Abstract
Background & Aims
The impact of chronic liver disease on outcomes in patients with COVID-19 is uncertain. Hence, we aimed to explore this association.
Methods
We explored the outcomes of all adult inpatients with COVID-19 in France, in 2020. We computed adjusted odds ratios to measure the associations between chronic liver disease, alcohol use disorders, mechanical ventilation and day-30 in-hospital mortality.
Results
The sample comprised 259,110 patients (median [IQR] age 70 (54–83) years; 52% men), including 15,476 (6.0%) and 10,006 (3.9%) patients with chronic liver disease and alcohol use disorders, respectively. Death occurred in 38,203 (15%) patients, including 7,475 (28%) after mechanical ventilation, and 2,941 (19%) with chronic liver disease. The adjusted odds ratios for mechanical ventilation and day-30 mortality were 1.54 (95% CI 1.44–1.64, p <0.001) and 1.79 (1.71–1.87, p <0.001) for chronic liver disease; 0.55 (0.47–0.64, p <0.001) and 0.54 (0.48–0.61, p <0.001) for mild liver disease; 0.64 (0.53–0.76; p <0.001) and 0.71 (0.63–0.80, p <0.001) for compensated cirrhosis; 0.65 (0.52–0.81, p <0.001) and 2.21 (1.94–2.51, p <0.001) for decompensated cirrhosis; 0.34 (0.24–0.50; p <0.001) and 1.38 (1.17–1.62, p <0.001) for primary liver cancer; and 0.82 (0.76–0.89; p <0.001) and 1.11 (1.05–1.17; p <0.001) for alcohol use disorders. Chronic viral hepatitis; non-viral, non-alcoholic chronic hepatitis; organ, including liver, transplantation, and acquired immunodeficiency syndrome were not associated with COVID-19-related death.
Conclusion
Chronic liver disease increased the risk of COVID-19-related death in France in 2020. Therapeutic effort limitation may have contributed to COVID-19-related death in French residents with a liver-related complication or an alcohol use disorder.
Lay summary
We studied the outcomes, including mechanical ventilation and day-30 mortality, of all adults with COVID-19 who were discharged from acute and post-acute care in France in 2020 (N = 259,110). Patients with mild liver disease; compensated cirrhosis; organ, including liver, transplantation; or acquired immunodepression syndrome were not at increased risk of COVID-19-related mortality. Patients with alcohol use disorders, decompensated cirrhosis, or primary liver cancer were at increased risk of COVID-19-related mortality but were less likely to receive mechanical ventilation. Our results suggest that therapeutic effort limitation may have contributed to the excess mortality in French residents with a liver-related complication or an alcohol use disorder.
Keywords: chronic liver disease; COVID-19; Respiration, Artificial; mortality; Withholding Treatment; alcohol use disorders
Graphical abstract
Introduction
In late-December 2019, the first case COVID-19 was described in the city of Wuhan in Hubei province, central China.1 In 2020, COVID-19 became a global pandemic, and was associated, at the beginning of 2021, with more than 2.4 million deaths.2 Risk factors for severe COVID-19 and COVID-19 mortality, including older ages, male sex, hypertension, obesity and severe comorbidities, have been consistently reported since the beginning of the pandemic.3 , 4 It is uncertain whether chronic liver disease associates with COVID-19 outcome. In a meta-analysis pooling 904 patients from the first pandemic wave in China, chronic liver disease was not associated with COVID-19 mortality.5 In a more recent, global, meta-analysis pooling 90,095 patients, chronic liver disease patients were at increased risk of severe COVID-19 (pooled effect size 1.52, 95% CI 1.14–2.02) and COVID-19 mortality (pooled effect size 1.36, 95% CI 1.22–1.53).6 The term ‘chronic liver disease’ comprises a spectrum of conditions, including underlying hepatopathy, such as alcohol use disorders or viral hepatitis, liver disease stage, such as compensated or decompensated cirrhosis, immune status, such as chemotherapy for cancer or immunosuppression for liver transplantation. This heterogeneity may differently affect COVID-19 outcomes. In 2 early retrospective studies from China, metabolic liver disease was associated with progressive COVID-19.7 , 8 In a cohort study from the United States, alcohol-related liver disease and hepatocellular carcinoma, but not viral hepatitis, were associated with COVID-19 mortality.9 In 2 European cohorts, liver transplant recipients were at increased risk of requiring mechanical ventilation, but not of COVID-19 mortality.[10], [11], [12] In a cohort of 932 patients with chronic liver disease, autoimmune hepatitis was associated with a higher risk of admission and not with mechanical ventilation and mortality.13 In a retrospective cohort of 50 patients with cirrhosis from Lombardy (Italy), during the first pandemic wave, acute respiratory failure was the main cause of death and mortality was 35%.14 In a retrospective cohort of 37 and 108 North American COVID-19 patients with and without cirrhosis, respectively, the rate of invasive mechanical ventilation for respiratory failure was identical, and cirrhosis was not associated with in-hospital mortality.15 These uncertainties prompted us to investigate the association between chronic liver disease and outcomes, including death and mechanical ventilation (a surrogate of COVID-19 respiratory failure), in the 259,110 adult inpatients with COVID-19 during the 2 pandemic waves in France in 2020.
Patients and methods
Data source
The data source was the French National Hospital Discharge database (Programme de Médicalisation des Systèmes d’Information), which contains all public and private claims for acute inpatient/day case hospital admissions and post-acute care since 2011. The anonymized standardized discharge summaries include: demographics (age, sex, postal code of residency); primary and associated discharge diagnosis codes according to the WHO International Classification of Diseases, tenth revision (ICD-10); medical procedures performed, including mechanical ventilation, and renal replacement therapy; length of stay, entry and discharge modes (including in-hospital death). Using unique anonymous identifiers, we traced back the hospital trajectory of each selected patient and recorded his/her previous/underlying conditions.16 The French Programme de Médicalisation des Systèmes d’Information coding system was shown to be 100% specific for hard outcomes, including decompensated cirrhosis, primary liver cancer, solid-organ transplantation, renal replacement therapy, mechanical ventilation, and in-hospital mortality.17 , 18 The study was approved by the Institut national des données de santé (INDS, registration number 917240) and authorized by the Commission nationale de l’informatique et des libertés (CNIL, registration number DR-2017–404). The requirement for informed consent was waived because the study used de-identified data.
Study population
As of January 1, 2021, there were 67,422,000 residents in France.19 On March 31, 2021 we selected 266,604 patients discharged from 2,187 hospitals or hospital groups after COVID-19 (ICD-10 codes: U0710, U0711, U0712, U0714, U0715) between January 1 and December 31, 2020. We removed 1,986 patients with inconsistent discharge modes. Patients younger than 18 years (n = 5,508) were not included in the analysis. We also collected the vital status of day case and post-acute care patients.
Outcome measures
The main outcomes were mechanical ventilation and day-30 COVID-19 mortality. Liver disease progression was a composite outcome of decompensated cirrhosis, defined by the onset of a liver-related event (defined as any of ascites, variceal bleeding, hepatic encephalopathy or non-obstructive jaundice) in a patient with otherwise compensated cirrhosis before COVID-19.18
Exposures
We investigated the relationship between chronic liver disease and mechanical ventilation for COVID-19 and COVID-19 mortality. We considered that intubation and mechanical ventilation was a surrogate of COVID-19 severity.20 Chronic liver disease included mild liver disease (chronic liver disease without cirrhosis); compensated cirrhosis; decompensated cirrhosis (cirrhosis with a liver-related complication before COVID-19); alcoholic liver disease; chronic hepatitis B; chronic hepatitis C; other, non-viral, non-alcoholic, causes of chronic liver disease; primary liver cancer; and liver transplantation.17 , 18 Comorbidities of COVID-19 were alcohol use disorders; past or current smoking; obesity; essential (primary) hypertension; diabetes mellitus; chronic kidney disease, without kidney transplantation; chronic obstructive pulmonary disease; organ transplant recipient, without liver transplantation; solid tumor, without liver cancer, localized or advanced; and acquired immunodeficiency syndrome. The Charlson comorbidity index was used to capture the risk associated with multiple comorbidities before COVID-19.21 The Charlson comorbidity index is the most extensively studied comorbidity score and has been widely used to predict mortality and disability outcomes, including in the setting of COVID-19 in patients with cirrhosis.15 , 22 It includes 19 major comorbidities such as chronic pulmonary disease, diabetes, and kidney or liver disease. The Charlson comorbidity index was previously adapted for use with ICD-10 from administrative inpatient or outpatient data,23 which have been reported to have good agreement with medical chart review of intensive care unit patients.23 In the study, the Charlson comorbidity index was calculated without age and liver disease in order to isolate their respective effects in the binary logistic regression models. Complications after COVID-19 were acute respiratory failure, including mechanical ventilation; acute kidney injury, including renal replacement therapy; acute liver failure; pulmonary embolism; portal vein thrombosis; and liver disease progression to decompensated cirrhosis in an otherwise compensated patient.
Statistical analysis
The strengths of associations with mechanical ventilation and day-30 mortality were estimated with multivariate binary logistic regressions. All statistical tests were based on 2-tailed p values, with p <0.05 considered to indicate statistical significance. All analyses were performed using RStudio statistical software (Version 1.4.869 © 2009-2020 RStudio, Inc).
Results
Sample characteristics
The sample consisted of 259,110 adult patients (median [IQR] age, 70 (54–83) years; 52% men). A total of 15,476 (6.0%) patients had chronic liver disease, including 3,623 (23.4%) with alcohol-related liver disease; 820 (5.3%) with chronic hepatitis B; 711 (4.6%) with chronic hepatitis C; 2,299 (14.9%) with a non-viral, non-alcohol-related, cause of chronic liver disease; 719 (4.6%) with primary liver cancer; and 329 (2.1%) with a liver transplant.
Table 1 details the characteristics of the cohort by chronic liver disease. Patients with chronic liver disease were more often males (p <0.001). The age distribution was also different (p <0.001), with a median (IQR) age of 70 (54–83) and 69 (58–79) years for patients without and with chronic liver disease, respectively. Patients with chronic liver disease more frequently had (p <0.001) alcohol use disorders (~one-fourth of patients); current or past tobacco use (14% of patients); obesity (~one-fourth of patients); hypertension (57% of patients); and diabetes mellitus (~40% of patients), including 38% with complications of diabetes mellitus. Major comorbidities were common in the cohort (58% of patients) and were over-represented (p <0.001) in patients with chronic liver disease, except dementia (see Table S1).
Table 1.
Characteristics of 2020 French COVID-19 patients by chronic liver disease.
| Characteristic | Overall, N = 259,1101 |
Chronic liver disease |
p value2 | |
|---|---|---|---|---|
| No, n = 243,634 (94%)1 | Yes, n = 15,476 (6.0%)1 | |||
| Sex | <0.001 | |||
| Male | 135,173 (52%) | 125,659 (52%) | 9,514 (61%) | |
| Female | 123,936 (48%) | 117,974 (48%) | 5,962 (39%) | |
| Age | 70 (54, 83) | 70 (54, 83) | 69 (58, 79) | <0.001 |
| Age category, years | <0.001 | |||
| [18,30) | 12,499 (4.8%) | 12,122 (5.0%) | 377 (2.4%) | |
| [30,40) | 16,222 (6.3%) | 15,582 (6.4%) | 640 (4.1%) | |
| [40,50) | 21,245 (8.2%) | 20,104 (8.3%) | 1,141 (7.4%) | |
| [50,60) | 33,054 (13%) | 30,824 (13%) | 2,230 (14%) | |
| [60,70) | 42,088 (16%) | 38,613 (16%) | 3,475 (22%) | |
| [70,80) | 49,658 (19%) | 45,855 (19%) | 3,803 (25%) | |
| [80,90) | 57,512 (22%) | 54,544 (22%) | 2,968 (19%) | |
| [90,Inf) | 26,832 (10%) | 25,990 (11%) | 842 (5.4%) | |
| Alcohol use disorders | 10,006 (3.9%) | 6,383 (2.6%) | 3,623 (23%) | <0.001 |
| Current or past tobacco use | 15,049 (5.8%) | 12,817 (5.3%) | 2,232 (14%) | <0.001 |
| Obesity | 43,124 (17%) | 39,145 (16%) | 3,979 (26%) | <0.001 |
| Essential (primary) hypertension | 113,407 (44%) | 104,564 (43%) | 8,843 (57%) | <0.001 |
| Diabetes mellitus | 61,664 (24%) | 55,753 (23%) | 5,911 (38%) | <0.001 |
| Modified Charlson comorbidity index | <0.001 | |||
| [0,2) | 170,308 (66%) | 164,165 (67%) | 6,143 (40%) | |
| [2,4) | 57,876 (22%) | 52,764 (22%) | 5,112 (33%) | |
| [4,6) | 20,893 (8.1%) | 18,423 (7.6%) | 2,470 (16%) | |
| [6,8) | 7,651 (3.0%) | 6,404 (2.6%) | 1,247 (8.1%) | |
| [8,Inf) | 2,382 (0.9%) | 1,878 (0.8%) | 504 (3.3%) | |
| Acute respiratory distress syndrome | 67,006 (26%) | 63,041 (26%) | 3,965 (26%) | 0.5 |
| Mechanical ventilation | 18,049 (7.0%) | 16,449 (6.8%) | 1,600 (10%) | <0.001 |
| Acute kidney injury | 12,461 (4.8%) | 11,208 (4.6%) | 1,253 (8.1%) | <0.001 |
| Renal replacement therapy | 2,892 (1.1%) | 2,441 (1.0%) | 451 (2.9%) | <0.001 |
| Pulmonary embolism | 8,179 (3.2%) | 7,647 (3.1%) | 532 (3.4%) | 0.039 |
| Acute liver failure | 1,222 (0.5%) | 552 (0.2%) | 670 (4.3%) | <0.001 |
| Portal vein thrombosis | 148 (<0.1%) | 53 (<0.1%) | 95 (0.6%) | <0.001 |
| Liver disease progression | 17 (<0.1%) | 0 (0%) | 17 (0.1%) | <0.001 |
| Day-30 post-COVID mortality | 38,203 (15%) | 35,262 (14%) | 2,941 (19%) | <0.001 |
Data are for patients who were discharged after COVID-19 between February 1 and December 31, 2020 in France. The 2011-2020 French National Patient Registry was used to indentiy underlying conditions before COVID-19. The Charlson comorbodity index predicts 10-year survival in patients with multiple comorbidities, and ranges from 0 to 30, without entering age and liver disease, with higher scores indicating higher frailty.
n (%); Median (IQR).
Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test.
Chronic liver disease and risks of organ failure
Chronic liver disease patients were at increased risk (p <0.001) of mechanical ventilation (10% of patients), but not of acute respiratory distress syndrome (p = 0.5). Acute kidney injury (8.1% of patients; p <0.001); renal replacement therapy (~one-third of patients with acute kidney injury; p <0.001); and pulmonary embolism (3.4% of patients; p = 0.039) were associated with chronic liver disease. Acute liver failure was ~20 times more frequent (p <0.001) in patients with chronic liver disease and occurred in 4.3% of this group. Portal vein thrombosis after COVID-19 was rare (<0.1%) and was more common (p <0.001) in patients with chronic liver disease, occurring in 0.6% of this group. Liver disease progression to decompensated cirrhosis was also rare and was only recorded in 17 (0.1%) patients with compensated disease before COVID-19.
Risk of mechanical ventilation after COVID-19
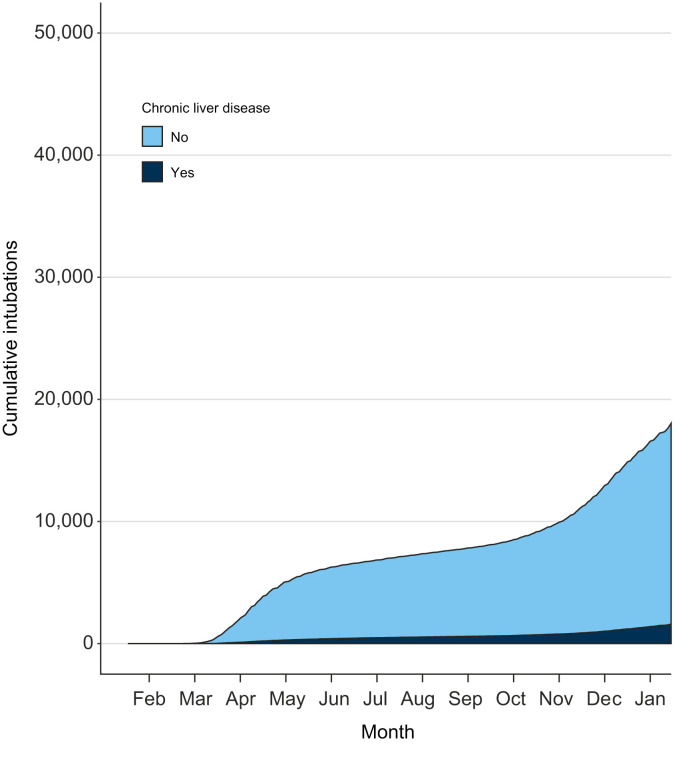
The contribution of chronic liver disease to mechanical ventilation is presented in Fig. 1 . The risks are detailed in Table 2 . Male sex and younger ages were risk factors. There was an over-representation (78%) of the [50-80) age category in the mechanical ventilation group, and an under-representation of the [18-50) and [80-Inf) age categories. Alcohol use disorders; current or past tobacco use; obesity; hypertension; diabetes mellitus; alcohol-related liver disease; chronic viral hepatitis; non-alcoholic, other non-metabolic causes of chronic liver disease; mild liver disease, compensated cirrhosis and decompensated cirrhosis; organ, including liver, transplantation; and acquired immunodeficiency syndrome were all associated (all p values <0.001) with mechanical ventilation. Patients with a modified Charlson comorbidity index ≥2 were also at increased risk. Patients with a primary liver cancer were not (p = 0.039) at increased risk of mechanical ventilation unless they were exposed to cancer treatment (p = 0.001). All outcomes, including acute liver failure and portal vein thrombosis, but not liver disease progression, were associated (p <0.001) with mechanical ventilation. Day-30 mortality was ~twice as high (28%) in patients with mechanical ventilation than in patients without mechanical ventilation (p <0.001).
Fig. 1.
Contribution of chronic liver disease to the burden of mechanical ventilation for severe COVID-19 in France, 2020.
Data are for patients who were discharged after COVID-19 between February 1 and December 31, 2020 in Metropolitan France. The 2011-2020 French National Patient Registry was used to identify chronic liver disease before COVID-19.
Table 2.
Characteristics of 2020 French COVID-19 patients by invasive mechanical ventilation.
| Characteristic | Overall N = 259,1101 |
Invasive mechanical ventilation |
p value2 | |
|---|---|---|---|---|
| No n = 232,053 (90%)1 | Yes n = 27,057 (10%)1 | |||
| Sex | <0.001 | |||
| Male | 135,173 (52%) | 116,302 (50%) | 18,871 (70%) | |
| Female | 123,936 (48%) | 115,751 (50%) | 8,185 (30%) | |
| Age | 70 (54, 83) | 71 (54, 84) | 68 (59, 75) | <0.001 |
| Age category, years | <0.001 | |||
| [18,30) | 12,499 (4.8%) | 12,173 (5.2%) | 326 (1.2%) | |
| [30,40) | 16,222 (6.3%) | 15,448 (6.7%) | 774 (2.9%) | |
| [40,50) | 21,245 (8.2%) | 19,483 (8.4%) | 1,762 (6.5%) | |
| [50,60) | 33,054 (13%) | 28,637 (12%) | 4,417 (16%) | |
| [60,70) | 42,088 (16%) | 34,074 (15%) | 8,014 (30%) | |
| [70,80) | 49,658 (19%) | 41,108 (18%) | 8,550 (32%) | |
| [80,90) | 57,512 (22%) | 54,576 (24%) | 2,936 (11%) | |
| [90,Inf) | 26,832 (10%) | 26,554 (11%) | 278 (1.0%) | |
| Alcohol use disorders | 10,006 (3.9%) | 7,956 (3.4%) | 2,050 (7.6%) | <0.001 |
| Current or past tobacco use | 15,049 (5.8%) | 11,745 (5.1%) | 3,304 (12%) | <0.001 |
| Obesity | 43,124 (17%) | 34,002 (15%) | 9,122 (34%) | <0.001 |
| Essential (primary) hypertension | 113,407 (44%) | 97,607 (42%) | 15,800 (58%) | <0.001 |
| Diabetes mellitus | 61,664 (24%) | 51,950 (22%) | 9,714 (36%) | <0.001 |
| Chronic liver disease | 15,476 (6.0%) | 12,233 (5.3%) | 3,243 (12%) | <0.001 |
| Alcoholic liver disease | 3,623 (1.4%) | 2,769 (1.2%) | 854 (3.2%) | <0.001 |
| Chronic hepatitis B | 820 (0.3%) | 667 (0.3%) | 153 (0.6%) | <0.001 |
| Chronic hepatitis C | 711 (0.3%) | 570 (0.2%) | 141 (0.5%) | <0.001 |
| Other causes of chronic liver disease | 2,299 (0.9%) | 1,864 (0.8%) | 435 (1.6%) | <0.001 |
| Mild liver disease | 2,567 (1.0%) | 2,147 (0.9%) | 420 (1.6%) | <0.001 |
| Compensated cirrhosis | 1,951 (0.8%) | 1,632 (0.7%) | 319 (1.2%) | <0.001 |
| Advanced cirrhosis | 1,256 (0.5%) | 950 (0.4%) | 306 (1.1%) | <0.001 |
| Primary liver cancer | 719 (0.3%) | 627 (0.3%) | 92 (0.3%) | 0.039 |
| Primary liver cancer with specific treatment | 383 (0.1%) | 324 (0.1%) | 59 (0.2%) | 0.001 |
| Liver transplantation | 329 (0.1%) | 210 (<0.1%) | 119 (0.4%) | <0.001 |
| Transplant recipient (without liver transplantation) | 2,290 (0.9%) | 1,682 (0.7%) | 608 (2.2%) | <0.001 |
| Acquired immunodeficiency syndrome | 1,118 (0.4%) | 920 (0.4%) | 198 (0.7%) | <0.001 |
| Modified Charlson comorbidity index | <0.001 | |||
| [0,2) | 170,308 (66%) | 155,300 (67%) | 15,008 (55%) | |
| [2,4) | 57,876 (22%) | 50,497 (22%) | 7,379 (27%) | |
| [4,6) | 20,893 (8.1%) | 17,904 (7.7%) | 2,989 (11%) | |
| [6,8) | 7,651 (3.0%) | 6,420 (2.8%) | 1,231 (4.5%) | |
| [8,Inf) | 2,382 (0.9%) | 1,932 (0.8%) | 450 (1.7%) | |
| Acute respiratory distress syndrome | 67,006 (26%) | 48,531 (21%) | 18,475 (68%) | <0.001 |
| Acute kidney injury after COVID-19 | 12,461 (4.8%) | 7,282 (3.1%) | 5,179 (19%) | <0.001 |
| Renal replacement therapy for COVID-19 | 2,892 (1.1%) | 294 (0.1%) | 2,598 (9.6%) | <0.001 |
| Pulmonary embolism | 8,179 (3.2%) | 6,418 (2.8%) | 1,761 (6.5%) | <0.001 |
| Acute liver failure after COVID-19 | 1,222 (0.5%) | 712 (0.3%) | 510 (1.9%) | <0.001 |
| Portal vein thrombosis | 148 (<0.1%) | 109 (<0.1%) | 39 (0.1%) | <0.001 |
| Liver disease progression | 17 (<0.1%) | 13 (<0.1%) | 4 (<0.1%) | 0.094 |
| Day-30 post-COVID mortality | 38,203 (15%) | 30,728 (13%) | 7,475 (28%) | <0.001 |
Data are for patients who were discharged after COVID-19 between February 1 and December 31, 2020 in France. The 2011-2020 French National Patient Registry was used to indentiy underlying conditions before COVID-19. Mild liver disease was chronic liver disease without cirrhosis. Advanced cirrhosis was cirrhosis with ascites, hypertensive bleeding, non-obstructive jaundice, or encephalopathy. The Charlson comorbodity index predicts 10-year survival in patients with multiple comorbidities, and ranges from 0 to 30, without entering age and liver disease, with higher scores indicating higher frailty.
n (%); Median (IQR).
Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test.
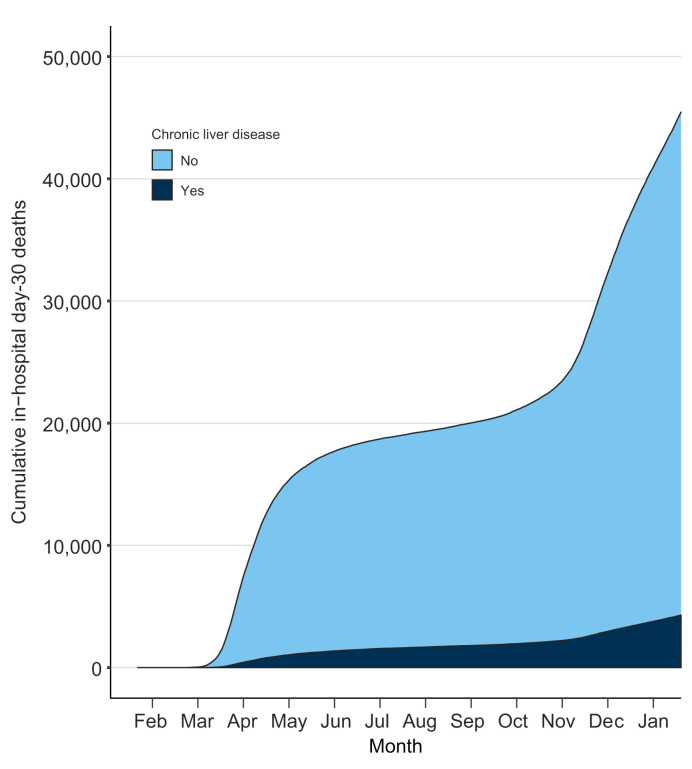
Risks of day-30 mortality after COVID-19
The contribution of chronic liver disease to the 38,203 (15%) deaths within 30 days after admission for COVID-19 was 2,941 (7.7%; p <0.001) and is presented in Fig. 2 . Overall, in-hospital mortality after COVID-19 was 45,453 (17.5%). The risks for day-30 mortality are detailed in Table 3 . Male sex and older ages (median [IQR] 83 (75–89) years) were risk factors, with an over-representation (63%) of the [80, Inf) age category. Alcohol use disorders and alcohol-related liver disease; hypertension (more than half of patients); diabetes mellitus (30% of deceased patients); compensated cirrhosis; advanced cirrhosis; and primary liver cancer, with or without a specific treatment, were also risk factors (p <0.001). A modified Charlson comorbidity index ≥2 was associated with mortality (p <0.001). Mild liver disease; chronic viral hepatitis; non-alcoholic, other non-metabolic causes of chronic liver disease; organ, including liver, transplantation; acquired immunodeficiency syndrome; and smoking were not associated with day-30 mortality. All outcomes, except liver disease progression (the number of events was low) were associated (p <0.001) with mortality.
Fig. 2.
Contribution of chronic liver disease to the death toll of COVID-19 in France, 2020.
Data are for patients who were discharged after COVID-19 between February 1 and December 31, 2020 in Metropolitan France. The 2011-2020 French National Patient Registry was used to identify chronic liver disease before COVID-19.
Table 3.
Characteristics of 2020 French COVID-19 patients by day-30 mortality.
| Characteristic | Overall N = 259,1101 |
Day-30 mortality |
p value2 | |
|---|---|---|---|---|
| No n = 220,907 (85%)1 | Yes n = 38,203 (15%)1 | |||
| Sex | <0.001 | |||
| Male | 135,173 (52%) | 112,741 (51%) | 22,432 (59%) | |
| Female | 123,936 (48%) | 108,165 (49%) | 15,771 (41%) | |
| Age | 70 (54, 83) | 67 (52, 81) | 83 (75, 89) | <0.001 |
| Age category, years | <0.001 | |||
| [18,30) | 12,499 (4.8%) | 12,466 (5.6%) | 33 (<0.1%) | |
| [30,40) | 16,222 (6.3%) | 16,095 (7.3%) | 127 (0.3%) | |
| [40,50) | 21,245 (8.2%) | 20,898 (9.5%) | 347 (0.9%) | |
| [50,60) | 33,054 (13%) | 31,810 (14%) | 1,244 (3.3%) | |
| [60,70) | 42,088 (16%) | 38,205 (17%) | 3,883 (10%) | |
| [70,80 | 49,658 (19%) | 41,268 (19%) | 8,390 (22%) | |
| [80,90) | 57,512 (22%) | 42,050 (19%) | 15,462 (40%) | |
| [90,Inf) | 26,832 (10%) | 18,115 (8.2%) | 8,717 (23%) | |
| Alcohol use disorders | 10,006 (3.9%) | 8,296 (3.8%) | 1,710 (4.5%) | <0.001 |
| Current or past tobacco use | 15,049 (5.8%) | 12,996 (5.9%) | 2,053 (5.4%) | <0.001 |
| Obesity | 43,124 (17%) | 37,551 (17%) | 5,573 (15%) | <0.001 |
| Essential (primary) hypertension | 113,407 (44%) | 91,281 (41%) | 22,126 (58%) | <0.001 |
| Diabetes mellitus | 61,664 (24%) | 50,184 (23%) | 11,480 (30%) | <0.001 |
| Chronic liver disease | 15,476 (6.0%) | 12,535 (5.7%) | 2,941 (7.7%) | <0.001 |
| Alcoholic liver disease | 3,623 (1.4%) | 2,840 (1.3%) | 783 (2.0%) | <0.001 |
| Chronic hepatitis B | 820 (0.3%) | 744 (0.3%) | 76 (0.2%) | <0.001 |
| Chronic hepatitis C | 711 (0.3%) | 605 (0.3%) | 106 (0.3%) | >0.9 |
| Other cause of chronic liver disease | 2,299 (0.9%) | 1,955 (0.9%) | 344 (0.9%) | 0.8 |
| Mild liver disease | 2,567 (1.0%) | 2,282 (1.0%) | 285 (0.7%) | <0.001 |
| Compensated cirrhosis | 1,951 (0.8%) | 1,601 (0.7%) | 350 (0.9%) | <0.001 |
| Advanced cirrhosis | 1,256 (0.5%) | 873 (0.4%) | 383 (1.0%) | <0.001 |
| Primary liver cancer | 719 (0.3%) | 522 (0.2%) | 197 (0.5%) | <0.001 |
| Primary liver cancer with specific treatment | 383 (0.1%) | 283 (0.1%) | 100 (0.3%) | <0.001 |
| Liver transplantation | 329 (0.1%) | 286 (0.1%) | 43 (0.1%) | 0.4 |
| Transplant recipient (without liver transplantation) | 2,290 (0.9%) | 1,929 (0.9%) | 361 (0.9%) | 0.2 |
| Acquired immunodeficiency syndrome | 1,118 (0.4%) | 1,029 (0.5%) | 89 (0.2%) | <0.001 |
| Modified Charlson comorbidity index | <0.001 | |||
| [0,2) | 170,308 (66%) | 153,214 (69%) | 17,094 (45%) | |
| [2,4) | 57,876 (22%) | 45,065 (20%) | 12,811 (34%) | |
| [4,6) | 20,893 (8.1%) | 15,315 (6.9%) | 5,578 (15%) | |
| [6,8) | 7,651 (3.0%) | 5,615 (2.5%) | 2,036 (5.3%) | |
| [8,Inf) | 2,382 (0.9%) | 1,698 (0.8%) | 684 (1.8%) | |
| Acute respiratory distress syndrome | 67,006 (26%) | 45,321 (21%) | 21,685 (57%) | <0.001 |
| Acute kidney injury | 12,461 (4.8%) | 7,519 (3.4%) | 4,942 (13%) | <0.001 |
| Renal replacement therapy | 2,892 (1.1%) | 1,533 (0.7%) | 1,359 (3.6%) | <0.001 |
| Pulmonary embolism | 8,179 (3.2%) | 6,811 (3.1%) | 1,368 (3.6%) | <0.001 |
| Acute liver failure | 1,222 (0.5%) | 762 (0.3%) | 460 (1.2%) | <0.001 |
| Portal vein thrombosis | 148 (<0.1%) | 108 (<0.1%) | 40 (0.1%) | <0.001 |
| Liver disease progression | 17 (<0.1%) | 17 (<0.1%) | 0 (0%) | 0.2 |
Data are for patients who were discharged after COVID-19 between February 1 and December 31, 2020 in France. The 2011-2020 French National Patient Registry was used to indentiy underlying conditions before COVID-19. Mild liver disease was chronic liver disease without cirrhosis. Advanced cirrhosis was cirrhosis with ascites, hypertensive bleeding, non-obstructive jaundice, or encephalopathy. The Charlson comorbodity index predicts 10-year survival in patients with multiple comorbidities, and ranges from 0 to 30, without entering age and liver disease, with higher scores indicating higher frailty.
n (%); Median (IQR).
Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test.
Mechanical ventilation and mortality relationships
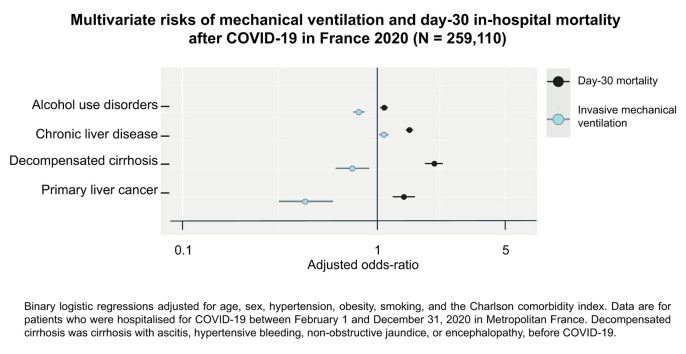
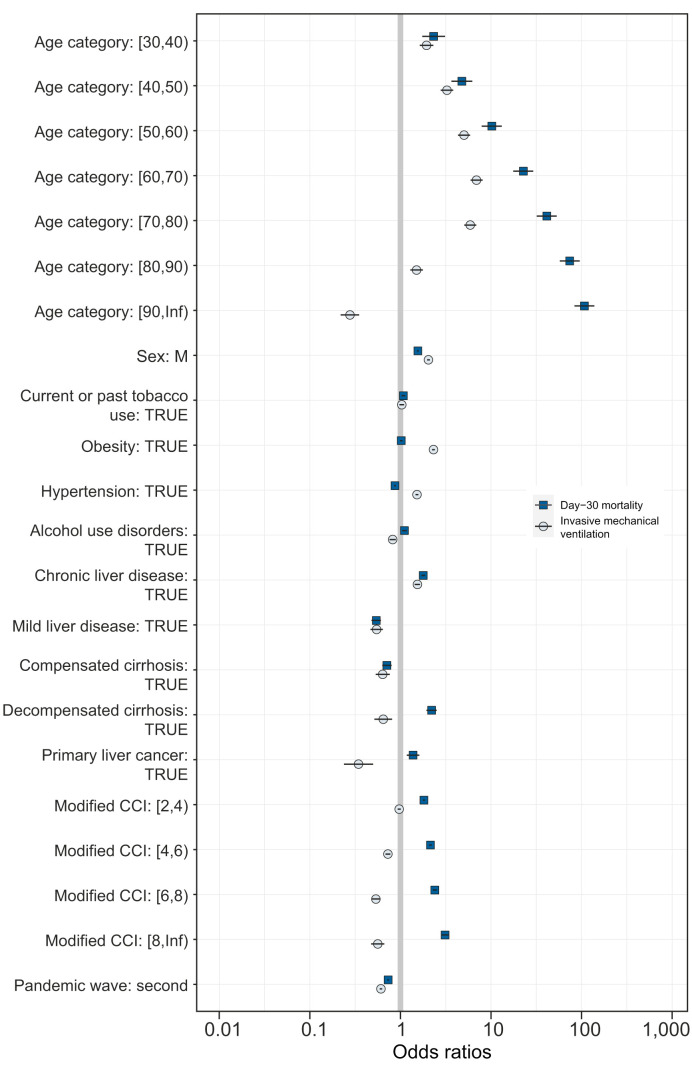
Fig. 3 presents the multivariate associations for mechanical ventilation and for day-30 mortality (see Tables S2 and S3 for the corresponding adjusted odds ratios). The adjusted risk for mechanical ventilation (in blue) was similar or higher than the risk of dying with COVID-19 (in black) for male sex, smoking, obesity, hypertension, mild liver disease, and compensated cirrhosis. The risk of mechanical ventilation was lower than the risk of day-30 mortality for patients with chronic liver disease. The risk of mechanical ventilation was negative, and the risk of COVID-19 death was positive for patients with alcohol use disorders; decompensated cirrhosis; or primary liver cancer, unless they were exposed to liver cancer treatment (adjusted odds ratio for mechanical ventilation and for day-30 mortality 0.78 [95% CI 0.47–1.49, p = 0.472] and 0.79 [95% CI 0.59–1.05, p = 0.109]). The risk for mechanical ventilation was also inconsistent with mortality for patients with primary liver cancer in a propensity-matched sample of 1,182 individuals (see Table S4). The adjusted risk of mechanical ventilation remained relatively stable within the [30, 70) age categories. The adjusted risk for mechanical ventilation remained stable and negative for patients with a modified Charlson comorbidity index ≥4. The adjusted odds ratios for mechanical ventilation and for day-30 mortality were 0.61 (95% CI 0.59–0.63; p <0.001) and 0.73 (95% CI 0.72–0.75; p <0.001), respectively, for the second pandemic wave. The risk of mechanical ventilation remained below the risk of COVID-19 mortality for patients with alcohol use disorders during the 2 pandemic waves (see Fig. S1).
Fig. 3.
Independent risks of mechanical ventilation and of day-30 mortality after COVID-19.
Odds ratios are shown on a log scale. Error bars represent the limits of the 95% CI for the odds ratio. Data are for patients who were discharged after COVID-19 between February 1 and December 31, 2020 in Metropolitan France. The 2011-2020 French National Patient Registry was used to identify underlying conditions before COVID-19. The Charlson comorbodity index was modified to isolate the effects of age and of chronic liver disease. Associations were computed with multivariate binary logistic regression models. Reference for age categories was [18-30).
Discussion
Our study shows that patients with chronic liver disease, including those with decompensated cirrhosis, primary liver cancer, and patients with alcohol use disorders, were at an increased adjusted risk for COVID-19 death and at a reduced, sometimes negative, adjusted risk for mechanical ventilation, during the 2020 pandemic waves in France. Patients with mild liver disease; compensated cirrhosis; chronic viral hepatitis; non-viral, non-alcoholic causes of chronic liver disease; acquired immunodeficiency syndrome; organ (including liver) transplantation; and smokers were not at increased risk of COVID-19 mortality but were more likely to need mechanical ventilation. Overall, our results suggest that chronic liver disease per se contributed marginally, despite higher rates of organ failures, to the death toll of COVID-19 in France in 2020. A limitation of therapeutic efforts, including reduced access to mechanical ventilation, may have accounted for the excess mortality of patients with cirrhosis and a liver-related complication or an alcohol use disorder. This is the largest study on the risks of COVID-19 severity and mortality.
There is a risk of reporting erroneous findings and inappropriate conclusions when measuring mortalities without taking into account the therapeutic effort, especially in a critical care setting.14 The relationship between COVID-19 mortality and resource allocation was mentioned early at the beginning of the SARS-CoV-2 pandemic.24 , 25 In our study, we observed, as in other cohorts, higher risks of COVID-19 death for patients with alcohol use disorders, advanced liver disease, including hepatocellular cancer and decompensated cirrhosis,9 , 13 and for older patients.26 The inverse relationship between mortality and access to mechanical ventilation was obvious, sometimes negative, for these patients. Our estimates suggest that previous measures of the risk of COVID-19 mortality in patients with chronic liver disease were limited by selection bias, or inappropriate surrogates of disease severity, including an absence of measurement of the therapeutic effort,13 , 27 such as access to organ, including pulmonary, support.9 , 14 In line with our findings, a retrospective, North American, cohort study, reported that cirrhosis was not associated with COVID-19 death, when patients had the same rate of mechanical ventilation.15 In general, advanced liver disease and alcohol use disorders associate with deprivation. Deprivation and a limited access to care could also have accounted for previous estimates on COVID-19 death in other national studies, as in the United Kingdom, or in the United States.9 , 28 , 29
Overall, our findings suggest that access to care and to advanced ventilatory support, including mechanical ventilation for severe SARS-CoV-2 pneumonia, is the cornerstone of COVID-19 outcome, including in patients with advanced chronic liver disease or patients with alcohol use disorders. Our findings do not support an excess in COVID-19 severity for patients with chronic liver disease, alcohol use disorders, cirrhosis, or primary liver cancer. The risk of mechanical ventilation remained high across all categories of the Charlson comorbidity index, suggesting that resource allocation in France was more a function of age, of liver disease stage, and of alcohol use disorders, than of other comorbidities. Nevertheless, COVID-19 patients with chronic liver disease were at increased adjusted risk for organ failure, including acute kidney injury, renal replacement therapy, acute liver failure, and liver disease progression, as reported for chronic liver disease patients with other, non-COVID, causes of acute respiratory distress syndrome.30
This is the largest cohort study on the prognosis of chronic liver disease patients with COVID-19. Compared with other studies, selection bias was limited because all residents in France have universal access to hospital care, although a significant number of patients died outside of the hospital with COVID-19 during the study period (19,863 patients during the first wave, for example).2 The 10-year backwards retrospective collection of COVID-19 comorbidities in the French national discharge database reduced the risk of information bias and of reverse associations. Since this is a hospital study, the estimates may only apply to inpatients: the risk of admission and intubation for outpatients with COVID-19 was not measured. Information was collected from a claim database, and not from patient-level medical records, therefore, information was limited to hard outcomes, including mechanical ventilation, which has been consistently used as a surrogate of COVID-19 severity,20 and in-hospital death. Other markers of COVID-19 severity could not be recorded.
In conclusion, our results suggest that the prognosis of COVID-19 patients with chronic liver disease or with alcohol use disorders could be more related to the therapeutic effort, including mechanical ventilation, and less to liver disease progression or to ethanol toxicity.9 Future studies should investigate the relationship between therapeutic effort limitations and outcomes in patients with COVID-19, including those with chronic conditions, and deprived individuals.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
VM, SP: conception of the study, analysis and interpretation of the data, draft of the manuscript; SB: acquisition, edition of the manuscript., NB: analysis of the data, edition of the manuscript. PS: analysis and interpretation of the data, edition of the manuscript. The authors declare they have seen and approved the final version of the manuscript. All members of the Demosthenes group facilitated the study.
Data availability statement
No additional data available (access to the French National Hospital Discharge database is restricted by law).
Transparency declaration
The lead author (VM) affirms that this manuscript is a honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
We thank Doctor Michaël Schwarzinger and Pierre Belnou for their help with the statistical code. We also thank Professor Fabrice Carrat for his comments on our work.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.04.052.
Contributor Information
Demosthenes research group:
Vincent Mallet, Nathanaël Beeker, Samir Bouam, Hélène Fontaine, Marion Corouge, Anaïs Vallet Pichard, Clémence Hollande, Loriane Lair Mehiri, Philippe Sogni, and Stanislas Pol
Supplementary data
The following are the supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus tracker: the latest figures as countries fight the COVID-19 resurgence. Financial Times [Internet]. Accessed March 1, 2021. Available from: https://www.ft.com/content/a2901ce8-5eb7-4633-b89c-cbdf5b386938.
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G., de Oliveira M.H.S., Henry B.M. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID-19): a pooled analysis. Eur J Gastroenterol Hepatol. 2021;33(1):114–115. doi: 10.1097/MEG.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H., Xu J., Liang X., Shi L., Wang Y. Chronic liver disease independently associated with COVID-19 severity: evidence based on adjusted effect estimates. Hepatol Int. 2021:1–6. doi: 10.1007/s12072-020-10133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y., et al. Implication of non-alcoholic fatty liver diseases (NAFLD) in patients with COVID-19: a preliminary analysis. J Hepatol. 2020;73(2):451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y.J., Zheng K.I., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: a multicenter preliminary analysis. J Hepatol. 2020;73(3):719–721. doi: 10.1016/j.jhep.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D., Adeniji N., Latt N., Kumar S., Bloom P.P., Aby E.S., et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colmenero J., Rodriguez-Peralvarez M., Salcedo M., Arias-Milla A., Munoz-Serrano A., Graus J., et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74(1):148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb G.J., Marjot T., Cook J.A., Aloman C., Armstrong M.J., Brenner E.J., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb G.J., Moon A.M., Barnes E., Barritt ASt, Marjot T. Age and comorbidity are central to the risk of death from COVID-19 in liver transplant recipients. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marjot T., Buescher G., Sebode M., Barnes E., Barritt ASt, Armstrong M.J., et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iavarone M., D'Ambrosio R., Soria A., Triolo M., Pugliese N., Del Poggio P., et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73(5):1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajaj J.S., Garcia-Tsao G., Biggins S.W., Kamath P.S., Wong F., McGeorge S., et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70(3):531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agence Technique de l’Information sur l’Hospitalisation . 2014. Aide à l’utilisation des informations de chaînage [How to use de-identified patient information] Lyon, France: agence technique de l'information sur l'hospitalisation.http://www.atih.sante.fr/aide-lutilisation-des-informations-de-chainage [updated October 6, 2015. Available from: [Google Scholar]
- 17.Goutte N., Sogni P., Bendersky N., Barbare J.C., Falissard B., Farges O. Geographical variations in incidence, management and survival of hepatocellular carcinoma in a Western country. J Hepatol. 2017;66(3):537–544. doi: 10.1016/j.jhep.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzinger M., Baillot S., Yazdanpanah Y., Rehm J., Mallet V. Contribution of alcohol use disorders on the burden of chronic hepatitis C in France, 2008-2013: a nationwide retrospective cohort study. J Hepatol. 2017;67(3):454–461. doi: 10.1016/j.jhep.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Institut National de la Statistique et des Etudes Economiques. Demographic balance sheet 2020. Accessed March 1, 2021. Available from: https://www.insee.fr/fr/statistiques/5012724.
- 20.Berlin D.A., Gulick R.M., Martinez F.J. Severe COVID-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 21.Quan H., Li B., Couris C.M., Fushimi K., Graham P., Hider P., et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 22.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Stavem K., Hoel H., Skjaker S.A., Haagensen R. Charlson comorbidity index derived from chart review or administrative data: agreement and prediction of mortality in intensive care patients. Clin Epidemiol. 2017;9:311–320. doi: 10.2147/CLEP.S133624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? The Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z.Y., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese center for disease control and prevention. Jama-Journal Am Med Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 27.Singh S., Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter Research network study. Gastroenterology. 2020;159(2) doi: 10.1053/j.gastro.2020.04.064. 768-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821) doi: 10.1038/s41586-020-2521-4. 430-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiley Z., Ross-Driscoll K., Wang Z., Smothers L., Mehta A.K., Patzer R.E. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monchi M., Bellenfant F., Cariou A., Joly L.-M., Thebert D., Laurent I., et al. Early predictive factors of survival in the acute respiratory distress syndrome: a multivariate analysis. Am J Respir Crit Care Med. 1998;158(4):1076–1081. doi: 10.1164/ajrccm.158.4.9802009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data available (access to the French National Hospital Discharge database is restricted by law).