Abstract
In a community-based sample of seropositive adults (n = 1101), we found that seropositive individuals who lived with a known coronavirus disease 2019 (COVID-19) case exhibited higher blood anti–severe acute respiratory syndrome coronavirus 2 spike receptor-binding domain immunoglobulin G concentrations and greater symptom severity compared to seropositive individuals who did not live with a known COVID-19 case.
Keywords: immunity, transmission, severe acute respiratory syndrome coronavirus 2
Exposure intensity may contribute to severity of SARS-CoV-2 infection. Seropositive participants who had lived with a known COVID-19 case had higher antibody concentrations and greater symptom severity compared to seropositive participants who had not lived with a known COVID-19 case.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections are highly variable in both disease severity and subsequent immunoglobulin G (IgG) concentrations [1–3]. Greater disease severity is associated with higher blood antibody concentrations in the convalescent phase of infection, as we and others have reported previously [4–7]. Our earlier work, consistent with the other studies cited here, found that those who reported more symptoms had higher concentrations of IgG antibodies directed against the SARS-CoV-2 spike receptor-binding domain (RBD) in a community-based sample of seropositive adults [4–7].
It has been hypothesized that the magnitude of SARS-CoV-2 viral exposure (amount of virus inoculum, closer contact, more prolonged exposure) may contribute to disease severity [8–10]. This relation between exposure dose and disease severity has been demonstrated experimentally for other respiratory viruses, including influenza [11]. In addition, higher SARS-CoV-2 viral load during acute infection in humans has been linked to greater disease severity [12]. Respiratory virus exposures within the household may often be more intense than respiratory virus exposures outside of the household [8, 10]. For example, studies of measles epidemics have reported that cases acquired from a cohabitant have higher age-specific case fatality ratios compared to cases acquired outside the home [13, 14]. Exposure to SARS-CoV-2 within the household may be more prolonged, physically proximate, and unmitigated by personal protective equipment compared to transient public exposures [8, 10]. While some may attempt to isolate from cohabitants when they develop symptoms of coronavirus disease 2019 (COVID-19), it has been demonstrated that high levels of SARS-CoV-2 viral shedding occur prior to the onset of symptoms [15]. Furthermore, isolation within the household may not be achievable in many living situations.
The aim of this article is to compare symptom severity and SARS-CoV-2 IgG antibody concentrations in seropositive individuals who cohabitated with a known COVID-19 case vs seropositive individuals who did not cohabitate with a known COVID-19 case.
METHODS
The analytic sample in this report (n = 1101) featured participants who screened positive for anti–SARS-CoV-2 antibody in the Screening for Coronavirus Antibodies in Neighborhoods (SCAN) study [2, 7, 16, 17]. SCAN has administered online surveys and collected dried blood spot (DBS) samples from >5000 participants. Recruitment messages were disseminated through social media, email blasts, print flyers, newspaper advertisements, and local press coverage. Participants were recruited from neighborhoods throughout the Chicago area and from personnel of the Northwestern University Feinberg School of Medicine (FSM) in Chicago. Eligible participants who consented to participate completed an online survey and received a kit for self-collection of a DBS sample. DBS kits were either sent to participants through the mail or, for FSM participants, made available for pickup. All study protocols were approved by the institutional review board at Northwestern University (numbers STU00212457 and STU00212472).
IgG antibodies to the RBD of SARS-CoV-2 were quantified using an enzyme-linked immunosorbent assay that has received emergency use authorization from the US Food and Drug Administration (COVID-SeroKlir, Kantaro Biosciences) [18, 19]. We adapted and validated this assay for use with DBS samples (methods described in detail in [15], including quantification using dilutions of CR3022, an IgG with defined affinity to RBD) [16]. The cutoff for seropositivity was set at the optical density value for the 0.39 μg/mL calibrator [16].
Participants were presented a checklist of symptoms and asked to report whether they had experienced each symptom since 1 March 2020. In a previous study, we identified a cluster of 8 symptoms that were associated with higher SARS-CoV-2 IgG concentrations [7]. To provide an empirical basis for estimating symptom severity, we weighted each of these symptoms by its regression coefficient in a simple regression model with the symptom as the independent variable (1 = present, 0 = absent) and log2 IgG concentration as the dependent variable. Symptoms were assigned the following weights: loss of taste/smell = 1.05, fever = 0.69, muscle/body aches = 0.61, shortness of breath = 0.49, fatigue/excessive sleepiness = 0.46, diarrhea/nausea/vomiting = 0.43, cough = 0.41, and headache = 0.26. The resulting symptom severity score ranged from 0 to 4.40 (mean = 1.10, standard deviation [SD] = 1.22). Similar weighting schemes have been used in prior research to generate quantitative symptom severity scores [20, 21].
Exposure to cohabitants with COVID-19 was assessed by asking the following question, “Since 1 March 2020, has anyone in your household been told by a healthcare provider that they have, or likely have, COVID-19? Do not include yourself when answering this question.” We created a 3-category variable to represent household exposure (1 = lived with a known COVID-19 case, 2 = lived with at least 1 other person but did not live with a known COVID-19 case, 3 = lived alone).
The covariates in our statistical models included age, sex assigned at birth, racial/ethnic identity, chronic preexisting conditions (having 1 or more of the following: chronic kidney disease, chronic lung disease, diabetes mellitus, cardiovascular disease, or body mass index >30 kg/m2), and number of cohabitants in the household. We also asked whether they had used tobacco or worked outside the home in close proximity to others since 1 March 2020. To document date of inclusion in the study, we calculated the number of days after 1 March 2020 when the completed DBS kit was received at the laboratory.
We fitted ordinary least squares regression models with exposure to cohabitants with COVID-19 as the independent variable, with the covariates described above, and with symptom severity scores and log2 IgG concentrations as the dependent variables. These models were fitted using the “lm” function in base R (version 4.0.3).
RESULTS
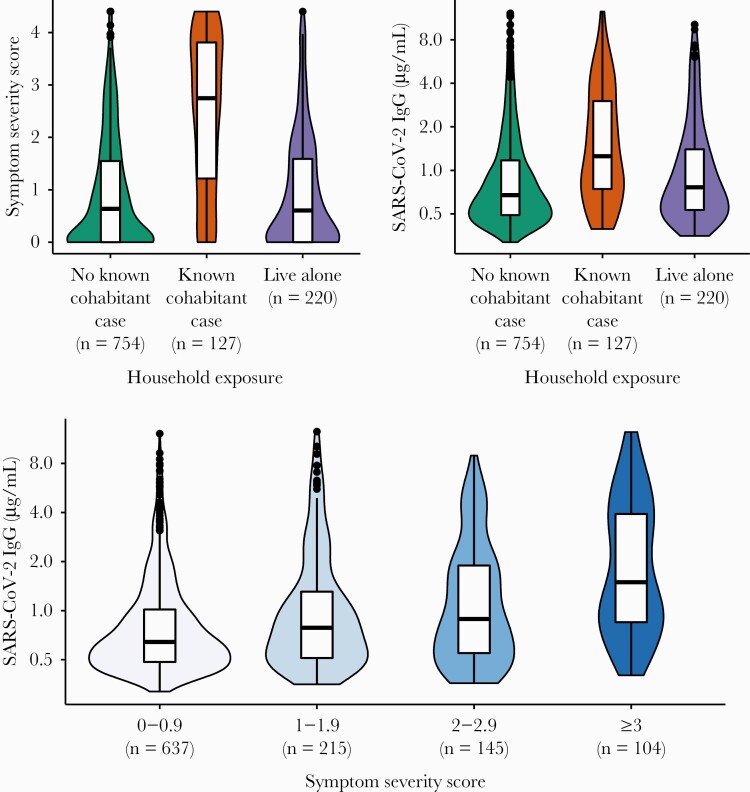
Out of 1101 seropositive participants, 42.7% identified as non-Hispanic white, 23.9% as Hispanic/Latinx, 8.5% as non-Hispanic black, 18.9% as Asian, and 6% selected other response categories for race/ethnicity. The mean age was 38.62 (SD = 12.55), and 56.2% were assigned female at birth. Figure 1 depicts symptom severity and SARS-CoV-2 IgG levels by household exposure status.
Figure 1.
Associations between exposure to a cohabitant with coronavirus disease 2019, symptom severity scores, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody concentrations. The y-axis for SARS-CoV-2 immunoglobulin G (IgG) concentrations is presented on a log2 scale. Data are from a community-based sample of seropositive adults from the Chicago area (N = 1011). The median date of inclusion in the study was 23 October 2020 (range, 30 June 2020 to 20 January 2021). None of the study participants reported having been vaccinated for SARS-CoV-2 at the time of survey completion. The distribution for each category is represented by smoothed kernel density plots overlaid with boxplots depicting interquartile ranges. This figure was created using the R package “ggplot2” (version 3.3.2).
In a regression model adjusted for age, sex, race/ethnicity, chronic preexisting conditions, tobacco use, working in proximity to others, number of cohabitants, and date of inclusion in the study, we found that those who lived with a known COVID-19 case exhibited greater symptom severity compared to those who did not live with known COVID-19 case (β = 1.358, standard error [SE] = 0.107; P < .0001) and those who lived alone (β = 1.298, SE = 0.144; P < .0001) (Table 1).
Table 1.
Unstandardized Coefficients, With Standard Errors in Parentheses, From Ordinary Least Squares Regression Models With Symptom Scores and Log2 Severe Acute Respiratory Syndrome Coronavirus 2 Immunoglobulin G Levels as Outcome Variables
| Characteristic | Outcome Variable | |
|---|---|---|
| Symptom Severity Score, β Coefficient (SE) | Log2 IgG Concentration, µg/mL, β Coefficient (SE) | |
| Age | –0.002 (0.003) | 0.007 (0.003) |
| Assigned female at birth | 0.091 (0.069) | 0.129 (0.068) |
| Race/ethnicity (ref = white) | ||
| Hispanic/Latinx | 0.270 (0.087) | 0.180 (0.086) |
| Black | 0.175 (0.126) | 0.234 (0.124) |
| Asian | –0.170 (0.093) | –0.166 (0.092) |
| Other | 0.263 (0.145) | 0.252 (0.143) |
| Chronic preexisting condition | 0.369 (0.078) | 0.036 (0.077) |
| Tobacco use | 0.225 (0.134) | 0.039 (0.132) |
| Working in proximity to others | –0.094 (0.069) | 0.012 (0.068) |
| Number of cohabitants in household | 0.012 (0.029) | –0.017 (0.029) |
| Date of inclusion in the study | 0.002 (0.001) | 0.002 (0.001) |
| Household exposure statusa | ||
| Known cohabitant case vs no known cohabitant case | 1.358 (0.107) | 0.718 (0.105) |
| Known cohabitant case vs people who live alone | 1.298 (0.144) | 0.536 (0.141) |
| No known cohabitant case vs people who live alone | –0.060 (0.104) | –0.182 (0.103) |
| Intercept | 0.364 (0.216) | –1.103 (0.213) |
| Observations, No. | 1101 | 1101 |
| R 2 | 0.208 | 0.087 |
Data are from a sample of adults seropositive for severe acute respiratory syndrome coronavirus 2 in the Chicago area (n = 1101). Table created using the R package “Stargazer” (version 5.2.2). Coefficients statistically significant at P < .05 are shown in bold.
Abbreviations: IgG, immunoglobulin G; SE, standard error.
aReferent group was rotated to depict all pairwise contrasts.
To evaluate whether this pattern was robust to a different method of operationalizing symptoms, we repeated these analyses with symptom level operationalized as a simple count variable in a Poisson regression model with the same covariates depicted in Table 1. Those who lived with a known COVID-19 case had higher symptom counts compared to those who did not live with a known COVID-19 case (β = 0.740, SE = 0.051; P < .0001) and those who lived alone (β = 0.709, SE = 0.080; P < .0001).
In a regression model adjusted for age, sex, race/ethnicity, chronic preexisting conditions, tobacco use, working in proximity to others, number of cohabitants, and date of inclusion in the study, we found that those who lived with a known COVID-19 case had higher log2 anti–SARS-CoV-2 RBD IgG levels compared to those who did not live with a known COVID-19 case (β = 0.718, SE = 0.105; P < .0001) and those who lived alone (β = 0.536, SE = 0.141; P = .0002) (Table 1).
DISCUSSION
In this study, we found that seropositive participants who lived with a known COVID-19 case had greater symptom severity and higher anti–SARS-CoV-2 RBD IgG levels compared to seropositive individuals who did not live with a known COVID-19 case. These results call for further research on whether the magnitude or duration of viral exposure explains the relation between household exposure, symptom severity, and antibody concentrations.
Notably, working in close proximity to others, which has been reported to increase risk for COVID-19 [22], was not associated with symptom severity or IgG concentration here. However, we did not ask whether participants had worked in close proximity with a known COVID-19 case.
This study design does not allow us to determine whether the cohabitant diagnosed with COVID-19 was the first SARS-CoV-2 case in the household. If the study participant was infected prior to the cohabitant, the association observed here may be due to greater risk of secondary spread to a cohabitant from a serosurvey participant. However, seropositive participants who did not live with a known case and seropositive participants who lived alone had similarly low levels of symptom severity and IgG concentrations. Those who lived alone could not have transmitted SARS-CoV-2 to a cohabitant. This suggests that similarly low levels of symptom severity and IgG concentrations in these 2 groups may be explained by their shared lack of exposure to another COVID-19 case within the household. Future studies should sample all household members and track timing of cases to confirm, or refute, the hypothesis that cohabitation with a COVID-19 case leads to more prolonged exposure or a higher virus inoculum that causes increased antibody responses, as well as greater symptom severity.
Regardless of which individual in the household was infected first, our results suggest that addressing transmission within households should be a critical area of focus for public health efforts to prevent symptomatic COVID-19. Policies and interventions that apply only to public places (eg, mask mandates, business capacity limits) may be improved in effectively preventing spread if they are combined with measures that also reduce transmission within households (eg, intensive testing, contact tracing, and isolation programs). The results of our study demonstrate the importance of sampling an adequate range of variation in exposure context, symptom severity, and antibody level quantitation to better understand transmission dynamics and inform policy [23].
Notes
Author contributions. J. M. S. conceptualized the paper, conducted the data analysis, and wrote the original draft of the manuscript. B. M., A. R. D., R. D., E. M. M., M. E. N., N. B., and T. W. M. contributed to study design and revised the manuscript critically for important intellectual content. R. S., D. T. R., L. A. V., M. P. V., N. R., and R. H. contributed to data acquisition and reviewed the manuscript.
Disclaimer. The funding sources had no role in the study design, data collection, analysis, interpretation, or writing of the report.
Financial support. This material is based upon work supported by the National Science Foundation (grant number 2035114 to T. W. M., R. D., E. M. M., B. M., and A. R. D.). Additional support was provided by the Northwestern University Office of Research, the Northwestern University Clinical and Translational Sciences Institute (National Institutes of Health grant number UL1TR001422), and a generous gift from Dr Andrew Senyei and Noni Senyei.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med 2021; 27:28–33. [DOI] [PubMed] [Google Scholar]
- 2. Demonbreun AR, McDade TW, Pesce L, et al. Patterns and persistence of SARS-CoV-2 IgG antibodies in a US metropolitan site. medRxiv [Preprint]. Posted online 18 November 2020. doi:10.1101/2020.11.17.20233452. [Google Scholar]
- 3. Rodebaugh TL, Frumkin MR, Reiersen AM, et al. Acute symptoms of mild to moderate COVID-19 are highly heterogeneous across individuals and over time. Open Forum Infect Dis 2021; 8:ofab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeewandara C, Jayathilaka D, Gomes L, et al. SARS-CoV-2 neutralizing antibodies in patients with varying severity of acute COVID-19 illness. Sci Rep 2021; 11:2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett M, Yoder S, Brady E, et al. A high-throughput liquid bead array assay confirms strong correlation between SARS-CoV-2 antibody level and COVID-19 severity. iScience 2021; 24:102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kowitdamrong E, Puthanakit T, Jantarabenjakul W, et al. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS One 2020; 15:e0240502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McDade TW, Schrock JM, D’Aquila R, et al. Symptoms of COVID-19 infection and magnitude of antibody response in a large community-based study. medRxiv [Preprint]. Posted online 8 February 2021. doi:10.1101/2021.2002.2004.21251170. [Google Scholar]
- 8. Guallar MP, Meirino R, Donat-Vargas C, et al. Inoculum at the time of SARS-CoV-2 exposure and risk of disease severity. Int J Infect Dis 2020; 97:290–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imai M, Iwatsuki-Horimoto K, Hatta M, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci U S A 2020; 117:16587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Damme W, Dahake R, van de Pas R, et al. COVID-19: Does the infectious inoculum dose-response relationship contribute to understanding heterogeneity in disease severity and transmission dynamics? Med Hypotheses 2021; 146:110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han A, Czajkowski LM, Donaldson A, et al. A dose-finding study of a wild-type influenza A(H3N2) virus in a healthy volunteer human challenge model. Clin Infect Dis 2019; 69:2082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020; 11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aaby P. Determinants of measles mortality: host or transmission factors? In: de la Maza LM, Peterson EM, eds. Medical Virology 10. Boston, MA: Springer US; 1991:83–116. [Google Scholar]
- 14. Wolfson LJ, Grais RF, Luquero FJ, et al. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int J Epidemiol 2009; 38:192–205. [DOI] [PubMed] [Google Scholar]
- 15. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. [DOI] [PubMed] [Google Scholar]
- 16. McDade TW, McNally EM, Zelikovich AS, et al. High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay. PLoS One 2020; 15:e0237833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mustanski B, Saber R, Ryan DT, et al. Geographic disparities in COVID-19 case rates are not reflected in seropositivity rates using a neighborhood survey in Chicago. medRxiv [Preprint]. Posted online 5 March 2021. doi:10.1101/2021.2003.2002.21252767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020; 26:1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kantaro Biosciences. Kantaro receives FDA emergency use authorization for semi-quantitative COVID-19 antibody test kit that detects the presence and level of SARS-CoV-2 IgG antibodies. 2021. Available at: https://kantarobio.com/kantaro-receives-fda-emergency-use-authorization-for-semi-quantitative-covid-19-antibody-test-kit-that-detects-the-presence-and-level-of-sars-cov-2-igg-antibodies/. Accessed 8 March 2021.
- 20. Bucks RS, Gidron Y, Harris P, et al. Selective effects of upper respiratory tract infection on cognition, mood and emotion processing: a prospective study. Brain Behav Immun 2008; 22:399–407. [DOI] [PubMed] [Google Scholar]
- 21. Orts K, Sheridan JF, Robinson-Whelen S, et al. The reliability and validity of a structured interview for the assessment of infectious illness symptoms. J Behav Med 1995; 18:517–29. [DOI] [PubMed] [Google Scholar]
- 22. Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants [manuscript published online ahead of print 9 December 2020]. Occup Environ Med 2020. doi:10.1136/oemed-2020-106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America guidelines on the diagnosis of coronavirus disease 2019 (COVID-19): serologic testing [manuscript published online ahead of print 12 September 2020]. Clin Infect Dis 2020. doi:10.1093/cid/ciaa1343. [DOI] [PMC free article] [PubMed] [Google Scholar]