Abstract
Environmental pollutants, such as mycotoxins, pesticides, and pharmaceuticals, are a group of contaminates that occur naturally, while others are produced from anthropogenic sources. With increased research on the adverse ecological and human health effects of these pollutants, there is an increasing need to regularly monitor their levels in food and the environment in order to ensure food safety and public health. The application of magnetic nanomaterials in the analyses of these pollutants could be promising and offers numerous advantages relative to conventional techniques. Due to their ability for the selective adsorption, and ease of separation as a result of magnetic susceptibility, surface modification, stability, cost-effectiveness, availability, and biodegradability, these unique magnetic nanomaterials exhibit great achievement in the improvement of the extraction of different analytes in food. On the other hand, conventional methods involve longer extraction procedures and utilize large quantities of environmentally unfriendly organic solvents. This review centers its attention on current applications of magnetic nanomaterials and their modifications in the extraction of pollutants in food commodities.
Keywords: environment, food commodities, pollutants, magnetic nanomaterial, extraction
1. Introduction
Mycotoxins, pesticides, and pharmaceuticals are groups of pollutants that contaminate water, and food and feed samples, posing a health threat to consumers of the contaminated products. These pollutants or contaminants enter the environment through numerous pathways. Pharmaceutical compounds are often partially metabolized in humans and animals, and they are then excreted through urine or feces. Eventually, these compounds reach wastewater treatment plants where they are only partially removed, or transformed, and then released into receiving water bodies. Besides pharmaceuticals, wastewater treatment plants are typically unable to completely remove several classes of pollutants, such as personal care products, industrial run-offs or chemicals, micro/nano-plastics, and many others [1,2,3,4,5].
Other pathways for the movement of pollutants and contaminants into the environment include run-offs and wastewater from farms [6,7], storm water from urban and semi-urban areas [8], hospital discharges [2,5,9], and other non-point sources. Once these pollutants enter surface or ground water resources, they can eventually contaminate drinking and agricultural water systems, thus finding their way into various food commodities. The presence of these pollutants and contaminants in food and the environment has received considerable attention in terms of research in identifying, monitoring, technologies for their removal, and implementing legislation to address the issue [2,4,5,10,11,12,13,14].
The sheer volume in terms of the quantity and type of chemicals and materials that are currently used daily, as well as new chemicals and materials developed each year, further exacerbate the problems associated with pollutants and contaminants of emerging concern. For example, some reports estimate that the European Union has registered over 100,000 chemicals for industrial, commercial, and personnel use with ~400 million ton of them produced globally [15]. EUROSTAT publishes statistics on the amounts of chemicals produced in the EU that are hazardous to the environment and health, and over 70% of these are chemicals with important environmental concern [16].
Due to the prevalence and harmful effects of these pollutants to humans, animals, and the environment, it is critical to regularly monitor their levels in food and the environment in order to adopt appropriate mitigation interventions to limit their occurrence and associated deleterious effects. In this regard, analysis plays a vital role, thus the constant need for improved and efficient analytical methods. Conventional methods of extraction and analysis of these pollutants are often expensive, inefficient, tedious, and utilize large volumes of harmful organic solvents. Hence, there is need for greener, cheaper, quicker, and more efficient approaches. Magnetic nanomaterials (MNMs) seem very promising in this regard and are, thus, proposed for the extraction of these pollutants for analysis. This review describes the extraction of these pollutants using MNMs, with a focus on mycotoxins, pesticides, and pharmaceuticals. The design of these extraction materials and their recent applications in different scientific fields are also discussed.
2. Occurrence and Significance of Pollutants in Food Commodities
Access to safe and quality food with adequate nutritional value is a priority for every food consumer; however, along the food production chain, most foods often become contaminated by pollutants, such as pesticides, mycotoxins, and pharmaceuticals, before reaching the consumers. Contamination of these pollutants begins on the farm, and continues during pre- and postharvest, as well as during storage, making it a global challenge, particularly due to their various adverse health effects in humans and animals [17]. In this regard, various food safety authorities have introduced regulatory guidelines to limit their exposure in consumers [18]. The United States’ Food and Drug Administration (USFDA), and Environmental Protection Agency/United State Department of Agriculture (USEPA/USDA), together with the Joint FAO/WHO Expert Committee on Food Additives (JECFA), European Union (EU), and other regulatory agencies, have set up these guidelines and maximum limits [19,20], as shown in Table 1.
Table 1.
The regulatory limits of pollutants in food commodities.
| Food Commodities | Pollutants | Regulatory Limits | Reference |
|---|---|---|---|
| Mycotoxins (μg/kg) | |||
| Processed cereal | OTA | 3 (EU) | [34] |
| Unprocessed cereals (raw cereals and grains) | 5 (EU) | [34] | |
| Unprocessed cereals (wheat, barley and rye) | 5 (JECFA) | [35] | |
| Milk | AFM1 | 0.5 (JECFA) | [36] |
| Processed cereals (tree nuts, dried fruits, rice, peanuts, maize) |
AFB1 | 2 (EU) | [37] |
| Cereals and processed cereal products, except corn and rice |
2 (EU) | [34] | |
| Corn and rice | 5 (EU) | [34] | |
| All foodstuffs | 5 (SA) | [38] | |
| All foodstuffs | Total AFs | 10 (SA) | [38] |
| Ground nuts and processed cereals | 4 (EU) | [37] | |
| Cereals and processed cereal products, except corn and rice |
4 (EU) | [34] | |
| Food | 20 (USFDA) | [34] | |
| Corn and rice | 10 (EU) | [34] | |
| All foods except milk | 20 (USA) | [38] | |
| All foodstuffs | PAT | 50 (SA) | [38] |
| Fruit nectar and fruit juices specifically fruit juice ingredi- ents in other beverages and apple juice |
50 (EU) | [38] | |
| Apple juice component of a food that contains apple juice as ingredient, apple juice, and apple juice concentrate |
50 (USA) | [38] | |
| Processed cereals/grains(flour, semolina, meals, flakes de- rived from barley, maize, and wheat) |
DON | 1000 (JECFA) | [35] |
| Processed wheat-based products | 1000 (USFDA) | [34] | |
| Processed grain (wheat, maize, and barley) | 2000 (JECFA) | [35] | |
| Unprocessed oat, durum wheat, and maize | 1750 (EU) | [34,37] | |
| Cereals ready for direct human consumption and other unprocessed cereals |
1250 (EU) | [34,37] | |
| Cereal flour (raw materials in food products) | 750 (EU) | [34] | |
| Processed grains (maize meal and flour) | FB1 and FB2 | 2000 (JECFA) | [35] |
| Unprocessed maize grain | 4000 (JECFA) | [35] | |
| Processed corn (corn meal, flour, and grits) | 1000 (EU) | [34] | |
| Unprocessed corn | 4000 (EU) | [34] | |
| Corn-based breakfast cereals and snacks | 800 (EU) | [34] | |
| Clean (processed) corn ready for mass production | Total FUMs | 4000 (USFDA) | [34] |
| Clean corn for popcorn | 3000 (USFDA) | [34] | |
| Dry milled corn bran | 4000 (USFDA) | [34] | |
| Degermed dry milled corn products | 2000–4000 (USFDA) | [34] | |
| Unprocessed maize intended for wet milling | 4000 (EU) | [37] | |
| Processed maize (flour, grit, meal, and semolina) | 1000 (EU) | [37] | |
| Corn flour | ZEN | 200 (EU) | [34] |
| Corn-based snacks and breakfast cereals | 100 (EU) | [34] | |
| Unprocessed cereals other than corn | 100 (EU) | [34] | |
| Unprocessed maize | 350 (EU) | [34] | |
| Cereal flour other than corn flour | 75 (EU) | [34,37] | |
| All product derived from unprocessed cereals intended for direct consumption (excluding processed corn-based foods) |
50 (EU) | [34] | |
| Pesticides (mg/kg) | |||
| Sweet potatoes | Oxamyl | 2.00 (USDA) | [39] |
| Dichloran | 10.00 (USDA) | [39] | |
| Parathion | 0.05 (SA, EU) | [40] | |
| Deltamethrin | 0.05 (SA), 0.01 (EU) | [40] | |
| Fludioxonil | 10.00 (SA, EU, CODEX) | [40] | |
| Triazophos | 0.05 (SA), 0.01 (EU) | [40] | |
| Azoxystrobin | 0.03 (SA), 1.00 (EU) | [40] | |
| Pineapples | Malathion | 2.00 (SA), 0.02 (EU),1.00 (CODEX), 8.00 (USA) | [40] |
| Oxamyl | 0.05 (SA), 0.01 (EU), 1.00 (USA) | [40] | |
| Isozofos | 0.05 (SA), 0.01 (EU) | [40] | |
| Fosety-al | 20.00 (SA), 50.0 (EU) | [40] | |
| Thia-bendazole | 10.00 (SA, USA), 0.01 (EU) | [40] | |
| Tomatoes | Malathion | 0.05 (SA), 0.02 (EU), 0.50 (CODEX) | [40] |
| Oxamyl | 0.02 (SA), 0.01 (EU, CODEX) | [40] | |
| Parathion | 0.10 (SA), 0.05(EU), 1.00 (CODEX) | [40] | |
| Chlorpyrifos | 0.50 (SA, CODEX), 0.30 (EU) | [40] | |
| Mangoes | Chlorpyrifos | 0.01 (SA, EU) | [40] |
| Fenvalerate | 0.05 (SA), 1.50 (EU, CODEX) | [40] | |
| Malathion | 2.00 (SA), 0.02 (EU), 0.10 (USA) | [40] | |
| Deltamethrin | 0.05 (SA), 0.01 (EU) | [40] | |
| Parathion | 0.10 (SA), 0.05 (EU) | [40] | |
| Azoxystrobin | 0.10 (SA), 0.70 (EU, CODEX), 2.00 (USA) | [40] | |
| Strawberries | Azoxystrobin | 5.00 (SA), 10.00 (EU, CODEX, USA) 0.50 (SA) |
[40] |
| Dimethoate | 0.10 (SA), 0.50 (EU), 2.00 (CODEX) | [40] | |
| Difenoconazole | 2.50 (USA) | [40] | |
| Captan | 15.00 (SA, CODEX), 6.00 (EU), 20.00 (USA) | [40] | |
| Emamectin benzoate | 0.04 (SA), 0.05 (EU) | [40] | |
| Banana | Thiabendazole | 3.00 (SA), 5.00 (CODEX) | [40] |
| Triazophos | 2.00 (SA) | [40] | |
| Chlorpyrifos | 1.00 (SA), 2.00 (CODEX) | [40] | |
| Dichlorvos | 0.10 (SA) | [40] | |
| Fenamiphos | 0.05 (SA, CODEX) | [40] | |
| Citrus | Azoxytrobin | 0.50 (SA), 15.00 (EU, CODEX) | [40] |
| Buprofezin | 0.05 (SA), 1.00 (EU, CODEX) | [40] | |
| Azinphos-methyl | 2.00 (SA), 0.05 (EU), 1.00 (CODEX) | [40] | |
| Chlorpyrifos | 0.30 (SA), 1.50 (EU) 1.00 (CODEX) | [40] | |
| Dimethoate | 2.00 (SA), 0.01 (EU), 5.00 (CODEX) | [40] | |
| Emamectin benzoate | 0.01 (SA, EU) | [40] | |
| Table grapes | Fenvalerate | 0.05 (SA), 0.30 (EU) | [40] |
| Fenthion | 0.50 (SA), 0.01 (EU) | [40] | |
| Dimethoate | 2.00 (SA), 0.02 (EU) | [40] | |
| Chlorpyrifos | 0.01 (EU, USA), 0.50 (CODEX) | [40] | |
| Azoxystrobin | 1.00 (SA), 3.00 (EU), 2.00 (CODEX, USA) | [40] | |
| Acephate | 1.50 (SA, USA), 0.01 (EU) | [40] | |
| Deltamethrin | 0.10 (SA), 0.20 (EU, CODEX) | [40] | |
Note: CODEX: FOA/WHO; OTA: Ochratoxin A; DON: deoxynivalenol; ZEN: Zearalenone; PAT: Patulin; Total AFs: sum of AFB1, AFB2, AFG1, and AFG2; Total FUMs: sum of Fumonisin B1, Fumonisin B2, and Fumonisin B3.
Mycotoxins are among the naturally occurring environmental pollutants of concern in food and feed. These are toxic biochemical compounds synthesized during the metabolic activities of mycotoxigenic fungal species. Exposure to them among humans occurs mainly via ingestion of mycotoxin contaminated food [21] with dermal, aerosol, and parental routes as other avenues. The significant mycotoxins, i.e., aflatoxins (AFs), ochratoxins (OTs), fumonisins (FUMs), trichothecenes (THs), and zearalenone (ZEN), either cause cancer, liver, and kidney damage and or compromise immune and nervous system function, which could be accompanied by vomiting and death in various animal species [22]. In Malawi, high occurrence of esophageal cancer has been reported to correlate with high consumption of maize contaminated with FUMs [23]. The occurrence of mycotoxins in grains (sorghum, maize, wheat, and their products) at concentrations exceeding the maximum limits recommended by the European Commission and the South African Government has been reported [24]. Elsewhere, Onyedum et al. [25] observed the occurrence of economically significant mycotoxins in maize, cassava flake (garri), millet, yam flour, sorghum, and rice from North-Central Nigeria. In that study, levels of AFs, the most potent group of naturally occurring carcinogens exceeded the maximum recommended limits established by the European Commission in some of the foodstuff. Despite several efforts to manage mycotoxin contamination in food, their prevalence in staple food products still persist indicating a critical food safety and public health concern, which must not be ignored.
Pesticides and pharmaceuticals on their part are other groups of environmental pollutants that, heretofore, remain elusive to most food and environmental safety interventions. Pesticides, in particular, are of much concern today as the world shifts towards mechanized farming—increasingly adopting inorganic agricultural practices with little regard to environmental safety and sustainability. Pesticides potentiate carcinogenic, gastrointestinal, reproductive, neurological, respiratory, and dermatological properties that manifest in humans, particularly in pregnant women, children, and older people [26]. Recent research data show high prevalence of pesticides in many food commodities intended for direct human and animal consumption, despite the various regulatory frameworks to control their applications in agriculture. For example, Galani et al. [27] investigated the occurrence of 99 pesticides in 72 samples of 12 agricultural products from Cameroon. The authors reported that at least 21 of these pesticides occurred at levels exceeding the EU maximum regulatory limits. The occurrence of pesticides in wheat samples was also reported in Algeria, and about 5% of samples contained levels above maximum residue limits [28]. The accumulation of organochlorine pesticides in fish species in South Africa was reported, and levels recovered in most samples were above those regulated by EU [29].
More recently, pharmaceuticals have gained increasing attention as environmental pollutants of concern due to their unintended effects on the environments, such as the conference of antimicrobial resistance in some pathogenic microorganisms by antibiotics. Indeed, many of these active ingredients from pharmaceutical products enter the environment as trace pollutants largely from their intended use in veterinary and human medical practices, personal care, and agriculture; nonetheless, their prevalence in recent times is concerning to public health, especially as more research reveals their potential hazards. According to Küster and Adler [30], approximately 10% of pharmaceutical products are of note regarding their potential environmental risks. Bommuraj et al. [31] reported the occurrence of three pharmaceuticals (ibuprofen, bezafibrate, and caffeine) in diary milk from Israel, while Goldstein et al. [32] reported the uptake of pharmaceutical by vegetables, such as tomatoes and cucumbers, irrigated with wastewater contaminated with pharmaceuticals. These pharmaceuticals causes tooth discoloration, vision problems, and allergic reactions in humans [33].
Indeed, the persistent occurrence of pollutants, such as mycotoxins, pesticides, and pharmaceuticals, in food, feed, water, and the environment is now of a global public health significance, as such requiring intensified efforts in order to control their prevalence. One of the ways to adequately manage and control these pollutants is by routine and efficient analysis to determine their incidence and levels to facilitate adoption of necessary combative interventions. In the analyses of contaminants, efficient extraction is a critical and often unavoidable step. Many of the conventional extraction methods for these pollutants are limited in one way or another, and there is a continuous quest for more effective analytical procedures to detect and quantify these pollutants. The application of nanomaterials as adsorbents for the extraction/removal of mycotoxins, pesticides, pharmaceutical, and other pollutants of concern in the environment has been identified as promising, effective, fast, and environmentally friendly. In the subsequent sections of this paper, we explored in detail the prospects and applications of MNMs for the extraction of various environmental contaminants, with focus on mycotoxins, pesticides, and pharmaceuticals in food commodities.
3. Nanomaterials
Nanomaterials have attracted tremendous attention in research and their industrial applications recently. They allow engineers, chemists, scientists, and physicians to work at cellular and molecular levels to produce efficient developments in the healthcare and life sciences, as well as other technological applications. The design and synthesis of nanostructure materials and nanoparticles for the removal of environmental contaminants ensure public health, environmental safety, and sustainability. They are significantly considered as great adsorbents because of their unique structure, extremely small size, functional features, and high surface area, which allow for their pre-concentration and efficient extraction of pollutants in food [41]. There are various types of nanomaterials, which include quantum dots, metal nanomaterials, carbon nanomaterial, and magnetic nanomaterials; in this review, emphasis is placed on MNMs.
3.1. Magnetic Nanomaterials (MNMs)
Magnetic nanomaterials (MNMs) are a category of nanoparticles with magnetic properties manipulated using magnetic fields. The utilization of their electrical, magnetic, chemical, and thermal properties in various analytical processes, such as extraction, pre-concentration, and clean-up (sample treatment), detection, and chromatographic techniques, helps in the development of new analytical strategies or the enhancement of traditional ones, with particular benefits of cost-effectiveness, improved extraction recoveries, selectivity, precision, and overall speed of extraction. The synthesis of these MNMs involves the use of various magnetic materials, which include iron (magnetite and maghemite), cobalt (Co), and nickel (Ni), with their various derivative compounds [42,43]. Among these materials, iron (Fe) oxides, such as Fe2O3 and Fe3O4, and their associated ferrite derivatives, such as CoFe2O4 and MnFe2O4, are the most widely used in the production of MNMs. This is due to the relative simplicity of their preparation, chemical stability, high magnetic moments, and compatibility with the various biological systems when compared with other metals/metallic alloys, such as FePt, Mn3O4, Ni, and Co [44].
Indeed, it is worth noting that, despite the reported efficiency and efficacy, applications of MNMs are usually targeted at compounds of interest. MNMs can be functionalized/modified with different chemical groups to achieve selective interaction or extraction of analytes of interest. This represents a major advantage of MNMs because their surface chemistry is usually tailored toward or favourable in the extraction of specific groups of compounds. Iron oxides are known to degrade organic compounds, decompose under acidic conditions, and easily react with O2 in air, and, as such, it is necessary to coat them with different protective layers of material, such as carbon nanomaterials, polymers, noble metals, or silica, that help improve their stability and also to introduce new functionalities and surface features [45]. The functionalization of the produced MNMs surface with different functional groups is simple and institutes various physicochemical properties on the materials in order to enhance their analytical applicabilities [46]. In the proceeding section of this review, we discuss different types of MNMs used for extraction.
3.1.1. Maghemite (γ-Fe2O3) and Magnetite (Fe3O4)
Maghemite and magnetite are the two major groups of iron oxide, which occur naturally with attractive magnetic features that are promising for different applications. They are both soft ferrimagnetic materials with similar structures, except that, in maghemite, the Fe cation is in trivalent states, while magnetite has cations of Fe2+ and Fe3+ [47]. Magnetite and maghemite are common components of MNMs used in magnetic solid phase extraction (MSPE). Different methods for synthesis of these Fe oxides have been described in the literature, such as hydrothermal method, solvothermal method, flow injection synthesis, oxidation of magnetic nanomaterials, co-precipitation, flame spray pyrolysis, sol-gel synthesis, and thermal decomposition of organic precursors at high temperatures and microemulsion [47,48]. Maghemite is a good absorbent for the removal of heavy metals due to its low cost, efficiency, safety, ease of separation and recovery, ability to adsorb, large surface site, simple synthesis availability, and superparamagnetic features [49,50,51]. Tuutijärvi et al. [52] successfully used maghemite to extract arsenic (V) in water. The removal of chromium (VII) by maghemite from contaminated water was also reported [53]. Narimani-Sabegh and Noroozian [51] synthesized a maghemite-based nanoparticle from lepidocrocite through calcination and extracted antimony (Sb) from aqueous media (soft drinks, bottle alcohol, water, non-alcoholic beers, and orange drinks). Research conducted by Devatha and Shivani [54] reported a novel application of maghemite nanomaterial coupled with bacteria (Bacillus substilis) for the extraction of cadmium (II) ion in aqueous media with a recovery of 76.4%. Rajput et al. [55] used maghemite nanomaterial synthesized by flame spray pyrolysis for the extraction of copper (II) ion and lead (II) ion in water.
Magnetite is also a promising absorbent applied in various scientific fields, most especially in the extraction of environmental pollutants. For instance, Piovesan et al. [56] synthesized magnetite nanomaterial coated with chitosan (Fe3O4@CS) to extract parathion in food commodities (tomato, carrot, rice, orange, and lettuce). Similarly, González-Jartín et al. [57] also synthesized this nanomaterial for the removal of mycotoxins in liquid food products (beverages). In addition, the application of molecularly imprinted polymer magnetic nanomaterial (Fe3O4@EGDMA) for OTA removal in grape juice was reported by Turan and Şahin [58].
3.1.2. Neodymium MNMs
Neodymium, a member of the rare-earth metal (Lanthanide group) has drawn attention in different studies due to its strong magnetic properties (perhaps one of the strongest known to man). It is presently utilized in the fabrication of permanent magnets that are applied in wind turbine, spindles for computer hard drives and electric motors [59]. Neodymium-based MNMs are synthesized using various methods, including gel combustion, hydrothermal method, solution co-precipitation, hydrogen plasma-metal reaction, and thermal decomposition and microemulsion [60]. Ahmad et al. [61] synthesized NdCl3 (neodymium (iii) chloride) embedded with OMC (ordered mesoporous carbon) for the removal of sunset yellow from aqueous solution. Similarly, the synthesis of Nd2O3 nanomaterial for extracting acid dye from aqueous media was reported [62]. In 2020, Chen and coworkers [63] prepared neodymium sesquioxide coated with graphene oxide nanocomposite and modified with glassy carbon electrode (GCE) for the determination of anti-cancer drug (raloxifene) in biological samples.
3.2. Magnetic Alloy Nanomaterials
A combination of different metallic compounds (i.e., alloys) have also been utilized to synthesize nanomaterials with unique physicochemical properties. Some of these magnetic nanoalloys are discussed.
3.2.1. Iron-Nickel (FeNi) Alloy MNMs
Iron-nickel (FeNi) alloy MNMs exhibit attractive magnetic features and are studied widely for various applications. FeNi3 has large saturation magnetization, high thermal stability, and permeability [64]. These alloy MNMs are synthesized using various methods, such as hydrothermal reduction technique, sol-gel method, spray pyrolysis, coordinated coprecipitation, and chemical reduction method [65,66]. It has become popular among analytical scientists due to ease of synthesis and application, separation of magnetic nanomaterial easily by an external magnet, and efficiency in extracting a wide range of organic compounds under different extraction conditions [67]. Khodadadi et al. [68] successfully synthesized FeNi3@SiO2 magnetic nanomaterial catalyst for tetracycline degradation in a neutral environment. Farooghi and coworkers [69] used FeNi3@SiO2 magnetic nanomaterial for the removal of lead in aqueous solution. Research by Nasseh et al. [70] reported the extraction of metronidazole in neutral environment using FeNi3@SiO2 magnetic nanocomposite.
3.2.2. Iron-Cobalt (FeCo) Alloy MNMs
Iron-cobalt (FeCo) alloy MNMs are soft ferromagnetic nanomaterials with special features, such as low coercivity, large saturation magnetization, high Curie temperature, high permeability, high anisotropy constant, and high anisotropy energy [71,72,73,74,75]. These magnetic alloy nanomaterials are utilized in different technological applications, including microwave devices, magnetic recording media and exchange-coupled nanocomposite magnets, hyperthermia, and drug delivery [76,77]. FeCo magnetic alloy nanomaterials are synthesized using techniques, such as mechanical ball milling, interfacial diffusion, chemical vapor deposition, chemical co-precipitation, pulse laser ablation deposition (PLAD), pyrolysis, and reductive decomposition of iron (iii) acetylacetonate and cobalt (ii) acetylacetonate [78,79,80].
3.2.3. Iron-Platinum (FePt) Alloy MNMs
Iron-platinum (FePt) are hard MNMs that have recently gained popularity amongst researchers due to the outstanding magnetic features they exhibit, such as great magnetocaloric effects, strong chemical stability, large saturation magnetization, magnetic imaging, and high magneto-crystalline anisotropy [81,82,83]. FePt magnetic materials are broadly applied in the biomedical field, magnetic data storage, large permanent magnet performance, electrocatalysis, nanobiotechnology, magnetic recording media, and biological research [84,85,86,87]. These alloys are synthesized through heat treatment (thermal decomposition) of Fe(Co)5 (iron pentacarbonyl), polyol process (reduction of platinum acetylacetonate in a mixed surfactants and polyol), and reduction of Fe salts and Pt (acac)2 [81].
3.2.4. Iron-Palladium (FePd) Alloy MNMs
Iron-palladium (FePd) alloy MNMs are hard MNMs because of their large magneto-crystalline anisotropy energy [88,89] synthesized using several methods, such as microwave irradiation, modification of chemical synthesis from iron-platinum synthesis procedure, modification of polyol procedure, and epitaxial growth electron beam deposition [90]. A Pd-rich iron-palladium (FePd) alloy material functions as an excellent hydrogen absorption kinetic and as a catalyst. These alloy MNMs are applied in ultrahigh magnetic recording media [91] and biomedicals [89]. Fe70Pd30 material being amongst the different systems has wide popularity among researchers due to its magnetic shape memory (MSM) and martensitic conversion effect. Different shapes of these nano-sized materials, such as nanorods, nanohelices, nanospheres, and nanotubes, have recently been reported [89].
3.3. Advantages and Limitations of MNMs
Magnetic nanomaterials exhibit advantageous features when compared to other non-MNMs. Such features include good dispersibility in solvents (facilitated by their size), high surface area, and possibility of functionalization/modification of their surface for improved specificity, range of sorbents, and/or adsorption efficiency, and ease of separation using an external magnet from complex matrices without the need for centrifugation or filtration steps [92,93]. The materials are also highly recyclable/reusable usually after appropriate rinsing, with analytes adsorbed by processes, such as sonication [92,94] use less amount of organic solvents and are often cost-effective [93,94,95].
Despite their many merits, MNMs have some limitations and one of which is their agglomeration and aggregation in media causing reduction in their intrinsic magnetic (superparamagnetic or ferromagnetic) features. This problem is solved by modification/functionalization of the MNM with different materials, such as silica oxide, graphene oxide, carbon nanotube, metal organic frameworks, molecularly imprinted polymers, covalent organic frameworks, aptamers, and immunoassay, amongst others [92,96,97]. Such action can increase the rate of transfer of electron due to their conductivity compared to unmodified MNMs [95]. For example, Xu et al. [19] modified MNM with carbon nanotubes for extracting pollutants in egg. The modification of MNM with molecularly imprinted polymer for the analysis of thiamethoxam and thiacloprid in honey was also reported [98]. Other limitations of MNMs are that bare MNM can easily be oxidized and form hydrated oxides in acidic atmospheres or, when exposed to air [92], the thermally unstable and stationary phase components of the MNM can completely or partly degrade during the desorption process at high temperature, which leads to reduction in precision and accuracy of the analysis. In fact, at pH < 4, the degradation of any magnetite present can take place allowing the formation of chelates between the free ions and the target analytes. Alternatively, magnetite particles acquire a negative charge at pH > 9 due to the binding of OH groups causing electrostatic repulsion between the adsorbent and anionic forms of target analytes [94].
In addition, due to the size of the nano adsorbents, they can be difficult to separate from complex matrices when compared to larger particles, particularly when the sorbent is non-magnetic. Larger particles can easily be filtered and, if denser than the samples, can be centrifuged to separate the analyte from the sample. MNMs are advantageous in this regard, and, despite their nanosizes, they can easily be extracted from the sample using an external magnet.
4. Extraction of Mycotoxins, Pesticides, and Pharmaceuticals
Extraction is the major step involved in analyzing these pollutants. Different conventional methods have been used to pre-concentrate and extract these pollutants from environmental samples and food commodities before quantification using GC-MS and LC-MS. The various steps used for the extraction processes are subsequently discussed.
4.1. Steps for Regular Sample Extraction
4.1.1. Liquid-Liquid Extraction
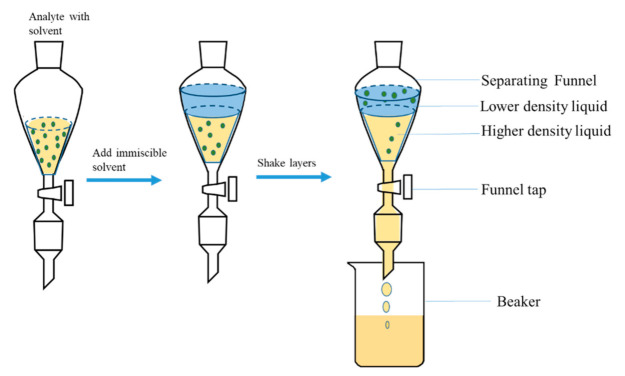
Liquid-Liquid extraction (LLE), also called solvent extraction, is a common technique used in extracting and purifying analytes for further analysis. It is one of the oldest extraction methods but still the most frequently used. This extraction approach is based on two immiscible solvents, the aqueous solvent and organic solvent. The solvent containing the analyte is placed in a funnel, and an immiscible solvent is added, forming two layers which are shaken together [99,100]. The analyte then migrates from the initial solvent to the second solvent based on their relative solubility in the solvent [100] (Figure 1). However, this method uses large amounts of organic solvents (such as dichloromethane, sulfuric acid, potassium chloride, and acetonitrile), a laborious process, and it involves longer extraction time.
Figure 1.
Diagrammatic illustration of liquid-liquid extraction (adapted from Nichols [100]).
4.1.2. Solid Phase Extraction
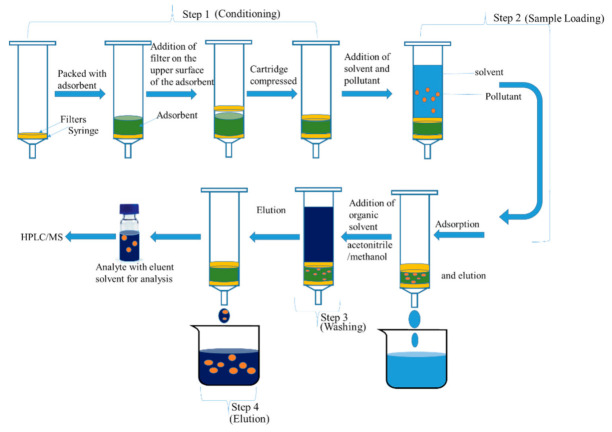
Solid phase extraction (SPE) is a frequently used method for extracting pollutants in aqueous samples. It basically involves four steps. The first step involves the use of cartridge filled with various types of particles with adsorption and adsorptive characteristics. It is loaded with sample in the second step, where the analytes of interest are retained. It is further washed with solvent to remove impurities (third step), and the analytes are eluted with a suitable solvent (fourth step) and kept for analysis using HPLC/MS [101,102] (Figure 2). SPE is mostly used for pre-concentration and clean-up of extracts in emerging pollutant analysis [103]. This method usually eliminates the need for expensive and environmentally sensitive solvents. However, there are limitations with this technique, such as loss of a compound of interest when loading the sample onto the sorbent, which causes clogging of cartridges by the sample’s suspended matter with the possibility of obtaining low recoveries by sorbent interaction towards the analytes [104,105].
Figure 2.
Diagrammatic steps of solid phase extraction (adapted from Badawy et al. [101]).
4.1.3. Quick, Easy, Cheap, Effective, Rugged, and Safe Extraction
This extraction method is an inexpensive and fast method for extracting pollutants in food using acetonitrile followed by dispersive solid phase extraction (DSPE). It is an effective sample preparation technique involving two steps. The first step is salting out to facilitate the equilibrium between the organic phase and aqueous phase, and the second step is clean-up employed by DSPE [106,107,108,109]. This technique is included as part of green analytical procedure due to it being an environmentally and user-friendly method. It uses little solvent, generates less waste [107], and can easily be modified using solvents, such as ethyl acetate and methanol [105], and clean up followed by filtering, SPE, freezing out, performing extra dilution, or LLE [106]. In 2018, Fernande et al. [110] used modified QuEChERS to analyze organophosphorus pesticides in strawberries with recovery values of 72–115%, limit of detection (LOD) and limit of quantification (LOQ) values ranging 3.64–10.38 µg/kg recorded. Similarly, the modified QuEChERS method for analyzing organophosphate pesticides in fruits and vegetables and recovery values in the range of 76.89–110.3 µg/kg, together with LOD and LOQ values from 0.1–1.0 µg/kg and 0.5–5 µg/kg, respectively, were reported [111]. More detailed reviews on QuEChERS, and its use on the analysis of various pollutants in various food commodities, have been reported in the literature [107,108,109].
4.1.4. Immunoaffinity Column Extraction
Immunoaffinity column extraction is an antibody-based separation technique that involves the use of a stationary phase that is made-up of an antibody or antibody related reagent linked to a chromatographic matrix or magnetic beads and exploit the selectivity and strong binding ability of the antibodies to their target [112]. For instance, the extraction of AFB1 requires loading the sample extract in the column. As the extract passes through the column, the target analyte (AFB1) is retained by the antibody in the column. The step is followed by washing to remove impurities, and the analyte is eluted using an elution solvent that disrupts the binding between the AFB1 and the antibody [113]. This method attains selective and efficient enrichment in only one step as compared with other extraction techniques, but the production of the antibodies is time consuming, expensive, and difficult. In addition, it causes long waiting times for sample analysis as there is possible congestion and limited flow rate in the column [106,114,115]. Ye et al. [116] used immunoaffinity magnetic beads for analysis of OTA in oil and cereals. The results reported recovery values of 86.3–95.4% and LOD and LOQ values of 0.24 µg/kg and 0.80 µg/kg, respectively. A magnetic-separation-based homogeneous immunosensor for DON from wheat and sauce samples was reported alongside the LOD and recovery values (0.5 and 3.0 ng/mL) and (78.7–88.5%), respectively, recorded [117].
4.1.5. Magnetic Solid Phase Extraction—Dispersive Liquid-Liquid Microextraction (MSPE-DLLME)
DLLME is the latest development in liquid phase micro-extraction. It involves the rapid injection of a dispersive and an extraction solvent into an aqueous solvent forming a cloudy solution, which produce microdroplets of the extraction solvent dispersed in the sample solution. The role of the dispersive solvent is to ensure that there is miscibility between the extraction solvent and the sample solution. The formation of a cloudy solution is to allow the instant separation of the analyte from the sample solution into the extraction phase. The cloudy solution is then centrifuged, and the extraction solvent containing the analyte of interest is collected by a microsyringe for analysis [118,119,120]. This extraction method has its advantages, such as minimal volume of solvent use, low-cost, simplicity, and high-speed extraction, but it also has such a drawback as low extraction efficiency [121]. This method is recently combined with other analytical techniques, such as MSPE-DLLME, SPE-DLLME, vortex-assisted-DLLME, air-assisted-DLLME, and ultrasound-assisted-DLLME to obtain extracts that are cleaner, have higher pre-concentration factor, and have better LOD values [119]. Yuan et al. [122] used MSPE-DLLME for extraction of herbicide in food (millet, oatmeal, barley rice, and soy), with good recoveries with LOD and LOQ values being 0.19–0.80 ng/g and 0.61–2.66 ng/g, respectively. Similarly, the use of MSPE-DLLME for extraction of three tetracyclines in milk was also reported with good recovery values of 70.6–121.5% recorded, together with LOD and LOQ values, respectively, of 1.8–2.9 µg/L and 6.1–9.7 µg/L [123].
4.1.6. Magnetic Nanomaterials for Analytical Extraction
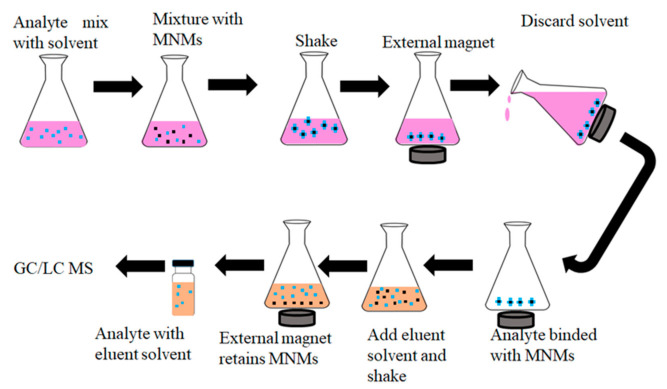
The practical aspects of MSPE are made up of five steps, i.e., sample preparation, adsorption, extraction, desorption, and detection. MNMs have been used for the removal of various chemical compounds in different matrices. Magnetic solid phase extraction has advantages over conventional methods in many ways, such as high enrichment factor, shorter extraction time, fast separation using an external magnet, and easy operation [124,125]. For example, using an adsorption method on MNMs, major and minor pollutants are removed, where less than 1 g of the MNM is introduced into aqueous samples containing the dissolved pollutants [126]. This process of adsorption promises to show faster extraction than most of the other methods. The MNM is analytically maintained as a limiting reactant, so that the rate of extraction of pollutants depends on the amount of MNM used. The sorbent and the analyte are made to interact effectively for a certain period of time, and then the sorbent material adsorbs the analyte on its surface. After this, it is separated from the solvent by means of an external magnet. This shows that both the adsorption and desorption processes employed are easier to achieve as compared to other methods. Other methods require filtration and centrifugation steps, but MNMs avoid such steps and use an external magnet. Subsequently, a suitable eluent is used to wash the analyte from the magnetic nanomaterial before analysis. This helps eliminate the use of a solid phase extraction column [124,127,128,129]. Below is an illustration in Figure 3. Dispersive solid phase extraction is one of the commonly used extraction processes for the application of magnetic nanomaterials. This technique has been applied in determining pesticides and other pollutants, including mycotoxins in food and environmental samples [110,130,131].
Figure 3.
Diagrammatic illustration of magnetic solid phase extraction process for extraction of emerging pollutants.
4.2. Analytical Characteristics and Efficiency Parameters of Magnetic Nanomaterials
4.2.1. Recovery
Recovery is one of the most important analytical parameters for studies involving extraction. It evaluates the closeness of agreement between acceptable values, and the experimentally observed values. Accurate quantification of pollutants in food commodities is essential in order to assess the compliance of the contamination levels of the pollutants in the sample with respect to the legal limits [132]. The EU recommended recovery values for mycotoxin is between 60–130% [132,133], pesticides from 70–120% [134,135,136], and pharmaceuticals between 80–120% [137]. The CODEX recommends 80–110% recovery values for mycotoxin [35] and 70–120% for pesticides [138]. The AOAC recommends recovery values between 70–125% for mycotoxins [132,139], while USFDA recommends 80–110% for mycotoxins [140] and 70–120% for pharmaceuticals residues at 10 ppb level [141]. USDA recommends recovery value for pesticide between 50–150% [141].
In the analysis of these pollutants, analytical approaches that yield recovery values which fall outside the recommended ranges as stated above are considered inaccurate and inadequate, which has been a recurring challenge of most conventional methods. Studies have reported low recovery values of 23.25–48.11% for some of the pesticides analyzed using the SPE method by Badawy et al. [101]. Likewise, low recovery values of 40–80% were reported for extracting pharmaceuticals using SPE approach [142]. In literature, effective recovery of emerging pollutants using MSPE method has been reported. For example, Ma et al. [143] reported recovery values of 80.2–108.3% for a simple magnetic solid phase extraction coupled to high performance liquid chromatography for the analysis of four heterocyclic pesticides from water. Similarly, the use of magnetite coupled to reduced graphene oxide nanocomposite for the extraction of isocarbophos in various sample matrices were also reported, with recovery values ranging from 81.0–108.5% [144]. The low recovery values reported by the conventional method is one of the SPE limitations, which is sometimes due to the blockage of the SPE column [101].
4.2.2. Matrix Effect
Often, during extraction experiments, components of a sample other than the analyte(s) are co-extracted alongside the analytes, and they frequently interfere with analytical signals, thus compromising result quality. Matrix effect can compromise the precision, selectivity, reproducibility, sensitivity, linearity, and accuracy of the performance of bioanalysis assays leading to erroneous quantification [145]. Yavuz and coworkers [146] synthesized magnetic nanomaterial coated with polydopamine (Fe3O4@PDA) for the magnetic dispersive solid phase extraction of copper from food products and reported that the method had a good matrix interference tolerance. Peng et al. [147] used magnetic dispersive solid phase extraction for the analysis of five bisphenol compounds and reported a significant decreased matrix effect after clean-up by the prepared material.
4.2.3. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
Limit of detection (LOD) and the limit of quantitation (LOQ) are two important performance characteristics in analytical method development and validation. In the scientific literature, there are some vigorous debates on the use of both LOD and LOQ, in terms of definitions, calculations, and applications [148,149]. An acceptable general definition for LOD includes the lowest amount of analyte in a sample that can be detected by the instrument but not necessarily quantified [148,149,150]. On the other hand, LOQ is the lowest concentration of an analyte in a sample that can be detected and quantified by the instrument with suitable accuracy and precision [148,149,150]. These parameters can be determined by using the signal to noise ratio of approximately ≥3 for LOD and ≥10 for LOQ. In the case of LOD, the use of several blank samples and the resulting standard deviation from the measurements can be considered for techniques, such as LC/MS, where, in some cases, a reliable S/N cannot be determined [149]. Similarly, the LOQ can be determined using the slope of the calibration curve and the standard deviation of the response in the low concentration range [149]. For more detailed information and reviews on determining LOD and LOQ, as well as critical aspects on the use of LOD and LOQ, the reader is referred to the references [148,149,150].
The use of MSPE for determining ammonium compounds in vegetable and fruit puree samples using Fe3O4@NH2@G2 (cynanuric chloride-imidazole dendrimer functionalized iron oxide nanoparticles) was conducted. The study reported LOD and LOQ values of 0.05–0.50 µg/kg and 0.20–2.00 µg/kg [151]. Li et al. [152] synthesized amphiphilic block copolymer-grafted with magnetic multi-walled carbon nanotubes for the analysis of mycotoxins and pesticides using modified the QuEChERS method. The result showed LOD values of 0.00015–1.3 µg/kg. In 2021, the determination of ZEN in corn oil using magnetic molecularly imprinted polymer (Fe3O4@PDA@MIPS) was conducted with LOD value of 0.68 ng/mL recorded [153]. Fu et al. [154], on the synthesis of MCNTs (magnetic carbon nanotubes) to extract sulfonamides from milk following MSPE, found LOD and LOQ values of 0.002–0.01 ng/mL and 0.01–0.03 ng/mL, respectively. Table 2 shows different extraction methods and respective LODs and LOQs of pollutants analyzed. From the literature reviewed herein, it is observed that MSPE and QuEChERS methods have lower LODs for these groups of analytes compared to the other extraction methods.
Table 2.
Current applications of MNMs in extracting pollutants from food commodities.
| Type of Nanomaterial | Type of Modification | Morphological Characteristics/Size | Extraction Technique | Pollutants | Matrix | Recovery | LOD(ng/mL) | LOD Method | LOQ(ng/mL) | LOQ Method | Analytical Techniques | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nanosensor | Apt-PLNPs@cDNA-Fe3O4 | TEM ZGO:Mn 48 ± 5 nm × 12 ± 1 nm ZGGO:Cr 20 ± 1 nm NH2-Fe3O4 25 ± 3 nm |
MSPE | AFB1 and ZEN | Grains (corn, rice, oats, wheat, millet, and corn grit) | 93.6–105.1% | 0.00022–0.00029 | 3s | Not provided | Not provided | Spectrophotometer | [155] |
| Nanocellulose | Fe3O4@Cellulose | Not provided | DMSPE | ENNs and BEAUs | Spice (paprika) | 89.5–97.7% | 2.8–3.0 | 3s | 9.5–9.9 | 10s | UHPLC-MS/MS | [130] |
| Magnetic nanomaterial | g-C3N4/Fe3O4 (Graphitic carbon nitride/Fe3O4) | Not provided | Modified QuEChERs | 27 mycotoxins | Maize | 77.81–115.21% | 0.004–0.6226 | Not provided | 0.0014–2.0753 | Not provided | UPLC-MS/MS | [156] |
| Magnetic nanomaterial | Polypyrrole magnetic microsphere | SEM 2.81 ± 0.25 µm |
MSPE | Carbaryl, carbofuran, and methomyl | Fruits and vegetables | 81.6–108.3% | 0.00137–0.0101 | 3s | 0.00457–0.0331 | 10s | HPLC-DAD | [157] |
| Molecular imprinted polymers | Magnetic molecularly-imprinted polymer nanoparticles | SEM 2 µm |
MMIP | Thiamethoxam and thiacloprid | Light and dark honey | 96.8–106.5% | 0.045–0.070 | 3s | 0.07–0.1 | 10s | UHPLC-MS/MS | [98] |
| Magnetic nanomaterial | HCP/Fe3O4 (hypercrosslinked polystyrene/Fe3O4) | Not provided | MSPE | Nitrofuran metabolites | Honey | 91–102% | 0.1–0.3 | 3s | 0.3–1.0 | 10s | LC-MS/MS | [158] |
| Magnetic bead | Fe3O4@AMP&ZnCl2@McAbs | Not provided | Immunoaffinity column | DON, ZEN, HT2, and T-2 | Corn flour, oats, and wheat flour | 76.60–99.47% | 2–5 | Not provided | 5–20 | Not provided | LC-MS | [159] |
| Carbon nanotubes | PEG-MWCNTs- MNPs | TEM 200 nm |
MSPE | Mycotoxins | Milk | 81.8–106.4% | 0.005–0.050 | 3s | 0.015–0.150 | 10s | UHPLC-Q Extractive HRMs | [160] |
| Magnetic nanomaterial | OAcFe-MNPs (Oleic acid/Fe3O4) | Not provided | DMSPE | Fenazaquin | Almonds | 91.2–109.2% | 0.06 | 3s | 0.21 | 10s | GC-MS | [161] |
| Magnetic nanomaterial | Fe3O4@CTS@Apt (Aptamer-functionalized chitosan magnetic nanoparticles) | SEM/TEM Fe3O4: 13.2 nm Fe3O4@CTS: 18.5 nm |
Magnetic extraction and immunoaffinity chromatography extraction | OTA | cornmeal | 91.3–99.1% | Not provided | Not provided | Not provided | Not provided | HPLC | [162] |
| Carbon nanotubes | Fe3O4@MWCNTs@copolymer | TEM/SEM Fe3O4: 20–30 nm Coated with polymer: 5 nm |
QuEChERs | mycotoxins and pesticides | Grains | 60–108% | 0.0002–1.3 | 3s | Not provided | Not provided | HPLC-MS/MS | [152] |
| Immunomagnetic nanomaterial | Fe3O4@CTS | TEM 400 nm |
Immunomagnetic extraction | ZEN | cornmeal | 91.7–104.3% | Not provided | Not provided | Not provided | Not provided | HPLC | [115] |
| Magnetic nanomaterial | MGO@LaP (lanthanum phosphate nanoparticles doped on magnetic graphene oxide) | SEM LaP nanoparticles: 20 nm |
MD-µ-SPE | Chlorpyrifos and hexaconazole | Fruits juices | 78–120% | 0.67–0.89 | 3s | 2.22–2.94 | 10s | GC-ECD | [163] |
| Magnetic nanomaterial | COF(TpPa-1)@ Fe3O4 | Not provided | MSPE | Fluoroquinolones | Milk | 90.4–101.2% | 0.05–0.20 | 3s | 0.19–0.71 | 10s | HPLC | [164] |
| Magnetic nanomaterial | SiO2-TiO2-NH2@Fe3O4 | TEM 20 nm |
MSPE | Malathion, chlorpyrifos, hexaconazole, and atrazine | Green and roasted coffee beans | 74–113% | 1.33–1.43 | 3s | 4.45–4.77 | 10s | GC-ECD | [165] |
| Carbon sheet | Fe3O4-Cs (magnetic carbon nanotubes) | FE-SEM 15 nm |
MD-µ-SPE | Malathion, chlorpyrifos, and fenthion | Vegetables and environmental samples | 70–81% | 0.46–1 | 3s | 2–5 | 10s | GC-IMS | [166] |
| Magnetic nanomaterial | Fe3O4@GO/Apt | Not provided | MSPE | Chloramphenicol | Honey and milk | 80.5–105.0% | 0.24 | 3s | 0.79 | 10s | HPLC | [167] |
| Magnetic nanomaterial | 3DPCMs (Macroporous magnetic 3D photonic crystal microspheres) | Not provided | Immunoaffinity column | AFB1, OTA, and ZEN | Corn, rice, wheat | 70.01–100.12% | Not provided | Not provided | Not provided | Not provided | HPLC–FLD | [168] |
| Carbon nanotube | Fe3O4@MWCNTs | Not provided | Modified QuEChERS | Pesticides, mycotoxins, and veterinary drugs | Eggs | 60.5–114.6% | Not provided | Not provided | 0.1–17.3 | 10s | UPLC–MS/MS | [19] |
| Carbon nanotubes | Fe3O4@MWCNTs | TEM 100–200 nm |
Modified QuEChERs | Mycotoxins | Grains | 73.5–112.9% | 0.0006–1.6337 | Not provided | 0.0021–5.4457 | Not provided | UPLC-MS/MS | [169] |
| Metal organic framework | Fe3O4/MIL-101 (Cr) (magnetic metal-organic framework MIL-101(Cr)) | Not provided | MSPE | Triazine | rice | 79.3–116.7% | 0.00108–0.0181 | 3s | 0.0036–0.0602 | 10s | HPLC-MS/MS | [170] |
| Quantum dot | Fe3O4@ MPA-CdTe QDs (mercaptopropionic acid-capped CdTe quantum dots) | Not provided | MNP-based immunoassay | Alternariol monomethyl ether (AME) | Fruits (cherry, apple, and orange) | 87.2–92.0% | 0.0003 | Not provided | Not provided | Not provided | HPLC-MS | [171] |
| Metal organic framework | M-IRMOF | TEM Fe3O4: 15 nm |
MDSPE | Epoxiconazole, fenbuconazole, difenoconazole, thiabendazole, and pyraclostrobin | Lettuce vegetables | 74.8–99.5% | 0.21–1.0 | 3s | Not provided | Not provided | HPLC-MS/MS | [172] |
| Polymer coated magnetic nanomaterial | TPN@Fe3O4@GO | FE-SEM Fe3O4: 22–50 nm TPN@Fe3O4:26–70 nm |
MSPE | Imidacloprid and 2,4-dichlorophenoxyacetic acid (2,4-D) pesticides | Tomato, cucumber, and water | 91.2–102.4% | 0.17 | 3s | 0.5–5.0 | 10s | HPLC-UV | [173] |
| Carbon nanotubes | Fe3O4@MWCNTs-NH2 | Not provided | MSPE | AFB1 and ZEN | Wheat flour | 88.8–96.0% | 0.15–0.24 | 3s | 0.52–0.83 | 10s | HPLC | [174] |
| Magnetic nanomaterial | Fe3O4-SP/GO (magnetite-sporopollenin/graphene oxide) | Not provided | MSPE | Organophosphorus pesticides (phenthoate, dimethoate, and phosphamidon) | Vegetables (green long pepper, reddish tomato, cucumber, and green long beans) | 81–120% | 0.02–0.05 | 3s | 0.10–0.17 | 10s | GC-µECD | [175] |
| Magnetic nanomaterial | Fe3O4@SiO2@GO-β-CD (β-cyclodextrin) | Not provided | MSPE-DLLME | Oxytetracycline, doxycycline, and tetracycline | Bovine milk | 70.6–121.5% | 1.8–2.9 | 3s | 6.1–9.7 | 10s | HPLC-UV | [123] |
| Magnetic nanoparticles | Fe3O4@rGO@ β- CD | Not provided | MSPE | Organochlorine pesticides | Honey | 78.8–116.2% | 0.00052–0.00321 | 3s | 0.00173–0.01072 | 10s | GC-ECD | [176] |
| Magnetic nanomaterial | Fe3O4@GO | TEM 10–15 nm |
MSPE | Patulin | Apple juice | 68.7–83.6% | 2.3 | 3s | 7.7 | 10s | HPLC-UV | [177] |
| Carbon nanotubes | Fe3O4@MWCNTs | Not provided | MSPE | ZEN and its derivatives | Maize | 75.8–104.1% | 0.03–0.04 | 3s | 0.07–0.10 | 10s | UHPLC-MS/MS | [178] |
| Magnetic nanomaterial | MG@SiO2-TMSPED (magnetic graphene-based silica-N-[3-(trimethoxysilyl)propyl] ethylenediamine) | TEM 10–30 nm |
MSPE | Pesticides | Tomatoes and grape | 82–113% | 0.23–0.30 | 3s | 0.76–1.0 | 10s | GC-µECD | [179] |
| Magnetic nanomaterial | Fe3O4@PAS-C18 (3-(N,N-diethylamino)propyltrimethoxysilane | Not provided | MSPE | Pesticides | Rice | 82.2–125% | 0.24–2.05 | 3s | 2.0–14.9 | Not provided | LC-MS/MS | [180] |
| Magnetic nanomaterial | Fe3O4@pDA (poly(dopamine)) | Not provided | m-μdSPE | Estrogenic mycotoxin | Yogurt and milk | 70–120% | 0.21–4.77 | 3s | 0.98–19.40 | 10s | LC-MS | [181] |
| Magnetic nanomaterial | Fe3O4@GO-β cyclodextrin | TEM 10 ± 3 nm |
MSPE | Tetracycline and doxycycline | Milk | 92.1–105.0% | 0.00018 | 3s | 0.00056 | 10s | Voltammetry | [182] |
| Magnetic nanomaterial | Fe3O4@G-CNPrTEOS | Not provided | MSPE | Organophosphorus pesticides | Cow milk | 82–94% | 0.01–0.6 | 3s | 0.05–1.9 | 10s | GC-µECD | [183] |
| Molecularly imprinted polymers | Fe3O4@SiO2-MIPs (dummy molecular imprinted polymers) | TEM Fe3O4: 200 nm md-MIP: 20 nm |
MSPE | Aflatoxin B1, B2, G1, and G2 | Corn and tea leaves | 75.6–94.8% | 0.05–0.1 | 3s | Not provided | Not provided | UHPLC-MS/MS | [184] |
| Magnetic nanomaterial | Fe3O4@MDN (tetraethyl orthosilicate (TEOS) and methacrylic acid-3-(trimethoxysilyl) propyl ester (MPS)) | SEM/TEM500–600 nm | MSPE | Triazole pesticides | Honey | 90.5–105.9% | 1.1–3.2 | 3s | 3.3–10.1 | 10s | HPLC-MS/MS | [185] |
Note: ENNs and BEAUs: enniatins and beauvericins; Apt-PLNPs@cDNA-Fe3O4: aptamer-modified persistent luminescence nanoparticles coated with complementary DNA-modified magnetic nanoparticle; ZGO:Mn and ZGGO:Cr (two types of PLNPs); TPN@Fe3O4@GO: triazine-based polymeric network modified magnetic nanoparticles/graphene oxide nanocomposite; Fe3O4@AMP&ZnCl2@McAbs: immunomagnetic bead based on the metal–organic framework materials conjugated with monoclonal antibodies coated with Fe3O4; PEG-MWCNTs- MNPs: PEGylated multi-walled carbon nanotubes magnetic nanoparticles; M-IRMOF: magnetic amino-functionalized zinc metal-organic framework; MDSPE: magnetic dispersive solid phase extraction; MSPE-DLLME: magnetic solid phase extraction- dispersive liquid-liquid microextraction; MMIP: magnetic molecularly imprinted polymer; MD-µ-SPE: magnetic dispersive micro-solid phase extraction; GC-ECD: gas chromatography electron capture detection; GC-µECD: gas chromatography microelectron capture detection; UHPLC-MS/MS: ultra-high performance liquid chromatography mass spectrometry; UPLC-MS/MS: ultra-performance liquid chromatography mass spectrometry; HPLC-MS/MS: high performance liquid chromatography mass spectrometry; HPLC-FLD: high-performance liquid chromatography with fluorescence detector; LC-MS/MS: liquid chromatography mass spectrometry; TEM: transmission electron microscopy; SEM: scanning electron microscope; FE-SEM: field emission scanning electron microscope‘ md-MIPs: magnetic dummy molecularly imprinted polymers.
4.2.4. Reusability
Reusability is a desirable quality of extraction adsorbents. The reusability of the MNMs or nanocomposite is obtained by washing the materials several times with suitable solvents and then drying the materials for reuse. These materials are reused for as many times as possible, but still, demonstrate no obvious changes in the analyte recoveries [186,187]. The stability of the magnetic nanocomposite (PANI-Fe3O4) was studied under optimized conditions by evaluating the change in the analyte recoveries through various sorption-elution cycles, and the results indicated that the material might be reused fifty times [188]. Zhou et al. [186] reported the stability of Fe@MgAl-LDH nanocomposite during the magnetic solid phase extraction process with good reusability. Similarly, Zhou et al. [187] made similar observations with this magnetic nanocomposite (Fe@SiO2@p (NIP AM-co-MAA), in addition to good absorbent ability, which demonstrate that the materials have wide applications in analyzing real samples. In addition, a 10-time reusability of Fe3O4@SiO2@GO@PEA (phenylethylamine) was reported for the extraction of pesticides [189]. In another study by Wanjeri et al. [190], Fe3O4@SiO2@GO-MWCNT was reported to be reused up to 5 times for similar pesticides extraction. Both studies showed that the magnetic nanomaterials could be reused without a significant loss of adsorption.
4.2.5. Extraction Time
An important factor in an effective MSPE adsorption process is the extraction time, which ultimately affects analytical output. Often, to reach the adsorption equilibrium, adequate contact time between the adsorbent and analytes is required. Wang et al. [191] reported optimized extraction time of 5 min for the extraction to be carried out. The extraction of AFB1 and ZEN in wheat flour by magnetic nanocomposite (Fe3O4@MWCNTs-NH2) was reported, and optimal extraction time for the study was 25 min [174]. Markus et al. [175] reported 5 min as the optimum extraction time in the adsorption of polar organophosphorus pesticides in vegetables.
4.2.6. Quantity of Extraction Material (Adsorbent Dosage)
An advantage of magnetic solid phase microextraction is the use of relatively little amounts of the adsorbent. This essentially contributes to lower costs and less waste generation. In the extraction of pesticides from grape and tomato, a maximum adsorbent dosage of 30 mg was achieved and used in the study [179]. Zhou et al. [187] investigated the effect of adsorbent amount (20–60 mg) on analytical performance and observed that recoveries remained constant at 40 mg. This showed that the analytes could be completely adsorbed by the adsorbent at 40 mg, which shows that a higher sensitivity was achieved when 40 mg of the nanocomposite were used in the experiment.
5. Applications of Magnetic Nanomaterials for Extraction of Pollutants in Food Commodities
In food analysis, magnetic nanomaterials are of great interest because of the unique features they exhibit, which include ease of separation with an external magnet, large unique surface site, and high charge transfer capacity [192]. The use of magnetic nanomaterials in food commodities is widely increasing and becoming of interest to researchers. They are modified with various functional compounds, chemicals, and groups, which include molecular imprinted polymers, ionic liquids, graphene, graphene oxides, multi-walled carbon nanotubes, and silica oxides, amongst others, to ease the extraction of pollutants from food samples. The applications of these various MNMs with their modifications are shown in Table 2.
6. MNMs for Non-Target Analysis
Non-target analysis refers to the rapid detection/quantification or screening of both known and unknown analytes in a matrix. It is a key way in identifying emerging pollutants or contaminants by screening the matrices without reference standards, and there are no limitations to the number of analytes that can be detected. This analysis uses high-resolution mass spectrometry (HRMS), specifically quadrupole, with time-of-flight hybridization and Orbitrap™ instruments [130]. The key features of this analysis is that a fingerprint known as a total ion chromatograph is obtained for each sample, which is used to compare with existing sample profiles to reveal deviations or helps in the identification of unknowns [193]. The raw data obtained from previous analysis can be used to re-look at analytes that were not of interest, or not known, search for new compounds, or re-sampling or re-analysis of stored samples.
In non-target analysis, different extraction sorbents are used [194], but our focus will be on MNMs sorbents. For example, Jin et al. [195] did non-target analysis in vegetables using hierarchial micro and mesosphere metal-organic framework coated with magnetic nanosphere (H-MOF@Fe3O4). Non-target analysis was conducted using magnetic nanocomposite based on cellulose (Fe3O4@Cellulose) and different metabolites of enniatins and beauvericins screened in paprika samples [130]. In another study, a non-target analysis of over 204 pesticides was carried out by developing a magnetic blade-spray tandem mass spectrometry assay (MBS-MS/MS) [196]. In addition, the identification of pesticides and phytochromes from vegetables using heteropore covalent organic framework coated with magnetic nanosphere was reported [197]. The studies highlight the potential application of MNMs for sample pretreatment for non-target analytes. In addition, since MNMs can have their surface chemistry tailored and altered in a relatively short period of time, MNMs can provide new and rapid methods in achieving high performance sample preparation, and identifying new emerging contaminants of concern in various matrices, especially in a very wide range of food commodities.
7. Conclusions/Future Trends
The applications of MNMs in food analysis is of great interest to the scientific community because of their chemical and physical properties, which give them an edge to other adsorbent materials, thus being of great benefit to food safety. They have commonly been used in the MSPE process because of the ease of separation and recovery of analytes by an external magnet and the possibility of reusing them several times. As reviewed herein, MNMs can easily be modified using different materials, including graphene, ionic liquids, carbon nanotubes, and polymers, amongst others, to increase their surface area, thus enhancing their extraction efficiency and enriching the trace level of the analytes targeted in complex food matrices. Their specific features in terms of extraction capacity enable them have high surface site, and charge transfer capacity coupled to their ease of modifications, drawing a huge number of their applications in extraction. These MNMs and their nanocomposites have shown great potential for extracting various pollutants and could be applicable for efficient analysis of emerging pollutants and other important chemical contaminants in food and the environment. Despite numerous achievements in extracting pollutants using MNMs and their alloys, there are still gaps on the use of some alloys (i.e., FeCo, FePt, and FePd) for extraction, although they have been utilized in such fields as biomedical, microwave absorption, catalysis, magnetic data storage, nanobiotechnology, and magnetic recording media. There are therefore knowledge gaps in the applicability of MNMs for extracting environmental pollutants (particularly emerging pollutants). Other areas of interest could be the use of modern synthetic pathways, such as environmentally friendly solvents (ethanol and water) and naturally-derived plant extracts to synthesize and/or modify MNMs.
Acknowledgments
The National Research Foundation (TWAS/NRF) is gratefully acknowledged for granting S.T. a fellowship to pursue Ph.D. studies at University of Johannesburg, South Africa. S.T. would like to acknowledge Oluwaseyi Damilare Saliu and Sefater Gbashi for review and proof reading the first draft of the manuscript. All authors gratefully acknowledge the Centre for Nanomaterials Science Research at the University of Johannesburg for support.
Author Contributions
Writing—reviewing, and editing S.T., P.B.N. and P.G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the University of Johannesburg’s Faculty of Science Research Committee funding.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Quesada H.B., Baptista A.T.A., Cusioli L.F., Seibert D., de Oliveira Bezerra C., Bergamasco R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere. 2019;222:766–780. doi: 10.1016/j.chemosphere.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 2.K’oreje K.O., Okoth M., Van Langenhove H., Demeestere K. Occurrence and treatment of contaminants of emerging concern in the African aquatic environment: Literature review and a look ahead. J. Environ. Manag. 2020;254:109752. doi: 10.1016/j.jenvman.2019.109752. [DOI] [PubMed] [Google Scholar]
- 3.Rogowska J., Cieszynska-Semenowicz M., Ratajczyk W., Wolska L. Micropollutants in treated wastewater. Ambio. 2020;49:487–503. doi: 10.1007/s13280-019-01219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hube S., Wu B. Mitigation of emerging pollutants and pathogens in decentralized wastewater treatment processes: A review. Sci. Total Environ. 2021;779:146545. doi: 10.1016/j.scitotenv.2021.146545. [DOI] [PubMed] [Google Scholar]
- 5.Yadav D., Rangabhashiyam S., Verma P., Singh P., Devi P., Kumar P., Hussain C.M., Gaurav G.K., Kumar K.S. Environmental and health impacts of contaminants of emerging concerns: Recent treatment challenges and approaches. Chemosphere. 2021;272:129492. doi: 10.1016/j.chemosphere.2020.129492. [DOI] [PubMed] [Google Scholar]
- 6.Cheng D., Ngo H.H., Guo W., Chang S.W., Nguyen D.D., Liu Y., Wei Q., Wei D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020;387:121682. doi: 10.1016/j.jhazmat.2019.121682. [DOI] [PubMed] [Google Scholar]
- 7.Snow D.D., Cassada D.A., Bartelt-hunt S.L., Li X., Zhang Y., Zhang Y., Yuan Q., Sallach J.B. Detection, Occurrence and Fate of Emerging Contaminants in Agricultural Environments. Water Environ. Res. 2012;84:764–785. doi: 10.2175/106143012X13407275694635. [DOI] [Google Scholar]
- 8.Spahr S., Teixidó M., Sedlak D.L., Luthy R.G. Hydrophilic trace organic contaminants in urban stormwater: Occurrence, toxicological relevance, and the need to enhance green stormwater infrastructure. Environ. Sci. Water Res. Technol. 2020;6:15–44. doi: 10.1039/C9EW00674E. [DOI] [Google Scholar]
- 9.Khan M.T., Shah I.A., Ihsanullah I., Naushad M., Ali S., Shah S.H.A., Mohammad A.W. Hospital wastewater as a source of environmental contamination: An overview of management practices, environmental risks, and treatment processes. J. Water Process Eng. 2021;41:101990. doi: 10.1016/j.jwpe.2021.101990. [DOI] [Google Scholar]
- 10.Vymazal J., Březinová T. The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: A review. Environ. Int. 2015;75:11–20. doi: 10.1016/j.envint.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Angeles L.F., Singh R.R., Vikesland P.J., Aga D.S. Increased coverage and high confidence in suspect screening of emerging contaminants in global environmental samples. J. Hazard. Mater. 2021;414:125369. doi: 10.1016/j.jhazmat.2021.125369. [DOI] [PubMed] [Google Scholar]
- 12.NORMAN—Network Reference Laboratories for Monitoring Emerging Substances. John Wiley & Sons; Hoboken, NJ, USA: 2008. [Google Scholar]
- 13.Tijani J.O., Fatoba O.O., Babajide O.O., Petrik L.F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environ. Chem. Lett. 2016;14:27–49. doi: 10.1007/s10311-015-0537-z. [DOI] [Google Scholar]
- 14.Cunha D.L., de Araujo F.G., Marques M. Psychoactive drugs: Occurrence in aquatic environment, analytical methods, and ecotoxicity—A review. Environ. Sci. Pollut. Res. 2017;24:24076–24091. doi: 10.1007/s11356-017-0170-4. [DOI] [PubMed] [Google Scholar]
- 15.Vargas-Berrones K., Bernal-Jácome L., de León-Martínez L.D., Flores-Ramírez R. Emerging pollutants (EPs) in Latin América: A critical review of under-studied EPs, case of study-Nonylphenol. Sci. Total Environ. 2020;726:138493. doi: 10.1016/j.scitotenv.2020.138493. [DOI] [PubMed] [Google Scholar]
- 16.Gavrilescu M., Demnerová K., Aamand J., Agathos S., Fava F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015;32:147–156. doi: 10.1016/j.nbt.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Tang Y., Yin M., Yang W., Li H., Zhong Y., Mo L., Liang Y., Ma X., Sun X. Emerging pollutants in water environment: Occurrence, monitoring, fate, and risk assessment. Water Environ. Res. 2019;9:984–991. doi: 10.1002/wer.1163. [DOI] [PubMed] [Google Scholar]
- 18.Omar T.F.T., Aris A.Z., Yusoff F.M., Mustafa S. Occurrence and level of emerging organic contaminant in fish and mollusk from Klang River estuary, Malaysia and assessment on human health risk. Environ. Pollut. 2019;248:763–773. doi: 10.1016/j.envpol.2019.02.060. [DOI] [PubMed] [Google Scholar]
- 19.Xu X., Xu X., Han M., Qiu S., Hou X. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS. Food Chem. 2019;276:419–426. doi: 10.1016/j.foodchem.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 20.Jia M., Liao X., Fang L., Jia B., Liu M., Li D., Zhou L., Kong W. Recent advances on immunosensors for mycotoxins in foods and other commodities. Trac Trends Anal. Chem. 2021;136:116193. doi: 10.1016/j.trac.2021.116193. [DOI] [Google Scholar]
- 21.Omotayo O.P., Omotayo A.O., Mwanza M., Babalola O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019;35:1–7. doi: 10.5487/TR.2019.35.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nleya N., Adetunji M.C., Mwanza M. Current status of mycotoxin contamination of food commodities in Zimbabwe. Toxins. 2018;10:89. doi: 10.3390/toxins10050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matumba L., Monjerezi M., Biswick T., Mwatseteza J., Makumba W., Kamangira D., Mtukuso A. A survey of the incidence and level of aflatoxin contamination in a range of locally and imported processed foods on Malawian retail market. Food Control. 2014;39:87–91. doi: 10.1016/j.foodcont.2013.09.068. [DOI] [Google Scholar]
- 24.Tebele S.M., Gbashi S., Adebo O., Changwa R., Naidu K., Njobeh P.B. Quantification of multi-mycotoxin in cereals (maize, maize porridge, sorghum and wheat) from Limpopo province of South Africa. Food Addit. Contam. Part A. 2020;37:1922–1938. doi: 10.1080/19440049.2020.1808715. [DOI] [PubMed] [Google Scholar]
- 25.Onyedum S.C., Adefolalu F.S., Muhammad H.L., Apeh D.O., Agada M.S., Imienwanrin M.R., Makun H.A. Occurrence of major mycotoxins and their dietary exposure in North-Central Nigeria staples. Sci. Afr. 2020;7:e00188. [Google Scholar]
- 26.Elfikrie N., Ho Y.B., Zaidon S.Z., Juahir H., Tan E.S.S. Occurrence of pesticides in surface water, pesticides removal efficiency in drinking water treatment plant and potential health risk to consumers in Tengi River basin, Malaysia. Sci. Total Environ. 2020;712:136540. doi: 10.1016/j.scitotenv.2020.136540. [DOI] [PubMed] [Google Scholar]
- 27.Galani J.H., Houbraken M., Wumbei A., Djeugap J.F., Fotio D., Spanoghe P. Evaluation of 99 pesticide residues in major agricultural products from the western highlands zone of Cameroon using QuEChERS method extraction and LC-MS/MS and GC-ECD analyses. Foods. 2018;7:184. doi: 10.3390/foods7110184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mebdoua S., Ounane G. Evaluation of pesticide residues in wheat grains and its products from Algeria. Food Addit. Contam. Part B. 2019;12:289–295. doi: 10.1080/19393210.2019.1661529. [DOI] [PubMed] [Google Scholar]
- 29.Buah-Kwofie A., Humphries M.S., Pillay L. Bioaccumulation and risk assessment of organochlorine pesticides in fish from a global biodiversity hotspot: iSimangaliso wetland park, South Africa. Sci. Total Environ. 2018;621:273–281. doi: 10.1016/j.scitotenv.2017.11.212. [DOI] [PubMed] [Google Scholar]
- 30.Küster A., Adler N. Pharmaceuticals in the environment: Scientific evidence of risks and its regulation. Philos. Trans. R. Soc. B Biol. Sci. 2014;369:20130587. doi: 10.1098/rstb.2013.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bommuraj V., Chen Y., Gal O., Ben Ari J., Kertsnus-Banchik E., Barel S., Shimshoni J.A. Human pharmaceutical and pesticide residues in Israeli dairy milk in association with dietary risk assessment. Food Addit. Contam. Part B. 2020;13:233–243. doi: 10.1080/19393210.2020.1764114. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein M., Shenker M., Chefetz B. Insights into the uptake processes of wastewater-borne pharmaceuticals by vegetables. Environ. Sci. Technol. 2014;48:5593–5600. doi: 10.1021/es5008615. [DOI] [PubMed] [Google Scholar]
- 33.Pizan-Aquino C., Wong A., Avilés-Félix L., Khan S., Picasso G., Sotomayor M.D. Evaluation of the performance of selective M-MIP to tetracycline using electrochemical and HPLC-UV method. Mater. Chem. Phys. 2020;245:122777. doi: 10.1016/j.matchemphys.2020.122777. [DOI] [Google Scholar]
- 34.Lee H.J., Ryu D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017;65:7034–7051. doi: 10.1021/acs.jafc.6b04847. [DOI] [PubMed] [Google Scholar]
- 35.Alimentarius C. [(accessed on 13 July 2021)];General Standard for Contaminants and Toxins in Food and Feed (Codex STAN 193-1995) 2015 :1–59. Available online: http://www.fao.org/fileadmin/user_upload/livestockgov/documents/1_CXS_193e.pdf.
- 36.Joint FAO. World Health Organization. WHO Expert Committee on Food Additives . Evaluation of Certain Contaminants in Food: Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization; Geneva, Switzerland: 2017. pp. 1–182. (WHO Technical Report Series). [Google Scholar]
- 37.Luo S., Du H., Kebede H., Liu Y., Xing F. Contamination status of major mycotoxins in agricultural product and food stuff in Europe. Food Control. 2021;127:108120. doi: 10.1016/j.foodcont.2021.108120. [DOI] [Google Scholar]
- 38.Van Egmond H.P., Jonker M.A. Regulations and limits for mycotoxins in fruits and vegetables. In: Barkai-Golan R., Paster N., editors. Mycotoxins in Fruits and Vegetables. Academic Press; Cambridge, MA, USA: 2008. pp. 45–74. [Google Scholar]
- 39.Relangi P.K. Ph.D. Thesis. Texas Southern University; Houston, TX, USA: 2019. LC/LC-MS Methods for Detection of Multiple Pesticide Residues and Endocrine Disrupting Chemicals: Effects of Ozonization on Degradation and Removal. [Google Scholar]
- 40.DALRRD . Food Safety and Quality Assurance. DALRRD; Pretoria, South Africa: 2020. [Google Scholar]
- 41.Azzouz A., Kailasa S.K., Lee S.S., Rascón A.J., Ballesteros E., Zhang M., Kim K.H. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. Trac Trends Anal. Chem. 2018;108:347–369. doi: 10.1016/j.trac.2018.08.009. [DOI] [Google Scholar]
- 42.Reyes-Gallardo E.M., Lasarte-Aragonés G., Lucena R., Cárdenas S., Valcárcel M. Hybridization of commercial polymeric microparticles and magnetic nanoparticles for the dispersive micro-solid phase extraction of nitroaromatic hydrocarbons from water. J. Chromatogr. A. 2013;1271:50–55. doi: 10.1016/j.chroma.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 43.Mirzajani R., Karimi S. Ultrasonic assisted synthesis of magnetic Ni-Ag bimetallic nanoparticles supported on reduced graphene oxide for sonochemical simultaneous removal of sunset yellow and tartrazine dyes by response surface optimi-zation: Application of derivative spectrophot. Ultrason. Sonochem. 2019;50:239–250. doi: 10.1016/j.ultsonch.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 44.Rios A., Zougagh M. Recent advances in magnetic nanomaterials for improving analytical processes. Trac Trends Anal. Chem. 2016;84:72–83. doi: 10.1016/j.trac.2016.03.001. [DOI] [Google Scholar]
- 45.De Souza K.C., Andrade G.F., Vasconcelos I., de Oliveira Viana M., Fernandes C., de Sousa E.M.B. Magnetic solid-phase extraction based on mesoporous silica-coated magnetic nanoparticles for analysis of oral antidiabetic drugs in human plasma. Mater. Sci. Eng. 2014;40:275–280. doi: 10.1016/j.msec.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Ramadan M.M., Mohamed M.A., Almoammar H., Abd-Elsalam K.A. Magnetic nanomaterials for purification, detection, and control of mycotoxins. In: Rai M., Abd-Elsalam K.A., editors. Nanomycotoxicology. Academic Press; Cambridge, MA, USA: 2020. pp. 87–114. [Google Scholar]
- 47.Martinez A.I., Garcia-Lobato M.A., Perry D.L. Study of the properties of iron oxide nanostructures. Res. Nanotechnol. Dev. 2009;19:184–193. [Google Scholar]
- 48.Yoon S. Preparation and physical characterizations of superparamagnetic maghemite nanoparticles. J. Magn. 2014;19:323–326. doi: 10.4283/JMAG.2014.19.4.323. [DOI] [Google Scholar]
- 49.Lin S., Lu D., Liu Z. Removal of arsenic contaminants with magnetic γ-Fe2O3 nanoparticles. Chem. Eng. J. 2012;211:46–52. doi: 10.1016/j.cej.2012.09.018. [DOI] [Google Scholar]
- 50.Roy A., Bhattacharya J. Removal of Cu(II), Zn(II) and Pb(II) from water using microwave-assisted synthesized maghemite nanotubes. Chem. Eng. J. 2012;211:493–500. doi: 10.1016/j.cej.2012.09.097. [DOI] [Google Scholar]
- 51.Narimani-Sabegh S., Noroozian E. Magnetic solid-phase extraction and determination of ultra-trace amounts of antimony in aqueous solutions using maghemite nanoparticles. Food Chem. 2019;287:382–389. doi: 10.1016/j.foodchem.2019.02.112. [DOI] [PubMed] [Google Scholar]
- 52.Tuutijärvi T., Lu J., Sillanpää M., Chen G. As (V) adsorption on maghemite nanoparticles. J. Hazard. Mater. 2009;166:1415–1420. doi: 10.1016/j.jhazmat.2008.12.069. [DOI] [PubMed] [Google Scholar]
- 53.Jiang W., Pelaez M., Dionysiou D.D., Entezari M.H., Tsoutsou D., O’Shea K. Chromium (VI) removal by maghemite nanoparticles. Chem. Eng. J. 2013;222:527–533. doi: 10.1016/j.cej.2013.02.049. [DOI] [Google Scholar]
- 54.Devatha C.P., Shivani S. Novel application of maghemite nanoparticles coated bacteria for the removal of cadmium from aqueous solution. J. Environ. Manag. 2020;258:110038. doi: 10.1016/j.jenvman.2019.110038. [DOI] [PubMed] [Google Scholar]
- 55.Rajput S., Singh L.P., Pittman C.U., Jr., Mohan D. Lead (Pb2+) and copper (Cu2+) remediation from water using superparamagnetic maghemite (γ-Fe2O3) nanoparticles synthesized by Flame Spray Pyrolysis (FSP) J. Colloid Interface Sci. 2017;492:176–190. doi: 10.1016/j.jcis.2016.11.095. [DOI] [PubMed] [Google Scholar]
- 56.Piovesan J.V., Haddad V.F., Pereira D.F., Spinelli A. Magnetite nanoparticles/chitosan-modified glassy carbon electrode for non-enzymatic detection of the endocrine disruptor parathion by cathodic square-wave voltammetry. J. Electroanal. Chem. 2018;823:617–623. doi: 10.1016/j.jelechem.2018.07.007. [DOI] [Google Scholar]
- 57.González-Jartín J.M., de Castro Alves L., Alfonso A., Piñeiro Y., Vilar S.Y., Gomez M.G., Osorio Z.V., Sainz M.J., Vieytes M.R., Rivas J., et al. Detoxification agents based on magnetic nanostructured particles as a novel strategy for mycotoxin mitigation in food. Food Chem. 2019;294:60–66. doi: 10.1016/j.foodchem.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 58.Turan E., Şahin F. Molecularly imprinted biocompatible magnetic nanoparticles for specific recognition of Ochratoxin A. Sens. Actuators B Chem. 2016;227:668–676. doi: 10.1016/j.snb.2015.12.087. [DOI] [Google Scholar]
- 59.Riaño S., Binnemans K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015;17:2931–2942. doi: 10.1039/C5GC00230C. [DOI] [Google Scholar]
- 60.Zinatloo-Ajabshir S., Mortazavi-Derazkola S., Salavati-Niasari M. Schiff-base hydrothermal synthesis and characterization of Nd2O3 nanostructures for effective photocatalytic degradation of eriochrome black T dye as water contaminant. J. Mater. Sci. Mater. Electron. 2017;28:17849–17859. doi: 10.1007/s10854-017-7726-4. [DOI] [Google Scholar]
- 61.Ahmad Z.U., Yao L., Wang J., Gang D.D., Islam F., Lian Q., Zappi M.E. Neodymium embedded ordered mesoporous carbon (OMC) for enhanced adsorption of sunset yellow: Characterizations, adsorption study and adsorption mechanism. Chem. Eng. J. 2019;359:814–826. doi: 10.1016/j.cej.2018.11.174. [DOI] [Google Scholar]
- 62.Ahmadi S., Mohammadi L., Rahdar A., Rahdar S., Dehghani R., Igwegbe C.A., Kyzas G.Z. Acid Dye Removal from Aqueous Solution by Using Neodymium (III) Oxide Nanoadsorbents. Nanomaterials. 2020;10:556. doi: 10.3390/nano10030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen T.W., Merlin J.P., Chen S.M., Anandaraj S., Elshikh M.S., Tseng T.W., Wang K., Qi D., Jiang J. Sonochemical synthesis and fabrication of neodymium sesquioxide entrapped with graphene oxide based hierarchical nanocomposite for highly sensitive electrochemical sensor of anti-cancer (raloxifene) drug. Ultrason. Sonochem. 2020;64:104717. doi: 10.1016/j.ultsonch.2019.104717. [DOI] [PubMed] [Google Scholar]
- 64.Zheng J., Dong Y., Wang W., Ma Y., Hu J., Chen X., Chen X. In Situ loading of gold nanoparticles on Fe3O4@SiO2 magnetic nanocomposites and their high catalytic activity. Nanoscale. 2013;5:4894–4901. doi: 10.1039/c3nr01075a. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y.C., Zheng F.C., Min Y.L., Wang T., Zhao Y.G. Synthesis and properties of magnetic FeNi3 alloyed microchains obtained by hydrothermal reduction. Solid State Sci. 2012;14:809–813. doi: 10.1016/j.solidstatesciences.2012.04.006. [DOI] [Google Scholar]
- 66.dos Santos C.M., Martins A.F.N., Costa B.C., Ribeiro T.S., Braga T.P., Soares J.M., Sasaki J.M. Synthesis of FeNi Alloy Nanomaterials by proteic sol-gel method: Crystallographic, morphological, and magnetic Properties. J. Nanomater. 2016;2016:9. doi: 10.1155/2016/1637091. [DOI] [Google Scholar]
- 67.Mohammed A.A., Brouers F., Sadi S.I.A., Al-Musawi T.J. Role of Fe3O4 magnetite nanoparticles used to coat bentonite in zinc (II) ions sequestration. Environ. Nanotechnol. Monit. Manag. 2018;10:17–27. doi: 10.1016/j.enmm.2018.04.004. [DOI] [Google Scholar]
- 68.Khodadadi M., Panahi A.H., Al-Musawi T.J., Ehrampoush M.H., Mahvi A.H. The catalytic activity of FeNi3@SiO2 magnetic nanoparticles for the degradation of tetracycline in the heterogeneous Fenton-like treatment method. J. Water Process Eng. 2019;32:100943. doi: 10.1016/j.jwpe.2019.100943. [DOI] [Google Scholar]
- 69.Farooghi A., Sayadi M.H., Rezaei M.R., Allahresani A. An efficient removal of lead from aqueous solutions using FeNi3@SiO2 magnetic nanocomposite. Surf. Interfaces. 2018;10:58–64. doi: 10.1016/j.surfin.2017.11.005. [DOI] [Google Scholar]
- 70.Nasseh N., Taghavi L., Barikbin B., Nasseri M.A., Allahresani A. FeNi3/SiO2 magnetic nanocomposite as an efficient and recyclable heterogeneous fenton-like catalyst for the oxidation of metronidazole in neutral environments: Adsorption and degradation studies. Compos. Part B Eng. 2019;166:328–340. doi: 10.1016/j.compositesb.2018.11.112. [DOI] [Google Scholar]
- 71.Rafique M.Y., Pan L., Iqbal M.Z., Qiu H., Farooq M.H., Guo Z. 3-D flower like FeCo alloy nanostructures assembled with nanotriangular prism: Facile synthesis, magnetic properties, and effect of NaOH on its formation. J. Alloys Compd. 2013;550:423–430. doi: 10.1016/j.jallcom.2012.10.120. [DOI] [Google Scholar]
- 72.Gupta V., Patra M.K., Shukla A., Saini L., Songara S., Jani R., Vadera S.R., Kumar N. Synthesis and investigations on microwave absorption properties of core–shell FeCo (C) alloy nanoparticles. Sci. Adv. Mater. 2014;6:1196–1202. doi: 10.1166/sam.2014.1889. [DOI] [Google Scholar]
- 73.Braga T.P., Dias D.F., de Sousa M.F., Soares J.M., Sasaki J.M. Synthesis of air stable FeCo alloy nanocrystallite by proteic sol–gel method using a rotary oven. J. Alloys Compd. 2015;622:408–417. doi: 10.1016/j.jallcom.2014.10.074. [DOI] [Google Scholar]
- 74.Li X., Feng J., Du Y., Bai J., Fan H., Zhang H., Peng Y., Li F. One-pot synthesis of CoFe2O4/graphene oxide hybrids and their conversion into FeCo/graphene hybrids for lightweight and highly efficient microwave absorber. J. Mater. Chem. A. 2015;3:5535–5546. doi: 10.1039/C4TA05718J. [DOI] [Google Scholar]
- 75.Zhou J., Shu X., Wang Z., Liu Y., Wang Y., Zhou C., Kong L. Hydrothermal synthesis of polyhedral FeCo alloys with enhanced electromagnetic absorption performances. J. Alloys Compd. 2019;794:68–75. doi: 10.1016/j.jallcom.2019.04.217. [DOI] [Google Scholar]
- 76.Abbas M., Islam M.N., Rao B.P., Ogawa T., Takahashi M., Kim C. One-pot synthesis of high magnetization air-stable FeCo nanoparticles by modified polyol method. Mater. Lett. 2013;91:326–329. doi: 10.1016/j.matlet.2012.10.019. [DOI] [Google Scholar]
- 77.Çelik Ö., Fırat T. Synthesis of FeCo magnetic nanoalloys and investigation of heating properties for magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2018;456:11–16. doi: 10.1016/j.jmmm.2018.01.090. [DOI] [Google Scholar]
- 78.Wang W., Jing Y., He S., Wang J.P., Zhai J.P. Surface modification and bioconjugation of FeCo magnetic nanoparticles with proteins. Colloids Surf. B Biointerfaces. 2014;117:449–456. doi: 10.1016/j.colsurfb.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 79.Galiote N.A., Oliveira F.E.R., Lima F.H.B.D. FeCo-NC oxygen reduction electrocatalysts: Activity of the different compounds produced during the synthesis via pyrolysis. Appl. Catal. B Environ. 2019;253:300–308. doi: 10.1016/j.apcatb.2019.04.057. [DOI] [Google Scholar]
- 80.Kishimoto M., Latiff H., Kita E., Yanagihara H. Morphology and magnetic properties of FeCo particles synthesized with different compositions of Co and Fe through co-precipitation, flux treatment, and reduction. J. Magn. Magn. Mater. 2019;476:229–233. doi: 10.1016/j.jmmm.2018.12.089. [DOI] [Google Scholar]
- 81.Bai L., Wan H., Street S.C. Preparation of ultrafine FePt nanoparticles by chemical reduction in PAMAM-OH template. Colloids Surf. A Physicochem. Eng. Asp. 2009;349:23–28. doi: 10.1016/j.colsurfa.2009.07.041. [DOI] [Google Scholar]
- 82.Ovejero J.G., Velasco V., Abel F.M., Crespo P., Herrasti P., Hernando A., Hadjipanayis G.C. Colloidal Nanoparticle Clusters to produce large FePt nanocrystals. Mater. Des. 2017;113:391–396. doi: 10.1016/j.matdes.2016.10.042. [DOI] [Google Scholar]
- 83.Duan X., Wu C., Wang X., Tian X., Pei W., Wang K., Wang Q. Evolutions of microstructure and magnetic property of wet-chemical synthesized FePt nanoparticles assisted by high magnetic field. J. Alloys Compd. 2019;797:1372–1377. doi: 10.1016/j.jallcom.2019.05.218. [DOI] [Google Scholar]
- 84.Liu Y., Yang K., Cheng L., Zhu J., Ma X., Xu H., Li Y., Guo L., Gu H., Liu Z. PEGylated FePt@Fe2O3 core-shell magnetic nanoparticles: Potential theranostic applications and in vivo toxicity studies. Nanomed. Nanotechnol. Biol. Med. 2013;9:1077–1088. doi: 10.1016/j.nano.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Shi Y., Lin M., Jiang X., Liang S. Recent advances in FePt nanoparticles for biomedicine. J. Nanomater. 2015;2015:2. doi: 10.1155/2015/467873. [DOI] [Google Scholar]
- 86.Akdeniz M.V., Mekhrabov A.O. Size dependent stability and surface energy of amorphous FePt nanoalloy. J. Alloys Compd. 2019;788:787–798. doi: 10.1016/j.jallcom.2019.02.271. [DOI] [Google Scholar]
- 87.Meng Z., Wei Z., Fu K., Lv L., Yu Z.Q., Wong W.Y. Amphiphilic bimetallic polymer as single-source precursors for the one-pot synthesis of L10-phase FePt nanoparticles. J. Organomet. Chem. 2019;892:83–88. doi: 10.1016/j.jorganchem.2019.04.015. [DOI] [Google Scholar]
- 88.Meng Z., Li G., Zhu N., Ho C.L., Leung C.W., Wong W.Y. One-pot synthesis of ferromagnetic FePd nanoparticles from single-source organometallic precursors and size effect of metal fraction in polymer chain. J. Organomet. Chem. 2017;849:10–16. doi: 10.1016/j.jorganchem.2017.06.006. [DOI] [Google Scholar]
- 89.Yamamoto S., Takao S., Muraishi S., Xu C., Taya M. Synthesis of Fe70Pd30 nanoparticles and their surface modification by zwitterionic linker. Mater. Chem. Phys. 2019;234:237–244. doi: 10.1016/j.matchemphys.2019.05.011. [DOI] [Google Scholar]
- 90.Van N.T.T., Trung T.T., Nam N.H., Phu N.D., Hai N.H., Luong N.H. Hard magnetic properties of FePd nanoparticles. Eur. Phys. J. Appl. Phys. 2013;64:10403. doi: 10.1051/epjap/2013130010. [DOI] [Google Scholar]
- 91.Luong N.H., Trung T.T., Loan T.P., Kien L.M., Hong T.T., Nam N.H. Magnetic properties of FePd nanoparticles prepared by sonoelectrodeposition. J. Electron. Mater. 2016;45:4309–4313. doi: 10.1007/s11664-016-4565-7. [DOI] [Google Scholar]
- 92.Khan F.S.A., Mubarak N.M., Khalid M., Walvekar R., Abdullah E.C., Mazari S.A., Nizamuddin S., Karri R.R. Magnetic nanoadsorbents’ potential route for heavy metals removal—A review. Environ. Sci. Pollut. Res. 2020;27:24342–24356. doi: 10.1007/s11356-020-08711-6. [DOI] [PubMed] [Google Scholar]
- 93.Faraji M., Yamini Y. Application of magnetic nanomaterials in food analysis. In: Ahmadi M., Afkhami A., Madrakian T., editors. Magnetic Nanomaterials in Analytical Chemistry. Elsevier; Amsterdam, The Netherlands: 2021. pp. 87–120. [Google Scholar]
- 94.Andrade-Eiroa A., Canle M., Leroy-Cancellieri V., Cerdà V. Solid-phase extraction of organic compounds: A critical review (Part I) Trac Trends Anal. Chem. 2016;80 doi: 10.1016/j.trac.2015.08.015. [DOI] [Google Scholar]
- 95.Ahmadi M., Ghoorchian A., Dashtian K., Kamalabadi M., Madrakian T., Afkhami A. Application of magnetic nanomaterials in electroanalytical methods: A review. Talanta. 2021;225:121974. doi: 10.1016/j.talanta.2020.121974. [DOI] [PubMed] [Google Scholar]
- 96.Mehdinia A., Khodaee N., Jabbari A. Fabrication of graphene/Fe3O4@polythiophene nanocomposite and its application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Anal. Chim. Acta. 2015;868:1–9. doi: 10.1016/j.aca.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 97.Faraji M., Shirani M., Rashidi-Nodeh H. The recent advances in magnetic sorbents and their applications. Trac Trends Anal. Chem. 2021;141:116302. doi: 10.1016/j.trac.2021.116302. [DOI] [Google Scholar]
- 98.Abdulhussein A.Q., Jamil A.K.M., Bakar N.K.A. Magnetic molecularly imprinted polymer nanoparticles for the extraction and clean-up of thiamethoxam and thiacloprid in light and dark honey. Food Chem. 2021;359:129936. doi: 10.1016/j.foodchem.2021.129936. [DOI] [PubMed] [Google Scholar]
- 99.Marsousi S., Karimi-Sabet J., Moosavian M.A., Amini Y. Liquid-liquid extraction of calcium using ionic liquids in spiral microfluidics. Chem. Eng. J. 2019;356:492–505. doi: 10.1016/j.cej.2018.09.030. [DOI] [Google Scholar]
- 100.Nichols L. Overview of Extraction. [(accessed on 14 May 2021)]; Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Organic_Chemis-try_Lab_Techniques_(Nichols)/04%3A_Extraction/4.02%3A_Overview_of_Extraction.
- 101.Badawy M.E., El-Nouby M.A., Marei A.E.S.M. Development of a solid-phase extraction (SPE) cartridge based on chitosan-metal oxide nanoparticles (Ch-MONPs) for extraction of pesticides from water and determination by HPLC. Int. J. Anal. Chem. 2018;2018:1–17. doi: 10.1155/2018/3640691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosero-Moreano M. New trends in chemical analysis of disinfection by-products. In: Kırmusaoğlu S., editor. Disinfection. IntechOpen; London, UK: 2018. pp. 57–77. [Google Scholar]
- 103.Shimelis O.I., Dumas C., Claus J. Analysis of aflatoxins in hops after solid phase extraction cleanup. Report. Food Beverage. 2016;34:11–13. [Google Scholar]
- 104.Zdravkovic S. Solid Phase Extraction: Realizing the Advantages of an Underutilized Technique. [(accessed on 1 September 2019)]; Available online: https://www.ppdi.com/Blog/2017/May/Solid-phase-extraction-May-2017.
- 105.Narenderan S.T., Meyyanathan S.N., Babu B. Review of pesticide residue analysis in fruits and vegetables. Pre-treatment, extraction and detection techniques. Food Res. Int. 2020;133:109141. doi: 10.1016/j.foodres.2020.109141. [DOI] [PubMed] [Google Scholar]
- 106.Atapattu S.N., Poole C.F. Recent advances in analytical methods for the determination of citrinin in food matrices. J. Chromatogr. A. 2020;1627 doi: 10.1016/j.chroma.2020.461399. [DOI] [PubMed] [Google Scholar]
- 107.Varela-Martínez D.A., González-Sálamo J., González-Curbelo M.Á., Hernández-Borges J. Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) Extraction. In: Poole C.F., editor. Liquid-Phase Extraction. Elsevier; Amsterdam, The Netherlands: 2020. pp. 399–437. [Google Scholar]
- 108.Kim L., Lee D., Cho H.-K., Choi S.-D. Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019;22:e00063. doi: 10.1016/j.teac.2019.e00063. [DOI] [Google Scholar]
- 109.Musarurwa H., Chimuka L., Pakade V.E., Tavengwa N.T. Recent developments and applications of QuEChERS based techniques on food samples during pesticide analysis. J. Food Compos. Anal. 2019;84:103314. doi: 10.1016/j.jfca.2019.103314. [DOI] [Google Scholar]
- 110.Fernandes V.C., Freitas M., Pacheco J.P.G., Oliveira J.M., Domingues V.F., Delerue-Matos C. Magnetic dispersive micro solid-phase extraction and gas chromatography determination of organophosphorus pesticides in strawberries. J. Chromatogr. A. 2018;1566 doi: 10.1016/j.chroma.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 111.Narenderan S.T., Meyyanathan S.N., Karri V.V.S.R., Babu B., Chintamaneni P. Multivariate response surface methodology assisted modified QuEChERS extraction method for the evaluation of organophosphate pesticides in fruits and vegetables cultivated in Nilgiris, South India. Food Chem. 2019;300 doi: 10.1016/j.foodchem.2019.125188. [DOI] [PubMed] [Google Scholar]
- 112.Abi-Ghanem D.A., Berghman Luc R. Immunoaffinity chromatography: A review. In: Magdeldin S., editor. Affinity Chromatography. InTech; London, UK: 2012. pp. 91–106. [Google Scholar]
- 113.Zhang K., Banerjee K. A review: Sample preparation and chromatographic technologies for detection of aflatoxins in foods. Toxins. 2020;12:539. doi: 10.3390/toxins12090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xie J., Jiang H., Shen J., Peng T., Wang J., Yao K., Sun S., Shao B., Tang J. Design of multifunctional nanostructure for ultrafast extraction and purification of aflatoxins in foodstuffs. Anal. Chem. 2017;89:10556–10564. doi: 10.1021/acs.analchem.7b02777. [DOI] [PubMed] [Google Scholar]
- 115.Wang S., Niu R., Yang Y., Wang Y. Development and evaluation of a rapid immunomagnetic extraction for effective detection of zearalenone in agricultural products. Food Control. 2020;110:106973. doi: 10.1016/j.foodcont.2019.106973. [DOI] [Google Scholar]
- 116.Ye J., Xuan Z., Zhang B., Wu Y., Li L., Wang S., Xie G., Wang S. Automated analysis of ochratoxin A in cereals and oil by immunoaffinity magnetic beads coupled to UPLC-FLD. Food Control. 2019;104:57–62. doi: 10.1016/j.foodcont.2018.11.006. [DOI] [Google Scholar]
- 117.Guo J.-B., Wei T.-L., He Q.-H., Cheng J.-S., Qiu X.-Z., Liu W.-P., Lan X.-Q., Chen L.-F., Guo M. A magnetic-separation-based homogeneous immunosensor for the detection of deoxynivalenol coupled with a nano-affinity cleaning up for LC-MS/MS confirmation. Food Agric. Immunol. 2021;32:204–220. doi: 10.1080/09540105.2021.1886254. [DOI] [Google Scholar]
- 118.Belay K. Advanced analytical microextraction techniques and there applications: A review. Environ. Pollut. 2016;6:13–20. [Google Scholar]
- 119.Quigley A., Cummins W., Connolly D. Dispersive liquid-liquid microextraction in the analysis of milk and dairy products: A review. J. Chem. 2016;2016:1–12. doi: 10.1155/2016/4040165. [DOI] [Google Scholar]
- 120.Tan Y.H., Chai M.K., Wong L.S. A review on extraction solvents in the dispersive liquid-liquid microextraction. Malays. J. Anal. Sci. 2018;22:166–174. doi: 10.17576/mjas-2018-2202-01. [DOI] [Google Scholar]
- 121.Samsidar A., Siddiquee S., Shaarani S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018;71:188–201. doi: 10.1016/j.tifs.2017.11.011. [DOI] [Google Scholar]
- 122.Yuan X., Liu C., Zhao J., Zhao P., Zhao L. A novel magnetic multi-walled carbon nanotube-based magnetic solid-phase extraction combined with dispersive liquid–liquid microextraction method for the determination of four phenoxy carboxylic acid herbicides in food crops by using ultra-high performanc. Anal. Methods. 2018;10:3263–3272. doi: 10.1039/C8AY00684A. [DOI] [Google Scholar]
- 123.Al-Afy N., Sereshti H., Hijazi A., Nodeh H.R. Determination of three tetracyclines in bovine milk using magnetic solid phase extraction in tandem with dispersive liquid-liquid microextraction coupled with HPLC. J. Chromatogr. B. 2018;1092:480–488. doi: 10.1016/j.jchromb.2018.06.049. [DOI] [PubMed] [Google Scholar]
- 124.Herrero-Latorre C., Barciela-García J., García-Martín S., Peña-Crecente R.M., Otárola-Jiménez J. Magnetic solid-phase extraction using carbon nanotubes as sorbents: A review. Anal. Chim. Acta. 2015;892:10–26. doi: 10.1016/j.aca.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 125.Wan Ibrahim W.A., Nodeh H.R., Aboul-Enein H.Y., Sanagi M.M. Magnetic solid-phase extraction based on modified ferum oxides for enrichment, preconcentration, and isolation of pesticides and selected pollutants. Crit. Rev. Anal. 2015;45:270–287. doi: 10.1080/10408347.2014.938148. [DOI] [PubMed] [Google Scholar]
- 126.Niu M., Li Z., Zhang S., He W., Li J., Lu R., Gao H., Zeng A., Zhou W. Hybridization of Metal-Organic Frameworks with attapulgite for magnetic solid phase extraction and determination of benzoylurea insecticides in environmental water samples. Microchem. J. 2020;159:105392. doi: 10.1016/j.microc.2020.105392. [DOI] [Google Scholar]
- 127.Li X., Xiao W., He G., Zheng W., Yu N., Tan M. Pore size and surface area control of MgO nanostructures using a surfactant-templated hydrothermal process: High adsorption capability to azo dyes. Colloids Surf. A Physicochem. Eng. Asp. 2012;408:79–86. doi: 10.1016/j.colsurfa.2012.05.034. [DOI] [Google Scholar]
- 128.Zhu G.T., Li X.S., Gao Q., Zhao N.W., Yuan B.F., Feng Y.Q. Pseudomorphic synthesis of monodisperse magnetic mesoporous silica microspheres for selective enrichment of endogenous peptides. J. Chromatogr. A. 2012;1224:11–18. doi: 10.1016/j.chroma.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 129.Hashemi M., Taherimaslak Z., Rashidi S. Application of magnetic solid phase extraction for separation and determination of aflatoxins B1 and B2 in cereal products by high performance liquid chromatography-fluorescence detection. J. Chromatogr. B. 2014;960:200–208. doi: 10.1016/j.jchromb.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 130.García-Nicolás M., Arroyo-Manzanares N., Campillo N., Viñas P. Cellulose-ferrite nanocomposite for monitoring enniatins and beauvericins in paprika by liquid chromatography and high-resolution mass spectrometry. Talanta. 2021;226:122144. doi: 10.1016/j.talanta.2021.122144. [DOI] [PubMed] [Google Scholar]
- 131.Kaw H.Y., Jin X., Liu Y., Cai L., Zhao X., Wang J., Zhou J.L., He M., Li D. Gas-liquid microextraction coupled with magnetic-assisted dispersive solid-phase extraction clean-up for multi-residue pesticide analysis in fatty foods of animal origin. LWT. 2021;137:110448. doi: 10.1016/j.lwt.2020.110448. [DOI] [Google Scholar]
- 132.Gbashi S., Njobeh P.B., De Saeger S., De Boevre M., Madala N.E. Development, chemometric-assisted optimization and in-house validation of a modified pressurized hot water extraction methodology for multi-mycotoxins in maize. Food Chem. 2020;307:125526. doi: 10.1016/j.foodchem.2019.125526. [DOI] [PubMed] [Google Scholar]
- 133.Commission Regulation (EC) No. 401/2006 of Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. European Commission; Brussels, Belgium: 2006. [Google Scholar]
- 134.Cieślik E., Sadowska-Rociek A., Ruiz J.M.M., Surma-Zadora M. Evaluation of QuEChERS method for the determination of organochlorine pesticide residues in selected groups of fruits. Food Chem. 2011;125:773–778. doi: 10.1016/j.foodchem.2010.09.019. [DOI] [Google Scholar]
- 135.European Commission . Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. European Commission; Brussels, Belgium: 2019. pp. 1–52. [Google Scholar]
- 136.Pano-Farias N.S., Ceballos-Magaña S.G., Muñiz-Valencia R., Jurado J.M., Alcázar Á., Aguayo-Villarreal I.A. Direct immersion single drop micro-extraction method for multi-class pesticides analysis in mango using GC–MS. Food Chem. 2017;237:30–38. doi: 10.1016/j.foodchem.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 137.Moudgil P., Bedi J.S., Aulakh R.S., Gill J.P.S., Kumar A. Validation of HPLC multi-residue method for determination of fluoroquinolones, tetracycline, sulphonamides and chloramphenicol residues in bovine milk. Food Anal. Methods. 2019;12:338–346. doi: 10.1007/s12161-018-1365-0. [DOI] [Google Scholar]
- 138.Sang Z.Y., Wang Y.T., Tsoi Y.K., Leung K.S.Y. CODEX-compliant eleven organophosphorus pesticides screening in multiple commodities using headspace-solid phase microextraction-gas chromatography–mass spectrometry. Food Chem. 2013;136:710–717. doi: 10.1016/j.foodchem.2012.08.060. [DOI] [PubMed] [Google Scholar]
- 139.AOAC . A Method for the Determination of Multiple Mycotoxins in Feeds and Raw Grains Intended for Feeds. AOAC; Rockville, MD, USA: 2009. [Google Scholar]
- 140.Orlandi P.A., Flynn W.T., Zink D.L., Norris P.E., Bunning V.K., Mcgrath T., Torrence M.E., editors. FDA Guidelines for the Validation of Chemical Methods for the FDA FVM Program. US Food and Drug Administration, Office of Foods and Veterinary Medicine; Silver Spring, MD, USA: 2015. [(accessed on 13 July 2021)]. Available online: https://www.fda.gov/media/81810/download. [Google Scholar]
- 141.AOAC . Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. AOAC; Rockville, MD, USA: 2002. pp. 1–38. [Google Scholar]
- 142.Krakkó D., Licul-Kucera V., Záray G., Mihucz V.G. Single-run ultra-high performance liquid chromatography for quantitative determination of ultra-traces of ten popular active pharmaceutical ingredients by quadrupole time-of-flight mass spectrometry after offline preconcentration by solid phase extraction. Microchem. J. 2019;148:108–119. doi: 10.1016/j.microc.2019.04.047. [DOI] [Google Scholar]
- 143.Ma J., Wu G., Li S., Tan W., Wang X., Li J., Chen L. Magnetic solid-phase extraction of heterocyclic pesticides in environmental water samples using metal-organic frameworks coupled to high performance liquid chromatography determination. J. Chromatogr. A. 2018;1553:57–66. doi: 10.1016/j.chroma.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 144.Yan S., Qi T.T., Chen D.W., Li Z., Li X.J., Pan S.Y. Magnetic solid phase extraction based on magnetite/reduced graphene oxide nanoparticles for determination of trace isocarbophos residues in different matrices. J. Chromatogr. A. 2014;1347:30–38. doi: 10.1016/j.chroma.2014.04.073. [DOI] [PubMed] [Google Scholar]
- 145.Jiang H., Cao H., Zhang Y., Fast D.M. Systematic evaluation of supported liquid extraction in reducing matrix effect and improving extraction efficiency in LC–MS/MS based bioanalysis for 10 model pharmaceutical compounds. J. Chromatogr. B. 2012;891:71–80. doi: 10.1016/j.jchromb.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 146.Yavuz E., Tokalıoğlu Ş., Patat Ş. Core–shell Fe3O4 polydopamine nanoparticles as sorbent for magnetic dispersive solid-phase extraction of copper from food samples. Food Chem. 2018;263:232–239. doi: 10.1016/j.foodchem.2018.04.134. [DOI] [PubMed] [Google Scholar]
- 147.Peng G., Lu Y., You W., Yin Z., Li Y., Gao Y. Analysis of five bisphenol compounds in sewage sludge by dispersive solid-phase extraction with magnetic montmorillonite. Microchem. J. 2020;157:105040. doi: 10.1016/j.microc.2020.105040. [DOI] [Google Scholar]
- 148.Raposo F., Ibelli-Bianco C. Performance parameters for analytical method validation: Controversies and discrepancies among numerous guidelines. Trac Trends Anal. Chem. 2020;129:115913. doi: 10.1016/j.trac.2020.115913. [DOI] [Google Scholar]
- 149.Kruve A., Rebane R., Kipper K., Oldekop M.-L., Evard H., Herodes K., Ravio P., Leito I. Tutorial review on validation of liquid chromatography–mass spectrometry methods: Part I. Anal. Chim. Acta. 2015;870:29–44. doi: 10.1016/j.aca.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 150.Evard H., Kruve A., Leito I. Tutorial on estimating the limit of detection using LC-MS analysis, part I: Theoretical review. Anal. Chim. Acta. 2016;942:23–39. doi: 10.1016/j.aca.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 151.Zhao T., Zhang M., Ma L., Ma L., Shi H., Kang W., Xu X. Cyanuric chloride-imidazole dendrimer functionalized nanoparticles as an adsorbent for magnetic solid phase extraction of quaternary ammonium compounds from fruit and vegetable puree based infant foods. J. Chromatogr. A. 2021;1636:461735. doi: 10.1016/j.chroma.2020.461735. [DOI] [PubMed] [Google Scholar]
- 152.Li N., Qiu J., Qian Y. Amphiphilic block copolymer–grafted magnetic multi-walled carbon nanotubes as QuEChERS adsorbent for simultaneous determination of mycotoxins and pesticides in grains via liquid chromatography tandem mass spectrometry. Microchim. Acta. 2020;187:648. doi: 10.1007/s00604-020-04632-w. [DOI] [PubMed] [Google Scholar]
- 153.Hu M., Ge W., Liu X., Zhu Y. Preconcentration and determination of zearalenone in corn oil by a one-step prepared polydopamine-based magnetic molecularly imprinted polymer (MIP) with high-performance liquid chromatography with fluorescence (HPLC-FLD) detection. Anal. Lett. 2021;2021:1–12. doi: 10.1080/00032719.2021.1931268. [DOI] [Google Scholar]
- 154.Fu L., Zhou H., Miao E., Lu S., Jing S., Hu Y., Wei L., Zhan J., Wu M. Functionalization of amino terminated carbon nanotubes with isocyanates for magnetic solid phase extraction of sulfonamides from milk and their subsequent determination by liquid chromatography-high resolution mass spectrometry. Food Chem. 2019;289:701–707. doi: 10.1016/j.foodchem.2019.03.097. [DOI] [PubMed] [Google Scholar]
- 155.Jiang Y.-Y., Zhao X., Chen L.-J., Yang C., Yin X.-B., Yan X.-P. A dual-colored persistent luminescence nanosensor for simultaneous and autofluorescence-free determination of aflatoxin B1 and zearalenone. Talanta. 2021;232:122395. doi: 10.1016/j.talanta.2021.122395. [DOI] [PubMed] [Google Scholar]
- 156.Ma S., Pan L.G., You T., Wang K. g-C3N4/Fe3O4 nanocomposites as adsorbents analyzed by UPLC-MS/MS for highly sensitive simultaneous determination of 27 mycotoxins in maize: Aiming at increasing purification efficiency and reducing time. J. Agric. Food Chem. 2021;69 doi: 10.1021/acs.jafc.1c00141. [DOI] [PubMed] [Google Scholar]
- 157.Changsan T., Wannapob R., Kaewpet M., Shearman K., Wattanasin P., Cheung Mak W., Kanatharana P., Thavarungkul P., Thammakhet-Buranachai C. Magnetic microsphere sorbent on CaCO3 templates: Simple synthesis and efficient extraction of trace carbamate pesticides in fresh produce. Food Chem. 2021;342 doi: 10.1016/j.foodchem.2020.128336. [DOI] [PubMed] [Google Scholar]
- 158.Melekhin A.O., Tolmacheva V.V., Shubina E.G., Dmitrienko S.G., Apyari V.V., Grudev A.I. Determination of nitrofuran metabolites in honey using a new derivatization reagent, magnetic solid-phase extraction and LC–MS/MS. Talanta. 2021;230:122310. doi: 10.1016/j.talanta.2021.122310. [DOI] [PubMed] [Google Scholar]
- 159.Han Q., Sun Y., Ding K., Chen X., Han T. Preparation of multitarget immunomagnetic beads based on metal–organic frameworks and their application in food samples. J. Chromatogr. B. 2020;1158:122341. doi: 10.1016/j.jchromb.2020.122341. [DOI] [PubMed] [Google Scholar]
- 160.Zhao Y., Yuan Y.-C., Bai X.-L., Liu Y.-M., Wu G.-F., Yang F.-S., Liao X. Multi-mycotoxins analysis in liquid milk by UHPLC-Q-Exactive HRMS after magnetic solid-phase extraction based on PEGylated multi-walled carbon nanotubes. Food Chem. 2020;305:125429. doi: 10.1016/j.foodchem.2019.125429. [DOI] [PubMed] [Google Scholar]
- 161.Turan N.B., TuğbaZaman B., Bakırdere S. Application of oleic acid functionalized magnetic nanoparticles for a highly sensitive and efficient dispersive magnetic solid phase extraction of fenazaquin in almond samples for determination by gas chromatrography mass spectrometry. Microchem. J. 2020;153:104329. doi: 10.1016/j.microc.2019.104329. [DOI] [Google Scholar]
- 162.Wang S., Niu R., Yang Y., Zhou X., Luo S., Zhang C., Wang Y. Aptamer-functionalized chitosan magnetic nanoparticles as a novel adsorbent for selective extraction of ochratoxin A. Int. J. Biol. Macromol. 2020;153:583–590. doi: 10.1016/j.ijbiomac.2020.03.035. [DOI] [PubMed] [Google Scholar]
- 163.Asadi M., Sereshti H., Rashidi Nodeh H. Development of magnetic dispersive microsolid-phase extraction using lanthanum phosphate nanoparticles doped on magnetic graphene oxide as a highly selective adsorbent for pesticide residues analysis in water and fruit samples. Res. Chem. Intermed. 2020;46:2789–2803. doi: 10.1007/s11164-020-04121-y. [DOI] [Google Scholar]
- 164.Guan S., Wu H., Yang L., Wang Z., Wu J. Use of a magnetic covalent organic framework material with a large specific surface area as an effective adsorbent for the extraction and determination of six fluoroquinolone antibiotics by HPLC in milk sample. J. Sep. Sci. 2020;43:3375–3784. doi: 10.1002/jssc.202000616. [DOI] [PubMed] [Google Scholar]
- 165.Asadi M., Sereshti H. Magnetic amino-functionalized hollow silica-titania microsphere as an efficient sorbent for extraction of pesticides in green and roasted coffee beans. J. Sep. Sci. 2020;43:2115–2124. doi: 10.1002/jssc.201901135. [DOI] [PubMed] [Google Scholar]
- 166.Kermani M., Jafari M.T., Saraji M. Porous magnetized carbon sheet nanocomposites for dispersive solid-phase microextraction of organophosphorus pesticides prior to analysis by gas chromatography-ion mobility spectrometry. Microchim. Acta. 2019;186:88. doi: 10.1007/s00604-018-3215-6. [DOI] [PubMed] [Google Scholar]
- 167.Tu C., Guo Y., Dai Y., Wei W., Wang W., Wu L., Wang A. Determination of chloramphenicol in honey and milk by HPLC coupled with Aptamer-functionalized Fe3O4/Graphene oxide magnetic solid-phase xtraction. J. Food Sci. 2019;84:3624–3633. doi: 10.1111/1750-3841.14955. [DOI] [PubMed] [Google Scholar]
- 168.Liu Y., Li W., Ding Z., Li Q., Wang X., Liu J., Zhuo S., Shao R., Ling Q., Zheng T., et al. Three-dimensional ordered macroporous magnetic photonic crystal microspheres for enrichment and detection of mycotoxins (II): The application in liquid chromatography with fluorescence detector for mycotoxins. J. Chromatogr. A. 2019;1604:460475. doi: 10.1016/j.chroma.2019.460475. [DOI] [PubMed] [Google Scholar]
- 169.Ma S., Wang M., You T., Wang K. Using magnetic multiwalled carbon nanotubes as modified QuEChERS adsorbent for simultaneous determination of multiple mycotoxins in grains by UPLC-MS/MS. J. Agric. Food Chem. 2019;67:8035–8044. doi: 10.1021/acs.jafc.9b00090. [DOI] [PubMed] [Google Scholar]
- 170.Jiang Y., Piao H., Qin Z., Li X., Ma P., Sun Y., Wang X., Song D. One-step synthesized magnetic MIL-101(Cr) for effective extraction of triazine herbicides from rice prior to determination by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2019;42:2900–2908. doi: 10.1002/jssc.201900345. [DOI] [PubMed] [Google Scholar]
- 171.Man Y., Jin X., Fu H., Pan L. A magnetic nanoparticle based immunoassay for alternariol monomethyl ether using hydrogen peroxide-mediated fluorescence quenching of CdTe quantum dots. Microchim. Acta. 2019;186:221. doi: 10.1007/s00604-019-3334-8. [DOI] [PubMed] [Google Scholar]
- 172.Liu G., Huang X., Lu M., Li L., Li T., Xu D. Facile synthesis of magnetic zinc metal-organic framework for extraction of nitrogen-containing heterocyclic fungicides from lettuce vegetable samples. J. Sep. Sci. 2019;42:1451–1458. doi: 10.1002/jssc.201801169. [DOI] [PubMed] [Google Scholar]
- 173.Shahrebabak S.M., Saber-Tehrani M., Faraji M., Shabanian M., Aberoomand-Azar P. Simultaneous magnetic solid phase extraction of acidic and basic pesticides using triazine-based polymeric network modified magnetic nanoparticles/graphene oxide nanocomposite in water and food samples. Microchem. J. 2019;146:630–639. doi: 10.1016/j.microc.2019.01.047. [DOI] [Google Scholar]
- 174.Li W.K., Zhang H.X., Shi Y.P. Simultaneous determination of aflatoxin B1 and zearalenone by magnetic nanoparticle filled amino-modified multi-walled carbon nanotubes. Anal. Methods. 2018;10:3353–3363. doi: 10.1039/C8AY00815A. [DOI] [Google Scholar]
- 175.Markus A., Gbadamosi A.O., Yusuff A.S., Agi A., Oseh J. Magnetite-sporopollenin/graphene oxide as new preconcentration adsorbent for removal of polar organophosphorus pesticides in vegetables. Environ. Sci. Pollut. Res. 2018;25:35130–35142. doi: 10.1007/s11356-018-3402-3. [DOI] [PubMed] [Google Scholar]
- 176.Mahpishanian S., Sereshti H. One-step green synthesis of β-cyclodextrin/iron oxide-reduced graphene oxide nanocomposite with high supramolecular recognition capability: Application for vortex-assisted magnetic solid phase extraction of organochlorine pesticides residue from honey sam. J. Chromatogr. A. 2017;1485:32–43. doi: 10.1016/j.chroma.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 177.Wang Y., Wen Y., Ling Y.C. Graphene oxide-based magnetic solid phase extraction combined with high performance liquid chromatography for determination of patulin in apple juice. Food Anal. Methods. 2017;10:210–218. doi: 10.1007/s12161-016-0570-y. [DOI] [Google Scholar]
- 178.Han Z., Jiang K., Fan Z., Di Mavungu J.D., Dong M., Guo W., Fan K., Campbell K., Zhao Z., Wu Y. Multi-walled carbon nanotubes-based magnetic solid-phase extraction for the determination of zearalenone and its derivatives in maize by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Control. 2017;79:177–184. doi: 10.1016/j.foodcont.2017.03.044. [DOI] [Google Scholar]
- 179.Nodeh H.R., Sereshti H., Gaikani H., Kamboh M.A., Afsharsaveh Z. Magnetic graphene coated inorganic-organic hybrid nanocomposite for enhanced preconcentration of selected pesticides in tomato and grape. J. Chromatogr. A. 2017;1509:26–34. doi: 10.1016/j.chroma.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 180.Liu Z., Qi P., Wang X., Wang Z., Xu X., Chen W., Wu L., Zhang H., Wang Q., Wang X. Multi-pesticides residue analysis of grains using modified magnetic nanoparticle adsorbent for facile and efficient cleanup. Food Chem. 2017;230:423–431. doi: 10.1016/j.foodchem.2017.03.082. [DOI] [PubMed] [Google Scholar]
- 181.González-Sálamo J., Socas-Rodríguez B., Hernández-Borges J., Rodríguez-Delgado M.Á. Core-shell poly (dopamine) magnetic nanoparticles for the extraction of estrogenic mycotoxins from milk and yogurt prior to LC–MS analysis. Food Chem. 2017;215:362–368. doi: 10.1016/j.foodchem.2016.07.154. [DOI] [PubMed] [Google Scholar]
- 182.Yakout A.A., El-Hady D.A. A combination of β-cyclodextrin functionalized magnetic graphene oxide nanoparticles with β-cyclodextrin-based sensor for highly sensitive and selective voltammetric determination of tetracycline and doxycycline in milk samples. RSC Adv. 2016;6:41675–41686. doi: 10.1039/C6RA03787A. [DOI] [Google Scholar]
- 183.Nodeh H.R., Ibrahim W.A.W., Sanagi M.M., Aboul-Enein H.Y. Magnetic graphene-based cyanopropyltriethoxysilane as an adsorbent for simultaneous determination of polar and non-polar organophosphorus pesticides in cow’s milk. RSC Adv. 2016;6:24853–24864. doi: 10.1039/C5RA26742K. [DOI] [Google Scholar]
- 184.Tan L., He R., Chen K., Peng R., Huang C., Yang R., Tang Y. Ultra-high performance liquid chromatography combined with mass spectrometry for determination of aflatoxins using dummy molecularly imprinted polymers deposited on silica-coated magnetic nanoparticles. Microchim. Acta. 2016;183:1469–1477. doi: 10.1007/s00604-016-1790-y. [DOI] [Google Scholar]
- 185.Miao Q., Wang J., Nie J., Wu H., Liu Y., Li Z., Qian M. Magnetic dispersive solid-phase extraction based on a novel adsorbent for the detection of triazole pesticide residues in honey by HPLC-MS/MS. Anal. Methods. 2016;8:5296–5303. doi: 10.1039/C6AY00376A. [DOI] [Google Scholar]
- 186.Zhou Q., Lei M., Li J., Zhao K., Liu Y. Sensitive determination of bisphenol A, 4-nonylphenol and 4-octylphenol by magnetic solid phase extraction with Fe@ MgAl-LDH magnetic nanoparticles from environmental water samples. Sep. Purif. Technol. 2017;182:78–86. doi: 10.1016/j.seppur.2017.01.071. [DOI] [Google Scholar]
- 187.Zhou Q., Lei M., Wu Y., Zhou X., Wang H., Sun Y., Sheng X., Tong Y. Magnetic solid phase extraction of bisphenol A, phenol and hydroquinone from water samples by magnetic and thermo dual-responsive core-shell nanomaterial. Chemosphere. 2020;238:124621. doi: 10.1016/j.chemosphere.2019.124621. [DOI] [PubMed] [Google Scholar]
- 188.Rezvani M., Asgharinezhad A.A., Ebrahimzadeh H., Shekari N. A polyaniline-magnetite nanocomposite as an anion exchange sorbent for solid-phase extraction of chromium (VI) ions. Microchim. Acta. 2014;181:1887–1895. doi: 10.1007/s00604-014-1262-1. [DOI] [Google Scholar]
- 189.Wanjeri V.W.O., Sheppard C.J., Prinsloo A.R.E., Ngila J.C., Ndungu P.G. Isotherm and kinetic investigations on the adsorption of organophosphorus pesticides on graphene oxide based silica coated magnetic nanoparticles functionalized with 2-phenylethylamine. J. Environ. Chem. Eng. 2018;6:1333–1346. doi: 10.1016/j.jece.2018.01.064. [DOI] [Google Scholar]
- 190.Wanjeri V.W.O., Gbashi S., Ngila J.C., Njobeh P., Mamo M.A., Ndungu P.G. Chemical vapour deposition of MWCNT on silica coated Fe3O4 and use of response surface methodology for optimizing the extraction of organophosphorus pesticides from water. Int. J. Anal. Chem. 2019;2019:4564709. doi: 10.1155/2019/4564709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang Y., Liu L., Xiao C., Chen L., Yang P., Liu Q., Wang J., Liu X. Rapid determination of trace sulfonamides in milk by graphene oxide-based magnetic solid phase extraction coupled with HPLC–MS/MS. Food Anal. Methods. 2016;9:2521–2530. doi: 10.1007/s12161-016-0433-6. [DOI] [Google Scholar]
- 192.Cao M., Li Z., Wang J., Ge W., Yue T., Li R., Colvin V.L., Yu W.W. Food related applications of magnetion iron oxide nanoparticles: Enzyme immobilization, protein purification, and food analysis. Trends Food Sci. Technol. 2012;27:47–56. doi: 10.1016/j.tifs.2012.04.003. [DOI] [Google Scholar]
- 193.Ballin N.Z., Laursen K.H. To target or not to target? Definitions and nomenclature for targeted versus non-targeted analytical food authentication. Trends Food Sci. Technol. 2019;86:537–543. doi: 10.1016/j.tifs.2018.09.025. [DOI] [Google Scholar]
- 194.Milman B.L., Zhurkovich I.K. The chemical space for non-target analysis. Trac Trends Anal. Chem. 2017;97:179–187. doi: 10.1016/j.trac.2017.09.013. [DOI] [Google Scholar]
- 195.Jin Y., Qi Y., Tang C., Shao B. Hierarchical micro-and mesoporous metal–organic framework-based magnetic nanospheres for the nontargeted analysis of chemical hazards in vegetables. J. Mater. Chem. A. 2021;9:9056–9065. doi: 10.1039/D1TA00120E. [DOI] [Google Scholar]
- 196.Rickert D.A., Singh V., Thirukumaran M., Grandy J.J., Belinato J.R., Lashgari M., Pawliszyn J. Comprehensive analysis of multiresidue pesticides from process water obtained from wastewater treatment facilities using solid-phase microextraction. Environ. Sci. Technol. 2020;54:15789–15799. doi: 10.1021/acs.est.0c04875. [DOI] [PubMed] [Google Scholar]
- 197.Li W., Jiang H.-X., Geng Y., Wang X.-H., Gao R.-Z., Tang A.-N., Kong D.-M. Facile removal of phytochromes and efficient recovery of pesticides using heteropore covalent organic framework-based magnetic Nanospheres and electrospun filmn. ACS Appl. Mater. Interfaces. 2020;12:20922–20932. doi: 10.1021/acsami.0c01608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.