Abstract
Introduction: To explore the efficacy of urine cytology, ureteroscopic biopsy, and a combination of both in detecting HG UTUC. Methods: We searched our institutional pathology database for cases (2008-2018) with urine cytology and subsequent histologic follow-up based on ureteroscopic biopsy and extirpative resection performed within 6 months of the urine cytology. Sensitivity, specificity, positive predictive value and negative predictive value were calculated for the specific diagnostic categories. Results: A cohort of 226 cases with both urine cytology and histologic follow-up based on ureteroscopic biopsies and/or surgical resection were included in the study. 144/226 cases (64%) had both urine cytology and extirpative resection, among which 57 (25%) cases also have ureteroscopic biopsy preoperatively. The sensitivities for urine cytology or ureteroscopic biopsy alone for a surgically confirmed HG UTUC were 64.7% and 59.2% respectively. Among 49 cases diagnosed as HG-UTUC by extirpative resection, 42 cases were diagnosed as HG-UTUC by either urine or biopsy diagnosis. The sensitivity of combining urine cytology and ureteroscopic biopsy was 85.7%, which was significantly higher than either method alone (P < 0.05). Conclusion: Our findings show urine cytology and ureteroscopic biopsy have comparable sensitivity in diagnosing HG UTUC. However, by combining urine cytology with ureteroscopic biopsy, there is a significant increase in the sensitivity for detecting HG UTUC and should therefore be incorporated into routine clinical practice.
Keywords: High grade upper tract urothelial carcinoma, urine cytology, ureteroscopic biopsy, sensitivity, specificity
Introduction
Urothelial carcinomas of the upper tract (UTUC), which includes ureter and/or renal pelvis, are relatively uncommon and account for only 5% of urothelial carcinomas (UC) and 10% of renal tumors in the United States [1]. The gold standard treatment for HG UTUC is radical nephroureterectomy with excision of the bladder cuff regardless of tumor location in the upper urinary tract [1,2]. Nephron sparing treatments, such as ureteroscopic ablation and segmental ureteral resection, are generally utilized in patients with low grade UTUC (LG UTUC) or with compulsory indications [3]. In addition, patients with HG UTUC are often offered neoadjuvant chemotherapy given the risk of invasive, and/or advanced disease at the time of diagnosis and the risk of chronic kidney disease following nephroureterectomy [4,5]. Therefore, accurate preoperative diagnosis of UTUC including grade assignment is essential for appropriate patient management. Nevertheless, high level evidence recommendations and guidelines for the diagnosis, treatment and follow-up of patients with UTUC, especially HG UTUC, are lacking [1].
Urine cytology has been used in the diagnosis and surveillance of urothelial carcinoma of bladder and UTUC as an inexpensive and noninvasive tool for detecting HG UTUCs [6,7]. Recently ureteroscopically obtained biopsies of the upper tract have played an important role in the detection of HG and LG UTUC. Ureteroscopic biopsies are considered to be the highest yield diagnostic procedures available and are recommended in guidelines published by various international urology societies [1,8]. However, ureteroscopic biopsy specimens are often limited in diagnostic value [9-11]. Processing difficulties and the presence of procedure induced cautery artifact make it challenging for pathologists to establish an accurate diagnosis and moreover assign tumor grade. Technical challenges for the urologist due to limited access to more proximal portions of the ureter and renal pelvis may impede ureteroscopic biopsy sampling in a subset of cases. Due to the limitations of both urine cytopathology and ureteroscopic biopsies, we chose to evaluate the diagnostic value of both methods for the detection of HG UTUC. Our study aimed to evaluate the accuracy of urine cytology alone, biopsy alone, and in combination for detecting HG UTUC.
Materials and methods
This retrospective study was approved by New York University Langone Health Institutional Review Board and ethics committee (i18-01599). A search was performed of the electronic pathology data system over a 10 year period (2008-2018). Cases were identified with both urine cytology specimen (voided and upper tract urine) and a subsequent histologic follow-up as represented by ureteroscopic biopsies and/or extirpative surgical specimens (segmental ureterectomy or nephroureterectomy specimens). Cases with histologic follow-up within 6 months of collected urine cytology were included in the study. The highest degree of abnormality was selected in cases where multiple specimens were available. Excluded from the study were cases diagnosed as non-urothelial origin malignancy by histologic follow-up or cases with previous or concomitant diagnosis of bladder urothelial carcinoma.
Urine cytology results were based on the original cytopathologic reports and classified as: (1) Positive for malignancy, high grade urothelial carcinoma (HG UC); (2) Atypical urothelial cells, suspicious for high grade urothelial carcinoma (AUC-H); (3) Positive for malignancy, low grade urothelial carcinoma (LG UC); (4) Atypical urothelial cells, suspicious or favor low grade urothelial carcinoma (AUC-L); (5) Atypical urothelial cells (AUC); (6) Negative for high grade urothelial carcinoma or negative for malignancy. For this study, we simplified the categories into four groups based on the Paris system [12]: (1) HG UTUC (combining categories of HG UC and AUC-H); (2) LG UTUC (combining LG UC and AUC-L); (3) AUC; (4) Negative (for HG UTUC). UC was diagnosed and graded on surgical specimens according to the 2004 World Health Organization (WHO)/1998 International Society of Urologic Pathology consensus classification, which is equivalent in the 2016 WHO classification [13].
Sensitivity, specificity, positive predictive value and negative predictive value were calculated for the specific diagnostic categories. Comparisons were performed by Fisher exact test, based on 2 × 2 contingency tables (2 sided test with null hypothesis). For all comparisons p-value < 0.05 indicated statistical significance. Calculations were conducted by GraphPad Prism 7 software (GraphPad Software, San Diego, CA, USA).
Results
A total of 226 cases with both urine cytology and histologic follow-up by ureteroscopic biopsy and/or extirpative surgical resection (segmental ureterectomy or nephroureterectomy specimens) were included in the study (Figure 1). 144/226 (64%) cases had histologic follow-up via surgical resection, among which 57 (25%) cases also have ureteroscopic biopsy preoperatively. Figure 2 shows urine cytology, ureteroscopic biopsy and nephroureterectomy from a representative case of HG UTUC in the renal pelvis.
Figure 1.
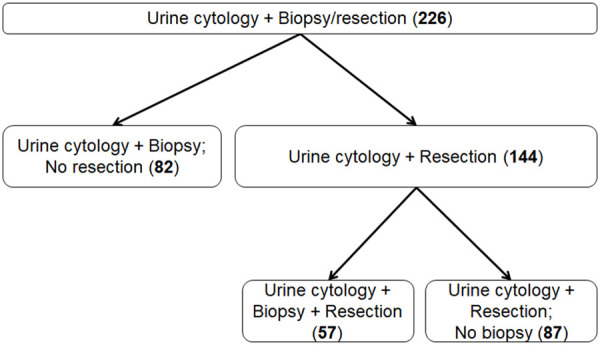
Flowchart of selecting cohorts for the study.
Figure 2.
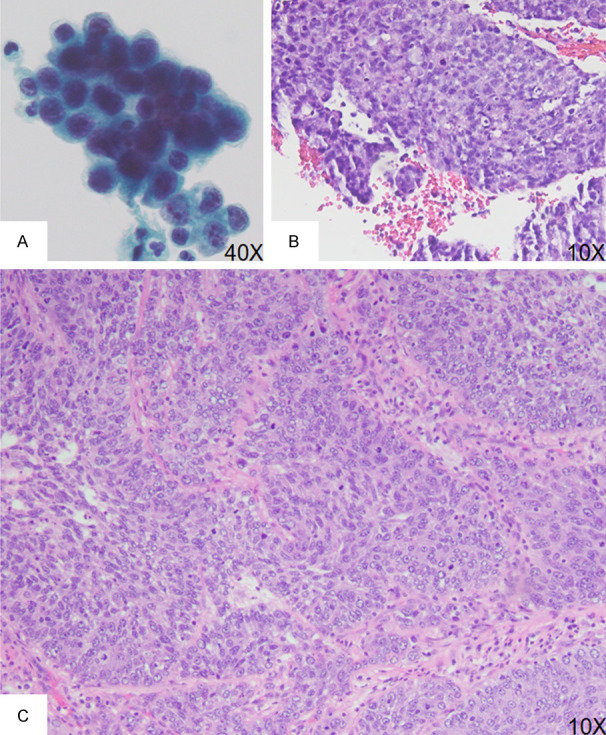
Urine cytology, ureteroscopic biopsy and nephroureterectomy from a representative case of HG UTUC in the renal pelvis. A. Urine cytology specimen shows tumor cells with enlarged nuclei, nuclear hyperchromasia, irregular nuclear membrane and increased nuclear-to-cytoplasmic ratio (> 0.7). B. Ureteroscopic biopsy shows papillary architecture with loss of cellular polarity, enlarged nuclei with nuclear hyperchromasia, and increased mitotic activity and apoptotic bodies. C. Section of tumor from a nephroureterectomy specimen shows invasive tumor nests with disorganized tumor cell arrangement with enlarged nuclei, prominent nucleoli, and increased mitotic activity.
First, we calculated the accuracy of urine cytology using surgical resection as confirmatory diagnosis in the 144 cases (Table 1). There were 81 (56%) cases classified as HG UTUC by cytology, 75 were confirmed by surgery as such. Twenty-one (15%) cases were classified as negative for HG UTUC on cytology (7 cases were confirmed to be negative; 5 cases were LG UTUC and 9 cases were HG UTUC upon surgery). Of the 39 (27%) cases with AUC, 32 cases were upgraded to HG UTUC by surgery. Four cases were categorized as LG UTUC on cytology and three cases were subsequently upgraded to HG UTUC following surgery. The overall sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for diagnosing HG UTUC by urine cytology in this group were 64.7%, 64.3%, 93.8% and 18% respectively if AUC category is considered as “negative”. The overall accuracy is 64.6%. Sensitivity, specificity, PPV, NPV and accuracy were 92.2%, 50%, 93.9%, 43.8% and 87.7% if AUC is counted as “positive”, and were 89.2%, 58.3%, 93.8%, 43.8% and 85.4% if AUC is excluded from this calculation. For the 11 cases diagnosed as LG UTUC upon surgical pathology evaluation, only one (9%) case was correctly diagnosed as such preoperatively by cytology, whereas 5 (45%) cases were diagnosed as negative, 4 (36%) cases as AUC and one case (9%) was over-diagnosed as HG UTUC by urine cytology.
Table 1.
Urine cytology and histology correlation in 144 cases with extirpative surgery
| Urine diagnosis categories | Surgery diagnoses | |||
|---|---|---|---|---|
|
| ||||
| Negative/Non-neoplastic | Malignant | Total | ||
|
| ||||
| LG UTUC | HG UTUC | |||
| Negative | 7 | 5 | 9 | 21 |
| AUC | 2 | 4 | 32 | 38 |
| LG UTUC | 0 | 1 | 3 | 4 |
| HG UTUC | 5 | 1 | 75 | 81 |
| Total | 14 | 11 | 119 | 144 |
In this cohort, a total of 82 cases had both urine cytology and ureteroscopic biopsy but did not undergo surgical resection at our institution (Table 2). Fifty-two cases (63.4%) showed complete concordance by urine cytology and ureteroscopic biopsy diagnosis. These diagnoses included negative for malignancy (n = 21); AUC/atypia (n = 1); LG UTUC (n = 6) and HG UTUC (n = 24). When we divided these cases into two major clinical categories: HG UTUC and non-HG UTUC (including negative, AUC/atypia and LG UTUC), we demonstrate an even greater concordance rate, with 68 (83%) cases in matching categories by urine cytology and ureteroscopic biopsy. Discrepancies in major clinical category assignment between urine cytology and ureteroscopic biopsy were observed in 14 (17%) cases. The majority involved grading, with 8 (57%) HG urine cytology cases showing LG by corresponding biopsies. Overall, ureteroscopic biopsy identified similar number of cases of HG UTUC as compared to cytology evaluation (29 vs 33), but diagnosed a much higher number of LG UTUC cases (23 vs 6). The combination of both methods enabled the identification of 38 cases of HG UTUC, which was much higher than each method alone. Although we do not have surgical resection confirmation for these cases, the data suggest utilization of both methods in combination may help to identify a larger number of HG UTUC cases.
Table 2.
Urine cytology and ureteroscopic biopsy correlation in 82 cases
| Urine diagnosis categories | UT biopsy diagnosis | ||||
|---|---|---|---|---|---|
|
| |||||
| Negative | Atypical | Malignant | Total | ||
|
| |||||
| LG UTUC | HG UTUC | ||||
| Negative | 21 | 2 | 1 | 1 | 25 |
| AUC | 3 | 1 | 8 | 4 | 16 |
| LG UTUC | 0 | 2 | 6 | 0 | 8 |
| HG UTUC | 1 | 0 | 8 | 24 | 33 |
| Total | 25 | 5 | 23 | 29 | 82 |
Next we studied the 57 patients in the cohort that had urine cytology and ureteroscopic biopsies, as well as surgical resection serving as a “gold standard” (Table 3), by which we calculated the sensitivity of each methods alone and in combination. Among the 49 cases with surgically diagnosed HG UTUC, urine cytology correctly assigned 31 cases and ureteroscopic biopsies diagnosed 29 as HG. 5/57 cases were noted to be negative for malignancy or to have no residual carcinoma upon surgical resection, while 2 and 4 of these cases were preoperatively diagnosed as HG UTUC by urine cytology and ureteroscopic biopsy respectively. The overall sensitivity and PPV for diagnosing HG UTUC by urine cytology were 63.3% and 93.9%; and by ureteroscopic biopsies were 59.2% and 87.9% respectively. There was no statistical difference in detection of HG UTUC by urine cytology or by ureteroscopic biopsy alone (P > 0.99). Of the 3 LG UTUC cases diagnosed by surgical resection, ureteroscopic biopsy correctly diagnosed all 3 cases, but only one of them were identified by urine cytology. For the 49 HG UTUC patients confirmed by extirpative surgery, we compared their preoperative urine cytology and ureteroscopic biopsy diagnoses. Although 22/49 cases (45%) showed diagnostic concordance between cytology and biopsy diagnoses (18 HG UTUC, 2 LG UTUC and 2 AUC/atypia), 11 (22%) cases with HG UTUC biopsy showed discordant urine cytology, including AUC (n = 10) and negative (n = 1). 13/49 cases (27%) with HG urine cytology diagnoses were classified as negative (n = 4), atypical (n = 8) or LG (n = 1) on ureteroscopic biopsy. Overall 42 cases were diagnosed as HG by either urine cytology or biopsy diagnosis. The sensitivity by combining urine cytology and ureteroscopic biopsy diagnoses was 85.7%, which was significantly better than by either method alone (P < 0.05).
Table 3.
Urine cytology, ureteroscopic biopsy and final histology correlation in 57 cases with extirpative surgery
| Surgery Diagnoses | |||
|---|---|---|---|
|
| |||
| Negative/Dysplasia | LG UTUC | HG UTUC | |
| Urine Diagnosis | |||
| Negative | 2 | 1 | 2 |
| AUC | 1 | 1 | 15 |
| LG UTUC | 0 | 1 | 1 |
| HG UTUC | 2 | 0 | 31 |
| Biopsy Diagnosis | |||
| Negative | 1 | 0 | 5 |
| AUC | 0 | 0 | 11 |
| LG UTUC | 0 | 3 | 4 |
| HG UTUC | 4 | 0 | 29 |
Discussion
Urine cytology is widely used for detecting HG UTUC preoperatively. Our study demonstrated an overall sensitivity for detecting HG UTUC by urine cytology is 64.7%. The specificity and PPV were 64.3% and 93.8% respectively when AUC is considered as “negative”. Our results are comparable to previous studies reporting sensitivities for detecting HG UTUC by urine cytology ranging from 43% to 89% [10,14,15]. Several reasons may account for the variations in sensitivities including methods of sampling urine, and the lack of uniform criteria for identification and categorization of AUC, especially before the era of the Paris system. We included both voided and upper tract urine cytology specimens in this study. Prior studies have shown that selective upper tract urines yield higher accuracy than voided urine in detecting HG UTUC [14,16], suggesting that the sensitivity and specificity may increase by evaluating upper tract cytology samples. For patients underwent ureteroscopy procedure for biopsy, a selective upper tract urine can be easily collected, which may help to improve the test accuracy.
The clinical management of patient with AUC category remains controversial [17]. At most institutions, management of patients with an AUC diagnosis will include routine follow-up, akin to the “negative” category [18]. However, in our study, 32 cases with AUC noted on cytology were upgraded to HG UTUC upon surgical resection, suggesting that AUC may have a stronger correlation with HG UTUC. Previous studies [19,20] have also noted that the diagnosis of AUC is associated with a higher rate of malignancy and should therefore be investigated more aggressively. Inclusion of AUC with positive cytology improves the sensitivity for high-grade and muscle invasive UTUC [14,21]. We compared the accuracy of urine cytology for detection of HG UTUC using the AUC category as a variable. When AUC was considered “positive”, urine cytology exhibited much higher sensitivity (from 64.7% to 92.2%), but relatively lower specificity (from 64.3% to 50%). The goal of increasing sensitivity to not miss HG lesions comes at the expense of some specificity. It is worthwhile to note that the low NPV in our cohort was partially due to patients with negative cytology that were subsequently managed with a surgical procedure, had other clinical indications for malignancy, for example, hydronephrosis or filling defect on imaging. The NPV is therefore likely under-estimated. Nevertheless, our results support the notion that the AUC category is associated with a higher rate of HG UTUC and should prompt further evaluation. The workup for AUC should be individualized based on the risk assessment of the patients. With the introduction of the Paris system in 2016, the diagnosis of AUC requires one major and one minor criterion [12]. The major criterion is the presence of nonsuperficial and nondegenerated urothelial cells with an increased nuclear cytoplasmic ratio (N:C > 0.5). The minor criteria include: (1) mild nuclear hyperchromasia, (2) irregular nuclear membranes, and (3) irregular, coarse, clumped chromatin. Studies also showed the AUC category under the Paris system is associated with higher rate of HG UC [22].
Ureteroscopic evaluation and histologic examination of suspected upper tract lesions play an important role in the diagnosis and management of UTUC. Previous studies have noted upper tract biopsy accuracy rates range from 43% to 92% for HG UTUC [10,23,24]. Our study revealed a sensitivity of 59.2%, which falls within the above range. An upgrade in diagnosis from the biopsy to the resection specimen was observed in 20 of 49 biopsies (40.8%), including 4 cases upgraded from LG to HG UTUC and 11 cases from atypia to HG UTUC. Secondary review of these cases revealed possible explanations based on either limited size of biopsy material, lack of proper tissue orientation, absence of papillary fronds, crush artifact and/or distorted architecture. Tumor heterogeneity presents another challenge for grading in preoperative ureteroscopic biopsies as demonstrated by studies showing that up to 32% of pTa papillary urothelial carcinoma and 43% of muscle-invasive urothelial carcinoma present different tumor grades within the same tumor [25,26]. The limited sampling provided by ureteroscopic biopsies may not represent the grade representative of the entire tumor. Multiple studies have shown similar rates of upgrading from biopsy to surgery [11]. The coexistence of a positive urine cytology diagnosis is also associated with an increased risk of upgrading. On the other hand, mimickers for urothelial carcinoma, including strips of urothelium without well-developed fibrovascular cores, polypoid ureteritis/pyelitis, and reactive urothelium, can lead to a false positive diagnosis [9].
We evaluated whether there is an improvement in the sensitivity for the detecting HG UTUC by combining the results from urine cytology and ureteroscopic biopsy. Previous studies have demonstrated that selective upper tract urine cytology has a comparable sensitivity for the detection of HG UTUC to that of ureteroscopic biopsy [10,19,27]. Similarly, our study revealed no statistically significant difference in sensitivity between urine cytology and biopsy in detecting HG UTUC. However, each method identified HG UTUC cases that were missed or under-called by the other. Cases with negative biopsy and a concurrent positive urine sample do not necessarily indicate a “false-negative” biopsy diagnosis, as urine cytology is based on analysis of more widely sampled specimen derived from the entire urinary tract including the bladder. Cases with negative urine cytology but a positive biopsy sample may relate to sampling error explained by variable tumor shedding into the urine, lysis of tumor cells during urine processing, dilution of sample and obscuring of tumor cells by non-neoplastic exfoliated cells or inflammatory cells. Cases with more subtle cytologic changes, such as in LG UTUC, focal HG in the background of LG UTUC or features intermediate between LG and HG UTUC can also be missed by urine cytology. This illustrates the necessity for close follow up in patients with equivocal findings by either modality, and for utilizing both methods rather than one alone in the diagnosis of HG UTUC. Combining two methods may significantly increase the sensitivity for detecting HG UTUC, as proved by this study as well as previous ones [28]. Of note, all surgically proven HG UTUC cases in this cohort were called atypical/AUC or higher by both urine cytology and ureteroscopic biopsy. Therefore, we propose that both a negative urine cytology and biopsy predicts a low probability for diagnosing HG UTUC.
We acknowledge several limitations in this study. This is a retrospective study and our findings should be confirmed by prospective evaluation. Our study focused on the detection of HG UTUC by urine cytology and ureteroscopic biopsy. Other diagnostic modalities that have the potential to collectively increase the sensitivity for the detection of UTUC were not assessed in this study, and should be considered in future studies. It has been suggested that preoperative imaging, biopsy grading and urine cytology can be combined to identify advanced UTUC [23,29,30]. UroVysion/fluorescent in-situ hybridization (FISH) has been widely used in urothelial carcinoma surveillance. Studies have shown that a combination of FISH and cytology can offer a higher detection of bladder urothelial carcinoma but has limited value in UTUC surveillance [31-33]. A variety of molecular and proteomic markers have been investigated as putative biomarkers for the diagnosis of UTUC, such as TP53 and MDM2 gene alterations for high-grade and advanced disease, and FGFR3 mutations for low grade cancer [34]. A large scale, prospective clinical trial which includes imaging, urine cytology, ureteroscopic biopsy and molecular markers may be warranted to assess the roles of contemporary methods for diagnosis and risk stratification in UTUC.
Conclusion
This study demonstrates that urine cytology and ureteroscopic biopsy have comparable sensitivity in diagnosing HG UTUC, but each has limitations when utilized alone. The sensitivity for the detection of HG UTUC may be significantly improved when these two diagnostic modalities are combined. The combined data can aid urologists in making important management decisions in patient care, and therefore should be routinely utilized in patients with suspected UTUC.
Disclosure of conflict of interest
None.
Abbreviations
- UTUC
upper tract urothelial carcinoma
- HG
high grade
- LG
low grade
- UC
urothelial carcinoma
- AUC
atypical urothelial cells
References
- 1.Baard J, de Bruin DM, Zondervan PJ, Kamphuis G, de la Rosette J, Laguna MP. Diagnostic dilemmas in patients with upper tract urothelial carcinoma. Nat Rev Urol. 2017;14:181–191. doi: 10.1038/nrurol.2016.252. [DOI] [PubMed] [Google Scholar]
- 2.Zigeuner R, Pummer K. Urothelial carcinoma of the upper urinary tract: surgical approach and prognostic factors. Eur Urol. 2008;53:720–731. doi: 10.1016/j.eururo.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Painter DJ, Denton K, Timoney AG, Keeley FX. Ureteroscopic management of upper-tract urothelial cancer: an exciting nephron-sparing option or an unacceptable risk? J Endourol. 2008;22:1237–1239. doi: 10.1089/end.2008.0187. [DOI] [PubMed] [Google Scholar]
- 4.Kaag MG, O’Malley RL, O’Malley P, Godoy G, Chen M, Smaldone MC, Hrebinko RL, Raman JD, Bochner B, Dalbagni G, Stifelman MD, Taneja SS, Huang WC. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58:581–587. doi: 10.1016/j.eururo.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng X, Chao B, Vijay V, Silver H, Margolin EJ, Balar A, Taneja SS, Shah O, Bjurlin MA, Anderson CB, Huang WC. High response rates to neoadjuvant chemotherapy in high-grade upper tract urothelial carcinoma. Urology. 2019;129:146–152. doi: 10.1016/j.urology.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Lee PJ, Owens CL, Lithgow MY, Jiang Z, Fischer AH. Causes of false-negative for high-grade urothelial carcinoma in urine cytology. Diagn Cytopathol. 2016;44:994–999. doi: 10.1002/dc.23621. [DOI] [PubMed] [Google Scholar]
- 7.Thiryayi SA, Rana DN. Urine cytopathology: challenges, pitfalls, and mimics. Diagn Cytopathol. 2012;40:1019–1034. doi: 10.1002/dc.21769. [DOI] [PubMed] [Google Scholar]
- 8.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Bohle A, Van Rhijn BW, Kaasinen E, Palou J, Shariat SF. European association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015;68:868–879. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Tavora F, Fajardo DA, Lee TK, Lotan T, Miller JS, Miyamoto H, Epstein JI. Small endoscopic biopsies of the ureter and renal pelvis: pathologic pitfalls. Am J Surg Pathol. 2009;33:1540–1546. doi: 10.1097/PAS.0b013e3181aec42a. [DOI] [PubMed] [Google Scholar]
- 10.Renshaw AA. Comparison of ureteral washing and biopsy specimens in the community setting. Cancer. 2006;108:45–48. doi: 10.1002/cncr.21456. [DOI] [PubMed] [Google Scholar]
- 11.Smith AK, Stephenson AJ, Lane BR, Larson BT, Thomas AA, Gong MC, Jones JS, Campbell SC, Hansel DE. Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology. 2011;78:82–86. doi: 10.1016/j.urology.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Barkan GA, Wojcik EM, Nayar R, Savic-Prince S, Quek ML, Kurtycz DFI, Rosenthal DL. The Paris System for Reporting Urinary Cytology: the quest to develop a standardized terminology. J Am Soc Cytopathol. 2016;5:177–188. doi: 10.1016/j.jasc.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. 2016;70:106–119. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 14.Messer J, Shariat SF, Brien JC, Herman MP, Ng CK, Scherr DS, Scoll B, Uzzo RG, Wille M, Eggener SE, Steinberg G, Terrell JD, Lucas SM, Lotan Y, Boorjian SA, Raman JD. Urinary cytology has a poor performance for predicting invasive or high-grade upper-tract urothelial carcinoma. BJU Int. 2011;108:701–705. doi: 10.1111/j.1464-410X.2010.09899.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, He H, Zarka MA, Zhou M, Magi-Galluzzi C. Upper tract urinary cytology to detect upper tract urothelial carcinoma: Using the Johns Hopkins Hospital template and evaluation of its feasibility. Cytojournal. 2015;12:17. doi: 10.4103/1742-6413.161608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang ML, Rosenthal DL, VandenBussche CJ. Upper urinary tract washings outperform voided urine specimens to detect upper tract high-grade urothelial carcinoma. Diagn Cytopathol. 2017;45:700–704. doi: 10.1002/dc.23746. [DOI] [PubMed] [Google Scholar]
- 17.McCroskey Z, Bahar B, Hu Z, Wojcik EM, Barkan GA. Subclassifying atypia in urine cytology: what are the helpful features? J Am Soc Cytopathol. 2015;4:183–189. doi: 10.1016/j.jasc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Barkan GA, Wojcik EM, Nayar R, Savic-Prince S, Quek ML, Kurtycz DF, Rosenthal DL. The paris system for reporting urinary cytology: the quest to develop a standardized terminology. Acta Cytol. 2016;60:185–197. doi: 10.1159/000446270. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Pambuccian SE, Wojcik EM, Barkan GA. Diagnosis of upper tract urothelial carcinoma-a comparative study of urinary cytology and surgical biopsy. J Am Soc Cytopathol. 2015;4:3–9. doi: 10.1016/j.jasc.2014.09.203. [DOI] [PubMed] [Google Scholar]
- 20.Muus Ubago J, Mehta V, Wojcik EM, Barkan GA. Evaluation of atypical urine cytology progression to malignancy. Cancer Cytopathol. 2013;121:387–391. doi: 10.1002/cncy.21278. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X, Si Q, Du D, Harshan M, Zhang Z, Haines K 3rd, Shi W, Chhieng DC. The Paris System for urine cytology in upper tract urothelial specimens: a comparative analysis with biopsy and surgical resection. Cytopathology. 2018;29:184–188. doi: 10.1111/cyt.12505. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Auger M, Kanber Y, Caglar D, Brimo F. Implementing The Paris System for Reporting Urinary Cytology results in a decrease in the rate of the “atypical” category and an increase in its prediction of subsequent high-grade urothelial carcinoma. Cancer Cytopathol. 2018;126:207–214. doi: 10.1002/cncy.21958. [DOI] [PubMed] [Google Scholar]
- 23.Brien JC, Shariat SF, Herman MP, Ng CK, Scherr DS, Scoll B, Uzzo RG, Wille M, Eggener SE, Terrell JD, Lucas SM, Lotan Y, Boorjian SA, Raman JD. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J Urol. 2010;184:69–73. doi: 10.1016/j.juro.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Keeley FX Jr, Bibbo M, Bagley DH. Ureteroscopic treatment and surveillance of upper urinary tract transitional cell carcinoma. J Urol. 1997;157:1560–1565. [PubMed] [Google Scholar]
- 25.Cheng L, Neumann RM, Nehra A, Spotts BE, Weaver AL, Bostwick DG. Cancer heterogeneity and its biologic implications in the grading of urothelial carcinoma. Cancer. 2000;88:1663–1670. doi: 10.1002/(sici)1097-0142(20000401)88:7<1663::aid-cncr21>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Kruger S, Thorns C, Bohle A, Feller AC. Prognostic significance of a grading system considering tumor heterogeneity in muscle-invasive urothelial carcinoma of the urinary bladder. Int Urol Nephrol. 2003;35:169–173. doi: 10.1023/b:urol.0000020305.70637.c6. [DOI] [PubMed] [Google Scholar]
- 27.Malm C, Grahn A, Jaremko G, Tribukait B, Brehmer M. Diagnostic accuracy of upper tract urothelial carcinoma: how samples are collected matters. Scand J Urol. 2017;51:137–145. doi: 10.1080/21681805.2017.1295102. [DOI] [PubMed] [Google Scholar]
- 28.Williams SK, Denton KJ, Minervini A, Oxley J, Khastigir J, Timoney AG, Keeley FX Jr. Correlation of upper-tract cytology, retrograde pyelography, ureteroscopic appearance, and ureteroscopic biopsy with histologic examination of upper-tract transitional cell carcinoma. J Endourol. 2008;22:71–76. doi: 10.1089/end.2007.9853. [DOI] [PubMed] [Google Scholar]
- 29.Roupret M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, Babjuk M, Oosterlinck W. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011;59:584–594. doi: 10.1016/j.eururo.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 30.Horovitz D, Meng Y, Joseph JV, Feng C, Wu G, Rashid H, Messing EM. The role of urinary cytology when diagnostic workup is suspicious for upper tract urothelial carcinoma but tumour biopsy is nonconfirmatory. Can Urol Assoc J. 2017;11:E285–E290. doi: 10.5489/cuaj.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johannes JR, Nelson E, Bibbo M, Bagley DH. Voided urine fluorescence in situ hybridization testing for upper tract urothelial carcinoma surveillance. J Urol. 2010;184:879–882. doi: 10.1016/j.juro.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds JP, Voss JS, Kipp BR, Karnes RJ, Nassar A, Clayton AC, Henry MR, Sebo TJ, Zhang J, Halling KC. Comparison of urine cytology and fluorescence in situ hybridization in upper urothelial tract samples. Cancer Cytopathol. 2014;122:459–467. doi: 10.1002/cncy.21414. [DOI] [PubMed] [Google Scholar]
- 33.Gomella LG, Mann MJ, Cleary RC, Hubosky SG, Bagley DH, Thumar AB, McCue PA, Lallas CD, Trabulsi EJ. Fluorescence in situ hybridization (FISH) in the diagnosis of bladder and upper tract urothelial carcinoma: the largest single-institution experience to date. Can J Urol. 2017;24:8620–8626. [PubMed] [Google Scholar]
- 34.Bagrodia A, Cha EK, Sfakianos JP, Zabor EC, Bochner BH, Al-Ahmadie HA, Solit DB, Coleman JA, Iyer G, Scott SN, Shah R, Ostrovnaya I, Lee B, Desai NB, Ren Q, Rosenberg JE, Dalbagni G, Bajorin DF, Reuter VE, Berger MF Collaborators. Genomic biomarkers for the prediction of stage and prognosis of upper tract urothelial carcinoma. J Urol. 2016;195:1684–1689. doi: 10.1016/j.juro.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


