Abstract
Background
Data regarding the association of antibody levels elicited after immunization with the BNT162b2 mRNA COVID-19 vaccine with epidemiological and clinical parameters are limited.
Methods
We prospectively measured the total (TAbs-RBD) and the neutralizing antibodies (NAbs-RBD) against the receptor binding domain (RBD) of SARS-CoV-2 spike protein in a cohort of 268 Healthcare workers before immunization, 20 days after the 1st dose and 30 days after the 2nd dose of the BNT162b2 vaccine. A statistical analysis for possible association of antibodies’ levels with epidemiological and clinical parameters was performed.
Results
The mean age (±SD) of the participants was 45.45 years (±11.93) (range: 24–70 years) and 211 (79.9%) were females. Statistically significant differences were detected regarding both TAbs-RBD and NAbs-RBD between the first and second doses of the vaccine (P < 0.001). The median (IQR) percentage (%) of NAbs-RBD after the 1st dose was 51.07% (31.60%) and after the 2nd dose 95.31% (3.70%) (P < 0.001). The correlation between the TAbs-RBD and NAbs-RBD was after the 1st dose, Spearman’s, rho: 0.861 (P < 0.001) and after the 2nd dose rho: 0.989 (P < 0.001). Twenty days after the 1st dose, 56/264 (21.2%) of the participants had low levels of NAbs-RBD, while one month after the 2nd dose all of them had protective levels of NAbs-RBD. After the 2nd vaccine dose, a statistically significant negative association of TAbs-RBD was detected for age (P < 0.001), smoking (P = 0.011), and immunosuppressive medications (P < 0.001), while a positive association was detected for BMI (P = 0.004) and systemic adverse events after immunization (P = 0.001).
Conclusion
A significant correlation of TAbs-RBD and NAbs-RBD was detected after both vaccine doses. Older age, smoking, and immunosuppressive medications negatively affected the final antibody level after SARS-CoV-2 immunization. Our findings emphasize the significance of the 2nd vaccine dose especially in the older age groups.
Keywords: SARS-CoV-2, COVID-19, BNT162b2, Antibody, Immunity, Spike protein
1. Introduction
The speed of development and approval of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines in response to the COVID-19 pandemic represent an extraordinary scientific accomplishment [1], [2]. Several vaccine designs have been developed based on different platforms and mRNA SARS-CoV-2 vaccines were the first to be authorized [3], [4]. Clinical trials as well as post-trials data of the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) have been shown that is a highly efficacious and safe vaccine [4], [5], [6]. Data from a mass vaccination program in Israel revealed that the effectiveness of the BNT162b2 vaccine more than 7 days after the second dose was 94% against symptomatic infection and 92% against severe disease with COVID-19 [6].
However, several questions regarding the immunological response to SARS-CoV-2 vaccines have not been answered yet. Establishing the immunological correlates of protection from SARS-CoV-2 infection and the clinical and epidemiological factors that affecting them are important for public health considerations, evaluation of new vaccines and patient management [7].
Real world data regarding the association of antibody levels elicited after immunization with the BNT162b2 mRNA COVID-19 vaccine with detailed epidemiological and clinical parameters are limited.
In the present study, we aimed to investigate the association of total and neutralizing antibodies against the receptor binding domain (RBD) of SARS-CoV-2 spike protein with epidemiological and clinical parameters in a cohort of healthcare workers (HCWs) after the 1st and 2nd dose of the BNT162b2 vaccine.
2. Materials and methods
2.1. Study design and participants
This was a prospective cohort study involving healthcare workers (HCWs) of the largest tertiary pediatric hospital in Athens, Greece (“Aghia Sophia” Children’s Hospital). The cohort of the study included healthcare professionals (medical doctors, nurses, technicians) and nonmedical personnel of the hospital who were vaccinated for SARS-CoV-2 virus with the Pfizer/BioNTech BNT162b2 vaccine in January 2021 and who were voluntarily tested for their humoral adaptive immune response to SARS-CoV-2 vaccine.
Blood sampling was performed at 3 time points for all participants. The first time point was before the vaccination to investigate the possibility of a past COVID-19 infection, the second was taken 20 days after the 1st dose of the vaccine administration (1 day before the 2nd dose) and the third one month after the 2nd dose. The determination of seropositivity and neutralization activity of antibodies were conducted in the Infectious Diseases Laboratory, First Department of Pediatrics, Medical School, National and Kapodistrian University of Athens, “Aghia Sophia” Children’s Hospital.
A form containing demographic and clinical data as well as adverse events (AEs) after each COVID-19 vaccination dose was completed by each participant. Data included gender, age, blood type and rhesus, smoking, Body Mass Index (BMI; underweight ≤ 18.5 kg/m2, Normal weight = 18.5–24.9 kg/m2, overweight = 25–29.9 kg/m2 and obesity ≥ 30 kg/m2), history of underlying diseases (cardiac, pulmonary, hyperlipidemia and hypertension, Type 1 diabetes mellitus, Hashimoto’s thyroiditis, rheumatoid arthritis, psoriasis, inflammatory bowel diseases, autoimmune hepatitis etc.), allergies (to any kind of allergen and in any manner of manifestation), use of immunosuppressive medications and history of pneumococcal or influenza vaccination within the last three months. Local AEs including pain, edema, pruritus, erythema, or systemic AEs such as fatigue, fever, headache, lymphadenopathy, myalgias, arthralgias etc., were also recorded after each dose of the vaccine.
The study protocol was approved by the scientific and bioethics committee of “Aghia Sophia” Children’s Hospital and informed consent was obtained from all participants.
2.2. RBD-specific antibody detection
Serum samples were tested using the Elecsys® Anti-SARS-CoV-2 S (Roche Diagnostics, Basel, Switzerland) reagent on a Cobas e 411 immunoassay analyzer for the semiquantitative detection of TAbs-RBD (IgA, IgM and IgG) of S1 subunit of SARS-CoV-2 spike protein according to the manufacturer’s instructions. The Elecsys® Anti-SARS-CoV-2 S is an Electrochemiluminescence Immunoassay (ECLIA), which is based on a double-antigen sandwich Enzyme-linked Immunosorbent Assay (ELISA) methodology. Values of ≥ 0.8 U/ml are positive.
2.3. Anti-RBD-specific neutralization assay
Determination of anti-RBD neutralization titers were conducted using the commercially available and Food and Drug Administration (FDA) approved cPassTM SARS-CoV-2 Neutralization Antibody Detection kit (GenScript Biotech Corporation, Piscataway, New Jersey, USA) according to the manufacturer’s instructions. This kit is based on a blocking ELISA using Horseradish Peroxidase (HRP) conjugated recombinant SARS-CoV-2 RBD fragment and the human Angiotensin-Converting Enzyme 2 (ACE2) receptor protein. The optical density (OD) was measured at 450 nm in the Labtech LT-4500 microtiter plate reader and the percentage of RBD-specific neutralization antibodies was calculated by the following type: Percentage signal inhibition (%) = (1- OD value of sample/OD value of negative control)*100. Percentages of ≥ 30% were considered positive.
2.4. Statistical methods
Absolute and relative frequencies (%) were used to describe the qualitative variables such as demographic characteristics, while mean, standard deviation (SD), median (Mdn), and interquartile range (IQR) were used for quantitative data. Differences between two or more independent samples were assessed with Mann-Whitney U test or Kruskal-Wallis H test, respectively. Post-hoc analysis was performed using Tukey-Kramer’s test. Spearman rho correlation coefficient was used for associations between continuous variables. Multiple linear regressions were assessed after variables’ logarithmic transformations. The assumption of normality was checked through kurtosis and skewness, Kolmogorov-Smirnov, and Shapiro-Wilk tests. Statistical significance level was set at P < 0.05. Statistical analysis was performed using SPSS version 26.0 (IBM Corp., Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
3. Results
3.1. Study population
A total of 268 HCWs who had 2 doses of the BNT162b2 vaccine according to the recommended schedule (21 days apart) were included in the study analysis. Four participants had previously confirmed SARS-CoV-2 infection and their antibody response was analyzed separately. The mean age (±SD) of the participants (n = 264) was 45.45 years (±11.93) (range: 24–70 years) and 211 (79.9%) were females. The mean (±SD) BMI of the participants was 24.68 kg/m3 (±4.20 kg/m2), 67 (25.8%) were smokers, 121 (46.5%) had history of underlying diseases and 6 (2.3%) were taking immunosuppressive medications.
3.2. Safety data
Solicited local or systemic adverse events (AEs) mostly observed within the first 3 days after each vaccine dose and are presented in Supplementary Table 1. The most common local self-limited reactions were pain (76.2–84.7%) and edema (13.06–14.55%) at the site of injection. Most common systemic AEs after 1st and 2nd vaccine dose, respectively, included fatigue (14.55% and 42.54%), headache (12.31% and 28.73%), myalgias (7.46% and 25%) and fever (1.11% and 19.78%). An enhancement of systemic AEs was noticed after the 2nd dose of vaccine. There was not any serious allergic reaction among the participants. Most serious AEs included a 45- year-old man who experienced Bell’s palsy 20 days after the 2nd dose and a 60-year-old woman who experienced serious tinnitus with temporary hearing loss 5 days after the 1st dose that lasted for over a month, with its intense being increased after the 2nd dose that finally responded to systemic use of corticosteroids.
3.3. Immunogenicity
Statistical differences were found between the TAbs-RBD after the 1st and 2nd vaccine doses (P < 0.001); TAbs-RBD after the 1st vaccine dose were median (IQR): 31.13 (63.59) U/ml, while one month after the 2nd dose were 1288.00 (1376.95) U/ml (Table 1 ). The same pattern was observed for NAbs-RBD; median (IQR): 51.07% (31.6) and 95.31% (3.70) after the first and second vaccine dose respectively (P < 0.001).
Table 1.
Differences in median values of TAbs-RBDand NAbs-RBD (%) SARS-CoV-2 spike – Receptor Binding Domain antibodies after the 1st and 2nd dose of BNT162b2 vaccine regarding demographic and clinical characteristics of the study population (n = 264). Values refer to median (interquartile range).
|
After 1st dose |
After 2nd dose |
||||
|---|---|---|---|---|---|
| TAbs-RBD (U/ml) | NAbs-RBD (%) | TAbs-RBD (U/ml) | NAbs-RBD (%) | ||
| Total population (N = 264) | 31.13 (63.59) | 51.07 (31.60) | 1288.00 (1376.95) | 95.31 (3.70) | |
| Gender |
Male 53 (20.1%) |
24.61 (44.69) | 48.57 (24.88) | 1092.00 (1304.30) | 94.54 (4.65) |
|
Female 211 (79.9%) |
33.98 (64.72) | 53.14 (32.27) | 1309.00 (1359.70) | 95.45 (3.62) | |
| P-value | 0.084 | 0.168 | 0.214 | 0.163 | |
| Age (years) |
20+ 35 (13.3%) |
33.98 (138.23) | 56.09 (28.21) | 1428.00 (1019.00) | 95.79 (2.13) |
|
30+ 59 (22.3%) |
53.18 (127.27) | 62.39 (34.94) | 1803.00 (2204.90) | 96.08 (3.69) | |
|
40+ 69 (26.1%) |
32.56 (44.46) | 50.04 (24.97) | 1237.00 (850.60) | 95.13 (3.24) | |
|
50+ 77 (29.2%) |
23.60 (52.79) | 47.30 (39.07) | 1243.00 (1573.30) | 95.32 (4.19) | |
|
60+ 24 (9.1%) |
12.39 (29.43) | 37.26 (37.82) | 657.30 (574.15) | 91.10 (8.61) | |
| P-value | .001c,e | .001c,e | <.001a,b,c,d,e,f | .001a,b,c,d | |
| Smoking |
No 193 (74.2%) |
33.89 (75.63) | 53.41 (29.97) | 1311.00 (1501.30) | 95.56 (3.33) |
|
Yes 67 (25.8%) |
22.47 (36.45) | 46.45 (34.32) | 1222.00 (1232.00) | 94.41 (5.67) | |
| P-value | 0.033 | 0.015 | 0.041 | 0.002 | |
| Underlying disease (4 missing values) |
No 139 (53.5%) |
34.45 (67.88) | 54.33 (27.33) | 1365.00 (1447.30) | 95.58 (3.37) |
|
Yes 121 (46.5%) |
25.89 (58.81) | 49.60 (41.82) | 1229.00 (1299.50) | 95.21 (5.34) | |
| P-value | 0.102 | 0.010 | 0.339 | 0.218 | |
| Immunosuppressive medications |
No 258 (97.7%) |
32.52 (65.35) | 51.45 (30.80) | 1307.00 (1382.00) | 95.35 (3.54) |
|
Yes 6 (2.3%) |
3.46 (12.54) | 19.68 (21.18) | 277.10 (371.30) | 86.26 (9.28) | |
| P-value | 0.002 | 0.003 | <0.001 | 0.001 | |
Abbreviations: TAbs-RBD; Total SARS-CoV-2 spike – Receptor Binding Domain antibodies, NAbs-RBD; Neutralizing antibodies (%), BMI; Body Mass Index. P-value of Mann-Whitney U test or Kruskal-Wallis H test. Post-Hoc Tukey-Kramer’s test showed differences between a60 + vs. 40+, b60 + vs. 20+, c60 + vs. 30+, d60 + vs. 50+, e50 + vs. 30 + and f40 + vs. 30 + . Statistically significant values are marked in bold.
The correlation between the TAbs-RBD and NAbs-RBD (%) SARS-CoV-2 spike antibodies was after the 1st dose, Spearman’s rho: 0.642 (P < 0.001) and after the 2nd dose rho: 0.0.694 (P < 0.001).
Twenty days after the 1st dose, 56/264 (21.2%) of the participants had NAbs-RBD below the positivity level (<30%), while 1 month after the 2nd dose all HCWs had NAbs-RBD above the positivity level. However, there was still a statistically significant difference in NAbs-RBD between HCWs with initially low NAbs-RBD (<30%) with others (P < 0.001). HCWs who had low NAbs-RBD after the 1st dose (n = 56) had median age 52 (IQR: 14) years compared to the rest with median age 44 (IQR: 19) years (P < 0.001).
HCWs who reported confirmed previous SARS-CoV-2 infection (n = 4) had higher TAbs-RBD after the 1st dose with median (IQR): 3915 (1834) U/ml and after the 2nd dose: 6908 (6205) U/ml (P < 0.001).
3.4. Association of immune response with epidemiological and clinical characteristics
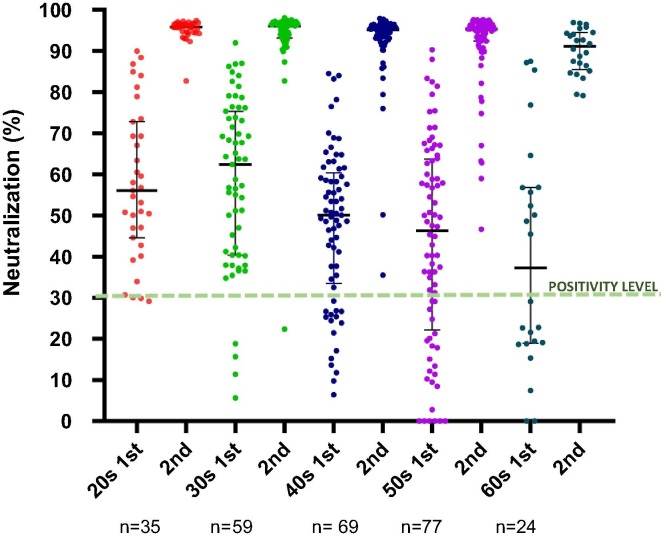
There was not a statistically significant difference in immune response between genders regarding either TAbs-RBD or NAbs-RBD for both vaccines’ doses (Table 1). In contrast statistically significant differences among different age groups were detected with younger age groups having higher TAbs-RBD and NAbs-RBD especially when compared with participants greater than 60 years old (Table 1 and Fig. 1 ). A negative correlation of antibody response with age was detected for both vaccine doses regarding both TAbs-RBD and NAbs-RBD (Supplementary Table 2).
Fig. 1.
Kinetics of SARS-CoV-2 neutralizing – receptor binding domain antibodies (%) in healthcare workers 20 days after vaccination with the first dose and one month after the second dose of the BNT162b2 vaccine. Black lines represent median (IQR) values.
HCWs who were smokers had a statistically significant lower antibody response for TAbs-RBD and NAbs-RBD after both the 1st and 2nd vaccine doses (P = 0.033, P = 0.015, P = 0.041, P = 0.002 respectively). Medical history of underlying diseases was associated only with lower NAbs-RBD after the 1st dose (P = 0.010) but did not affect the final antibody response (Table 1). In contrast, HCWs who were receiving immunosuppressive medications (n = 6) elicited lower levels of TAbs-RBD and NAbs-RBD after both doses (Table 1). There was not any significant correlation of elicited antibody response with weight or BMI (Supplementary Table 2). Non-significant associations with a history of seasonal flu or pneumococcal immunization, allergies, or blood group type were detected (data not shown).
Number and type of local AEs did not associate with the levels of TAbs-RBD and NAbs-RBD (Table 2 ). However, participants who experienced three or more systemic AEs elicited higher final levels of TAbs-RBD and NAbs-RBD than HCWs who experienced one or they did not have systemic AEs (Table 2).
Table 2.
Differences in median values of TAbs-RBD and NAbs-RBD (%) SARS-CoV-2 spike – Receptor Binding Domain antibodies after the 1st and 2nd dose of BNT162b2 vaccine regarding local or systemic side effects after immunization in healthcare workers (n = 264). Values refer to median (interquartile range).
|
After 1st dose |
After 2nd dose |
||||
|---|---|---|---|---|---|
| TAbs-RBD | NAbs-RBD (%) | TAbs-RBD | NAbs-RBD (%) | ||
| Local AEs | 0 | 26.54 (23.86) | 48.94 (34.15) | 1276.00 (1498.30) | 95.41 (4.86) |
| 1 | 33.85 (65.52) | 51.18 (31.64) | 1248.00 (1322.40) | 95.21 (3.76) | |
| 2+ | 23.60 (92.47) | 54.48 (24.27) | 1567.00 (1421.40) | 95.64 (3.16) | |
| P-value | 0.420 | 0.558 | 0.778 | 0.985 | |
| Systemic AEs | 0 | 27.45 (63.56) | 51.12 (31.64) | 1211.00 (937.40) | 94.79 (4.12) |
| 1 | 44.07 (60.24) | 50.51 (27.98) | 1054.50 (1333.30) | 94.98 (4.88) | |
| 2 | 41.54 (73.39) | 56.45 (24.99) | 1530.50 (1622.40) | 95.83 (2.86) | |
| 3+ | 22.18 (70.52) | 36.26 (47.08) | 1946.00 (1751.00) | 96.09 (2.64) | |
| P-value | 0.761 | 0.448 | <.001a,b | <.004a,b | |
Abbreviations: TAbs-RBD; Total SARS-CoV-2 spike – Receptor Binding Domain antibodies, NAbs-RBD; Neutralizing antibodies (%), AE; adverse events. P-value of Kruskal-Wallis H test. Post-Hoc Tukey-Kramer’s test showed differences between a0 vs. 3 + and b1 vs. 3 + side effects. Statistically significant values are marked in bold.
Multiple linear regressions for parameters associated with the levels of NAbs-RBD were established for NAbs-RBD after the first and second vaccine dose (Table 3 ). After the 1st vaccine dose, a statistically significant negative association of TAbs-RBD was detected for age (P < 0.001), smoking (P = 0.012) and immunosuppressive medications (P < 0.001). After the 2nd vaccine dose, a statistically significant negative association of TAbs-RBD was detected for age (P < 0.001), smoking (P = 0.011), and immunosuppressive medications (P < 0.001), while a positive association was detected for BMI (P = 0.004) and systemic adverse events after immunization (P = 0.001).
Table 3.
Multiple linear regression of parameters affecting total SARS-CoV-2 spike – Receptor Binding Domain antibodies levels after the 1st and 2nd dose of BNT162b2 vaccine in healthcare workers (n = 264).
| Coefficients | Unstandardized Coefficients β | Std. Error | t | P-value |
|---|---|---|---|---|
| TAbs-RBD after 1st dose | ||||
| (Constant) | 1.788 | 0.250 | 7.165 | <0.001 |
| Age (years) | −0.014 | 0.003 | −4.023 | <0.001 |
| ΒΜΙ | 0.017 | 0.010 | 1.744 | 0.082 |
| Smoking | −0.227 | 0.090 | −2.533 | 0.012 |
| Underlying disease | −0.099 | 0.082 | −1.216 | 0.225 |
| Immunosuppressive medications | −0.967 | 0.253 | −3.824 | <0.001 |
| Systemic AEs | −0.047 | 0.047 | −0.997 | 0.320 |
| R2 = 0.159, ANOVA; F = 7.625, P < 0.001 | ||||
| TAbs-RBD after 2nd dose | ||||
| (Constant) | 3.183 | 0.136 | 23.421 | <0.001 |
| Age (years) | −0.011 | 0.002 | −5.846 | <0.001 |
| ΒΜΙ | 0.016 | 0.005 | 2.948 | 0.004 |
| Smoking | −0.122 | 0.048 | −2.563 | 0.011 |
| Underlying disease | −0.030 | 0.043 | −0.702 | 0.483 |
| Immunosuppressive medications | −0.562 | 0.134 | −4.190 | <0.001 |
| Systemic side AEs | 0.057 | 0.018 | 3.229 | 0.001 |
| R2 = 0.267, ANOVA; F = 14.729, P < 0.001 | ||||
Abbreviations: TAbs-RBD; Total SARS-CoV-2 spike – Receptor Binding Domain antibodies, BMI; Body Mass Index, AE; adverse events. Logarithmic transformations are established to TAbs-RBD variables. Statistically significant coefficients are marked in bold.
During the study analysis period, almost 40 days after the 2nd vaccine dose, two participants with good levels of neutralizing activity (95% and 90% respectively) developed confirmed SARS-CoV-2 infection with mild self-limited symptoms including low grade fever and nasal congestion for 2 days.
4. Discussion
In the present study, we aimed to detect possible associations of total and neutralizing antibodies against the receptor binding domain (RBD) of SARS-CoV-2 spike protein with epidemiological and clinical characteristics after immunization with the 1st and 2nd doses of the BNT162b2 vaccine. A good correlation of TAbs-RBD and NAbs-RBD was detected after both vaccine doses. Detection of TAbs-RBD is less costly and easier to measure compared to NAbs-RBD and may be a good substitute in population Abs screening is required after SARS-CoV-2 immunization.
Given the current global vaccine shortage, some researchers have proposed the delay of the administration of the 2nd vaccine dose to ensure partial immunity to more individuals [8]. In the present study, twenty days after the 1st dose, a significant percentage of the participants (21.2%) did not have detectable NAbs-RBD, while one month after the 2nd dose all of them mounted NAbs-RBD. Our findings support the timely administration of the 2nd vaccine dose especially in the older age groups.
Parameters in the present study that negatively affected the final antibody level after SARS-CoV-2 immunization were older age, smoking, and immunosuppressive medications. Recent studies have detected a negative association of age with NAbs-RBD, which is in accordance with our results, but also a sex dependent response, which was not the case in our study [9].
To the best of our knowledge this is the first study associating smoking status with lower TAbs-RBD as well as NAbs-RBD responses after both doses of BNT162b2 vaccine, which was detected in the single-factor analysis and confirmed in the regression analysis of the study parameters. Further studies are required to confirm the finding and form a pathophysiologic basis for this finding.
Recent studies have shown that seropositive people after SARS-CoV-2 infection, who receive a single dose of BNT162b2 vaccine, mount much higher antibody responses (also detected in our study) that may be sufficient to prevent infection [10], [11]. However, whether a single dose of mRNA vaccine provides adequate induction of T-cell responses and effective long-term protection in seropositive persons requires further investigation [12], [13].
Emerging variants of SARS-CoV-2 are of clinical concern and vaccine breakthrough infections with SARS-CoV-2 variants in previously fully immunized individuals have been described [14]. These SARS-CoV-2 variants could have a direct impact on the available COVID-19 vaccines, as they can alter the neutralizing activity of vaccine-elicited antibodies resulting in variable loss of efficacy [15], [16]. However, it is not clear in such cases if it is the lower neutralizing activity of SARS-CoV-2 antibodies, inadequate T-cell responses, or genetic novelty of SARS-CoV-2 variants that caused these infections. In our cohort, two participants with good levels of NAbs-RBD developed mild self-limited COVID-19 infection.
Defining immunological correlates of protection and a protective antibody threshold would be important for public health decisions and for the development of new SARS-CoV-2 vaccines, as they can substitute large and costly field efficacy trials with smaller immunogenicity-based phase 3 trials [7], [17].
The administration of the BNT162b2 vaccine had a good safety profile in our cohort with most vaccinees developing local self-limited reactions and limited systemic reactions that were more frequent after the 2nd vaccine dose. Indeed, experiencing 3 or more systemic AEs was associated with higher NAbs-RBD after the 2nd vaccine dose, possibly indicating higher immune induction. There was not any serious allergic or anaphylactic reaction, while there was one episode of Bell’s palsy and one episode with serious tinnitus and deafness that could not be determined if they were vaccine related. It is important after the emergent authorization of COVID-19 vaccines, to maintain public confidence for the safety of immunization with post-vaccination surveillance and documentation of AEs, to achieve high immunization coverage [18], [19].
Limitations of present study include that there was a limited number of participants with previous SARS-CoV-2 infection, or under immunosuppressive medications so these results and comparisons should be interpreted cautiously. In conclusion, in the present study several clinical parameters were associated with SARS-CoV-2 antibody responses. Long term studies following the antibody kinetics, T-cell responses and real-life protection are important to determine mass vaccination policies and if there will be need for additional doses or modified mRNA vaccines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.07.067.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Fauci A.S. The story behind COVID-19 vaccines. Science. 2021;372(6538):109. doi: 10.1126/science.abi8397. [DOI] [PubMed] [Google Scholar]
- 2.Ball P. The lightning-fast quest for COVID vaccines - and what it means for other diseases. Nature. 2021;589(7840):16–18. doi: 10.1038/d41586-020-03626-1. [DOI] [PubMed] [Google Scholar]
- 3.Creech C.B., Walker S.C., Samuels R.J. SARS-CoV-2 Vaccines. JAMA. 2021;325(13):1318. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 4.Lamb Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs. 2021;81(4):495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krammer F. Correlates of protection from SARS-CoV-2 infection. Lancet. 2021;397(10283):1421–1423. doi: 10.1016/S0140-6736(21)00782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skowronski D.M., De Serres G. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med. 2021;384:1576–1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 9.Terpos E., Trougakos I.P., Apostolakou F., Charitaki I., Sklirou A.D., Mavrianou N., et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021;96(7):E257–E259. doi: 10.1002/ajh.26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saadat S., Rikhtegaran Tehrani Z., Logue J., Newman M., Frieman M.B., Harris A.D., et al. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA. 2021;325(14):1467. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prendecki M., Clarke C., Brown J., Cox A., Gleeson S., Guckian M., et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. The Lancet. 2021;397(10280):1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. The Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacisuleyman E., Hale C., Saito Y., Blachere N.E., Bergh M., Conlon E.G., et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N Engl J Med. 2021;384(23):2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharun K., Tiwari R., Dhama K., Emran T.B., Rabaan A.A., Al M.A. Emerging SARS-CoV-2 variants: impact on vaccine efficacy and neutralizing antibodies. Hum Vaccin Immunother. 2021:1–4. doi: 10.1080/21645515.2021.1923350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalkanen P., Kolehmainen P., Häkkinen H.K., Huttunen M., Tähtinen P.A., Lundberg R., et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-24285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch T, Mellinghoff SC, Shamsrizi P, Addo MM, Dahlke C. Correlates of Vaccine-Induced Protection against SARS-CoV-2. Vaccines (Basel). 2021;9(3):238. [DOI] [PMC free article] [PubMed]
- 18.Krause PR, Gruber MF. Emergency Use Authorization of Covid Vaccines - Safety and Efficacy Follow-up Considerations. N Engl J Med. 2020;383:e107. [DOI] [PubMed]
- 19.Longo D.L., Castells M.C., Phillips E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N Engl J Med. 2021;384(7):643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.