Abstract
Without a doubt, the current global pandemic affects all walks of our life. It affected almost every age group all over the world with a disease named COVID-19, declared as a global pandemic by WHO in early 2020. Due to the high transmission and moderate mortality rate of this virus, it is also regarded as the panic-zone virus. This potentially deadly virus has pointed up the significance of COVID-19 research. Due to the rapid transmission of COVID-19, early detection is very crucial. Presently, there are different conventional techniques are available for coronavirus detection like CT-scan, PCR, Sequencing, CRISPR, ELISA, LFA, LAMP. The urgent need for rapid, accurate, and cost-effective detection and the requirement to cut off shortcomings of traditional detection methods, make scientists realize to advance new technologies. Biosensors are one of the reliable platforms for accurate, early diagnosis. In this article, we have pointed recent diagnosis approaches for COVID-19. The review includes basic virology of SARS-CoV-2 mainly clinical and pathological features. We have also briefly discussed different types of biosensors, their working principles, and current advancement for COVID-19 detection and prevention.
c: COVID-19, Biomarker, Biosensors, SARS-CoV 2, Immunoassay, Diagnosis, Point of care
1. Introduction
The novel form of coronavirus, whose existence was recognized in 2019 is commonly known as COVID-19 infection, a highly transmitted disease otherwise called severe acute respiratory syndrome infected every age group throughout the globe [[1], [2]]. The outbreak of this Virus was firstly identified in the Wuhan district (China) from the patient with unusual pneumonia-like symptoms, which later spread uncontrollably throughout the planet [2]. It was identified as COVID-19 (earlier known as 2019-nCov) (novel coronavirus) on January 12, 2020 by WHO (World Health Organization) [2]. ICTV (International Committee on Taxonomy of Viruses) annotated the COVID-19 causing virus as SARS-CoV-2 on Feb 11, 2020 [1]. In terms of transmissibility and outbreak region, SARS-CoV-2 simply transcended previous coronavirus outbreaks with SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory symptoms) [3]. Due to the rate of transmission and an increasing number of cases WHO announced a global pandemic for COVID-19 on March 12, 2020. [4,5]. According to WHO published data 160,813,869 confirmed cases and 3,339,002 deaths were reported till May 15, 2021 [4,5].
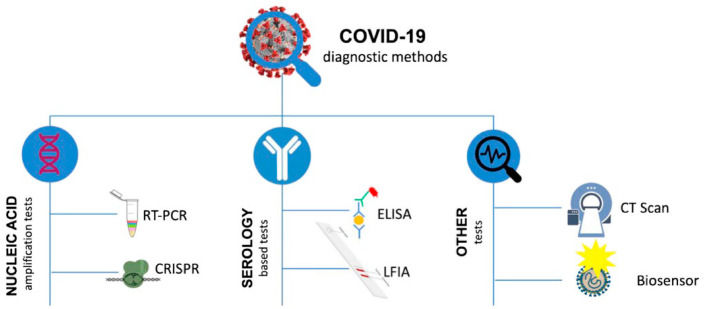
Based on its infection rate and impact on the socio-economic condition, the utmost concern is to develop tools, techniques, and strategies to overcome this pandemic [144]. Multiple surveillance and screening tools are still needed to develop for early detection of the novel coronavirus and its mutated versions. In only two-decade five pandemics have already hit our community which is SARS, MERS, Swine Flu, Ebola, and SARS-CoV 2. For that reason, early identification of such viruses will not only prevent the spreading of infection but also helps in the socio-economic benefit of humankind [144]. In recent times during the COVID-19 Pandemic, there is some preexisting diagnostic approach for detection and screening of the virus which is also considered the gold standard in the field of medical microbiology. Previously X-rays were used to take images of the chest for identification of chest infection during the initial stage of the pandemic when there was a lack of information about novel coronavirus [6]. When the data became available related to structural and genomics study with the help of microscope and sequencing, various molecular tools like PCR (Polymerase chain reaction), serologic tests were developed and optimized for COVID-19 screening. In case of PCR (Polymerase chain reaction) various sequence of the virus was targeted based upon the genomics sequence available in the database with specific primers and probes [7]. In the case of serological test kits, antigen or antibodies from the blood samples were used for diagnosis of the virus [8]. In most of the case, the serological kits available in the market are based on LFA (Lateral flow assay) or ELISA (Enzyme-linked immunosorbent assay) as shown Table 3.
Table 3.
A different source of Kit based on Immunoassay with their Target analyte and method of detection (EUA authorized FDA approved, European Union Conformity Marked) [79].
| Source | Principle | Target Antibody |
|---|---|---|
| Autobio diagnostic co Ltd | LFA | Immunoglobulin G, Immunoglobulin M |
| DiaSorin Inc | CIA | Immunoglobulin G |
| Euroimmun US Inc | ELISA | Immunoglobulin G |
| Healgen Scientific | LFA | Immunoglobulin G, Immunoglobulin M |
| Jiangsu Macro & Micro-Test | Gold in form of colloid | Immunoglobulin G, Immunoglobulin M |
| Ortho Clinical | LFA | Immunoglobulin G, Immunoglobulin M |
| Quidel Corporation | LFA | Nucleocapsid protein |
| Autobio Diagnostics | Immunology based | – |
| Siemens Healthcare Diagnostics Inc | Immunology based Chemiluminescence | Total Antibody |
| Vibrant America Clinical Laboratories | Immunology based Chemiluminescence | Immunoglobulin G, Immunoglobulin M |
| Assure Tech | Immunology based | Immunoglobulin G, Immunoglobulin M |
| Hangzhou Biotest Biotech Co., Ltd. | LFA | Immunoglobulin G, Immunoglobulin M |
| Beijing Decombio Biotechnology | Immunology based | Immunoglobulin G, Immunoglobulin M |
| BiOSCiENCE | Immunology based | Immunoglobulin G, Immunoglobulin M |
| BTNX Inc | Immunology based | Immunoglobulin G, Immunoglobulin M |
| Abbott Laboratories, Inc | Immunology based Chemiluminescent | Immunoglobulin G |
| Cellex | Immunology based | Immunoglobulin G, Immunoglobulin M |
| ChemBio diagnostics | Immunology based | Immunoglobulin G, Immunoglobulin M |
| Core Technology | Immunology based | Immunoglobulin G, Immunoglobulin M |
| Roche Diagnostics | Immunology based | IL-6 |
| PharmaTech | Immunology based | Immunoglobulin G, Immunoglobulin M |
| Snibe Diagnostic | Immunology based | Immunoglobulin G, Immunoglobulin M |
| Wadsworth Center | Microsphere based Immunology | Total Antibody |
| NanoResearch | Immunology based | Immunoglobulin G, Immunoglobulin M |
| Mount Sinai Laboratory | Immunology based | Immunoglobulin G |
To address the limitations associated with traditional detection methods, multiple physical diagnostic tools have developed in recent times which are mostly based on biosensors due to their beneficial properties like high sensitivity, high specificity, low cost, and easy to use [9]. Biosensors are the device having both organic and inorganic components which analyze and provide qualitative and quantitative information about the specific type of analyte in the provided samples [8]. The organic component of the biosensors are biological elements which can be antibody or aptamer, and the inorganic components are transducer which generates signals in the sensor [10]. Based on these types of components used in biosensors they can be immune-sensors, Enzyme based sensors, Electrochemical sensors, Optical sensors, FRET-based sensors, Colorimetric sensors, Piezoelectric sensors, Magnetoelastic sensors, and others [11]. Advancement in the field of nanotechnology and microfluidics is creating numerous pathways in developing biosensors with multidimensional properties [12,13]. This not only helps in miniaturization in the size of biosensors but also increases the accessibility to common people in the pandemic situation. These physicochemical-based biosensors in the field of diagnostics, aside from been considered as the first line of defense and also in high demand, due to their flexible properties.
These biosensors are already present in the market as rapid diagnostic kits and some are still in the phase of development which are mostly based on the principle of CRISPR technology, microarray, microfluidics, isothermal amplification, and others which are assumed to be promising technologies that can diagnose the virus in the early stage of the infection [14].
This review presents a broad insight into the pathogenic mechanism of SARS-CoV-2 and significant virulence factors. Besides, potential interventions and conventional diagnosis methods to arrest COVID-19 transmission are also discussed. We further pointed up the advancement of recent technologies in the field of biosensors that have sped up the rapid, accurate diagnosis into practice.
2. Details on COVID-19
2.1. COVID-19 and their clinical significance
It is found that several clinical signs are associated with COVID-19 infection wherein most of the case symptoms appear within 3–7 days [15]. Fever, diarrhea, runny nose, pharyngological myodynia-like symptoms appear in the first week which can create hypoxia and dyspnea in the later week of infection. In the critical condition of this infection, the disease can be progressed as ARDS (acute respiratory distress syndrome), septic shock, and coagulation disorder [15]. In some cases, gastrointestinal discomfort, vomiting, depression, shortness of breath is seen [16].
The infection is transmitted due to inhalation of the droplet which can be arisen by sneezing, coughing, or talking with infected Pearson [17]. It can be also spread by direct or indirect contact where the virus can enter inside the body through the mouth, eyes, nose, or other mucosal layers [17]. It is found that viral load is higher in the nasal cavity compared to the throat [3].
Apart from symptomatic, patients can be classified into asymptomatic and presymptomatic. In true asymptomatic cases, COVID-19 positive patients do not experience any symptoms though, in the case of presymptomatic cases, patients may not develop any symptoms before diagnosis but symptoms can come up within 14 days of diagnosing positive [18].
2.2. Basic biology of SARS-CoV-2
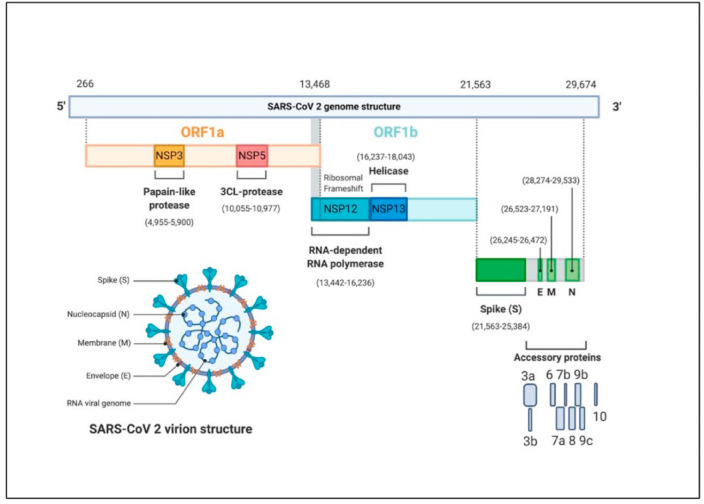
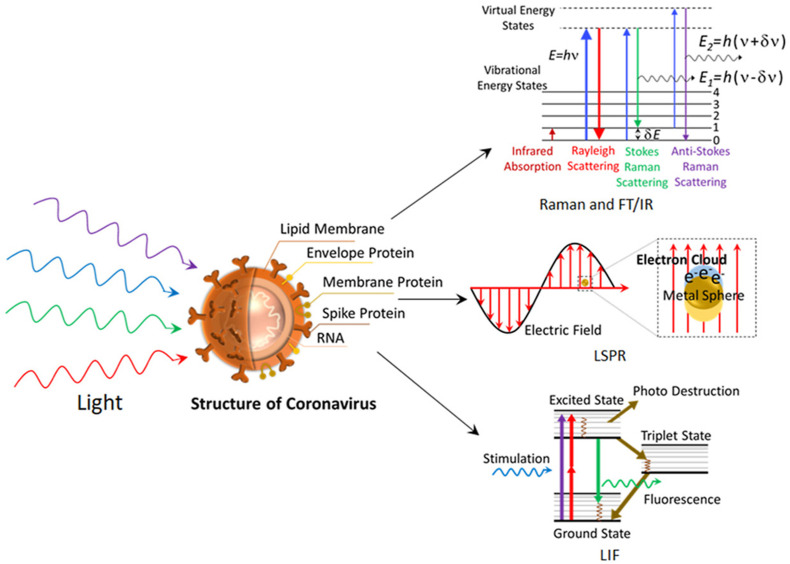
Coronaviruses are a class of ss (Single-stranded) RNA containing enveloped viruses as shown in Fig. 1 , that possess an evolutionary rate of 4–10 nucleotides per site per year [17]. Corona has been categorized into four broad genera which are the ɑ-coronavirus, β-coronavirus, γ-coronavirus, and δ-coronavirus [3]. The most common example of alpha coronaviruses is human-associated coronavirus NL63, 229E, Porcine epidemic diarrhea virus [17]. While, beta coronavirus 1, human coronavirus, SARS, SARS-CoV 2 are in beta coronavirus genera and porcine coronavirus HKU1 is in delta genera [17]. Amidst, ɑ and β-coronaviruses mainly transmit the disease to mammals, γ coronaviruses affect birds and δ-coronaviruses can transmit to both mammals and birds [3].
Fig. 1.
The genomic framework of SARS-CoV-2. (A) Representation of structural component of SARS-C0V-2 virus encoding various structural proteins (membrane, spike, envelop, nucleocapsid). SARS-CoV-2 seems more or less spherical in shape and has spike glycoproteins covered the whole envelope. (B) Schematic of the genomic organization of SARS-CoV-2. Its genome is single-stranded linear RNA (29,884 kb). The whole genome comprises around 14 open reading frames (ORF). 5′ end containing ORFs, ORF1a, ORF1b (21,563) encode polyproteins which further modified to form different nonstructural proteins (Papaine like protease, 3CL protease, RNA dependent RNA polymerase, Helicase), and accessory proteins. Reproduced from open access article [66] under the terms of Creative Commons CC BY.
SARS-CoV-2 is the causing virus for COVID-19 which is more dangerous than the Middle East respiratory syndrome coronaviruses (MERS-CoV) and SARS-CoV, in terms of pathogenicity [19]. Understanding the virulence and pathogenic mechanism associated with SARS-CoV-2 is the urgent research priority to accelerate COVID-19 therapeutic interventions.
The size of the COVID-19 virus is in the range of 60–140 nm diameter. The genetic material of this virus is comprised of single-stranded positive-sense RNA with almost 29,884 nucleotide sizes [21]. The genome of the virus codes for twenty-seven types of proteins. Some of them are helicase, papain-like protease, 3CL protease, RNA-dependent RNA polymerase, and four structural proteins required during virus assembly, etc [21]. Almost two-thirds of the SARS-CoV-2 genome consists of ORF1a (13,468 kb) and ORF1b (21,563 kb). These 5′ ended genes express polyprotein 1a and polyprotein 1b. Whilst at the 3′ end SARS-CoV-2 encode structural proteins comprise of spike surface glycoprotein (S), Small envelope protein(E), matrix protein (M), and nucleocapsid protein(N) [19]. Additionally, the SARS-CoV-2 genome also codes for several accessories’ proteins like 3a, 3b, 6, 7a, 7b, 8, 9b, 9c, and 10 as shown in Figs. 1 and 3. These proteins are required during viral replication [20].
Fig. 3.
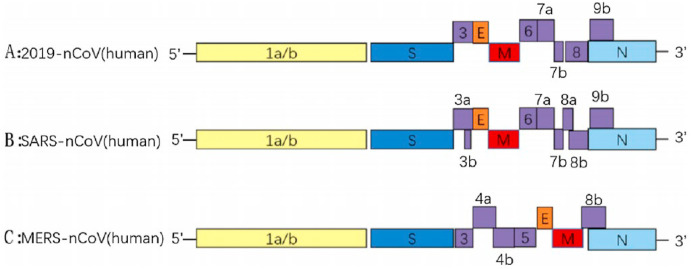
Representation of genome similarity from 5′UTR (Untranslated region) to 3′UTR (Untranslated region) of SARS-CoV-2 with SARS-CoV and MERS-Cov, where E, M, N represent envelop protein-coding gene, a membrane protein-coding gene, and nucleocapsid protein-coding gene respectively. The numbers like 3, 7, 8, 9, 10B, 13, 14 in COVID-19 genome, 3A, 3B, 6, 7, 7B, 8, 8A, 9B in SARS-CoV genome and 3, 4A, 4B, 5, 8 in MERS-CoV genome represent accessories protein codding region present in the genome. Reproduced from an open-access article [27] under the terms of Creative Commons CC BY.
2.3. Binding and pathogenicity
Coronaviruses follow a complex receptor-mediated host cell machinery to invade the host cell [17]. For instance, alpha coronavirus like human coronavirus NL63 (HCoV-NL63) recognize aminopeptidase N, beta coronavirus like MERS-CoV recognize serine peptidase or dipeptidyl transferase 4 (DPP4) [3]. Mouse hepatitis virus (MHV) mainly interacts with host cell adhesion glycoprotein cell-adhesion glycoprotein carcinoembryonic antigen-related cell adhesion molecule 1 [22,23]. Further, HCoV-NL63, SARS-CoV-2 can recognize angiotensin-converting enzyme 2 (ACE2) in host cells [24,142]. Coronaviruses utilize diverse host receptor recognizing groups for invasion. Other than receptor recognizing coronavirus spike protein machinery carry out multiple viral physiological functions. The particular configuration of spike protein assists the interaction between receptor binding motif (RBM) of S1 protein and ACE2 ectodomain [19].
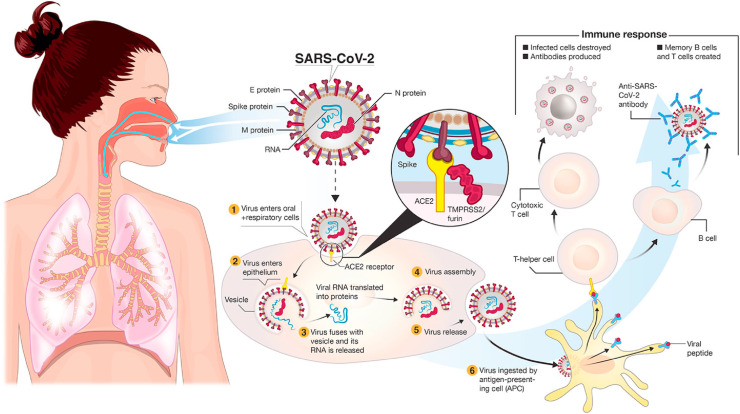
ACE2 receptors are frequently found in type II alveoli cells of lungs and other tissues. The binding affinity of this receptor SARS-CoV-2 virus spike protein is very high [25]. So, it is considered that the cells which express the ACE2 receptor are the main target for the viral infection as shown in Fig. 2 . This ACE2 also expresses cells in oral tissues which suggest the oral cavity can be the route of entry of the virus [26]. Basigin also is known as CD147 encoded by the BSG gene, can be also used by SARS-CoV-2 as an entry site where spike protein binds with this receptor [26]. The attachment of SARS-CoV-2 S protein with host ACE2 activates cellular TMPRSS2 protease which opens the spike protein and exposes fusion protein and, in that way, the virus is endocytosed inside the host cell [26]. Inside the human cell, the virus uncoats the N protein to release the single-stranded (ss) RNA [27]. Through the activity of replicase and transcriptase gene, viral genetic material replicates that translated later to form major viral proteins (S, M, N, E) [27]. Those viral proteins are processed through the endoplasmic-Golgi network to finally self-assemble to form a complete virion [28]. During infection, SARS-CoV-2 hijacks different host cell machinery, one such factor is the activation of Caspase 8, a key factor of extrinsic apoptosis that leads to host cell death [29].
Fig. 2.
The viral spike proteins bind with host lung epithelial and vascular endothelial cells expressing ACE2 receptor in cooperation with TMPRSS2. Finally, the membrane fusion leads to endocytosis of virion inside the human cytoplasm. Reproduced from open-access article [49] under the terms of Creative Commons CC BY.
2.4. Similarity with other viruses
Based on host type, coronaviruses can be classified into animal coronaviruses and human coronaviruses. The human coronaviruses can be divided into 4 different subfamilies: alpha, beta, gamma, and delta [3]. The subfamilies can further be classified into subgroups. Though the genome organization is similar but consecutive mutation at S protein and open reading frame (ORF) makes one coronavirus different from the other one [27]. Current SARS-CoV-2 has sequence similarity with SARS-CoV of 76 % whereas BatCoV-RaTG13, the suspected precursor of SARS-CoV-2 has sequence closeness of around 97 % [30].
The viral disease is highly contagious as transmitted by airborne, droplets, and touch-based [31]. Results of comparative analysis support SARS-CoV2 virus contains RNA as genetic material as other retroviruses. It is also found 91.2 % genetic similarity of nCOVID and Pan-SL-CoV which are found in pangolin of Guandong province and 85.4 % with Pan-SL-Cov virus present in pangolins of Guangxi province [32]. The spike protein of the virus differs by only one amino acid of Spike protein present in pangolin coronavirus [32].
Moreover, regarding transmissibility, the Influenza virus shares a lot of similarities with SARS-CoV-2 [33]. Nevertheless, genomic analysis suggests MERS-CoV shares only 50 % of sequence similarity, the asymptomatic transmission of SARS-CoV-2 is very similar to MERS-CoV [33]. Structural mutation at S protein led the SARS to detect host ACE2 and CD147 receptor, while MERS recognize only DPP4 and SARS-CoV-2 detect ACE2 [28].
As shown in Fig. 3, the genome encoding for various nonstructural proteins, structural proteins (Spike, envelop, nucleocapsid and membrane) and accessories proteins are similar in the case of COVID-19, SARS, and MERS virus with minor differences.
2.5. COVID-19, a panic zone virus
SARS-CoV-2 symptoms bear a resemblance to the common cold and other viral infections and thusly, it masked itself and deceived medical professionals initially, maintaining a medium Reproduction rate of 2.25 and a medium Mortality rate of 5.7 % till June 7, 2020 [34]. Most importantly, the current novel coronavirus has never been seen before in humans which is why the human immune system was completely uneducated about it. It cannot draw public attention in the initial phase of a pandemic. In a comparison with Death Zone viruses like Ebola and smallpox, this virus has a very less mortality rate but a higher infection rate, and due to which it is spreading so quickly. In addition to that, asymptomatic transmission is another big issue, unwittingly patients are spreading the virus. Since it becomes a prime issue so quickly, generates a huge medical equipment supply shortage in the initial phase to fight against SARS-CoV 2. Until now, more than a hundred variants of SARS-CoV-2 have appeared to spread with high transmission efficiency [35]. Virologists announced recent coronavirus strain delta plus as a ‘variant of concern’ for its high transmission efficiency and great immune escape ability [35]. Thence, developing countries like India faced over 300,000 active cases in the month of May–June, affected by extreme oxygen shortage. Above that several life-threatening fungal variants are affecting coronavirus patients or patients who had it earlier. In between extreme challenges, now and then, it is very important to take COVID-19 protective measures very seriously.
3. Biomarker associated
The COVID-19 emergency makes the scientists to commit accelerating safe and productive vaccine development. Understanding the pathogenicity and particular biomarkers associated with SARS-CoV-2 benefitting as the knight in shining armor for vaccine development [142]. Moreover, reliable SARS-CoV-2 biomarker identification is required not only for covid- 19 disease diagnosis but also to identify the pathogenicity level [142]. Biomarkers can also help doctors to classify patients in various risk groups based on pathogenicity of virus and disease progression to provide better treatment. Based on the pattern of Biomarker (Hematological, Biochemical, Inflammatory, and immune) identified in the patient, the severity of the disease can be also identified and monitored accordingly [25]. The biomarker found in the COVID-19 patient can be categorized as stated in Table 1 . Biomarkers can be both quantitative or qualitative [36]. In the case of hematological biomarkers, the number of various blood cells is the indicator of the severity of disease but in the case of inflammatory markers (Interleukin, C reactive proteins) and biochemical biomarkers (Total bilirubin, Blood urea nitrogen, LDH, Creatinin, Cardiac troponin, Aminotransferase, Creatine kinase), both qualitative and quantitative parameters are important to understand the severity of the disease [25,33].
Table 1.
Categorization of Potential Biomarkers used for diagnosis of COVID-19 infection based upon their properties [37].
| Biomarker's type | Up-regulated | Downregulated |
|---|---|---|
| Hematological | WBC count Neutrophil count Neutrophilic - lymphocyte ratio Monocyte-lymphocyte ratio Erythrocyte sedimentation rate |
T cell count B cell count Platelet count Eosinophil count Lymphocyte count NK cell count |
| Inflammatory | C reactive protein Interleukin class (2, 6, 8, 10) Serum ferritin |
|
| Biochemical | Total bilirubin Blood urea nitrogen LDH Creatinin Cardiac troponin Aminotransferase Creatine kinase |
Albumin |
| Blood coagulators | D- Dimer Pro-thrombin time |
|
| Potential new | Angiotensin II Homocysteine |
Almandine Angiotensin-(1–9) |
4. Type of diagnostic test for COVID-19
SARS-CoV-2 infected patients most commonly have a fever, dry cough, tiredness, dyspnea, etc.
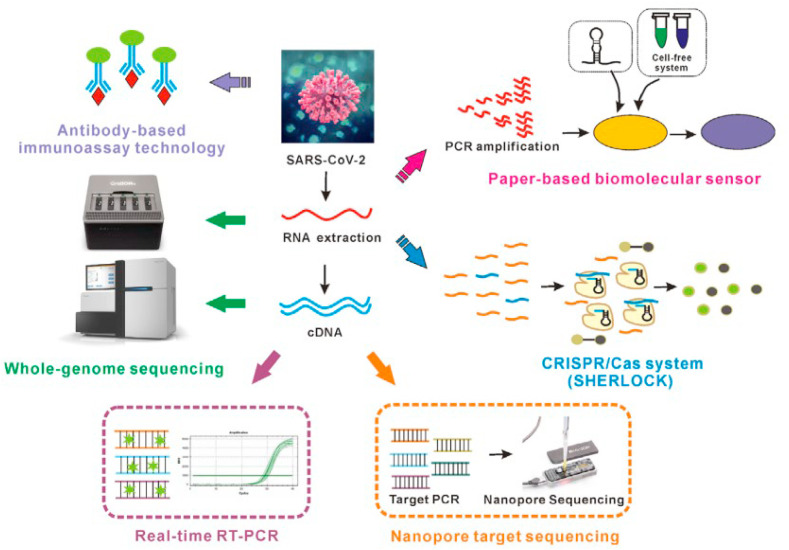
In one study guan et al. found that 44 % of 1099 patients had a fever while 89 % developed fever before and after entering the hospital, cough (38 %), Sputum production in 34 %, and 19 % of patients with shortness of breath in China [20]. Because of, similar disease presentation many of the COVID-19 symptoms can be also be correlated with various other respiratory diseases such as influenza, SARS, and MERS [33]. Testing methods and tools used for COVID-19 diagnoses are also based on the type of samples isolated from the patient and the target molecule to be identified in samples (Fig. 5). If the sample taken is oropharyngeal and nasopharyngeal swab, in that case, the viral antigen can be detected by ELISA and LFA-based methods and the viral genome can be identified by PCR and sequencing method [36]. But if the sample is patient blood, then the diagnosis method will be restricted to blood cell counts, viral antigen, and antibody against the virus as shown in Fig. 4 [36]. Although, RT-PCR (Reverse transcription-polymerase chain reaction) exhibit a gold standard in COVID-19 diagnoses [38], other conventional COVID-19 diagnosis method includes immunological detection method like ELISA, examination of the pathophysiological condition of the affected organ by chest CT (Computed tomography) scan, NGS (Next-generation sequencing), etc [36].
Fig. 5.
Overview on boon or bane of different COVID-19 detection methods. Extraction of RNA from clinical specimen followed decontamination to involve RT-PCR for reverse transcription of target RNA to complementary DNA (cDNA). The amplified cDNA fragments are used for quantification of viral load. For antigen-based testing, sometimes due to unavailability of proper antibodies, specific aptamers are also used. Sequencing of whole viral genome guide for designing proper clinical diagnostic kit. Reproduced from an open-access article [36] under the terms of Creative Commons CC BY.
Fig. 4.
Different COVID-19 diagnosis methods based on the type of sample isolated from the patient infected and other criteria. Reproduced from an open-access article [39] under the terms of Creative Commons CC BY.
4.1. CT scan
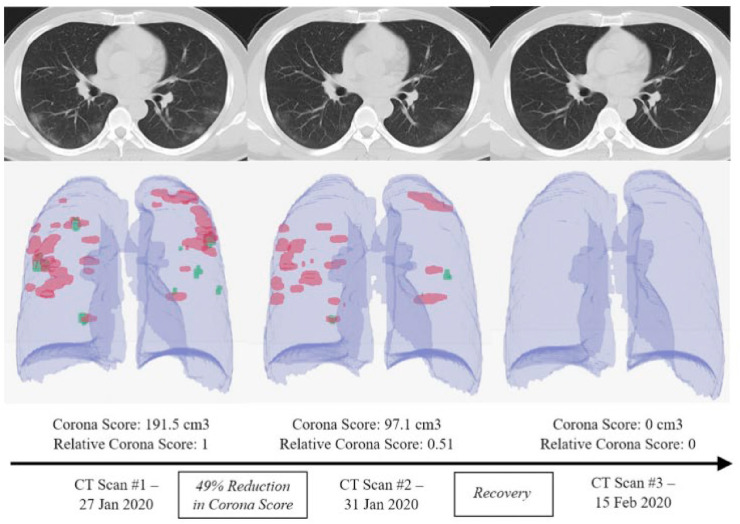
A CT or computed tomography scan permits doctors to examine the internal organs of the body. In, CT scan, a large number of chest x-ray are taken, and cross-sectional 3D images are developed, analyzed to detect abnormalities to verify the presence of COVID-19 infections [40]. Ground-glass opacity appears in the lower part of lobes and consolidation of the lungs [41]. In the early cases, small patchy pulmonary lesions appear. According to one report, a CT scan was found to be more sensitive than RT-PCR at a very early stage of infection [41]. It can be deduced from their findings that a CT scan conjointly with any other standard diagnosis technique can achieve excellent accuracy. CT scan not only helps to identify the infection due to COVID-19 (Fig. 6 ) but also helps to understand the condition of lungs and severity of disease, which guides the physicians to manage the patients related to infection [42].
Fig. 6.
Schematic illustration of diagnosing COVID-19 complications through chest CT scan. RAD logics algorithm model is considered superior in classifying CT images in categorizing into COVID-negative or positive. Reproduced from an open-access article [42] under the terms of Creative Commons CC BY.
4.1.1. Challenges with CT scan
CT has very low specificity (25 %) for COVID-19. The diagnostic imaging with CT overlaps with another disease like pneumonia [43]. Further, there is a considerable amount of uncertainty with CT in the case of asymptomatic patients [7]. In addition to that, repeated exposure to radiation can impose a biological impact in a long run [43]. CT system is a very expensive tool and requires an expert to operate and analyze the data so, there is a need to develop other tools. The key challenges and advantages are mentioned in SWOT 1.
4.2. Nucleic acid-based test
4.2.1. Sequencing based
Sequence-specific detection of SARS-CoV-2 can be performed through nucleic acid sequencing, one of the highly specific molecular techniques to detect novel Coronavirus. This method is used to analyze the complete genetic material of the virus [1]. It is not only useful in diagnosis but also to evaluate the mutation pattern of the virus. Further, genomic analysis is very crucial in designing different molecular assays such as primer designing for PCR. Most of the current COVID-19 research uses Sanger sequencer and next-generation sequencing [44]. In the case of sanger sequencing, the patient sample is lysed for viral RNA isolation and some spike-in control RNA is mixed along with Viral RNA [45]. The spiked RNA is used for the normalization and quantification of the virus present in the sample [45]. A specific set of primers allowed the PCR amplification to facilitate the differentiation of SARS-CoV-2 viral RNA and spike in RNA upon electropherogram analysis [45]. On the other hand, most next-generation sequencing-based SARS-CoV-2 diagnoses mainly rely on Illumina sequencing and oxford nanopore sequencing [44]. Unlike sanger sequencing, next-generation sequencing can target billions of target sequences simultaneously [44]. Genomic and sub genomic data analysis enables understanding the molecular features of pathogenesis and to development of a different diagnostic approach for accurate SARS-CoV-2 detection from a patient sample. It is reported that sanger sequencing can run 4000 tests/day with a single instrument which is 30 times faster than RT-PCR [46]. Understanding the different SARS-CoV-2 variants is very critical to follow the effectiveness of different vaccines. Moreover, it will be very tough to determine the viral mutation and infection progress without sequencing. The gold standard qRT-PCR can only be modified and adapted based upon sequencing. In addition to that sanger sequencing can deliver proper diagnosis at a 30 times faster rate than RT-PCR [44]. To test one sample with Sanger sequencing the running cost approximated to be less than 20$ [44]. The sequencing-related advantages and disadvantages are mentioned in SWOT 2.
4.2.2. PCR based detection
PCR is a molecular technology that amplifies the segment of the targeted genome present in the sample. RT-PCR continues to be a benchmark in COVID-19 diagnosis. As the global outbreak of COVID-19 is based on a newly discovered SARS virus, the handy diagnostic approach appears less. With the metagenomic RNA sequencing method, the proteogenomic composition of SARS-CoV-2 was found quickly on March 25, 2020. Then, the RNA Sequence (29870 bp) of COVID-19 was shared with the public and added to the GenBank sequence repository on Jan 10, 2020 [21]. After onwards, more than 1000 sequence was shared and added in the GISAID database from various scientific community all across the world [21]. Depending upon the complete genomic information, scientists developed an accurate diagnostic tool for targeting specific regions of the viral genome. SARS-CoV-2 is mainly a positive-sense single-stranded RNA virus, so the most appropriate method for diagnosis based on PCR is RT-PCR, where RNA is first converted to complementary DNA and then amplified for detection [21]. RT-PCR-based SARS-CoV-2 detection, mainly target, RNA dependent RNA polymerase gene (RdRp), ORF1ab, N, E, and S portion of genomic sequence [38]. Except for the RdRp, the sensitivity of other targeted genes is in a comparable range. While, for RdRp, slightly lower sensitivity is mostly caused by the reverse primer-template mismatches [38].
SARS-CoV-2 can be detected directly from collected biological fluid which has to be preprocessed for viral RNA isolation that needs to be converted to cDNA in presence of reverse transcriptase [47]. The converted cDNA of the virus is targeted now, and a segment of the viral genome is amplified with PCR [47]. Additional components (probe) are also added to situate a foundation that hybridized with the complementary cDNA segment for amplification. The single-step Taqman probe allows real-time quantitative monitoring of the PCR cycle [43]. The result of the PCR amplification is analyzed based on control (positive control, negative control, and internal control) used in the reactions and the Ct value (Threshold value) obtained during amplification of the viral genome (Fig. 7 ). The COVID-19 positivity criteria depend on measuring the Ct value. The Ct value is the number of PCR cycles required to cross the threshold fluorescent signal value. In most cases of COVID-19 infection, the cut-off Ct value is between 35 and 40 [48]. A patient with a Ct value lower than 35 is considered as COVID-19 positive, whereas above 40 Ct value, clinically considered as negative [48]. A Higher Ct value represents a lower viral load in the sample [47]. Various FDA-approved RT-PCR kits are listed in Table 4 for SARS-CoV-2 detection.
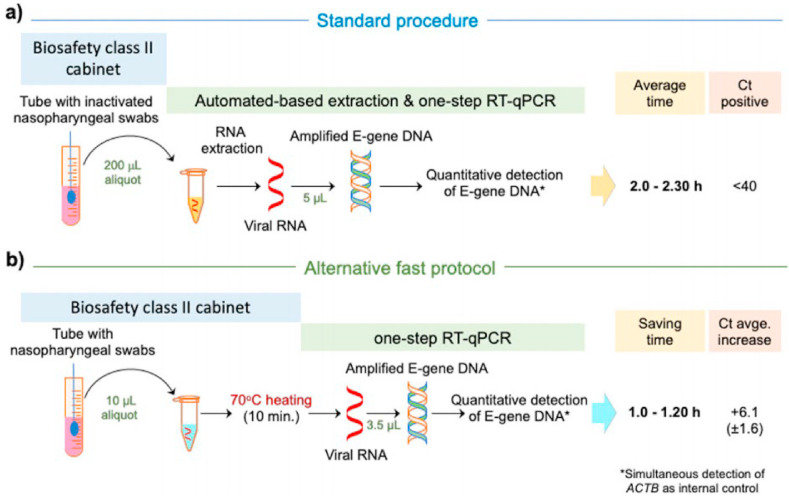
Fig. 7.
Reverse transcription quantitative polymerase chain reaction of nasopharyngeal swab is the conventional method of COVID-19 confirmation. (A) Typically, RNA is extracted from clinical sample to amplify with qRT-PCR. (B) Various ongoing research used qRT-PCR for clinical diagnosis without any pre sample treatment. The assay sensitivity is in comparable range in both the cases however average threshold cycle value (Ct) increases in direct testing [52]. Reproduced with the permission from the Elsevier.
Table 4.
List of nucleic acid-based diagnostic tools developed on the basis of emergency use authorized by FDA [79].
| Source | Mechanism of Detection | Site/antibody Target | Time limit | Detection limit | Point of care |
|---|---|---|---|---|---|
| Thermo Fisher | qReverse transcriptase -PCR | Open reading frame1ab, Nucleoprtein coding gene, Surface protein coding-gene | >2h | 10 copies/microliter | no |
| Roche | qReverse transcriptase -PCR | Open reading frame 1a | >2h | 9 copies/microliter | no |
| PerkinElmer | qReverse transcriptase -PCR | Open reading frame 1 ab, Nucleoprotein coding gene | >2h | 0.025 copies/microliter | no |
| Sherlock Bioscience | CRISPR + LFA | Surface protein-coding gene, Open reading frame1ab | 60 min | 10-100 copies/microliter | yes |
| Cepheid | qReverse transcriptase -PCR | Nucleoprotein coding gene, Envelope protein coding-gene | 45 min | 0.25 copies/microliter | yes |
| BioFire | qReverse transcriptase -PCR | Open reading frame 8, Open reading frame 1 ab | 50 min | 0.33 copies/microliter | yes |
| Mesa Biotech | Reverse transcriptase -PCR + LFA | Nucleoprotein coding gene | 30 min | 200 copies/reaction | yes |
| Abbott | Isothermal nucleic acid amplification | RNA dependent RNA polymerase-gene | 5/13 min | 0.125 copies/microliter | yes |
| CDC | qReverse transcriptase -PCR | Nucleoprotein coding gene | >2h | 3.2 copies/microliter | no |
| Mammoth Bioscience | CRISPR + LFA | Envelope protein-coding gene, Nucleoprtein gene | 30 min | 70-300 copies/microliter | yes |
4.2.2.1. Challenges with PCR
There are many possible causes of false-negative cases in PCR-based SARS-CoV-2 detection. Firstly, during the viral replication cycle sometimes RNA gets damaged due to lower stability, which leads to fragmentation of the viral genome in the bloodstream that imposes a major challenge for RT-PCR-based detection [47]. Secondly, the exact severity of lung infection and associated viral load cannot be measured by examining any type of body fluid [47]. Thirdly, PCR as a diagnostic tool requires benchtop size instruments, a large number of PCR reagents, long detection time, which limits its usage in under-equipped rural areas [2]. No test is 100 % accurate. In one COVID-19 diagnosis-related study, the false-negative rate for qRT-PCR was found to be 9.3% while sensitivity was 90.7 % [50]. Other than mentioned issues related to qRT-PCR, sample cross-talk, software problems may decrease the sensitivity. In another large-scale study in China, the false positive rate was found to be 30 % of the total patients diagnosed [51]. The major advantages and drawbacks are presented in SWOT-3.
4.2.3. CRISPR based
Recent progress in CRISPR (Clustered regularly interspaced short palindromic repeats) research aside from revolutionizes genome engineering also opens up a new area for nucleic acid diagnostics-based pathogen detection. CRISPR is mainly a bacterial defense system against the infected virus. CRISPR RNA (crRNA) guides the CRISPR protein to destroy the target sequence that is attached with a complementary crRNA sequence [53]. Each CRISPR protein has its signature cleavage ability that makes one unique from the other. For instance, Cas9 binds with target dsDNA while Cas13a cleaves SSDNA. Cas14 can recognize ssDNA more efficiently than dsDNA [53]. The current, global pandemic escalates the interest to use CRISPR tool inaccurate point of care COVID-19 diagnosis.
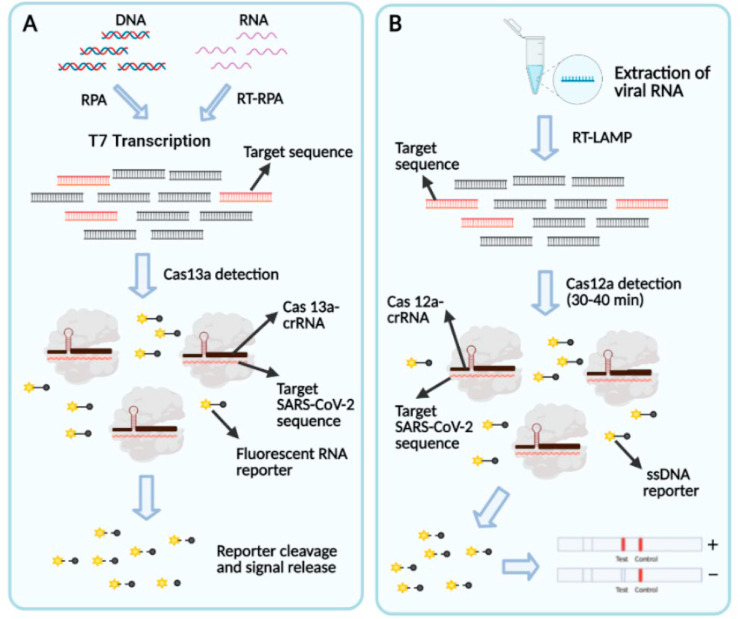
Two highly renowned companies Mammoth Bioscience and Sherlock Bioscience are harnessing the power of CRISPR to develop a cost-effective, rapid, specific diagnostic tool for COVID-19 (Fig. 8 ).
Fig. 8.
(A) Schematic of SHERLOCK based COVID-19 detection. The point of care method follows three simple steps and can be finished with an hour. (i) An isothermal amplification of extracted RNA (ii) Detection with Cas13-crRNA complex (iii) Visual readout in a paper strip. (B) Schematic of DETECTR based SARS-CoV-2 detection. Extracted RNA from nasopharyngeal swab is amplified by RT-LAMP. Followed by Cas12 based cleavage of target sequence and visual detection on lateral flow reader. Reproduced from open-access article [65] under the terms of Creative Commons CC BY.
Mammoth bioscience is working on a CRISPR tool developed by Jennifer lab that uses the Cas12a enzyme for cleaving and degradation of ssRNA and fluorescent reporter activation [54].
Feng Zhang and his colleagues developed SHERLOCK system which uses Cas13 enzyme along with a programmed CRISPR system, which can target specific gene sequence for cleavage, degradation of target strand and activation of the fluorescent reporter [55]. In both systems when the guide RNA finds the specific sequence in the sample, it cleaves the identified sequence and gives fluorescence. These two CRISPR-based biosensors work is on the principle of optical biosensors, which will capture the fluorescence during COVID-19 detection [54,55]. Different CRISPR-based COVID-19 detection methods are summarized in Table 2 . Further main merits and demerits of CRISPR related to SARS-CoV-2 detection are mentioned in SWOT-4.
Table 2.
Some important CRISPR based biosensors for SARS-CoV-2 detection.
| SN | Name | Target Sequence | Cas | LOD | |
|---|---|---|---|---|---|
| 1 | SHERLOCK | SARS-CoV-2 S & ORF1ab gene | Cas13a | 10-100 copies/μl | [56] |
| 2 | DETECTR | SARS-CoV-2 N & E gene | Cas12a | 10 copies/μl | [54] |
| 3 | CREST | SARS-CoV-2 N gene (N1,N2, N3 segment) | Cas13a | 10 copies/μl | [57] |
| 4 | CASdetec | SARS-CoV-2 RNA dependent RNA polymerase | Cas12b | 1 × 104 copies/mL | [58] |
| 5 | iSCAN | SARS-CoV-2 N&E genes | Cas12a | 10 RNA copies/reaction | [59] |
| 6 | ENHANCE | SARS-CoV-2 N gene | Cas12a | [60] | |
| 7 | AIOD-CRISPR | SARS-CoV-2 N gene | Cas12a | 1.3 copies | [61] |
| 8 | FELUDA | SARS-CoV-2 N & NSP8 | Cas9 | [62] | |
| 9 | CRISPR-FDS | SARS-CoV-2 ORF1ab | Cas12a | 108amplicons/sample | [63] |
| 10 | CONAN | SARS-CoV-2 N gene | Cas3 | 102copies/sample | [64] |
4.3. Immunoassay
Apart from direct detection, SARS-CoV-2 can also be diagnosed indirectly considering the immune response of an infected individual. Nevertheless, recent research has shown serological detection of COVID-19 with different fluids including saliva samples [52]. Rather than nasopharyngeal swab, serological diagnosis mainly targets blood samples for virus detection [36]. It was found that blood enriched by the detectable concentration of antibodies within 7 days of infection in 50 % of affected individuals and within 14 days for all individuals. This primary immune response detection is very important to inspect community transmission. Analysis of IgG and IgM are two main antibodies for detection. The bioanalytical method that relies on antigen-antibody-based interaction is termed immunoassay.
Traditionally LFA and ELISA, are two main immunoassay-based serological diagnostic methods. Specific antigens or antibodies are targeted for COVID-19 diagnosis. The stage of infection is crucial in deciding the diagnostic approach for COVID-19 detection. According to various studies, a minimum of 5 days is required for sufficient viral load and a minimum of 7 days is required for antibody development in the system. However, antibody concentration may decline after 7 days of infection. Many immunoassay platforms have been developed so far for rapid COVID-19 detection.
4.3.1. Challenges with immunoassay
The viral load does not remain constant throughout the infection which hinders the sensitivity of the test [67]. Other coronaviridae family viruses share similarities with the COVID-19 virus that causes cross-reactivity during the test to give a false-positive result [67]. Even being a less time-consuming, cost-effective method, the immunoassay-based test is less sensitive than a molecular test like PCR [66]. SARS-CoV-2 detection-related advantages and disadvantages are listed in SWOT-5.
4.3.2. Lateral flow immunoassay
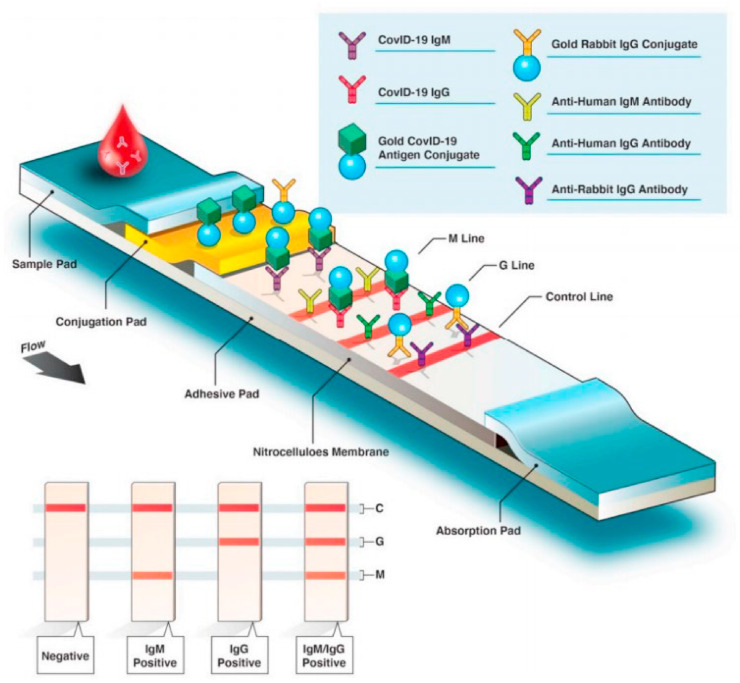
Lateral flow immunoassay (LFA) is a paper build qualitative analyte detection technique that uses a coating of antigen or antibody on the surface of a strip which bound to either viral protein or antibody developed against the virus [68,69]. Some lateral flow immuno-assay also uses aptamers as bioreceptor. When the sample from the infected person having SARS-CoV-2 is placed on a strip coated with antibody, the antibody binds with COVID-19 protein and shows the color change on a paper strip [69]. Generally, LFA is a qualitative test. As indicated in Fig. 5, the sample is mounted at one end of the paper strip, preferentially on the sample pad. Due to capillary interaction, a sample containing target analyte migrates towards a conjugation pad, that is coated with the probe, specific against the target analyte of interest. Upon the formation of a conjugate complex, it migrates towards the detection pad. Some lines in the detection pad are there to monitor the antibody-antigen reactions and are termed as M line, where some act as a control for the test, and some for guidance (G line) as shown in Fig. 9 [68]. The particular analyte recognition on the detection pad produces color read-out to be detected with the naked eye. The efficiency of color read-out can be adjusted by using different nanoparticles like colloid gold modified probe on conjugation pad [70].
Fig. 9.
Lateral flow-based immunoassay for the serological test from a blood sample for COVID-19 detection showing various components of lateral flow-based assay kits where M line is a monitor line and G line is a guideline. Reproduced from an open-access article [71] under the terms of Creative Commons CC BY.
In comparison with the gold standard, PCR test, lateral flow immunoassay based serological detection require less complex technical setup, rapid detection, and cost-efficient [70]. While inefficacy of early infection detection limits the ‘immunity passport’ detection using LFA [13].
There are various Point of care based rapid diagnostic kits, based on LFA that have already been developed by several companies worldwide for COVID-19 detection as stated in Table 3, Table 5 .
Table 5.
List of Immunological assay-based tools supplier along with its sensing method, target, time used, sensitivity, and limit of detection in percentages (Emergency use authorization from FDA) [79].
| Source | Mechanism of Detection | Site/antibody Target | Time limit | Detection limit | Point of care |
|---|---|---|---|---|---|
| Abbott | Chemiluminescent microparticle immunoassay | Immunoglobulin G | 29 min | 100 % | no |
| Quidel | LFA | Nucleoprotein coding gene | 15 min | 80% | yes |
| Sugentech | LFA | Immunoglobulin M, Immunoglobulin G | 15 min | 94 % | yes |
| Pharmact | LFA | Immunoglobulin M, Immunoglobulin G | 20 min | 70 %-98.6 % | yes |
| Bio-Rad | ELISA | Immunoglobulin M, Immunoglobulin G | <44 min | 98 % | no |
| Roche | Electrochemiluminescence immunoassay | Immunoglobulin G | 18 min | 100 % | no |
4.3.3. ELISA immunoassay
ELISA-based IgM, IgG detection exhibited a very specific and sensitive detection of COVID-19 so far.
Enzyme-linked immunosorbent assay is commonly known as ELISA because in this process enzymes are conjugated with specific antibodies to detect various proteins and pathogens present in the system [72]. It can work either way whether to detect COVID-19 viral antigen or antibody developed against the COVID-19 inside the host body. This system uses microtiter plates containing 96 wells, where the antibody is coated or fixed at the surface and then the sample containing the specific analyte (virus, proteins, antigens) is added, the fixed antibody with the analyte conjugate is identified by enzyme tagged antibody in presence of specific substrate which produces color, fluorescence or luminescence that can be visually identified [72]. The same procedure is followed for antibody detection for COVID-19 [73,74]. Companies like BioRad and Euroimmun US Inc have already developed ELISA-based methods for IgG and IgM detection for COVID-19 infection diagnosis as stated in Tables 3 and 5.
4.3.3.1. Antigen based
The antigen-based detection technique is important for the diagnosis of COVID-19, as it gives the direct result of the presence of virus inside the system and also helps in early detection of COVID-19 to prevent further infection [75]. There are multiple proteins like envelop, spike, nucleocapsid, matrix, and others associated with the COVID-19 virus, which can act as potential biomarkers for COVID-19 diagnoses [76]. The problem with antigen detection is, as the viral load reduces after the first week of infection, the amount of viral protein also reduces and becomes difficult to detect. C-reactive proteins and D-dimer along with other various biomarkers associated with COVID-19 used as promising antigens candidates for the diagnosis of this infection [37]. Cytokines which are also considered the inflammatory biomarker can be also detected. Other than performing individual immunoassays with ELISA, the principle of ELISA can also be integrated with biosensors for rapid and sensitive diagnosis.
4.3.3.2. Antibody-based testing
Development of antibodies in the human host in case of COVID-19 infections has been shown after a week of the first symptom appear in the patient. A high quantity of IgA and IgM has been identified using recombinant viral protein within 3 days of first symptom development [77]. It is also found that the specificity for specific antibodies against COVID-19 in ELISA is greater than 80 % [78]. Spike protein 1 has shown promising results in binding COVID-19 antibodies [78]. Whereas the case of COVID-19 diagnoses with both ELISA and colloidal gold immunochromatographic kit shows 100 % sensitivity and similar specificity [77]. There are more than 100 kits available in the market for the detection of antibodies specific to COVID-19 in an emergency. This type of test is rapid and cost-effective in comparison to other molecular tools. The antibody-based test also helps in identifying the history of pre-exposure to the virus of the patient. These tests will be useful in the re-opening of social engagement where a strong immune response against the virus can be detected.
4.3.3.2.1. Challenge with an antibody test
Detection of antibodies will be only beneficial for those post-symptomatic patients who have developed antibodies against COVID-19, but it is not advised particularly when the patient is in critical condition. These tests showed lower sensitivity in comparison to the PCR test [36]. It has been also seen many false-negative cases in antibody testing against COVID-19, can be due to lower titer value of the antibody, homologous protein present in the sample, or low sensitivity of the instrument [77].
4.4. Point of care test
Combining the rapid detection of disease-causing agents, with a pandemic preparedness plan can strengthen the healthcare sector to check future pandemic outbreaks.
In a situation like a pandemic, point of care testing (POC) is very crucial in terms of disease management [143]. The priority is to understand the pathogen before develop any suitable diagnostic tool. It allows rapid detection of analyte near to the patient that enables diagnosis at a very early stage and routine monitoring to improve treatment at right time. Market analytics expect the POC diagnostic market to grow $81.37 billion by 2028 at a compound annual growth rate of 9.4 % [80]. Analyzing the recent trend, we can definitely say that COVID-19 is the real game-changer in POC diagnostic market. Since new technologies are coming very often in this COVID-19 situation, POC as a whole moving towards a crucial point in the diagnosis and healthcare sector. Tables 3 and 5 consist of such point-of-care-based tests, which are FDA approved for emergency use. These tools are either based on the detection of viral RNA or Immunological components like antigen or antibodies.
5. Biosensors and their properties
Biosensors are analytical tools, a combination of organic (Biological), inorganic (transducer), and signal processing component, which can detect an analyte of interest and provide quantitative or qualitative information. It is a sensing tool that contains a biological component as the receptor and a physical element as the transducer, for the detection of a specific molecule [10]. It is generally composed of three important components which are Bioreceptors, electrochemical interference, and transducer [11]. Specific bioreceptor in a sensitive biosensor interacts with a particular analyte, while electrochemical interference provides a surface for reaction and helps in the generation of a biochemical signal into an electrical signal for effective detection [67]. An accurate, rapid biosensor must fulfill all WHO prescribed ASSURED criteria (Affordable, Sensitive, Specific, User-friendly, Rapid and Robust, Equipment-free, and Deliverable to end-users) to become a useful and unique tool [81].
5.1. Designing a biosensor
A marvelous advancement in the healthcare sector has been made right after the invention of the glucose biosensor [83]. Both in terms of innovation and application, biosensor has adapted many technical strategies to enlarge as micro size, cost-effective, sensitive, multifunctional, and powerful analytical device (Fig. 10 ) [87]. Current biosensor research very much emphasizes improving biosensor designing so to address the shortcomings [84]. Every biosensor has two parts as bio elements and sensor elements. Bio-element acts as the receptor of Biosensors which determines the specificity of biosensors, whereas sensing elements are used in signal transduction in biosensors [85,86].
Fig. 10.
Schematic representation of the features required in the biosensor for effective results, easy utility, and wide application in the field of diagnostics. Reproduced from an open-access article [82] under the terms of Creative Commons CC BY.
There are various methods proposed for combining bio-elements with sensor elements of biosensors, here only four methods of coupling are focused which are mostly used in the development of biosensors [86,87]. They can be as stated below.
-
a)
Matrix entrapment
-
b)
Membrane immobilization
-
c)
covalent amalgamation
-
d)
physical adsorption encapsulation
5.2. Classification of Biosensors
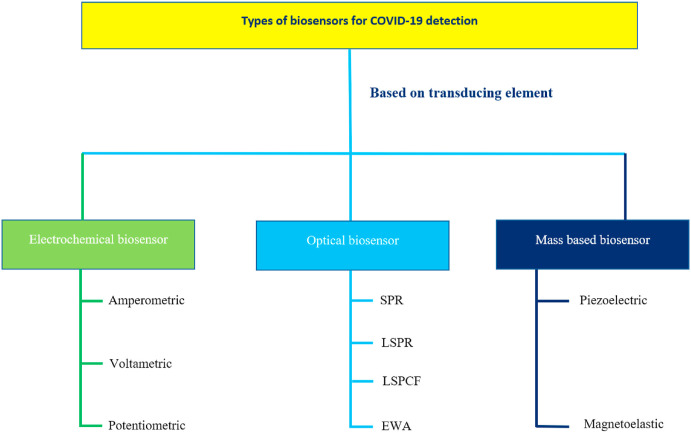
Biosensors can be divided into various classes based on multiple scales. Based on signals sensing method and transduction principles they can be classified as electrochemical, optical, radiation-sensitive, mass sensitive, and many more (Fig. 11 ) [88]. Based on the Biorecognition element prospective biosensors can be grouped as protein-based, ligand-based, enzyme-based, nucleic acid-based, saccharides-based, oligonucleotide-based, aptamers-based as shown in Fig. 7 [89]. The biosensor performance depends upon these immobilized components, to a great extent [146]. Further, based on the analyte to be detected it can be classified as DNA, Toxins, Drugs, Proteins, Enzymes, Glucose, Mycotoxin's biosensor [90].
Fig. 11.
Classification of Biosensors based on the transducing element used in SARS-CoV-2 detection.
5.2.1. Immunosensors
Immunosensors utilize the concept of biochemical immunoassay where mainly
Antibody-antigen interactions are identified on the surface of the transducer and an output signal is measured [1]. In this type of sensor, both monoclonal and polyclonal antibodies can be used which binds with the complementary antigen for detection of specific pathogens. Immunosensors are mainly classified into two broad categories: labeled and label-free immunosensor [70]. Various immunosensors have been developed by multiple companies on an immediate basis for COVID-19 diagnosis. Some are listed in Tables 3 and 5. An immunosensor measures the biochemical interaction between the target analyte and specific receptor through various detection methods like optical, electrochemical, fluorescence, or other detection methods [11]. Though label-based immunosensors can seriously improve the sensitivity and detection efficiency however wipe out of enzyme labels, purification, and washing steps increase the complexity and cost factor [91]. In addition to that nonspecific binding, stability of major concern [91]. Inadequacy to combine the sensing and detection area to a single platform is a serious problem in the commercialization of immunosensors.
5.2.2. Enzyme based biosensor
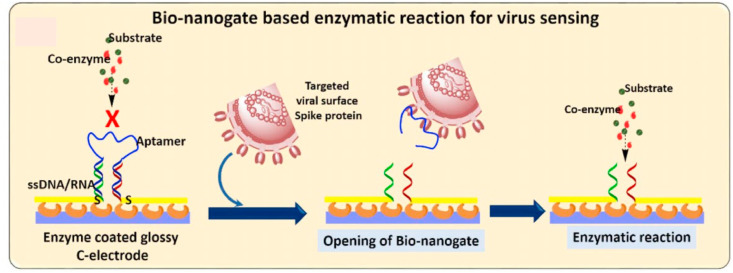
Enzymes are often used as biorecognition elements for biosensor detection. These immobilized enzymes are very specific to the analyte of interest and catalyze biochemical reactions for successful detection. The most efficacious commercially available biosensors are for evaluating blood glucose levels, developed by Guilbault [83]. Enzymatic biosensors are more stable, non-toxic, sensitive, and can easily be tagged with a radiolabeled and fluorescent molecule [92]. Different types of enzymes can be immobilized on the sensor surface. HRP (Horseradish Peroxide) is a commonly used enzyme in a sensor like this [92]. Various factors influence the performance of enzyme-based biosensors such as medium pH, temperature, cofactor, etc [93]. In affinity-based sensors, the utilization of enzymes is very common. ELISA-based kits developed by various companies mostly use enzymes as the sensing tool. Other than traditional ELISA, diagnosis of SARS-CoV-2 very much rely on enzyme-labeled antibodies that mediate signal generation and amplification for selective identification. Due to high catalytic efficiency and specificity towards analyte identification, enzyme-based biosensors conceived an extensive usage in biosensors [93]. The antibody-tagged specific enzyme has a set of substrates or particular substrates to catalyze and different detection methods can be engrossed for detection of the catalyzed product (Fig. 12 ). Such as in a recent publish electrochemical detection of SARS-CoV-2 spike (S) protein employed alkaline phosphatase enzyme tagged secondary antibody to catalyze 1 naphthyl phosphate substrate on the surface of a screen-printed carbon electrode (SPCE). [94]. However, due to the instability of enzymes depending on the environmental condition, nanotechnology stole the limelight in biosensor design [93,141]. Above all of that, the capacity to work with small sample volume, sensor robustness, and high substrate specificity will always employ enzymes as across-the-board sensing elements [93]. Various advantages and disadvantages of the enzyme-based biosensor are listed in SWOT 6 in consequence of COVID-19 diagnosis.
Fig. 12.
Enzymatic biosensor for SARS-CoV detection. Enzymatic reaction upon aptamer-viral spike protein interaction useful for virus detection based on electrochemical measurement. Reproduced from open-access article [95] under the terms of Creative Commons CC BY.
5.2.3. Optical biosensor
Due to simple, straightforward, cost-effective techniques, optical biosensors have the potential to become a major device in COVID-19 diagnosis [146].
These are the sensors in which labeled analyte excited with a laser and the signals are generated [96]. The first optical biosensor was based on fiber optics. The optical biosensor is divided into several types based on the light used and absorbent, UV–Visible sensor, surface plasmon resonance, FRET (Fluorescence Resonance Energy Transfer) sensors, and others are common optical sensors [11,96]. Changes in the intensity of light are detected by this type of sensor. LSPR (Localized surface plasmon resonance) based optical biosensors for SARS-CoV-2 detection have been developed where the viral genome is bound to gold nanoparticles and then illuminated with a light source as shown in Fig. 13 [96]. The heat generated is detected by LSPR detectors, the signals generated by the detector are used to analyze the presence of SARS-CoV-2 in the sample [97].
Fig. 13.
Localized surface plasmon resonance-based diagnosis of COVID-19 [97]. Reproduced with permission from Elsevier.
Melting temperature analysis is very crucial in qRT-PCR. The primer annealing temperature is usually set up slightly lower than melting temperature to stimulate successful primer binding. Qiu et al. followed the same idea to develop a plasmonic photothermal biosensor for Covid detection [98]. They thiol fabricated cDNA on the gold chip to immobilize the complementary SARS-CoV-2 RdRp sequence at a temperature rise of 41° [98]. The photothermal energy generated on gold nanochip is further converted to plasmon energy and transduced for sensing. Based on the signal-to-target sample size relationship the LOD for this dual-functional biosensor was found to be 2.26 × 104 copies [98]. An extensive study on sandwich assay-based optical detection has substantially developed the scientific research towards combating this deadly disease. Ramakrishna et al. have developed a fiber-optic biosensor for the detection of COVID-19. They immobilized the capture antibody on optical fiber and ultimately a sandwich was formed on the U bent fiber probe [99]. AuNPs were tagged as a plasmonic label for the evanescent wave absorbance sensing to diagnose the target analyte [99]. Positive and negative consequences related to SARS-CoV-2 detection are listed in SWOT-7.
5.2.4. FRET biosensor
Fluorescence resonance energy transfer (FRET) is one type of non-radiative energy transfer event between the donor and accepted fluorophore separated between a distance of 1–10 nm [100]. It is a very important analytical tool, particularly in the field of biological research to quantify invivo protein to protein interaction [100]. High sensitivity towards the intermolecular separation between fluorophore pairs, makes the FRET biosensor a powerful analytical tool. In one experiment Weng et al. developed a sandwiched FRET biosensor for the detection of avian coronavirus. They used molybdenum disulfide (MoS2) fluorophore modified antibody to bind with target antigen while Alexa fluor 488 labeled antibodies to form a sandwich between MoS2-Ab and target analyte [101]. Based on intermolecular distance-dependent energy transfer between Alexa-fluor-488 and MoS2, they detected avian coronavirus in the linear range of 102- 106 EID50 and limit of detection of 4.6 × 102 EID50 [101]. The development of a promising FRET design can certainly be a powerful device for SARS-CoV-2 detection. In another experiment, Brown et al. developed a FRET biosensor based on the proteolytic cleavage of the dipeptide of two fluorogenic peptide molecules, fabricated on the sensor surface. Shown in Fig. 14 [102]. The peptide hydrolyzation leads to a notable increase in fluorescence signal. The increased signal is directly related to the concentration of SARS-CoV-2 extracellular protease. This extremely sensitive biosensor can achieve a LOD of 9.7 ± 3 pfu/mL [102]. The main advantages and drawbacks associated with the FRET biosensor are listed in SWOT- 8.
Fig. 14.
Schematic of sensitive, fast FRET assay for SARS-CoV-2 detection. (A) Due to internal quenching of fluorogenic peptides screen high FRET signal. (B) Enzymatic hydrolysis of peptide bonds increases the intermolecular distances and eventually the FRET signal decreases. Reproduced from open-access article [102] under the terms of Creative Commons CC BY.
5.2.5. Colorimetric biosensors
To address the COVID-19 problems, mass testing is very crucial. Considering that, the colorimetric test is very simple, and qualitative or quantitative results can be identified by naked eyes.
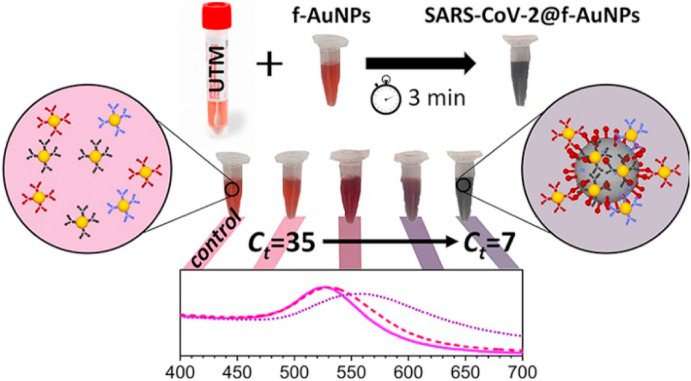
Colorimetric biosensors are tools that work on the principle in which color change is detected associated with specific biochemical reactions between analyte and sensing receptors on the biosensor surface [103]. Detection of color can be done easily without expensive tools [103]. Sensitivity can be also increased by using nanomaterials such as colloidal golds [104]. In the case of COVID-19 detection, mainly viral structural antigen is targeted with a specific antibody. When the reaction between the antigen (viral protein) and antibody takes place, the particular color on the strip of the biosensor is generated [104]. The color that appeared on the strip can be visualized by naked eyes or can be detected by detectors present in biosensors. Nanotechnology has marked tremendous advantages in colorimetric assays [141]. AuNP is very convenient because of its attractive optical properties, tunable surface plasmon, and exceptional photostability [105]. Recently, a colorimetric bioassay has been developed based on the detection of color change with the naked eye. The SARS-CoV-2 antisense N oligonucleotide tagged with AuNP that in presence of complementary interaction change its surface plasmon can be detected (Fig. 15 ). Further to visual detection, endonuclease enzyme RNase H was used to visually detect the precipitation of AuNP [105]. As given in Table 3, there are industries like Jiangsu Macro & Micro-test and others that have used colloidal gold to enhance the sensitivity of LFA-based colorimetric biosensors. Even having many advantages, colorimetric biosensors contain several disadvantages, listed in SWOT-9, as well.
Fig. 15.
Schematic reveals a colorimetric biosensor for the SARS-CoV-2 detection. Anti-SARS-CoV-2 spike, membrane, envelope modified AuNP form a complex with viral particles upon incubation with a universal transport medium (UTM) solution containing viral particles. The f-AuNP-SARS-CoV-2 interaction leads to change the free oscillation of electrons and consequently a color change that can be detected visually. Reproduced from open-access article [106] under the terms of Creative Commons CC BY. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
5.2.6. Electrochemical biosensors
The idea of electrochemical biosensors mainly emerges from the classical discovery of glucose biosensors. An electrochemical advanced transduction system can detect electrochemical responses where the biochemical information is transduced to an electric signal [96]. High sensitivity, easy miniaturization, small volume requirement, low cost makes this an excellent choice for analyte detection [107]. Electrochemical sensors are such an analytical platform that can be used for both qualitative and quantitative analyte detection [107]. These sensors can further be classified into various groups based on the sensing method, amperometric, voltammetric, potentiometric [1]. Amperometric biosensors are commonly used sensing tools for virus and pathogen detection. It detects the change in current at constant potential during the electrochemical reaction [9]. This tool is highly sensitive, rapid-response time, easy to use, and cheaper than other tools. Potentiometric biosensors are a type of electrochemical sensor to detect potential differences, due to chemical reactions [9]. This type of sensor mostly uses enzymes as a biorecognition element that senses the hydrogen (Proton) ions that are released or absorbed during reaction causing the change in potential of the surrounding environment on sensors [107]. Whereas impedimetric electrochemical biosensors depend on the change in impedance on the sensor surface [9]. Mainly, recognition of target analyte by biorecognition element contributes to the change in impedance, which is detected by electroactive species [108].
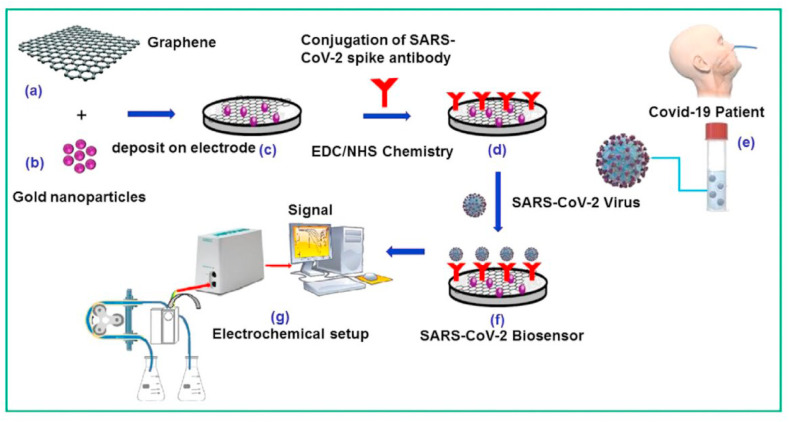
Designing suitable electrode material is very essential in the case of the electrochemical biosensor [145]. Electrically conductive solid materials are generally used such as gold, copper, carbon-based, nickel, etc. Further, advancement in nanotechnology manifested a huge significance in an electrochemical sensor. Nanoparticles modify the transducer part of a biosensor to make it more electrokinetically active [11]. As the knowledge of SARS-CoV-2 has increased, it opens several new ways of accurate detection. A hypothetical schematic of an electrochemical biosensor is exhibited in Fig. 16 for the successful detection of SARS-CoV-2 on the graphene surface. A novel sensor based on the fabrication of AuNP on the surface of fluorine-loaded tin oxide doped electrodes has been successfully developed for SARS-CoV-2 detection [109]. They mainly immobilized antibodies against SARS-CoV-2 spike protein. In the quest for rapid diagnosis, this biosensor can accurately detect SARS-CoV-2 S protein within 10–30 s [109]. Though antibodies are very specific towards target analyte, in terms of stability, immobilization efficiency, aptamer shows superiority over antibodies. In one experiment, Tian et al. developed a dual aptamer-based detection of SARS-CoV-2 N protein on the gold electrode surface. They used Au@Pt/MIL-53 as multifunctional catalysts for the generation of the electrochemical signal [110]. This electrochemical aptasensor can detect 8.33 pg/ml of viral particles in real-time [110]. Here, we have listed some of the electrochemical attempts to detect SARS-CoV-2 in Table 6 . The advantages and limitations of SARS-COV-2 specific electrochemical biosensors are listed in SWOT- 10.
Fig. 16.
Schematic illustration of designing of electrochemical immunosensor for SARS-CoV-2 detection. Deposition of graphene (a) and gold nanoparticle (b) on electrode surface increases overall surface area and conductivity of sensor surface (c). Immobilization of anti-SARS-CoV-2 spike antibody (d) on sensor surface for the attachment of SARS-CoV-2 (f) on incubation of clinical sample (e) electrochemical set up and signal processing unit for detection (g) [116]. Reproduced with the permission from the Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 6.
Various electrochemical biosensors for the detection of SARS-CoV-2.
| SN | Target | Recognition element | Detection method | LOD | |
|---|---|---|---|---|---|
| 1 | S Protein | SpAb-AuNPs-FDTO | DPV | 90 fM | [112] |
| 2 | S Protein | G-PBASE-anti-S Ab | FET based detection | 242 copies/ml | [113] |
| 3 | N protein | PmPD-Au printed board | DPV | 27 fM | [114] |
| 4 | S & N Protein | Sandwich assay on SPE | DPV | 8 fM(N protein) 19 fM(S Protein) |
[94] |
| 5 | N protein | Anti N Ab on SPE | SWV | 0.8 pg/ml | [115] |
| 6 | S Protein | SpAb-PBASE-Graphene | FET | 16 pfu/ml | [112] |
| 7 | N gene | PCB | DPV | 10 pg/ml | [111] |
5.2.7. Piezoelectric biosensors
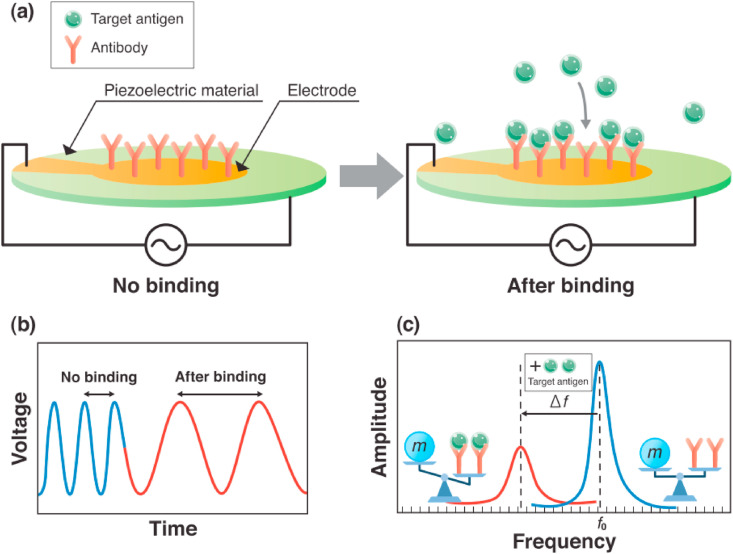
A piezoelectric biosensor is a type of label-free sensor that works on change in oscillation frequency upon biorecognition event [118].
These types of biosensors are based on piezoelectric materials, that can generate voltage when mechanically stressed. Such a sensitive sensor can even sense a very low femtogram of mass [118]. The surface of these piezoelectric crystals coated with receptors which on binding with the analyte, reduces the oscillation frequency of quartz-crystal material (Fig. 17 ) [117]. It is also used in the diagnosis of pathogenic microorganisms from various clinical samples [117]. In this current global pandemic situation, SARS-CoV-2 has spread almost everywhere. In such a scenario, the piezoelectric biosensor is an alluring device as it doesn't need any power source.
Fig. 17.
An overview on main approach for SARS-CoV-2 detection. (a) Cantilever as resonant sensor (piezoelectric material) detect the target viral antigen. Due to adsorption of viral antigen on fabricated antibody the mass changes lead to cantilever bending. That changes the signal. (b) voltage against time (c) amplitude against frequency. Reproduced from open-access article [117] under the terms of Creative Commons CC BY.
5.2.8. Magnetoelastic biosensor
Magnetic biosensors start to get some particular attention in the last few years.
These sensors are made from a ferromagnetic amorphous alloy. Wireless Sensors use a coil system where the signal is generated away from the coil and sensing is done remotely when the sample is subjected to the sensor [117]. The change in magnetic field causes magnetoelastic resonance, which is detected by a non-contact signal collector coil, [21]. Pietschmann et al. have developed a magnetic SARS-CoV-2 antibody detection set up based on immobilization of S protein on the sensor surface. They found the limit of detection of 3.36 ng/ml in the serum sample while 2.96 ng/ml in PBS [119]. Even so, having several advantages, magnetic immunosensors are usually ignored when it comes to POC diagnostics. One main disadvantage, associated with the magnetoelastic sensor is the dependence of magnetic labels for detection [117]. Nevertheless, scientists are trying to optimize that part so to make a quality POC market for magnetoelastic biosensors.
5.2.9. FET biosensor
The enzyme-electrode biochemical-based biosensor was first developed by Clark and Lyons in the year 1962 [147]. After that, several types of sensing mechanisms have been developed. Among these, a unique kind of sensing approach was discovered, i.e. FET-based biosensor because of rapid growth in solid-state technologies [148]. Field-effect transistor (FET) biosensors grew to be one of the most promising alternatives due to their advantages like being fast, low-cost, and simple. At the same time, the ability to make highly sensitive and instantaneous measurements using small amounts of analytes makes them a suitable candidate for use in point-of-care testing and diagnosis [149]. The very first FET biosensor was developed by Bergveld approximately fifty years ago and evolving since 1970 in different forms and is an ideal approach for swift and precise detection of various analytes [150].
To define, “a solid-state device in which the electroconductivity of the semiconductor between the source and drain terminals is regulated by a third gate electrode through an insulator is called a FET” [151]. Generally, there are two kinds of FETs based on prime charge carriers namely n-type and p-type, where electrons and holes are the charge carriers, respectively. In the case of a positively charged target molecule, there will be a drastic increase in conductance in the n-type sensor due to electron aggregation. In contrast, the conductance will decrease if the target is negatively charged. The reverse will be applicable for a p-type biosensor. To enhance the sensitivity, nano materials are deposited on the structure [152].
Seo et al. [153] have developed a graphene-based FET biosensor for rapid detection of COVID-19. This device can detect SARS-CoV-2 spike protein at concentrations of 1 fg/mL in phosphate-buffered saline and 100 fg/mL clinical transport medium. The limit of detection was 1.6 × 101 pfu/mL (in culture medium) and 2.42 × 102 copies/mL (in clinical samples). Additionally, this device needs no labeling process or sample pretreatment. 1-pyrenebutyric acid N-hydroxysuccinimide ester (PBASE) was used as a probe linker to immobilize the SARS-CoV-2 spike antibody with the graphene. Lastly, the device could distinguish the SARS-CoV-2 antigen protein from those of MERS-CoV. Zhang et al. [154] also fabricated graphene FET structure for the detection of SARS-CoV-2 spike protein S1 within 2 min with a minimum detection limit of 0.2 pM.
5.2.10. Microfluidic biosensor
Microfluidics refers to the technology for the fabrication of microminiaturized devices having micrometer-sized channels and chambers, in order to allow small volumes of fluids to flow [155,156]. In recent years, there has been a tremendous increase in the integration of biosensing with microfluidics. Advantages like small sample volumes, rapid turnaround times, ease of multiplexing, and high portability offered by microfluidics, which make it suitable for biosensing applications. Precise control of droplets gives rise to miniaturization and rapid processing of samples for the application of lab-on-a-chip diagnostics [157]. These microfluidic-based point-of-care (PoC) devices can detect analytes and may provide rapid diagnostics even in remote regions and near a patient with limited-resource or non-existing healthcare settings [158]. This happened because of the integration of POC devices with smartphones and wireless communications. The selection of materials for fabrication should be such that, it should be optically transparent, easily modifiable and mechanically strong, and thermally stable. A single material cannot fulfil all these requirements.
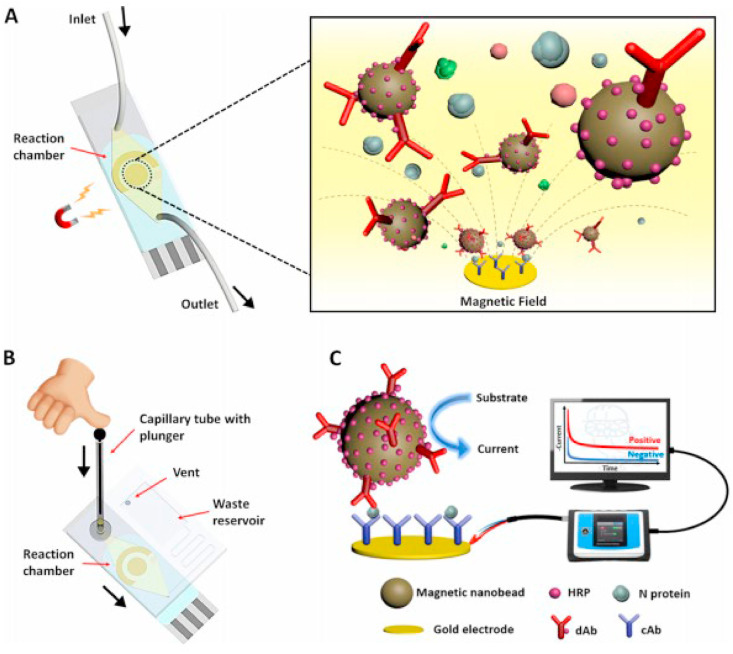
A unique sensing scheme was developed by Lillehoj and group [159] which utilizes dually-labeled magnetic nanobeads for immunomagnetic enrichment and signal amplification on a microfluidic chip to detect SARS-CoV-2 nucleocapsid protein (Fig. 18 ). It provides advantages of enhanced detection sensitivity, minimal sample, reagent consumption, and simplified sample handling. It could detect concentrations as low as 50 pg/mL in whole serum within 1 h and 10 pg/mL in 5 × diluted serum within half an hour. To add to its portability, the chip was connected to a smartphone using a smart potentiostat, which does not require external power sources.
Fig. 18.
Schematic illustrations of (A) microfluidic immunosensor chip highlighting the magnetic concentration of DMBs to the sensor surface, (B) microfluidic immunosensor chip for the smartphone-based diagnostic device, and (C) experimental setup and electrochemical sensing scheme using the PalmSens4-based sensing platform. Reprinted with permission from (Li, J.; Lillehoj, P. B. Microfluidic Magneto Immunosensor for Rapid, High Sensitivity Measurements of SARS-CoV-2 Nucleocapsid Protein in Serum. ACS Sensors2021, 6 (3), 1270–1278). Copyright (2021), American Chemical Society.
Hwang-soo et al. [160] proposed a different procedure to detect SARS-CoV-2 using isothermal amplification of nucleic acids by utilizing a mesh having multiple pores. Blockage of pore takes place by the formation of DNA hydrogel when hybridization of pathogen and probe DNA occurs. It has a limit of detection (LOD) of 0.7 aM at 15-min incubation for SARS-CoV-2. But, it is necessary to optimize the mesh material, pore size, and precision microfluidics for achieving such value.
6. Overview of biosensing platforms for SARS-CoV-2 detection
In view of supply chain issues related to testing reagents and sample transportation, the current era imposes a serious emergency condition for global supplies. The US Centers for Disease Control and Prevention (CDC) has updated the guidelines for clinical sample collection and transportation. CDC recommends the clinical sample to store at 2–8 °C for 72 h and afterward sample storage in particular viral transportation media at −70 °C [121]. These guidelines impose a huge difficulty to reach mass testing. Portable, automatic point of care devices eliminate the requirement for sample storage and transportation. Various smart POCT devices not only allow fast data acquisition but facilitate at-home diagnosis [143]. The gold standard qRT-PCR test prices RS 2500–3200 ($42.96) depending upon various states of India while the accurate paper-based ‘FELUDA’ costs only RS 500 ($6.80) [120]. Further scientists from IIT-Bombay have developed a printed circuit board (PCB) electrode-based electrochemical detection of SARS-CoV-2 with RS 40 ($0.55) [111]. Considering the current scenario, ICMR has approved a rapid antigen test which costs RS 250 ($3,32) and provides a result within 15 min [122]. In comparison with RT-PCR that even takes 3–4 days to get the result, the POCT platform is very fast and has opened the opportunity for quick mass diagnosis [143].
Further advancement of nano biosensors can exponentiate remaining detection-related concerns. Other than the decoration of sensor surface with nanoparticles, discussed above, polymer-coated biosensors were found to be very convenient for fast, accurate detection [11]. Polymers containing acrylic groups are mainly favored for biosensor fabrication.
Above all mentioned methods of SARS-CoV-2 LFA biosensors is the key player with a view to the commercial market. After the integration of powerful CRISPR-Cas technique with LFA has been in the focus of public attention. Such biosensor not only reveals high specificity and sensitivity but being a low cost and on-site diagnostic tool, a non-specialist person can perform the test in real-time. As a point of care, diagnostics can't be standalone with 100 % accuracy, it is better to run such a rapid antigen test, combined with gold-standard RT-PCR. To combat concerned issues, researchers are going to develop a ‘sample in -result out’ POC nucleic acid detection system. Such as IIT- Kharagpur scientists develop an isothermal nucleic acid testing-based SARS-CoV-2 detection platform, called COVIRAP, where the result can be interpreted with a free smartphone app [123]. To accelerate the detection and to come down with a more economical platform, microfluidics also joins the race. Researchers are trying to reduce the intricacy of PCR temperature control systems, with the help of a microfluidics system [124]. Here we have discussed some recent biosensors important for SARS-CoV-2 detection in Table 7 .
Table 7.
Some important biosensors developed recently for the detection of SARS-CoV-2.
| S.N | Detection method | Material | Analyte | Detection time | LOD | Detection range | |
|---|---|---|---|---|---|---|---|
| 1 | Magnetosensor | Glossy carbon electrodes and magnetic beads | Viral S/N protein | 30 min | 8 ng/ml for S protein 19 ng/ml for N protein |
[94] | |
| 2 | Electrochemical sensor | Gold electrode modified with Fe3O4 | RNA genome | Real time | 200 copies/ml | 10−17-10−12M | [125] |
| 4 | Optical biosensor | Optical fiber and gold nanoparticle modified protein | N protein | – | 1 pg/ml | 0.100 pg/ml −1 ng/ml | [126] |
| 5 | Field effect transistor | Graphene layer | S proteins | Real time | 1 fg/ml | [113] | |
| 6 | Breath sensor | ABI prism 700 instrument | N protein | 10 s | – | 10-106copies/ml | [127] |
| 7 | Piezoelectric immunosensor | Quartz crystal and magnetic particle | Helicase protein | 1 min | 3.5 ng/ml | 0.050–1.00 mg/ml | [128] |
| 8 | Laser scanning microscopy (Optical sensor) | Quantum dot fabricated aptamer | N protein | – | 0.1 pg/ml | 0.1–50 pg/ml | [129] |
| 9 | SPR biosensor | Gold chip/aptamer | RNA genome | Real time | 2 nM | 1nM/1 μM | [130] |
| 10 | Paper based sensor | COVID-19 ePad | Antibody | 45 min | 1 μg/ml | 0.1–50 μg/ml | [131] |
| 11 | SPR based colorimetric sensor | Oligonucleotide tagged gold nanoparticle | RNA genome | 10 min | 3.6–3.9 copies/ml | 0.2–3 ng/ml | [105] |
| 12 | Electrochemical biosensor | Reduced graphene oxide nanoflakes | Antibody | Within minutes | 2.8 × 1015 M (Spike protein) 16.9 × 1015 M (Receptor binding protein antibodies) | – | [132] |
7. Futuristic strategy for COVID-19 detection
There is a large number of kits and tools already present in the market but due to the lack of suitable properties as run time, cost, and complexity of technique it is important to develop more advanced POC tools. Among newly diagnostic tools developed for nucleic acid identification of COVID-19, LAMP (Loop mediated isothermal amplification) shows promising results compare to RT-PCR. Where PCR requires a sophisticated tool and facilities, this can be done at a constant temperature at 40-65-degree Celsius in the water bath [133].
RT-LAMP is based on the same principle where RNA of COVID-19 viruses is converted in cDNA and then amplified using 4 to 6 primers. LAMP is also integrated by Zhang's group [134] and Chiu's group [135] with CRISPR technology for the detection of COVID-19 viruses with the sensitivity of 10 copies/mL in POC format.
Another approach to develop a diagnostic kit can be based on Rolling Circle Amplification (RCA) [78]. This type of genome amplification method is generally found in viruses, which is also an isothermal method for nucleic acid identification in the sample. In this process, DNA is first circularized and then amplified with the high processive polymerase to achieve higher sensitivity. This method is used to develop a kit for COVID-19 diagnoses with higher sensitivity [21].
Lateral Flow-based assay for nucleic acid identification based on isothermal amplification can be an innovative option in developing diagnosis tools. There is a paper-based lateral flow assay (LFA) which have gain people's trust and popularity in the field of COVID-19 diagnoses [136]. Paper plastic-based LFA has been developed for malaria which uses folded paper for genome isolation and LAMP for genome amplification. 2D paper network-based LFAs have been also developed for COVID-19 detections.
The major problem associated with the immune-associated LFA is having less sensitivity [136]. It can be solved by various approaches some of them can be the use of colloidal gold particles. Solvent evaporation, Analyte pre-concentration, Ion concentration polarization methods. Another Novel approach to increase sensitivity, specificity, and rapidity of tests various approaches like proximity ligation Assay where aptamers and antibody used to target the viral genome [137]. DNA microarray-based tools can be also used for COVID-19 detection [138].
The rapid growth in the rate of infection due to COVID-19 every day, scarcity of appropriate diagnostic tools for early diagnosis of virus is an important concern for controlling the disease and preventing further spreading in society. The need for early diagnosis at a very low cost even in remote areas for global screening with an effective result is required, which can be achieved by the biosensor-based tool. These tools target specific molecules like protein or nucleic acid of the COVID-19 virus with high specificity and sensitivity [139]. The challenge with biosensors is to convert the complex principle into simpler forms that can not only decrease the cost of technology but also increase the demand in day-to-day life [140,146]. These tools must be user-friendly, as well. Despite the presence of several tools like CT scan, RT-PCR, Sequencing, and other, rapid diagnostic kits are essential in global screening and controlling the virus. Due to the various pros and cons of an existing tool for COVID-19 diagnosis, there is a huge need for further development of a biosensor that can fulfill the need of the present time to control and prevent the COVID-19 virus. There are multiple strategies developed in the field of a biosensor that can enhance the sensitivity and efficacy of the sensor. The parameter of these biosensors can be enhanced by either changing the surface property of the sensor, electrochemical property, and binding receptor of the sensor [141,142].
8. Conclusion
The advancement of diagnostic tools in COVID-19 diagnosis has enabled physicians and epidemiologists to screen and prevent COVID-19 from the spread on large scale. Various methods of COVID-19 diagnosis are developed and optimized based on the experience of previous pandemics due to SARS 2002 and MARS. Researchers and physicians from various geographical regions came together to solve the problem related to COVID-19 and each community helps to provide data and information related to COVID-19 on a common platform so that it became easy to develop and optimize various kits and tools for diagnosis. The sequence of the virus, later on, used to develop various PCR assays in the field of diagnostics which act as the first line of defense during the pandemic. In the second stage of the pandemic, various serological kits were developed from various industries as rapid diagnostic kits as stated in Table 3. These kits were based on the detection of either antibodies against the virus from the blood sample or antigen of the virus in the blood. Later during the pandemic, several other biosensors were developed which were mostly based on microfluidics, aptamer-based, probe-based, Barcoding, nanotechnology, isothermal amplification, CRISPR- Cas, which are easy to use and rapid in screening the SARS-CoV-2 virus. These Biosensors are considered promising tools for diagnostics and decision-making to save millions of lives during pandemics. The tools and advancements in the field of nanotechnology and microfluidics can help to develop various biosensors which will enable us to diagnose more rapidly with high sensitivity and specificity at the point of sit with a multidimensional approach to solve various problems during the pandemic. It has been observed that the rate of mutation in this virus is very high, so there is a higher chance of reemerging this virus from various mutant strains. The need for the biosensor in the field of diagnostics is highly demanding and will be very effective in the early detection of the virus.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Authors gratefully acknowledge the Start-Up Research Grant (SRG) funded by Science & Engineering Research Board (SERB) (SRG/2020/000712), Department of Science and Technology (DST) (Government of India, Ministry of Science and Technology, (Technology Development and Transfer, TDP/BDTD/12/2021/General), Indo-German Science & Technology Center (IGSTC) (IGSTC/Call 2019/NOMIS/22/2020–21/164), and Institute Scheme for Innovative Research and Development (ISIRD) (IIT/SRIC/ISIRD/2019–2020/17), Indian Institute of Technology Kharagpur (IIT Kharagpur), India for the financial support.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Tan W. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothan A.H., Byrareddy N.S. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Who Coronavirus disease 2019 (Covid-19): situation report − 52. https://www.who.int/docs/default-source/coronavirus/situation-reports/20200312-sitrep-52-Covid-19. Pdf ?sfvrsn=e2bfc9c0_4 2020-04-15.
- 5.Who Coronavirus disease 2019 (Covid-19): situation report − 69. https://www.who.int/docs/default-source/coronavirus/situation-reports/20200329-sitrep-69-Covid-19. pdf?sfvrsn=8d6620fa_4 2021-05-15.
- 6.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W., Tao Q., Sun Z., Xia L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J., Liu J., Li S., Peng Z., Xiao Z., Wang X., Yan R., Luo J. Detection and analysis of nucleic acid in various biological samples of COVID-19 patients. Trav. Med. Infect. Dis. 2020;101673 doi: 10.1016/j.tmaid.2020.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mobed A., Sepehri Shafigh E. Biosensors promising bio-device for pandemic screening “COVID-19“. Microchem. J. 2021;164:106094. doi: 10.1016/j.microc.2021.106094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhalla N., Jolly P., Formisano N., Estrela P. Introduction to biosensors. Essays Biochem. 2016;60(1):1–8. doi: 10.1042/ebc20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sangadevan S., Periasamy M. Recent trends in biosensors and their application. Revised Advance Material Science. 2014;36:62–69. doi: 10.1016/2Fj.jobcr.2015.12.002. [DOI] [Google Scholar]
- 11.Iravani S. Nano- and biosensors for the detection of SARS-CoV-2: challenges and opportunities. Mater. Adv. 2020;1(9):3092–3103. doi: 10.1039/D0MA00702A. [DOI] [Google Scholar]
- 12.Dutta G., Regoutz A., Moschou D. Enzyme-assisted glucose quantification for a painless Labon-PCB patch implementation. Biosens. Bioelectron. 2020;167:112484. doi: 10.1016/j.bios.2020.112484. [DOI] [PubMed] [Google Scholar]
- 13.Park S., Kim J., Ock H., Dutta G., Seo J., Shin E.C., Yang H. Sensitive electrochemical detection of vaccinia virus in a solution containing a high concentration of L-ascorbic acid. Analyst. 2015;140:5481–5487. doi: 10.1039/C5AN01086A. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen T., Bang D.D., Wolff A. Novel coronavirus disease (COVID-19): paving the road for rapid detection and point-of-care diagnostics. Micromachines. 2019;11(3):306. doi: 10.3390/mi11030306. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eastin C., Eastin T. Clinical characteristics of coronavirus disease 2019 in China. J. Emerg. Med. 2020;58(4):711–712. doi: 10.1016/j.jemermed.2020.04.004. [DOI] [Google Scholar]
- 16.Zeng L.K., Tao X.W., Yuan W.H., Wang J., Liu X., Liu Z.S. First case of neonate infected with novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58 doi: 10.3760/cma.j.issn.0578-1310.2020.0009. E009. [DOI] [PubMed] [Google Scholar]
- 17.Lu C.W., Liu X.F., Jia Z.F. nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/s0140-6736(20)30313-5. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savvides C., Siegel R. Asymptomatic and presymptomatic Transmission of SARS-CoV-2: a systematic review. Epidemiology. 2020;2:1–27. doi: 10.1101/2020.06.11.20129072. Preprint. [DOI] [Google Scholar]
- 19.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021;54(2):159–163. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu N., Zhang D., Wang W., Li X., Yang B., Zhan F., Ma X., Wang D. China novel coronavirus investigating and research team, A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Gao Z., Jin Q., Wang J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dveksler G.S., Pensiero M.N., Cardellichio C.B., Williams R.K., Jiang G.S., Holmes K.V., Dieffenbach C.W. Cloning o the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J.Virol. 1991;65(12):6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams R.K., Jiang G.S., Holmes K.V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc. Natl. Acad. Sci. Unit. States Am. 1991;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultze B., Gross H.-J., Brossmer R., HERRLERl G. The S protein of bovine coronavirus is a hemagglutinin recognizing 9-0-acetylated sialic acid as a receptor determinant. J. Virol. 1991;65:6. doi: 10.1128/jvi.65.11.6232-6237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu A., Peng Y., Huang B., Ding X. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rambaut A., Drummond A.J., Xie D., Baele G., Suchard M.A. Posterior summarization in bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018;67(5):901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funk C.D., Laferrière C., Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front. Pharmacol. 2020;11:937. doi: 10.3389/fphar.2020.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S., Zhang Y., Guan Z., Li H., Ye M., Chen X., Shen J., Zhou Y., Shi Z.-L., Zhou P., Peng K. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Sig Transduct Target Ther. 2020;5(1):235. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H., Dai H., Xie X. Solid organ transplantation during the COVID-19 pandemic. Front. Immunol. 2020;11:1392. doi: 10.3389/fimmu.2020.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mousavizadeh L., Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021;54(2):159–163. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdelrahman Z., Li M., Wang X. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza A respiratory viruses. Front. Immunol. 2020;11:552909. doi: 10.3389/fimmu.2020.552909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baud D., Qi X., Saines K.N., Musso D., Pomar L., Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. 2020;20:773. doi: 10.1016/s1473-3099(20)30195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.https://www.who.int/news-room/q-a-detail/sars-cov-2-evolution
- 36.Liu R., Fu A., Deng Z., Li Y., Liu T. Promising methods for detection of novel coronavirus SARS-CoV-2. View. 2020;1(1) doi: 10.1002/viw2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab Sci. 2020;57(6):389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren X., Liu Y., Chen H., Liu W., Guo Z., Zhang Y., Chen C., Zhou J., Xiao Q., Jiang G., Shan H. Application and optimization of RT-PCR in diagnosis of SARS-CoV-2 infection. medRxiv. 2020;1:1–29. doi: 10.1101/2020.02.25.20027755. Preprint. [DOI] [Google Scholar]
- 39.Giri B., Pandey S., Shrestha R., Pokharel K., Ligler F.S., Neupane B.B. Review of analytical performance of COVID-19 detection methods. Anal. Bioanal. Chem. 2021;413(1):35–48. doi: 10.1007/s00216-020-02889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Law J.W., Chan K.G., Lee L.H. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front. Microbiol. 2015;5:770. doi: 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernheim A., Mei X. Chest CT findings in coronavirus disease-19 (Covid-19): relationship to duration of infection. Radiology. 2020;295:685–691. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alafif T., Tehame A.M., Bajaba S., Barnawi A., Zia S. Machine and deep learning towards COVID-19 diagnosis and treatment: survey, challenges, and future directions. IJERPH. 2021;18(3):1117. doi: 10.3390/ijerph18031117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: what we know. Int. J. Infect. Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu C.Y., Chan K.G., Yean C.Y., Ang G.Y. Nucleic acid-based diagnostic tests for the detection SARS-CoV-2: an update. Diagnostics. 2021;11(1):53. doi: 10.3390/diagnostics11010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Instructions for use: qSanger-COVID-19 assay. http://www.billiontoone.com
- 46.Bueno O., Atay D., Tsao S. A highly scalable and rapidly deployable RNA extraction-free COVID-19 assay by quantitative sanger sequencing. bioRxiv. 2020;1:1–15. doi: 10.1101/20204.07.029199. Preprint. [DOI] [Google Scholar]
- 47.Udugama B., Kadhiresan P., Gubbay J., Chan Diagnosing covid-19: the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 48.https://indianexpress.com/article/explained/explained-the-ct-value-in-a-covid-test-7291682
- 49.Funk C.D., Laferrière C., Ardakani A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front. Pharmacol. 2020;11:937. doi: 10.3389/fphar.2020.00937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanji J.N., Zelyas N., MacDonald C., Pabbaraju K., Khan M.N., Prasad A., Hu J., Diggle M., Berenger B.M., Tipples G. False negative rate of COVID-19 PCR testing: a discordant testing analysis. Virol. J. 2021;18(1):13. doi: 10.1186/s12985-021-01489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao A.T., Tong Y.X., Gao C., Zhu L., Zhang Y.J., Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in wuhan, China: a descriptive study. J. Clin. Virol. 2020;127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alcoba-Florez J., González-Montelongo R., Íñigo-Campos A., de Artola D.G.-M., Gil-Campesino H. The microbiology technical support team. Int. J. Infect. Dis. 2020;97:66–68. doi: 10.1016/j.ijid.2020.05.099. Ciuffreda, L.; Valenzuela-Fernández, A.; Flores, C. Fast SARS-CoV-2 Detection by RT-QPCR in Preheated Nasopharyngeal Swab Samples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganbaatar U., Liu C. CRISPR-based COVID-19 testing: toward next-generation point-of-care diagnostics. Front. Cell. Infect. Microbiol. 2021;11:663949. doi: 10.3389/fcimb.2021.663949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.-Y., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. CRISPR–Cas12-Based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38(7):870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahimi H., Salehiabar M., Barsbay M., Ghaffarlou M., Kavetskyy T. CRISPR systems for COVID-19 diagnosis. ACS Sens. 2021;6(4):1430–1445. doi: 10.1021/acssensors.0c0231. [DOI] [PubMed] [Google Scholar]
- 56.Zhang, F.; Abudayyeh, O. O.; Gootenberg, J. S. A Protocol for Detection of COVID-19 Using CRISPR Diagnostics. vol. 8.
- 57.Rauch J.N., Valois E., Solley S.C., Braig F., Lach R.S., Audouard M., Ponce-Rojas J.C., Costello M.S., Baxter N.J., Kosik K.S., Arias C., Acosta-Alvear D., Wilson M.Z. A scalable, easy-to-deploy protocol for cas13-based detection of SARS-CoV-2 genetic material. J. Clin. Microbiol. 2021;59(4):8. doi: 10.1128/JCM.02402-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo L., Sun X., Wang X., Liang C., Jiang H., Gao Q., Dai M., Qu B., Fang S., Mao Y., Chen Y., Feng G., Gu Q., Wang R.R., Zhou Q., Li W. SARS-CoV-2 detection with CRISPR diagnostics. Cell Discov. 2020;6(1):34. doi: 10.1038/s41421-020-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali Z., Aman R., Mahas A., Rao G.S., Tehseen M., Marsic T., Salunke R., Subudhi A.K., Hala S.M., Hamdan S.M., Pain A., Alofi F.S., Alsomali A., Hashem A.M., Khogeer A., Almontashiri N.A.M., Abedalthagafi M., Hassan N., Mahfouz M.M. ISCAN: an RT-LAMP-coupled CRISPR-cas12 module for rapid, sensitive detection of SARS-CoV-2. Virus Res. 2020;288:198129. doi: 10.1016/j.virusres.2020.198129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen L.T., Smith B.M., Jain P.K. Enhancement of trans-cleavage activity of Cas12a with engineered CrRNA enables amplified nucleic acid detection. Nat. Commun. 2020;11(1):4906. doi: 10.1038/s41467-020-18615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding X., Yin K., Li Z., Liu C. All-in-One dual CRISPR-cas12a (AIOD-CRISPR) assay: a Case for rapid, Ultrasensitive and visual Detection of novel coronavirus SARS-CoV-2 and HIV virus; preprint. Biochemistry. 2020;1:1–19. doi: 10.1101/2020.03.19.998724. [DOI] [Google Scholar]
- 62.Azhar Mohd, Phutela R., Ansari A.H., Sinha D., Sharma N., Kumar M., Aich M., Sharma S., Rauthan R., Singhal K., Lad H., Patra P.K., Makharia G., Chandak G.R., Chakraborty D., Maiti S. Rapid, field-deployable nucleobase Detection and identification using FnCas9. Mol. Biol. 2020;1:1–28. doi: 10.1101/2020.04.07.028167. Preprint. [DOI] [Google Scholar]
- 63.Huang Z., Tian D., Liu Y., Lin Z., Lyon C.J., Lai W., Fusco D., Drouin A., Yin X., Hu T., Ning B. Ultra-sensitive and high-throughput CRISPR-p owered COVID-19 diagnosis. Biosens. Bioelectron. 2020;164:112316. doi: 10.1016/j.bios.2020.112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mashimo T. Rapid and accurate detection of novel coronavirus SARS-CoV-2 using CRISPR-cas3. medRxiv. 2020;1:1–30. doi: 10.1101/2020.06.02.20119875. Preprint. [DOI] [Google Scholar]
- 65.Shaffaf T., Ghafar-Zadeh E. COVID-19 diagnostic strategies. Part I: nucleic acid-based technologies. Bioengineering. 2021;8(4):49. doi: 10.3390/bioengineering8040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alanagreh L., Alzoughool F., Atoum M. The human coronavirus disease COVID-19: its origin, characteristics, and insights into potential Drugs and its mechanisms. Pathogens. 2020;9(5):331. doi: 10.3390/pathogens9050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kassanjee Reshma. Viral load criteria and threshold optimization to improve HIV incidence assay characteristics. AIDS (London, England) 2016;30:2361–2371. doi: 10.1097/qad.0000000000001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bosch I., de Puig H., Hiley M., Carré-Camps M., Gehrke L. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci. Transl. Med. 2017;9:1589. doi: 10.1126/scitranslmed.aan1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dutta G., Lillehoj P.B. Wash-free, label-free immunoassay for rapid electrochemical detection of PfHRP2 in whole blood samples. Sci. Rep. 2018;8(1):17129. doi: 10.1038/s41598-018-35471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lassaunière R., Frische A., Harboe Z.B., Nielsen A.C.Y., Fomsgaard A., Krogfelt K.A., Jørgensen C.S. Evaluation of nine commercial SARS-CoV-2 immunoassays. Infectious Diseases (except HIV/AIDS) 2020;1:1–15. doi: 10.1101/2020.04.09.20056325. Preprint. [DOI] [Google Scholar]
- 71.Ghaffari A., Meurant R., Ardakani A. COVID-19 serological tests: how well do they actually perform? Diagnostics. 2020;10(7):453. doi: 10.3390/diagnostics10070453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dutta G., Kim S., Park S., Yang H. Washing-free heterogeneous immunosensor using proximity-dependent electron mediation between an enzyme label and an electrode. Anal. Chem. 2014;86:4589. doi: 10.1021/ac5006487. [DOI] [PubMed] [Google Scholar]
- 73.Dutta G., Park S., Singh A., Seo J., Kim S., Yang H. Low-interference washing-free electrochemical immunosensor using glycerol-3-phosphate dehydrogenase as an enzyme label. Anal. Chem. 2015;87:3574. doi: 10.1021/ac504485a. [DOI] [PubMed] [Google Scholar]
- 74.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Chen L., Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C., Cai J.P., Chan J.M., Chik T.S., Lau D.P., Choi C.Y., Chen L.L., Yuen K.Y. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiang J., Yan M., Li H., lin C. Evaluation of enzyme-linked immunoassay and colloidal GoldImmunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (Covid-19) medRxiv. 2020;1:1–13. doi: 10.1101/2020.02.27.20028787. Preprint. [DOI] [Google Scholar]
- 78.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Emergency use authorization from FDA. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics euashttp://ph.china-embassy.org/eng/sgdt/t1760281
- 80.https://www.fortunebusinessinsights.com/industry-reports/point-of-care-diagnostics-market-101072
- 81.Land K.J., Boeras D.I., Chen X.-S., Ramsay A.R., Peeling R.W. REASSURED diagnostics to inform disease control strategies, strengthen Health systems and improve patient outcomes. Nat Microbiol. 2019;4(1):46–54. doi: 10.1038/s41564-018-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maddali H., Miles C.E., Kohn J., O'Carroll D.M. Optical biosensors for virus detection: prospects for SARS-CoV-2/COVID-19. Chembiochem. 2021;22(7):1176–1189. doi: 10.1002/cbic.202000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoo E.-H., Lee S.-Y. Glucose biosensors: an overview of use in clinical practice. Sensors. 2010;10(5):4558–4576. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chao J., Zhu D., Zhang Y., Wang L., Fan C. DNA nanotechnology-enabled biosensors. Biosens. Bioelectron. 2016;76:68–79. doi: 10.1016/j.bios.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Doretti L., Ferrara D., Lora S., Schiavon F., Veronese F.M. Acetylcholine biosensor involving entrapment of acetylcholinesterase and poly(ethylene glycol)-modified choline oxidase in a poly(vinyl alcohol) cryogel membrane. Enzym. Microb. Technol. 2000;27:279–285. doi: 10.1016/s0141-0229(00)00210-6. [DOI] [PubMed] [Google Scholar]
- 86.Gupta R., Chaudhury N.K. Entrapment of biomolecules in sol-gel matrix for applications in biosensors: problems and future prospects. Biosens. Bioelectron. 2007;22:2387–2399. doi: 10.1016/j.bios.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 87.Rajesh V. Bisht, Takashima W., Kaneto K. An amperometric urea biosensor based on covalent immobilization of urease onto an electrochemically prepared copolymer poly (N-3-aminopropyl pyrrole-co-pyrrole) film. Biomaterials. 2005;26:3683–3690. doi: 10.1016/j.biomaterials.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 88.Thévenot D.R., Toth K., Durst R.A., Wilson G.S. Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron. 2001;16:121–131. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 89.Mohanty S.P., Kougianos E. Biosensors: a tutorial review. IEEE Potentials. 2006;25:35–40. doi: 10.1109/MP.2006.1649009. [DOI] [Google Scholar]
- 90.Turner A.P. Biosensors-sense and sensitivity. Science. 2000;290:1315–1317. doi: 10.1126/science.290.5495.1315. [DOI] [PubMed] [Google Scholar]
- 91.Antiochia R. Developments in biosensors for CoV detection and future trends. Biosens. Bioelectron. 2021;173:112777. doi: 10.1016/j.bios.2020.112777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma H., Mutharasan R. Review of Biosensors for food borne pathogens and toxins. Sensor. Actuator. B Chem. 2013;183:535–549. doi: 10.1016/j.snb.2013.03.137. [DOI] [Google Scholar]
- 93.Imran S., Ahmadi S., Kerman K. Electrochemical biosensors for the detection of SARS-CoV-2 and other viruses. Micromachines. 2021;12(2):174. doi: 10.3390/mi12020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fabiani L., Saroglia M., Galatà G., De Santis R., Fillo S., Luca V., Faggioni G., D'Amore N., Regalbuto E., Salvatori P., Terova G., Moscone D., Lista F., Arduini F. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: a reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021;171:112686. doi: 10.1016/j.bios.2020.112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Samson R., Navale G.R., Dharne M.S. Biosensors: frontiers in rapid detection of COVID-19. 3 Biotech. 2020;10(9):385. doi: 10.1007/s13205-020-02369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Majumdar T., Raychaudhuri U., Chakraborty R. Detection of food borne pathogens. Int J Adv Biol Res. 2015;5:96–107. doi: 10.12691/nnr-6-1-1. [DOI] [Google Scholar]
- 97.Lukose J., Chidangil S., George S.D. Optical technologies for the detection of viruses like COVID-19. Progress and prospects. 2021;178:11304. doi: 10.1016/j.bios.2021.113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 99.Ramakrisna B., Sai V.V.R. Vol. 226. 2016. pp. 184–190. (Evanescent wave absorbance based U-bent fiber probe for immuno biosensor with gold nanoparticle labels). [DOI] [Google Scholar]
- 100.http://zeiss-campus.magnet.fsu.edu/tutorials/spectralimaging/fretbiosensors/indexflash.html
- 101.Weng X., Neethirajan S. Vol. 18. 2018. pp. 4358–4363. (Immunosensor Based on Antibody-Functionalized MoS2 for Rapid Detection of Avian Coronavirus on Cotton Thread). 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown A.S., Ackerley D.F., Calcott M.J. High-throughput screening for inhibitors of the SARS-CoV-2 protease using a FRET-biosensor. Molecules. 2020;25(20):4666. doi: 10.3390/molecules25204666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rubab M., Shahbaz H.M., Olaimat A.N., Oh D.H. Biosensors for rapid and sensitive detection of Staphylococcus aureus in food. Biosens. Bioelectron. 2018;105:49–57. doi: 10.1016/j.bios.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 104.Yuan J., Wu S., Duan N., Ma X., Xia Y., Chen J., Ding Z., Wang Z. A sensitive gold nanoparticle-based colorimetric aptasensor for Staphylococcus aureus. Talanta. 2014;127:163–168. doi: 10.1016/j.talanta.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 105.Moitra P., Alafeef M., Dighe K., Frieman M.B., Pan D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano. 2020;14(6):7617–7627. doi: 10.1021/acsnano.0c03822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ventura B.D., Cennamo M., Minopoli A., Campanile R., Censi S.B., Terracciano D., Portella G., Velotta R. Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. ACS Sens. 2020;5(10):3043–3048. doi: 10.1021/acssensors.0c01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahshid S.S., Flynn S.E., Mahshid S. The potential application of electrochemical biosensors in the COVID-19 pandemic: a perspective on the rapid diagnostics of SARS-CoV-2. Biosens. Bioelectron. 2021;176:112905. doi: 10.1016/j.bios.2020.112905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naik M.K., Srinivas D., Sasi B., Jakeer Basha S.K. Biosensors in food processing-A review. Int J Pure App Biosci. 2017;5:1219–1227. [Google Scholar]
- 109.Mahari S., Roberts A., Shahdeo D., Gandhi S. Bioengineering; 2020. ECovSens-Ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of NCovid-19 antigen, a spike protein domain 1 of SARS-CoV-2; preprint. [DOI] [Google Scholar]
- 110.Tian J., Liang Z., Hu O., He Q., Sun D., Chen Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta. 2021;387:138553. doi: 10.1016/j.electacta.2021.138553. [DOI] [Google Scholar]
- 111.Kumar M.S., Nandeshwar R., Lad S.B., Megha K., Mangat M., Butterworth A., Knapp C.W., Knapp M., Hoskisson P.A., Corrigan D.K., Ward A.C., Kondabagil K., Tallur S. Electrochemical sensing of SARS-CoV-2 amplicons with PCB electrodes. Sensor. Actuator. B Chem. 2021;343:130169. doi: 10.1016/j.snb.2021.130169. [DOI] [Google Scholar]
- 112.Imran S., Ahmadi S., Kerman K. Electrochemical biosensors for the detection of SARS-CoV-2 and other viruses. Micromachines. 2021;12(2):174. doi: 10.3390/mi12020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., Lee C.-S., Jun S., Park D., Kim H.G., Kim S.-J., Lee J.-O., Kim B.T., Park E.C., Kim S.I. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 114.Raziq A., Kidakova A., Boroznjak R., Reut J., Öpik A., Syritski V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021;178:113029. doi: 10.1016/j.bios.2021.113029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eissa S., Zourob M. Development of a low-cost cotton-tipped electrochemical immunosensor for the detection of SARS-CoV-2. Anal. Chem. 2021;93(3):1826–1833. doi: 10.1021/acs.analchem.0c04719. [DOI] [PubMed] [Google Scholar]
- 116.Srivastava A.K., Dwivedi N., Dhand C., Khan R., Sathish N., Gupta M.K., Kumar R., Kumar S. Potential of graphene-based materials to combat COVID-19: properties, perspectives, and prospects. Materials Today Chemistry. 2020;18:100385. doi: 10.1016/j.mtchem.2020.100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Narita F., Wang Z., Kurita H., Li Z., Shi Y., Jia Y., Soutis C. A review of piezoelectric and magnetostrictive biosensor materials for detection of COVID-19 and other viruses. Adv. Mater. 2021;33(1):2005448. doi: 10.1002/adma.202005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pohanka M. Overview of piezoelectric biosensors. Immunosensors and DNA Sensors and Their Applications. 2018;18:448. doi: 10.3390/ma11030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pietschmann J., Vöpel N., Spiegel H., Krause H.-J., Schröper F. Brief communication: magnetic immuno-Detection of SARS-CoV-2 specific antibodies. Mol. Biol. 2020;1:1–16. doi: 10.1101/2020.06.02.131102. Preprint. [DOI] [Google Scholar]
- 120.https://www.milenia-biotec.com/en/feluda-test-rapid-covid-detection
- 121.https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
- 122.https://www.hindustantimes.com/india-news/india-approves-home-test-for-covid-19-to-cost-250-per-kit-101621452395819.html
- 123.https://www.thehindu.com/sci-tech/health/iit-kharagpur-launches-covirap-diagnostic-technology/article34376251.ece
- 124.Wu J., Kodzius R., Xiao K., Qin J., Wen W. Fast detection of genetic information by an optimized PCR in an interchangeable chip. Biomed. Microdevices. 2012;14(1):179–186. doi: 10.1007/s10544-011-9595-6. [DOI] [PubMed] [Google Scholar]
- 125.Zhao H., Liu F., Xie W., Zhou T.-C., OuYang J., Jin L., Li H., Zhao C.-Y., Zhang L., Wei J., Zhang Y.-P., Li C.-P. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sensor. Actuator. B Chem. 2021;327:128899. doi: 10.1016/j.snb.2020.128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang J.C., Chang Y.-F., Chen K.-H., Su L.-C., Lee C.-W., Chen C.-C., Chen Y.-M.A., Chou C. Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in human serum using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosens. Bioelectron. 2009;25(2):320–325. doi: 10.1016/j.bios.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li X., Li J., Ge Q., Du Y., Li G., Li W., Zhang T., Tan L., Zhang R., Yuan X., Zhang H., Zhang C., Liu W., Ding W., Sun L., Chen K., Wang Z., Shen N., Lu J. Detecting SARS-CoV-2 in the breath of COVID-19 patients. Front. Med. 2021;8:604392. doi: 10.3389/fmed.2021.604392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Albano D.R.B., Shum K., Tanner J.A., Fung Y.S. Proceedings IMCS 2018; AMA Service GmbH, Von-Münchhausen-Str. vol. 49. Wunstorf; Germany: Vienna, Austria: 2018. BS5.3 - piezoelectric quartz crystal aptamer biosensor for detection and quantification of SARS CoV helicase protein; pp. 211–213. 31515. [DOI] [Google Scholar]
- 129.Roh C., Jo S.K. Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip. J. Chem. Technol. Biotechnol. 2011;86(12):1475–1479. doi: 10.1002/jctb.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shi L., Sun Q., He J., Xu H., Liu C., Zhao C., Xu Y., Wu C., Xiang J., Gu D., Long J., Lan H. Development of SPR biosensor for simultaneous detection of multiplex respiratory viruses. BME. 2015;26(s1):S2207–S2216. doi: 10.3233/BME-151526. [DOI] [PubMed] [Google Scholar]
- 131.Yakoh A., Pimpitak U., Rengpipat S., Hirankarn N., Chailapakul O., Chaiyo S. Paper-based electrochemical biosensor for diagnosing COVID-19: detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021;176:112912. doi: 10.1016/j.bios.2020.112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ali Md A., Hu C., Jahan S., Yuan B., Saleh M.S., Ju E., Gao S., Panat R. Sensing of COVID-19 antibodies in seconds via aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes. Adv. Mater. 2021;33(7):2006647. doi: 10.1002/adma.202006647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Afzal A. Molecular diagnostic technologies for COVID-19: limitations and challenges. J. Adv. Res. 2020;26:149–159. doi: 10.1016/j.jare.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Guevara H., Wadford Da J.S., Chen C.Y., Chiu C.Y. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38(7):870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.oczula K.M., Gallotta A. Lateral flow assays. Essays Biochem. 2016;60:111–120. doi: 10.1042/ebc20150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Greenwood C., Ruff D., Kirvell S., Johnson G., Dhillon H.S., Bustin S.A. Proximity assays for sensitive quantification of proteins. Biomol Detect Quantif. 2015;4:10. doi: 10.1016/j.bdq.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khan M.J., Trabuco A.C., Alfonso H.L., Figueiredo M.L., Batista W.C., Badra S.J., Figueiredo L.T., Lavrador M.A., Aquino V.H. DNA microarray platform for detection and surveillance of viruses transmitted by small mammals and arthropods. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dutta G., Lillehoj P.B. An ultrasensitive enzyme-free electrochemical immunosensor based on redox cycling amplification using methylene blue. Analyst. 2017;142:3492. doi: 10.1039/C7AN00789B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dutta G., Jallow A., Paul D., Moschou D. Label-Free electrochemical detection of S. Mutans exploiting commercially fabricated printed circuit board sensing electrodes. Micromachines. 2019;10:575. doi: 10.3390/mi10090575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rangayasami A., Kannan K., Murugesan S., Radhika D., Sadasivuni K.K., Reddy K.R., Raghu A.V. Influence of nanotechnology to combat against COVID-19 for global Health emergency: a review. Sensors International. 2021;2:100079. doi: 10.1016/j.sintl.2020.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Agrahari R., Mohanty S., Vishwakarma K., Nayak S.K., Samantaray D., Mohapatra S. Update vision on COVID-19: structure, immune pathogenesis, treatment and safety assessment. Sensors International. 2021;2:100073. doi: 10.1016/j.sintl.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Konwar A.N., Borse V. Current status of point-of-care diagnostic devices in the Indian healthcare system with an update on COVID-19 pandemic. Sensors International. 2020;1:100015. doi: 10.1016/j.sintl.2020.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mishra N.P., Das S.S., Yadav S., Khan W., Afzal M., Alarifi A., kenawy E.-R., Ansari M.T., Hasnain M.S., Nayak A.K. Global impacts of pre- and post-COVID-19 pandemic: focus on socio-economic consequences. Sensors International. 2020;1:100042. doi: 10.1016/j.sintl.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chandra P. Miniaturized label-free smartphone assisted electrochemical sensing approach for personalized COVID-19 diagnosis. Sensors International. 2020;1:100019. doi: 10.1016/j.sintl.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Behera S., Rana G., Satapathy S., Mohanty M., Pradhan S., Panda M.K., Ningthoujam R., Hazarika B.N., Singh Y.D. Biosensors in diagnosing COVID-19 and recent development. Sensors International. 2020;1:100054. doi: 10.1016/j.sintl.2020.100054. [DOI] [Google Scholar]
- 147.Clark L.C., Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962;102(1):29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 148.Syu Y.-C., Hsu W.-E., Lin C.-T. Review—field-effect transistor biosensing: devices and clinical applications. ECS J. Solid State Sci. Technol. 2018;7(7):Q3196–Q3207. doi: 10.1149/2.0291807jss. [DOI] [Google Scholar]
- 149.Syedmoradi L., Ahmadi A., Norton M.L., Omidfar K. A review on nanomaterial-based field effect transistor technology for biomarker detection. Microchim. Acta. 2019;186(11) doi: 10.1007/s00604-019-3850-6. [DOI] [PubMed] [Google Scholar]
- 150.Bergveld P. Development of an ion-sensitive solid-state. IEEE Trans. Biomed. Eng. 1970:70–71. doi: 10.1109/tbme.1970.4502688. January. [DOI] [PubMed] [Google Scholar]
- 151.Gubala V., Harris L.F., Ricco A.J., Tan M.X., Williams D.E. Point of care diagnostics: status and future. Anal. Chem. 2012;84(2):487–515. doi: 10.1021/ac2030199. [DOI] [PubMed] [Google Scholar]
- 152.Vu C. vol. 22. 2019. (Field-E Ff Ect Transistor Biosensors for Biomedical). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seo G., Lee G., Kim M.J., Baek S.H., Choi M., Ku K.B., Lee C.S., Jun S., Park D., Kim H.G., Kim S.J., Lee J.O., Kim B.T., Park E.C., Kim S. Il. Rapid Detection of COVID-19 Causative Virus (Sars-CoV-2) in Human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 154.Zhang X., Qi Q., Jing Q., Ao S., Zhang Z., Ding M., Wu M., Liu K., Wang W., Ling Y., Zhang Z., Fu W. Electrical probing of COVID-19 spike protein receptor binding domain via a graphene field-effect transistor. 2020;1–20 [Google Scholar]
- 155.Arduini F., Cinti S., Scognamiglio V., Moscone D., Palleschi G. Analytica chimica acta how cutting-edge technologies impact the design of electrochemical ( bio ) sensors for environmental analysis . A review. Anal. Chim. Acta. 2017;959:15–42. doi: 10.1016/j.aca.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 156.Id B.K.G., Id A.R.J., Id C.J.L., Goenner B.L. A review of current methods in microfluidic device fabrication and future commercialization prospects. 2018. [DOI]
- 157.Review F., Oliveira O.N. Plasmonic biosensing focus review. 2018. [DOI] [PubMed]
- 158.Estrela P., Kennedy R.O. 2015. Point-of-Care diagnostics in low resource settings: present status and future role of microfluidics; pp. 577–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li J., Lillehoj P.B. Microfluidic Magneto immunosensor for rapid, high sensitivity measurements of SARS-CoV-2 nucleocapsid protein in serum. ACS Sens. 2021;6(3):1270–1278. doi: 10.1021/acssensors.0c02561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim H., Abbas N., Shin S. Biosensors and bioelectronics A rapid diagnosis of SARS-CoV-2 using DNA hydrogel formation on microfluidic pores. Biosens. Bioelectron. 2021;177(January):113005. doi: 10.1016/j.bios.2021.113005. [DOI] [PMC free article] [PubMed] [Google Scholar]