Introduction
Two single-dose COVID-19 vaccines utilizing the adenoviral vector platform, ChAdOx1 nCoV-19 (AstraZeneca) and Ad26.COV2.S (Janssen), have recently been associated with rare immune-mediated thrombotic events and thrombocytopenia.1, 2, 3 In response to 6 reports on Ad26.COV2.S-associated thrombosis, the US Food and Drug Administration recommended a brief pause in the use of the Ad26.COV2.S vaccine on April 13, 2021. After further investigation, the US Food and Drug Administration and Centers for Disease Control and Prevention concluded that the vaccine's benefits outweigh the low risk of thrombosis and recommended the resumption of Ad26.COV2.S vaccination with a manufacturer warning for possible thrombosis. Although available evidence clearly demonstrates that both vaccines are efficacious and otherwise safe, further characterization of any vaccine-associated adverse events is necessary for public health and future vaccine development. We report a case of cutaneous small vessel vasculitis (CSVV) in a SARS-CoV-2− patient 1 week after inoculation with Ad26.COV2.S that rapidly resolved following treatment with systemic and topical corticosteroids.
Case report
A 65-year-old African American man with a history of hypertension, hyperlipidemia, and mechanical aortic valve replacement (on warfarin) presented to the emergency department with a pruritic and painful purpuric rash on his left arm, lower extremities, and abdomen. The patient first observed the rash on the morning of presentation when he noticed localized pruritus and redness of the upper portion of the left extremity. Over the course of the day, a pruritic and painful red rash developed in the lower portions of his bilateral extremities and abdomen, leading him to present to the emergency department that evening. On review of systems, he noted “pins and needles” in his feet but denied respiratory, abdominal, musculoskeletal, and genitourinary symptoms. He denied the initiation of new medications; however, on further inquiry, he acknowledged receiving the Ad26.COV2.S vaccine for the first time 7 days prior to presentation. He denied any prior vaccine or medication-associated adverse events except for a minor rash to ibuprofen several decades ago.
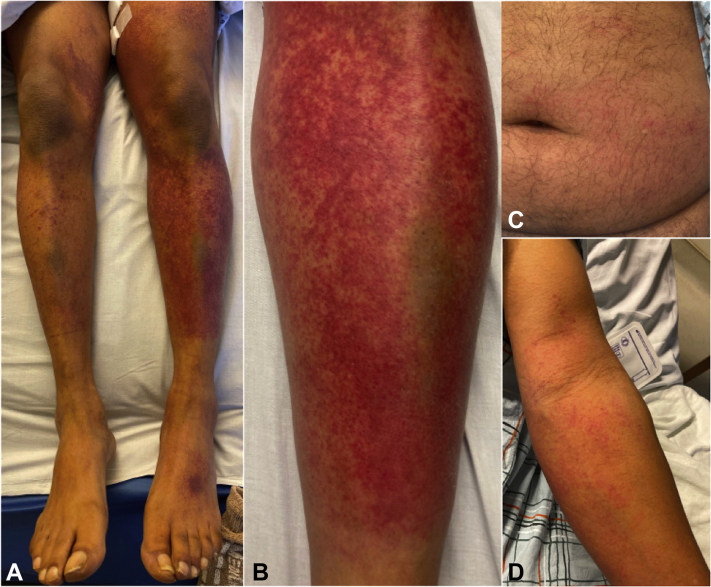
Examination revealed exquisitely tender petechiae and purpura on the lower portions of bilateral extremities (Fig 1, A and B), scattered petechiae on the lower portion of the abdomen (Fig 1, C), and palpable purpura on the left arm (Fig 1, D). No mucosal or palmar/plantar involvement, livedo reticularis, skin ulcerations, skin nodules, or lymphadenopathy were apparent. All vital signs were within normal limits. Significant laboratory abnormalities included a C-reactive protein level of 86 mg/L and an erythrocyte sedimentation rate of 34 mm/hr. Complete blood cell counts were within the normal ranges. International normalized ratio was therapeutic. Urinalysis, chest x-ray, and venous Doppler of the lower portion of the extremity were unremarkable. He was admitted for further workup and management.
Fig 1.
Clinical images on the day of admission to the hospital. A, Petechiae and purpura on the lower portions of bilateral extremities and dorsal side of the left foot. B, Petechiae and purpura on the anterior aspect of the left leg. C, Scattered petechiae on the lower portion of the abdomen. D, Petechiae and palpable purpura on the left arm.
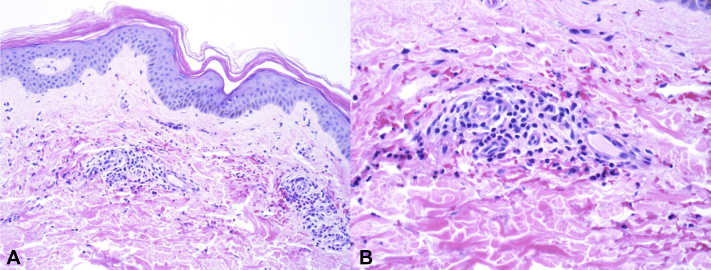
Two 3-mm punch biopsy specimens were obtained from the left thigh and were placed in formalin (for hematoxylin-eosin staining) and Michel media (for direct immunofluorescence). Extensive serologic workup revealed serum IgG, 3310 mg/dL (reference range, 537-1535 mg/dL); serum immunoglobulin M, 369 mg/dL (reference range, 35-242 mg/dL); fibrinogen, 581 mg/dL (reference range, 220-480 mg/dL); D-dimer, 0.72 μg/mL (age-adjusted normal value, <0.65 μg/mL); lactate dehydrogenase, 407 IU/L (reference range, 98-192 IU/L); haptoglobin, 11 mg/dL (reference range, 41-203 mg/dL); rheumatoid factor, 271.0 IU/mL (reference value, <15.0 IU/mL); and reactive syphilis antibody. Reflexive rapid plasma reagin was negative, and the patient denied a previous history of syphilis or any genital lesions. Normal serologic results included complement levels, serum IgA, heparin-induced platelet antibody (Lifecodes IgG assay, Immucor), indirect/direct antiglobulin, antinuclear antibody, perinuclear antineutrophilic cytoplasmic antibody, cytoplasmic antineutrophilic cytoplasmic antibody, cryoglobulin, and cyclic citrullinated peptide antibody. Infectious workup, including hepatitis panel, HIV antigen/antibody, SARS-CoV-2 polymerase chain reaction, and blood cultures, was negative. Histopathologic results of skin biopsy revealed superficial dermal small vessels with endothelial cell swelling, neutrophils within the vessel wall, some cellular debris, and extravasated red blood cells consistent with leukocytoclastic vasculitis (Fig 2, A and B). Direct immunofluorescence was negative.
Fig 2.
Histopathology of lesion tissue. A and B, Histopathologic image of lesion specimen demonstrated superficial dermal small vessels with endothelial cell swelling, neutrophils within the vessel wall, some cellular debris, and extravasated red blood cells. (A and B, Hematoxylin-eosin stain; original magnifications: A, ×100; B, ×200.)
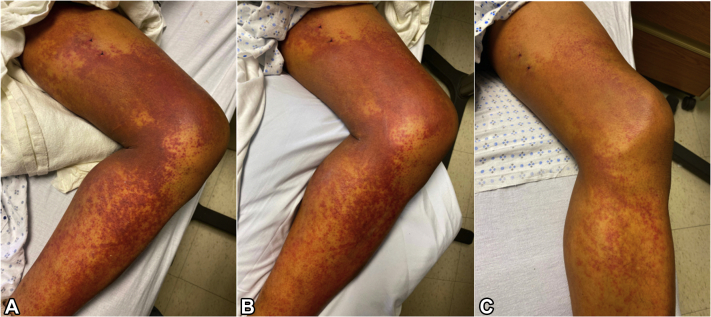
The patient was treated with prednisone 60 mg daily, triamcinolone 0.1% cream twice daily, and analgesics (eg, hydromorphone and oxycodone) for pain as needed. During the first 24 hours, his rash coalesced into larger, more violaceous purpuric patches and he required escalated doses of intravenous analgesics for pain control. No additional skin sites became involved. His symptoms and rash progressively improved over the course of 4 days after initiation of oral and topical corticosteroids (Fig 3, A to C). He was discharged on a prednisone taper (5 mg decrease daily) and triamcinolone 0.1% cream twice a day for 2 weeks. Follow-up with dermatology and rheumatology was arranged 1 week after discharge; however, the patient, unfortunately, did not follow up in either clinic. At 2 months, the patient was reached via telephone at which point he confirmed the completion of his treatment regimen and noted a complete resolution of his rash without any residual skin changes or symptoms. The etiology of the patient's CSVV was attributed to the Ad26.COV2.S vaccine. A report of an adverse event was submitted to the vaccine adverse event reporting system, and the patient was instructed to never undergo repeat vaccination with Ad26.COV2.S.
Fig 3.
Clinical response to systemic corticosteroids. A, Image of patient's left leg on the day of presentation, (B) 24 hours after prednisone initiation, and (C) 72 hours after prednisone initiation.
Discussion
Our patient's clinical picture and the temporal association are supportive of a causal relationship between Ad26.COV2.S and CSVV. To our knowledge, no prior studies have reported this relationship; however, CSVV has been reported in association with COVID-19.4 Immune stimulation causing the production of pathologic IgG antibodies to platelet factor 4 has been suggested as the pathophysiology of the newly designated vaccine-induced thrombotic thrombocytopenia.1, 2, 3 Although our patient's CSVV occurred in the absence of thrombocytopenia, clinically apparent thrombosis, and anti–platelet factor 4 antibodies, the increased immunoglobulin levels detected in our patient's serum may suggest immune stimulation secondary to vaccine administration similar to that reported in vaccine-induced thrombotic thrombocytopenia. Whether this immune stimulation is due to the antigenic crossover between the SARS-CoV-2 and human tissues5 or neoantigen formation due to vaccine components remains to be elucidated.6 Furthermore, whether prior systemic anticoagulation may have influenced our patient's clinical course remains unclear.
Clinicians need to be aware of Ad26.COV2.S-induced CSVV as a potential complication of the adenoviral vaccine. Further investigation of any additional cases will be crucial for estimating the frequency and pathophysiology of Ad26.COV2.S-induced CSVV. Furthermore, additional studies are needed to determine whether patients with severe adverse events to Ad26.COV2.S, including vaccine-induced thrombotic thrombocytopenia, should avoid future inoculation with alternative COVID-19 vaccines. Vaccine-related adverse events remain rare, and the benefits of Ad26.COV2.S vaccination for protection against severe COVID-19 clearly outweigh the extremely low risk of complications.7
Conflicts of interest
None disclosed.
Acknowledgments
We thank the patient for his cooperation and willingness to publish this case report. We appreciate Dr Mitchell Sternlieb (MD, Delaware Valley ID Associates), Dr Khan Thieu (MD, Innovative Dermatology), and Dr Gary Gordon (MD, Main Line Rheumatology) for their assistance in the care of this patient.
Footnotes
Funding sources: Lankenau Medical Center, Department of Internal Medicine.
IRB approval status: Not applicable.
References
- 1.Cines D.B., Bussel J.B. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384(23):2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muir K.L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384(20):1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez-Santas M., Diaz-Guimaraens B., Garcia Abellas P., Moreno-Garcia Del Real C., Burgos-Blasco P., Suarez-Valle A. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) J Eur Acad Dermatol Venereol. 2020;34(10):e536–e537. doi: 10.1111/jdv.16663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A., Selleng K., Mayerle J. Anti-SARS-CoV-2 spike protein and anti-platelet factor 4 antibody responses induced by COVID-19 disease and ChAdOx1 nCov-19 vaccination. Research Square. April 9, 2021 doi: 10.21203/rs.3.rs-404769/v1. [DOI] [Google Scholar]
- 7.Sadoff J., Gray G., Vandebosch A. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]