Abstract
A 66-year-old man with refractory multiple myeloma presented with acute severe aortic insufficiency leading to cardiogenic shock and multiorgan failure. After comprehensive heart team evaluation, he underwent successful JenaValve transcatheter aortic valve (JenaValve Technology, Inc., Irvine, California) implantation resulting in resolution of his aortic insufficiency and improvement in his clinical status. (Level of Difficulty: Advanced.)
Key Words: aortic valve, endocarditis, valve replacement
Abbreviations and Acronyms: AI, aortic insufficiency; AS, aortic stenosis; TAVR, transcatheter aortic valve replacement; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography
Central Illustration
History Of Presentation
Our patient is a 66-year-old man with a history of hypertension, atrial fibrillation, and refractory immunoglobulin A kappa multiple myeloma despite chemotherapy and stem cell transplantation who presented with acute shortness of breath. The patient had dyspnea on exertion that progressively worsened to shortness of breath at rest over the course of a week.
Past Medical History
Two months before his presentation, the patient was admitted for Streptococcus pneumoniae meningitis and bacteremia. He completed a 2-week course of intravenous antibiotics. He had remained afebrile and blood culture negative after his antibiotic treatment.
Learning Objectives
-
•
To recognize the current indications and limitations of current TAVR devices.
-
•
To describe how patients with pure AI can be treated with new TAVR technology.
Investigations
The patient’s infectious work-up, including blood cultures, was negative. Over the course of several days, his renal and liver function worsened (Table 1). His earlier transthoracic echocardiogram (TTE) demonstrated normal left ventricular function and moderate aortic insufficiency (AI) (Video 1A). However, during this presentation, his TTE revealed newly depressed left ventricular function (ejection fraction of 30%) and significant worsening of his AI (Figure 1A, Video 1B and 1C) with diastolic flow reversal in the aorta (Figure 1B). There were no signs of active endocarditis on transesophageal echocardiography (TEE); however, there was regurgitant flow through the aortic noncoronary cusp, consistent with a leaflet perforation and partial prolapse resulting from his previous bacteremia (Figures 1C and 1D). The aortic valve annular area measured 519 mm2, and the perimeter was 83.6 mm. He underwent right-sided heart catheterization, which demonstrated elevated filling pressures with a mean pulmonary artery pressure of 50 mm Hg. His pulmonary artery saturation was 38%, and his Fick cardiac output was 3.6.
Table 1.
Trend of Patient’s Renal and Liver Function Over the Course of His Hospitalization
| BUN, mg/dl | Cr, mg/dl | AST, U/l | ALT, U/l | |
|---|---|---|---|---|
| Baseline | 34 | 1.05 | 15 | 16 |
| Day 1 | 29 | 1.84 | — | — |
| Day 2 | 42 | 2.35 | 326 | 336 |
| Day 3 (dialysis started) | 34 | 2.04 | 342 | 461 |
| Day 4 | 29 | 2.43 | 510 | 697 |
| Procedure (dialysis stopped) | 26 | 1.96 | 773 | 1,036 |
| Post-operative day 1 | 30 | 1.83 | 223 | 715 |
| Post-operative day 2 | 28 | 1.72 | 143 | 555 |
| Post-operative day 3 | 18 | 1.57 | 103 | 417 |
| Post-operative day 4 | 18 | 1.49 | 63 | 177 |
| 1-yr follow-up | 12 | 1.03 | 24 | 38 |
ALT = alanine transaminase; AST = aspartate transaminase; BUN = blood urea nitrogen; Cr = creatinine.
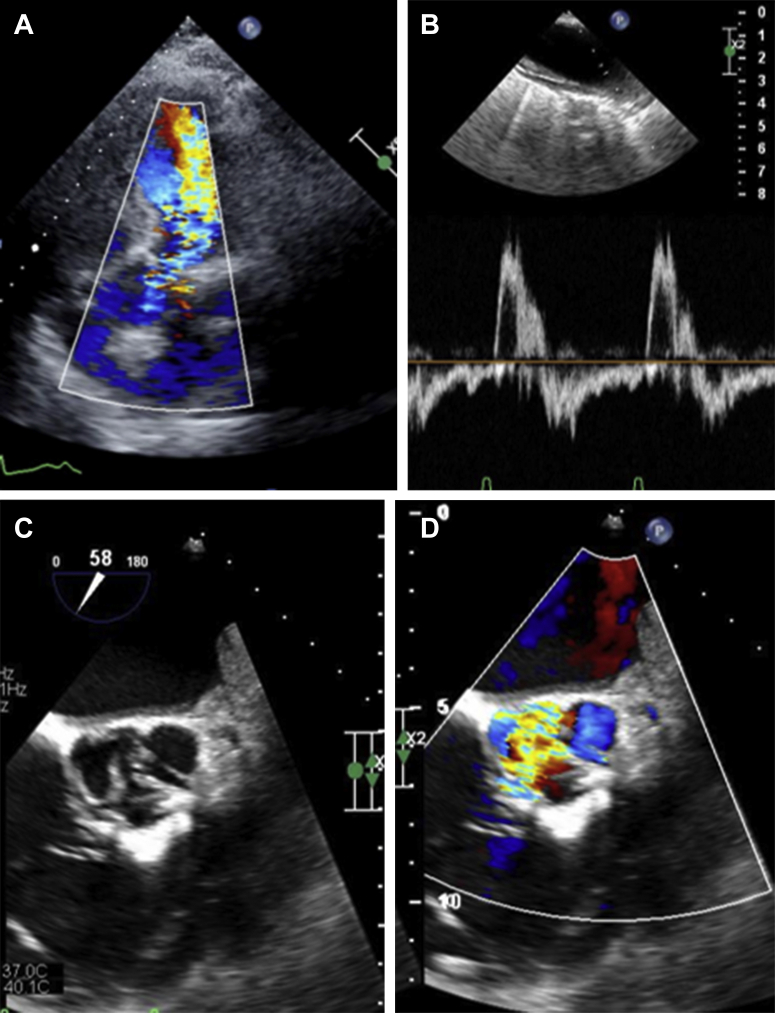
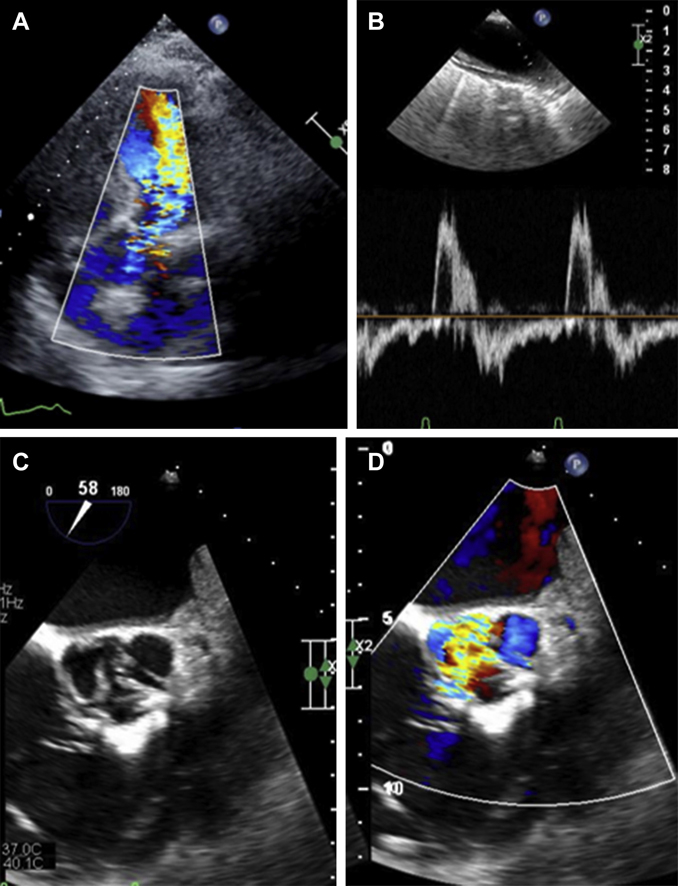
Figure 1.
Pre-Procedural Imaging
(A) Transthoracic echocardiographic imaging demonstrating severe aortic insufficiency. (B) Diastolic flow reversal in the aorta. (C) Transesophageal echocardiographic short-axis imaging of native aortic valve demonstrating perforated noncoronary cusp leaflet. (D) Transesophageal echocardiography with color-flow Doppler demonstrating flow through the perforated noncoronary cusp leaflet.
Management
Despite inotropic medications and high doses of diuretic agents, the patient became oliguric, and he was started on dialysis to optimize his volume status. He was evaluated on an emergency basis by our structural heart team. Because of his refractory multiple myeloma with ongoing therapy, multiorgan failure, and cardiogenic shock, he was deemed an extreme-risk surgical candidate, and surgery was not an option. As such, the decision was made to perform a transcatheter aortic valve replacement (TAVR) with a JenaValve (JenaValve Technology, Inc., Irvine, California) (Figure 2) under an emergency use protocol. Given the patient’s worsening renal function, his pre-procedural computed tomography scan was performed without a contrast medium to evaluate access. The TAVR procedure was performed with the patient under general anesthesia to minimize contrast use and ensure accurate positioning of the valve under TEE guidance. A transvenous pacer was inserted in the left femoral vein. The 27-mm valve was introduced through an 18-F sheath in the right femoral artery and was advanced over a Safari XS wire (Boston Scientific, Marlborough, Massachusetts). The valve was positioned across the aortic valve and was slowly unsheathed. After the valve locators were positioned within each coronary cusp under TEE and fluoroscopic guidance (Figures 3A to 3B and 4A to 4D, Videos 2A to 2C), the valve was fully deployed (Figure 4E to 4F). Final TEE analysis demonstrated excellent valve function with an effective orifice area of 3.0 cm2, a mean gradient of 3 mm Hg, and a trace paravalvular leak (Figures 3C and 3D).
Figure 2.
JenaValve Design
JenaValve (JenaValve Technology, Inc., Irvine, California). THV = transcatheter heart valve.
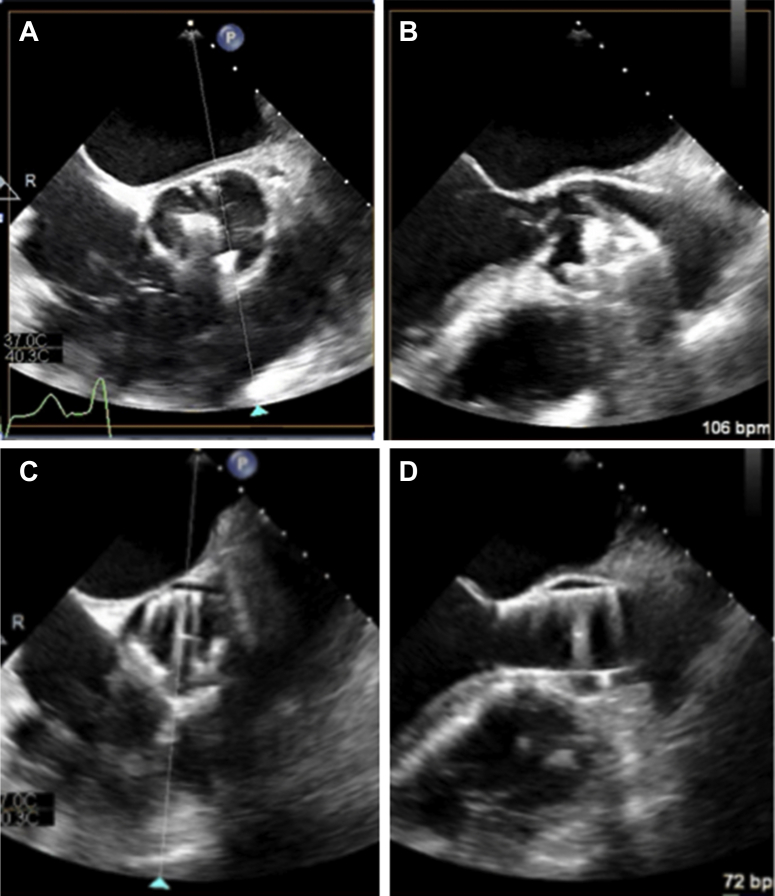
Figure 3.
Intraprocedural Imaging
(A) Intraprocedural transesophageal echocardiographic localization of JenaValve (JenaValve Technology, Inc., Irvine, California) locators (arrowhead) in each coronary cusp using short-axis imaging. (B) Intraprocedural transesophageal echocardiographic localization of JenaValve locators (arrowhead) in each coronary cusp using long-axis imaging. (C) Final intraprocedural transesophageal echocardiographic imaging of deployed JenaValve in short axis. (D) Final intraprocedural transesophageal echocardiographic imaging of deployed JenaValve in long axis.
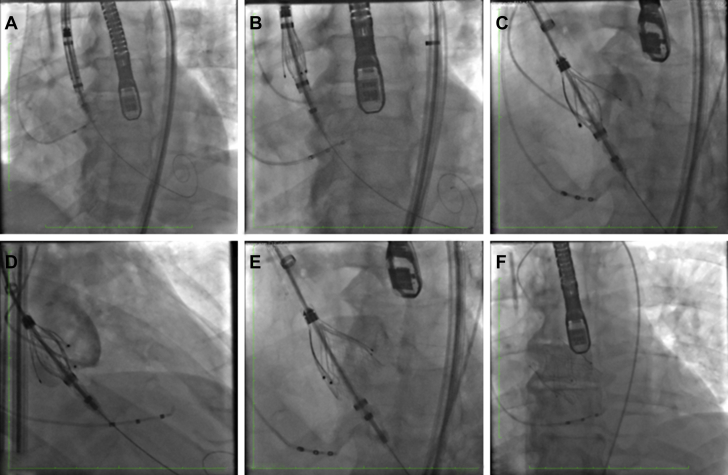
Figure 4.
JenaValve Deployment
(A) Sheath and valve are positioned in the ascending aorta. (B) Locators are exposed by retracting the sheath. (C) Locators are aligned with the left coronary cusp. (D) Locators in right and left coronary cusps. (E) Deployment of sealing ring. (F) Fully deployed JenaValve (JenaValve Technology, Inc., Irvine, California).
Discussion
TAVR implantation for the treatment of aortic stenosis (AS) has evolved from an alternative therapy for extreme-risk and high-risk patients to a well-established treatment option for patients across all risk strata. Despite the adoption of TAVR as a mainstay therapy for AS, surgical aortic valve replacement continues to be the gold standard therapy for patients with symptomatic severe AI (1). Unfortunately, many patients with severe AI do not undergo surgical intervention because they are at excessive surgical risk. Current U.S. Food and Drug Administration–approved TAVR prostheses rely on calcification of the native aortic valve to anchor the prosthesis in place. Although TAVR devices have been used off-label to treat selected patients with AI, these TAVR devices cannot be reliably implanted in the native valve AI annuli because of challenges with device placement and anchoring. Thus, it is not surprising that TAVR outcomes in patients with pure AI are worse compared with outcomes in patients with AS (2), with complications including inadequate valve sealing with significant paravalvular leak and valve embolization (3). Operators adapting current TAVR technology to patients with AI frequently rely on oversizing of the TAVR device to improve valve anchoring (2). Self-expanding transcatheter heart valves have been preferred in the treatment of pure native valve AI cases because of their ability to oversize the valve prosthesis by relying on the radial force of the valve while minimizing the risk of annular rupture (2).
The valve used in this patient is a TAVR prosthesis that does not rely on aortic valve calcification for its anchoring. Instead, this porcine pericardium valve is mounted on a nitinol frame and has a cliplike mechanism to hold onto the native valve leaflets themselves. A first-generation device designed for transapical implantation was evaluated in the JUPITER (JenaValve Evaluation of Long Term Performance and Safety in Patients With Severe Aortic Stenosis of Aortic Insufficiency) registry. In a series of 31 patients, the procedural success was 96.7%, and all-cause mortality was 10% at 1-year follow-up (4). The current valve TAVR system used in our patient has a novel valve design and a transfemoral delivery system. This device is currently under investigation in the ALIGN-AR study (JenaValve Pericardial TAVR Aortic Regurgitation Study; NCT04415047).
Follow-Up
Our patient experienced symptomatic improvement on post-operative day 1 with decreased dyspnea. Over the course of 4 days, his renal and hepatic function improved, and he was discharged on post-operative day 4 (Table 1). At 30-day follow-up, he reported that his dyspnea had completely resolved, and he returned to his baseline functional status. Over the course of a year, the patient continued to feel well, without any recurrence of bacteremia. His follow-up TTE demonstrated improved left ventricular function (ejection fraction 50%) and a well-functioning aortic valve prosthesis without valvular stenosis and trace AI (Videos 3A and 3B).
Conclusions
In this emergency case of severe native valve AI in a high-risk patient, we were able to treat our patient successfully with the JenaValve prosthesis, which uses a unique clip mechanism that does not rely on aortic annular calcification. Importantly, active endocarditis must be ruled out before considering TAVR in patients with acute AI.
Funding Support and Author Disclosures
Dr. Khalique is director of the core laboratory that has a contract with JenaValve; however, he does not receive direct compensation. Dr. Vahl has received institutional funding to Columbia University Irving Medical Center from JenaValve, Boston Scientific, Edwards Lifesciences, and Medtronic; and has personally received consulting fees from Abbott Vascular, Boston Scientific, and Siemens Healthineers. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Echocardiographic Imaging Demonstrating Patient’s Aortic Insufficiency. (A) Baseline transthoracic echocardiographic imaging demonstrating moderate aortic insufficiency. (B) Transthoracic echocardiographic imaging at time of presentation demonstrating severe aortic insufficiency (C) Transesophageal echocardiography demonstrating flow through the noncoronary cusp.
Echocardiographic Imaging Demonstrating Patient’s Aortic Insufficiency. (A) Baseline transthoracic echocardiographic imaging demonstrating moderate aortic insufficiency. (B) Transthoracic echocardiographic imaging at time of presentation demonstrating severe aortic insufficiency (C) Transesophageal echocardiography demonstrating flow through the noncoronary cusp.
Echocardiographic Imaging Demonstrating Patient’s Aortic Insufficiency. (A) Baseline transthoracic echocardiographic imaging demonstrating moderate aortic insufficiency. (B) Transthoracic echocardiographic imaging at time of presentation demonstrating severe aortic insufficiency (C) Transesophageal echocardiography demonstrating flow through the noncoronary cusp.
JenaValve Positioning. (A) Transesophageal echocardiographic localization of JenaValve (JenaValve Technology, Inc., Irvine, California) locators (white arrows) in each coronary cusp using short axis imaging of aortic valve. (B) Transesophageal echocardiographic localization of JenaValve locators (white arrows) in coronary cusps using long axis imaging of aortic valve. (C) Fluoroscopic localization of JenaValve locators in coronary cusps. Multipurpose diagnostic catheter sequentially placed into each coronary cusp to aid with positioning.
Transthoracic Echocardiographic Imaging of JenaValve at Follow-Up Demonstrating Stable Aortic Valve Position and Trace Aortic Insufficiency. JenaValve (JenaValve Technology, Inc., Irvine, California). (A) Aortic valve long axis. (B) Aortic valve short axis.
Transthoracic Echocardiographic Imaging of JenaValve at Follow-Up Demonstrating Stable Aortic Valve Position and Trace Aortic Insufficiency. JenaValve (JenaValve Technology, Inc., Irvine, California). (A) Aortic valve long axis. (B) Aortic valve short axis.
References
- 1.Nishimura R.A., Otto C.M., Bonow R.O. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 2.Anwaruddin S., Desai N.D., Szeto W.Y. Self-expanding valve system for treatment of native aortic regurgitation by transcatheter aortic valve implantation (from the STS/ACC TVT registry) Am J Cardiol. 2019;124:781–788. doi: 10.1016/j.amjcard.2019.05.045. [DOI] [PubMed] [Google Scholar]
- 3.De Backer O., Pilgrim T., Simonato M. Usefulness of transcatheter aortic valve implantation for treatment of pure native aortic valve regurgitation. Am J Cardiol. 2018;122:1028–1035. doi: 10.1016/j.amjcard.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Silaschi M., Conradi L., Wendler O. The JUPITER registry: one-year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter Cardiovasc Interv. 2018;91:1345–1351. doi: 10.1002/ccd.27370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiographic Imaging Demonstrating Patient’s Aortic Insufficiency. (A) Baseline transthoracic echocardiographic imaging demonstrating moderate aortic insufficiency. (B) Transthoracic echocardiographic imaging at time of presentation demonstrating severe aortic insufficiency (C) Transesophageal echocardiography demonstrating flow through the noncoronary cusp.
Echocardiographic Imaging Demonstrating Patient’s Aortic Insufficiency. (A) Baseline transthoracic echocardiographic imaging demonstrating moderate aortic insufficiency. (B) Transthoracic echocardiographic imaging at time of presentation demonstrating severe aortic insufficiency (C) Transesophageal echocardiography demonstrating flow through the noncoronary cusp.
Echocardiographic Imaging Demonstrating Patient’s Aortic Insufficiency. (A) Baseline transthoracic echocardiographic imaging demonstrating moderate aortic insufficiency. (B) Transthoracic echocardiographic imaging at time of presentation demonstrating severe aortic insufficiency (C) Transesophageal echocardiography demonstrating flow through the noncoronary cusp.
JenaValve Positioning. (A) Transesophageal echocardiographic localization of JenaValve (JenaValve Technology, Inc., Irvine, California) locators (white arrows) in each coronary cusp using short axis imaging of aortic valve. (B) Transesophageal echocardiographic localization of JenaValve locators (white arrows) in coronary cusps using long axis imaging of aortic valve. (C) Fluoroscopic localization of JenaValve locators in coronary cusps. Multipurpose diagnostic catheter sequentially placed into each coronary cusp to aid with positioning.
Transthoracic Echocardiographic Imaging of JenaValve at Follow-Up Demonstrating Stable Aortic Valve Position and Trace Aortic Insufficiency. JenaValve (JenaValve Technology, Inc., Irvine, California). (A) Aortic valve long axis. (B) Aortic valve short axis.
Transthoracic Echocardiographic Imaging of JenaValve at Follow-Up Demonstrating Stable Aortic Valve Position and Trace Aortic Insufficiency. JenaValve (JenaValve Technology, Inc., Irvine, California). (A) Aortic valve long axis. (B) Aortic valve short axis.