Abstract
Valve disease in the presence of porcelain aorta and severe peripheral artery disease challenge physicians in choosing the appropriate therapy. We used a total transcatheter approach, simultaneously implanting a dedicated mitral and aortic valve prosthesis treating a patient with mitral and aortic valve disease at an extremely high surgical risk. (Level of Difficulty: Advanced.)
Key Words: mitral valve insufficiency, transapical, transcatheter aortic valve implantation, transcatheter mitral valve implantation
Abbreviations and Acronyms: CT, computer tomography; MAC, mitral annular calcification; MR, mitral valve regurgitation; TAVR, transcatheter aortic valve replacement; TMVR, transcatheter mitral valve replacement
Central Illustration

History of Presentation
A 78-year-old man with severe mitral valve regurgitation (MR), congestive heart failure, in New York Heart Association functional class III was referred to our institution. Because the patient presented with porcelain aorta, the heart team concluded that conventional mitral valve surgery and replacement of the ascending aorta with a no-clamp technique in hypothermic circulatory arrest would harbor an unacceptable risk. Potential interventional mitral valve therapies were evaluated. Restrictive posterior mitral valve leaflet prohibited an edge-to-edge repair. Mitral annulus calcium distribution was not suitable for implantation of a transcatheter aortic valve in mitral position. Consequently, the patient was approved for transcatheter mitral valve replacement (TMVR) using the Abbott Tendyne valve (Abbott Vascular, Santa Clara, California). At the time of hospital readmission for TMVR, echocardiography revealed a worsening of aortic valve regurgitation from moderate to severe. Because of this change in severity, the decision was made to simultaneously treat the aortic valve with a transcatheter aortic valve replacement (TAVR). Because of severe peripheral artery disease a transfemoral access for TAVR was not available. Alternative vascular access routes, including the transcaval approach, were also excluded because of severe calcification of both subclavian arteries, carotid artery plaques, the abdominal aorta, and the porcelain aorta. Hence, the decision was made to simultaneously treat the mitral and aortic valve using a transcatheter approach via a transapical access.
Learning Objectives
-
•
To be able to analyze treatment options for patients with porcelain aorta and valvular disease.
-
•
To understand the challenges in transapical access utilization.
Past Medical History
Past medical history included coronary artery disease with extensive previous stenting, severe peripheral artery disease including bilateral vascular surgery, atrial fibrillation with previous atrial appendage closure with an AMPLATZER Amulet device (Abbott Vascular), moderate aortic valve regurgitation, and chronic obstructive pulmonary disease. The logistic EuroScore was 14.6%, and EuroScore II was 3.8%.
Differential Diagnosis
Differential diagnoses included rheumatic mitral valve disease and degenerative MR.
Investigations
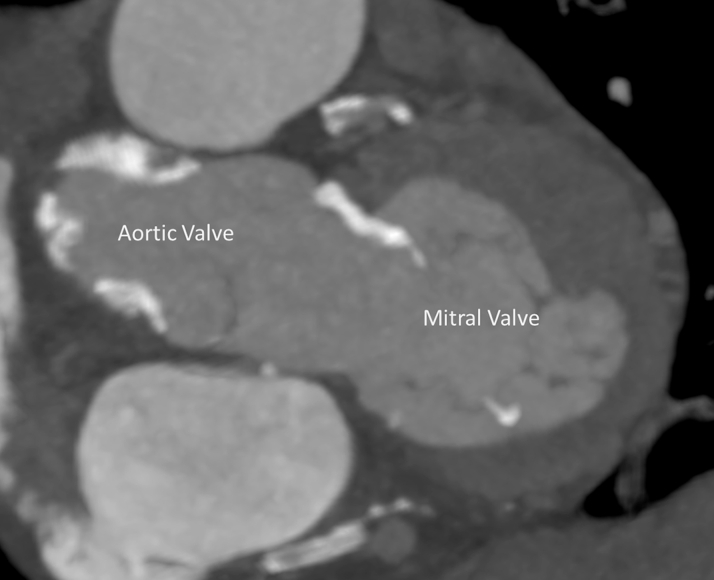
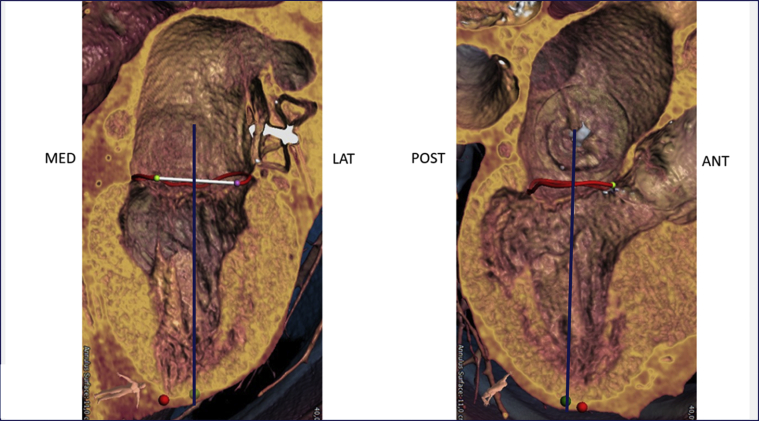
Echocardiography showed severe functional MR with a restricted and short posterior leaflet (MR ERO 0.11 cm2, dPmean 2 mm Hg). The aortic valve displayed severe insufficiency and mild stenosis. Multislice computed tomography (CT) imaging proved a suitable aortic valve anatomy for TAVR (perimeter 7.9 cm, diameter 25.2 mm, area 476 mm2, adequate coronary distance). Analysis of the mitral valve showed an ample estimated neo left ventricular outflow tract (>320 mm2 in systole and >400 mm2 in diastole) (Figure 1), an anterior-posterior distance of 31 mm, and an intercommisural distance of 42 mm allowing for approximately 15% oversizing using the 35M low-profile Tendyne valve. Simulation of TAVR implantation was not included. According to the scoring system proposed by Guerrero et al. (1), the patient had a MAC score of 3, mild calcification, most prominently with a sporn on the anterior-medial trigone (Figure 2). The suggested left ventricular apical access point for the TMVR deviated only 1 cm from the usual transapical TAVR access point, suggesting a favorable access for both the TMVR and the TAVR (Figure 3). Patient consent for publication of this case was obtained.
Figure 1.
Calculation of Neo-LVOT
Calculation of neo-LVOT in systole (A) and diastole (B), based on computed tomography images. Image courtesy of Abbott Vascular (Santa Clara, California). LVOT = left ventricular outflow tract.
Figure 2.
Mitral Valve Calcification
Figure 3.
Apical Access Point Prediction
The apical access point (green dot) is derived from 2 perpendicular axes through the mitral valve. In this patient the apical access point is slightly lateral and posterior from the true apex (red dot). Image courtesy of Abbott Vascular (Santa Clara, California).
Management
After left anterolateral thoracotomy, the CT-derived apical access site appropriate for mitral and aortic valve access was confirmed via transesophageal echocardiography. After placement of 2 pledgeted U-stiches, a 26-mm Edwards Sapien 3 Ultra valve (Edwards Lifescience Corp., Irvine, California) was implanted via the 21-F Edwards eSheath, in the usual manner achieving stable intra-annular valve anchoring. A high TAVR-implantation was not strived for, because an interference with the Tendyne was not presumed probable due to the low height of the 26-mm Sapien 3 Ultra valve and the avoidance of valve embolization in leading aortic valve regurgitation had priority. Transprosthetic gradient measured 3 mm Hg with no insufficiency.
Via the Edwards eSheath, the mitral valve was crossed with a standard J-wire. “Flossing” with a Fogarty catheter ruled out any entanglement with mitral valve chordae. Because of a stiff anterior leaflet and a MAC score of 3, a valvuloplasty with a 28-mm True Dilatation Balloon (Bard Peripheral Vascular, Tempe, Arizona) was done. The eSheath was exchanged for the 34-F Tendyne delivery system. Under echocardiographic guidance, the Tendyne valve was implanted after being repositioned once. The apical pad was fixed according to echocardiographic analysis and tether-tension measurements (Video 1). Severe apical bleeding, caused by a ventricular tear from the apical insertion point, was treated with multiple pledgeted sutures. Overall the patient received 2 U of red blood cells and an autologous blood transfusion of approximately 3 l. The patient was hemodynamically stable throughout the procedure.
Intraoperative echocardiography showed no paravalvular leak on the mitral prosthesis, a mean transprosthetic mitral gradient of 1 to 2 mm Hg, and no left ventricular outflow tract gradient. The patient was extubated 15 h after the procedure.
Discussion
To our knowledge, this is the first-in-man report of simultaneous transcatheter double-valve intervention via a transapical access, using the Abbott Tendyne for mitral insufficiency and the Edwards Sapien 3 Ultra for combined aortic valve disease.
This patient presented with a porcelain aorta, complicating conventional surgical strategies. A possible surgical procedure in the presence of porcelain aorta would include mitral and aortic valve replacement and ascending aorta replacement under hypothermic cardiac arrest. Thirty-day mortality and the risk for neurological dysfunctions are high in patients undergoing hypothermic circulatory arrest, with age being a risk factor for severe adverse events (2). The patient and the heart team declined this treatment option, citing the unacceptable risk. Dedicated transcatheter mitral valves can help address an unmet need in patients at high-risk for surgery or not qualified for edge-to-edge repair techniques.
The rationale for the mitral valve valvuloplasty was to accommodate complete Tendyne frame expansion, support intra-annular valve positioning, and allow optimal sealing. This concept was based solely on clinical experience from previous Tendyne implantations and data supporting this concept need to be established.
In this patient, the apical access site displayed severe bleeding, in part caused by a ventricular tear. Multiple factors may have contributed to this complication. First, the need for 2 valves required an exchange of large-bore devices. Possibly, in cases with an exchange of large sheaths, additional surgical measures must be taken to better secure the access site. The severity of this complication was not anticipated. Second, the implantation of a valve in mitral and aortic position requires different angulations. Although CT planning suggested a small deviation of the access point from the standard localization for the TAVR procedure, the apex may have been placed under excessive stress, promoting bleeding and the subsequent tear. In contrast, data from a small cohort of double-valve implantations from the MAC-registry, showed no higher complication rates for this group (3). Larger studies are needed to better understand complication rates and outcomes for this type of therapy. Transapical left ventricular puncture is an attractive solution to gain access for interventional valve procedures in aortic and mitral valve disease. However, because surgical exposure is limited, one has to be aware that even a minor problem or a progressing ventricular tear, requiring surgical treatment, may extend to a lethal catastrophe instantaneously. Furthermore, placement of sutures can be cumbersome because of the apical pad hindering direct visualization of the ventricular lesion.
Overall, at the moment, this severe complication and the possible confounders emphasize the need to critically assess the risk-benefit ratio for this procedure. In our patient, after diligent screening, we assumed this to be the best possible treatment. Nonetheless, it is surely a procedure only reserved for selected patients.
Follow-Up
The patient was intermittently on the intensive care unit for 24 days and was released from the hospital on day 35. Discharge was delayed because of a post-operative delirium. CT imaging showed no signs of intracranial ischemia or bleeding. Post-operative echocardiography showed an excellent function of the aortic (dPmean 8 mm Hg, minimal paravalvular regurgitation) and mitral transcatheter heart valve (dPmean 3 mm Hg, no paravalvular regurgitation).
Conclusions
Limited to a transapical access, only a simultaneous TMVR using the Abbott Tendyne valve and TAVR using the Edwards Sapien 3 Ultra valve enabled a treatment of the double-valve disease with an excellent hemodynamic and overall clinical result. Even so, a severe bleeding complication should raise awareness for meticulous patient selection.
Funding Support and Author Disclosures
Dr. Lange holds shares of HighLife Medical. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank Dr. Christian Noebauer.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Procedural Video
References
- 1.Guerrero M., Wang D.D., Pursnani A. A cardiac computed tomography-based score to categorize mitral annular calcification severity and predict valve embolization. J Am Coll Cardiol Img. 2020;13:1945–1957. doi: 10.1016/j.jcmg.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Khaladj N., Shrestha M., Meck S. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg. 2008;135:908–914. doi: 10.1016/j.jtcvs.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 3.Guerrero M., Dvir D., Himbert D. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: results from the first multicenter global registry. J Am Coll Cardiol Intv. 2016;9:1361–1371. doi: 10.1016/j.jcin.2016.04.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Procedural Video