Abstract
The widespread extrapulmonary complications of coronavirus disease 2019 (COVID-19) have gained momentum; the pancreas is another major target for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Here, we take a closer look into potential pathological interactions. We provide an overview of the current knowledge and understanding of SARS-CoV-2 infection of the pancreas with a special focus on pancreatic islets and propose direct, indirect, and systemic mechanisms for pancreas injury as result of the COVID-19–diabetes fatal bidirectional relationship.
Keywords: COVID-19, SARS-CoV-2, islets, β-cell, pancreas, inflammation, diabetes, ACE2, insulin
COVID-19 caused by SARS-CoV-2 has resulted in widespread global morbidity and mortality and poses a serious threat to public health as a result of detrimental defects on pulmonary, immune, endocrine, and homeostatic regulation. Although at the beginning of the outbreak, COVID-19 appeared as pulmonary disease targeting lung alveolar epithelial cells, we now know of many other target cells and organs as well as extrapulmonary complications caused by SARS-CoV-2, with long-term devastating consequences of heterogenous symptoms (‘long COVID’), especially critical for metabolic diseases and pathological impacts on metabolically active tissues such as fat, liver, and pancreas at the cellular level.
COVID-19 and metabolic derangement: a two-way road
Not only an immune but a metabolic network at both the systemic and the cellular level is instrumental for the host to cope with pathogens. SARS-CoV-2 highjacks the cellular machinery to replicate. On its way, it can alter both host metabolic responses and adaptive mechanisms, which are primarily in place to defend against infection, but through massive inflammatory responses, they can trigger chronic collateral damage to metabolic health and result in multiorgan failure. The important bidirectional relationship between COVID-19 and patients’ metabolic status has been observed in numerous studies, such that: (i) patients with obesity, type 2 diabetes (T2D), and the metabolic syndrome suffer more severe and critical COVID-19 disease; and (ii) COVID-19 induces severe metabolic complications in pre-existing diabetes and is even associated with persistent insulin resistance, new-onset hyperglycemia, and β-cell dysfunction and subsequently diabetes at an alarming rate [1], as observed from several other viral infections although not as apparently and severely [2].
Substantial epidemiological, clinical, and retrospective data have shown obesity and T2D, two frequently coexisting chronic metabolic disorders, as risk factors for severe COVID-19 and higher mortality. The strong association between higher COVID-19 mortality and obesity indicates that obesity shifts COVID-19 towards fatal disease with adverse outcomes in all populations. Underlying disease mechanisms are complex and multifactorial, similar to the metabolic syndrome itself, including abnormally elevated glucose metabolism and resultant intensified viral replication, impaired innate and/or adaptive immune responses, endothelial dysfunction, insulin resistance, and last but not least the excessive chronic systemic inflammation referred to as ‘cytokine storm’ in COVID-19. All of these mechanisms show shared pathophysiology to some degree and are likely to act synergistically in exacerbating the severity of SARS-CoV-2 infection and compromising metabolic health, which is supported by the observation that tight glycemic control could minimize the severity of the disease and that glucose levels directly correlate with COVID-19 severity [1].
Reciprocally, metabolic disturbance has frequently been observed during COVID-19 disease with alert glucose metabolism, ketoacidosis, and hyperglycemia in patients with and without pre-existing diabetes or the development of new-onset diabetes as well as pancreas injury [1]. Besides endocrine disorders, several reports indicate SARS-CoV-2-associated acute pancreatitis and exocrine derangements [3].
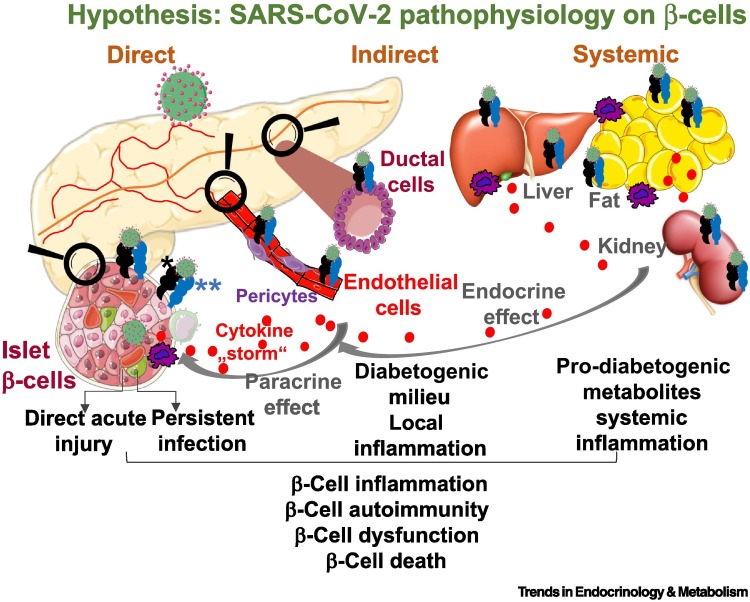
For many years, clinical, epidemiological, pathological, and in vitro studies have implicated enteroviruses as initiators of autoimmunity and β-cell failure in genetically susceptible individuals [2]. Enteroviruses could trigger β-cell autoimmunity or exert direct cytotoxicity on pancreatic β-cells [2]. Whether (i) SARS-CoV-2 is also a diabetogenic virus initiating direct destruction of β-cells, whether (ii) SARS-CoV-2 causes pleiotropic alterations of glucose metabolism that could trigger diabetes, or whether (iii) the exocrine pancreas is the major SARS-CoV-2 target and would then secondarily lead to disturbance in the endocrine pancreas is under debate (Figure 1 ).
Figure 1.
Proposed model of how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) targets islet β-cells.
SARS-CoV-2 may interact with β-cells in islets through three different mechanisms. (i) Directly: virus entry through several viral receptors in β-cells and their subsequent injury as a result of direct acute viral damage or long-term persistent presence of uncleared SARS-CoV-2. In both scenarios, SARS-CoV-2 directly induces β-cell dysfunction and death or acts as an initiator of β-cell autoimmunity. (ii) Indirectly: SARS-CoV-2 infects viral receptor-expressing pancreatic cells such as ductal or endothelial cells and pericytes in the microvasculature resulting in their structural and functional transformation, leading to local inflammation and cytokine and chemokine release as well as the generation of a prodiabetic milieu that can perturb the integrity of neighboring non-infected β-cells in a paracrine fashion and potentially leads to β-cell loss or dysfunction. (iii) Systemically: SARS-CoV-2 targets putative viral receptor-expressing cells in metabolic organs such as liver, fat, and kidney, causing loss of disease-tolerance mechanisms, metabolic derangement, and maladaptive functions. This can lead to systemic inflammation and the accumulation of prodiabetic metabolites and ultimately damage β-cells, constituting another possible mechanism of SARS-CoV-2 infection-related islet damage. *Potential SARS-CoV-2 viral entry factors such as angiotensin-converting enzyme 2 (ACE2) (Table 1), neuropilin 1 (NRP1), TFRC, FURIN, and dipeptidyl peptidase-4 (DPP4). **Associated proteases such as TMPRSS2 (Table 1) and CTSL. Created using Smart Servier Medical Art under https://creativecommons.org/licenses/by/3.0/.
SARS-CoV-2 entry receptors in pancreatic cells: twilight zone
Angiotensin-converting enzyme 2 (ACE2), an integral component of the renin-angiotensin-aldosterone system (RAAS), is the key entry receptor for SARS-CoV-2. In principle, SARS-CoV-2 cell entry requires engagement of the virus’s spike subunit with ACE2 following priming of the spike protein by the SARS-CoV-2 entry-associated cellular protease TMPRSS2 or CTSL. Cellular coexpression of ACE2 and TMPRSS2 has been considered critical for efficient SARS-CoV-2 uptake. Entering through the respiratory tract, SARS-CoV-2 initially destroys ACE2 receptor-expressing alveolar cells. However, ACE2 is widely expressed in other organs like the heart, kidney, gut, and pancreas [4]. Multiple independent laboratories have investigated whether the canonical SARS-CoV-2 cell-entry machinery is present in human pancreatic cells, which resulted in many studies that did find pancreatic islet ACE2 or TMPRSS2 expression [3,5., 6., 7., 8., 9., 10., 11., 12.], while other studies did not [4,13,14] (Table 1 ). A more consistent result among different groups is the substantial ACE2 expression in pancreatic ductal cells and in the microvasculature. In particular, this has been confirmed by extensive profiling using multiple complementary approaches from single-cell RNA-seq to fluorescence in situ hybridization and immunohistochemistry (IHC) [4,13,14], together with extremely low to undetectable expression levels in islets from donors with and without diabetes. By contrast, others have demonstrated that primary human or stem cell-derived β-cells show considerable expression, albeit heterogeneously, of ACE2 or TMPRSS2 (Table 1) [5., 6., 7., 8., 9., 10., 11., 12.]. To add to the controversy, conflicting data were also obtained in regard to a potential correlation of ACE2 or TMPRSS2 expression with body mass index (BMI) [6,7,13,14] or diabetes [6,13]. While Taneera et al. [6] reported significant upregulation of ACE2 in diabetic donors, Coate et al. [13] and Wu et al. [11] challenged such findings by showing no differences in ACE2 expression between non-diabetic donors and individuals with T2D. The rationale behind such analysis is that potential upregulation of islet ACE2 expression in diabetes may foster SARS-CoV-2’s entry and subsequent replication, compromising a natural cellular defense response, which would accelerate excessive local inflammation and β-cell demise.
Table 1.
Viral factor expression and SARS-CoV-2 tropism in various pancreatic endocrine and exocrine cells extracted from recent literature
| Human pancreatic cell type | ACE2 expression | TMPRSS2 expression | Viral tropism |
|---|---|---|---|
| Endocrine β-cell | Expressed [3,5., 6., 7., 8., 9., 10., 11.] Extremely low or undetected [4,13,14] |
Expressed [5,6,8,10,11] Extremely low or undetected [13,14] |
Entered and/or infected [5,8., 9., 10., 11., 12.] None [14] |
| Stem cell-derived β-cell | Expressed [5,9] | NDa | Entered and/or infected [5,9] |
| Endocrine α-cell | Expressed [5., 6., 7., 8.,11] Extremely low or undetected [13,14] |
Expressed [5,6,8,11] Extremely low or undetected [13] |
Entered and/or infected [5,11,12] |
| Exocrine acinar cell | Expressed [3,5,9,13,14] Extremely low or undetected [8] |
Expressed [5,9,13,14] Extremely low or undetected [8] |
Entered and/or infected [9,12] |
| Exocrine ducts | Expressed [3., 4., 5.,7., 8., 9.,13,14] | Expressed [5,8,13,14] | Entered and/or infected [8,9,12,14] |
| Microvasculature | Expressed [7,10,13,14] | Entered and/or infected [10,12] |
Abbreviation: ND, not determined.
Differences in methodological or technical approaches such as tissue processing and preservation, imprecise reagents, or biological variations including heterogeneity in islet and pancreas organ donors and low sample sizes as well as rapid data evaluation and publication turnover may explain the discrepancy in results. Islets are well known for unspecifically picking up immunoglobulins, which had led to several misinterpretations. It is important to note that none of the studies that had validated ACE2 antibody specificity obtained samples from ACE2 loss-of-function systems [i.e., from ACE2-knockout (KO) mice or virally depleted ACE2 in human isolated islets], which could critically be useful in the future to resolve the discrepancy.
As ACE2 is needed for canonical SARS-CoV-2 cell entry, most of the initial aforementioned studies set ACE2 together with the protease TMPRSS2, although dispensable [12], as primary indicators for SARS-CoV-2 entry into pancreatic islets and as a proof of concept for islet tropism towards SARS-CoV-2. However, other SARS-CoV-2 entry factors, such as neuropilin 1 (NRP1), the transferrin receptor TFRC, the pro-protein convertase FURIN, another protease CTSL, and dipeptidyl peptidase-4 (DPP4), which is used as an entry receptor by MERS-CoV, are expressed in pancreatic islets, with particularly high expression of NRP1 and TFRC in human β-cells [10., 11., 12.], which may explain some of the tropism of SARS-CoV-2 in islets for β-cells in COVID-19 patients [12].
In addition to such previous expression studies, further in-depth analyses are still needed to identify the functional SARS-CoV-2 cellular entry and alternative receptors or mechanisms (e.g., HMGB1, cellular heparan sulfate, circulating soluble ACE2 [15]), as there is productive SARS-CoV-2 infection throughout the pancreas in vivo in COVID-19 patients as well as ex vivo in infected human islets.
SARS-CoV-2 infection of pancreatic cells
Our understanding of the mechanisms and consequences of SARS-CoV-2 infection, its pathogenic drivers, and its target cell types, including SARS-CoV-2 infection and tropism in various endocrine islet cell models, has evolved rapidly since the onset of the pandemic.
Early striking observations show SARS-CoV-2 infection of hPSC-derived islet organoids, hPSC-derived islet xenografts, and primary human α- and β-cells [5] and iPSC-derived pancreatic endocrine and exocrine cells [9] and human islets [8] resulting in cellular as well as transcriptional alterations of genes linked to β-cell function and the upregulation of interferon (IFN)-mediated inflammatory signatures, markers of oxidative stress, and cell death [5,8,9], the latter reminiscent of the transcriptional upregulation of cytokines and chemokines in lung autopsy samples from COVID-19 patients [5] as well as in SARS-CoV-2 active viral replication [8], which is blocked by the RNA polymerase inhibitor remdesivir, which had provoked hope for its use in therapy. Support for SARS-CoV-2-mediated islet damage is provided by notable morphological and functional alterations including reduced numbers of insulin-secretory granules in β-cells, loss of insulin gene transcription, impairment in insulin secretion, and higher numbers of bihormonal insulin/glucagon-positive cells, all collectively reflecting a path towards β-cell degranulation, dedifferentiation, and loss [8,11,12]. SARS-CoV-2 infection of human islets leads to lower insulin gene expression in β-cells, while glucagon and other α-cell as well as acinar cell markers are upregulated in β-cells. Such loss in insulin as well as an unusual presence of trypsin/insulin-double-positive cells were confirmed in autopsy samples from COVID-19 patients, hinting at SARS-CoV-2-induced β-cell transdifferentiation [12]. Facing the low capacity of β-cell turnover as well as the rarely seen α-to-β-cell differentiation, it is unclear at this stage whether such Janus-faced cells may ever return to fully functional β-cells.
Furthermore, through intrinsic alterations in the cellular inflammatory status, SARS-CoV-2 virus-mediated pancreatic damage may initiate the recruitment of tissue-resident immune cells, which constitutes a path towards eventual β-cell autoimmunity. This merits future long-term epidemiological and clinical observations, since despite a high prevalence of mostly asymptomatic SARS-CoV-2 infection in children, there is so far no association between SARS-CoV-2 antibodies and type 1 diabetes (T1D) autoimmunity, which was confirmed this year by national registries.
Importantly, several independent groups have attempted to identify and localize SARS-CoV-2 RNA or protein in pancreases from deceased COVID-19 patients, so far with relatively low-sample sizes. The virus nucleocapsid protein SARS-CoV-2-N was found in the exocrine-interlobular ductal compartment, often in close proximity to islets [8,14], or in insulin-negative, β-cell marker NKX6.1-positive cells within islets, and rarely and occasionally, capsid/insulin-double-positive cells were observed [8].
By contrast, several other studies [9., 10., 11., 12.] revealed the presence of SARS-CoV-2 protein in β-cells in the islets of autopsy pancreases from COVID-19 patients, albeit with heterogeneous patterns and variations among pancreases, which indicates β-cell infection during the course of disease; whether infection persists needs to be identified. Resulting islet damage is suggested by apoptotic or necroptotic cell death in islets from infected human islets or COVID-19 pancreases [10,11], reminiscent of islets in T1D. Consistently, albeit assessed in only two COVID-19 patients, islets show immune cell infiltration with a general increase in interislet CD45-positive cells [10].
Although histopathological observations from all of these previous studies did not achieve consistent results and range from an infected exocrine compartment close to the islets to clear infection of β-cells, they indicate – despite the different methodologies used in the studies – very heterogeneous disease mechanisms, similar to what is seen in diabetes itself.
A noticeable infection of the exocrine pancreas, in particular in ductal structures, raises the question whether there is another causal link between SARS-CoV-2 and acute pancreatitis and even new-onset diabetes. However, the examination of a few pancreatic tissues from COVID-19 patients did not show pathological evidence of pancreatic inflammation [13]. From more comprehensive pancreatic histopathological analyses of stool and blood in association with enterovirus infection in the past, it became clear that an interplay of environmental factors (i.e., viral infection together with a strong genetic predisposition towards virus-associated pathways) may finally result in autoimmunity and T1D; however, a single virus infection has not been sufficient to cause autoimmunity or T1D [2] and thus a definitive conclusion on the COVID-19–diabetes association from so few pancreas studies without the availability of respective genetic analyses and long-term assessments is currently impossible.
Certain pathophysiological features, among them chronic inflammation with immune cell activation, unite pancreatic diseases including pancreatitis and all forms of diabetes. Therefore, the pathogenesis of the viral infection is dependent on the mechanism of action of the virus on target cells during the course of the infection and the viral persistence in the target cells thereafter. More comprehensive studies in larger patient cohorts of different demographics, ethnicities, and geographic location with long-term clinical follow-up are needed together with the exact cellular expression, frequency, and specific pattern of infection and the virus-initiated cellular interplay with the immune system. At this point, we hypothesize an evolving SARS-CoV-2-targeted pancreas pathology in a direct, indirect, and/or systemic manner (see Figure 1 for a proposed model).
In concert, results obtained during the 2019–2021 pandemic represent: (i) intriguing SARS-CoV-2 effects on the pancreas; and (ii) a possible fatal consequence of SARS-CoV-2 infection on endocrine islets and interconnected metabolic derangements. Detailed mechanistic knowledge is critically important to determine the susceptibility of pancreatic cells to SARS-CoV-2 in the context of COVID-19, as well as defense strategies to protect vulnerable cells to prevent a severe SARS-CoV-2 disease outcome, including long COVID.
Acknowledgments
Acknowledgments
Work conducted in the authors’ laboratory is supported by the German Research Foundation (DFG) and the JDRF. We apologize that we had to focus only on islet studies and were unable to cite many primary references due to strict limitations of 15 references in this format.
Declaration of interests
The authors declare no interests.
References
- 1.Montefusco L., et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021;3:774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geravandi S., et al. Enteroviruses and T1D: is it the virus, the genes or both which cause T1D. Microorganisms. 2020;8:1017. doi: 10.3390/microorganisms8071017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F., et al. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 2020;18:2128–2130.e2. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hikmet F., et al. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16 doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L., et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136.e7. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taneera J., et al. Expression profile of SARS-CoV-2 host receptors in human pancreatic islets revealed upregulation of ACE2 in diabetic donors. Biology (Basel) 2020;9:215. doi: 10.3390/biology9080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fignani I., Dotta F. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 is expressed in human pancreatic islet β-cells and is upregulated by inflammatory stress. Front. Endocrinol. 2020 doi: 10.3389/fendo.2020.596898. Published online November 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller J.A., et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021;3:149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 9.Shaharuddin H., et al. Deleterious effects of SARS-CoV-2 infection on human pancreatic cells. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.678482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steenblock C., et al. Viral infiltration of pancreatic islets in patients with COVID-19. Nat. Commun. 2021;12:3534. doi: 10.1038/s41467-021-23886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C.T., et al. SARS-CoV-2 infects human pancreatic beta cells and elicits beta cell impairment. Cell Metab. 2021;33:1565–1576.e5. doi: 10.1016/j.cmet.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang X., et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2021;33:1577–1591.e7. doi: 10.1016/j.cmet.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coate K.C., et al. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in beta cells. Cell Metab. 2020;32:1028–1040.e4. doi: 10.1016/j.cmet.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusmartseva I., et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020;32:1041–1051.e6. doi: 10.1016/j.cmet.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung M.L., et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin–angiotensin system. Cell. 2021;184:2212–2228.e12. doi: 10.1016/j.cell.2021.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]