Abstract
Although the left ventricular assist device is an important bridge to heart transplantation for patients with end-stage heart failure, it can also be a source of embolic stroke. We present a case of late intracranial mechanical thrombectomy performed for embolic stroke beyond the recommended 6 h, thus allowing for heart transplantation 4 days after intracranial mechanical thrombectomy. (Level of Difficulty: Advanced.)
Key Words: collateral circulation, heart, ischemic stroke, left ventricular assist device, mechanical thrombectomy, pediatric, transplantation
Abbreviations and Acronyms: CICU, cardiac intensive care unit; CT, computed tomography; LVAD, left ventricular assist device; MT, mechanical thrombectomy; PedNISS, Pediatric National Institutes of Health Stroke Scale
Central Illustration
History of Presentation
A 14-year-old boy was referred to our pediatric hospital (Hospital Necker-Enfants-Malades, AP-HP, Paris, France) for end-stage heart failure. Despite maximal medical treatment, including intravenous inotropic drugs, the patient finally required cardiac assistance by a left ventricular assist device (LVAD) while awaiting orthotopic heart transplantation. Anticoagulant treatment using intravenous heparin was maintained, and antiplatelet therapy (aspirin and dipyridamole) was added as a course of treatment after the Berlin Heart EXCOR pediatric LVAD (Berlin Heart, Berlin, Germany) was implanted. At day 3 post-LVAD implantation, the anti–factor Xa level was within the target range, and the LVAD was free of any visible clot or deposit. However, he suddenly presented with isolated speech disturbances. Four hours later, aphasia and right motor deficit were reported (Pediatric National Institutes of Health Stroke Scale [PedNIHSS] = 11). We first suspected an ischemic stroke, considering the worsening neurological assessment and the previous LVAD implantation.
Learning Objectives
-
•
To suspect a related embolic stroke in the context of neurological deterioration in patients with LVADs.
-
•
To understand that LVAD-related embolic stroke management is particularly challenging because all patients receive antithrombotic treatment, which is a contraindication to intravenous tissue plasminogen activator treatment.
-
•
To be aware that LVAD blood pump replacement may be urgently required as a result of potential embolic recurrence risk.
-
•
To discover that there are no validated guidelines for children, unlike in adults, for MT beyond the recommended 6 h, thereby reinforcing the need for onsite multidisciplinary expertise.
Past Medical History
The patient had a medical history of idiopathic dilated cardiomyopathy.
Differential Diagnosis
Sedation side effects were initially suspected as the cause of phasic disorders because the remaining neurological examination was normal. In this context of anticoagulant and antiplatelet treatment, a hemorrhagic stroke could also be suspected.
Investigations
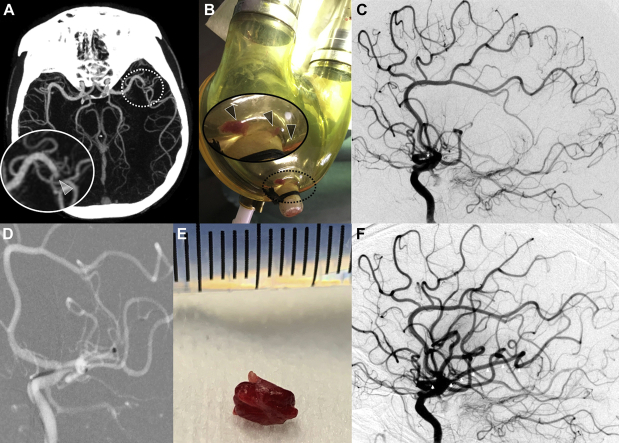
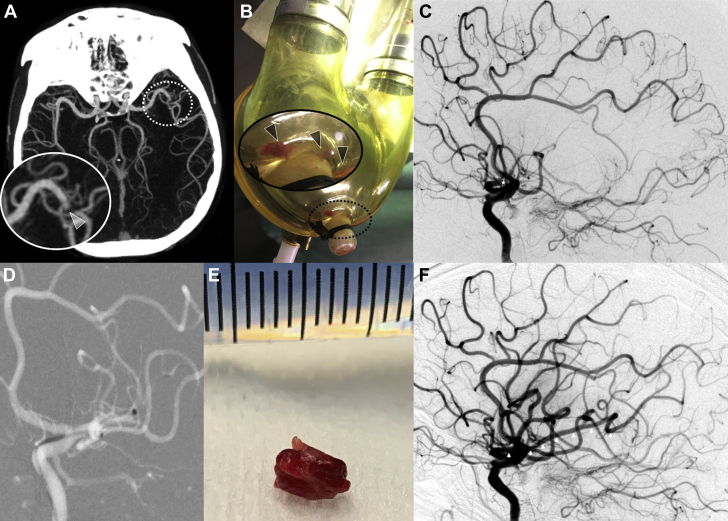
Brain computed tomography (CT) scan and CT angiography (64-detector high-speed HD Lightspeed VCT system, GE Healthcare, Barrington, Illinois) revealed an occlusion of the proximal M2 segment of the left middle cerebral artery (Figure 1A) without detectable early ischemic changes. CT angiography showed good collateral circulation of the left brain hemisphere, defined as the filling of >50% of the middle cerebral artery peripheral arterial circulation (1).
Figure 1.
Pediatric M2 Left Middle Cerebral Artery MT for Embolic Stroke From LVAD
(A) Computed tomography angiography showing an occlusion of the M2 segment of the left middle cerebral artery with good collateral circulation, defined as the filling of >50% of the middle cerebral artery peripheral arterial circulation. (B) Removed left ventricular assist device (LVAD) blood pump revealed a large clot and fibrin deposits. See zoomed area (corresponding to dotted circle) into the removed LVAD blood pump revealing a large clot and fibrin deposits (arrowheads). (C) Basal digital subtraction angiography with occlusion of the left M2 middle cerebral artery (star). (D) Aspiration catheter deployment up to the proximal face of the thrombus into the M2 left middle cerebral artery. (E) A 5-mm mixed clot removed in a single pass. (F) Final digital subtraction angiography showing a Thrombolysis in Cerebral Infarction 3 recanalization 8.7 h after initial onset with a completely permeable left middle cerebral artery (arrowheads). MT = mechanical thrombectomy.
Management
Considering the patient’s recent cardioembolic ischemic stroke with a favorable brain parenchymal imaging profile, the multidisciplinary team opted to maintain the decision to perform mechanical thrombectomy (MT) during an immediate round. To prevent immediate embolic recurrence risk, the team performed LVAD blood pump replacement on an emergency basis in the cardiac intensive care unit (CICU) before transporting the patient to the angiography suite. Visual examination of the removed LVAD blood pump revealed a large clot and multiple fibrin deposits (Figure 1B). Intracranial MT was then performed while the patient was under general anesthesia, with groin puncture at 8.5 h after onset of symptoms. Through a short 8-F right femoral inserter, a long sheath was advanced into the left internal carotid artery (Neuron Max 6-F 088 inches, Penumbra, Alameda, California). Selective injection into the left internal carotid artery confirmed the left proximal M2 segment occlusion (Figure 1C). A large-bore 6-F aspiration catheter (Ace 68, Penumbra) and a microcatheter (3Max, Penumbra) guided by a 0.14-inch microwire were then introduced through the long sheath. The aspiration catheter was advanced up to the proximal face of the thrombus over the 3Max, which was subsequently removed (Figure 1D). The thrombus aspiration was performed using a pump system for approximately 1 min. A 5-mm mixed clot (Figure 1E) was removed in a single pass, thus allowing complete flow reperfusion 8.7 h after the initial onset (modified Thrombolysis in Cerebral Infarction score of 3) (Figure 1F).
Discussion
Thromboembolic complications of both end-stage heart failure and LVAD use are well documented, and they occur in 29% of children supported with the Berlin Heart EXCOR LVAD (2). In this setting, embolic stroke management is particularly challenging because all patients receive antithrombotic treatment, which is a contraindication to intravenous tissue plasminogen activator treatment. Conversely, neurological disabilities following cerebral embolism may represent a contraindication to heart transplantation, thereby leading to a medical deadlock. Over the last decade, very few cases of successful MT performed in patients with an LVAD and cardioembolic stroke were reported (3), with procedures performed by a stent retriever or aspiration (4) technique, even in children. In a recent review of 14 cases, Colletta et al. (4) reported successful recanalization in 71% of procedures performed after a median delay of 184 min from the first symptoms, with a good functional outcome achieved in 57% of patients.
The American Heart Association stroke guidelines recommend the completion of endovascular therapy within 6 h of symptom onset, in addition to intravenous thrombolysis within 4.5 h in the absence of contraindications (5). This strategy is judged reasonable for patients younger than 18 years of age who present with a large-vessel occlusion acute ischemic stroke, even if benefits are not established in children (6). For patients presenting late after stroke symptom onset, a thorough individual selection process that is based on clinical and advanced imaging criteria is recommended in adults (6, 7, 8). Imaging criteria are not validated in children. Delayed stroke diagnosis is not uncommon in the setting of CICU because patients in CICUs are typically sedated and have comorbidities. Emergency stroke management in these patients requires a pediatric stroke protocol and a multidisciplinary decision-making process to combine the expertise of cardiologists, intensivists, cardiac surgeons, pediatric neurologists, radiologists, and interventional radiologists. In this case, we decided to treat the patient aggressively with MT because of the following considerations: 1) his imaging profile was favorable (initial CT did not detect irreversible ischemic changes in the brain and showed good collateral circulation); 2) LVAD-associated antithrombotic treatments were contraindications to systemic and in situ thrombolysis; and 3) an irreversible neurologic disorder would have rendered the patient ineligible for any future heart transplantation project, which was the only reasonable treatment for the patient’s end-stage cardiomyopathy. Although LVAD blood pump replacement was necessary before MT, thus increasing treatment delay, this management seemed to have a relatively good risk-to-benefit balance, which proved to be effective.
Follow-Up
Neurological assessments after the MT procedure showed rapid improvement of motor symptoms and mild aphasia (PedNIHSS = 1 at 2 days after the procedure). Brain CT obtained at day 3 post-procedure showed no hemorrhagic transformation. The patient underwent cardiopulmonary bypass for heart transplantation 4 days after successful removal of the thrombus. Interestingly, visual examination of the explanted heart also revealed the presence of several thrombi (measured in centimeters) in the left ventricle that had been suspected on previous repeated transthoracic echocardiograms. The patient was discharged to a rehabilitation facility. Although he had normal heart function and no significant motor or sensitive deficit, he did display persistent mild aphasia and mild cognitive impairment.
Conclusions
MT is a lifesaving procedure for selected pediatric patients experiencing LVAD-related embolic stroke, even when MT is performed beyond the recommended 6 h. For patients with end-stage heart failure, LVAD use has become an important aspect of bridge therapy before heart transplantation (9), and we recommend MT as an adjunct to reinforce the foundation of this bridge in case of life-threatening embolic cerebral complications.
FUNDING SUPPORT AND Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Goyal M., Demchuk A.M., Menon B.K. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 2.Jordan L.C., Ichord R.N., Reinhartz O. Neurological complications and outcomes in the Berlin Heart EXCOR® pediatric investigational device exemption trial. J Am Heart Assoc. 2015;4:2001429. doi: 10.1161/JAHA.114.001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mufti F., Bauerschmidt A., Claassen J. Neuroendovascular interventions for acute ischemic strokes in patients supported with left ventricular assist devices: a single-center case series and review of the literature. World Neurosurg. 2016;88:199–204. doi: 10.1016/j.wneu.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 4.Colletta K.L., Bar B., Liebo M.J. Thrombectomy of ventricular assist device-originated embolic stroke: a clinical decision model. J Neuroimaging. 2019;29:423–430. doi: 10.1111/jon.12621. [DOI] [PubMed] [Google Scholar]
- 5.Powers W.J., Rabinstein A.A., Ackerson T. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 6.Wilson J.L., Amlie-Lefond C., Abruzzo T. Survey of practice patterns and preparedness for endovascular therapy in acute pediatric stroke. Childs Nerv Syst. 2019;35:2371–2378. doi: 10.1007/s00381-019-04358-y. [DOI] [PubMed] [Google Scholar]
- 7.Albers G.W., Lansberg M.G., Kemp S. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3) Int J Stroke. 2017;12:896–905. doi: 10.1177/1747493017701147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jovin T.G., Saver J.L., Ribo M. Diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo (DAWN) trial methods. Int J Stroke. 2017;12:641–652. doi: 10.1177/1747493017710341. [DOI] [PubMed] [Google Scholar]
- 9.Slaughter M.S., Pagani F.D., McGee E.C. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32:675–683. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]