Abstract
The aim of this study is to evaluate the prognostic value of the vertebral bone mineral density (BMD) on chest computed tomography (CT) in COVID-19 patients. The chest CT of hospitalized patients with COVID-19 pneumonia were evaluated for Pneumonia Severity Score (PSS) as the ratio of the volume of involved lung parenchyma to the total lung volume. In addition, BMD was manually measured from the vertebral corpus using axial CT images. The relationships of clinical variables, PSS and vertebral BMD with patient outcomes, namely mortality, intensive care unit (ICU) admission and mechanical ventilation were investigated. Lower BMD was defined as ≤100 HU. The study included 209 patients (118 males, 56.4%). As a result of the univariate analysis, the rates of mortality, ICU admission and mechanical ventilation were 17.2% (n = 36), 24.8% (n = 52), and 20.6% (n = 43), respectively, and they were significantly higher among the patients with lower BMD (38.1 vs 13.0%, p < 0.001; 33.4 vs 21.2%, p = 0.002; and 38.1 vs 8.2%, p < 0.001, respectively). In the mortality group, PSS was significantly higher (median, 9 vs 5; p < 0.001) and vertebral BMD was significantly lower (median, 83 vs 139; p < 0.001). Severe clinical incidence was significantly higher in patients with lower BMD compared to those with higher BMD (39.7 vs 24.7% and p = 0.028). There was a significant correlation between clinical classification and lower BMD (r = 0.152 and p = 0.028). The multivariate analysis revealed vertebral BMD [odds ratio (OR), 1.028; 95% CI, 1.011−1.045, p = 0.001) and lower BMD (OR, 4.682; 95% CI, 1.784−12.287, p = 0.002) as significant independent predictors of mortality. Vertebral BMD is a strong independent predictor of mortality that is reproducible and can be easily evaluated on the chest CT images of COVID-19 patients.
Key Words: Bone mineral density, Computed tomography, COVID-19, Osteoporosis, Prognosis
Introduction
Coronavirus disease 2019 (COVID-19) that emerged as a pneumonia outbreak in Wuhan, China, in the last month of 2019 and was found to be caused by the newly identified severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been declared a global public health emergency by the World Health Organization (WHO) considering its pandemic potential 1, 2, 3. Although the reverse transcription polymerase chain reaction (RT-PCR) test is the gold standard for the diagnosis of COVID-19, in cases of COVID-19 presenting with pneumonia, the computed tomography (CT) of the chest provides valuable information about diagnosis, disease severity, and prognosis 4, 5, 6, 7, 8. In addition, chest CT plays an important role in the diagnosis process and determining the prognosis, especially in patients with respiratory failure and those requiring hospitalization. The typical CT findings of COVID-19 guide treatment at an early stage and assist in the prompt initiation of preventive quarantine ( 9 ).
Quantitative measurements using CT in various tissues, such as the liver (e.g., fat quantification for steatosis), vasculature (e.g., aortic and coronary artery calcification scoring for atherosclerosis) and fat (e.g., size and attenuation for adiposity) can be used as independent predictors of specific clinical outcomes, and such findings can have prognostic power ( 10 ). In addition, the most commonly studied biomarkers in the musculoskeletal system are bones (e.g., attenuation for osteoporosis) and muscles (e.g., size and attenuation for sarcopenia) ( 10 ). It has been shown that frailty caused by decreased function and strength of the musculoskeletal system due to aging affects the prognosis of various diseases ( 11 ). Recent feasibility studies suggest that vertebral attenuation can be used to assess the loss of bone mineral density (BMD) in the screening of osteoporosis characterized by low bone mass and quality, increasing bone fragility and fracture risk ( 12,13 ). A low BMD value has been reported to be a critical prognostic factor in many benign and malignant diseases such as coronary artery disease, chronic obstructive pulmonary disease (COPD), cerebrovascular conditions, and breast cancer 13, 14, 15. In addition, there is pre-existing evidence indicating that in the presence of systemic inflammation, bone loss and reduced BMD can be seen due to the interaction of inflammatory mediators (interleukin, cytokines, etc.) with bone cells leading to the activation of osteoclasts through the receptor activator of nuclear factor-κB ligand (RANKL) system ( 16 ). Many studies have measured cardiac markers, such as the cardiothoracic ratio, pulmonary artery diameter, epicardial adipose tissue and coronary artery calcification, liver density for hepatic steatosis, and muscle volume in the musculoskeletal system in the prediction of prognosis in the CT scans of patients with COVID-19. Thus, predictive factors for the prognosis of COVID-19 have been explored by performing many quantitative measurements, and some of these factors have also been associated with vertebral BMD ( 9,17, 18, 19 ). Despite all this information, the prognostic value of BMD in COVID-19 has not yet been adequately investigated.
The purpose of the current study was to evaluate the efficacy of the vertebral BMD value on chest CT in the prediction of the prognosis and outcomes of COVID-19 patients.
Material and Method
Approval for this retrospective study was obtained from the Ethics Committee of Harran University (Number: HRU/21.02.19 and date: 18.01.2021), and written informed consent was waived.
Study Population
This retrospective cohort study was conducted in a tertiary care center in Mehmet Akif Inan Training and Research Hospital located in Southeast Turkey. All adult patients with a diagnosis of COVID-19 confirmed by the RT-PCR test and hospitalized for at least 24 hours between July 1, 2020 and August 20, 2020 were analyzed. Of these patients, those that underwent non-contrast chest CT were included in the study. Pediatric patients (under 18 years of age), those with major motion artifacts on CT, and cases in which a non-standardized CT protocol was used (e.g., tube voltage other than 120 kV and intravenous contrast imaging). In addition, patients with pathologies related to the vertebral corpus (e.g., implants, focal lesions, and bone island) were excluded from the analysis. Lastly, patients with mild symptoms of COVID-19 and no signs of pneumonia on CT, who only required outpatient treatment were not included in the study. Figure 1 presents the flow chart of the inclusion and exclusion criteria.
Fig. 1.
Flow chart of the inclusion and exclusion criteria. CT, Computed tomography; RT-PCR, Reverse transcription-polymerase chain reaction.
CT indications were determined according to the COVID-19 patient management algorithm of the Turkish Ministry of Health ( 20 ) based on the presence of one of the following clinical conditions in adult patients with symptoms and suspicion of COVID-19 pneumonia: respiratory distress or tachypnea (>22/min), tachycardia, and oxygen saturation (SpO2) < 93%. For those without clinical findings, the CT indication was being over 50 years and having comorbidities or having a worsening clinical status ( 20 ).
Chest CT Protocol
Chest CT was performed in all patients with a 16-detector multi-slice CT device (Siemens Healthineers; Erlangen, Germany). CT images were obtained in supine position during deep inspiration without the use of any intravenous contrast material. The main scanning parameters were as follows: tube current-time product, 50−350 mAs; tube voltage, 120 kV; pitch, 1.25; matrix, 512 × 512; slice collimation, 16 × 0.75 mm; slice thickness, 3 mm; and reconstructed slice thickness, 1 mm. Raw data were reconstructed with the standard ''bone tissue'' reconstruction kernel.
CT Image Evaluation
Prior to the measurements, the quality and suitability of the CT images were evaluated by a radiologist (MT) with eight-year experience in thoracoabdominal imaging, and the CT images that were not suitable for the study were excluded. Then, all the measurements of the vertebral BMD and lung were simultaneously assessed by two independent radiologists with eight and nine years of experience in thoracoabdominal imaging (MT and EK, respectively), and the average of the 2 separate measurements of the same parameters was recorded as the final value. All significant discrepancies in measurements between the two radiologists were analyzed and evaluated again in the presence of a third radiologist (Y.A.) with 10 years of experience in thoracoabdominal imaging. Furthermore, the vertebral BMD and PSS of a random sample of 140 patients were determined by a different radiologist (NK) with 12 years of experience in thoracoabdominal imaging to evaluate the interobserver variability of the measurements. The measurements were performed blinded to the clinical and laboratory information of the patients. Before the measurements were taken, both researchers participated in an interactive training session to master the measurement technique.
Assessment of Vertebral BMD and Placement of Regions of Interest (ROI)
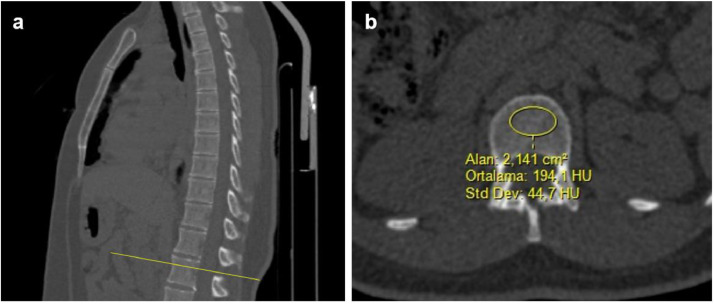
The researchers first measured vertebral BMD in the bone window (window level: 300 and window width: 1600 Hounsfield units) on non-contrast CT. All the measurements were undertaken in axial sections. The mean CT attenuation was measured in Hounsfield units (HU). The measurement site was determined as the area between the superior endplate of the first lumbar vertebra (L1) and the entrance of the veins in the midportion. Then, circular ROI were placed in the homogeneous trabecular bone area in the middle of the vertebral corpus, avoiding the posterior basivertebral venous plexus and cortical bone (Fig. 2 ). If the L1 vertebra was non-homogeneous, not visualized, or fractured, the measurement was performed from the next intact vertebra (thoracic 12th vertebra) ( 21 ). Based on previous studies, a vertebral BMD value of 100 HU was accepted as lower density ( 13,22 ).
Fig. 2.
Computed tomography (CT) images showing region of interest (ROI) placement in the first lumbar vertebra (L1) of a patient. Sagittal multiplanar reformatting CT images (A) show L1 vertebra level and measurement localization. In this selected level, a ROI was placed in the upper part of L1 between the endplate and the entrance of vessels at the midportion (B). The ROI was as large as possible and positioned in a homogenous area without including the cortical bone.
Assessment of Pneumonia Severity Score (PSS)
The PSS of each patients was evaluated on the lung window (window level: -500 HU and window width: 1500 HU) of the CT images. PSS is a semi-quantitative scoring method in which CT images are evaluated visually. PSS was first defined by Chung et al ( 23 ) and has been used in previous studies ( 17 ). This score is calculated as follows: each of the 3 lobes in the right lung and 2 lobes in the left lung is scored separately, and the sum of the scores obtained from the 5 lobes forms PSS. For each lobe, 0 point is given if there is no pneumonia (0%), 1 point if there is minimal involvement (1%–25% volume of the lung lobe), 2 points if mild involvement (26%–50%), 3 points if moderate involvement (51%–75%), and 4 points if severe involvement (76%–100%) ( 17,23 ).
Clinical Examination
Comorbidities such as diabetes mellitus, hypertension, coronary artery disease, cardiac failure, chronic lung disease (COPD and asthma), chronic renal failure, immunosuppression, and malignancy were recorded from the electronic medical records of the patients. The patients were divided into three categories according to their clinical severity: (1) common disease (symptomatic patients with pneumonia signs on chest CT, who did not require oxygen support; (2) severe disease [signs of respiratory infection and respiratory distress, and having an increased respiratory rate (≥30 breaths/min), decreased oxygen saturation in room air (SpO2 ≤ 93 %), and/or partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤ 300 mm Hg]; and (3) critical disease (respiratory failure requiring mechanical ventilation, septic shock, and other organ failure requiring intensive care unit (ICU) monitoring and treatment ( 24 ).
Data on the length of hospital stay, mechanical ventilation requirement, ICU admission, and mortality development were obtained from the electronic medical records using a standardized data collection form. Prolonged hospital stay was defined as more than 10 days of hospitalization ( 17 ). The most severe outcome was evaluated as the presence of multiple hospital presentations due to COVID-19. For example, patients who were followed up at home after first admission but then presented to the hospital again, required longer hospitalization and died, were assigned to the mortality group.
Statistical Analysis
All statistical analyses were performed using SPSS v. 20.0 (SPSS Inc., Chicago, IL). Variables were divided into 2 as categorical and continuous. Categorical variables were given as numbers and percentages (%) and compared with the pearson's chi-square or Fisher's exact test. Continuous variables were expressed as mean ± standard deviation or median [interquartile range (IQR)], depending on the normality of the distribution. The Kolmogorov-Smirnov test was conducted to determine whether the data were normally distributed, and the data with a p value greater than 0.05 were defined as normally distributed. Normally distributed data were compared using the independent-samples t-test, and non-normally distributed data using the Mann-Whitney U test. The binominal logistic regression analysis was performed with the data found significant in the univariate analysis to identify the independent predictors of mortality. Pearson's correlation analysis was used to examine the relationship between continuous variables. Interobserver agreement for the vertebral BMD and PSS measurements was tested using the intra-class correlation coefficient (ICC). An ICC value of <0.4 was considered to indicate poor agreement, 0.4–0.75 moderate agreement, 0.75–0.9 good agreement, and >0.9 excellent agreement ( 17 ). Statistically, a p value of <0.05 was considered significant for all analyses.
Results
Baseline Characteristics
A total of 209 patients with COVID-19 pneumonia who were hospitalized for treatment and met the eligibility criteria were included in the study. The characteristics of all patients are summarized in Table 1 . Of the patients, 56.4% (n = 118) were male, and the mean age was 61.0 ± 16.2 years. At least one comorbidity was present in 65.1% of the patients (n = 135), with the most common being hypertension (n = 71, 34.0%).
Table 1.
Characteristics of the Patients With Lower (≤100 HU) and Higher (>100 HU) Vertebral BMD
| Variables | All patients (n = 209) | Lower BMD (≤100 HU)(n = 63) | Higher BMD (>100 HU)(n = 146) | p value |
|---|---|---|---|---|
| Age (yr), mean ± SD | 61.0 ± 16.2 | 72.1 ± 12.6 | 56.3 ± 15.3 | <0.001* |
| Older age (≥65 yr), n (%) | 92 (44.0) | 47 (74.6) | 45 (30.8) | <0.001* |
| Male gender, n (%) | 118 (56.4) | 31 (49.2) | 87 (59.6) | 0.165 |
| Pneumonia Severity Score, median (IQR) | 5 (1-17) | 6 (1-17) | 5 (1-17) | 0.150 |
| C-reactive protein (mg/L), median (IQR) | 121 (4-379) | 244 (9-304) | 69 (4-379) | 0.001 |
| Comorbidity, n (%) | 136 (65.1) | 52 (82.5) | 84 (57.5) | <0.001* |
| Hypertension | 71 (34.0) | 28 (44.4) | 43 (29.5) | 0.027* |
| Diabetes mellitus | 67 (32.1) | 24 (38.1) | 43 (29.5) | 0.143 |
| Coronary artery disease | 32 (15.3) | 15 (23.8) | 17 (11.6) | 0.023* |
| Chronic lung disease | 25 (12) | 8 (12.7) | 17 (11.6) | 0.497 |
| Cardiac failure | 9 (4.3) | 4 (6.3) | 5 (3.4) | 0.270 |
| Chronic kidney failure | 11 (5.3) | 2 (3.2) | 9 (6.2) | 0.303 |
| Other | 14 (6.8) | 6 (9.5) | 8 (5.2) | 0.193 |
| Clinical classification, n (%) | ||||
| Common | 143 (68.4) | 36 (57.1) | 107 (73.3) | 0.021* |
| Severe | 61 (29.2) | 25 (39.7) | 36 (24.7) | 0.028* |
| Critical | 5 (2.4) | 2 (3.2) | 3 (2.1) | 0.627 |
| Clinical outcomes, n (%) | ||||
| Prolonged hospital stay | 77 (36.8) | 26 (41.3) | 51 (34.9) | 0.383 |
| Mechanic ventilation | 43 (20.6) | 24 (38.1) | 19 (13.0) | <0.001* |
| Intensive care unit admission | 52 (24.8) | 21 (33.4) | 31 (21.2) | 0.002* |
| Mortality | 36 (17.2) | 24 (38.1) | 12 (8.2) | <0.001* |
Statistically significant. Abbr: BMD, bone mineral density; IQR, interquartile range; SD, standard deviation.
Relationship Between Vertebral BMD and Clinical Variables
As shown in Table 1, the patients with COVID-19 were classified into two groups as lower (≤100 HU) and higher (>100 HU) vertebral BMD. The group with low BMD had significantly higher age (72.1 ± 12.6 vs 56.3 ± 15.3 years; p < 0.001) and included significantly more patients over 65 years (74.6 vs 30.8%, p < 0.001) compared to the higher BMD group. The incidence of hypertension was also significantly higher in the lower BMD group compared to the patients with higher BMD (44.4 vs 29.5%, p = 0.027). The rates of mechanical ventilation, ICU admission and mortality were significantly higher among the patients with lower BMD (38.1 vs 13.0%, p < 0.001; 33.4 vs 21.2%, p = 0.002; and 38.1 vs 8.2%, p < 0.001, respectively). There was a significant correlation between clinical classification and lower BMD (r = 0.152 and p = 0.028).
Relationship Between PSS and Clinical Variables
There was an excellent agreement between the two observers both for the vertebral BMD measurements (ICC, 0.931; 95% CI, 0.904–0.951) and for PSS (ICC, 0.986; 95% CI, 0.980–0.990). The median PSS of the total study population was 5 (IQR, 1–17), and a PSS of >5 was defined as high (Table 2 , Figure 3). No differences were detected between the patients with low and high PSS with respect to the demographic data or rate of comorbidities. The rates of mechanical ventilation, ICU admission and mortality were significantly higher in the high PSS group (26.9 vs 10.1%, p = 0.004; 31.5 vs 13.9%, p = 0.001; and 23.8 vs 6.3%, p < 0.001, respectively). There was a significant correlation between clinical classification and PSS (r = 0.591 and p < 0.001).
Table 2.
Comparison of the Demographic and Clinical Characteristic of the Patients According to PSS
| Variables | All patients (n = 209) | Low PSS (≤5) (n = 79) | High PSS (>5) (n = 130) | p value |
|---|---|---|---|---|
| Age (yr), mean ± SD | 61.0 ± 16.2 | 59.8 ± 16.8 | 61.8 ± 15.9 | 0.401 |
| Older age (≥65 yr), n (%) | 92 (44.0) | 31 (39.2) | 61 (46.9) | 0.278 |
| Male gender, n (%) | 118 (56.4) | 38 (48.1) | 80 (61.5) | 0.057 |
| Comorbidity, n (%) | 136 (65.1) | 46 (58.2) | 90 (69.2) | 0.071 |
| Hypertension | 71 (34.0) | 24 (30.4) | 47 (36.2) | 0.393 |
| Diabetes mellitus | 67 (32.1) | 22 (27.8) | 45 (34.6) | 0.194 |
| Coronary artery disease | 32 (15.3) | 9 (11.4) | 23 (17.7) | 0.152 |
| Chronic lung disease | 25 (12) | 11 (13.9) | 14 (10.8) | 0.319 |
| Cardiac failure | 9 (4.3) | 4 (5.1) | 5 (3.9) | 0.392 |
| Chronic kidney failure | 11 (5.3) | 3 (3.8) | 8 (6.2) | 0.346 |
| Other | 14 (6.8) | 6 (7.7) | 8 (6.2) | 0.614 |
| Clinical classification, n (%) | ||||
| Common | 143 (68.4) | 70 (88.6) | 73 (56.2) | <0.001* |
| Severe | 61 (29.2) | 8 (10.1) | 53 (40.8) | <0.001* |
| Critical | 5 (2.4) | 1 (1.3) | 4 (3.1) | 0.406 |
| Clinical outcomes, n (%) | ||||
| Prolonged hospital stay | 77 (36.8) | 25 (31.6) | 52 (40.0) | 0.225 |
| Mechanic ventilation | 43 (20.6) | 8 (10.1) | 35 (26.9) | 0.004* |
| Intensive care unit admission | 52 (24.8) | 11 (13.9) | 41 (31.5) | 0.001* |
| Mortality | 36 (17.2) | 5 (6.3) | 31 (23.8) | 0.001* |
Statistically significant. Abbr: PSS, Pneumonia Severity Score; SD, standard deviation.
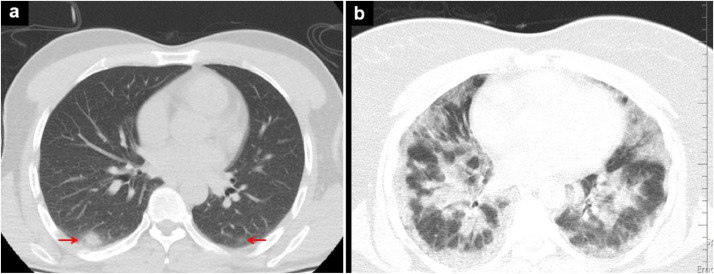
Fig. 3.
Chest CT images of 2 different patients with a low (A) and high (B) pneumonia severity score (PSS): a) small ground glass opacities (arrows) in the lower lobes of both lungs and b) diffuse infiltrates in both lungs.
Association Between Vertebral BMD and Patient Mortality
The characteristics of the patients who were discharged from hospital and those that developed in-hospital mortality are shown in Table 3 . The univariate analysis showed that the age of the patients who died was higher compared to those that survived (78.9 ± 6.4 vs 58.1 ± 15.9 years; p < 0.001), and there were significantly more patients aged ≥65 years in the mortality group (75.0 vs 37.6%, p < 0.001). In addition, the mean vertebral BMD value was significantly lower in the mortality group (83 vs 139 HU, p < 0.001, respectively). Prolonged hospital stay (52.8 vs 33.5%, p = 0.025) and severe disease according to clinical classification (63.9 vs 22.0%, p < 0.001) were also significantly more common in the mortality group (Table 3). The data that were determined to be significant for mortality in the univariate analysis were further analyzed using the regression analysis (Table 4 ). In the multivariate analysis, vertebral BMD [odds ratio (OR), 1.028; 95% CI, 1.011–1.045, p = 0.001) and lower density (OR, 4.682; 95% CI, 1.784–12.287, p = 0.002) were significant independent predictors of mortality.
Table 3.
Comparison of the Discharged Patients and Those That Developed In-Hospital Mortality
| Variables | Discharge(n = 173) | Mortality(n = 36) | p value |
|---|---|---|---|
| Age (yr), mean ± SD | 58.1 ± 15.9 | 78.9 ± 6.4 | <0.001* |
| Older age (≥65 yr), n (%) | 65 (37.6) | 27 (75.0) | <0.001* |
| Male gender, n (%) | 95 (54.9) | 23 (63.9) | 0.212 |
| PSS, median (IQR) | 5 (1-17) | 9 (2-17) | <0.001* |
| High PSS (>5), n (%) | 99 (57.2) | 31 (86.1) | 0.001* |
| Vertebral BMD (HU), median (IQR) | 139 (28-296) | 83 (61-221) | 0.005* |
| Lower BMD (<100 HU), n (%) | 39 (22.5) | 24 (66.7) | <0.001* |
| Prolonged hospital stay, n (%) | 58 (33.5) | 19 (52.8) | 0.025* |
| C-reactive protein (mg/L), median (IQR) | 62 (4-379 | 235 (52-304) | <0.001* |
| Comorbidity, n (%) | 106 (61.3) | 30 (83.3) | 0.008* |
| Hypertension | 55 (31.8) | 16 (44.4) | 0.104 |
| Diabetes mellitus | 53 (30.6) | 14 (38.9) | 0.219 |
| Coronary artery disease | 21 (12.1) | 11 (30.6) | 0.008* |
| Chronic lung disease | 22 (12.7) | 3 (8.3) | 0.340 |
| Cardiac failure | 8 (4.6) | 1 (2.8) | 0.521 |
| Chronic kidney failure | 7 (4.0) | 4 (11.1) | 0.100 |
| Other | 11 (6.4) | 3 (8.4) | 0.533 |
| Clinical classification, n (%) | |||
| Common | 133 (76.9) | 10 (27.8) | <0.001* |
| Severe | 38 (22.0) | 23 (63.9) | <0.001* |
| Critical | 2 (1.1) | 3 (8.3) | 0.01* |
Statistically significant. Abbr: BMD, bone mineral density; HU, Hounsfield unit; IQR, interquartile range; PSS, Pneumonia Severity Score; SD, standard deviation.
Table 4.
Binominal Logistic Regression Analysis of the Data Found Significant in the Univariate Analysis for the Prediction of Mortality in Patients With COVID-19
| Variables | Odds ratio (OR) | 95% confidence interval (CI) | P value |
|---|---|---|---|
| Age (yr) | 1.055 | 0.985-1.131 | 0.125 |
| Older age (≥65 yr) | 1.185 | 0.203-6.928 | 0.851 |
| PSS | 1.049 | 0.928-1.187 | 0.443 |
| High PSS (score >5) | 1.717 | 0.404-7.300 | 0.464 |
| Vertebral BMD | 1.028 | 1.011-1.045 | 0.001* |
| Lower BMD (≤100 HU) | 4.682 | 1.784-12.287 | 0.002* |
| Coronary artery disease | 1.713 | 0.558-5.258 | 0.347 |
| Clinical classification | |||
| Common | 0.166 | 0.015-1.844 | 0.144 |
| Severe | 0.745 | 0.081-6.829 | 0.795 |
| Critical | 6.012 | 0.542-66.631 | 0.144 |
| Prolonged hospital stay | 1.766 | 0.690-4.519 | 0.235 |
Statistically significant. Abbr: PSS, Pneumonia Severity Score; BMD, bone mineral density; HU, Hounsfield unit.
Discussion
Our results in the current study revealed that PSS, vertebral BMD values, and lower BMD (≤100 HU) measured on chest CT were significantly associated with mortality. In addition, age, coronary artery disease, prolonged hospital stay, and clinical severity were found to be significantly associated with mortality. Furthermore, when age, PSS, vertebral BMD, coronary artery disease, and clinical severity were included in the regression analysis, a low BMD value (≤100 HU) was observed to increase the risk of mortality by 5 times (OR, 4.682; 95% CI, 1.784–12.287, p = 0.002), independent of the remaining parameters. In addition, several adverse outcomes such as low vertebral BMD, which can be easily measured on CT, mechanical ventilation (38.1 vs 13.0%, p < 0.001), and ICU admission (33.4 vs 21.2%, p = 0.002) were related to mortality. To the best of the authors’ knowledge, this is the first study that evaluated the role of quantitative vertebral BMD and PSS obtained from CT and their relationship with mortality, which is an indicator of the worst clinical outcome in COVID-19 patients.
It is becoming increasingly common to measure attenuation from the L1 vertebra included in the examination on both chest and abdominal CT and to report values less than 100 HU as they indicate possible osteoporosis. It has also been reported that lower BMD is significantly associated with an increased risk of all-cause and cardiovascular mortality in the general population ( 14,22 ). The presence of calcium deposits in coronary arteries on CT has been accepted as a marker of atherosclerotic coronary artery disease and plays an important role in predicting cardiac events ( 10 ). Similarly, the measurement of vertebral BMD has been used as a predictor of various clinical situations in previous studies ( 10,14,25 ). However, the efficacy of a lower vertebral BMD value obtained from chest CT, which is a predictor of osteoporosis, in COVID-19 was previously unknown. We measured the L1 vertebral density in COVID-19 patients who underwent chest CT at the time of hospitalization and showed that low BMD (≤100 HU) had a significant relationship with mortality. We consider vertebral density can be easily measured on the chest CT scans of COVID-19 patients without additional radiation, scanning, or patient burden, and it can be an important parameter in predicting the prognosis of these patients. However, further studies are needed to validate this finding.
Recent clinical studies show that some inflammatory diseases are associated with increased bone loss and increased fracture rate. Current data in the field of osteoimmunology, referring to the reciprocal communication between inflammatory cells and bone cells, offer some insight into the complex pathogenesis of bone loss in systematic inflammatory diseases. Fundamental Basic studies have clarified that upregulated RANKL followed by activated osteoclastogenesis is an important determinant of bone loss ( 26 ). In addition to local pathological changes, immunological factors, such as excessive immune response (cytokine storm), which in are directly related to morbidity and mortality in some cases, play an important role in COVID-19 ( 27 ). Our study revealed that COVID-19 cases with low BMD had a poor prognosis. This shows the necessity of directing the systemic inflammatory response of COVID-19 to the osteoimmunological field in order to better elucidate its pathophysiology.
Chung et al. ( 23 ), who evaluated PSS using the chest CT of COVID-19 patients, reported that high PSS was correlated with a high clinical severity score. Although PSS is a semi-quantitative scoring, excellent interobserver agreement has been reported in previous studies (ICC range, 0.957–0.985), indicating that this measurement can be used as an objective criterion among radiologists ( 17,28 ). In addition, Ufuk et al ( 17 ) demonstrated a significant correlation between PSS and clinical disease score in COVID-19 patients (p < 0.001, r = 0.609). Similarly, in the current study, the agreement between the 2 observers for PSS were excellent with the ICC value being calculated as 0.986 (95% CI, 0.980–0.990), and there was a correlation between PSS and clinical severity score (r = 0.591 and p < 0.001).
Many studies have shown that advanced age and underlying comorbidities are associated with mortality and poor prognosis in patients with COVID-19 (17,29,30). In a study examining 5,279 COVID-19 patients in the United States (US), advanced age (>75 years), malignancies, cardiac failure, and male gender were reported to be independent factors of mortality ( 29 ). Similarly, Du et al ( 30 ), evaluating 179 patients with COVID-19, reported advanced age (> 65 years) and cardiovascular or cerebrovascular disease to be strong independent predictors of mortality. In a different study investigating 143 patients with COVID-19 in Turkey, advanced age (>65 years) was a significant parameter for mortality according to the univariate analysis but it was not an independent predictor in the multivariate analysis ( 17 ). The differences between studies can be attributed to the effect of different geographic regions and patient populations studied. The current study that was also conducted in Turkey similarly showed that advanced age (>65 years) and presence of coronary artery disease were significant for mortality in the univariate analysis but they were not significant predictors according to the regression analysis. However, low vertebral BMD was determined to be a strong and independent predictor of mortality.
In various studies, the mortality rate of COVID-19 patients is reported in a wide range from 1.4 to 20.3%, with marked differences 31, 32, 33, 34. Piroth et al. ( 32 ) investigated 89,530 COVID-19 patients in France and reported the prevalence of in-hospital mortality as 16.9% (n = 15,104) and the rate of ICU admission as 16.3% (n = 14,585). In contrast, Guan et al. ( 31 ) stated that of the 1,099 COVID-19 patients, 5% (n = 55) were admitted to ICU and 1.4% (n = 15) died. These differences between studies may be due to demographic characteristics and comorbidities of the patients, such as age and gender, geographic region where the study was conducted, and hospital and ICU capacities. Another possible explanation for the higher mortality rate of COVID-19 in some areas may be the sudden patient flow in a short period of time creating medical structural constraints and care teams being directed to prioritize patients according to clinical status and prognosis. In our study, the in-hospital mortality rate of COVID-19 patients was 17.2% However, unlike previous studies, only patients with chest CT were investigated and analyzed in the current study. The exclusion of patients with mild COVID-19 who did not have a CT scan may have led to differences in mortality rates.
The current study has certain limitations. First, it solely focused on hospitalized patients that underwent chest CT, and therefore our findings cannot be generalized to asymptomatic patients or those with only mild symptoms of COVID-19. Second, the study had a retrospective and single-center design, and the sample size was relatively small. However, it is the first most comprehensive study investigating the relationship between vertebral BMD and COVID-19 based on quantitative data obtained from CT and reveal its prognostic significance. Third, only laboratory-confirmed COVID-19 patients were analyzed, with those having negative test results but typical clinical symptoms being excluded from the sample. Fourth, dual-energy X-ray absorptiometry (DEXA), which is the gold standard for osteoporosis, was not performed on the patients. However, it is very difficult to apply this test during a pandemic. Finally, patients included in the current study were treated in a single tertiary hospital, and all patients were from a single geographic area. Factors associated with adverse outcomes may differ in other regions and populations.
In conclusion, this study revealed that the quantitatively obtained vertebral BMD in hospitalized COVID-19 patients was a strong predictor of mortality. In addition, PSS was determined to be associated with various negative outcomes, such as ICU admission, mechanical ventilation, and mortality. These parameters are reproducible and can be easily evaluated using the chest CT images of COVID-19 patients, and we consider that they may be useful in routine clinical practice due to their prognostic value and requirement of no additional examination.
Authors’ Contributions
MT, NK, YA, and EK designed the study. MT and NK contributed equally to this work. MT analyzed and interpreted the data with NK, YA, EK. MT and FS wrote the manuscript. YKI and MG provided scientific support and valuable advice. All authors proofread the manuscript and revised it critically. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or national research committees and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The research project was approved by Harran University Institutional Review Board.
Acknowledgments
Not applicable
The authors declare that they received no funding for this study.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. https://linkinghub.elsevier.com/retrieve/pii/S0140673620301835 [Internet]Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salehi S, Abedi A, Balakrishnan S, et al. (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020 Jul;215(1):87–93. doi: 10.2214/AJR.20.23034. https://www.ajronline.org/doi/10.2214/AJR.20.23034 [Internet] Available from: Accessed March 15, 2021. [DOI] [PubMed] [Google Scholar]
- 4.Alpdagtas S, Ilhan E, Uysal E, et al. Evaluation of current diagnostic methods for COVID-19. APL Bioeng. 2020;4(4) doi: 10.1063/5.0021554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gietema HA, Zelis N, Nobel JM, et al. CT in relation to rt-PCR in diagnosing covid-19 in the netherlands: a prospective study. PLoS One [Internet] 2020;15:1–10. doi: 10.1371/journal.pone.0235844. (7 July) Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo H. Contribution of CT Features in the diagnosis of COVID-19. Can Respir J. 2020;2020(September) doi: 10.1155/2020/1237418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, et al. Vol. 296. 2020 Jul. (The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society). p. 172–180. Radiology [Internet] Available from: http://pubs.rsna.org/doi/10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tahtabaşı M. COVID-19 pneumonia: experiences regarding the use of computed tomography in diagnosis and follow-up. J Mol Virol Immunol. 2020 Sep;1(2):51–53. [Google Scholar]
- 9.Zimmermann GS, Fingerle AA, Müller-Leisse C, et al. Coronary calcium scoring assessed on native screening chest CT imaging as predictor for outcome in COVID-19: An analysis of a hospitalized German cohort. PLoS One. 2020;15:1–10. doi: 10.1371/journal.pone.0244707. (12 December) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boutin RD, Lenchik L. Value-added opportunistic CT: Insights into osteoporosis and sarcopenia. Am J Roentgenol. 2020;215(3):582–594. doi: 10.2214/AJR.20.22874. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16–31. doi: 10.1093/ageing/afy169. https://academic.oup.com/ageing/article/48/1/16/5126243 [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang S, Graffy PM, Ziemlewicz TJ, et al. Opportunistic osteoporosis screening at routine abdominal and Thoracic CT: Normative L1 trabecular attenuation values in more than 20 000 adults. Radiology. 2019;291(2):360–367. doi: 10.1148/radiol.2019181648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SH, Jeong YM, Lee HY, et al. Opportunistic use of chest CT for screening osteoporosis and predicting the risk of incidental fracture in breast cancer patients: a retrospective longitudinal study. PLoS One [Internet] 2020;15:1–12. doi: 10.1371/journal.pone.0240084. (10 October) Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang HJ, Lee SM, Seo JB, et al. Quantitative vertebral bone density seen on chest CT in chronic obstructive pulmonary disease patients: association with mortality in the korean obstructive lung disease cohort. Korean J Radiol. 2020;21(7):880–890. doi: 10.3348/kjr.2019.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veronese N, Stubbs B, Crepaldi G, et al. Relationship between low bone mineral density and fractures with incident cardiovascular disease: a systematic review and meta-analysis. J Bone Miner Res. 2017;32(5):1126–1135. doi: 10.1002/jbmr.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fauny M, Albuisson E, Bauer E, et al. Study of vertebral fracture and Scanographic Bone Attenuation Coefficient in rheumatoid arthritis and ankylosing spondylitis vs. controls. Sci Rep. 2019 Dec 16;9(1):13323. doi: 10.1038/s41598-019-49712-x. http://www.nature.com/articles/s41598-019-49712-x [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ufuk F, Demirci M, Sagtas E, et al. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur J Radiol. 2020 Oct;131 doi: 10.1016/j.ejrad.2020.109271. https://linkinghub.elsevier.com/retrieve/pii/S0720048×20304605 [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eslami V, Abrishami A, Zarei E, et al. The Association of CT-measured cardiac indices with lung involvement and clinical outcome in patients with COVID-19. Acad Radiol. 2021 Jan;28(1):8–17. doi: 10.1016/j.acra.2020.09.012. https://linkinghub.elsevier.com/retrieve/pii/S1076633220305511 [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tahtabasi M, Hosbul T, Karaman E, et al. Frequency of hepatic steatosis and its association with the pneumonia severity score on chest computed tomography in adult COVID-19 patients. World J Crit Care Med. 2021 May 9;10(3):47–57. doi: 10.5492/wjccm.v10.i3.47. https://www.wjgnet.com/2220-3141/full/v10/i3/47.htm [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Republic of Turkey Ministry of Health's COVID-19 patient management algorithm [Internet]. 2021 [cited 2021 Jan 3]. Available from: https://hsgm.saglik.gov.tr/depo/covid19/Ingilizce/Algoritmalar/COVID19-PLKACILHASTAYONETIMI_ENG.pdf. Accessed March 15, 2021.
- 21.Pompe E, de Jong PA, de Jong WU, et al. Inter-observer and inter-examination variability of manual vertebral bone attenuation measurements on computed tomography. Eur Radiol [Internet] 2016;26(9):3046–3053. doi: 10.1007/s00330-015-4145-x. Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiechter M, Bengs S, Roggo A, et al. Association between vertebral bone mineral density, myocardial perfusion, and long-term cardiovascular outcomes: a sex-specific analysis. J Nucl Cardiol. 2020;27(3):726–736. doi: 10.1007/s12350-019-01802-z. [DOI] [PubMed] [Google Scholar]
- 23.Chung M, Bernheim A, Mei X, et al. Vol. 295. 2020 Apr. (CT imaging features of 2019 Novel Coronavirus (2019-nCoV). Radiology [Internet]). p 202–207. Available from: http://pubs.rsna.org/doi/10.1148/radiol.2020200230. Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Tao Z-W, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) [Internet]. 2020 May 5;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. https://journals.lww.com/10.1097/CM9.0000000000000775 Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang HJ, Lee SM, Seo JB, Kim J-E, Choi HY, Kim N, et al. Quantitative vertebral bone density seen on chest CT in chronic obstructive pulmonary disease patients: association with mortality in the korean obstructive lung disease cohort. Korean J Radiol [Internet] 2020;21(7):880. doi: 10.3348/kjr.2019.0551. https://kjronline.org/DOIx.php?id=10.3348/kjr.2019.0551 Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bultink IEM, Vis M, van der Horst-Bruinsma IE, et al. Inflammatory rheumatic disorders and bone. Curr Rheumatol Rep. 2012 Jun 3;14(3):224–230. doi: 10.1007/s11926-012-0252-8. http://link.springer.com/10.1007/s11926-012-0252-8 [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui KPY, Cheung M-C, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med. 2020 Jul;8(7):687–695. doi: 10.1016/S2213-2600(20)30193-4. https://linkinghub.elsevier.com/retrieve/pii/S2213260020301934 [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020 Aug 25;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. http://link.springer.com/10.1007/s00330-020-06817-6 [Internet]Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020 May 22 doi: 10.1136/bmj.m1966. [Internet]m1966. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du R-H, Liang L-R, Yang C-Q, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020 May;55(5) doi: 10.1183/13993003.00524-2020. http://erj.ersjournals.com/lookup/doi/10.1183/13993003.00524-2020 [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. http://www.nejm.org/doi/10.1056/NEJMoa2002032 [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piroth L, Cottenet J, Mariet A-S, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2020 Dec doi: 10.1016/S2213-2600(20)30527-0. https://linkinghub.elsevier.com/retrieve/pii/S2213260020305270 [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenthal N, Cao Z, Gundrum J, et al. Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Netw Open [Internet]. 2020 Dec 10;3(12) doi: 10.1001/jamanetworkopen.2020.29058. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2773971 Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nachtigall I, Lenga P, Jóźwiak K, et al. Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID-19 in Germany: an observational study. Clin Microbiol Infect. 2020 Dec;26(12):1663–1669. doi: 10.1016/j.cmi.2020.08.011. https://linkinghub.elsevier.com/retrieve/pii/S1198743×20304936 [Internet] Available from: Accessed March 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]