Abstract
Urochloa (syn. Brachiaria) is the most popular fodder of livestock farmers in Cameroon for hay and seed productions. Farmers in Cameroon have been producing Brachiaria seeds for decades for own uses and surplus are sold to neighbours, and to traders from Cameroon and neighbouring countries. However, there is no information available about qualities of these seeds. Fifteen Urochloa seeds samples were collected from farmers and/or government stations in five regions (Adamaoua, East, North, North West, and West) and analysed for major seed quality parameters along with seeds of improved Urochloa cultivar Basilisk imported from Brazil as a check. Study showed significant differences among treatments for various seed quality parameters tested (P < 0.0001). The highest thousand grains weight was recorded in Basilisk (5.685 g), followed by W12 (3.555 g), A05 (3.153 g) and N01 (2.655 g). Caryopsis number and caryopsis weight were highest in Basilisk followed by E09, A06, and W12. Of three conditions tested for seed germination, mean germination was the highest in greenhouse (7.39%) where Basilisk had the highest germination (25.5%) followed by N01 (18.50%), A05 (14.50%) and W12 (12.75%). The seed viability ranged from 18% (E09) to 81% (N01), and there were a positive and highly significant relationships between seed germination and viability traits (r = 0.883; P < 0.0001). This study showed a marked difference in seed quality parameters of Urochloa grass seeds produced in Cameroon, and the potential of developing Urochloa grass seed business in the Northern, Adamaoua and Western regions of Cameroon.
Subject terms: Ecology, Plant sciences
Introduction
Urochloa grass is one of the most important tropical forages native to Africa. The genus Urochloa consists of about 100 documented species1 of which seven perennial species of African origin (Urochloa arrecta, Urochloa brizantha, Urochloa decumbens, Urochloa dictyoneura, Urochloa humidicola, Urochloa mutica and Urochloa ruziziensis) are used as fodder plants2. Urochloa grass was introduced to other parts of the world in different occasions3–8. Urochloa grass is the most extensively cultivated forage in tropical Americas9–12, and it is among the most widely cultivated forages in Asia, the South Pacific and Australia13.
In Africa, the evaluations of Urochloa grass for pasture improvement started in 1950s. Early studies confirmed a high biomass production and broad adaptation of some Urochloa species14. However, none of these Urochloa species were used for commercial pasture production in Africa essentially due to no role of sown pasture in the livestock production except in the smallholder dairies in the highlands14. A gradual intensification in the livestock production system in Africa has spurred demand for high quality feeds and improved forage including Urochloa grass. Improved forages have been one of the most preferred feed resources to livestock farmers as they provide quality feed option at a low cost and are resilient to the climate change.
The initial introduction and evaluations of improved Urochloa grass cultivars in Africa started in the early 2000s for ruminant nutrition, pest management (push–pull-system) and conservation agriculture15. Subsequently, additional hybrids and improved cultivars were introduced and evaluated for ruminant nutrition16–18. The Climate-smart Brachiaria Program (2012–2016) evaluated several improved Urochloa cultivars for adaptation, agronomy, and livestock performance in the East Africa. Urochloa grass when fed to livestock increased milk production in between 15–40% and there was over 50% increase in body weight in heifers19,20. Due to an excellent adaptation, desirable agronomic attributes, and substantial benefits to livestock productivity there is high demand for Urochloa grass from farmers, therefore many livestock development initiatives in the region have been promoting improved Urochloa grass. However, the pace of dissemination and adoption of Urochloa grass has been very slow in Africa due to unavailability and restricted access to seeds. Therefore, African farmers have been relying on rooted tillers as planting material from neighbouring farmers. This enables them to establish small-scale Urochloa pasture but the large-scale cultivation in wider geographical areas using rooted tillers is less practical as it requires substantial investment in buying, movement, and handling of bulky planting materials. Availability of rooted tillers in large quantity has been another bottleneck for wider adoption of Urochloa grass.
At present, Urochloa grass productions in Africa rely on imported seeds, and rooted tillers. A wide scale adoption of improved Urochloa grass is necessary to realize full benefits of this forage on livestock productivity in Africa. To achieve this, African farmers should have timely access to Urochloa seeds at affordable price. Regulatory restrictions on seed import, long-distance shipment, and small trade volume are major causes for high seed price and limited access to Urochloa seeds to farmers. A sustainable supply of Urochloa seeds in Africa requires lessening dependence on imported seeds and establishing an effective and commercial Urochloa seed production system in the continent. This proposition is realisable as other studies report success in Urochloa seed production in Cameroon and Kenya21,22.
In Cameroon, the introduction, and evaluations of Urochloa grass for fodder production started in the1960’s23. Urochloa is a popular forage promoted in Cameroon for hay and seeds production22. Farmers in Cameroon have been producing Urochloa seed for years for own uses and surplus seeds are sold to neighbours and vendors from other parts of the country and neighbouring countries like Nigeria and Central African Republic. Urochloa growers in Cameroon uses seeds rates of 50 to100 kg/ha, which is several folds higher than recommended seed rates of 3–7 kg/ha24. This practice of high seed rates used in Cameroon is likely due to a low-quality of locally produced Urochloa seeds. However, there is no information available on the qualities of Urochloa seeds produced in Cameroon. Therefore, this study aims to assess the qualities of Urochloa seeds produced in Cameroon and identify the suitable niches for Urochloa seed production in the country.
Methods
Description of the study area
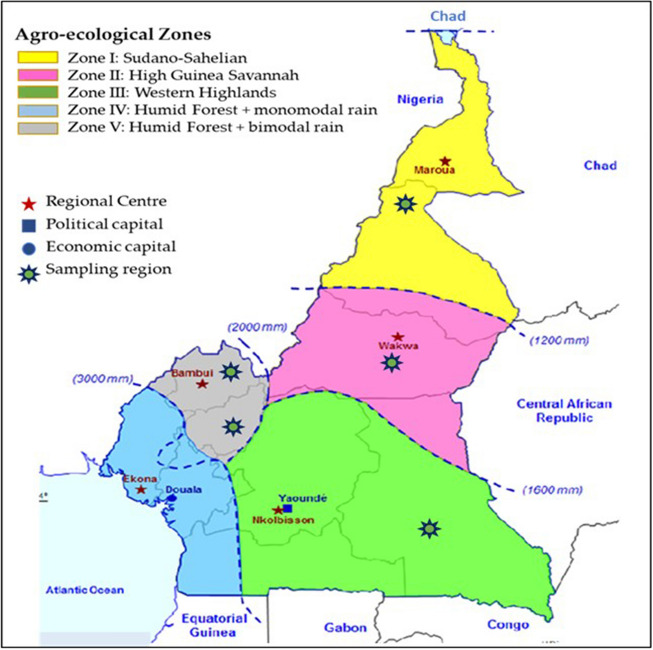
Cameroon is located in between Equatorial Africa in the South and Tropical Africa in the North. It is also known as Miniature Africa due to a high geographical, climatic, and ecological diversities. It covers 475,650 km2 land mass and borders with Nigeria, Chad, Central African Republic, Congo Brazzaville, Gabon, and Equatorial Guinea. Cameroon can be divided into five agroecological zones; Sudano-Sahelian in the North and extreme North region, Sudano-guinea in the Adamaoua Plateau, Western High Plateau in West and North-west region, Humid Forest with unimodal rainfalls in the Littoral and South-west region, and the Humid Forest with bimodal rainfalls in Central and Eastern part of the country25 (Fig. 1). Livestock are important in the Western Highlands, Adamaoua Plateau and North26.
Figure 1.
A map of Cameroon showing agroecological zones, regional centres, political and economic capitals and Urochloa seeds sample collection regions (https://agritrop.cirad.fr/556139/1/document_556139.pdf ).
Source of Urochloa seeds
Urochloa seed samples were collected from the North, Adamawa, East, West, and North-west regions that represent four agroecological zones in Cameroon. Selection of these regions was based on the history of Urochloa cultivation for hay and seeds production and the importance of the livestock. A total of 15 Urochloa seeds samples were collected from farmers and government’s agricultural stations and used for different analysis following the guidelines of Institute of Agricultural Research for Development (IRAD), Cameroon. The amounts of seeds collected per sample ranged in between 150 and 300 g. Although no official information available about Urochloa grass species/cultivar grown by farmers in Cameroon, it is most likely to be U. ruziziensis that has been vulgarized in the country following the early introduction and evaluations22. A recent agro-morphological characterization of four landraces of Urochloa grass from two regions of Cameroon has tentatively identified them as U. ruziziensis (Ntane Ojong et al. unpublished results) Other details about sample collection locality are presented in Table 1. Urochloa decumbens cv. Basilisk seeds imported from Marangatu Seeds, Brazil was used as a check in the study.
Table 1.
Origin of Urochloa seeds samples collected in Cameroon for seed quality analysis.
| Sample ID | Region | Sites | Altitude (m asl) | Rainfall (mm) | GPS Coordinates |
|---|---|---|---|---|---|
| N1 | North | Garoua | 248 | 1500 | N09.35100/E013.28667 |
| N2 | North | Louguere | 475 | 1500 | N09.93060/E013.77282 |
| N3 | North | Louguere | 388 | 1500 | N09.93060/E013.77282 |
| N4 | North | Louguere | 412 | 1500 | N09.93060/E013.77282 |
| A5 | Adamaoua | Wakwa | 1143 | 2000 | N07.13600/E13.34600 |
| A6 | Adamaoua | Banyo | 1250 | 2000 | N06.28000/E11.33000 |
| A7 | Adamaoua | Banyo | 1200 | 2000 | N06.49000/E12.00000 |
| A8 | Adamaoua | Banyo | 1250 | 2000 | N06.74960/E11.80360 |
| E9 | East | Bertoua | 717 | 2500 | N04.34382/E13.41452 |
| E10 | East | Bertoua | 717 | 2500 | N04.35590/E13.43599 |
| E11 | East | Bertoua | 717 | 2500 | N04.343820/ E13.41045 |
| W12 | West | Bangangté | 1205 | 3000 | N05.10389/E10.33961 |
| NW13 | North West | Bambui | 1288 | 3000 | N06.01941/E10.05720 |
| NW14 | North West | Bambui | 1601 | 3000 | N05. 59767/E10.16859 |
| NW15 | North West | Bambui | 1281 | 3000 | N06. 01962/E10.05752 |
Harvesting and processing of seed samples
All 15 seed samples from Cameroon were harvested manually in between November and December 2017. For seed samples W12, NW13, NW14 and NW15, inflorescences were cut at maturity, stored inside bag for three days and hand-shaken, whereas other eleven seed samples were collected by shaking inflorescence within a basket or bag. Seeds were manually cleaned and stored in jute bags on the bamboo ceilings or floors of houses at ambient temperature. In August, seeds were transported to the Bioscience eastern and central Africa—International Livestock Research Institute (BecA-ILRI) Hub, further cleaned to remove debris (if any), placed inside airtight plastic containers (Lock & Lock Interlock Door Pocket of one litre capacity). About 200 g seeds were put in each container and stored at 22 to 24 °C and ambient humidity of min. 58% and max. 75% until further analysis starting in September 2018. Otherwise stated, all empty to fully filled seeds in a sample were used in seed quality analysis. Basilisk seeds used as check in this study were produced in the State of Mato Grosso do Sul, Brazil in 2017 crop season and harvested at about 10% moisture content using tropical forages harvesting machine. Seeds were checked for qualities, processed using air and screens and gravity separator and stored in the warehouse. These Basilisk seeds were not scarified.
Thousand grains weight
Manually cleaned seeds of each sample were counted into four batches of one thousand seeds and weighed on a digital balance (KERN PCB, Germany) for thousand grains weight (TGW) determination.
Caryopsis percentage and weight
The caryopsis count was performed on 25 seeds per sample in four replicates. The seeds were counted in a batch of 25 seeds, placed in between wet double layer paper towels then kept inside a Ziploc plastic bag. The Ziploc bag was sealed and incubated in dark at room temperature for 44 h to soften the husk cover. Following the incubation each seed was dehusked by separating lemma and palea using forceps under dissecting microscope, and the presence/absence of endosperm/caryopsis was recorded. After the caryopsis count, caryopses were wrapped into a paper towel, dried at 40 °C for 72 h and weighed in precision digital balance (KERN PCB, Germany) for caryopsis weight determination.
Seed germination test
Germination tests were performed in three different environments i.e., laboratory, greenhouse, and field. In laboratory tests, 100 Urochloa seeds of each seed sample was randomly arranged on a double layer paper towel in a petri dish in four replications. The paper towels were soaked with sterile water at saturation. Petri-dishes were placed into plastic bags, sealed, and incubated in dark at 28 °C. Germination data were recorded at 3, 7, 14 and 21 days after incubation. The cumulative germination data was used in analysis.
In greenhouse tests, a total of sixteen flat trays (48 cm × 16 cm × 15 cm) were filled with a mixture of sieved two parts of field soil and one part of well decomposed farmyard manure (volume/volume). One hundred seeds of each treatment were shown in a row at 2 cm depth, covered with soil and watered at saturation. Experiment was organized in randomized block design in four replicates. Trays were watered from the bottom as necessary, and germination data was recorded one week after seeding and at 14 days interval thereafter. The cumulative germination data was used in analysis.
In field test, seedbed was prepared by repeated digging and mixing well decomposed farmyard manure at the rate of 10 t/ha. Seeds from each sample were sown continuously in single row at two-centimetre depth with row to row spacing of 15 cm and in between the replication spacing of 50 cm. Each seed sample was sown in four replicates of 1000 seeds each. The seed bed was mulched for the first two weeks. The number of seedlings for each treatment was recorded at eight weeks.
Seed viability examination
A total of 100 bold seeds comprising 25 seeds/replicate of each seed sample was used for viability test. The tetrazolium solution was prepared by adding 0.1 g of triphenyl tetrazolium chloride (> 95%) into 100 ml of autoclaved distilled water in a beaker. The solution in beaker was wrapped by aluminium foil, stored at 4 °C and used within one week of preparation.
Seeds for viability test were placed in between two layers of paper towel, moistened with 2 ml of sterile water, transferred into a Ziploc bag, and incubated at 20 °C for 18 h. Seeds were cut longitudinally with scalpel blade under a dissecting microscope in a way that reveals the embryo cut in two equal halves. One half of each seed for each replicate was stained with 2 ml of 0.1% tetrazolium solution, covered with aluminium foil and incubated at 40 °C for 4 h. Then seeds were examined under a dissecting microscope for embryo staining. The embryo-coloured deep pink or red were recorded as viable and other colours such as brown, black, and whitish were recorded as non-viable.
A subset of 100 bold seeds from the seeds used for viability testing comprising 25 seeds per replication were sown in 15 cm pots filled with soil and compost mixture (2:1 volume/volume) in greenhouse for germination test. All pots were bottom watered, total number of seedlings was counted every week for eight weeks, and cumulative seed germination data was compared with seed viability tests data.
Experimental data analysis
A statistical software XLSTAT was used for the data analysis27. Data on thousand grain weight, caryopsis number, caryopsis weight, seed germination, and viability tests were subjected to analysis of variance (ANOVA). Where treatment effect was significant, treatment means were separated using Duncan's Multiple Range Test (MRT). A linear regression analysis was performed to observe the relationship between caryopsis number and caryopsis weight, and the relationships between seed germination and seed viability.
Results
One thousand grains weight
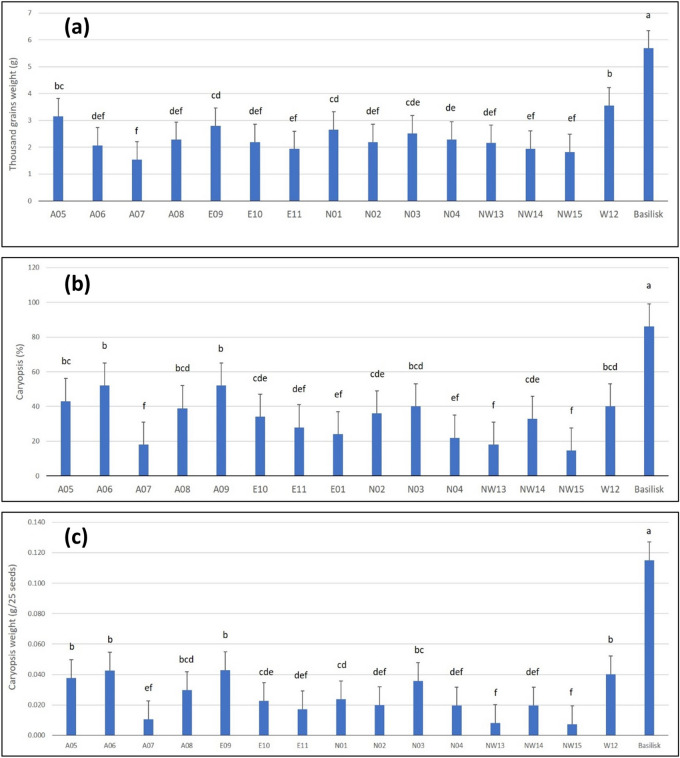
The seed samples deferred significantly for TGW (P < 0.0001). The TGW ranged from 1.538 g for seed sample A07 to 5.685 g for Basilisk with the mean of 2.549 g (Fig. 2a). Among the Urochloa seed samples from Cameroon W12 (3.555 g) and A05 (3.153 g) had the highest TGW.
Figure 2.
Quality attributes of 15 Urochloa seeds samples from Cameroon and seeds of Urochloa decumbens cv. Basilisk from Brazil, (a) thousand grain weight, (b) caryopsis percentage, and (c) caryopsis weight. Bars with different letters for each quality attribute are significantly different (P < 0.0001). The error bars are standard errors (SE).
Caryopsis percentage
Urochloa seed samples were significantly different for the caryopsis formation (P < 0.000). Caryopsis formation was highest in Basilisk (86.0%) and lowest in NW15 (14.7%) with the mean caryopsis value of 36.5%. Among the Cameroonian seed samples, the highest caryopsis formation was recorded in A06 and A09, both with caryopsis formation of 52% (Fig. 2b).
Caryopsis weight
As for TGW and caryopsis formation, seed samples differed significantly for caryopsis weight which ranged between 0.007 g/25 seeds (NW15) to 0.115 g/25 seeds (Basilisk) with mean of 0.031 g/25 seeds (P < 0.0001) (Fig. 2c). Simple linear regression analysis of caryopsis percentage and caryopsis weight showed significant and highly positive correlation between these two parameters (r = 0.951; P < 0.0001) and caryopsis percentage accounted for 90% of the variability in caryopsis weight.
Seed germination
In the laboratory, treatment differed significantly for germination which ranged in between zero (N3 and NW15) to 18% (Basilisk) with mean germination of 3.28% (Table 2). Among the seed samples from Cameroon, the highest germination was recorded in N01 (9.75%) and A05 (7.25%).
Table 2.
Germination of Urochloa grass seed samples from different regions of Cameroon at laboratory, greenhouse, and field conditions.
| Sample ID | Region | Site | Percent seed germination | ||
|---|---|---|---|---|---|
| Lab | Greenhouse | Field | |||
| A05 | Adamaoua | Wakwa | 7.25 bc | 14.50 bc | 9.24 c |
| A06 | Adamaoua | Banyo | 3.25 de | 9.25 de | 3.48 def |
| A07 | Adamaoua | Banyo | 0.50 de | 2.25 fg | 0.90 g |
| A08 | Adamaoua | Banyo | 4.00 cd | 8.00 e | 4.68 d |
| E09 | East | Bertoua | 0.25 de | 2.25 fg | 3.66 def |
| E10 | East | Bertoua | 1.00 de | 3.50 fg | 1.56 fg |
| E11 | East | Bertoua | 2.00 de | 3.25 fg | 3.00 defg |
| N01 | North | Garoua | 9.75 b | 18.50 b | 15.12 b |
| N02 | North | Louguere | 0.50 de | 2.00 fg | 1.62 fg |
| N03 | North | Louguere | 0.00 e | 0.75 g | 1.56 fg |
| N04 | North | Louguere | 1.00 de | 2.50 fg | 1.08 g |
| NW13 | Northwest | Bambui | 0.50 de | 5.50 ef | 4.26 de |
| NW14 | Northwest | Bambui | 1.40 de | 5.40 ef | 3.74 de |
| NW15 | Northwest | Bambui | 0.00 e | 2.33 fg | 2.08 efg |
| W12 | West | Baganté | 3.00 de | 12.75 cd | 7.68 c |
| Basilisk | – | – | 18.00 a | 25.50 a | 25.20 a |
| Mean | 3.275 | 7.390 | 5.554 | ||
| Probability | < 0.0001 | < 0.0001 | < 0.0001 | ||
Values with different letter(s) within a column are statistically difference.
As observed in laboratory, seed samples varied significantly for seed germination in greenhouse (Table 2; P < 0.0001). The seed germination ranged in between 0.75% (N03) to 25.50% (Basilisk) with mean germination of 7.39%. From 15 seed samples studied, N01 (18.50%) and A05 (14.50%) had the highest germinations rates.
There was significant difference among the seed samples in field germination (Table 2). The germination rate was the highest in Basilisk (25.20%) and was the lowest in A07 (0.90%) with mean germination of 5.55%. The seed samples N01 (15.12%) and A05 (9.24%) had highest germinations rates among the seed samples from Cameroon.
Seed viability tests
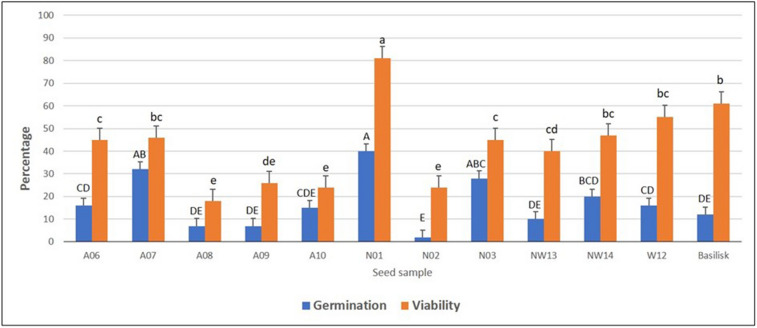
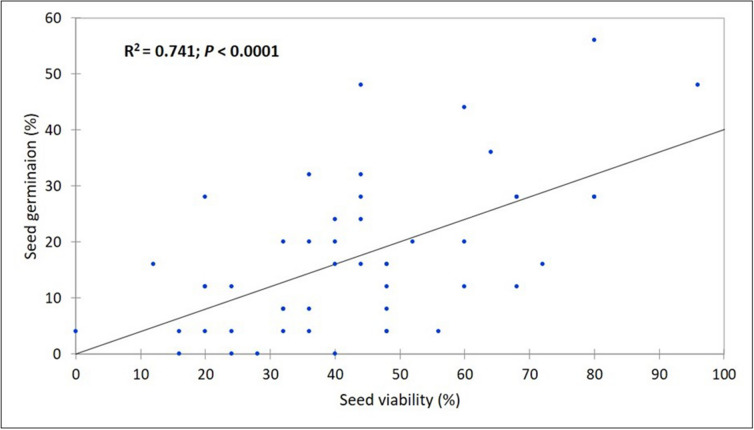
The tetrazolium test was effective in differentiating between viable and nonviable Urochloa grass seeds (Fig. 3). A significant difference was observed among the 12 seed samples tested for viability (Fig. 4). The seed viability ranged from 18% in E09 to 81% in N01 with mean viability of 42.67%. The viability of improved U. decumbens cultivar Basilisk was 61%. The subsets of seed samples tested for viability were also tested for germination. The germination test showed average germination of 17.08% with a range of 2% (N02) to 40% (N01). The differences among the seed samples for germination were significant. A simple regression analysis of seed germination and viability tests data showed positive and significant association between these two parameters (Fig. 5).
Figure 3.
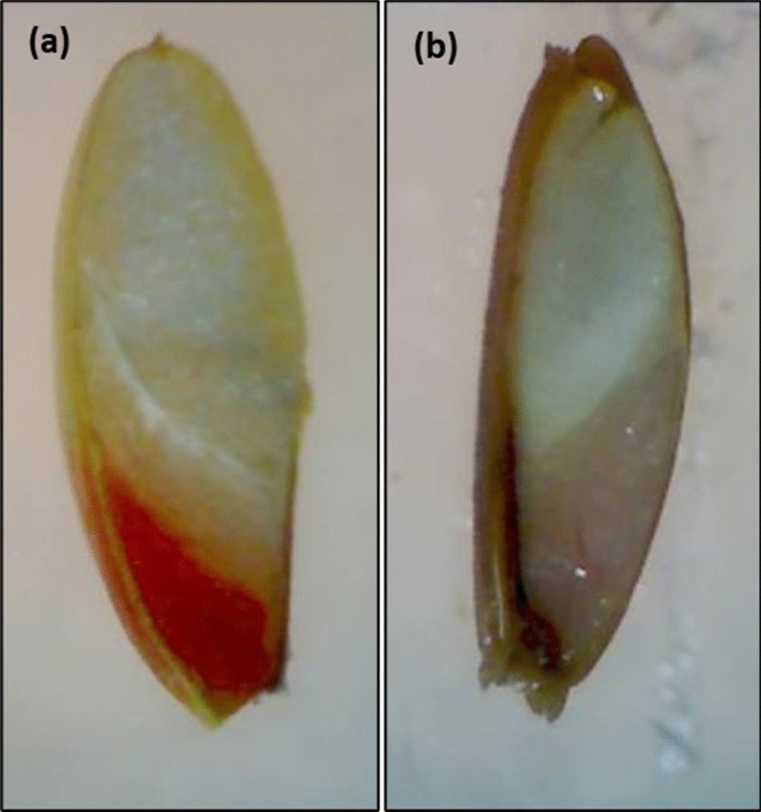
Urochloa grass seeds stained with 0.1% tetrazolium solution, (a) viable seed showing red embryo, and (b) dead seed showing brown embryo.
Figure 4.
Germination and viability percentage for 12 Urochloa spp. seeds samples from Cameroon and seeds of Urochloa decumbens cv. Basilisk from Brazil. Bars of same colour with different letters are significantly different (P < 0.0001). The error bars are standard errors (SE).
Figure 5.
Simple linear regression analysis of seed germination and seeds viability traits showing a significant and positives relationship between these two traits.
Discussion
In this study 15 Urochloa grass seeds samples were collected from Adamaoua, East, North, Northwest, and West regions of Cameroon, and analyzed for quality parameters along with the imported seeds of Urochloa decumbens cv. Basilisk from Brazil. As expected, imported Basilisk seeds were the best for almost all seed quality parameters examined. There was clear differences in seed quality attributes among the seed samples originating from different parts of Cameroon suggesting superiority of one site over another for seeds production.
The TGW is one of the important seed quality attributes that has direct relationships with seedling vigor, seedling growth, grain weight and other traits related to kernels and grain yield28–30. The variations in TGW within and between the cultivars of a crop are attributed to natural variation, human imposed selections31 and seed production environment. The seed samples analyzed in this study were significantly different for TGW. As expected, Basilisk seeds from Brazil had the highest TGW (5.685 g), and the best performing seeds samples from Cameroon i.e., W12 and A05 had TGW of 3.555 g and 3.153 g, receptively. The TGW for Basilisk recorded in the present study was comparable to previous study reporting TGW of 6.846 g for U. decumbens32. The TGW of 1.659 g, 5.581 g, 8.920 g and 8.600 g have been reported for U. brizantha, U. ruziziensis, Mulato and Mulato II respectively32,33. The difference in TGW for W12 and A05 (which are likely to be U. ruziziensis) and TGW reported for U. ruziziensis could be explained by the inclusion of empty seeds in our studies while recording TGW32. A study in tropical Savanna region of Brazil reports TGW of 2.94 to 4.28 g for U. humidicola and 1.78 to 4.77 g for U. decumbens cv. Basilisk depending on the harvest and year of harvest34. In general, we noticed a low TGW for the most seed samples from Cameroon which could be attributed to inclusion of empty seeds while recording TGW, difference in seed production practices, processing, and storage methods and variations in the seeds production environment.
The caryopsis percentage and caryopsis weight are other important seed quality traits that affect germination, vigour, plant growth as well as the crop performance. We observed marked difference among seed samples analysed in this study for caryopsis percentage and caryopsis weight. Basilisk had the highest caryopsis percent (86%) whereas it ranged in between 17 and 52% for the seed samples from Cameroon suggesting 48–83% of empty seeds that were unable to germinate and produce seedlings. These results were within the range (0–93%) reported for 31 Urochloa grass accessions of five Urochloa species35. The thousand caryopsis weight (TCW) computed for Basilisk (4.400 g) in our study was comparable to U. decumbens (4.082 g), and the TCW calculated for two best performing seed samples from Cameroon i.e., E09 and A06 (1.680 g for each) was higher than TCW reported for U. brizantha (0.948 g) but was lower than U. ruziziensis (4.542 g)32.
We used tetrazolium test to determine viability of Urochloa seeds, a test commonly used for rapid assessment of seed germination potential. The viability test provides information on number of live seeds and dormant seeds and it is also useful to determine level of damage on seeds during harvesting, processing, and storage. We observed differences among seeds samples for seed viability; three seed samples from Cameroon (A08, NW14 and W12) had similar viability to Basilisk seeds, and N01 had significantly higher seed viability than Basilisk (81% vs. 61%). In this study, mean viability was 2.5 folds higher than mean germination (42.67% vs. 17.08%) and as anticipated every seed sample had higher viability than germination (Fig. 4). Similar differences in seed germination percent (5–20%) and corresponding seed viability percent (70–90%) have been reported in some tropical pasture species36. A 2.5 folds differences between viability and germination percentages observed in our study could be explained by dormancy imposed by seed covering and embryo dormancy37,38. Indeed, all the seed samples from Cameroon were collected in the late 2017 and tested for viability and germination within first 15 months of harvest which may not have been sufficient period for overcoming seed dormancy39. The regression analysis revealed 74% variation in seed germination was accounted by seed viability suggesting 26% of variation coming from other factors including seed dormancy.
Seed germination test provides information on ability of the seed to emerge from the soil to produce a plant in the field under normal conditions. The initial seed germination percentage for a quality seed batch should exceed 85% but for plant species that do not reach high levels of germination, a lower germination parentage is acceptable40. In this study were performed germination tests in three environments i.e., laboratory, greenhouse, and field; the mean germination rate was the highest in greenhouse test which was followed field test and laboratory test. Except two landraces, the seed germination was consistently high in the greenhouse test. Basilisk seeds had the highest germination (25.5%), and germination for 15 seed samples from Cameroon ranged in between 0.75% to 18.5%. A study in India with four Urochloa species reported germination rates from 0.0% to 18.0%32. The lowest seed germination rate observed in the laboratory conditions in our study could be attributed to the germination test conditions i.e., constant temperature of 28 °C and continuous dark while other authors have reported the use of alternating day/night temperature and light in assessing seed germination of Brachiaria grass seeds32,41,42. We therefore, acknowledge some methodological deficiencies in the lab testing which was solely because of unavailability of incubator with provision of alternate light and temperature cycles. Noteworthy to mention here is that some authors have reported no difference between light and dark conditions for the germination of U. brizantha cv. Piata seeds43 and no statistical differences between alternating light/dark cycle and continuous dark condition for the germination of Brachiaria eruciformis seeds42.
Over all, a low germination rates of Urochloa seeds observed in our study and other studies could be attributed to various factors including immature embryo at the time of seed dispersal, post-harvest dormancy, storage conditions and length of storage period33,37,44,45. Use of about one year old seeds stored in ambient condition might have significantly contributed to a low germination. The practices including seed scarification, seed stratification, exposure of seeds to light, exogenous application of chemical stimulants, invigoration treatments, seed storage in a cool room and use of dry chain technologies including hermetic bags can be helpful to improve seed germination in Urochloa grass37,41,46–49. Several technologies are available to improve seed germination and other seed quality parameters but their use in many countries in Africa including in Cameroon particularly for tropical forage seed are rare/nonexistence due to limited awareness, low technical know-how, limited access to agricultural inputs and credits, low priority given to forage research and development activities, and subsistence nature of forage and forage seed production. Urochloa seeds in Cameroon are harvest during the dry months of November and December enabling farmers to harvest seeds at adequately safe moisture level. These seeds are stored in jute bags on the bamboo ceilings or earthen floors of houses at ambient conditions until June-July, the planting season of Urochloa grass). Any unused/surplus seeds are stored further for the next year planting. The quality of these seeds in the ambient storage conditions deteriorate over time because of exposure to high temperature, high humidity and fluctuations in temperature and humidity. The use of technologies like storage of seeds in hermetic super bags and in a cold chain could slow down deterioration of these seeds41,47.
The seed production potentials and seed quality attributes including dormancy and germination behaviors of tropical grass species are greatly affected by site characteristics and the prevailing climatic conditions50 as they play significant roles in plant growth, flowering, and seed setting. A suitable locality for Urochloa seed production should allow unrestricted crop growth with day length that satisfy photoperiod need for flowering and seed setting as well as should restrict continuous vegetative growth to reproductive phase particularly in long day taxa39. Such conditions prevail in between about the tenth parallel and Tropic of Cancer in either Hemisphere where most successful Urochloa seed production activities have been taking place35,38,44. Urochloa seed samples from Cameroon analyzed in this study were collected from latitude ranges in between 4.33820°N to 9.93060°N, and seeds samples with the highest germination i.e., N01, A05 and W12 were from Garoua (9.93060°N), Wakwa (7.13600°N) and Bangangte (5.10389°N), respectively. While looking at other seed quality parameters e.g., TGW and caryopsis traits, seeds from higher latitudes were not necessarily superior to seeds from the lower latitudes which could be explained by the difference between the sites in other factors such as soil fertility, amount of rainfall, and altitude39.
The five Urochloa seeds samples from Cameroon with the highest germination had germination in between 8.0 and 18.5% which was 31.4% to 72.5% of improved cultivar Basilisk from Brazil. In general, the seed germination rates observed in our study was low both for seed samples from Cameroon and Basilisk seeds. This could have been attributed to a low seed setting in many tropical forage species51, inherent dormancy in fresh seeds (for seed samples from Cameroon), and loss of germination of Basilisk seeds due to an extended storage period. The observed differences for seeds quality traits among the Urochloa seeds samples from Cameroon could be due to the agroclimatic differences among seeds production localities and the variations in practices among farmers in production, processing, and storage of those seeds.
Conclusions
Farmers in Cameroon mostly from the Adamaoua and North regions have been involved in Urochloa seed production for years, but no information was available about seed qualities. This study closes existing knowledge gaps about the qualities of Urochloa seeds produced in Cameroon. Our study showed marked differences among seed produced in different localities and regions of Cameroon for major seed quality parameters—thousand gains weight, caryopsis formation, caryopsis weight, seed germination and seed viability. Urochloa seeds from Garoua (North), Wakwa (Adamaoua), and Baganté (West) were of superior quality to seeds produced in other localities for most seed quality parameters assessed in the study suggesting possibility of exploring these localities and regions further for Urochloa seeds production at commercial scale. Mapping Urochloa seed producers in North, Adamaoua and West regions; quality analysis of the seeds from across these three regions; documentation of biophysical characteristics of seed production localities; understanding of farmers’ practices of production, processing, and storage of seeds; and the seed agronomy research are some areas suggested for future research that help to comprehend full potential of Cameroon for the commercial production of Urochloa seeds.
Acknowledgements
Authors are thankful to Collins Mutai and John Juma at BecA-ILRI Hub respectively for technical support in executing research activities and preliminary analysis of experimental data.
Author contributions
S.R.G. and E.K.A. conceived the project, S.R.G., E.K.A. and C.N designed experiment, C.N. performed field and laboratory work, and C.N. and S.R.G. wrote manuscript. All authors participated in review and approval of this manuscript.
Funding
This study was supported by the BecA-ILRI Hub through the Africa Biosciences Challenge Fund (ABCF) program. The ABCF Program is funded by the Australian Department for Foreign Affairs and Trade (DFAT) through the BecA-CSIRO partnership; the Syngenta Foundation for Sustainable Agriculture (SFSA); the Bill & Melinda Gates Foundation (BMGF); the UK Department for International Development (DFID) and the Swedish International Development Cooperation Agency (Sida).
Data availability
The data sets used and/analysed in this study are available from the corresponding author.
Competing interests
The authors declare no competing interests.
Permission for collection of seeds
The first author obtained appropriate permission for the collection of Urochloa grass seeds used in this study.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Renvoize, S., Clayton, W. D. & Kabuye C. H. S. In Brachiaria: Biology, Agronomy, and Improvement 1–15 (CIAT and Embrapa, 1996).
- 2.Keller-Grein, G., Maass, B. L. & Hanson, J. In Brachiaria: Biology, Agronomy, and Improvement 16–42 (CIAT and Embrapa, 1996).
- 3.Barnard, C. Herbage Plant Species 154 (Australian Herbage Plant Registration Authority, Canberra, Australia, 1969).
- 4.Roberts OT. A review of pasture species in Fiji 1. Grasses. Trop. Grassl. 1970;4:129–137. [Google Scholar]
- 5.Serrão, E. A. S. & Simão Neto, M. Informaçõessobreduasespécies de gramíneasforrageiras do gênero Brachiaria na Amazônia: B. decumbens Stapf, B. ruziziensis Germain et Everard. Estudossobreforrageirasna Amazônia (IPEAN, 1971)
- 6.Parsons JJ. Spread of African pasture grasses to the American tropics. J. Range. Manag. 1972;25:12–17. doi: 10.2307/3896654. [DOI] [Google Scholar]
- 7.Sendulsky T. Brachiaria: Taxonomy of cultivated and native species in Brazil. Hoehnea. 1978;7:99–139. [Google Scholar]
- 8.Oram, R. N. Register of Australian herbage plant cultivars. In Australian Herbage Plant Registration Authority, 304 (East Melbourne, Australia, 1990).
- 9.Argel, P. J. & Keller-Grein, G. In Brachiaria: Biology, Agronomy, and Improvement 205–224 (CIAT and Embrapa, 1996).
- 10.Pizarro, E. A., do Valle, C. B., Keller-Grein, G., Schultze-Kraft. R. & Zimmer, A. H. In Brachiaria: Biology, Agronomy, and Improvement 225–246 (CIAT and Embrapa, 1996).
- 11.Jank L, Barrios SC, Valle CB, Simeão RM, Alves GF. The value of improved pastures to Brazilian beef production. Crop. Pasture Sci. 2014;65:1132–1137. doi: 10.1071/CP13319. [DOI] [Google Scholar]
- 12.Rueda Thaiana, da Silva C, et al. Yield component responses of the Brachiaria brizantha forage grass to soil water availability in the Brazilian Cerrado. Agriculture. 2020;10(1):13. doi: 10.3390/agriculture10010013. [DOI] [Google Scholar]
- 13.Stür, W. W., Hopkinson, J. M. & Chen, C.P. In Brachiaria: Biology, Agronomy, and Improvement 258–271 (CIAT and Embrapa, 1996).
- 14.Ndikumana, J. & de Leeuw, P. N. In Brachiaria: Biology, Agronomy, and Improvement 247–257(CIAT and Embrapa, 1996).
- 15.Maass BL, et al. Home coming of Brachiaria: Improved hybrids prove useful for African animal agricultural. E. Afr. Agric. For. J. 2015;81:71–78. doi: 10.1080/00128325.2015.1041263. [DOI] [Google Scholar]
- 16.Mutimura M, Everson TM. On-farm evaluation of improved Brachiaria grasses in low rainfall and aluminum toxicity prone areas of Rwanda. Int. J. Biodivers. Conserv. 2012;4:137–154. doi: 10.5897/IJBC10.121. [DOI] [Google Scholar]
- 17.Njarui, D. M. G., Gatheru, M. & Ghimire, S. R. In African Handbook of Climate Change Adaptation 1–21 10.1007/978-3-030-42091-8_146-1 (2020).
- 18.Ghimire, S. et al. In Proceedings of 23rd International Grassland Congress 361–370 (Range Management Society of India, 2015).
- 19.Njarui, D. M. G., Gichangi, E. M., Ghimire, S. R. & Muinga, R. W. Climate smart Brachiaria Grass for Improving Livestock Production in East Africa—Kenya Experiences (KALRO, 2016).
- 20.Mutimura M, Ebong C, Rao IM, Nsahlai IV. Change in growth performance of crossbred (Ankole × Jersey) dairy heifers fed on forage grass diets supplemented with commercial concentrates. Trop. Anim. Health. Prod. 2016;48:741–746. doi: 10.1007/s11250-016-1019-4. [DOI] [PubMed] [Google Scholar]
- 21.Boonman JG. Experimental studies on seed production of tropical grasses in Kenya. 2. Tillering and heading in seed crops of eight grasses. Neth. J. Agric. Sci. 1971;19:237–249. [Google Scholar]
- 22.Pamo ET, Yonkeu S, Onana J. Evaluation des plantesfourragèresintroduites dans l’AdamaouaCamerounais. Cahiers d’Agric. 1997;6:203–207. [Google Scholar]
- 23.Borget M. Les recherchesfourragères à l'IRAT/Cameroun (Bilan à la mi-1968). L'AgronomieTropicale Série 2, Agronomie Générale. Etudes Techniques. 1968;23:1231–1241. [Google Scholar]
- 24.Husson, O. et al. Brachiaria sp., B. ruziziensis, B. brizantha, B. decumbens, B. humidicola. Manuel pratique du semis direct à Madagascar (CIRAD, 2008).
- 25.Ministry of Agriculture and Rural Development. The State of Biodiversity for Food and Agriculture in the Republic of Cameroon (MINADER, 2015), http://www.fao.org/3/CA3431EN/ca3431en.pdf
- 26.Institute of Agricultural Research for Development. Annual report. Regional Agricultural Research Centre (Wakwa, Ngaoundéré, Cameroon, 2015).
- 27.Addinsoft. XLSTAT Statistical and Data Analysis Solution. New York, USA. https://www.xlstat.com (2021).
- 28.Botwright TL, Condon AG, Rebetzke GJ, Richards RA. Field evaluation of early vigour for genic improvement of grain yield in wheat. Aust. J. Agric. Res. 2002;53:1137–1145. doi: 10.1071/AR02007. [DOI] [Google Scholar]
- 29.Dholakia BB, et al. Molecular marker analysis of kernel size and shape in bread wheat. Plant Breed. 2003;122:392–395. doi: 10.1046/j.1439-0523.2003.00896.x. [DOI] [Google Scholar]
- 30.Wu W, Zhou L, Chen J, Qiu Z, He Y, Gain TKW. A measurement system of thousand kernel weight based on the Android Platform. Agronomy. 2018;8(9):178. doi: 10.3390/agronomy8090178. [DOI] [Google Scholar]
- 31.Heimbach U. Variability of thousand grain weights of seed batches of important arable and some horticultural crops. J. für Kulturpflanzen. 2018;70:250–254. [Google Scholar]
- 32.Parihar SS, Pathak PS. Flowering phenology and seed biology of selected tropical perennial grasses. Trop. Ecol. 2006;47:81–87. [Google Scholar]
- 33.Hare M, Tatsapong PP, Phengphet S. Effect of storage duration, storage room and bag type on seed germination of Brachiaria hybrid cv. Mulato. Trop. Grassl. 2008;42:224–228. [Google Scholar]
- 34.de Andrade RP, Thomas D, Ferguson JE. Seed production of pasture species in a tropical savanna region of Brazil. II Grasses. Trop. Grassl. 1983;17:59–64. [Google Scholar]
- 35.Nakamanee, G. & Phaikaew, C. In Proceedings of the third regional meeting of the Forages for Smallholders Project 155–162 (CIAT, 1998).
- 36.Song L, Kalms I. Improving germination of tropical grasses with new seed-coating technologies. Trop. Grassl. 2007;41:242. [Google Scholar]
- 37.Adkins SW, Bellairs SM, Loch DS. Seed dormancy mechanisms in warm season grass species. Euphytica. 2002;126:13–20. doi: 10.1023/A:1019623706427. [DOI] [Google Scholar]
- 38.Simpson GM. Seed Dormancy in Grasses. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- 39.Hopkinson, J. M., de Souza, F. H. D., Diulgheroff, S., Ortiz, A. & Sanchez, M. In Brachiaria: Biology, Agronomy, and Improvement 124–140 (CIAT, 1996)
- 40.Food and Agriculture Organization. Genebank Standards for Plant Genetic Resources for Food and Agriculture (FAO, 2013).
- 41.Hare MD, Sutin N, Phengphet S, Songsiri T. Germination of tropical forage seeds stored for six years in ambient and controlled temperature and humidity conditions in Thailand. Trop. Grassl. Forrajes Trop. 2018;6:26–33. doi: 10.17138/TGFT(5)26-33. [DOI] [Google Scholar]
- 42.Mobli A, Mollaee M, Manalil S, Chauhan BS. Germination ecology of Brachiaria eruciformis in Australia and its implications for weed management. Agronomy. 2020;10:30. doi: 10.3390/agronomy10010030. [DOI] [Google Scholar]
- 43.Romani F, Inacio R, de Carvalho RIN. Break dormancy, germination and vigour of Brachiaria brizantha cv. Brs Piatã seeds. R. Eletr. Cient Uergs. Porto Alegre. 2016;2:235–239. doi: 10.21674/2448-0479.23.235-239. [DOI] [Google Scholar]
- 44.Pizarro EA, Hare MD, Mutimura M, Changjun B. Brachiaria hybrids: potential, forage use and seed yield. Trop. Grassl. Forrajes. Trop. 2013;1:31–35. doi: 10.17138/TGFT(1)31-35. [DOI] [Google Scholar]
- 45.Herrera J. Efecto de alguns tratamentos para interromper o resto em sementes de grama. II. Urochloa decumbens. Agron. Costarric. 1994;18:75–85. [Google Scholar]
- 46.Whiteman PC, Mendra K. Effects of storage and seed treatments on germination of Brachiaria decumbens. Seed Sci. Technol. 1982;10:233–242. [Google Scholar]
- 47.Bakhtavar MA, Afzal I. Preserving wheat grain quality and preventing aflatoxin accumulation during storage without pesticides using dry chain technology. Environ. Sci. Pollut. Res. Int. 2020;27:42064–42071. doi: 10.1007/s11356-020-10212-5. [DOI] [PubMed] [Google Scholar]
- 48.Batista TB, da Silva Binotti FF, Cardoso ED, Costa E, do Nascimento DM. Appropriate hydration period and chemical agent improve priming in Brachiaria seeds. Pesqui. Agropecu. Trop. 2016;46:350–356. doi: 10.1590/1983-40632016v4638422. [DOI] [Google Scholar]
- 49.Pereira SR, da Lima AES, Contreiras-Rodrigues APD, de Oliveira DR, Laura VA. Priming of Urochloa brizantha cv. Xaraes seeds. Afr. J. Agric. Res. 2018;13:2804–2807. doi: 10.5897/AJAR2018.13414. [DOI] [Google Scholar]
- 50.Ferguson, J. E., Thomas, D., de Andrade, R. P., Costa, N. S. & Jutzi, S. In Proceedings of XIV International Grassland Congress 275–278 (Westview Press, 1983)
- 51.Boonman JG. East Africa’s Grasses and Fodders: Their Ecology and Husbandry. The Netherlands: Kluwer Academic Publishers; 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/analysed in this study are available from the corresponding author.