Abstract
We present the case of a 60-year-old man who was successfully treated for obstructive fungal infective endocarditis of the ascending aorta caused by Geotrichum capitatum. This extremely rare cause of fungal infective endocarditis required surgical and prolonged medical management, facilitated by effective multidisciplinary cooperation. (Level of Difficulty: Intermediate.)
Key Words: cardiac surgery, congenital, fungal infective endocarditis, Geotrichum capitatum, β-D-glucan, multidisciplinary team
Abbreviations and Acronyms: BDG, 1,3-β-D-glucan; IE, infective endocarditis; TTE, transthoracic echocardiogram
Central Illustration
History of Presentation
A 60-year-old man was transferred to Royal Brompton Hospital, London, United Kingdom, with a 1-week history of night sweats and myalgia. On examination, his chest was clear on auscultation, with heart sounds S1, S2, and click. He had no focal signs of infection and no peripheral signs of infective endocarditis (IE). He was clinically stable on arrival; normopneic, normotensive, and mildly tachycardic at 92 beats/min.
Learning Objectives
-
•
For high-risk patients, such as those with congenital cardiac disease, early consideration of fungal infection is essential in the diagnosis and management of IE.
-
•
Treatment with an extended course isavuconazole was effective for a severe deep fungal infection caused by Geotrichum capitatum.
-
•
The use of multidisciplinary decision making should be advocated alongside multimodality imaging in complex cases of endocarditis, in the absence of clear guidelines.
Past Medical History
This patient was born with a Sievers type 0 (anteroposterior) bicuspid aortic valve. He underwent homograft aortic valve replacement for mixed aortic valve disease and aortic root replacement for a dilated ascending aorta in 1995. Subsequently, in 2016, he underwent a redo aortic valve and root replacement for severe aortic regurgitation. Recovery was complicated by ventricular fibrillation cardiac arrest secondary to severe left main stem stenosis, requiring emergency percutaneous coronary intervention. He had good functional cardiovascular capacity and spent the previous summer working in his garden, weeding and clearing dead leaves.
Differential Diagnosis
IE was strongly suspected, considering his congenital heart condition and the presence of a prosthetic valve. Another differential diagnosis was Mycobacterium chimaera infection following potential inadvertent exposure during cardiopulmonary bypass for his surgery in 2016. Sepsis secondary to community-acquired pneumonia or urinary tract infection was ruled out by the referring hospital.
Investigations
On admission, his blood values showed raised inflammatory markers (Table 1). Three sets of peripheral blood cultures from the referring hospital returned positive results for Geotrichum capitatum. Two further sets isolated the same organism. The microbiology department advised testing for 1,3-β-D-glucan (BDG), and the result was positive; the initial concentration was >500 pg/ml (normal <80 pg/ml).
Table 1.
Admission Blood Tests and Values
| Blood Tests | Result | Normal Value |
|---|---|---|
| White blood cell count, ×109/l | 14.1 | 4.4–10.1 |
| Neutrophil count, ×109/l | 12.7 | 2.1–6.7 |
| Eosinophil count, ×109/l | 0.0 | 0.1–0.5 |
| C-reactive protein, mg/l | 79 | 0–10 |
| Hemoglobin, g/l | 104.0 | 134–166 |
| Platelet count, ×109/l | 180 | 136–343 |
| Creatinine, μmol/l | 92 | 60–120 |
| Estimated glomerular filtration rate, ml/min/1.73 m2 | 73 | >60 |
An electrocardiogram showed normal sinus rhythm, a normal PR interval, a narrow QRS complex, and a nonprolonged QTc. Chest radiography revealed bilateral pleural effusions, but no focal consolidation (Figure 1). Sputum and urine cultures were negative for bacteria.
Figure 1.
Admission Chest Radiograph
Small bilateral pleural effusions, sternotomy wires and metallic aortic valve replacement. R = right.
The transthoracic echocardiogram (TTE) showed a severely dilated left ventricle with a moderately reduced ejection fraction of 35% to 40%. The aortic root and ascending aorta appeared normal, with a well-seated and unobstructed aortic valve replacement. However, the flow velocity and gradients in the ascending aorta had significantly increased compared with previous echocardiograms (Figures 2A and 2B, Table 2). Doppler study demonstrated turbulent flow in the ascending aorta. No obvious vegetation was seen on native or prosthetic valves.
Figure 2.
Transthoracic Echocardiogram Images
(A) Increased peak velocity and gradient in the ascending aorta detected from the suprasternal view using a nonimaging probe on admission. (B) Compared with 8 months earlier from the apical 5-chamber view. AV = aortic valve; AVA = aortic valve area; CW = continuous wave; max = maximum; PG = peak gradient; V = velocity; VTI = velocity time interval; WF = wall filter.
Table 2.
Comparison of Aortic Valve and LV TTE Measurements: On Admission in December 2019, Previous TTE in April 2019, and Following Successful Therapy in October 2020
| Measurement | April 2019 | December 2019 | October 2020 |
|---|---|---|---|
| Peak gradient, mm Hg | 35.0 | 76.0 | 20.5 |
| Mean gradient, mm Hg | 17.0 | 40.0 | 10.8 |
| Area (continuity), cm2 | 1.33 | 0.83 | 1.12 |
| Mitral regurgitation | Mild | Moderate | Mild |
| LVEF, % | 35–40 | 35–40 | 45–50 |
| LVEDD, cm | 5.83 | 6.03 | 5.23 |
| LVESD, cm | 5.20 | 4.54 | 4.82 |
LV = left ventricular; LVEDD = left ventricular end-systolic dimension; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic dimension; TTE = transthoracic echocardiogram.
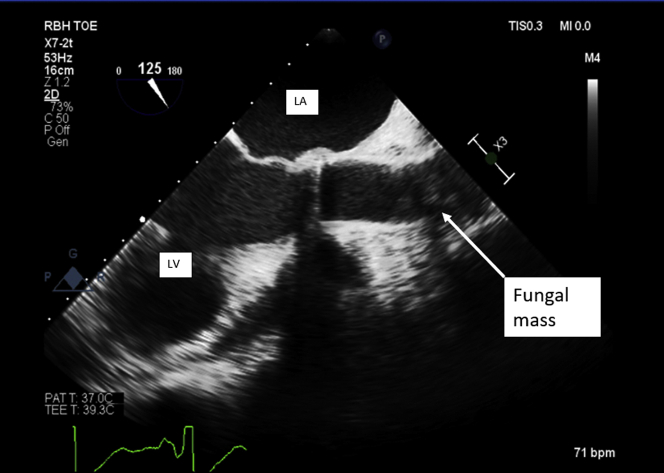
A transesophageal echocardiogram performed pre-operatively confirmed a normally functioning prosthetic valve with shadowing in ascending aorta, previously unseen on TTE (Figure 3, Videos 1 and 2). These images correlated with computed tomography findings, which identified a large, irregular filling defect in the aortic root (Figures 4A to 4D).
Figure 3.
Transesophageal Echocardiogram of Left Ventricular Outflow
Echo-dense mass partially visualized in the ascending aorta, likely at the suture line from a previous operation. bpm = beats/min; LA = left atrium; LV = left ventricle; MI = mechanical index; PAT = patient; RBH = Royal Brompton Hospital; TOE = transoesophageal echocardiogram; 2D = 2-dimensional.
Figure 4.
Computed Tomography Scan
Two-dimensional (A) axial and (B) sagittal images. Three-dimensional Reconstruction of a computed tomography (CT) scan of (C) the ascending aorta and (D) an obstructive mass in the ascending aorta. A, anterior; AVR = aortic valve replacement; F = feet; L = left; P = posterior; R = right.
Management
The patient was started on intravenous high-dose amphotericin B liposomal and flucytosine, in line with culture sensitivities. Multidisciplinary team discussions recommended urgent high-risk but lifesaving surgical intervention. During surgery, an incision through the Dacron (polyethylene terephthalate) component of the previous composite graft revealed a large mass obstructing the mechanical prosthesis in the aortic position (Figures 5A and 5B). The mass was completely removed, followed by hydrogen peroxide washout. The operation was high risk, with extensive adhesions and prolonged bypass time. Because the mechanical prosthesis was well functioning and macroscopically free of vegetations, an intraoperative decision was made to leave it in place. Microbiology samples from the excised mass confirmed growth of G. capitatum, consistent with peripheral blood cultures (Figure 6).
Figure 5.
Intraoperative Images
(A and B) A large, fungating ascending aortic mass almost obstructing the aortic lumen.
Figure 6.
Microbiology Samples
Geotrichum capitatum isolated from blood cultures and the explanted fungating mass.
His post-operative management was complicated by transient atrial fibrillation, a period of renal replacement therapy, and the development of new lateralizing neurological signs, diagnosed as subacute infarction on computed tomography, complicating immediate anticoagulation decisions. He completed a total course of combined intravenous high-dose amphotericin B liposomal and flucytosine for 8 weeks post-surgery. His response to treatment was monitored clinically, alongside inflammatory markers and BDG, which both showed a clear downtrend. He continued dual antifungal therapy, in addition to warfarin and optimal heart failure medications. He was safely discharged 4 months after his initial operation.
Discussion
This case demonstrates the severity and long-term complications of fungal IE. Only 2% to 4% of cases of endocarditis are caused by fungi. Patients with prosthetic valves are at higher risk for fungal IE, along with intravenous drug users and immunocompromised patients (1). Candida albicans is the most common causative organism, representing between 50% to 80% of cases. Most patients require combined surgical intervention and prolonged antifungal therapy, but some need long-term suppressive therapy, occasionally for life (2). There are no clear guidelines on antifungal treatment for these types of rare fungal invasive infections (3).
G. capitatum, previously known as Saprochaete capitata, and now renamed Magnusiomyces capitatus, is a ubiquitous fungus found in soil, water, air and plants. G. capitatum invasive infections are very rare and are almost exclusively reported in immunocompromised patients, with a poor prognosis and a mortality rate exceeding 50% despite appropriate treatment (4). Geotrichum spp. IE are extremely rare. A literature review returned a single reported case of Geotrichum candidum IE, in a 6-year-old child with pulmonary atresia. It is posited that our patient contracted the fungus while gardening because he worked without gloves and sustained blisters on his hands.
Symptoms of fungal IE overlap heavily with those of bacterial endocarditis, thus requiring a high index of suspicion for timely diagnosis. The most common symptoms are fever, new or changing heart murmur, and major peripheral embolization, most commonly cerebral and femoral. Classic signs of endocarditis such as Osler’s nodes, finger clubbing, splinter hemorrhages, and Roth spots are infrequent (5).
Peripheral blood cultures remain the cornerstone of diagnosis, by providing live organisms for identification and sensitivity testing. However, in the absence of positive microbiological findings, and if there is suspicion of fungal infection, a BDG level can be useful. BDG is a component of the cell wall of most fungi, yeasts, and molds. Antigen testing has a reported sensitivity of 75% and a specificity of 81% for invasive fungal infections (6). For patients with suspected IE, European Society of Cardiology guidelines recommend TTE as the first-line imaging modality, irrespective of the organism. For patients with prosthetic valves, or a high clinical suspicion despite negative TTE findings, a transesophageal echocardiogram is indicated (7).
Follow-Up
This patient returned to the hospital shortly after discharge with nausea, fatigue, and rapidly increasing C-reactive protein (4 to 98 mg/l). He was readmitted for suspected fungal infection relapse because his antifungal therapy had recently been altered. He subsequently tested positive for COVID-19 but remained hemodynamically stable and required no supplemental respiratory support. His antifungal treatment was changed to monotherapy with oral isavuconazole, and he was discharged with fortnightly monitoring of inflammatory markers, renal function, and BDG level. Six months post-surgery, the BDG level became negative and has remained negative, with no clinical evidence of infection recurrence. Because the mechanical aortic valve replacement was left in place during surgery, there remains a risk of fungal biofilm, which is difficult to treat and fully clear. A multidisciplinary meeting recommended prolonged antifungal therapy for more than a year post-operatively.
Conclusions
This case demonstrates the clinical presentation, diagnosis, and successful management of fungal IE, caused by G. capitatum, obstructing the ascending aorta. Fungal IE should be considered for higher-risk patients, especially those with prosthetic valves or immunocompromise. Management often requires surgical intervention and extensive antifungal therapy.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transesophageal Echocardiogram of Left Ventricular Outflow. Mobile mechanical aortic prosthesis discs with a shadow in the distal ascending aorta.
Transesophageal Echocardiogram of Ascending Aorta. Large mass obstructing the ascending aorta lumen. Confirmed post-operatively as a fungating mass.
References
- 1.Gould F.K., Denning D.W., Elliott T.S. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults. J Antimicrob Chemother. 2012;67:269–289. doi: 10.1093/jac/dkr450. [DOI] [PubMed] [Google Scholar]
- 2.Tattevin P., Revest M., Lefort A., Michelet C., Lortholary O. Fungal endocarditis: current challenges. Int J Antimicrob Agents. 2014;44:290–294. doi: 10.1016/j.ijantimicag.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Baddour L.M., Wilson W.R., Bayer A.S. Infective endocarditis in adults: diagnosis, antimicrobial therapy and management of complications. A scientific statement for healthcare professionals from the American Heart Association. Endorsed by the Infectious Diseases Society of America. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 4.Meena S., Singh G., Dabas Y., Rajshekhar P., Xess I. Geotrichum candidum in infective endocarditis. J Glob Infect Dis. 2017;9:127–128. doi: 10.4103/jgid.jgid_112_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis M.E., Al-Abdely H., Sandridge A., Greer W., Ventura W. Fungal endocarditis: evidence in the world literature, 1965-1995. Clin Infect Dis. 2001;32:50–62. doi: 10.1086/317550. [DOI] [PubMed] [Google Scholar]
- 6.He S., Hang J.P., Zhang L. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-β-D-glucan for invasive fungal infection: focus on cut-off levels. J Microbiol Immunol Infect. 2015;48:351–361. doi: 10.1016/j.jmii.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Habib G., Lancellotti P., Antunes M.J. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal Echocardiogram of Left Ventricular Outflow. Mobile mechanical aortic prosthesis discs with a shadow in the distal ascending aorta.
Transesophageal Echocardiogram of Ascending Aorta. Large mass obstructing the ascending aorta lumen. Confirmed post-operatively as a fungating mass.