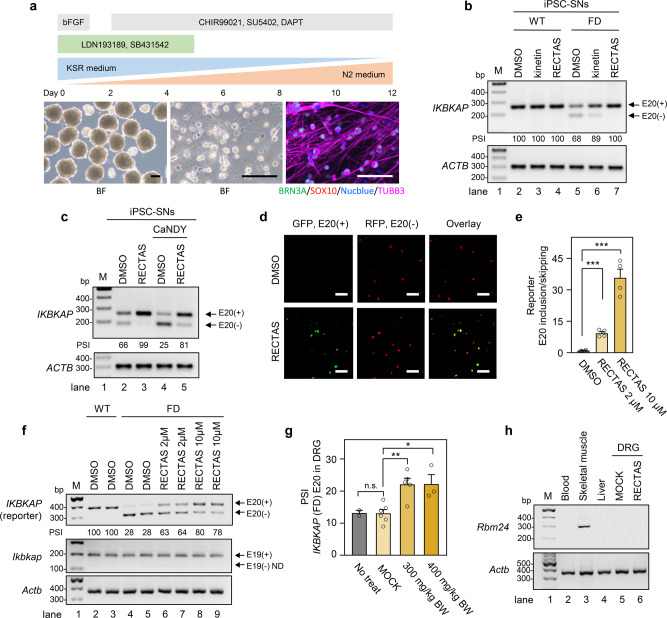
Having established the mechanism and target selectivity of RECTAS, we then assessed its therapeutic potential for FD, in two FD disease models: iPSC-SNs and IKBKAP-FD transgenic mice. As alternative splicing undergoes tissue-dependent regulation due to a varied distribution of RNA-binding proteins41, it is desirable to assess therapeutic potential in the context of sensory or autonomic neurons, which are affected in FD patients18–20. For efficient preparation of induced pluripotent stem cells-derived sensory neurons (SNs), we employed the embryoid-body method for the initial differentiation step (detailed in “Methods”). This modification resulted in higher viability and rapid derivation of the SNs (BRN3A + /TUBB3 + ) on day 12 (Fig. 4a) (see “Methods” for details). The effect of the compounds was tested by adding kinetin, RECTAS, or solvent only (0.1% dimethyl sulfoxide (DMSO)) to the culture media during the whole differentiation process. We found that 10 µM of RECTAS completely restored IKBKAP-FD exon 20 inclusion (lane 7 in Fig. 4b), while 10 µM of kinetin did not eliminate exon 20 skipping completely in patient iPSC-SNs (lane 6 in Fig. 4b). The effect of RECTAS on exon 20 inclusion was transient, and beginning around 2 h after the incubation and lasting for ~4 h after washout (Supplementary Fig. 3). We also examined the effect of CLK inhibition for RECTAS responsivity in iPSC-SNs. Consistent with observations in IKBKAP-FD reporter-transfected HeLa cells, iPSC-SNs showed impaired RECTAS activity by CLK inhibition with CaNDY treatment (Fig. 4c).
Fig. 4. RECTAS restores IKBKAP-familial dysautonomia (FD) exon 20 inclusion in patient induced pluripotent stem cells-derived sensory neurons (iPSC-SNs) and transgenic mice.
a Diagram for preparation of iPSC-SNs, showing time-course (upper), microscopic images of spheroids at day 2 (lower, left), and differentiated iPSC-SNs on day 5 (lower middle for brightfield and lower right for BRN3A/SOX10/Nucblue/TUBB3 immunocytostaining image). BF, bright field image; scale bars, 100 µm. b, c RT-PCR for IKBKAP exon 19–21 and ACTB as a loading control for differentiated iPSC-SNs from a healthy donor (WT) or FD patient (FD) on day 12 (b), and iPSC-SNs (day 12) treated with CaNDY (10 µM) or 0.1% DMSO for 2 h, followed by addition of RECTAS (10 µM) or 0.1% DMSO in media and subsequent incubation for 22 h (c). IKBKAP and ACTB were detected with primers oAM138 + oAM139 and oAM13 + oAM14, respectively. d, e Microscopic images (d) and quantification for the extent of IKBKAP-FD exon 20 inclusion (e), following treatment with RECTAS (10 µM for (d), and 2 and 10 µM for (e)) or solvent only (0.1% DMSO) for 24 h in neuro 2A cells transfected with the IKBKAP-FD reporter. Bars, 100 µm in (d). f RT-PCR for exogenous human IKBKAP (oAM124 + oAM126, primers designed for vector backbone) and endogenous mouse Ikbkap (oAM666 + oAM667, primers designed for exon 18 and 20) in neuro 2A cells transfected with IKBKAP-WT or -FD reporter and treated with 2 or 10 µM RECTAS or 0.1% DMSO for 24 h. Actb (oAM364 + oAM366) served as a loading control. g Quantification of PSI for IKBKAP-FD exon 20 in DRG from IKBKAP-FD-humanized transgenic mice administered RECTAS (300, or 400 mg/kg BW, p.o.) with a booster administration of the same dose at 4 h, and DRG were collected 8 h after the first administration to be applied for RT-PCR for IKBKAP exon 19–21 with primer set of HsIKAPRT18F + HsIKAPRT21R (n = 2 for no treatment, n = 6 for MOCK, n = 5 for 300 mg/kg BW RECTAS, and n = 3 for 400 mg/kg BW RECTAS). MOCK, control for the vehicle only. h RT-PCR for Rbm24 in blood cells, skeletal muscle, and liver tissues from 2-month-old B6 mouse, as well as DRG from MOCK or RECTAS (300 mg/kg BW)-administered IKBKAP-FD transgenic mice. Actb served as a loading control. Rbm24 and Actb were detected by primers Rbm24-F + Rbm24-R and oAM365 + oAM366, respectively. Data from three iPSC clones are shown in (a–c), four replicates were shown in (d–e), two replicates are shown in (f). Representative data from two experiments are shown in (h). E20 (+), exon 20 inclusion product; E20 (−), exon 20 skipping product; E19 (+), exon 19 inclusion product; E19 (−), exon 19 skipping product in (b), (c), and (f). Columns, mean; bars, SE; n.s., P ≥ 0.05; **P < 0.01; ***P < 0.001 for unpaired two-tailed t test in (e) (vs. DMSO control: 2 µM, P = 5.6 × 10−5; 10 µM, P = 1.5 × 10−4) and (g) (vs. MOCK control: no treat, P = 0.97; 300 mg/kg BW, 2.4 × 10−3; 400 mg/kg BW, 1.0 × 10−2); PSI, percent spliced-in in (b), (c), and (f); ND, not detectable in (f).
Next, we attempted to test the therapeutic potency of RECTAS in an FD mouse model. Prior to in vivo administration, we examined whether RECTAS also has an exon 20 inclusion-promotive effect in mice neural tissue culture, neuro 2A cells. We confirmed a marked induction of exon 20 inclusion in neuro 2A (Fig. 4d, e), indicating activity of RECTAS for human IKBKAP-FD exon 20 is retained in murine cells. RT-PCR was also confirmed for RECTAS-induced exon 20 inclusion for human IKBKAP-FD, without alteration in endogenous mouse Ikbkap exon 19 (Fig. 4f), which is homologous to exon 20 in human and lacking IVS20 + 6 T > C mutation (Supplementary Fig. 4). Then we measured the effect of per os (p.o.) administration of RECTAS, using previously established IKBKAP-FD transgenic mice, in which an IVS20 + 6 T > C-mutant human IKBKAP gene was introduced in a BAC clone, in the presence of the endogenous murine wild-type Ikbkap, as an asymptomatic splicing model42. RECTAS was orally administered at 300 or 400 mg/kg body weight (BW) with a booster administration at 4 h intervals. Eight hours after the first administration, total RNA was extracted from dorsal root ganglia (DRG) to assess the splicing profile in sensory neurons. RT-PCR revealed enhanced inclusion (about 70% increase in PSI from ~12.5 to ~22%) of IKBKAP-FD exon 20 following RECTAS administration at both doses in DRG (Fig. 4g). We subsequently conducted transcriptome analysis for DRG, excised from MOCK and RECTAS-treated (300 mg/kg BW) groups. We found IKBKAP-FD exon 20 inclusion among the highly promoted exons (13th highest alterations) (Supplementary Fig. 5 and Supplementary Data 2), consistent with its selective effect in patient primary fibroblasts (Fig. 1b, c and Supplementary Data 1). Moreover, we confirmed there was no Rbm24 expression detectable in DRG irrespective of RECTAS administration (Fig. 4h), ruling out the involvement of Rbm24 as shown for human tissue cultures (Fig. 1d). Finally, we assessed the preclinical profile of RECTAS, checking for effects on body weight change and food consumption, as well as conducting necropsy and hematology tests, both after single p.o. administration (0, 100, 300, 1000 mg/kg BW) and 14-day repetitive p.o. administration (0, 40, 200, and 1000 mg/kg BW/day) (Supplementary Table 1). There was no genotoxicity for RECTAS in the micronucleus and Ames tests (Supplementary Table 1). These preclinical data together indicate that there was no anticipated adverse effect associated with RECTAS administration, assumingly due to its high target selectivity for IKBKAP-FD exon 20 (Fig. 1b, c and Supplementary Fig. 5).
Discussion
Recent studies revealed that the prevalence of splicing mutations is much higher than previously thought, as ~30–50% of disease-associated mutations are estimated to cause abnormal splicing43,44, and the total number is further increasing due to the identification of deep-intronic splicing mutations through recent whole-genome sequencing and transcriptome analysis13,45–48. Therefore, an innovative therapeutic strategy to target misregulation of splicing is urgently needed. Small-molecule compounds that target trans-acting splice factors offer an opportunity to systematically target a particular set of mis-splicing events that are regulated by a similar splicing context. In this study, we identified alternative exons that show a dependency on a subset of SRSFs and CLK kinases are rational targets for selective manipulation in both promotive and suppressive ways (Fig. 5). Our data indicate that enhanced SRSF6 activity is a crucial aspect of the splicing environment induced by RECTAS treatment, and the requirement of CLKs in this process suggests that the direct interaction of RECTAS with CLK1 (Fig. 3c, d) sterically activates cellular CLK1. As a precedent for PDK1, the regulatory kinase of AGC kinases, there is an allosteric small-molecule activator that binds to the HM/PIF-pocket to induce a conformational change49. We assume that RECTAS binding modifies the intramolecular conformation of CLK1 to promote its catalytic activity or provide accessibility of a signal mediator upstream of CLK1. Understanding the binding mode of RECTAS to CLK1 and the activating pathway for CLKs in the context of suboptimal donor RNA recognition by SRSF6 will provide further insights into this regulation.
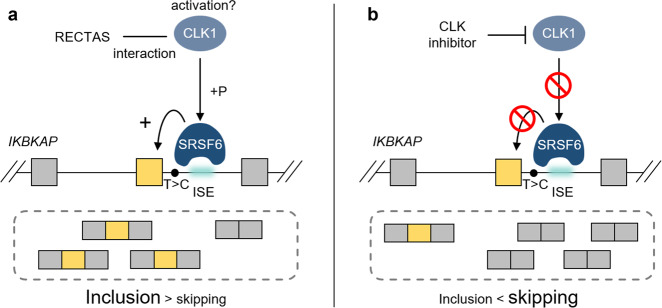
Fig. 5. Diagram for bidirectional control of IKBKAP-FD exon 20 by small molecules.
a SRSF6 binding at the ISE is a major factor in the therapeutic splicing environment induced by RECTAS treatment. The requirement of CLK1 for this process and the direct interaction between CLK1 and RECTAS implies a steric effect for the activation of cellular CLK1. b For its dependence on CLK1 and SRSF6 for recognition, IKBKAP-FD exon 20 splicing can be controlled in a reverse direction by CLK inhibitor treatment.
We found that RECTAS p.o. administration of 200–300 mg/kg BW for 8 h achieved ~22% PSI for exon 20 in DRG of IKBKAP-FD transgenic mice, versus ~12.5% baseline PSI in mock or untreated controls (Fig. 4g). In the recent report from Morini et al.50, as little as ~12.5% of IKBKAP exon 20 PSI (versus ~10% baseline) by kinetin administration (200 mg/kg BW/day in the diet for 6 months) was sufficient for the phenotypic rescue of sensory loss. Though these data were obtained with different transgenic mice and cannot be directly compared, we expect that RECTAS should exhibit higher therapeutic efficacy in vivo than kinetin, which already completed a phase 1 clinical trial in FD patients (NCT02274051 in ClinicalTrials.gov). Kinetin has been reported to have high target selectivity in FD patient fibroblasts, and our study revealed a similar target-selective profile for RECTAS. Other RECTAS-responsive exons, such as TATA-box binding protein associated factor, RNA polymerase I subunit A (TAF1A) exon 4 and par-3 family cell polarity regulator (PARD3) exon 21 (Supplementary Data 1) had no apparent effect on cell viability. Presumably, this is because alternative splicing occurs physiologically for most genes, and the safety profile determined in preclinical tests further confirmed that RECTAS is well-tolerated in rodents. In addition, we confirmed the higher activity of RECTAS than previously reported IKBKAP splice modifiers in a reporter assay (Fig. 2k), which further highlights the efficient activity of RECTAS for IKBKAP-FD exon 20 inclusion. These observations together suggest that RECTAS may prove to be a potent agent for the treatment of FD, and ongoing medicinal chemistry indicates further improvement in potency.
Searching in existing databases, there are numerous splicing mutations that resemble the context of IKBKAP-FD exon 20. For example, a splicing mutation in the potassium voltage-gated channel subfamily Q member 1 (KCNQ1), which is responsible for congenital long QT syndrome type I, has a similar splice enhancer motif, and the impaired exon of KCNQ1 is also manipulated bidirectionally in a similar manner as IKBKAP IVS20 + 6 T > C (our unpublished observations). The opportunity for bidirectional regulation by CLK inhibitors and agonists offers a strategic approach for individual splicing contexts, as promoting recognition of a target exon, on the one hand, provides a therapeutic effect for splicing mutations like the IVS20 + 6 T > C mutation of IKBKAP, whereas inhibition of a target exon, on the other hand, offers a therapeutic opportunity for splice-site-creating splicing mutations, as we previously demonstrated the therapeutic effects of CLK inhibitors in EDA-ID with the IVS6 + 866 C > T mutation of NEMO13 and cystic fibrosis with the c.3849 + 10 kb C > T mutation of CFTR11. CLK-targeting strategies may also become increasingly relevant, as recent whole-genome and transcriptome analyses highlighted the widespread presence of deep-intronic mutations that lead to the creation of cryptic pseudoexons13,45–48.
In addition, numerous splicing mutations have also been identified in recent cancer genomics studies51,52, although their individual contributions to malignancy remain largely unknown. CLK inhibitors (e.g., T-02553 and SM0850254) and SRPK inhibitors (e.g., SPHYNX55,56, SRPIN34035, and SRPKIN-157) described by other groups and us were recently reported to have anti-cancer activity in several cancer types. Moreover, small-molecule compounds that target the essential splice factor SF3B, such as spliceostatin A and E710758,59, have shown efficacy in leukemia models harboring mutations in the same or different trans-acting splice factors, namely SF3B1, SRSF2, or U2AF35, which sensitize the cells to the inhibitors60–63, though inhibition of SF3B also evokes global intron retention and cytotoxicity. In this regard, targeting CLKs by RECTAS, with a target selectivity sufficient for a favorable safety profile in preclinical tests, may provide an alternative therapeutic strategy to target cancer cells with vulnerable splicing machinery or CLK-dependent causal splicing misregulation.
Overall, our current observations with IKBKAP-FD exon 20 not only highlight RECTAS as a potential therapeutic agent for FD but also suggest the feasibility of chemical control of disease-associated missplicing by manipulating the splicing environment composed of SRSFs and CLKs in target cells. Facing the increasing prevalence of splicing mutations associated with human diseases, mechanism-oriented precision medicine for missplicing, as exemplified by this study, may provide a new framework for targeting such splicing diseases.
Methods
Cell lines
HeLa cells were obtained from the Japanese Collection of Research Bioresources (JCRB) Cell Bank of the National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN) (JCRB9004) (Osaka, Japan) and cultured in Dulbecco’s modified Eagle medium (DMEM) (Bibco of Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. Neuro 2A cells were obtained from JCRB Cell Bank of NIBIOHN (IFO50081), and cultured in DMEM (GIBCO of Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 1× GlutaMAXI (GIBCO of Thermo Fisher Scientific), 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. Fibroblasts from 19- and 26-year-old male patients with FD were provided by Coriell Institute (Camden, NJ, USA) (GM02342 (P1) and GM00850 (P2), respectively) and cultured in DMEM supplemented with 15% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. Primary fibroblasts from healthy donors (36-year-old male (TIG-114, C1) and 40-year-old female (TIG-108, C2)) were obtained from JCRB Cell Bank of NIBIOHN, and cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 µg/mL streptomycin. All cells were maintained in a cell incubator at 37 °C with 5% CO2. The tissue culture study was conducted under ethical approval by the ethics committee of Kyoto University Graduate School and Faculty of Medicine.
iPSCs
Control- and patient-derived iPSCs C1 and M1 were established previously24,64. iPSCs were maintained on Matrigel (Corning, #354277) as colonies in mTeSR1 medium (STEMCELL Technologies, #85850), according to the manufacturer’s protocol. iPSC-SNs were prepared following a published protocol24 with several modifications; the undifferentiated iPSCs were trypsinized to single cells and re-aggregated on AggreWell 800 plates (STEMCELL Technologies, 34815) in Primate embryonic stem cell medium (REPROCELL, RCHEMD001) with bFGF (5 ng/mL, WAKO, 062-06661) and Y-27632 (10 µM, 036-24023). On day 5, spheroids were transferred to Matrigel-coated cell culture plates and differentiated further. The medium was gradually changed to DMEM/F12 supplemented with N2 supplement, nonessential amino acids, and Glutamax supplement in the presence of LDN-193189 0.1 µM and SB431542 10 µM (Repro-nLS, Cellagen Technology, K6570-500) for days 0–4 and CHIR99021 3 µM, DAPT 10 µM, and SU5402 10 µM (n3i, Cellagen Technology, K6571-500) for days 2–11. On day 12, the medium was changed to a 1:1 mixture of the above N2 medium and Neurobasal medium supplemented with B27 supplement, and with brain-derived neurotrophic factor 10 ng/mL, glia-derived neurotrophic factor 10 ng/mL, and dbcAMP 1 mM for maturation. For immunocytochemistry, cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 in D-PBS (−) for 15 min, and blocked with 3% bovine serum albumin (BSA) in D-PBS (−) for 1 h. Then the cells were incubated with primary antibodies in 3% BSA in D-PBS (−) for 2 h, followed by visualization with appropriate secondary antibodies. The nuclei were counterstained with Hoechst 33342 (Thermo Fisher Scientific). Experiments using iPSCs were conducted under ethical approval by the ethics committee of Kyoto University Graduate School and Faculty of Medicine.
Antibodies
The following antibodies were used for Western blotting: anti-U1-70k mouse monoclonal antibody (9C4.1) (05-1588, Merk Millipore, Burlington, MA) at 1:500; anti-SmB/B′ mouse monoclonal antibody (Y12) (MA5-13449, Thermo Fisher Scientific) at 1:500; anti-CLK1 rabbit polyclonal antibody (ARP52021_P050, Aviva systems biology, San Diego, CA) at 1:500; anti-CLK2 rabbit polyclonal antibody (ab65082, Abcam, Cambridge, UK) at 1:500; anti-CLK3 rabbit polyclonal antibody (3256, Cell Signaling Technology, Danvers, MA) at 1:500; anti-CLK4 rabbit polyclonal antibody (ab104321, Abcam) at 1:500; anti-β-actin mouse monoclonal antibody (Ac-15) (sc-69879, Santa Cruz Biotechnology, Dallas, TX) at 1:4000; anti-SR protein (1H4G7) mouse monoclonal antibody (33–9400, Thermo Fisher Scientific) at 1:200 for phosphorylated SR proteins; anti-Lamin B1 (EPR8985) rabbit monoclonal antibody (ab133741, Abcam, Cambridge, UK) at 1:500. The following antibodies were used for immunocytostaining: anti-OCT4 rabbit monoclonal antibody (T.631.9) (MA5-14845, Thermo Fisher Scientific) at 1:400, anti-SSEA4 mouse monoclonal antibody (MC-813-70) (MA1-021, Thermo Fisher Scientific) at 1:500, anti-SOX10 rabbit monoclonal antibody (EPR4007) (ab155279, Abcam) at 1:250, anti-BRN3A mouse monoclonal antibody (5A3.2) (MAB1585, Merck Millipore) 1:200, and anti-beta III tubulin (TUBB3) rabbit monoclonal antibody (EP1569Y) (ab52623, Abcam) 1:500. Counterstaining was done with NucBlue cell stain (Thermo Fisher Scientific).
Compounds
Kinetin was purchased from Nacalai Tesque (Tokyo, Japan). (−)-epigallocatechin gallate (EGCG), daidzein, genistein, epoxomicin, and bortezomib were purchased from FUJIFILM Wako Pure Chemical (Osaka, Japan). δ-tocotrienol was produced by LKT Laboratories, Inc. (St. Paul, MN). Phosphatidylserine was produced by Larodan Fine Chemicals AB (Solna, Sweden). Digoxin was produced by Cayman Chemical (Ann Arbor, MI). Carfilzomib was purchased from Cell Signaling Technology. b-RECTAS was synthesized in-house from RECTAS. Compound stock solutions were prepared with DMSO, except for phosphatidylserine, which was dissolved in chloroform:methanol solution (95:5 %volume).
IKBKAP (IVS20+6T>C) humanized mice
The IKBKAP (IVS20 + 6 T > C) transgenic mouse strain used in this study was obtained from Drs. Pickel and Slaugenhaupt42. Mice were bred in the light/dark cycle of 12 h/12 h, 40–60% humidity and 69–72 °F temperature. RECTAS in suspension with 0.5% carboxymethyl cellulose was administered to more than 8-week-old mice p.o. at a dose of 300 or 400 mg/kg BW. Four hours later, an equivalent booster dose was given, and after another 4 h, the mice were euthanized and RNA extraction from dorsal root ganglia was performed. Extracted RNA was further analyzed by RT-PCR to measure the PSI value for exon 20 inclusion of IKBKAP. An animal study using IKBKAP (IVS20 + 6 T > C) mice was conducted under ethical approval by Cold Spring Harbor Laboratories.
Reverse transcription-coupled polymerase chain reaction (RT-PCR)
Total RNA purification was conducted by direct-zol RNA miniprep (zymo research, Irvine, CA) or RNeasy mini kit (Hilden, Germany) from a cell line or homogenized tissue block of C57BL/6 mouse in TRIzol (Thermo Fisher Scientific). Blood total RNA from C57BL/6 mouse was extracted by NucleoSpin RNA blood column (Macherey-Nagel, Dueren, Germany), followed by addition of TRIzol (Thermo Fisher Scientific) and another round of purification by direct-zol RNA miniprep (zymo research). Total RNAs from human liver (No. 636531), kidney (No. 636529), lung (No. 636524), heart (No. 636532), and skeletal muscle (No. 636534) were purchased from Takara Bio (Shiga, Japan). For RT-PCR, total RNA was applied for reverse transcription by PrimeStar Reverse Transcriptase (Takara Bio) with random hexamers, and the resulting products were amplified with ExTaq DNA polymerase (Takara Bio) with target-specific primer sets. Primers used for RT-PCR are listed in Supplementary Table 2. Detection was conducted with ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA) and analyzed by Image Lab software (6.0.1) (Bio-Rad).
RNAi
RNAi was conducted by lipofection of small interfering RNA (siRNA) (Silencer Select siRNA, Ambion of Thermo Fisher Scientific) targeting human SRSF1 (siRNA ID, s12725), SRSF2 (siRNA ID, s12728), SRSF4 (siRNA ID, s12735), SRSF6 (siRNA ID, s12741), SRSF7 (siRNA ID, s12743), SRSF8 (siRNA ID, s21495), SRSF9 (siRNA ID, s16546), SRSF10 (siRNA ID, s57794), SRSF11 (siRNA ID, s17770), SRSF12 (siRNA ID, s43932), CLK1 (siRNA ID, s3162), CLK2 (siRNA ID, s3165), CLK3 (siRNA ID, s3169), CLK4 (siRNA ID, s32988), DYRK1A (siRNA ID, s4400), DYRK1B (siRNA ID, s17490), DYRK2 (siRNA ID, s16027), and DYRK3 (siRNA ID, s16026) and a non-specific control (si-NS) (siRNA ID, 4390843) as a negative control. For simultaneous knockdown of CLK isoforms and DYRK isoforms, mixture of siRNAs targeting CLK1, 2, 3, and 4 and DYRK1A, 1B, 2, and 3 were transfected, respectively. siRNA transfection was conducted using Lipofectamine RNAiMAX (Thermo Fisher Scientific) with a final concentration of each siRNAs in the culture medium set to 100 nM. Knockdown effects at the protein level were confirmed for siRNAs targeting SRSF6, CLK1, CLK2, CLK3, and CLK4 (Supplementary Fig. 6).
Dual-fluorescence reporter system for splicing evaluation
We previously constructed the SPREADD reporter system for IKBKAP IVS20 + 6 T > C17, in which the splicing extent of IKBKAP exon 20 was quantified by the ratio of green fluorescent protein (GFP) and red fluorescent protein (RFP). For the reporter assay, the IKBKAP IVS20 + 6 T > C splicing reporter vector was transfected into HeLa cells (0.1 × 106 cells/well, seeded in a 12-well plate) with Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific) and changed to fresh media at 4–5 h after transfection to be incubated with or without compounds for 24 h before sample preparation or fixation with 4% paraformaldehyde in phosphate-buffered saline (pH 7.4). Fixed cells were then scanned with a BZ-X710 fluorescence microscope (Keyence, Osaka, Japan), and analyzed for fluorescence intensities with a hybrid-cell count software of BZ-X analyzer (1.4.0.1) (Keyence). The resulting GFP/RFP fluorescence ratio was then plotted to indicate the relative extent of IKBKAP exon 20 inclusion.
RNA pull-down assay and Western blot
Lysate for HeLa cell or primary fibroblasts lysate was prepared in lysis buffer (10 mM Tris-HCl; pH 7.4), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.25% sodium deoxycholate, and 10% glycerol with protease-inhibitor (Nacalai Tesque) and phosphatase-inhibitor (Sigma Aldrich)), followed by sonication, treatment with DNase I (Promega, Madison, WI) at 37 °C for 5 min, and centrifugation (18,500×g at 4 °C for 15 min). The supernatant was used as a soluble fraction for RNA pull-down assay and western blot. For western blot, soluble fraction was dissolved in Laemmli buffer and separated in 5–20% Tris-glycine polyacrylamide gel (FUJIFILM Wako Pure Chemical). Separated proteins were then transferred to the PVDF membrane, and processed for blocking with 5% skim milk in Tris-buffered saline and incubation with antibody. For RNA pull-down assay, 5′-biotin, 3′-dTdT-attached RNAs (5′-UUGGACAAGUAAGUGCCAUUGUACU-3′ (oAM153) for the IKBKAP exon 20 donor region of reference sequence, 5′-UUGGACAAGUAAGCGCCAUUGUACU-3′ (oAM154) for the IKBKAP exon 20 donor region with IVS20 + 6 T > C mutation, 5′-UUGUACUGUUUGCGACUAGUUAGCU-3′ (oAM155) for the site-a, 5′-GUUAGCUUGUGAUUUAUGUGUGAAG-3′ (oAM156) for the site-b, 5′-AAGACAAUAAGUAUUUUAUUACAAU-3′ (oAM209) for the site-c, and 5′-GUUAGCUUGUGAUUUAUAUACAAAG-3′ (oAM167) for the site-b(mt)) were incubated with HeLa cell lysate (for oAM155, oAM156, oAM209, and oAM167) or HeLa nuclear extract (for oAM153 and oAM154) in presence of 1% DMSO or 10 μM RECTAS (CILBIOTECH, s.a., Mons, Belgium) for 16 h with rotation at 4 °C, followed by washing with Tris-buffered saline three times and eluting with Laemmli buffer. Eluted proteins were then analyzed by Western blotting. Detection was conducted with ChemiDoc MP Imaging System (Bio-Rad) and analyzed with Image J (1.52a)65.
Compound pull-down assay
b-RECTAS was synthesized as a RECTAS analog with IKBKAP exon 20-targeting activity with covalent biotin labeling. The resulting b-RECTAS was used for the compound pull-down assay with neutravidin beads. Neutravidin beads were incubated with 0, 100, or 200 nM of biotin-conjugated RECTAS for 2 h at 4 °C. Following washing with Tris-buffered saline (pH 7.4), the biotin-RECTAS-bound beads were then incubated with HeLa cell lysate or GST-CLK1 recombinant protein expressed in Sf9 insect cells (ProQinase, Freiburg, Germany) for 16 h at 4 °C. For competition assays, 100 nmol RECTAS was incubated during the protein binding step, followed by washing and eluting with Laemmli buffer. Eluted samples were analyzed by western blotting.
Cell viability assay
For cell viability assay, HeLa cells were seeded in a 96-well plate in triplicate and incubated with compounds for 72 h. Cell viability was measured by WST-8 assay using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). Absorbance at 450 nm was measured with ARVO X5 multimode plate reader (Perkin Elmer, Waltham, MA).
RNA-Seq
Total RNA was extracted from FD patient fibroblasts or DRG from IKBKAP (IVS20 + 6 T > C) transgenic mice, using TRIzol (Thermo Fisher Scientific) with DNase treatment. For FD patient fibroblasts, total RNA (2.5 µg) was poly-A purified with a Dynabeads mRNA DIRECT Micro Kit (Thermo Fisher Scientific), and used for library preparation with an Ion Total RNA-seq Kit v2 (Thermo Fisher Scientific). Single-end sequencing was performed on an Ion Proton System (Thermo Fisher Scientific). For DRG from IKBKAP (IVS20 + 6 T > C) transgenic mice, total RNA (0.6–0.7 µg) was poly-A purified and the library was prepared with TruSeq stranded mRNA library kit (Illumina, Inc. San Diego, CA). RNA-Seq was then conducted with NovaSeq 6000 (Illumina, Inc.), and Real-Time Analysis (RTA) 1.9 software (Illumina, Inc.) was applied for primary data processing. Sequence reads were split into 60-base fragments from the 5′ end and mapped to the human genome (GRCh38) with STAR (ver. 2.4.1d), using the Ensemble annotation GRCh38.94 as reference. 66 Sequence reads with average Phred quality scores lower than 17, and those from rRNA, tRNA, snRNA, snoRNA, or repetitive elements in the Repbase database67 were eliminated. To calculate the PSI values, we used all internal exons from representative variants for each gene selected based on the nucleotide sequences of variants and coding sequences in UniProt68. The PSI value of the exon (n) was calculated as the number of junction reads of exon (n − 1) and (n) divided by the sum of junction reads of exon (n − 1) and (n) and exon (n − 1) and (n + 1). For analysis of FD patient fibroblasts, PSI values obtained from patient fibroblasts P1 and P2 were averaged, and exons with more than 15 reads per million junction read for the sum of their skipping forms and inclusion forms in DMSO and/or RECTAS-treatment conditions were used for the analysis. The Sashimi plots were generated using an Integrated Genomic Viewer (ver. 2.8.0)69. The RNA-seq data were deposited at the National Bioscience Database Center database for patient fibroblasts (accession: hum0180), and at the Gene Expression Omnibus of the National Center for Biotechnology Information for transgenic mice (accession: GSE161109).
Statistics
The student’s t test was used to determine the statistical difference between the two groups, and P values <0.05 were considered statistically significant. Regression analysis for determination of EC50 and CT50 was conducted by GraphPad Prism 7.05. (GraphPad Software, San Diego, CA).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We acknowledge Dr. Shuh Narumiya of Kyoto University Graduate School of Medicine for his encouragement and suggestions throughout the work, members of the Department of Anatomy and Developmental Biology and the Department of Drug Discovery Medicine of Kyoto University Graduate School of Medicine for fruitful discussions, and Ms. Chihiro Takashima and Ms. Chizuru Tsuzuki for technical supports. This study was supported by 15H05721 (to M.A., T.A., and M.H.) and 19K07367 (to M.A.) from the Japan Society for the Promotion of Science; JP18kk0305003h0003, 19ek0109327h0002, and 19lm0203054h0002 of the Japan Agency for Medical Research and Development (to M.A., T.A., and M.H.). A.R.K. and Y.J.K. acknowledge support from NIH grants R37-GM42699, F30HL137326-03, and the St. Giles Foundation.
Source data
Author contributions
M.A., T.A., Y.J.K., R.K., S.M., S.S., and T.S conducted biological experiments. K.I. and M.D. conducted and analyzed RNA-seq. N.T. conducted compound synthesis. R.S. provided patient iPS cells. A.R.K. provided technical interpretation. M.A. and M.H. wrote the paper.
Data availability
All relevant data are available from the authors. The original RNA-seq data were deposited at the National Bioscience Database Center database with the accession ID hum0180 for patient fibroblasts, and at the Gene Expression Omnibus of the National Center for Biotechnology Information for transgenic mice with the accession ID GSE161109. The Repbase database is available at https://www.girinst.org/repbase/. GRCh38 human reference genome and Ensemble annotation GRCh38.94 are available from the Ensembl database (https://ensembl.org). Source data are provided with this paper.
Competing interests
S.M. and S.S. are employees of Kyorin Pharmaceutical Co., Ltd. M.H. is a founder, shareholder, and member of the scientific advisory board of KinoPharma, Inc., and BTB Drug Development Research Center Co., Ltd. The remaining authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/12/2021
A Correction to this paper has been published: 10.1038/s41467-021-26287-8
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24705-5.
References
- 1.Hua Y, Vickers TA, Okunola HL, Bennett CF, Krainer AR. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am. J. Hum. Genet. 2008;82:834–848. doi: 10.1016/j.ajhg.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkel RS, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 2017;377:1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri E, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 2018;378:625–635. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 4.Naryshkin NA, et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345:688–693. doi: 10.1126/science.1250127. [DOI] [PubMed] [Google Scholar]
- 5.Ratni H, et al. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA) J. Med. Chem. 2018;61:6501–6517. doi: 10.1021/acs.jmedchem.8b00741. [DOI] [PubMed] [Google Scholar]
- 6.Sivaramakrishnan M, et al. Binding to SMN2 pre-mRNA-protein complex elicits specificity for small molecule splicing modifiers. Nat. Commun. 2017;8:1476. doi: 10.1038/s41467-017-01559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Schultz PG, Johnson KA. Mechanistic studies of a small-molecule modulator of SMN2 splicing. Proc. Natl Acad. Sci. USA. 2018;115:E4604–E4612. doi: 10.1073/pnas.1800260115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campagne S, et al. Structural basis of a small molecule targeting RNA for a specific splicing correction. Nat. Chem. Biol. 2019;15:1191–1198. doi: 10.1038/s41589-019-0384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohe K, Hagiwara M. Modulation of alternative splicing with chemical compounds in new therapeutics for human diseases. ACS Chem. Biol. 2015;10:914–924. doi: 10.1021/cb500697f. [DOI] [PubMed] [Google Scholar]
- 10.Muraki M, et al. Manipulation of alternative splicing by a newly developed inhibitor of Clks. J. Biol. Chem. 2004;279:24246–24254. doi: 10.1074/jbc.M314298200. [DOI] [PubMed] [Google Scholar]
- 11.Shibata, S., Ajiro, M. & Hagiwara, M. Mechanism-based personalized medicine for cystic fibrosis by suppressing pseudo exon inclusion. Cell Chem. Biol.10.1016/j.chembiol.2020.08.013 (2020). [DOI] [PubMed]
- 12.Nishida A, et al. Chemical treatment enhances skipping of a mutated exon in the dystrophin gene. Nat. Commun. 2011;2:308. doi: 10.1038/ncomms1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boisson B, et al. Rescue of recurrent deep intronic mutation underlying cell type-dependent quantitative NEMO deficiency. J. Clin. Investig. 2019;129:583–597. doi: 10.1172/JCI124011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroyanagi H, Kobayashi T, Mitani S, Hagiwara M. Transgenic alternative-splicing reporters reveal tissue-specific expression profiles and regulation mechanisms in vivo. Nat. Methods. 2006;3:909–915. doi: 10.1038/nmeth944. [DOI] [PubMed] [Google Scholar]
- 15.Kuroyanagi H, Ohno G, Sakane H, Maruoka H, Hagiwara M. Visualization and genetic analysis of alternative splicing regulation in vivo using fluorescence reporters in transgenic Caenorhabditis elegans. Nat. Protoc. 2010;5:1495–1517. doi: 10.1038/nprot.2010.107. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi A, Hosokawa M, Nojima T, Hagiwara M. Splicing reporter mice revealed the evolutionally conserved switching mechanism of tissue-specific alternative exon selection. PLoS ONE. 2010;5:e10946. doi: 10.1371/journal.pone.0010946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida M, et al. Rectifier of aberrant mRNA splicing recovers tRNA modification in familial dysautonomia. Proc. Natl Acad. Sci. USA. 2015;112:2764–2769. doi: 10.1073/pnas.1415525112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Axelrod FB. Familial dysautonomia. Muscle Nerve. 2004;29:352–363. doi: 10.1002/mus.10499. [DOI] [PubMed] [Google Scholar]
- 19.Axelrod FB, Goldberg JD, Ye XY, Maayan C. Survival in familial dysautonomia: impact of early intervention. J. Pediatr. 2002;141:518–523. doi: 10.1067/mpd.2002.127088. [DOI] [PubMed] [Google Scholar]
- 20.Slaugenhaupt SA, et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am. J. Hum. Genet. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmel I, Tal S, Vig I, Ast G. Comparative analysis detects dependencies among the 5′ splice-site positions. RNA. 2004;10:828–840. doi: 10.1261/rna.5196404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim EC, et al. Weak definition of IKBKAP exon 20 leads to aberrant splicing in familial dysautonomia. Hum. Mutat. 2007;28:41–53. doi: 10.1002/humu.20401. [DOI] [PubMed] [Google Scholar]
- 23.George L, et al. Familial dysautonomia model reveals Ikbkap deletion causes apoptosis of Pax3+ progenitors and peripheral neurons. Proc. Natl Acad. Sci. USA. 2013;110:18698–18703. doi: 10.1073/pnas.1308596110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeltner N, et al. Capturing the biology of disease severity in a PSC-based model of familial dysautonomia. Nat. Med. 2016;22:1421–1427. doi: 10.1038/nm.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohe K, et al. RBM24 promotes U1 snRNP recognition of the mutated 5′ splice site in the IKBKAP gene of familial dysautonomia. RNA. 2017;23:1393–1403. doi: 10.1261/rna.059428.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu XD, Ares M., Jr. Context-dependent control of alternative splicing by RNA-binding proteins. Nat. Rev. Genet. 2014;15:689–701. doi: 10.1038/nrg3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartegni L, Chew SL, Krainer AR. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat. Rev. Genet. 2002;3:285–298. doi: 10.1038/nrg775. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Shi H, Lee DK, Lis JT. Specific SR protein-dependent splicing substrates identified through genomic SELEX. Nucleic Acids Res. 2003;31:1955–1961. doi: 10.1093/nar/gkg286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. ESEfinder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31:3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha R, et al. Antisense oligonucleotides correct the familial dysautonomia splicing defect in IKBKAP transgenic mice. Nucleic Acids Res. 2018;46:4833–4844. doi: 10.1093/nar/gky249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano-Kobayashi A, et al. Prenatal neurogenesis induction therapy normalizes brain structure and function in Down syndrome mice. Proc. Natl Acad. Sci. USA. 2017;114:10268–10273. doi: 10.1073/pnas.1704143114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa Y, et al. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat. Commun. 2010;1:86. doi: 10.1038/ncomms1090. [DOI] [PubMed] [Google Scholar]
- 33.Sonamoto R, et al. Identification of a DYRK1A inhibitor that induces degradation of the target kinase using co-chaperone CDC37 fused with luciferase nanoKAZ. Sci. Rep. 2015;5:12728. doi: 10.1038/srep12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuhara T, et al. Utilization of host SR protein kinases and RNA-splicing machinery during viral replication. Proc. Natl Acad. Sci. USA. 2006;103:11329–11333. doi: 10.1073/pnas.0604616103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amin EM, et al. WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing. Cancer Cell. 2011;20:768–780. doi: 10.1016/j.ccr.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shetty RS, et al. Specific correction of a splice defect in brain by nutritional supplementation. Hum. Mol. Genet. 2011;20:4093–4101. doi: 10.1093/hmg/ddr333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slaugenhaupt SA, et al. Rescue of a human mRNA splicing defect by the plant cytokinin kinetin. Hum. Mol. Genet. 2004;13:429–436. doi: 10.1093/hmg/ddh046. [DOI] [PubMed] [Google Scholar]
- 38.Masaki S, et al. Design and synthesis of a potent inhibitor of class 1 DYRK kinases as a suppressor of adipogenesis. Bioorg. Med. Chem. 2015;23:4434–4441. doi: 10.1016/j.bmc.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Abergel A, et al. Ledipasvir-sofosbuvir in patients with hepatitis C virus genotype 5 infection: an open-label, multicentre, single-arm, phase 2 study. Lancet Infect. Dis. 2016;16:459–464. doi: 10.1016/S1473-3099(15)00529-0. [DOI] [PubMed] [Google Scholar]
- 40.Rubin BY, Anderson SL. IKBKAP/ELP1 gene mutations: mechanisms of familial dysautonomia and gene-targeting therapies. Appl Clin. Genet. 2017;10:95–103. doi: 10.2147/TACG.S129638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang ET, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hims MM, et al. A humanized IKBKAP transgenic mouse models a tissue-specific human splicing defect. Genomics. 2007;90:389–396. doi: 10.1016/j.ygeno.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manning KS, Cooper TA. The roles of RNA processing in translating genotype to phenotype. Nat. Rev. Mol. Cell Biol. 2017;18:102–114. doi: 10.1038/nrm.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sperling, R. The nuts and bolts of the endogenous spliceosome. Wiley Interdiscip Rev. RNA8, 10.1002/wrna.1377 (2017). [DOI] [PubMed]
- 45.Dufner-Almeida LG, do Carmo RT, Masotti C, Haddad LA. Understanding human DNA variants affecting pre-mRNA splicing in the NGS era. Adv. Genet. 2019;103:39–90. doi: 10.1016/bs.adgen.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Gonorazky H, et al. RNAseq analysis for the diagnosis of muscular dystrophy. Ann. Clin. Transl. Neurol. 2016;3:55–60. doi: 10.1002/acn3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan AO, et al. A deep intronic CLRN1 (USH3A) founder mutation generates an aberrant exon and underlies severe Usher syndrome on the Arabian Peninsula. Sci. Rep. 2017;7:1411. doi: 10.1038/s41598-017-01577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riant F, et al. Deep intronic KRIT1 mutation in a family with clinically silent multiple cerebral cavernous malformations. Clin. Genet. 2014;86:585–588. doi: 10.1111/cge.12322. [DOI] [PubMed] [Google Scholar]
- 49.Engel M, et al. Allosteric activation of the protein kinase PDK1 with low molecular weight compounds. EMBO J. 2006;25:5469–5480. doi: 10.1038/sj.emboj.7601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morini E, et al. ELP1 splicing correction reverses proprioceptive sensory loss in familial dysautonomia. Am. J. Hum. Genet. 2019;104:638–650. doi: 10.1016/j.ajhg.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey MH, et al. Comprehensive characterization of cancer driver genes and mutations. Cell. 2018;174:1034–1035. doi: 10.1016/j.cell.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jayasinghe RG, et al. Systematic analysis of splice-site-creating mutations in cancer. Cell Rep. 2018;23:270–281 e273. doi: 10.1016/j.celrep.2018.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwai, K. et al. Anti-tumor efficacy of a novel CLK inhibitor via targeting RNA splicing and MYC-dependent vulnerability. EMBO Mol. Med. 10, 10.15252/emmm.201708289 (2018). [DOI] [PMC free article] [PubMed]
- 54.Tam BY, et al. The CLK inhibitor SM08502 induces anti-tumor activity and reduces Wnt pathway gene expression in gastrointestinal cancer models. Cancer Lett. 2020;473:186–197. doi: 10.1016/j.canlet.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 55.Batson J, et al. Development of potent, selective SRPK1 inhibitors as potential topical therapeutics for neovascular eye disease. ACS Chem. Biol. 2017;12:825–832. doi: 10.1021/acschembio.6b01048. [DOI] [PubMed] [Google Scholar]
- 56.Tzelepis K, et al. SRPK1 maintains acute myeloid leukemia through effects on isoform usage of epigenetic regulators including BRD4. Nat. Commun. 2018;9:5378. doi: 10.1038/s41467-018-07620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatcher JM, et al. SRPKIN-1: a covalent SRPK1/2 inhibitor that potently converts VEGF from pro-angiogenic to anti-angiogenic isoform. Cell Chem. Biol. 2018;25:460–470 e466. doi: 10.1016/j.chembiol.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaida D, et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat. Chem. Biol. 2007;3:576–583. doi: 10.1038/nchembio.2007.18. [DOI] [PubMed] [Google Scholar]
- 59.Kotake Y, et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat. Chem. Biol. 2007;3:570–575. doi: 10.1038/nchembio.2007.16. [DOI] [PubMed] [Google Scholar]
- 60.Lee SC, et al. Modulation of splicing catalysis for therapeutic targeting of leukemia with mutations in genes encoding spliceosomal proteins. Nat. Med. 2016;22:672–678. doi: 10.1038/nm.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eskens FA, et al. Phase I pharmacokinetic and pharmacodynamic study of the first-in-class spliceosome inhibitor E7107 in patients with advanced solid tumors. Clin. Cancer Res. 2013;19:6296–6304. doi: 10.1158/1078-0432.CCR-13-0485. [DOI] [PubMed] [Google Scholar]
- 62.Hong DS, et al. A phase I, open-label, single-arm, dose-escalation study of E7107, a precursor messenger ribonucleic acid (pre-mRNA) splicesome inhibitor administered intravenously on days 1 and 8 every 21 days to patients with solid tumors. Investig. N. Drugs. 2014;32:436–444. doi: 10.1007/s10637-013-0046-5. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida K, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 64.Chambers SM, et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol. 2012;30:715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zerbino DR, et al. Ensembl 2018. Nucleic Acids Res. 2018;46:D754–D761. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao W, Kojima KK, Kohany O. Repbase update, a database of repetitive elements in eukaryotic genomes. Mob. DNA. 2015;6:11. doi: 10.1186/s13100-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.UniProt Consortium, T. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2018;46:2699. doi: 10.1093/nar/gky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson JT, Thorvaldsdottir H, Wenger AM, Zehir A, Mesirov JP. Variant review with the integrative genomics viewer. Cancer Res. 2017;77:e31–e34. doi: 10.1158/0008-5472.CAN-17-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All relevant data are available from the authors. The original RNA-seq data were deposited at the National Bioscience Database Center database with the accession ID hum0180 for patient fibroblasts, and at the Gene Expression Omnibus of the National Center for Biotechnology Information for transgenic mice with the accession ID GSE161109. The Repbase database is available at https://www.girinst.org/repbase/. GRCh38 human reference genome and Ensemble annotation GRCh38.94 are available from the Ensembl database (https://ensembl.org). Source data are provided with this paper.