Abstract
It has been shown that gut dysbiosis can be associated with the development of type 2 diabetes mellitus (T2DM). Consequently, intervention with probiotics may be a useful approach to improve metabolic variables in diabetes. The present study aimed to evaluate the efficacy of L. paracasei HII01 on glycemia in T2DM patients. In a randomized, double-blind, placebo-controlled study, 50 participants were allocated to receive L. paracasei HII01 (50 × 109 CFU/day) or a placebo (corn starch 10 mg/day). Blood and fecal samples were assessed at baseline and at the end of the trial. After 12 weeks of intervention, fasting blood glucose level had significantly decreased in the probiotic group compared with the placebo group. Importantly, probiotic supplementation significantly decreased the plasma levels of LPS, TNF-α, IL-6 and hsCRP compared the placebo group. Additionally, an increase in beneficial bacteria and a decrease in pathogenic bacteria, which related to the improvement of SCFAs, was found following L. paracasei HII01 supplementation. These findings demonstrated that L. paracasei HII01 improved hyperglycemia and inflammatory markers by favorably modifying gut microbiota and subsequently ameliorating the leaky gut and endotoxemia, thereby suggesting a potential role as an adjuvant treatment in type 2 diabetes.
Keywords: Lactobacillus paracasei, probiotics, type 2 diabetes mellitus, gut microbiota, glycemia, inflammation
1. Introduction
Type 2 diabetes mellitus (T2DM) is one of the most common metabolic disorders in the world. In 2019, it was estimated that 463 million people worldwide had diabetes. Moreover, the prediction found that the number of diabetic patients will reach to 578 million in 2030 and 700 million in 2045 [1]. T2DM is characterized by hyperglycemia due to either insulin insufficiency, insulin resistance or both [2]. Diabetes is an established major independent risk factor for several chronic diseases, such as ischemic heart disease, stroke and renal failure, which caused the death of 4.2 million people in 2019 [3]. These diabetic complications can be prevented or reduced by sustained control of blood glucose. Nowadays, drugs in the treatment of diabetes can trigger several serious side effects. Thus, there is still a need for safer and effective hypoglycemic agents.
Gut microbiota have been accepted as a key environmental factor contributing to the development of diabetes [4]. Alteration of gut microbiota (dysbiosis) can disrupt the gut’s tight junctions, leading to increased gut permeability and favoring lipopolysaccharide (LPS) translocation to blood circulation. Increased circulating LPS induces “metabolic endotoxemia,” which triggers inflammatory reactions and insulin resistance [5]. Modulation of gut microbiota via probiotics has been widely used to prevent the development of T2DM and its complications. Probiotics refer to microorganisms that confer health benefits to hosts when administered in adequate amounts [6]. Prebiotics is defined as a selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefits upon host health [7]. Lactobacillus and Bifidobacterium are the strains most commonly used as probiotics in functional foods and dietary supplements [8]. A clinical study demonstrated that treatment with probiotic yogurt (7.23 × 106 CFU of L. acidophilus La5 and 6.04 × 106 CFU of B. lactis Bb12) for 6 weeks reduced fasting blood glucose and HbA1c levels in T2DM patients [9]. The consumption of L. plantarum WCFS1 at a dose of 1012 CFU/day increased the tight junction protein, ZO-1, in healthy subjects [10]. Additionally, it has been reported that treatment with L. acidophilus La-5 and B. animalis subsp lactis BB-12 (109 CFU/day, each) decreased the blood level of inflammatory cytokines (TNF-α and resistin) and increased in the SCFAs level of acetic acid in the feces of T2DM patients [11]. Recently, Shaun et al. (2019) found that supplementation with probiotic mixture for 6 months reduced blood LPS level resulting in decreased fasting blood glucose, HOMA-IR, inflammatory markers (TNF-α, IL-6), C-reactive protein (CRP) and resistin in patients with diabetes [12]. However, the actual effects of probiotic supplementation on gut microbiota or glucose metabolism are still under debate in clinical studies.
Recently, a newly identified probiotic Lactobacillus paracasei HII01 (L. paracasei HII01), from the fermentation of northern Thai pickle, was found to have beneficial effects in an animal model of obesity and diabetes [13,14]. However, the hypoglycemic effect and its potential mechanism of L. paracasei HII01 in T2DM patients need to be validated. Therefore, the aim of the present study was to evaluate the efficacy of the probiotic L. paracasei HII01 on the treatment of glycemia in T2DM patients. In addition, we focused on the modulation of gut microbiota, gut permeability and its contribution to improving systemic inflammation.
2. Materials and Methods
The study protocol was approved by the Ethics Committee of Phrae Provincial Public Health Office (approval number: PPH No.1/2562) and conducted according to the guidelines of the National Policy Guidelines for Human Research 2015, National Research Council of Thailand. The study was performed under the supervision of physicians. Prior to the study, the purpose and methodology of the study were fully explained to the participants by the researchers, and all patients gave written informed consent before any study procedures were initiated.
A randomized, double-blind, placebo-controlled clinical trial was used in this study. in fasting plasma glucose concentrations as the primary outcome and the change of Bifidobacterium in feces as the secondary outcome [15]. To detect a 25% decrease in fasting plasma glucose concentrations after the intervention or an abundance of Bifidobacterium of the intervention group, which was 7.36 (standard deviation = 0.79) at pretreatment period and 7.94 (standard deviation = 0.87) at post-treatment period, an α value equal to 0.05 and a power of 80% were considered using the STATA program [15]. The maximum number of estimated sample size from each calculation was chosen and used in this study. Considering 20% probable drop in the sample, at least 50 participants were allocated to placebo and intervention groups (25 in each group).
2.1. Participants
The eligible participants consisted of individuals (men and women) recruited from health-promoting hospitals in Phrae Province, Thailand. The inclusion criteria were T2DM according to WHO criteria [16], being aged between 20 and 70 years and not having had any antibiotic treatment for 14 days prior to the start of the study to prevent the bactericidal effect. Although antibiotic drugs have a side effect to reduce the diversity of gut microbiome, the recovery of microbiome disturbances after antibiotics depends on many factors such as type of antibiotic drug, dosages, duration of taking or plasma half-life. For example, amoxicillin has been reported to not change total bacterial numbers and microbial diversity significantly [17]. The exclusion criteria were an abnormal liver or renal function test, a history of malignant, micro- and macrovascular complications, chronic alcoholism or heavy alcohol use (defined heavy alcohol use as binge drinking on 5 or more days in the past month) [18], being pregnant or breastfeeding, taking nonsteroidal anti-inflammatory drugs, heavy cigarette smoking or heavy smoker (defined as more than 20 cigarettes daily) [19] and hormone replacement therapy. Additionally, participants were excluded from the study if there was any change in medication or lifestyle, or they started to take antibiotics at any stage of the investigation.
2.2. Study Protocol
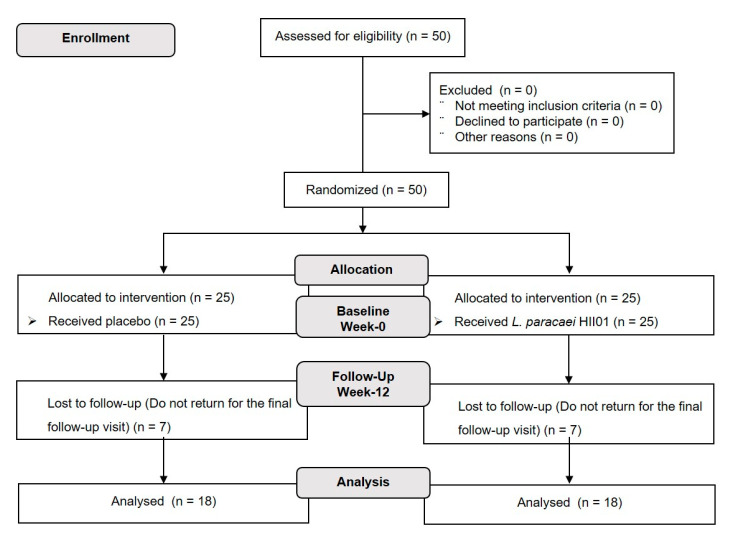
The participants (n = 50) were randomly assigned by blocked randomization at a ratio of 1:1 with a computer-generated assignment to either probiotic or placebo groups. The investigators, study staff and participants were blinded to the group assignment. Participants in the probiotic group received a probiotic L. paracasei HII01 50 × 109 CFU/day and the placebo group received corn starch 10 mg/day throughout the 12 weeks of intervention. The study design is presented in Figure 1. The probiotic L. paracasei HII01 was produced by LACTOMASON Company (Gyeongsangnam-do, Korea). Both the probiotic and the corn starch were contained within an aluminum foil envelope. All participants were required to:
Take one aluminum foil envelope per day (20 min before dinner or sleeping) with clean drinking water.
Store the probiotic or placebo in the fridge at 4–6 °C.
Record the number of aluminum foil envelopes taken each day in the study diary.
Avoid eating or drinking yoghurt, fermented food, dietary supplements (i.e., vitamins, minerals, nutraceuticals, herbal preparations, probiotics, prebiotics or fish oils).
Figure 1.
Flowchart of the study design.
The primary assessment of compliance was evaluated from the count of aluminum foils. In addition, participants’ recording books containing dietary and medication intake, physical activity, defecation and any undesirable side effects were considered.
2.3. Outcome Measures
The primary outcome measurements were fasting blood glucose (FBG) and HbA1c concentration at the end of the 12-week study period. The secondary outcomes included bacterial and SCFAs abundance in feces, gut permeability (plasma ZO-1), plasma LPS, plasma IgA, plasma inflammatory biomarkers (IL-1β, IL-6, IL-10, TNF-α and hsCRP), plasma lipid (TG, cholesterol, HDL and LDL) and plasma adipokines (leptin and adiponectin) levels.
2.4. Biochemical Measurements
After overnight fasting, blood collection was performed following the initial assessment (week-0) and at the end of the study (week-12) in ethylenediaminetetraacetic acid (EDTA) or heparin as appropriate. These blood samples were immediately stored at 4 °C and centrifuged for 15 min at 1000 g at 2–8 °C for 30 min. Plasma samples were aliquoted in pyrogen-free tubes and stored at −80 °C. Blood biochemistry parameters, including FBG, HbA1c and lipid levels were analyzed via the certified routine biochemistry laboratory service. The plasma leptin and adiponectin levels were determined using a sandwich ELISA kit (LINCO, Research, Saint Charies, MO, USA).
2.5. Gut Permeability
Biomarkers of gut permeability (ZO-1) and LPS were measured using commercial kits according to the manufacturers’ instructions. Serum LPS was assessed using a Pierce™ Limulus Amoebocyte Lysate chromogenic endotoxin quantification kit (Thermo Fisher, Sydney, NSW, Australia). The EDTA-plasma ZO-1 level was measured with a human haptoglobin ELISA kit (Abcam®, Sydney, NSW, Australia).
2.6. Inflammation
Biomarkers of inflammation were determined in serum. Serum hsCRP was measured with a human hsCRP ELISA kit (OriGene, Rockville, MD, USA). Serum tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), interleukin 1 beta (IL-1β) and interleukin 10 (IL-10) were quantified with an ELISA kit (Thermo Fisher, Sydney, NSW, Australia) according to the manufacturers’ instructions.
2.7. Fecal Analysis
Stool samples were collected following the initial assessment (week-0) and at the end of the study (week-12) using a stool specimen collection kit. This collection kit contained an instruction book for the stool sample collection and transportation, ice packs, gloves, a sterile container, a sealed plastic pouch, a cool box and an AnaeroGen™ Compact Sachet, which preserves the microbiological characteristics of the sample for 72 h. The containers were stored at 4 °C. The fecal sample was analyzed by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry within 24–48 h after collection. In addition, a 1 g sample was stored at −80 °C prior experiment for fecal microbiota. The fecal samples were extracted by QIAamp PowerFecal DNA/RNA kit (QIAGEN, Hidden, Germany). Fecal microbiome dataset was normalized by the total number of reads in each sample to remove potential biases related to different sequencing depth by Omics Sciences and Bioinformatics Center (OMICs, Chulalongkorn University, Thailand) using the Illumina MiSeq platform next generation sequencing system (Themo Fisher, Sydney, NSW, Australia). Sequencing data were processed using a quantitative analysis of fecal microbial ecology (QIIME II). Original sequencing reads that perfectly matched the barcode were assigned to the corresponding samples and identified as valid sequences. Low-quality sequences were filtered to remove.. After chimera detection, the remaining high-quality sequences were clustered into OTUs with 97% sequence identity. The default parameters were used to select the representative sequence from each OTU. OTUs with a total content of less than 0.001% in all samples were discarded to minimize sequencing depth differences across samples.
Short chain fatty acids (SCFAs) were measured using high-performance liquid chromatography (HPLC) according to the modified method of Nuntawat et al. (2019). Briefly, 1 g stool samples were homogenized in 0.15 mM sulfuric acid, pH 7, mixed and centrifuged at 10,000 g for 10 min at 4 °C. The supernatant was collected and filtered through a 0.22 um nylon syringe filter. The samples were analyzed with a Shimadzu-HPLC system using Shodex SUGAR SH1011 (SHOWA DENKO K.K., Tokyo, Japan). SCFA concentrations were quantified by comparing with the standard curve and the results were expressed as μmol/g sample [20].
2.8. Statistical Analysis
Data were presented as mean ± SD. After testing for normality of distribution, the characteristics and biochemical variables at the beginning of the study were compared among the two groups using independent t-test or Wilcoxon rank sum test, as appropriate. Differences in sex, education, smokers and alcoholics were evaluated by Fisher’s exact test. A paired t-test or Wilcoxon signed rank test were used to determine the treatment effects within group difference. Linear regression model was used to assess the treatment effects on study outcomes among the two groups after adjusting for the confounding parameters including age, sex, education, smoker, alcoholic, BMI and the baseline biochemical parameters. A p-value of less than 0.05 was considered statistically significant. Data analysis was performed using STATA Statistical Software version 15.1 (Brazos County, TX, USA).
3. Results
A total of 50 T2DM patients were screened for eligibility and enrolled in this present randomized, double-blind, placebo-controlled clinical trial. All participants were randomly allocated into two groups (n = 25/group) receiving either a placebo, corn starch 10 mg/day or probiotics, L. paracasei HII01, at a dose of 50 × 109 CFU/day for 12 weeks. Seven patients in the placebo (n = 7) and probiotics (n = 7) groups dropped out due to personal reason. Hence, 36 participants (72%) finished the study per protocol, reducing the power from 80 to 75.79. There were no statistically significant differences between the two groups regarding any of the baseline characteristics and biochemical parameters at the beginning of the study (Table S1 (Supplementary File)). There were no seriously adverse effects or symptoms among the participants and they all demonstrated good compliance. The medication of individual participants did not change during the study. (Table S2 (Supplementary File)).
Demographic characteristics of the participants are shown in Table 1. There was no significant difference in age, sex, education, smokers, alcoholics and BMI between placebo and probiotic group at the beginning of the study.
Table 1.
General characteristics of the type 2 diabetes participants.
| Characteristics | Placebo (n = 18) | Probiotic (n = 18) | p-Value |
|---|---|---|---|
| Age (year) | 61.78 ± 7.73 | 63.50 ± 5.94 | 0.459 a |
| Sex | 0.228 b | ||
| Male | 2 (11.11) | 6 (33.33) | |
| Female | 16 (88.89) | 12 (66.67) | |
| Education | 1.000 b | ||
| No education | 2 (11.11) | 3 (16.67) | |
| Primary | 14 (77.78) | 13 (72.22) | |
| Secondary | 2 (11.11) | 2 (11.11) | |
| Smokers | 0.486 b | ||
| No | 18 (100) | 16 (88.89) | |
| Yes | 0 (0) | 2 (11.11) | |
| Alcoholic | 1.000 b | ||
| No | 17 (94.44) | 18 (100) | |
| Yes | 1 (5.56) | 0 (0) | |
| BMI (kg/m2) | 23.05 ± 2.60 | 23.22 ± 2.72 | 0.852 a |
Data are mean ± SD. a p-value obtained from independent t-test, b p-value obtained from Fisher’s exact test.
3.1. The Effect of L. paracasei HII01 Supplementation on Blood Biochemical Parameters
Table 2 illustrates the biochemical parameters assessment of the two groups at baseline and at the end of the study with a comparison of within-group changes. At the end of the 12 weeks of intervention, there was no significant change in the level of all parameters detected in the placebo group compared to baseline. Interestingly, the FBG level in probiotic group at the end of the study significantly reduced compared to baseline (p < 0.05). In addition, the FBG level in probiotic group was also significantly reduced when comparing with placebo group at the end of study. (p < 0.05) (Table 3). As is well-known, adipokine plays an important role in regulating glucose metabolism. Thus, we next evaluated the plasma level of two adipokines, namely adiponectin and leptin. The results showed that there were no significant differences in either plasma adiponectin or leptin levels within the groups compared with the baseline values (Table 2). Regardless, the plasma leptin and adiponectin levels likely improve in the probiotics group. However, between-group comparisons showed no significant changes in these adipokines levels at the end of the intervention as shown in Table 3.
Table 2.
Blood levels of FBG, HbA1c, adiponectin, leptin and lipid profiles at baseline and at the end of the study.
| Parameters | Placebo (n = 18) | Probiotic (n = 18) | ||||
|---|---|---|---|---|---|---|
| Wk-0 | Wk-12 | p-Value | Wk-0 | Wk-12 | p-Value | |
| FBG (mg/dL) | 139.29 ± 49.77 | 149.12 ± 47.42 | 0.208 a | 129.18 ± 34.52 | 109.35 ± 15.56 | 0.005 a |
| HbA1c (%) | 6.64 ± 1.44 | 6.73 ± 1.29 | 0.837 a | 7.05 ± 1.85 | 6.46 ± 1.49 | 0.145 a |
| Adiponectin (ng/mL) | 27.70 ± 7.26 | 24.03 ± 12.52 | 0.465 b | 23.40 ± 8.75 | 30.73 ± 18.40 | 0.763 b |
| Leptin (ng/mL) | 15.56 ± 13.56 | 26.46 ± 8.49 | 0.095 b | 15.74 ± 9.51 | 14.60 ± 8.70 | 0.345 b |
| TG (mg/dL) | 147.78 ± 71.32 | 159.50 ± 67.87 | 0.581 a | 153.27 ± 55.46 | 144.53 ± 58.68 | 0.560 a |
| Cholesterol (mg/dL) | 190.83 ± 43.22 | 184.94 ± 46.65 | 0.314 a | 188.59 ± 34.21 | 182.88 ± 30.38 | 0.453 a |
| LDL (mg/dL) | 102.11 ± 32.60 | 90.61 ± 16.06 | 0.106 a | 109.24 ± 38.44 | 81.47 ± 33.53 | <0.00 a |
| HDL (mg/dL) | 62.87 ± 9.83 | 68.67 ± 13.99 | 0.145 a | 62.06 ± 14.84 | 72.06 ± 21.34 | 0.026 a |
Data are mean ± SD. Difference between baseline and the end of study for the within group comparisons. a p-value obtained from paired t-test, b p-value obtained from Wilcoxon signed rank test.
Table 3.
The differentiation of blood levels of FBG, HbA1c, adiponectin, leptin and lipid profiles between L. paracasei HII01 treatment and placebo group at the end of study.
| Comparison to Placebo at Week-12 | |||
|---|---|---|---|
| Probiotic Group | |||
| Parameters | Coef. | 95% Confidence Interval | p-Value |
| FBG (mg/dL) | −37.16 | (−60.35, −13.97) | 0.004 |
| HbA1c (%) | −1.50 | (−4.62, 1.62) | 0.252 |
| Adiponectin (ng/mL) | 6.03 | (22.64, 10.59) | 0.371 |
| Leptin (ng/mL) | −7.44 | (−26.02, 11.15) | 0.292 |
| TG (mg/dL) | −45.35 | (−119.26, 28.55) | 0.211 |
| Cholesterol (mg/dL) | −17.35 | (−40.33, 5.63) | 0.128 |
| LDL (mg/dL) | −18.54 | (−34.63, −2.44) | 0.0026 |
| HDL (mg/dL) | 14.53 | (−0.29, 28.77) | 0.046 |
Data are mean ± SD. Significant difference between groups at the end of study. p-values obtained from linear regression after adjusting for baseline characteristics and baseline of the corresponding parameter. Coefficient (Coef.) is defined as the difference in outcome measures between probiotic and placebo groups.
As can be seen in Table 2, the changes of the plasma TG and cholesterol levels were not observed in both placebo and probiotic group compared with the baseline and between-group comparisons. However, the plasma LDL level significantly decreased in the probiotic group between baseline and the end of the study (p < 0.05). A significant increase in the plasma HDL level was also exhibited in the L. paracasei HII01-treated group compared with the baseline (p < 0.05). In addition, we detected a significant decrease of LDL level together with increase HDL level in the probiotics group compared to placebo (p < 0.05) (Table 3). These findings may suggest that dyslipidemia in T2DM individuals was improved after the administration of L. paracasei HII01 for 12 weeks.
3.2. The Effect of L. paracasei HII01 Supplementation on the Level of ZO-1 and Inflammation Parameters
To evaluate the underlying mechanisms involving the possible beneficial effects of L. paracasei HII01 in improving gut permeability and endotoxemia, the levels of the tight junction protein, LPS, and pro-inflammatory cytokines were investigated and are presented in Table 4. Within group comparison showed that no differences were observed in all parameters in the placebo group. Interestingly, a significant decrease of the plasma LPS level was found in the probiotic L. paracasei HII01 group when compared with baseline (p < 0.05). Additionally, the levels of TNF-α and IL-6 at the end of the study had significantly decreased in the probiotic group compared to baseline (p < 0.05). Likewise, participants in the probiotic group also showed a significant reduction in the LPS, TNF-α and IL-6 levels after 12 weeks of intervention compared to participants in the placebo group (p < 0.05) (Table 5).
Table 4.
Blood levels of ZO-1 and inflammation parameters at baseline and at the end of the study.
| Parameters | Placebo (n = 18) | Probiotic (n = 18) | ||||
|---|---|---|---|---|---|---|
| Wk-0 | Wk-12 | p-Value | Wk-0 | Wk-12 | p-Value | |
| ZO-1 (ng/mL) | 1.80 ± 0.86 | 1.74 ± 0.77 | 0.455 a | 1.61 ± 0.53 | 1.45 ± 0.63 | 0.204 a |
| LPS (pg/mL) | 72.38 ± 40.67 | 69.36 ± 24.91 | 0.638 b | 92.05 ± 35.95 | 51.07 ± 20.26 | 0.002 b |
| TNF-α (ng/mL) | 11.75 ± 3.01 | 9.62 ± 1.37 | 0.252 a | 11.91 ± 0.72 | 6.16 ± 0.90 | 0.009 a |
| IL-1β (ng/mL) | 7.26 ± 2.49 | 6.17 ± 1.42 | 0.345 b | 8.21 ± 3.52 | 5.46 ± 3.24 | 0.109 b |
| IL-6 (ng/mL) | 11.84 ± 1.78 | 10.76 ± 4.91 | 0.549 a | 11.01 ± 1.91 | 7.85 ± 2.92 | 0.033 a |
| IL-10 (ng/mL) | 1.27 ± 0.15 | 6.04 ± 5.43 | 0.068 b | 1.12 ± 0.05 | 10.58 ± 0.1 | 0.180 b |
| IgA (ng/mL) | 660.68 ± 262.60 | 679.65 ± 338.66 | 0.867 a | 526.24 ± 249.61 | 707.02 ± 265.37 | 0.027 a |
| hsCRP (mg/L) | 0.0160 ± 0.0069 | 0.0158 ± 0.0059 | 0.950 a | 0.0143 ± 0.0024 | 0.0124 ± 0.0026 | 0.032 a |
Data are mean ± SD. Difference between baseline and the end of study for the within group comparisons. a p-value obtained from paired t-test, b p-value obtained from Wilcoxon signed rank test.
Table 5.
The differentiation of blood levels of ZO-1 and inflammation parameters between L. paracasei HII01 treatment and placebo at the end of study.
| Comparison to Placebo at Week-12 | |||
|---|---|---|---|
| Probiotic | |||
| Parameters | Coef. | 95% Confidence Interval | p-Value |
| ZO-1 (ng/mL) | −0.30 | (−0.69, −0.09) | 0.124 |
| LPS (pg/mL) | −26.46 | (−46.55, −6.37) | 0.020 |
| TNF-α (ng/mL) | −3.16 | (−5.50, −0.82) | 0.023 |
| IL-1β (ng/mL) | −2.13 | (−5.72, 1.46) | 0.125 |
| IL-6 (ng/mL) | −4.12 | (−5.68, −2.56) | 0.001 |
| IL-10 (ng/mL) | 5.61 | (−87.25, 98.47) | 0.060 |
| IgA (ng/mL) | 257.62 | (663.69, −148.45) | 0.188 |
| hsCRP (mg/L) | −0.004 | (−0.008, −0.001) | 0.026 |
Data are mean ± SD. Significant difference between groups at the end of study. p-values obtained from linear regression after adjusting for baseline characteristics and baseline of the corresponding parameter. Coefficient (Coef.) is defined as the difference in outcome measures between probiotic and placebo groups.
In this study, we also measured the plasma level of hsCRP, which is a biomarker of inflammation. There was no significant alteration in the plasma hsCRP level when compared with baseline in the placebo group. Remarkably, L. paracasei HII01 supplementation for 12 weeks in T2DM individuals significantly reduced the plasma hsCRP level compared with baseline (p < 0.05) (Table 4). Likewise, the reduction of hsCRP level was found in probiotic group compared with placebo group (p < 0.05) (Table 5). Furthermore, IgA, which is an antibody that plays a major role in the immune function, significantly increased in the probiotic group when compared with the baseline (p < 0.05) (Table 4). Together, these findings suggested that supplementation with L. paracasei HII01 attenuated endotoxemia and systemic inflammation in T2DM patients.
3.3. The Effect of L. paracasei HII01 Supplementation on the Level of SCFAs
It has been well established that gut microbiota also influences glucose metabolism partly via the production of SCFAs. Therefore, lactic, propionic, butyric and acetic acids, which are the most important SCFAs that affect glycemic control, were determined (Table 6). Interestingly, the results of the probiotic group demonstrated that the level of SCFAs, i.e., lactic, propionic, acetic and butyric acid, significantly increased within the group when compared with baseline (p < 0.05), while no significant difference was observed in the placebo group (p > 0.05) (Table 6). There were no significant differences between the two groups in those SCFAs levels at the end of the study. These findings indicated that administration of L. paracasei HII01 increased the level of SCFAs and may have contributed to the regulation of glucose metabolism in T2DM patients.
Table 6.
The levels of SCFAs in feces at baseline and at the end of the study.
| Parameters | Placebo (n = 18) | Probiotic (n = 18) | ||||
|---|---|---|---|---|---|---|
| Wk-0 | Wk-12 | p-Value | Wk-0 | Wk-12 | p-Value | |
| Lactic acid (μmol/g) | 22.60 ± 19.57 | 40.73 ± 25.80 | 0.068 b | 20.46 ± 16.08 | 51.00 ± 27.96 | 0.028 b |
| Propionic acid (μmol/g) | 42.83 ± 17.91 | 211.93 ± 30.28 | 0.109 b | 49.86 ± 94.36 | 307.64 ± 104.55 | 0.012 b |
| Acetic acid (μmol/g) | 359.40 ± 75.80 | 405.18 ± 71.08 | 0.217 a | 343.84 ± 46.56 | 512.54 ± 79.57 | 0.003 a |
| Butyric acid (μmol/g) | 51.46 ± 13.68 | 162.45 ± 57.14 | 0.109 b | 48.70 ± 29.76 | 181.80 ± 147.48 | 0.012 b |
Data are mean ±SD. Difference between baseline and the end of study for the within group comparisons. a p-value obtained from paired t-test, b p-value obtained from Wilcoxon signed rank test.
3.4. The Effect of L. paracasei HII01 Supplementation on Microbial Diversity
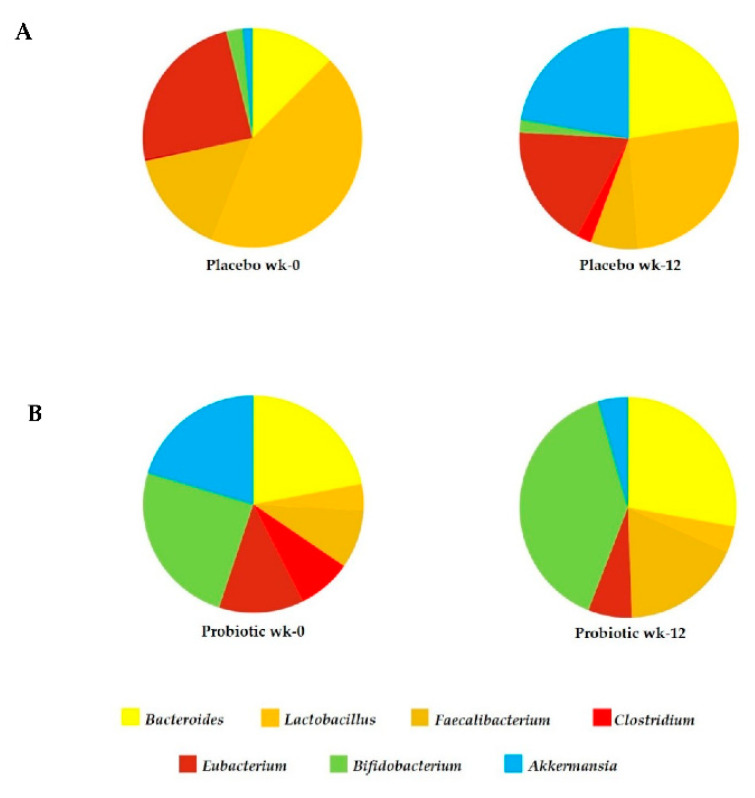
The relative abundance OTUsplot of bacterial genus diversity in feces is illustrated in Figure 2. In comparison with baseline, sequences were distributed among seven genera including Akkermansia, Bifidobacterium, Eubacterium, Clostridium, Faecalibacterium, Lactobacillus and Bacteroides. The abundance percentage level of beneficial bacteria, such as Lactobacillus, Faecalibacterium and Bifidobacterium markedly increased after probiotic L. paracasei HII01 supplementation. There was no significantly higher difference in the Lactobacillus composition. In contrast, the proportion of genus Bifidobacterium, Bacteroides and Faecalibacterium significantly increased in the probiotic L. paracasei HII01 supplementation group compared to placebo group (p < 0.05, 95% confidence interval). Bifidobacterium was the most altered among those fecal bacteria, varying from 17 to 29%, after L. paracasei HII01 administration. Notably, the pathogenic Clostridium abundance was increased only in the placebo group and this was not observed in the probiotic treatment group (Figure 2). These outcomes suggested that L. paracasei HII01 administration effectively enhanced some beneficial bacteria as well as reduced the pathogenic bacteria in T2DM patients.
Figure 2.
Seven genera: Akkermansia, Bifidobacterium, Eubacterium, Clostridium, Faecalibacterium, Lactobacillus, and Bacteroides dominated the majority of OTUs. 16S rRNA gene sequences were used for identification. The relative abundance of bacteria in feces of placebo group (A) and probiotic group (B) at baseline and at the end of the study.
4. Discussion
The main findings of this study were that intervention with L. paracasei HII01 for 12 weeks improved glycemia in T2DM patients. Importantly, L. paracasei HII01 supplementation was able to affect endotoxemia and improve inflammatory cytokines. The modifying fecal microbiome seemed to play a role in the facilitation and extent of these beneficial effects.
The improvement in glycemic control and other aspects of diabetes that were found in this present trial is in line with other studies. Supplementation with fermented milk with the L. acidophilus, L. casei and Bifidobacteria for 8 weeks reduced the level of FBG and HbA1c compared with the control group in T2DM patients in a randomized, double-blind, placebo-controlled clinical trial [21]. Sivieri K. and colleagues (2012) reported that daily consumption of 4 × 108 CFU/100 mL of L. acidophilus, 4 × 108 CFU/100 mL of B. bifidum and 1 g/100 mL of fructooligosaccharides for 30 days resulted in significantly decreased FBG in T2DM individuals [22]. Moreover, it has been reported that consumption of capsules containing 108 CFU of L. casei for 8 weeks significantly reduced FBG and insulin resistance in T2DM patients [23]. In contrast, Horvath A. et al. (2020) recently reported that there were no changes in glucose metabolism or mixed meal tolerance test responses in diabesity patients receiving a multispecies probiotic and a prebiotic for 6 months [24]. Possible explanations for the contradictory results from clinical studies are manifold and related to, among other things, individual participants, probiotic formulations, and the concentration and duration of the intervention. However, the precise mechanisms of the beneficial effects of probiotics on glucose metabolism remain unclear. Previous studies suggested that some kinds of probiotics could change, in particular, the composition of gut microbiota (i.e., increase the good bacteria and decrease the pathogenic bacteria), leading to reduced gut dysbiosis [25,26,27]. It has been shown that an increase in the level of the pathogenic bacteria Clostridium, together with a reduction in the level of beneficial bacteria, namely Bacteroides, Bifidobacterium and Actinobacteria, was experienced by T2DM patients [28,29]. Additionally, an association between the improvement of FBG and higher levels of Bacteroides, Bifidobacterium and Feacalibacterium has been reported [30,31]. In this study, the genus level changes of gut microbiota were explored. Even the abundance of those bacteria at baseline was not the same compared between the two groups but the resulted revealed that the percentage abundance of Bacteriodes, Lactobacillus, Faecalibacterium and Bifidobacterium increased after probiotic L. paracasei HII01 supplementation for 12 weeks in T2DM patients. Interestingly, the level of Clostridium evidently decreased in the probiotic group, while the opposite result was found in the placebo group. Ducarmon and coworkers (2019) reported the probiotic bacteria, such as Lactobacillus and Bifidobacterium had an effect against pathogenic bacteria via reducing the gut pH leading to inhibition of the growth of pathogenic bacteria or increased mucin secretion leading to increased mucus layer and prohibiting the colonization of pathogenic bacteria [32]. Although the probiotic L. paracasei HII01 was supplemented, the abundance OTUs of Lactobacillus was lower than the abundance of Bifidobacterium at week 12 in feces of T2DM patients. Lactobacillus strain was fed to human volunteers and then fecal microbiota were examined after 12 weeks of administration. While the administration continued, total Lactobacillus (flora and the administered strains) excretion slightly increased in feces but total Bifidobacterium was greatly increased. The results were that two probiotic bacterial genera can survive the passage through the gastrointestinal tract, but Lactobacillus do not colonize the gastrointestinal tract to a significant extent. Moreover, the fecal microbiome OTUs was determined at weeks 0 and 12 but the dynamic change of probiotic Lactobacillus colonization might increase in shorter term than 12 weeks. Further work on the kinetic changes of gut microbiota should be classified. However, the colonization of administered Lactobacillus may be unnecessary to achieve functional properties in probiotic therapy and promote symbiotic growth for good bacterial Bifidobacterium. Besides, the present study found that the participant’s microbiota had low diversity, was rich in members of Lactobacillus and Bifidobacterium, and had low numbers of Clostridium. There are many factors for reducing the abundance of Clostridium such as dietary intake, host genetics, age, residence area or medication [33]. Additionally, the evidence informed that some antidiabetic medications could be against the colonization of Clostridium. A previous study reported that treatment with metformin had a protective effect against the colonization of Clostridium difficile in diabetic patients [34]. On the other hand, the reduction of Gram-negative bacteria, for example Bacteroides, relating to their increased number of deaths, was noted in diabetic patients [4].
Gut microbiota may be involved in insulin resistance and type 2 diabetes mellitus through several probable mechanisms: for example, alteration of energy homeostasis or glucose metabolism and also low-grade inflammation [8]. One common theory is that bacterial LPS derives from the outer membranes of Gram-negative bacteria, which has been known to induce metabolic endotoxemia by promoting secretion of pro-inflammatory cytokines [35]. LPS can translocate through the damaged gut barrier. Then, LPS binds to TLR4 and activates the TLR4/CD14 complex, which activates pro-inflammatory pathways [36]. Chronic exposure to pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 activates the signaling proteins that block the activation of the insulin signaling cascade in the target organs of insulin, including skeletal muscle, adipose tissue and liver, leading to hyperglycemia [37]. To support this concept, our findings clearly showed that the systemic inflammation resulting from plasma endotoxemia or LPS levels was significantly reduced in the L. paracasei HII0-treated group, indicating decreased translocation of bacterial products. Additionally, supplementation with L. paracasei HII01 for 12 weeks decreased the plasma level of inflammatory cytokines, including TNF-α, IL-6 and hsCRP when compared to their baseline values. Another probable pathway mediating the connection between gut microbiota and metabolic endotoxemia is gut permeability [4]. Our previous study demonstrated that supplementation with the L. paracasei HII01 for 12 weeks in T2DM rat resulted in lessening plasma levels of DX-4000-FITC, suggesting an improvement of gut barrier integrity [38]. This effect could directly ameliorate systemic endotoxemia by reducing the leakage of LPS into systemic circulation.
Gut dysbiosis could induce abnormal immune responses such as the abnormal secretion of IgA [5]. Moreover, the presence of LPS in blood circulation not only acts as a potent inflammatory mediator and influences insulin sensitivity but also disturbs the functionality of the innate immune system [39]. In the present study, we found that the IgA levels in the L. paracasei HII01-treated group were increased when compared to baseline. Similarly, results from an in vivo study revealed that L. lactis increased the IgA secretion [40]. It has also been reported that the B. breve increased the level of IgA, leading to an increased humoral immune response [41].
Previous articles have reported that the antidiabetic effect of probiotics may be due to microbial metabolites such as SCFAs [42]. SCFAs are fermentation products of carbohydrates or proteins by probiotic bacteria. [43]. A reduction of SCFAs levels has been detected in T2DM [44]. Importantly, the results from this study demonstrated that the administration of L. paracasei HII01 for 12 weeks in T2DM patients effectively increased the number of those SCFAs in fecal content compared to baseline. Several mechanisms have been proposed to explain the effects of SCFAs in the improvement of diabetes. These SCFAs are not only of importance in gut health as signaling molecules but might also enter the systemic circulation and directly affect peripheral tissues via AMPK activation and subsequently GLUT4 translocation to membrane for uptake of glucose into cells [45]. In addition, our previous study found that supplementation of L. paracasei HII01 alleviated hyperglycemia in diabetic rats via increasing glucose uptake by the skeletal muscle which was mediated partly by PI3K/Akt and AMPK activation [38].
In this study, there were no significant differences in the plasma TG and cholesterol levels at the end of the study. These results were consistent with the previous study demonstrating that Lactobacillus supplementation significantly decreased the total cholesterol and LDL levels, while no significant effects were found on the TG and HDL levels [46]. A meta-analysis of 12 randomized controlled trials found that the effects of probiotics on the lipid profile were nonsignificant [47]. The authors suggested that the levels of lipid profiles were inconclusive due to various factors such as sample sizes, subject status, age and BMI. However, L. paracasei HII01 supplementation significantly reduced the LDL level together with enhanced HDL level after 12 weeks of the intervention. As mentioned earlier, the SCFAs can induce AMPK activation. Our data revealed that treatment of L. paracasei HII01 increased the level of SCFAs. AMPK activation in the liver leads to the stimulation of fatty acid oxidation and suppression of lipogenesis through the inhibition the enzymes 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase and acetyl-CoA carboxylase (ACC) [40]. Therefore, it may play a role in the regulation of lipid metabolism by probiotics.
This study has some limitations in the interpretation of our findings. Due to the methodology limitation, we did not characterize the gut microbiota at the level of species and thus, successful colonization of probiotic Lactobacillus paracasei HII01 in participants’ guts cannot be confirmed. Additionally, this study is relatively biased by the unequal sex distribution of the study participants. In total, 77.77% of the study participants were female, and this fact might be one of the confounding factors affecting the gut microbiome composition. In addition, dietary, physical activity assessment and other information including frequency and characteristic of feces in this study relied only on subjective reports which are not as accurate as objective methods for measuring their compliance.
In conclusion, the outcomes of this present trial firstly point to beneficial effects of L. paracasei HII01 supplementation in terms of glycemic improvement and other metabolic variables by favorably modifying the gut microbiota and subsequently ameliorating endotoxemia, which suggests a potential role of probiotic L. paracasei HII01 as a commercial probiotic product for an add-on treatment in subjects with T2DM.
Acknowledgments
We gratefully acknowledge the Research and Researchers for Industries (RRi) grant under the Thailand Science Research and Innovation (TSRI), the National Research Council of Thailand (NRCT) and Chiang Mai University for their financial support.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10071455/s1. Table S1: Baseline biochemical parameters at the beginning of the study; Table S2: Oral antidiabetic drugs used by participants.
Author Contributions
Conceptualization, C.C. and N.L.; funding acquisition, C.C. and N.L.; writing—original draft, P.T.; investigation, P.T., N.K. and S.S.; methodology, P.T., N.K. and S.S.; formal analysis, P.T., N.K. and N.L.; writing—review and editing, C.C. and N.L.; project administration, N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research and Researchers for Industries (RRi) grant to P.T. and N.L. (PHD60I0085) under the Thailand Science Research and Innovation (TSRI) and the National Research Council of Thailand (NRCT) for their financial support to N.L. (Grant No. 270390), and Chiang Mai University for partial support.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the Ethics Committee of Phrae Provincial Public Health Office (protocol code; PPH No.1/2562 and date of approval; 10 May 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Diabetes Federation . IDF Diabetes Atlas. 9th ed. IDF; Brussels, Belgium: 2019. [Google Scholar]
- 2.American Diabetes Association Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:13–27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 3.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pr. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 4.Gomes A.C., Bueno A.A., Machado de Souza R.G., Mota J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014;17:60–72. doi: 10.1186/1475-2891-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho B.M., Saad M.J.A. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediat. Inflamm. 2013;2013:986734. doi: 10.1155/2013/986734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 7.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 8.Plaza-Diaz J., Ruiz-Ojeda F.J., Gil-Campos M., Gil A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019;10:49–66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ejtahed H.S., Mohtadi-Nia J., Homayouni-Rad A., Niafar M., Asghari-Jafarabadi M., Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–543. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Karczewski J., Troost F.J., Konings I., Dekker J., Kleerebezem M., Brummer R.J., Wells J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:851–859. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 11.Tonucci L.B., Olbrich Dos Santos K.M., Licursi de Oliveira L., Machado Rocha Ribeiro S., Stampini Duarte Martino H. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017;36:85–92. doi: 10.1016/j.clnu.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Sabico S., Al-Mashharawi A., Al-Daghri N.M., Wani K., Amer O.E., Hussain D.S., Ansari M.G.A., Masoud M.S., Alokail M.S., McTernan P.G. Effects of a 6-month multistrain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019;38:1561–1569. doi: 10.1016/j.clnu.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Wanchai K., Yasom S., Tunapong W., Chunchai T., Eaimworawuthikul S., Thiennimitr P., Chaiyasut C., Pongchaidecha A., Chatsudthipong V., Chattipakorn S., et al. Probiotic Lactobacillus paracasei HII01 protects rats against obese-insulin resistance-induced kidney injury and impaired renal organic anion transporter 3 function. Clin. Sci. 2018;132:1545–1563. doi: 10.1042/CS20180148. [DOI] [PubMed] [Google Scholar]
- 14.Thiennimitr P., Yasom S., Tunapong W., Chunchai T., Wanchai K., Pongchaidecha A., Lungkaphin A., Sirilun S., Chaiyasut C., Chattipakorn N., et al. Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats. Nutrition. 2018;54:40–47. doi: 10.1016/j.nut.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Peña F., Mizgier M.L., Morales P., Rios I., Carrasco-Pozo C., Diaz E., Brunser O., Gotteland M. Effect of the Synbiotic (B. animalis spp. lactis Bb12 + Oligofructose) in Obese Subjects. A Randomized, Double-Blind, Controlled Clinical Trial. J. Food Nutr. Res. 2014;2:491–498. doi: 10.12691/jfnr-2-8-10. [DOI] [Google Scholar]
- 16.Classification of Diabetes Mellitus 2019. WHO; Geneva, Switzerland: 2019. pp. 1–36. [Google Scholar]
- 17.Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:36. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian J., Cai M., Gao J., Tang S., Xu L., Critchley J.A. Trends in smoking and quitting in China from 1993 to 2003: National Health Service Survey data. Bull. World Health Organ. 2010;88:769–776. doi: 10.2471/BLT.09.064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Substance Abuse and Mental Health Services Administration Alcohol Use Facts & Resources. [(accessed on 18 May 2021)]; Available online: https://www.samhsa.gov/sites/default/files/alcohol-use-facts-resources-fact-sheet.pdf.
- 20.Khat-udomkiri N., Toejing P., Sirilun S., Chaiyasut C., Lailerd N. Antihyperglycemic effect of rice husk derived xylooligosaccharides in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rat mod. Food Sci. Nutr. 2019;8:428–444. doi: 10.1002/fsn3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostadrahimi A., Taghizadeh A., Mobasseri M., Farrin N., Payahoo L., Gheshlaghi Z.B., Vahedjabbari M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health. 2015;44:228237. [PMC free article] [PubMed] [Google Scholar]
- 22.Moroti C., Souza Magri L.F., Costa M.R., Cavallini D.C., Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012;11:29–36. doi: 10.1186/1476-511X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalili L., Alipoor B., Jafarabadi M.A., Faraji I., Sani M.A. The effects of Lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with type 2 diabetes mellitus: A randomized controlled trial. Iran. Biomed. J. 2018;23:68–77. doi: 10.29252/ibj.23.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath A., Leber B., Feldbacher N., Tripolt N., Rainer F., Blesl A., Trieb M., Marsche G., Sourij H., Stadlbauer V. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: A randomized, double-blind, placebo-controlled pilot study. Eur. J. Nutr. 2020;59:2969–2983. doi: 10.1007/s00394-019-02135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuke N., Nagata N., Suganuma H., Ota T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients. 2019;11:2277. doi: 10.3390/nu11102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cândido T.L.N., Bressan J., Alfenas R.C.G. Dysbiosis and metabolic endotoxemia induced by high-fat diet. Nutr. Hosp. 2018;35:1432–1440. doi: 10.20960/nh.1792. [DOI] [PubMed] [Google Scholar]
- 27.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Shen D., Fang Z., Jie Z., Qiu X., Zhang C., Chen Y., Ji L. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PLoS ONE. 2013;8:e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 30.Ley R.E., Turnbaugh P.J.P., Klein S., Gordon J.I. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 31.Xu J., Liang R., Zhang W., Tian K., Li J., Chen X., Yu T., Chen Q. Faecalibacterium prausnitzii-derived microbial anti-inflammatory molecule regulates intestinal integrity in diabetes mellitus mice via modulating tight junction protein expression. J. Diabetes. 2020;12:224–236. doi: 10.1111/1753-0407.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ducarmon Q.R., Zwittink R.D., Hornung B.V.H., Schaik W., Young V.B., Kuijpera E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019;83:e00007-19. doi: 10.1128/MMBR.00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen L., Duffy A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017;147:1468–1475. doi: 10.3945/jn.116.240754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eliakim-Raz N., Fishman G., Yahav D., Goldberg E., Stein G.Y., Zvi H.B., Barsheshet A., Bishara J. Predicting Clostridium difficile infection in diabetic patients and the effect of metformin therapy: A retrospective, case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:1201–1205. doi: 10.1007/s10096-015-2348-3. [DOI] [PubMed] [Google Scholar]
- 35.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 36.Allin K.H., Nielsen T., Pedersen O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2015;172:167–177. doi: 10.1530/EJE-14-0874. [DOI] [PubMed] [Google Scholar]
- 37.Rehman K., Akash K.S.H. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2006;23:87–104. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toejing P., Khat-Udomkiri N., Intakhad J., Sirilun S., Chaiyasut C., Lailerd N. Putative Mechanisms Responsible for the Antihyperglycemic Action of Lactobacillus paracasei HII01 in Experimental Type 2 Diabetic Rats. Nutrients. 2020;10:3015. doi: 10.3390/nu12103015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Creely S.J., McTernan P.G., Kusminski C.M., Fisher F.M., Da Silva N.F., Khanolkar M., Evans M., Harte A.L., Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007;292:740–747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 40.Villena J., Medina M., Vintiñi E., Alvarez S. Stimulation of respiratory immunity by oral administration of Lactococcus lactis. Can. J. Microbiol. 2008;54:630–638. doi: 10.1139/W08-052. [DOI] [PubMed] [Google Scholar]
- 41.Bodera P., Chcialowski A. Immunomodulatory effect of probiotic bacteria. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3:58–64. doi: 10.2174/187221309787158461. [DOI] [PubMed] [Google Scholar]
- 42.Markowiak-Kopeć P., Śliżewska K. The Effect of Probiotics on the Production of Short Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12:1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puddu A., Sanguineti R., Montecucco F., Viviani G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat. Inflamm. 2014;2014:162021. doi: 10.1155/2014/162021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim C.H. Microbiota or short-chain fatty acids: Which regulates diabetes? Cell Mol. Immunol. 2018;15:88–91. doi: 10.1038/cmi.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu G., Chen G., Xu H., Ge R., Lin J. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med. Hypotheses. 2010;74:123–126. doi: 10.1016/j.mehy.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y., Zhang Q., Ren Y., Ruan Z. Effect of probiotic Lactobacillus on lipid profile: A systematic review and meta-analysis of randomized, controlled trials. PLoS ONE. 2017;12:e0178868. doi: 10.1371/journal.pone.0178868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasinska M.A., Drzewoski J. Effectiveness of probiotics in type 2 diabetes: A meta-analysis. Pol. Arch. Intern. Med. 2015;125:803–813. doi: 10.20452/pamw.3156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.