Abstract
An 86-year-old man with paroxysmal atrial fibrillation on flecainide, a class IC antiarrhythmic, presented with cardiac arrest. The patient had extremely wide QRS complexes with inconsistent pacemaker capture on electrocardiography. Due to cardiac failure and renal failure, the patient developed progressive flecainide toxicity, which led to pacemaker failure, and ultimately, death. (Level of Difficulty: Beginner.)
Key Words: atrial fibrillation, cardiac pacemaker, complication
Abbreviations and Acronyms: AF, atrial fibrillation; ECG, electrocardiography; HF, heart failure; LVH, left ventricular hypertrophy
Central Illustration

History of Presentation
An 86-year-old man presented to the hospital after cardiac arrest. On the morning of the arrest, the patient collapsed while walking to the bathroom. Emergency medical services were called; they reportedly found the patient with pulseless electrical activity. Return of spontaneous circulation was achieved, and the patient was brought to the emergency department. Upon arrival, the patient arrested again with return of circulation after resuscitation.
Learning Objectives
-
•
To illustrate the mechanism by which flecainide slows phase 0 of the fast sodium channel, which leads to decreased conduction velocity of the conduction system with widening of the QRS and increased repolarization times.
-
•
To recognize the toxic effects of flecainide and its contraindications in patients with HF. and structural heart disease.
-
•
To understand that renal failure increases the half-life of flecainide dramatically, and therefore, a high index of suspicion for flecainide toxicity is needed in the setting of renal failure and a wide QRS.
-
•
To recognize that immediate actions are needed to support patients while awaiting reversal of flecainide toxicity to prevent hemodynamic collapse and death.
Past Medical History
The patient’s medical history included paroxysmal atrial fibrillation (AF), left ventricular hypertrophy (LVH) with a reported normal ejection fraction, and complete atrioventricular block; he had an Abbott/St. Jude Medical (Minneapolis, Minnesota) dual-chamber pacemaker. He had been started on flecainide 100 mg twice daily for his AF in the weeks preceding his admission. This was later increased to 150 mg due to an increased burden of paroxysmal AF. Concurrently, he developed heart failure (HF) symptoms that required escalating doses of furosemide.
Differential Diagnosis
The patient’s differential diagnosis for his pacemaker malfunction included medication toxicity, lead dislodgement, electrolyte abnormalities, and ischemia in the setting of known worsening HF. Due to his history, a probable inciting cause was flecainide toxicity.
The differential diagnosis for his arrest included bradycardia, negative inotropy, decreased cardiac output from dyssynchrony and HF, arrhythmia, or a combination of the preceding.
Initial Investigation
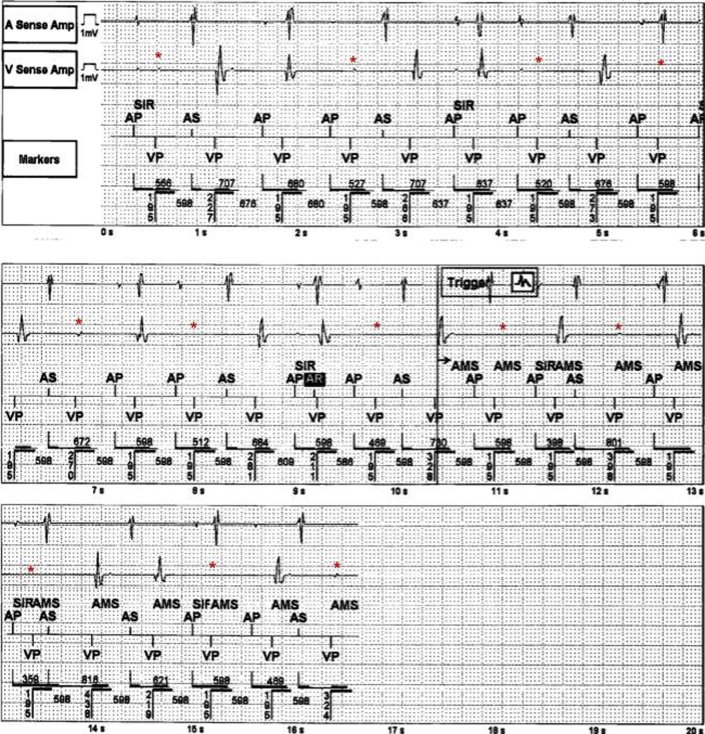
The initial physical examination was significant for an unresponsive man on mechanical ventilation. As per emergency room documentation, initial heart rate after resuscitation was approximately 30 beats/min, with a mean arterial pressure of 60 mm Hg. Cardiac auscultation revealed a slow but regular rhythm with normal S1 and S2 without significant murmurs or gallops. Electrocardiography (ECG) revealed a wide complex rhythm (QRS duration ∼320 ms) with intermittent capture of his pacemaker spikes (2:1 ventricular capture). The ventricular rate by ECG was approximately 40 beats/min (Figure 1A). The patient’s pacemaker interrogation revealed programming of DDDR 70 with increased ventricular thresholds from a baseline of 1 V at 0.4 ms to 1.75 V at 0.4 ms. This threshold was performed several hours after initial resuscitation. Ventricular outputs were 2.5 V at 0.4 ms but increased at the time of interrogation to 4 V to provide a 2:1 safety margin. There were clear episodes of loss of reliable ventricular and atrial capture that seemed to correlate temporally to the presumed time of the patient’s arrest (Figure 2). These events were incidentally captured as automatic mode switching events because of lack of capture and oversensing in the atrial channel. Thus, we did not know the nadir ventricular rate during arrest. No ventricular arrhythmias were detected. The initial lactic acid on admission was 6.4 with a pH of 7.05 and bicarbonate of 20 mmol/l; he subsequently developed acute renal failure. His initial flecainide level was elevated to 2.44 μg/ml, which was more than twice the upper limit of normal (0.99 μg/ml). Echocardiography after resuscitation showed an ejection fraction of 20% to 25% with marked asymmetric LVH, with a measured interventricular septum of 1.8 cm (Figure 3). Marked mechanical ventricular dyssynchrony was present (Video 1). Coronary angiography showed no evidence of obstructive coronary artery disease.
Figure 1.
Electrocardiograms
(A) Electrocardiogram after resuscitations shows very wide QRS (∼320 ms) with intermittent capture. The ventricular rate is 40 beats/min. (B) Electrocardiogram the next day shows underlying atrial flutter with ventricular paced rhythm. There is narrowing of QRS (200 ms) from admission and return of consistent ventricular capture.
Figure 2.
Device Interrogation From Around the Time of Event
Device interrogation from around the time of event shows loss of consistent ventricular capture (asterisk).
Figure 3.
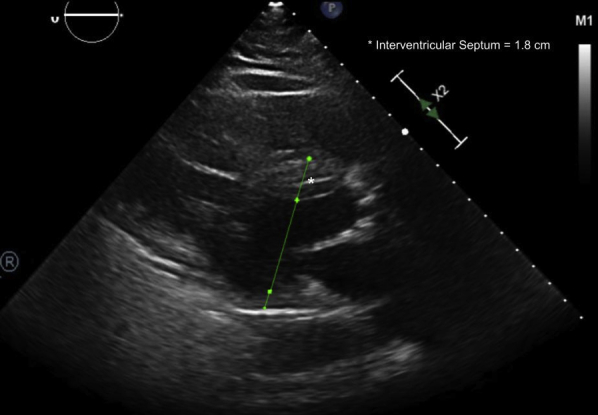
Echocardiogram After Arrest
The parasternal long-axis view of the left ventricle shows marked asymmetric left ventricular hypertrophy. The interventricular septum measures 1.8 cm.
Management and Interventions
The patient was treated with a sodium bicarbonate infusion, and the pacemaker’s pacing output was increased to 4 V with return of consistent capture. Within 24 h, the patient had a ventricular paced rhythm with a narrowing of his QRS duration to approximately 200 ms (Figure 1B), and the ventricular pacing thresholds returned to baseline.
Discussion
This case report illustrated the multiple cardiovascular effects of flecainide toxicity that could affect device therapy. When the patient first presented with loss of capture, the initial reflex was to search for an intrinsic malfunction of his pacemaker. However, there were no issues with the pacemaker’s placement or programming. Instead, flecainide toxicity had produced a dose-dependent decrease in intracardiac conduction, increased ventricular refractoriness, and had consequently affected the ability of the pacemaker to capture both the atrial and ventricular channels (1). The acute changes of the QRS and QTc width associated with pacemaker loss of capture were consistent with the acute flecainide toxicity confirmed by serum levels.
Flecainide is a Class IC antiarrhythmic that slows phase 0 of the fast sodium channel, which leads to decreased conduction velocity of the conduction system and myocardial electrical propagation (2). Specifically, its high affinity for open-state sodium channels with slow unbinding from these channels in diastole leads to prolonged ventricular refractoriness (3). Furthermore, it inhibits the rapid phase of the delayed rectifier potassium current (IKr), which leads to prolongation of ventricular action potentials (3). Therefore, toxic levels can lead to an extremely wide QRS and QTc, as seen in our patient. These decreased conduction times, with corresponding widening of the paced QRS morphology and increased refractoriness, resulted in intermittent capture. With standard programming, the timing of the pacing spike fell in a refractory period after the wide preceding ventricular paced complex. In addition, flecainide can increase the capture threshold of pacemakers by up to 200% (4), which leads to loss of capture with a correlation between the degree of QRS widening and the increase in pacing thresholds.
Flecainide has also been associated with increased dyssynchrony with decreased cardiac output in an animal model with baseline left bundle branch block (5). Furthermore, it has a known negative inotropic effect (6). Due to these effects and the increased mortality seen in the CAST (Cardiac Arrhythmia Suppression Trial) (7), current guidelines recommend that the drug be avoided in patients with structural heart disease or coronary artery disease (8).
Toxicity can be amplified by renal failure due to the marked prolongation in the half-life of the drug (9). Congestive HF and ischemia also promote sodium channel blockade by flecainide (1). Our patient had worsening HF before admission and developed acute renal failure.
In light of this, the cause of this patient’s cardiac arrest was likely multifactorial―bradycardia with loss of consistent capture, marked ventricular dyssynchrony, and negative cardiac inotropy against the backdrop of worsening HF likely triggered and accelerated by the high flecainide levels.
A high index of suspicion for toxicity is needed in patients with a device who have cardiac and metabolic disturbances on flecainide to preempt sequela of toxicity. Because flecainide is poorly dialyzable, therapies such as activated charcoal, intravenous sodium bicarbonate infusion, intravenous fat emulsion, increased pacing outputs, and mechanical support need to be initiated quickly to support the patient until the conduction system normalizes (2).
Follow-up
We were able to support the patient with increased pacing outputs until the conduction abnormalities caused by his flecainide toxicity were successfully reversed after sodium bicarbonate infusion. Unfortunately, the patient had already sustained anoxic brain injury, and care was withdrawn.
Conclusions
This case demonstrated the importance of approaching the interpretation of device therapies and programming algorithms with an understanding of the dramatic effect of antiarrhythmic medications on intrinsic conduction properties and refractoriness. Although flecainide has an overall favorable safety profile in patients with AF, caution must be taken in patients at risk for structural heart disease and avoided all together in patients, such as our older adult man, with risk for HF with LVH and a pacemaker. Moreover, under circumstances promoting toxicity, flecainide can cause major conduction abnormalities at the cellular level, culminating in inconsistent pacemaker capture and worsening hemodynamics. In pacemaker-dependent patients, this can have devastating consequences.
FUNDING SUPPORT AND Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Echocardiogram after Cardiac Arrest. The echocardiogram shows reduced ejection fraction and marked ventricular dyssynchrony.
References
- 1.Aliot E., Capucci A., Crijns H.J., Goette A., Tamargo J. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13:161–173. doi: 10.1093/europace/euq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valentino M.A., Panakos A., Ragupathi L., Williams J., Pavri B.B. Flecainide toxicity: a case report and systematic review of its electrocardiographic patterns and management. Cardiovasc Toxicol. 2017;17:260–266. doi: 10.1007/s12012-016-9380-0. [DOI] [PubMed] [Google Scholar]
- 3.Andrikopoulos G.K., Pastromas S., Tzeis S. Flecainide: current status and perspectives in arrhythmia management. World J Cardiol. 2015;7:76–85. doi: 10.4330/wjc.v7.i2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellestrand K.J., Burnett P.J., Milne J.R. Effect of the antiarrhythmic agent flecainide acetate on acute and chronic pacing thresholds. Pacing Clin Electrophysiol. 1983;6:892–899. doi: 10.1111/j.1540-8159.1983.tb04410.x. [DOI] [PubMed] [Google Scholar]
- 5.van Middendorp L.B., Strik M., Houthuizen P. Electrophysiological and haemodynamic effects of vernakalant and flecainide in dyssynchronous canine hearts. Europace. 2014;16:1249–1256. doi: 10.1093/europace/eut429. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmeister H.M., Hepp A., Seipel L. Negative inotropic effect of class-I-antiarrhythmic drugs: comparison of flecainide with disopyramide and quinidine. Eur Heart J. 1987;8:1126–1132. doi: 10.1093/oxfordjournals.eurheartj.a062178. [DOI] [PubMed] [Google Scholar]
- 7.Echt D.S., Liebson P.R., Mitchell L.B. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 8.Brandes A., Deirdre A., Lebeau J.-P. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2021;42:373–498. [Google Scholar]
- 9.Williams A.J., McQuinn R.L., Walls J. Pharmacokinetics of flecainide acetate in patients with severe renal impairment. Clin Pharmacol Ther. 1988;43:449–455. doi: 10.1038/clpt.1988.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiogram after Cardiac Arrest. The echocardiogram shows reduced ejection fraction and marked ventricular dyssynchrony.