Abstract
Despite reductions in malaria incidence and mortality across Sub-Saharan (SSA) countries, malaria control and elimination efforts are currently facing multiple global challenges such as climate and land use change, invasive vectors, and disruptions in healthcare delivery. Although relationships between malaria risks and socioeconomic factors have been widely demonstrated, the strengths and variability of these associations have not been quantified across SSA. In this study, we used data from population-based malaria indicator surveys in SSA countries to assess spatial trends in relative and absolute socioeconomic inequalities, analyzed as social (mothers’ highest educational level—MHEL) and economic (wealth index—WI) inequalities in malaria prevalence. To capture spatial variations in socioeconomic (represented by both WI and MHEL) inequalities in malaria, we calculated both the Slope Index of Inequality (SII) and Relative Index of Inequality (RII) in each administrative region. We also conducted cluster analyses based on Local Indicator of Spatial Association (LISA) to consider the spatial auto-correlation in SII and RII across regions and countries. A total of 47,404 participants in 1874 Primary Sampling Units (PSU) were analyzed across the 13 SSA countries. Our multi-country assessment provides estimations of strong socioeconomic inequalities between and within SSA countries. Such within- and between- countries inequalities varied greatly according to the socioeconomic metric and the scale used. Countries located in Eastern Africa showed a higher median Slope Index of Inequality (SII) and Relative Index of Inequality (RII) in malaria prevalence relative to WI in comparison to countries in other locations across SSA. Pockets of high SII in malaria prevalence in relation to WI and MHEL were observed in the East part of Africa. This study was able to map this wide range of malaria inequality metrics at a very local scale and highlighted the spatial clustering patterns of pockets of high and low malaria inequality values.
Subject terms: Malaria, Epidemiology
Introduction
In the last years, Sub-Saharan African (SSA) countries were on track for reductions in malaria incidence and mortality1,2. However, most of worldwide malaria cases (93%) and deaths (94%) still occur in this region, mainly (99.7%) caused by Plasmodium falciparum3. Previous studies highlighted the strong spatial heterogeneity in malaria transmission between- and within- SSA countries and highlighted the importance of pockets of malaria transmission at the macro- and micro- epidemiological level across stable and unstable malaria foci4,5. Ecological composition that favor micro-habitats for breeding sites of dominant malaria vectors (Anopheles gambiae, Anopheles coluzzii, Anopheles funestus, and Anopheles arabiensis)6 is a major driver to the scattered or clustered pattern of malaria transmission7–10. However, other important factors collide with such environmental determinants to intensify malaria heterogeneities, such as dwelling infrastructure11, human mobility12,13, and socioeconomic status14.
The malaria elimination efforts are currently facing multiple regional challenges. Climate and land use changes are major Regional health threats that in turn poses critical risks to achieving malaria elimination15,16. Changes in meteorological and ecological patterns influence the distribution of the vector, human hosts and alter the biological cycle of the parasite and vector17,18, that ultimately, may diverge current epidemiological trends in malaria-endemic countries19–21. Such as in other infectious diseases, socioeconomic inequalities were reported in malaria cases and deaths22–25. Socioeconomic status may influence malaria risks through multiple different mechanisms. For example, improved housing associated with increased wealth may substantially decrease child malaria risks26 and socioeconomic development may be one of the most effective interventions for malaria control27. However, the role of socioeconomic factors in reducing malaria risks may vary substantially between and within countries. While agricultural development is frequently associated with increased prosperity, the expansion of irrigated lands may increase vector densities and lead to counterintuitive associations between socioeconomic status and malaria risks28. Important evidence about the socioeconomic inequalities on the malaria risk was reported in previous studies done in SSA countries such as in Uganda22, Kenya23,29, Tanzania30, and Nigeria24. These studies highlighted the substantial heterogeneity between socioeconomic metrics at a subnational and local level and the utility of their identification to target malaria control interventions. However, no study comprehensively assessed malaria socioeconomic inequalities across many SSA countries at a fine spatial scale.
The Demographic and Health Surveys (DHS) and in particular the Malaria Indicator Survey (MIS) provides information about malaria screening and sociodemographic variables in multiple countries, moreover, when multi-country assessments in the SSA region are scant. This dataset represents a unique opportunity to capitalize on to identify such malaria heterogeneities across the socioeconomic gradient. In particular, the socioeconomic status (SES) implies multiple, yet related, mechanisms that may impact malaria inequalities. Income or wealth reflect the resources to afford the direct and indirect costs of a malaria infection and access to preventive measures for example31,32. On the other hand, maternal education (as a social dimension of SES) captures the receptiveness and knowledge capital of the family about preventive measures and early health-seeking behaviors33,34. Previous studies highlighted the role of the maternal education on the child’s health in developing countries35–37; however, few studies have examined independently the wealth and socioeconomic status inequalities in malaria38,39.
Commonly, the relative position in the distribution of wealth or socioeconomic indicator is not comparable across populations (i.e. geographical locations). The slope index of inequality (SII) and the relative index of inequality (RII) are two major indicators for the cross-population comparison and quantification of the wealth/socioeconomic gradient in the relative and absolute scale, respectively. Both indicators provide key and complementary information based on the relative socioeconomic position of the individual in the population, termed `socioeconomic rank`40–43. Particularly, studies in other infectious diseases such as in HIV showed contrasting results in the inequality interpretation based on the absolute or relative scale of the inequality metric44,45. Also, since both indicators compare hypothetical extremes of the socioeconomic rank and are estimated using a regression framework, the RII and SII are considered a summary measure rather than a true population parameter and could be used for comparisons between and within countries40. In this study, we used data from population-based malaria indicator surveys in thirteen SSA countries to assess spatial trends in relative and absolute social (mothers’ highest educational level—MHEL) and economic (wealth index—WI) inequalities in malaria prevalence. We aimed to extend these estimations at fine spatial scale in the SSA region. We also aimed to identify clusters of high or low SES malaria inequalities across SSA countries.
Methods
Study design and data description
Our study is a multi-country cross-sectional data analysis of the Malaria Indicator Survey (MIS) from thirteen SSA countries for which such data has been collected (Angola, Burkina Faso, Burundi, Kenya, Liberia, Madagascar, Malawi, Mali, Mozambique, Sierra Leone, Tanzania, Togo, and Uganda). We used data from the most recent MIS (from 2015 to 2018), developed by the Monitoring and Evaluation Working Group (MERG) of the Roll Back Malaria initiative. Data of malaria test results, WI, and MHEL were accessed through the DHS Program. The GPS coordinates of the Primary Sampling Units (PSU) were available with an injected 0–2 km displacements in urban clusters, and 0–5 km for rural clusters. In addition, the administrative boundaries were accessed via DHS spatial data repository (https://spatialdata.dhsprogram.com/). Admirative boundaries represent sub-national regions, commonly administrative level 1, and varies between survey years and countries. Two variables were analyzed for relative (i.e. RII) and absolute (i.e. SII) inequalities in malaria prevalence, the WI and MHEL. Inequality estimates were computed and mapped at the PSU and administrative level. For the main analyses, we considered PSU with a sample size higher than 10 and we conducted sensitivity analyses (see details below). In this paper, the main metric of interest is related to SES inequalities in malaria positive tests focusing on two distinct SES measures (WI and MHEL) and two scales to capture such inequalities.
Exposure and outcome definitions
We defined the malaria test result based on the malaria screening in blood samples conducted by rapid diagnostic test (RDT) during field work. No malaria species identification result was included in this study. A positive malaria test result was considered if the sample was positive to any malaria species, and negative otherwise.
The WI is a composite measure based on a Principal Component Analysis (PCA) of a household's cumulative living standard using household's ownership of selected assets, such as televisions and bicycles; materials used for housing construction; and types of water access and sanitation facilities as described elsewhere46. Finally, MHEL was also explored and was re-classified in four categories: no education, elementary/primary, secondary, and higher education. While MHEL was also measured in years of formal education completed by each woman, we reclassified MHEL into these four categories to have subgroups with homogenous sample sizes and reduce the risk of empty cells (i.e. MHEL subgroups with no positive malaria test).
Socio-economic inequality analysis
Two metrics—on the relative and absolute scales—were used to assess the SES inequalities in malaria prevalence at both the PSU and administrative levels relative to WI and MHEL. The SII and the RII41 were computed separately for WI and MHEL. In SII and RII calculation, SES variable was ranked from high to low, thus high values of SII and RII represents high malaria concentration among low-SES levels, and low values represents high malaria concentration among high-SES levels. SII represents the linear regression coefficient that shows the relation between malaria prevalence in each SES level and the hierarchical ranking of SES level. RII expresses the ratio of the predicted outcomes between population in the highest SES level and the lowest SES level. Both indicators were obtained by fitting a linear regression to estimate the coefficient between participants’ relative SES rank and malaria prevalence41. In addition, a third inequality metric, the concentration index (CI), was evaluated. CI computation details were presented in the supplementary information. For additional data exploration, Spearman's rank correlation coefficient (Spearman's ρ) was conducted to examine the association between different scales (SII/RII) of the same SES variable, and different SES measures (WI/MHEL) using the same scale of the inequality index.
Sensitivity analysis
Since the sample size in each PSU varies across countries, extreme malaria prevalence estimates may result from very small sample sizes (Supplementary Fig. 1). Sensitivity analyses were conducted by including only PSU with a sample size higher than 20 and 30.
Spatial analysis
Spatial autocorrelation of CI, SII, and RII was assessed using local Getis-Ord Gi* statistic (a type of Local Indicator of Spatial Association—LISA) to identify local patterns and clusters of high- and low-inequality across SSA countries. A distance-based neighborhood structure was used for Getis-Ord Gi* computation. Neighboring PSUs were defined based on the distance d that assigns at least one neighbor to each PSU (nearest neighbor). Gi* statistic was categorized based on the sign (cold- or hotspot) and percentile (90%, 95%, 99%) to prevent bias due to multiple and dependent tests47. Data assemble, statistical analyses, spatial data processing, and visualizations were performed in R software v.4.0.1 (R: A language and environment for statistical computing, R Core Team, R Foundation for Statistical Computing, Vienna, Australia (2021) http://www.R-project.org/).
Results
A total of 47,404 participants (a subset of 33,187 for the analysis of MHEL) in 1,874 (MHEL subset: 1,603) PSUs were analyzed across the 13 SSA countries. The sample size in the PSUs ranges from 10 to 114 (MHEL subset: 10 to 107) participants. Overall, a weighted prevalence of 36.44% was observed in all the SSA countries, ranging from 16.02% in Madagascar to 55.4% in Sierra Leone (Table 1). The sample size and prevalence distribution by PSU is presented in Supplementary Fig. 1.
Table 1.
Summary statistics of 13 Sub-Saharan African countries.
| Country | Sample size | Prevalence* | Wealth ^ | Maternal Education ^ | ||
|---|---|---|---|---|---|---|
| SII | RII | SII | RII | |||
| AO | 1,199 | 22.22% | − 0.014 | 0.944 | 0.003 | 1.013 |
| BF | 4,954 | 20.00% | 0.054 | 1.458 | 0.156 | 3.827 |
| BU | 2,099 | 16.67% | 0.053 | 1.455 | 0.154 | 3.161 |
| KE | 4,318 | 16.16% | 0.083 | 1.601 | 0.157 | 3.161 |
| LB | 2,685 | 52.98% | 0.090 | 1.225 | 0.197 | 1.609 |
| MD | 1,947 | 8.08% | 0.063 | 3.110 | 0.027 | 1.629 |
| ML | 6,534 | 31.83% | 0.040 | 1.126 | 0.092 | 2.029 |
| MW | 1,840 | 33.33% | 0.200 | 2.212 | 0.222 | 2.765 |
| MZ | 3,430 | 43.74% | 0.131 | 1.504 | 0.109 | 1.400 |
| SL | 7,406 | 60.00% | 0.064 | 1.112 | 0.084 | 1.172 |
| TG | 2,831 | 46.97% | 0.053 | 1.123 | 0.092 | 1.336 |
| TZ | 2,942 | 13.33% | 0.129 | 2.182 | 0.114 | 1.917 |
| UG | 5,219 | 26.06% | 0.072 | 1.391 | 0.086 | 1.632 |
Sample size, prevalence and median slope index of inequality (SII) and relative index of inequality (RII) by country.
*Weighted prevalence; ^ median values.
SES inequalities in malaria prevalence
Important heterogeneities on SES inequalities on malaria prevalence was observed across the SSA countries. Malawi, Tanzania, and Mozambique were the countries with the greatest median WI inequality in the absolute and relative scales, respectively (Supplementary Fig. 2 and Supplementary Fig. 3). Malawi was the country with the greatest median MHEL inequality in the absolute scale (Supplementary Fig. 4) followed by Liberia in the relative scales (Supplementary Fig. 5). Greater variability in SII for WI was observed in Togo (sd: 0.406) followed by Malawi (sd: 0.397) (Supplementary Fig. 2) and Liberia (sd: 0.397), and for MHEL in Sierra Leone (sd: 0.58) followed by Angola (sd: 0.56) and Malawi (sd: 0.54) (Supplementary Fig. 4). In comparison, the country where the greatest variably in RII for WI was observed is Tanzania (sd: 186.9) followed by Mali (sd: 156.3) and Madagascar (sd: 84.28) (Supplementary Fig. 3), and for MHEL was Burkina Faso (sd: 26.19) followed by Burundi (sd: 22.25) and Liberia (sd: 21.39) (Supplementary Fig. 5).
Overall, SES-inequality showed moderate correlation between WI and MHEL in the absolute (rho-SII: 0.12; p-value < 0.001) and relative (rho-RII: 0.16; p-value < 0.001) scales. Kenya is the only country with a negative correlation between WI/MHEL-SII and the strongest WI/MHEL-SII correlation was observed in Liberia. On the other hand, the strongest correlation in WI/MHEL-RII was observed in Angola, and Kenya, Burkina Faso, and Burundi showed a negative WI/MHEL-RII correlation (Supplementary Table 1). No significant patterns of SES-inequality in both scales (SII or RII) as a function of the PSU prevalence was observed for WI and MHEL (Supplementary Fig. 2 to Supplementary Fig. 5).
Absolute and relative inequality scales
The information of both scales, absolute and relative, provides complementary information about public health actions. Only a moderate correlation was observed between different scales (SII and RII) in the same SES variable (rho = 0.34 for WI and rho = 0.15 for MHEL) (Supplementary Fig. 6). While absolute scale provides a measure of the public health impact of the SES inequality, the relative scale provides a measure of the strength of the association between the SES variable and the disease outcome. The only countries with a negative correlation between SII and RII for WI are Madagascar (rho = − 0.31), Angola (− 0.09), Tanzania (− 0.06), and Burundi (− 0.02). Burkina Faso, Burundi, Madagascar, Tanzania, and Kenya showed a negative correlation between SII and RII for MHEL (Supplementary Table 2).
Spatial heterogeneity and clustering
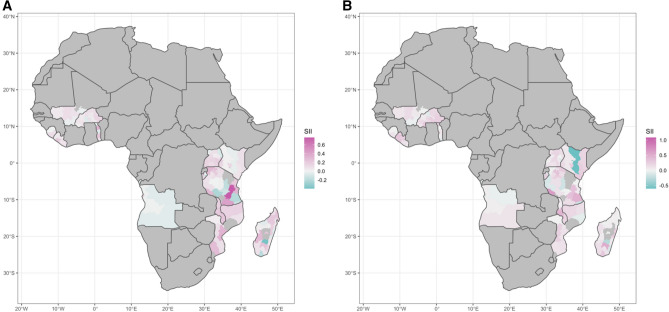
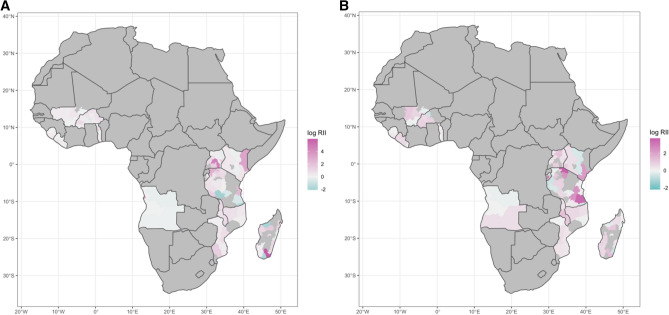
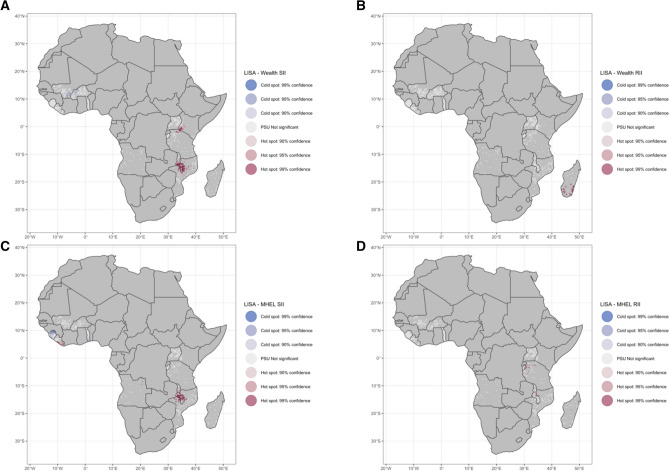
At the subnational level, contrasting patterns were observed between countries in SSA (Figs. 1 and 2). Countries located in Eastern Africa showed a higher median SII and RII in malaria prevalence relative to WI in comparison to countries in other locations across SSA when analyzing at administrative level (Figs. 1 and 2) and PSU level (Supplementary Fig. 7 and Supplementary Fig. 8). The clusters of high (hotspot) or low (coldspot) values of SES-inequality differ according to the SES variable and scale used (Fig. 3). Pockets of high SII in malaria prevalence in relation to WI and MHEL were observed in the East part of Africa, mainly close to Malawi and Uganda. On the other hand, pockets of low RII in malaria prevalence in relation to WI and MHEL were observed close to Burkina Faso (Fig. 3). Consistent patterns were observed when using CI as the inequality metric (Supplementary Fig. 9 to Supplementary Fig. 13).
Figure 1.
Distribution of the Slope Index of Inequality (SII) in malaria prevalence at the administrative level in Sub-Saharan African (SSA) countries. Relative to (A) wealth index (WI) and (B) mothers’ highest educational level (MHEL). The maps were generated using R software v.4.0.1 (R: A language and environment for statistical computing, R Core Team, R Foundation for Statistical Computing, Vienna, Australia (2021) http://www.R-project.org/) with ggplot2 3.3.2 (H. Wickham. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016.) and country boundaries from Natural Earth (https://www.naturalearthdata.com/) using the package spData 0.3.1 (https://nowosad.github.io/spData).
Figure 2.
Distribution of the Relative Index of Inequality (RII) in malaria prevalence at the administrative level in Sub-Saharan African (SSA) countries. Relative to (A) wealth index (WI) and (B) mothers’ highest educational level (MHEL). RII in logarithmic scale. The maps were generated using R software v.4.0.1 (R: A language and environment for statistical computing, R Core Team, R Foundation for Statistical Computing, Vienna, Australia (2021) http://www.R-project.org/) with ggplot2 3.3.2 (H. Wickham. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016.) and country boundaries from Natural Earth (https://www.naturalearthdata.com/) using the package spData 0.3.1 (https://nowosad.github.io/spData).
Figure 3.
Local spatial autocorrelation of malaria inequality as Local Getis-Ord Gi* at the Primary Sampling Unit (PSU) level. Malaria inequality relative to wealth index (WI) in the (A) absolute and (B) relative scales, and relative to mothers’ highest educational level (MHEL) in the (C) absolute and (D) relative scales. The maps were generated using R software v.4.0.1 (R: A language and environment for statistical computing, R Core Team, R Foundation for Statistical Computing, Vienna, Australia (2021) http://www.R-project.org/) with ggplot2 3.3.2 (H. Wickham. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016.) and country boundaries from Natural Earth (https://www.naturalearthdata.com/) using the package spData 0.3.1 (https://nowosad.github.io/spData).
Discussion
This study compared two dimensions of the socioeconomic status—the wealth index and maternal education—for measuring the inequalities in malaria prevalence across Sub-Saharan African (SSA) countries using an absolute and relative scale of inequality. Our multi-country assessment provides estimations of strong socioeconomic inequalities in malaria risks between and within SSA countries. Such within- and between-countries inequalities varied greatly according to the socioeconomic metric and the scale used. Finally, this study was able to map this wide range of malaria inequality metrics at a very local scale and highlighted the spatial clustering patterns of pockets of high and low malaria inequality values.
Mapping geographical inequalities at a fine spatial scale highlights important information to guide programmatic actions that would help reduce malaria inequalities, such as targeted provision of sanitation or control of infectious diseases48–50. Despite sparse studies that highlighted important socioeconomic inequalities regarding malaria prevalence in different contexts22,23,25, a comprehensive assessment of the malaria inequality landscape in SSA countries were not available. Understanding such malaria inequalities and their localization at a very fine scale is of particular interest in SSA where multiple contemporary challenges might synergistically impact in vulnerable populations and worsen their health status.
This paper constitutes the first assessment of malaria SES inequalities across many SSA countries while assessing the spatial distribution of such malaria inequalities. We emphasize that the malaria burden is not shared equally across SES strata in SSA (both within and between countries) and that such inequalities are not randomly distributed across space. We believe this work could be extremely beneficial for existing programs to incorporate an inequality focus into malaria prevention efforts and identify areas (at a fine geographical scale) where such inequalities are concentrated for informing targeted efforts. The results presented here showed the important and complementary information of the multiple dimensions of socioeconomic status. The fine-scale mapping showed in this study stressed out the contrasting spatial patterns of malaria inequalities in relation to wealth or maternal education. Previous studies showed that both maternal education and wealth strongly influence knowledge about and efforts to prevent and treat malaria in Madagascar51. Furthermore, a recent systematic review suggest that public policy measures that can improve economic and educational opportunities for the poor, will help in reducing the burden of malaria in SSA14. In addition, a multi-country study highlighted that maternal education and wealth are important components of a simplified SES index52. Taken together, the results of this study emphasize the multiple socioeconomic mechanisms that may impact in the uneven malaria distribution also at a very local level.
This study has a number of limitations. First, socioeconomic metrics such as the components of the wealth index and maternal education may be subject to measurement error due to recall bias, inaccurate reporting during interviews, and social desirability bias. Second, despite the fine spatial scale of this study, these results might not fully represent intra-urban malaria inequalities. Third, our analysis does not capture the impacts of conflicts or climate change-related meteorological events and disasters, and data for locations affected by these factors might not reflect current or seasonal conditions. Fourth, the spatial jittering injected to PSU’s GPS data (to preserve confidential data) may affect the spatial clustering analysis.
In conclusion, socioeconomic inequality of malaria in relation to wealth index and maternal education were strongly heterogeneous with pockets of high and low malaria inequality across SSA. The use of multiple metrics of socioeconomic status and scales of inequality metrics allows for a better characterization of the multiple mechanisms in place that originate such strong malaria heterogeneities observed in this study. Finally, historical malaria inequalities reflected in this study collide with current challenges that might synergistically and negatively impacts the health of vulnerable populations in SSA countries.
Supplementary Information
Author contributions
Conceived and designed the study: G.C.E., T.B. Data collection and analysis: G.C.E., and K.F. Wrote the manuscript: G.C.E., K.F., and T.B. All authors reviewed and approved the final manuscript.
Data availability
The datasets used in this study are available upon authorization from the DHS program and can be accessed on the website (https://dhsprogram.com/data/available-datasets.cfm).
Code availability
Codes to reproduce the inequalities indicators, the figures, and tables can be accessed at https://bookdown.org/gabc91/mal_ineq/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94601-x.
References
- 1.Battle KE, et al. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–17: A spatial and temporal modelling study. Lancet. 2019;394:332–343. doi: 10.1016/S0140-6736(19)31096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss DJ, et al. Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum, 2000–17: A spatial and temporal modelling study. Lancet. 2019;394:322–331. doi: 10.1016/S0140-6736(19)31097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization, W. H. World malaria report 2019. (2019).
- 4.Baidjoe AY, et al. Factors associated with high heterogeneity of malaria at fine spatial scale in the Western Kenyan highlands. Malar. J. 2016;15:307. doi: 10.1186/s12936-016-1362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awine T, Malm K, Peprah NY, Silal SP. Spatio-temporal heterogeneity of malaria morbidity in Ghana: Analysis of routine health facility data. PLoS ONE. 2018;13:e0191707. doi: 10.1371/journal.pone.0191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinka ME, et al. The dominant Anopheles vectors of human malaria in the Americas: Occurrence data, distribution maps and bionomic précis. Parasit. Vectors. 2010;3:72. doi: 10.1186/1756-3305-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson JC, et al. Spatio-temporal heterogeneity of malaria vectors in northern Zambia: Implications for vector control. Parasit. Vectors. 2016;9:510. doi: 10.1186/s13071-016-1786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkot TR, et al. Spatial-temporal heterogeneity in malaria receptivity is best estimated by vector biting rates in areas nearing elimination. Parasit. Vectors. 2018;11:606. doi: 10.1186/s13071-018-3201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank C, et al. Spatial heterogeneity of malaria in Ghana: A cross-sectional study on the association between urbanicity and the acquisition of immunity. Malar. J. 2016;15:84. doi: 10.1186/s12936-016-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soma DD, et al. Uneven malaria transmission in geographically distinct districts of Bobo-Dioulasso, Burkina Faso. Parasit. Vectors. 2018;11:296. doi: 10.1186/s13071-018-2857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tusting LS, et al. Housing improvements and malaria risk in sub-Saharan Africa: A multi-country analysis of survey data. PLOS Med. 2017;14:e1002234. doi: 10.1371/journal.pmed.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tessema S, et al. Using parasite genetic and human mobility data to infer local and cross-border malaria connectivity in Southern Africa. Elife. 2019;8:e43510. doi: 10.7554/eLife.43510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prosper O, Ruktanonchai N, Martcheva M. Assessing the role of spatial heterogeneity and human movement in malaria dynamics and control. J. Theor. Biol. 2012;303:1–14. doi: 10.1016/j.jtbi.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Degarege A, Fennie K, Degarege D, Chennupati S, Madhivanan P. Improving socioeconomic status may reduce the burden of malaria in sub Saharan Africa: A systematic review and meta-analysis. PLoS ONE. 2019;14:e0211205. doi: 10.1371/journal.pone.0211205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanser FC, Sharp B, le Sueur D. Potential effect of climate change on malaria transmission in Africa. Lancet. 2003;362:1792–1798. doi: 10.1016/S0140-6736(03)14898-2. [DOI] [PubMed] [Google Scholar]
- 16.Caminade C, et al. Impact of climate change on global malaria distribution. Proc. Natl. Acad. Sci. 2014;111:3286–3291. doi: 10.1073/pnas.1302089111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laneri K, Cabella B, Prado PI, Coutinho RM, Kraenkel RA. Climate drivers of malaria at its southern fringe in the Americas. PLoS ONE. 2019;14:2. doi: 10.1371/journal.pone.0219249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mordecai EA, et al. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol. Lett. 2013;16:22–30. doi: 10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]
- 19.Eikenberry SE, Gumel AB. Mathematical modeling of climate change and malaria transmission dynamics: A historical review. J. Math. Biol. 2018;77:857–933. doi: 10.1007/s00285-018-1229-7. [DOI] [PubMed] [Google Scholar]
- 20.Onyango EA, Sahin O, Awiti A, Chu C, Mackey B. An integrated risk and vulnerability assessment framework for climate change and malaria transmission in East Africa. Malar. J. 2016;15:551. doi: 10.1186/s12936-016-1600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tusting LS, et al. measuring socioeconomic inequalities in relation to malaria risk: A comparison of metrics in rural Uganda. Am. J. Trop. Med. Hyg. 2016;94:650–658. doi: 10.4269/ajtmh.15-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Were V, et al. Socioeconomic health inequality in malaria indicators in rural western Kenya: Evidence from a household malaria survey on burden and care-seeking behaviour. Malar. J. 2018;17:166. doi: 10.1186/s12936-018-2319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onwujekwe OE, Uzochukwu BS, Ezeoke OP. Socio-economic inequalities in cost of seeking treatment for malaria in south-east Nigeria. Int. J. Med. Health Dev. 2010;15:2–16. [Google Scholar]
- 25.Worrall E, Basu S, Hanson K. Is malaria a disease of poverty? A review of the literature. Trop. Med. Int. Health. 2005;10:1047–1059. doi: 10.1111/j.1365-3156.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 26.Tusting LS, et al. Housing and child health in sub-Saharan Africa: A cross-sectional analysis. PLOS Med. 2020;17:e1003055. doi: 10.1371/journal.pmed.1003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tusting LS, et al. Socioeconomic development as an intervention against malaria: A systematic review and meta-analysis. Lancet Lond. Engl. 2013;382:963–972. doi: 10.1016/S0140-6736(13)60851-X. [DOI] [PubMed] [Google Scholar]
- 28.Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: Paddies paradox. Med. Vet. Entomol. 2001;15:1–11. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- 29.Ilinca S, Di Giorgio L, Salari P, Chuma J. Socio-economic inequality and inequity in use of health care services in Kenya: Evidence from the fourth Kenya household health expenditure and utilization survey. Int. J. Equity Health. 2019;18:196. doi: 10.1186/s12939-019-1106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somi MF, et al. Use of proxy measures in estimating socioeconomic inequalities in malaria prevalence. Trop. Med. Int. Health. 2008;13:354–364. doi: 10.1111/j.1365-3156.2008.02009.x. [DOI] [PubMed] [Google Scholar]
- 31.Alonso S, et al. The economic burden of malaria on households and the health system in a high transmission district of Mozambique. Malar. J. 2019;18:360. doi: 10.1186/s12936-019-2995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tefera DR, Sinkie SO, Daka DW. Economic burden of malaria and associated factors among rural households in Chewaka District, Western Ethiopia. Clin. Outcomes Res. CEOR. 2020;12:141–152. doi: 10.2147/CEOR.S241590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racine AD, Joyce TJ. Maternal education, child immunizations, and public policy: Evidence from the US National Immunization Survey. Soc. Sci. Med. 2007;65:1765–1772. doi: 10.1016/j.socscimed.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Noor AM, Omumbo JA, Amin AA, Zurovac D, Snow RW. Wealth, mother’s education and physical access as determinants of retail sector net use in rural Kenya. Malar. J. 2006;5:5. doi: 10.1186/1475-2875-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cropley L. The effect of health education interventions on child malaria treatment-seeking practices among mothers in rural refugee villages in Belize Central America. Health Promot. Int. 2004;19:445–452. doi: 10.1093/heapro/dah406. [DOI] [PubMed] [Google Scholar]
- 36.Glewwe P. Why does Mother’s schooling raise child health in developing countries? Evidence from Morocco. J. Hum. Resour. 1999;34:124–159. doi: 10.2307/146305. [DOI] [Google Scholar]
- 37.Bicego GT, Boerma JT. Maternal education and child survival: A comparative study of survey data from 17 countries. Soc. Sci. Med. 1993;1982(36):1207–1227. doi: 10.1016/0277-9536(93)90241-U. [DOI] [PubMed] [Google Scholar]
- 38.Njau JD, Stephenson R, Menon MP, Kachur SP, McFarland DA. Investigating the important correlates of maternal education and childhood malaria infections. Am. J. Trop. Med. Hyg. 2014;91:509–519. doi: 10.4269/ajtmh.13-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siri JG. Independent Associations of Maternal Education and Household Wealth with Malaria Risk in Children. Ecol. Soc. 2014;19:2. doi: 10.5751/ES-06134-190133. [DOI] [Google Scholar]
- 40.Moreno-Betancur M, Latouche A, Menvielle G, Kunst AE, Rey G. Relative index of inequality and slope index of inequality: A structured regression framework for estimation. Epidemiology. 2015;26:518–527. doi: 10.1097/EDE.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 41.Regidor E. Measures of health inequalities: Part 2. J. Epidemiol. Community Health. 2004;58:900–903. doi: 10.1136/jech.2004.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackenbach JP, Kunst AE. Measuring the magnitude of socio-economic inequalities in health: An overview of available measures illustrated with two examples from Europe. Soc. Sci. Med. 1997;1982(44):757–771. doi: 10.1016/S0277-9536(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 43.Pamuk ER. Social class inequality in mortality from 1921 to 1972 in England and Wales. Popul. Stud. 1985;39:17–31. doi: 10.1080/0032472031000141256. [DOI] [PubMed] [Google Scholar]
- 44.King NB, Harper S, Young ME. Use of relative and absolute effect measures in reporting health inequalities: Structured review. BMJ. 2012;345:2. doi: 10.1136/bmj.e5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King NB, Kaufman JS, Harper S. Relative measures alone tell only part of the story. Am. J. Public Health. 2010;100:2014–2015. doi: 10.2105/AJPH.2010.203232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutstein, S. O. & Johnson, K. The DHS wealth index. DHS comparative reports no. 6. Calverton ORC Macro (2004).
- 47.de Caldas CM, Singer BH. Controlling the false discovery rate: A new application to account for multiple and dependent tests in local statistics of spatial association. Geogr. Anal. 2006;38:180–208. doi: 10.1111/j.0016-7363.2006.00682.x. [DOI] [Google Scholar]
- 48.Deshpande A, et al. Mapping geographical inequalities in access to drinking water and sanitation facilities in low-income and middle-income countries, 2000–17. Lancet Glob. Health. 2020;8:e1162–e1185. doi: 10.1016/S2214-109X(20)30278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pullan RL, Freeman MC, Gething PW, Brooker SJ. Geographical inequalities in use of improved drinking water supply and sanitation across SUB-Saharan Africa: Mapping and spatial analysis of cross-sectional survey data. PLOS Med. 2014;11:e1001626. doi: 10.1371/journal.pmed.1001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiner RC, et al. Mapping geographical inequalities in childhood diarrhoeal morbidity and mortality in low-income and middle-income countries, 2000–17: analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:1779–1801. doi: 10.1016/S0140-6736(20)30114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clouston SAP, Yukich J, Anglewicz P. Social inequalities in malaria knowledge, prevention and prevalence among children under 5 years old and women aged 15–49 in Madagascar. Malar. J. 2015;14:499. doi: 10.1186/s12936-015-1010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Psaki SR, et al. Measuring socioeconomic status in multicountry studies: results from the eight-country MAL-ED study. Popul. Health Metr. 2014;12:8. doi: 10.1186/1478-7954-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this study are available upon authorization from the DHS program and can be accessed on the website (https://dhsprogram.com/data/available-datasets.cfm).
Codes to reproduce the inequalities indicators, the figures, and tables can be accessed at https://bookdown.org/gabc91/mal_ineq/.