Abstract
Patent foramen ovale (PFO) is present in about one-quarter of the population and should be considered an anatomical variant rather than a malformation. The association of PFO with cryptogenic stroke, migraine, peripheral embolism and other pathologies is still controversial. The evaluation of anatomical complexity, and particularly the long-tunnel morphology, is crucial for the assessment of the risk profile and for a targeted therapeutic management. Long-tunnel PFOs seem to be more prone to clot formation and complications related to percutaneous closure procedures. Echocardiography is the most useful method to investigate anatomical complexity, confirm and reinforce the indication to treatment, select the appropriate device and guide the PFO closure towards a successful procedure.
Keywords: Patent foramen ovale, tunnel PFO, complex PFO, cryptogenic stroke, percutaneous PFO closure
Introduction
Patent foramen ovale (PFO) consists of a continuity solution in the interatrial septum, sited between the thin valve-shaped septum primum and the more rigid and thick annular-shaped septum secundum. Together with ductus arteriosus, PFO allows the blood flow from right heart to left heart in the fetal circulation, shunting the high-resistance pulmonary circle.
In adults PFO can be identified in about one-fourth of the population [1,2]. In recent years PFO has been associated with higher risk of cryptogenic stroke (CS) advocating that paradoxical embolism could be the most probable etiology. Consequently, several trials tried to demonstrated that a device-closure strategy could be superior to a medical-therapy strategy. After an initial fail related to some inconsistencies in the study and to errors in the selected population and follow-up, investigators demonstrated the advantage of closure over medical therapy. However, some uncertainties and challenges remain. First of all not all CS are actually PFO-related and, secondary, some anatomical features may hinder the closure procedure increasing the risk of complications and of incomplete closure. Long-tunnel morphology emerged as one of the most important findings influencing the probability of PFO-related CS and to difficulties in closure procedure.
Consequently, two aspects appear to be fundamental: obtaining an accurate analysis of specific anatomical and functional aspects of the PFO by mean of comprehensive imaging techniques and consequently selecting the most appropriate device and procedure aiming to the best fitting between the device and the PFO.
In this review we summarize the diagnostic strategies and the technical aspects which appear crucial to gain the best results in terms of safety and efficacy.
Patent foramen ovale: anatomical and physiological details
PFO represents the persistence of the physiological opening that allows a portion of the blood flow coming from inferior vena cava to bypass the pulmonary circulation during fetal life. The anatomy of atrial septum is characterized by the septum secundum, consisting of a roughly ring-shaped and thick border, and by septum primum which is a thin and relatively elastic curtain fixed to septum secundum in the posterior-inferior portion, while it is only lying on the border in anterior-superior portion where the PFO is usually located. At birth, the expansion of lungs causes the reduction of pulmonary resistances and a consequent drop in pressure of the right atrium; as flow through the pulmonary arterial circuit rises, left atrial pressure increases too. This gradient inversion fixes the whole septum primum on the septum secundum. Anatomical closure usually occurs within the first year of life in about two-thirds of the population while in the adult life it remains unclosed in almost one quarter; for this reason, PFO should be considered as an anatomical variant rather than a pathological condition [3]. The overlap between the septum primum and septum secundum is variable, resulting in a tunnel of different width and length. Ho et al. reported a length of 1-6 mm and widths of 5-13 mm in small series of heart specimens [4]. Usually the opening to the right atrium is located between the fixed antero-superior border of fossa ovalis and the thin flap valve posteriorly (Figure 1). On the left side, the entrance usually has a crescent shape and is bounded by the free edge of the flap valve. Usually, the flap valve of PFO allows only a right-to-left shunt, either during the brief phase of the cardiac cycle when the right atrial pressure exceeds the left one or following a straining maneuver. Conversely, in the presence of ASD, blood flows from left to right during most of the cardiac cycle, but the shunt can be reversed due to severe pulmonary hypertension [5].
Figure 1.
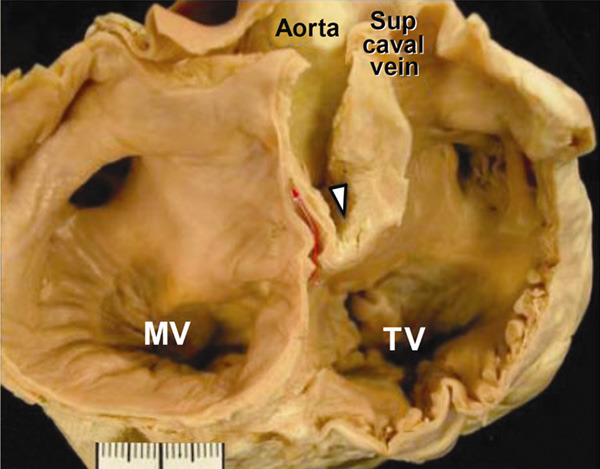
Cross-sectional view of the atrial septum showing the tunnel of the PFO (red arrow) passing between the infoding (triangle) of the right atrial wall (septum secundum) and the flap valve of the fossa (septum primum) in the antero-superior part of fossa ovalis. MV = mitral valve; TV = tricuspid valve; Sup caval vein = superior caval vein. (Reproduced with permission from Oxford University Press).
It is important to note that, in particular cases, the distinction between ASD and PFO is not simple; some authors proposed that defects characterized by large left and right atrial openings and short tunnel length should be more properly considered as ASD rather than PFO. Usually, these borderline defects present at rest left-to-right shunting, which is less common in PFOs [6].
Diagnosis
Detection and detailed analysis of PFO lies on several echocardiographic techniques, including transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), Intracardiac Echocardiography (ICE) and Transcranial Doppler Ultrasound (TCD). All these modalities can improve image quality with or without administration of a contrast agent, like agitated saline contrast solution or commercially available contrast agents [7,8], with an accuracy nearly comparable among all imaging modalities for moderate to large shunts, mildly superior for TCD and TEE in case of small shunts [9]. Valsalva maneuver is commonly required to provoke the detachment of the two septa; in sedated patients, an external abdominal pressure followed by release (after 20 seconds) can be equally effective [10].
TTE with harmonic imaging is first line method approaching a patient with a recent stroke or TIA, its main advantages are low-invasiveness and low cost [11-13]. Atrial septal aneurysm (ASA) is often easily detectable and is associated to presence of PFO and to paradoxical embolism [14]. The presence of right-to-left shunt may be revealed by using color Doppler and administration of microbubble contrast agents while the patients performs a Valsalva maneuver, increasing sensitivity [15]. To avoid misdiagnosis with intra-pulmonary shunts, only microbubbles appearing in the left atrium within 3 cardiac cycles from opacification of the right atrium should be considered diagnostic for the presence of PFO [16].
TCD with microbubbles injection, provides a semiquantitative estimation of right-to-left shunt. This technique investigates the passage of micro-air emboli in the middle cerebral artery. A study comparing transcranial Doppler with TEE as criterion standard, demonstrated a sensitivity of 96.8% and a specificity of 78.4% [17]. The amount of bubbles visualized in a limited range of cardiac cycles quantified the extent of the PFO. A large shunt is defined as composed by at least 20 bubbles. The principal limitation of transcranial Doppler is the difficulty to differentiate interatrial from intrapulmonary shunts, due to the overlap in time when microbubbles are detected in the middle cerebral artery in both situations, increasing the risk of false positive diagnosis [18]. Therefore, TEE is required if a shunt is suspected.
TEE provides the best direct visualization of atrial septum and PFO [19]. It also plays a pivotal role in guiding the percutaneous closure [20].
TEE offers the possibility to obtain high spatial resolution 3D images with detailed visualization of atrial septum and surrounding structures [21]. This technique provides an incremental value during the percutaneous closure procedure allowing an appropriate visualization of catheters and the correct positioning of closure devices, minimizing the likelihood of residual shunts and further complications [22]. Another relevant capability of TEE is the comprehensive assessment of the PFO-complexity; in fact, the most part of the criteria for the definition of PFO-complexity can be visualized only by TEE or ICE [23,24]. ICE is performed with ultrasonographic probes implanted on adapted intracardiac catheters. The main advantage of ICE is the possibility to avoid general anesthesia and invasive ventilation, resulting in improved compliance of the patient to the procedure [25]. Moreover, due to probe position close to the interatrial septum, ICE may occasionally provide better image quality than TEE (Figure 2), facilitating the procedure especially when long (continuous or repeated) viewings are required or in case of complications [26]. The main limitation to the full introduction of the technique in clinical practice is the relatively high cost [27]. The PFO tunnel is usually localized in the superior portion of the fossa ovalis and can be well visualized in the longitudinal plane (90-degrees) in the bicaval view (Figure 3A); in addition, an accurate sweep from 30-degrees to 120-degrees at mid-esophagus level is usually performed [28]. The use of color Doppler allows the visualization of flow through the tunnel and semi-quantification of the right-to-left shunt (Figure 3B).
Figure 2.
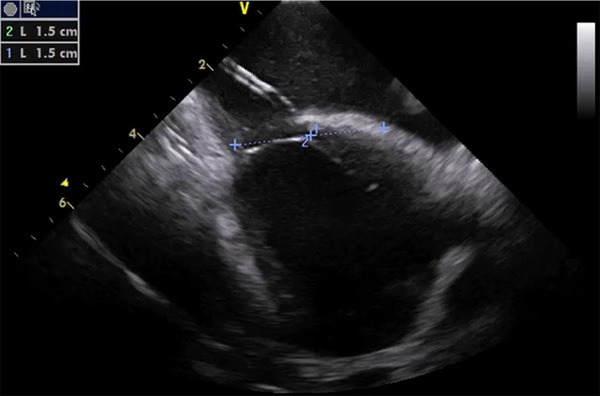
ICE imaging for guidance of PFO closure, showing the delivery catheter through a tunneled defect, positioned from right (above) to left (below) atrium. As showed, a proper sizing can be performed with two measurements drawn from the center (1 and 2), simulating the final position of the device (30 mm in this case).
Figure 3.
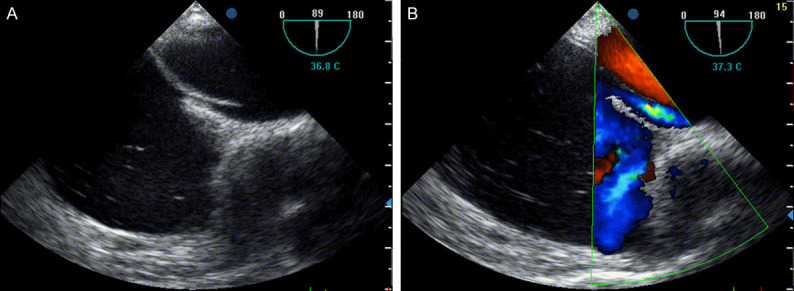
TEE in the 90-degree bicaval view showing a long-tunnel PFO in the superior portion of the fossa ovalis (A), typical localization of the tunnel. Right-to-left shunt, at rest or after Valsalva maneuver, can be revealed perfectly by color-Doppler (B).
Complex patent foramen ovale: focus on the long tunnel PFO
PFOs can present various anatomical patterns, shifting from simple forms to a wide range of complex structural conformation. Besides simple diagnosis of the presence of PFO, to define anatomical complexity is crucial because of association with higher risk of stroke and/or transient ischemic attack (TIA) and of implications in the closure procedures, particularly in the choice of the device [29,30]. Long-tunnel PFO are characterized by a significant long portion of overlap between septum primum and septum secundum, it has been defined in different ways depending on various studies, in the most part of them it was defined as a Tunnel length >8-10 mm (Figures 4 and 5). The assumption that long-tunnel morphology could be prone to clot formation has been supported by several authors. The hematic stasis within the tunneled portion could be a potential mechanism of arterial embolism associated with PFO. Goel et al. compared the morphological characteristics of PFO in patients with and without CS. They reported that the presence of atrial septal aneurysm (45% vs 21%, P<0.005), the size of PFO (3.9±1.6 vs 2.9±1.4 mm, P<0.001) and the length of the tunnel (14±6 vs 12±6 mm, P=0.05) were the most important risk factors in patients presenting stroke and TIA [31]. A similar result has been reported by a Polish group [29]. Recently, a Japanese study retrospectively analyzed PFO anatomy in more than 100 patients, with or without prior CS, defining a risk-score based on specific features: long-tunnel PFO, highly mobile interatrial septum, prominent Eustachian valve or Chiari network, large right-to-left shunt during Valsalva maneuver and low-angle PFO (≤10° from inferior vena cava) resulted independently related to CS. The presence of ≥2 or more of these characteristics on TEE could be useful to identify PFO patients with higher risk of CS [32].
Figure 4.
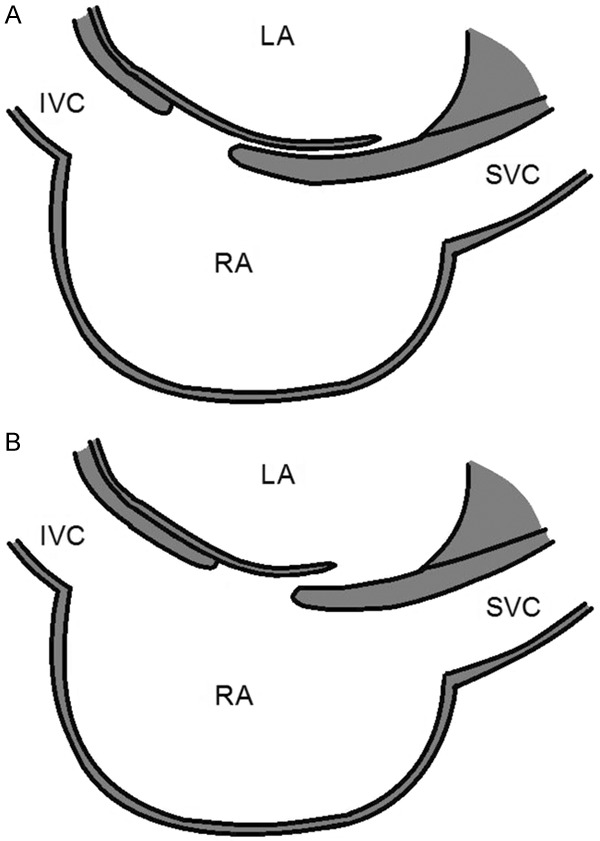
Tunnel PFO with long overlap between septum primum (flap) and septum secundum (A). PFO with short overlap length and flap valve (B). LA = left atrium; IVC = inferior vena cava; RA = right atrium; SVC = superior vena cava.
Figure 5.
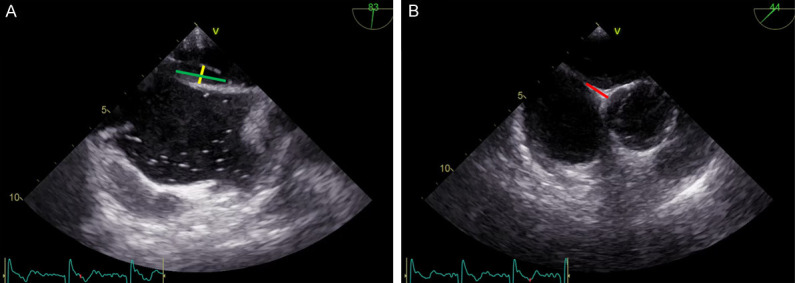
TEE in the 90-degree bicaval view showing a long-tunnel PFO (Panel A); this technique provides additional information, like length and width of the tunnel (green and yellow lines), useful to plan the therapeutic approach, as well as the length of the aortic rim in the 45-degree short-axis view (B-red line).
Besides tunnel length, the definition of anatomically complex PFO take in count other elements including: multiple openings on the left atrial side; presence of atrial septal aneurysm or excessive septum excursion; association with other defects of the fossa ovalis (hybrid defect); excessive thickening of septum secundum (≥10 mm); presence of Eustachian ridge/valve, or Chiari network, size of the opening, burden of the shunt at rest and after Valsalva maneuver and opening of the angle between PFO and inferior vena cava and PFO (Table 1) [23,32,33].
Table 1.
Characteristics of simple and complex PFOs
| PFO category | Anatomical characteristics |
|---|---|
| Simple: | ● Standard anatomy, i.e. none of the below |
| Complex: | ● Long tunnel length (≥8-10 mm) |
| ● Multiple openings into left atrium | |
| ● Atrial septal aneurysm | |
| ● Excessive movement of atrial septum | |
| ● Large opening | |
| ● Shunt at rest | |
| ● Hybrid defect | |
| ● Thick septum secundum (≥10 mm) | |
| ● Prominent Eustachian ridge | |
| ● Eustachian valve or Chiari network | |
| ● Angle between PFO and IVC ≤10° |
PFO: patent foramen ovale; IVC: inferior vena cava.
Clinical conditions associated with PFO
Several pathological conditions are associated with PFO, including cryptogenic stroke (CS), migraine, systemic arterial embolism, decompression illness and worsening of pre-existent chronic lung disease.
Cryptogenic stroke
About 800,000 strokes and 200,000 to 500,000 TIAs occur every year in the United States [34]. A clear cause is not identified in about 25 to 40% of strokes. These cases are defined as CS. The finding of a PFO has been considered as potential cause of CS calling in question a paradoxical embolism pathogenesis. Retrospective studies and meta-analysis reported a positive correlation between CS incidence and PFO [35,36], but, initially, prospective trials failed to demonstrate the superiority of closure over medical therapy [37]. Conversely, in a recent meta-analysis, PFO closure resulted to be superior to medical therapy for prevention of stroke [HR 0.32, (95% CI) 0.13-0.82; P = 0.018] with a greater benefit (HR 0.33, 95% CI 0.16-0.72; P = 0.005), in patients with moderate to large shunt; the incidence of AF was significantly increased after device closure, as expected [38].
Migraines
The association between migraine and PFO is based on the hypothesis that microemboli or humoral factors, normally degraded in lungs, could be delivered to cerebral circulation by the shunt. There is no consensus on this assumption with controversies between different studies [39,40]. Consequently, PFO closure in this setting should be considered only for patients enrolled in clinical trials or for compassionate use.
Systemic arterial embolism
Some authors described paradoxical arterial embolism to many districts including heart, limbs, kidneys or gut [41,42]. However, a clear association between cryptogenic arterial embolism and PFO has not yet been demonstrated and the indication to percutaneous closure in these patients remains uncertain [43].
Decompression illness
Decompression illness is a clinical condition caused by gas embolization in vessels and tissues; it can commonly occur in divers, pilots or astronauts during rapid shifting from high to lower atmospheric pressure.
The intracardiac shunt could make the pulmonary circulation unable to clear the nitrogen forming in the blood during the decompression.
Data showed a relationship between the size of the shunt with the severity and recurrence of the disease [44,45]. Despite the lack of randomized controlled trials, professional divers in which a PFO is associated with decompression illness should be recommended for closure in selected cases, after a proper evaluation of the causal role of PFO [46]. In the recent European Position Paper, Pristipino et al. stressed the importance of an individual assessment of these events, including a thorough investigation of the previous decompression phase, the size of the PFO, and the onset of the illness after the ascent, dives or flight (inversely related to the size of the PFO) [40].
Other conditions linked to PFO
Sleep apnea, platypnea-orthodeoxia and transient global amnesia have been associated with PFO and, in some cases, closure resulted in an improvement of the condition [47]. However, the lack of supporting data should deter from routinely perform the procedure. Considering individual characteristics and always involving the patient in the decision-making, highlighting the lack of evidences is crucial [40].
Clinical features of PFO-related stroke
Since PFO can be detected in about 25% of the general population while CS is relatively rare the possibility that the two events could be incidentally present in the same patient is not negligible. Consequently, some Authors tried to define clinical features associated to PFO-related stroke. There is no consensus on this argument however the main indication on this matter come from a retrospective analysis of 12 cohort studies of CS patients has been developed in the Risk of Paradoxical Embolism (RoPE) study, aiming to develop predictive models able to estimate the pathogenic or incidental role of a PFO diagnosed in these patients [48]. The study introduced the “RoPE score”, a 10-point index measured by decreasing the score by one for each given risk factor (diabetes, hypertension, smoking, previous TIA/stroke, cortical stroke at neuroimaging) as well as for each decade of age exceeding 20 years [49]. A high RoPE score can therefore identify patients with PFO-mediated CS, most likely to benefit from PFO closure to reduce the risk of stroke recurrence, becoming an important tool in patient selection [50]. The main limitation of RoPE score is not accounting for anatomical PFO features derived by echocardiography, it should be carefully used only in the context of a multiparametric evalutation.
Therapeutic management
The best therapeutic strategy in patients experiencing CS, associated with the finding of PFO, is still controversial. Moreover, the benefit of one strategy rather than another, in terms of lower incidence of recurrent stroke, is closely dependent on patient and PFO features.
Medical therapy has proven to be effective in secondary prevention of CS. Studies do not report superiority of vitamin K antagonist in comparison to antiplatelet drugs, therefore, the therapy is usually tailored on the basis of comorbidity and compliance of the patient [51]. Several randomized clinical trials have been carried out to demonstrate the superiority of percutaneous PFO closure in comparison to medical therapy in the prevention of recurrent CS [52]. The first trials failed in demonstrating this goal. The STARFlex Septal Closure System in Patients with a Stroke and/or TIA due to Presumed Paradoxical Embolism Through a PFO (CLOSURE I) Trial, designed to prove the efficacy of transcatheter PFO closure with STARFlex septal closure system vs best medical therapy, showed an insignificant difference in the two-year rate of recurrent stroke and TIA [53]. However, the design of CLOSURE I has been criticized, particularly for the inclusion of TIA as enrolling criteria. Similarly, Percutaneous Closure of PFO in CS (PC) Trial, also using TIA as inclusion criteria, failed to demonstrate the primary endpoint of reduction of stroke and death in patients treated with the Amplatzer PFO occluder (Abbott/St Jude Medical, St Paul, MN, USA) device [43]. The same device was tested in the Randomized Evaluation of recurrent Stroke comparing PFO closure to Established Current standard of care Treatment (RESPECT) Trial, which confirmed similar results, although patients with TIA were excluded; a significant superiority of percutaneous closure has been reported only in the pre-specified per-protocol (HR, 0.37; 95% CI, 0.14 to 0.96; P = 0.03) and as-treated analysis (HR, 0.27; 95% CI, 0.10 to 0.75; P = 0.007) [54]. Data from the extended follow-up (median 5.9 years) demonstrated a reduction in ischaemic stroke after interventional treatment (HR 0.55; 95% CI [0.31-0.99]; P = 0.046; NNT = 45) [55].
More recent randomized trials provided more conclusive data, confirming superiority of PFO closure over medical therapy. The Gore® Septal Occluder Device for PFO Closure in Stroke Patients (GORE REDUCE) trial showed a significant reduction in clinically relevant ischemic stroke (1.45 vs 5.5%; P = 0.002; NNT = 25) compared with antiplatelet regimen [58], results recently confirmed at 5 year follow-up [59]. In the PFO Closure or Anticoagulants Versus Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE) study, patients undergoing percutaneous PFO closure had no ischemic stroke, unlike those treated with antiplatelet drugs (HR 0.03; 95% CI [0.00-0.26]; P<0.001; NNT = 17) [60]. Last but not least, the Device Closure vs Medical Therapy for CS Patients With High-Risk PFO (DEFENSE-PFO) trial showed, at 2 years, a significant reduction of a composite endpoint (stroke, vascular death and TIMI-major bleeding) for interventional closure compared with medical therapy (0 vs 12.9%; P = 0.013; NNT = 8) [61].
Treatment issues connected to long-tunnel PFO
Most devices used for PFO are actually ASD closure devices slightly modified for PFO anatomy [62]. Correct device selection is crucial, considering the variable morphology of PFO and of surrounding structures [63]. Currently, the most widely used closure device is the Amplatzer PFO Occluder. Despite a statistically significant reduction of stroke over medical treatment, its usage in challenging septal anatomies showed significantly higher re-event rate [64,65]. Some basic consideration are important for all the devices, first of all the diameter of the device should be at least twice the maximum diameter of the PFO. A short septal length (<25 mm) should be easier treated with a small and/or soft device to prevent erosion of surrounding structures. Conversely, a firmer device could be needed in patients with a prominent Eustachian ridge to ensure the correct deployment of the right atrial disk [66]. A study by Vitarelli et al., comparing three different devices (Amplatzer PFO occluder, Figulla Occlutech - Helsinborg, Sweden - and Atriasept Cardia - Cardiac Inc., Eagan, MN, USA) in patients with simple vs complex PFO, showed larger sizes of the devices implanted in complex defects, with lower closure rate (persistence of residual shunt), unrelated to the device model [67].
While approaching the closure of a long-tunnel PFO it is worth to know that the rigid waist of some devices can cause the “bunching” of the flap, distorting the septum anatomy and resulting in unstable positioning. Although the lack of focused studies and clear guidelines to follow in this particular anatomical variant of PFO, Authors proposed some gimmicks to limit complications. One of these consists in delivering the device through a trans-septal puncture rather than directly within the tunnel, in order to improve the closure profile, reducing the risk of embolization and significant residual shunt [68,69]. A second option lays on a “detunnelisation” procedure by stepwise inflation of a low-pressure balloon, prior to closure with an umbrella device [70].
Another strategy implies the use of relatively new devices with features more adaptable to the long-tunnel morphology. The Gore Septal Occluder-GSO (W.L.Gore & Associates, Inc., Flagstaff, AZ, USA), evolution of the previous Gore HELEX, due to the flexible and soft consistency of its two disks (frame of five nickeltitanium nitinol wires with platinum core, covered by expanded polytetrafluoroethylene ePTFE) which allow asymmetrical opening, reduced the incidence of device misalignment showing improved deliverability and closure rates [71-73]. The studies published so far have consistently shown low periprocedural risk and good technical success when implanting the GSO in challenging PFO anatomies, such as ASAs and long-tunnel PFOs with tunnel length ≥20 mm [74,75].
The Premere (St Jude Medical, MN, USA), is able to adapt to the interatrial channel portion varying the distance between the disks; these devices showed low incidence of atrial fibrillation and thrombosis, probably due to the absence of septal distortion and the limited amount of material within the atria.
The Coherex FlatStent™ EF system (Coherex Medical, Salt Lake City, UT, USA), is a dedicated “in-tunnel” PFO closure device, composed of a radiopaque planar self-expanding nitinol lattice framework with an attached polyurethane foam. The device accommodates within the tunnel and is covered with a foam inducing the spontaneous adhesion of the two septa [76,77], despite the innovative and promising concept the experience with this device is still limited and it is not adaptable to PFO with moderate-severe ASA.
A Similar principle is at the basis of the SeptRX Occluder (Secant Medical, Perkasie, PA, USA), it is a self-expanding nitinol frame with flexible anchor struts to grip to hedges of the tunnel, the device is not provided of atrial occluders and the whole structure is delivered within the tunnel. The nitinol mesh is designed to stimulate the natural adhesion of the septum primum to septum secundum, definitively closing the PFO. The main limitation consists of the very low experience and to the difficulties to adapt the device to extreme lengths of the tunnel [78].
Recently, an innovative device gained visibility: the NobleStitch™ EL (HeartStitch, Inc., Fountain Valley, CA, USA), a suture-based percutaneous system, able to perform a “device-less” PFO closure by means of two polypropylene threads (one attached to the septum primum and one to the s. secundum), tied together by a specific sealing system. In a registry by Gaspardone et al. over 192 patients treated with suture-mediated PFO closure, the procedure resulted successful in 96%, with non-significant right-to-left shunt (grade ≤1) in almost 90% of patients and absence of device-related complications at follow-up (206±130 days) [79]. However, despite the innovative and minimalistic design, this system showed some limitations such as the potential occurrence of septal tears causing iatrogenic ASD [80]. Table 2 summarizes the different types of device currently available with main advantages and disadvantages.
Table 2.
Main advantages and disadvantages of different PFO-closure device available in commerce
| Device | Advantages | Disadvantages |
|---|---|---|
| Amplatzer PFO Occluder | ● Large experience and supporting trial | ● Rigid device |
| ● Rigid waist not fitting long-tunnel morphologies | ||
| ● High failure incidence in complex PFO | ||
| ● Complex transseptal puncture through the device | ||
| STARflex septal closure system | ● Large experience and supporting trial | ● Rigid waist not fitting long-tunnel morphologies |
| ● Soft structure limiting erosion | ● High failure incidence in complex PFO | |
| ● Recurrence of stoke/TIA and incidence of on-device Thrombosis not negligible | ||
| Gore Septal Occluder | ● Large experience and supporting trial | ● Risk of septal distortion in long-tunnel morphology significantly reduced but not abolished |
| ● Soft structure limiting erosion and adapting to PFO an surrounding structures anatomy | ||
| St Jude Premere PFO Closure system | ● Long and adaptable interatrial portion adapting to long-tunnel morphology | ● Low experience |
| ● No septal distortion | ● Not tested in moderate or severe ASA | |
| ● No post-procedural atrial fibrillation | ||
| ● Retrievable after delivery | ||
| Coherex Flatstent PFO occluder | ● In-tunnel structure without atrial occluders | ● Low experience |
| ● Designed for long-tunnel morphology | ||
| ● Very low risk of on-device thrombus | ||
| SeptRx PFO closure device | ● In-tunnel structure without atrial occluders | ● Low experience |
| ● Very low risk of on-device thrombus | ● Not adaptable to all the tunnel lengths | |
| Noble-Stitch | ● Device-less system | ● Low experience |
| ● No risk of on-device thrombus | ● Risk of septal tears | |
| ● No residual structures on the left side of the septum |
PFO: patent foramen ovale; TIA: transient ischemic attack; ASA: atrial septal aneurysm.
What appears clear is that every solution has its own advantages and pitfalls when approaching a complex PFO with a long-tunnel morphology. The corner stone for an adequate planning of the procedure mainly consists of an accurate anatomical evaluation by imaging in order to select the best device and the optimal procedure.
Regarding the long-tunnel morphology the most important aspects are the length of the tunnel and the size of the opening, influencing the device selection, and the presence of ASA or wide septal excursion, influencing the stability of the device.
In an interesting prospective multicenter study, the authors carefully evaluated the tunnels anatomy during TEE, proving that a thorough morphologic assessment was necessary for a successful placement, to improve closure rates and prevent complications. A classification of three distinct PFO tunnel morphologies was proposed (Table 3). A suboptimal closure was present only in type 3 PFO tunnel because the unstable length of the tunnel did not allow correct implantation of the device [81].
Table 3.
Classifications of three distinct PFO tunnel morphologies
| Type | Septum anatomy | Length of tunnel during cardiac cycle |
|---|---|---|
| Type 1 | Normal | Stable |
| Type 2 | Aneurysmal | Stable (≥4 mm) |
| Type 3 | Aneurysmal | Variable |
Anatomical features of PFO tunnels, classified by Sievert et al. The variability of tunnel length (Type 3) can be a limitation for correct implantation of new “in-tunnel” devices. Data from Sievert H et al. [68].
Conclusions
PFO closure represents nowadays a routinely performed procedure, in selected patients, with precise indications and standardized procedure. Proper assessment of complex anatomical features of a PFO, such as septal aneurysm and long-tunnel morphology, by means of multimodality imaging techniques is crucial for a correct estimation of the risk profile and to guide a targeted treatment. Several devices are currently available, supported by randomized and real-world data, presenting different characteristics and specific destination of use. It is necessary that the new devices designed to face the more complex anatomies would be tested in larger studies to provide stronger indications to their use in specific conditions.
Acknowledgements
The authors gratefully acknowledge Susan Nord and Jennifer Pfaff of Aurora Cardiovascular Services for editorial preparation of the manuscript, and Brian Miller and Brian Schurrer of Aurora Research Institute for their help in preparing figures.
Disclosure of conflict of interest
None.
Abbreviations
- 3D
Three dimensional
- ASD
atrial septal defects
- CS
cryptogenic strokes
- ICE
intracardiac echocardiography
- PFO
patent foramen ovale
- PH
pulmonary hypertension
- RoPE:
Risk of Paradoxical Embolism
- TCD
transcranial Doppler
- TEE
transesophageal echocardiography
- TIA
transient ischemic attack
- TTE
transthoracic echocardiography
References
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr. 2008;153:807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantinotti M, Assanta N, Murzi B, Lopez L. Controversies in the definition and management of insignificant left-to-right shunts. Heart. 2014;100:200–205. doi: 10.1136/heartjnl-2013-304372. [DOI] [PubMed] [Google Scholar]
- 3.Kutty S, Sengupta PP, Khandheria BK. Patent foramen ovale: the known and the to be known. J Am Coll Cardiol. 2012;59:1665–1671. doi: 10.1016/j.jacc.2011.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Ho SY, McCarthy KP, Rigby ML. Morphological features pertinent to interventional closure of patent oval foramen. J Interv Cardiol. 2003;16:33–38. doi: 10.1046/j.1540-8183.2003.08000.x. [DOI] [PubMed] [Google Scholar]
- 5.Krasuski RA. When and how to fix a ‘hole in the heart’: approach to ASD and PFO. Cleve Clin J Med. 2007;74:137–147. doi: 10.3949/ccjm.74.2.137. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen SG, Spruance SL, Smout R, Horn S. Transcranial Doppler quantification of residual shunt after percutaneous patent foramen ovale closure: correlation of device efficacy with intracardiac anatomic measures. J Interv Cardiol. 2012;25:304–312. doi: 10.1111/j.1540-8183.2011.00714.x. [DOI] [PubMed] [Google Scholar]
- 7.Falanga G, Carerj S, Oreto G, Khandheria BK, Zito C. How to understand patent foramen ovale clinical significance: part I. J Cardiovasc Echogr. 2014;24:114–121. doi: 10.4103/2211-4122.147202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pizzino F, Khandheria B, Carerj S, Oreto G, Cusma-Piccione M, Todaro MC, Oreto L, Vizzari G, Di Bella G, Zito C. PFO: button me up, but wait ... Comprehensive evaluation of the patient. J Cardiol. 2016;67:485–492. doi: 10.1016/j.jjcc.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Zito C, Dattilo G, Oreto G, Di Bella G, Lamari A, Iudicello R, Trio O, Caracciolo G, Coglitore S, Arrigo F, Carerj S. Patent foramen ovale: comparison among diagnostic strategies in cryptogenic stroke and migraine. Echocardiography. 2009;26:495–503. doi: 10.1111/j.1540-8175.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues AC, Picard MH, Carbone A, Arruda AL, Flores T, Klohn J, Furtado M, Lira-Filho EB, Cerri GG, Andrade JL. Importance of adequately performed Valsalva maneuver to detect patent foramen ovale during transesophageal echocardiography. J Am Soc Echocardiogr. 2013;26:1337–1343. doi: 10.1016/j.echo.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Cotter PE, Martin PJ, Belham M. Toward understanding the atrial septum in cryptogenic stroke. Int J Stroke. 2011;6:445–453. doi: 10.1111/j.1747-4949.2011.00647.x. [DOI] [PubMed] [Google Scholar]
- 12.Madala D, Zaroff JG, Hourigan L, Foster E. Harmonic imaging improves sensitivity at the expense of specificity in the detection of patent foramen ovale. Echocardiography. 2004;21:33–36. doi: 10.1111/j.0742-2822.2004.02168.x. [DOI] [PubMed] [Google Scholar]
- 13.Clarke NR, Timperley J, Kelion AD, Banning AP. Transthoracic echocardiography using second harmonic imaging with Valsalva manoeuvre for the detection of right to left shunts. Eur J Echocardiogr. 2004;5:176–181. doi: 10.1016/S1525-2167(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, DeGraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873–923. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 15.Ha JW, Shin MS, Kang S, Pyun WB, Jang KJ, Byun KH, Rim SJ, Huh J, Lee BI, Chung N. Enhanced detection of right-to-left shunt through patent foramen ovale by transthoracic contrast echocardiography using harmonic imaging. Am J Cardiol. 2001;87:669–671. A611. doi: 10.1016/s0002-9149(00)01455-7. [DOI] [PubMed] [Google Scholar]
- 16.Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, Habib G, Ringelstein EB, Sicari R, Zamorano JL, Sitges M, Caso P European Association of Echocardiography. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur J Echocardiogr. 2010;11:461–476. doi: 10.1093/ejechocard/jeq045. [DOI] [PubMed] [Google Scholar]
- 17.Caputi L, Carriero MR, Falcone C, Parati E, Piotti P, Materazzo C, Anzola GP. Transcranial Doppler and transesophageal echocardiography: comparison of both techniques and prospective clinical relevance of transcranial Doppler in patent foramen ovale detection. J Stroke Cerebrovasc Dis. 2009;18:343–348. doi: 10.1016/j.jstrokecerebrovasdis.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Jauss M, Zanette E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc Dis. 2000;10:490–496. doi: 10.1159/000016119. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Reyes R, Lopez-Fernandez T, Moreno-Yanguela M, Moreno R, Navas-Lobato MA, Refoyo E, Guzman G, Dominguez-Melcon F, Lopez-Sendon JL. Role of real-time three-dimensional transoesophageal echocardiography for guiding transcatheter patent foramen ovale closure. Eur J Echocardiogr. 2009;10:148–150. doi: 10.1093/ejechocard/jen214. [DOI] [PubMed] [Google Scholar]
- 20.Goel PK, Kapoor A, Batra A, Khanna R. Transcatheter retrieval of embolized AMPLATZER Septal Occluder. Tex Heart Inst J. 2012;39:653–656. [PMC free article] [PubMed] [Google Scholar]
- 21.Balzer J, Kuhl H, Rassaf T, Hoffmann R, Schauerte P, Kelm M, Franke A. Real-time transesophageal three-dimensional echocardiography for guidance of percutaneous cardiac interventions: first experience. Clin Res Cardiol. 2008;97:565–574. doi: 10.1007/s00392-008-0676-3. [DOI] [PubMed] [Google Scholar]
- 22.Rostamian A, Rathod A, Makkar RR, Siegel RJ. Three-dimensional echocardiographic assessment of patent foramen ovale in platypnea-orthodeoxia. Echocardiography. 2013;30:E239–242. doi: 10.1111/echo.12265. [DOI] [PubMed] [Google Scholar]
- 23.Rana BS, Shapiro LM, McCarthy KP, Ho SY. Three-dimensional imaging of the atrial septum and patent foramen ovale anatomy: defining the morphological phenotypes of patent foramen ovale. Eur J Echocardiogr. 2010;11:i19–25. doi: 10.1093/ejechocard/jeq122. [DOI] [PubMed] [Google Scholar]
- 24.Cunnington C, Hampshaw SA, Mahadevan VS. Utility of real-time three-dimensional intracardiac echocardiography for patent foramen ovale closure. Heart. 2013;99:1789–1790. doi: 10.1136/heartjnl-2013-304220. [DOI] [PubMed] [Google Scholar]
- 25.Medford BA, Taggart NW, Cabalka AK, Cetta F, Reeder GS, Hagler DJ, Johnson JN. Intracardiac echocardiography during atrial septal defect and patent foramen ovale device closure in pediatric and adolescent patients. J Am Soc Echocardiogr. 2014;27:984–990. doi: 10.1016/j.echo.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Bartel T, Muller S. Contemporary echocardiographic guiding tools for device closure of interatrial communications. Cardiovasc Diagn Ther. 2013;3:38–46. doi: 10.3978/j.issn.2223-3652.2013.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alboliras ET, Hijazi ZM. Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. Am J Cardiol. 2004;94:690–692. doi: 10.1016/j.amjcard.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Rana BS, Thomas MR, Calvert PA, Monaghan MJ, Hildick-Smith D. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging. 2010;3:749–760. doi: 10.1016/j.jcmg.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Komar M, Podolec P, Przewlocki T, Wilkolek P, Tomkiewicz-Pajak L, Motyl R. Transoesophageal echocardiography can help distinguish between patients with “symptomatic” and “asymptomatic” patent foramen ovale. Kardiol Pol. 2012;70:1258–1263. [PubMed] [Google Scholar]
- 30.Presbitero P, Lanzone AM, Albiero R, Lisignoli V, Zavalloni Parenti D, Gasparini GL, Lodigiani C, Barbaro C, Fappani A, Barberis G, Rossi ML, Pagnotta P. Anatomical patterns of patent foramen ovale (PFO): do they matter for percutaneous closure? Minerva Cardioangiol. 2009;57:275–284. [PubMed] [Google Scholar]
- 31.Goel SS, Tuzcu EM, Shishehbor MH, de Oliveira EI, Borek PP, Krasuski RA, Rodriguez LL, Kapadia SR. Morphology of the patent foramen ovale in asymptomatic versus symptomatic (stroke or transient ischemic attack) patients. Am J Cardiol. 2009;103:124–129. doi: 10.1016/j.amjcard.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama R, Takaya Y, Akagi T, Watanabe N, Ikeda M, Nakagawa K, Toh N, Ito H. Identification of high-risk patent foramen ovale associated with cryptogenic stroke: development of a scoring system. J Am Soc Echocardiogr. 2019;32:811–816. doi: 10.1016/j.echo.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Bayar N, Arslan S, Cagirci G, Erkal Z, Ureyen CM, Cay S, Koklu E, Yuksel IO, Kucukseymen S. Assessment of morphology of patent foramen ovale with transesophageal echocardiography in symptomatic and asymptomatic patients. J Stroke Cerebrovasc Dis. 2015;24:1282–1286. doi: 10.1016/j.jstrokecerebrovasdis.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 34.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2013;34:3342–3352. doi: 10.1093/eurheartj/eht285. [DOI] [PubMed] [Google Scholar]
- 36.Khan AR, Bin Abdulhak AA, Sheikh MA, Khan S, Erwin PJ, Tleyjeh I, Khuder S, Eltahawy EA. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2013;6:1316–1323. doi: 10.1016/j.jcin.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Spencer FA, Lopes LC, Kennedy SA, Guyatt G. Systematic review of percutaneous closure versus medical therapy in patients with cryptogenic stroke and patent foramen ovale. BMJ Open. 2014;4:e004282. doi: 10.1136/bmjopen-2013-004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad Y, Howard JP, Arnold A, Shin MS, Cook C, Petraco R, Demir O, Williams L, Iglesias JF, Sutaria N, Malik I, Davies J, Mayet J, Francis D, Sen S. Patent foramen ovale closure vs medical therapy for cryptogenic stroke: a meta-analysis of randomized controlled trials. Eur Heart J. 2018;39:1638–1649. doi: 10.1093/eurheartj/ehy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azarbal B, Tobis J, Suh W, Chan V, Dao C, Gaster R. Association of interatrial shunts and migraine headaches: impact of transcatheter closure. J Am Coll Cardiol. 2005;45:489–492. doi: 10.1016/j.jacc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 40.Pristipino C, Germonpré P, Toni D, Sievert H, Meier B, D’Ascenzo F, Berti S, Onorato EM, Bedogni F, Mas JL, Scacciatella P, Hildick-Smith D, Gaita F, Kyrle PA, Thomson J, Derumeaux G, Sibbing D, Chessa M, Hornung M, Zamorano J, Dudek D Collaborators; Joint task force of European Association of Percutaneous Cardiovascular Interventions (EAPCI), European Stroke Organisation (ESO), European HeartRhythm Association (EHRA), European Association for Cardiovascular Imaging (EACVI), European Paediatric and Congenital Cardiology (AEPC), ESC Working Group on Adult Congenital Heart Disease, ESC Working Group on Thrombosis, European Haematological Society (EHA), European Underwater and Baromedical Society (EUBS) Part II - Decompression sickness, migraine, arterial deoxygenation syndromes and select high-risk clinical conditions. Eur Heart J. 2021 EIJ-D-20-00785. [Google Scholar]
- 41.Dao CN, Tobis JM. PFO and paradoxical embolism producing events other than stroke. Catheter Cardiovasc Interv. 2011;77:903–909. doi: 10.1002/ccd.22884. [DOI] [PubMed] [Google Scholar]
- 42.Ramineni R, Daniel GK. Association of a patent foramen ovale with myocardial infarction and pulmonary emboli in a peripartum woman. Am J Med Sci. 2010;340:326–328. doi: 10.1097/MAJ.0b013e3181e732b2. [DOI] [PubMed] [Google Scholar]
- 43.Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, Wahl A, Windecker S, Jüni P PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–1091. doi: 10.1056/NEJMoa1211716. [DOI] [PubMed] [Google Scholar]
- 44.Torti SR, Billinger M, Schwerzmann M, Vogel R, Zbinden R, Windecker S, Seiler C. Risk of decompression illness among 230 divers in relation to the presence and size of patent foramen ovale. Eur Heart J. 2004;25:1014–1020. doi: 10.1016/j.ehj.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 45.Cartoni D, De Castro S, Valente G, Costanzo C, Pelliccia A, Beni S, Di Angelantonio E, Papetti F, Vitali Serdoz L, Fedele F. Identification of professional scuba divers with patent foramen ovale at risk for decompression illness. Am J Cardiol. 2004;94:270–273. doi: 10.1016/j.amjcard.2004.03.084. [DOI] [PubMed] [Google Scholar]
- 46.Wilmshurst P, Walsh K, Morrison L. Transcatheter occlusion of foramen ovale with a button device after neurological decompression illness in professional divers. Lancet. 1996;348:752–753. doi: 10.1016/S0140-6736(05)65638-3. [DOI] [PubMed] [Google Scholar]
- 47.Roxas-Timonera M, Larracas C, Gersony D, Di Tullio M, Keller A, Homma S. Patent foramen ovale presenting as platypnea-orthodeoxia: diagnosis by transesophageal echocardiography. J Am Soc Echocardiogr. 2001;14:1039–1041. doi: 10.1067/mje.2001.112968. [DOI] [PubMed] [Google Scholar]
- 48.Kent DM, Thaler DE RoPE Study Investigators. The Risk of Paradoxical Embolism (RoPE) Study: developing risk models for application to ongoing randomized trials of percutaneous patent foramen ovale closure for cryptogenic stroke. Trials. 2011;12:185. doi: 10.1186/1745-6215-12-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, Homma S, Di Angelantonio E, Di Tullio MR, Lutz JS, Elkind MS, Griffith J, Jaigobin C, Mattle HP, Michel P, Mono ML, Nedeltchev K, Papetti F, Thaler DE. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619–625. doi: 10.1212/WNL.0b013e3182a08d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thaler DE, Ruthazer R, Weimar C, Mas JL, Serena J, Di Angelantonio E, Papetti F, Homma S, Mattle HP, Nedeltchev K, Mono ML, Jaigobin C, Michel P, Elkind MS, Di Tullio MR, Lutz JS, Griffith J, Kent DM. Recurrent stroke predictors differ in medically treated patients with pathogenic vs other PFOs. Neurology. 2014;83:221–226. doi: 10.1212/WNL.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falanga G, Carerj S, Oreto G, Khandheria B, Zito C. How to understand patent foramen ovale clinical significance - part II: therapeutic strategies in cryptogenic stroke. J Cardiovasc Echogr. 2015;25:46–53. doi: 10.4103/2211-4122.161779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pristipino C, Sievert H, D’Ascenzo F, Louis Mas J, Meier B, Scacciatella P, Hildick-Smith D, Gaita F, Toni D, Kyrle P, Thomson J, Derumeaux G, Onorato E, Sibbing D, Germonpre P, Berti S, Chessa M, Bedogni F, Dudek D, Hornung M, Zamorano J, Evidence Synthesis T, Eapci Scientific D, Initiatives C, International E. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur Heart J. 2019;40:3182–3195. doi: 10.1093/eurheartj/ehy649. [DOI] [PubMed] [Google Scholar]
- 53.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L, Investigators CI. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 54.Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100. doi: 10.1056/NEJMoa1301440. [DOI] [PubMed] [Google Scholar]
- 55.Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL RESPECT Investigators. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032. doi: 10.1056/NEJMoa1610057. [DOI] [PubMed] [Google Scholar]
- 56.Furlan AJ, Jauss M. Patent foramen ovale and cryptogenic stroke: the hole story. Stroke. 2013;44:2676–2678. doi: 10.1161/STROKEAHA.113.001676. [DOI] [PubMed] [Google Scholar]
- 57.Alkhouli M, Sievert H, Holmes DR. Patent foramen ovale closure for secondary stroke prevention. Eur Heart J. 2019;40:2339–2350. doi: 10.1093/eurheartj/ehz157. [DOI] [PubMed] [Google Scholar]
- 58.Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, Spence JD, Thomassen L Gore REDUCE Clinical Study Investigators. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–1042. doi: 10.1056/NEJMoa1707404. [DOI] [PubMed] [Google Scholar]
- 59.Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D, Spence JD, Thomassen L Gore REDUCE Clinical Study Investigators. Five-year outcomes of PFO closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2021;384:970–971. doi: 10.1056/NEJMc2033779. [DOI] [PubMed] [Google Scholar]
- 60.Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O, Guidoux C, Canaple S, Vaduva C, Dequatre-Ponchelle N, Sibon I, Garnier P, Ferrier A, Timsit S, Robinet-Borgomano E, Sablot D, Lacour JC, Zuber M, Favrole P, Pinel JF, Apoil M, Reiner P, Lefebvre C, Guérin P, Piot C, Rossi R, Dubois-Randé JL, Eicher JC, Meneveau N, Lusson JR, Bertrand B, Schleich JM, Godart F, Thambo JB, Leborgne L, Michel P, Pierard L, Turc G, Barthelet M, Charles-Nelson A, Weimar C, Moulin T, Juliard JM, Chatellier G CLOSE Investigators. Patent foramen ovale closure or anticoagulation vs antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021. doi: 10.1056/NEJMoa1705915. [DOI] [PubMed] [Google Scholar]
- 61.Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, Song JM, Kang DH, Kwon SU, Kang DW, Lee D, Kwon HS, Yun SC, Sun BJ, Park JH, Lee JH, Jeong HS, Song HJ, Kim J, Park SJ. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO trial. J Am Coll Cardiol. 2018;71:2335–2342. doi: 10.1016/j.jacc.2018.02.046. [DOI] [PubMed] [Google Scholar]
- 62.Sievert H, Horvath K, Zadan E, Krumsdorf U, Fach A, Merle H, Scherer D, Schrader R, Spies H, Nowak B, Lissmann-Jensen H. Patent foramen ovale closure in patients with transient ischemia attack/stroke. J Interv Cardiol. 2001;14:261–266. doi: 10.1111/j.1540-8183.2001.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 63.Rigatelli G, Dell’Avvocata F, Ronco F, Giordan M, Cardaioli P. Patent oval foramen transcatheter closure: results of a strategy based on tailoring the device to the specific patient’s anatomy. Cardiol Young. 2010;20:144–149. doi: 10.1017/S1047951109990631. [DOI] [PubMed] [Google Scholar]
- 64.von Bardeleben RS, Richter C, Otto J, Himmrich L, Schnabel R, Kampmann C, Rupprecht HJ, Marx J, Hommel G, Munzel T, Horstick G. Long term follow up after percutaneous closure of PFO in 357 patients with paradoxical embolism: difference in occlusion systems and influence of atrial septum aneurysm. Int J Cardiol. 2009;134:33–41. doi: 10.1016/j.ijcard.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 65.Vizzari G, Pizzino F, Bajwa T, Ammar KA, Khandheria BK. Amplatzer(R) septal occluder device early embolization to left ventricular outflow tract in asymptomatic patient. Eur Heart J Cardiovasc Imaging. 2014;15:925. doi: 10.1093/ehjci/jeu049. [DOI] [PubMed] [Google Scholar]
- 66.Davison P, Clift PF, Steeds RP. The role of echocardiography in diagnosis, monitoring closure and post-procedural assessment of patent foramen ovale. Eur J Echocardiogr. 2010;11:i27–34. doi: 10.1093/ejechocard/jeq120. [DOI] [PubMed] [Google Scholar]
- 67.Vitarelli A, Mangieri E, Capotosto L, Tanzilli G, D’Angeli I, Toni D, Azzano A, Ricci S, Placanica A, Rinaldi E, Mukred K, Placanica G, Ashurov R. Echocardiographic findings in simple and complex patent foramen ovale before and after transcatheter closure. Eur Heart J Cardiovasc Imaging. 2014;15:1377–85. doi: 10.1093/ehjci/jeu143. [DOI] [PubMed] [Google Scholar]
- 68.McMahon CJ, El Said HG, Mullins CE. Use of the transseptal puncture in transcatheter closure of long tunnel-type patent foramen ovale. Heart. 2002;88:E3. doi: 10.1136/heart.88.2.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson AJ, Hagler DJ, Taggart NW. Transseptal puncture to facilitate device closure of “long-tunnel” patent foramen ovale. Catheter Cardiovasc Interv. 2015;85:1053–1057. doi: 10.1002/ccd.25723. [DOI] [PubMed] [Google Scholar]
- 70.Spence MS, Khan AA, Mullen MJ. Balloon assessment of patent foramen ovale morphology and the modification of tunnels using a balloon detunnelisation technique. Catheter Cardiovasc Interv. 2008;71:222–228. doi: 10.1002/ccd.21415. [DOI] [PubMed] [Google Scholar]
- 71.MacDonald ST, Daniels MJ, Ormerod OJ. Initial use of the new GORE((R)) septal occluder in patent foramen ovale closure: implantation and preliminary results. Catheter Cardiovasc Interv. 2013;81:660–665. doi: 10.1002/ccd.24405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomson JD, Hildick-Smith D, Clift P, Morgan G, Daniels M, Henderson R, Spence MS, Mahadevan VS, Crossland D, Ormerod O. Patent foramen ovale closure with the Gore septal occluder: initial UK experience. Catheter Cardiovasc Interv. 2014;83:467–473. doi: 10.1002/ccd.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lockhart CJ, Johnston NG, Spence MS. Experience using the new GORE Septal Occluder at the margins. Catheter Cardiovasc Interv. 2013;81:1244–1248. doi: 10.1002/ccd.24736. [DOI] [PubMed] [Google Scholar]
- 74.Knerr M, Bertog S, Vaskelyte L, Hofmann I, Sievert H. Results of percutaneous closure of patent foramen ovale with the GORE((R)) septal occluder. Catheter Cardiovasc Interv. 2014;83:1144–1151. doi: 10.1002/ccd.25336. [DOI] [PubMed] [Google Scholar]
- 75.Geis NA, Pleger ST, Katus HA, Hardt SE. Using the GORE(R) Septal Occluder (GSO) in challenging patent foramen ovale (PFO) anatomies. J Interv Cardiol. 2015;28:190–197. doi: 10.1111/joic.12181. [DOI] [PubMed] [Google Scholar]
- 76.Noc M, Cernic Suligoj N, Zvan B, Zorc M, Kar S. In-tunnel closure of patent foramen ovale with a FlatStent EF. Kardiol Pol. 2015;73:549–556. doi: 10.5603/KP.a2015.0026. [DOI] [PubMed] [Google Scholar]
- 77.Reinthaler M, Aggarwal SK, Mert A, Lim S, Skurk C, Landmesser U, Mullen MJ. Closure of long-tunnel PFOs with the coherex flatstent EF - a tailored approach. J Invasive Cardiol. 2015;27:E190–195. [PubMed] [Google Scholar]
- 78.Zimmermann WJ, Heinisch C, Majunke N, Staubach S, Russell S, Wunderlich N, Sievert H. Patent foramen ovale closure with the SeptRx device initial experience with the first “In-Tunnel” device. JACC Cardiovasc Interv. 2010;3:963–967. doi: 10.1016/j.jcin.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 79.Gaspardone A, De Marco F, Sgueglia GA, De Santis A, Iamele M, D’Ascoli E, Tusa M, Corciu A, Mullen M, Nobles A, Carminati M, Bedogni F. Novel percutaneous suture-mediated patent foramen ovale closure technique: early results of the NobleStitch EL Italian Registry. EuroIntervention. 2018;14:e272–e279. doi: 10.4244/EIJ-D-18-00023. [DOI] [PubMed] [Google Scholar]
- 80.Baldetti L, Ferri LA, Ancona M, Bellini B, Visco E, Melillo F, Beneduce A, Chieffo A, Ancona F, Agricola E, Montorfano M. Interatrial septal tear after patent foramen ovale closure with the noblestitch device. JACC Cardiovasc Interv. 2019;12:e139–e140. doi: 10.1016/j.jcin.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 81.Sievert H, Wunderlich N, Reiffenstein I, Ruygrok P, Grube E, Buellesfeld L, Meier B, Schofer J, Muller D, Jones RK, Gillam L. Initial clinical experience with the Coherex FlatStent and FlatStent EF PFO closure system for in-tunnel PFO closure: results of the Coherex-EU study. Catheter Cardiovasc Interv. 2014;83:1135–1143. doi: 10.1002/ccd.24565. [DOI] [PubMed] [Google Scholar]


