Abstract
Salivary gland regeneration is important for developing treatments for radiation-induced xerostomia, Sjögren’s syndrome, and other conditions that cause dry mouth. Culture conditions adopted from tissue engineering strategies have been used to recapitulate gland structure and function to study and regenerate the salivary glands. The purpose of this review is to highlight current trends in the field, with an emphasis on soluble factors that have been shown to improve secretory function in vitro. A PubMed search was conducted to identify articles published in the last 10 years and articles were evaluated to identify the most promising approaches and areas for further research. Results showed increasing use of extracellular matrix mimetics, such as Matrigel®, collagen, and a variety of functionalized polymers. Soluble factors that provide supportive cues, including fibroblast growth factors (FGFs) and neurotrophic factors, as well as chemical inhibitors of Rho-associated kinase (ROCK), epidermal growth factor receptor (EGFR), and transforming growth factor β receptor (TGFβR) have shown increases in important markers including aquaporin 5 (Aqp5); muscle, intestine, and stomach expression 1 (Mist1); and keratin (K5). However, recapitulation of tissue function at in vivo levels is still elusive. A focus on identification of soluble factors, cells, and/or matrix cues tested in combination may further increase the maintenance of salivary gland secretory function in vitro. These approaches may also be amenable for translation in vivo to support successful regeneration of dysfunctional glands.
Keywords: salivary gland, tissue engineering, cell culture, soluble cues, media optimization
1. Introduction
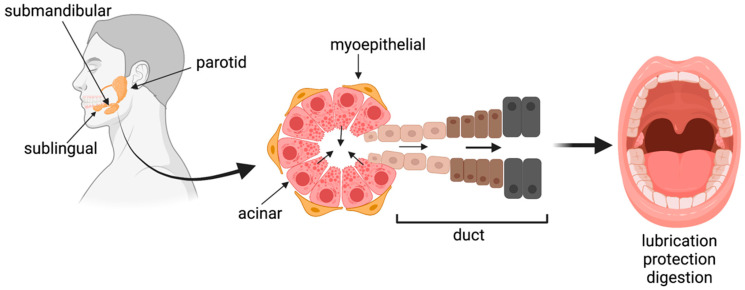
Salivary glands are organs that produce saliva, an aqueous fluid that contains enzymes, electrolytes, mucins, and other components that aid in lubricating the mouth, preliminary digestion, and antimicrobial and buffering activity to prevent oral infections [1]. There are three major pairs of salivary glands—the parotid, submandibular and sublingual—as well as numerous minor salivary glands located throughout the mouth [2]. The general gland structure consists of clusters of acinar cells, which secrete the proteins/fluid, and ductal cells, which modify the composition of the saliva and transport it into the oral cavity (Figure 1). There are also myoepithelial cells that surround the acinar units and other supportive tissues, including blood vessels, providing an exchange of nutrients and waste, and nerves, which play a key role in stimulating the glands to secrete [1]. More extensive reviews of salivary gland anatomy, physiology, and development can be found elsewhere [1,3,4,5].
Figure 1.
Schematic of the three major types of salivary glands, general gland structure, and major functions of saliva. Created with BioRender.com.
The normal function of the salivary glands can be diminished by several conditions, such as off-target damage from radiation treatment for head or neck cancer, the autoimmune disease, Sjögren’s syndrome, some systemic conditions including diabetes and neurological diseases, and a vast array of medications, including anti-hypertensives, β-blockers, antidepressants, and many others [2,6,7]. The mechanisms underlying salivary gland dysfunction are not well understood and may vary greatly depending on the cause [6]. Therefore, existing treatments, including lubricating mouthwashes, gels, chewing gum, and sprays only ameliorate symptoms and provide temporary relief. Sialogogues that stimulate parasympathetic pathways, such as pilocarpine and cevimeline, have also been used, but success requires the patient to have some residual salivary function and they can cause systemic side effects [6]. Specific to radiation-induced salivary gland dysfunction, prevention is feasible by treating with amifostine, a reactive oxygen species scavenger, and/or utilizing intensity-modulated radiation therapy (IMRT), which is a targeted approach to avoid direct radiation to the parotid glands, but these methods come with drawbacks as well [6,8]. For example, amifostine can cause severe adverse side effects, including nausea, vomiting, allergic reactions, and hypotension, resulting in the need to discontinue treatment in up to 40% of cases [9]. Additionally, while IMRT has shown a benefit in preserving the saliva flow rate, patients may still suffer symptoms of xerostomia, possibly due to changes in saliva composition [10] and further, IMRT may not be possible based on tumor location [11]. Ultimately, incomplete understanding of the mechanism leading to salivary gland dysfunction has hindered the development of alternative treatment strategies and radioprotective drugs.
The use of in vitro culture systems would be useful to gain more knowledge on the mechanisms leading to salivary gland dysfunction as well as enable the development of new therapies, such as cell transplantation, the discovery of new radioprotective drugs, and methods to regenerate the damaged gland. However, culture of salivary glands has historically been difficult due to the rapid loss of secretory phenotype and function in vitro. For example, the characteristics of secretory acinar cells, such as mucin biosynthesis [12] and amylase production [13], decrease significantly after 1 day in culture. Expression of Mist1 (bHLH15a), a transcription factor necessary to specify the acinar cell phenotype [14], drops significantly as well [15,16]. Thus, tissue engineering approaches have been leveraged to address the requirement of functional tissue to enable drug discovery and screening, regeneration, and fundamental studies. In this review, we will discuss trends in salivary gland tissue engineering over the past decade with advantages/disadvantages of different culture methods. We identify areas of research that provide opportunities to improve in vitro culture of functional salivary gland tissue.
2. Materials and Methods
A literature review was conducted by searching for “salivary gland culture in vitro” in the PubMed database on 4 March 2021. The results were restricted to the past 10 years, giving a total of 320 articles. The results were screened to remove review articles and articles that did not contain in vitro culture, used nonmammalian cells, cultured only unhealthy tissue (ex. samples from a Sjögren’s syndrome or salivary gland cancer patient), or used non-salivary gland cell sources (ex. adipose, bone marrow, dental pulp) to differentiate into salivary gland-like structures. While these approaches are beneficial in expanding knowledge on the salivary gland, they likely demand vastly different culture conditions.
From the remaining 170 articles, various parameters were identified and categorized, as listed in Table 1. Categories included cell source, cell maturity, cell species, and gland type. Cell source was divided into Primary cells, Cell line, or Both while cell maturity indicated whether the study used Adult, Embryonic, or Both types of tissue; cell lines were considered under the Adult category. Cell species was split into Human, Mouse, Rat, Other, and Multiple; Other consisted of rarely used species (porcine, rabbit) and Multiple contained studies that used more than one cell species. Gland type was classified as Submandibular, Parotid, Minor (i.e., labial or other minor salivary glands), Other, or Multiple. Other consisted of sublingual or unspecified gland types while Multiple referred to studies that used more than one gland type.
Table 1.
Categorization of information extracted from selected articles.
| Cell Source | Cell Maturity | Cell Species | Gland Type | Media Type | Substrate |
|---|---|---|---|---|---|
| Primary Cell line Both |
Adult Embryonic Both |
Human Mouse Rat Other Multiple |
Submandibular Parotid Minor Other Multiple |
Growth Factor Serum Commercial |
Matrix mimetic Plastic Transfer Specialized Multiple Membrane Unreported |
Media type was divided into three groups based on the use of Growth Factors, such as epidermal growth factor (EGF) or fibroblast growth factor (FGF), the use of Serum including fetal bovine serum (FBS) and fetal calf serum (FCS), or Commercial media that contains proprietary or specialized additives optimized for a specific cell type. Overlap between these categories was considered. Individual media components were also extracted and listed separately.
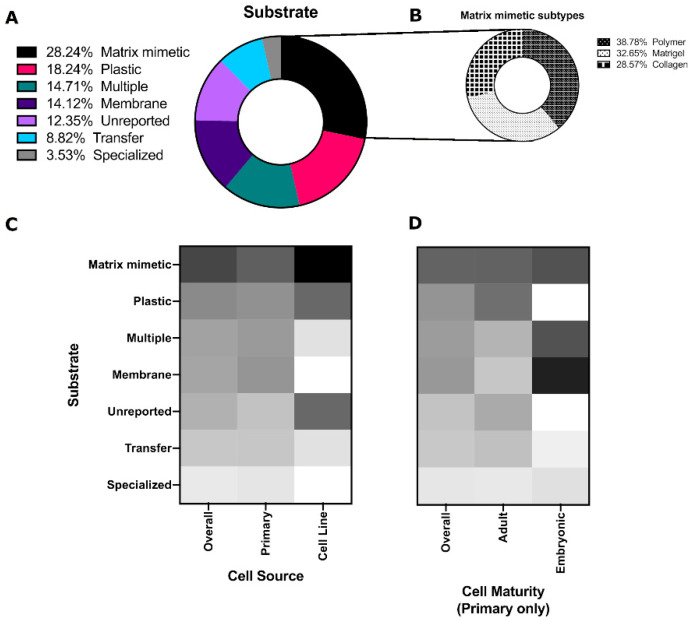
Substrates were categorized into six groups: Matrix mimetic, Plastic, Transfer, Specialized, Multiple, Membrane, and Unreported. Matrix mimetic refers to substrates that are intended to mimic the function of the extracellular matrix (ECM) and was further divided into collagen, Matrigel®, and polymer sub-categories. Plastic indicates any kind of tissue culture polystyrene substrate, such as tissue culture plates, culture flasks, and Petri dishes. No distinction was made between tissue culture-treated and suspension plates, as this was often not reported in the literature. Transfer refers to cells that were cultured on one substrate and then transferred to another (ex. plastic for 7 days, then transferred to Matrigel®). Specialized includes commercially available platforms, such as hanging drop and GravityTRAP™ plates for 3D cell culture. The Membrane category contains substrates such as Transwells® and Nuclepore™ polycarbonate filters. Multiple was used to classify articles that used multiple substrates or combined substrates from the other defined categories. Unreported was used to describe articles that did not clearly state the substrate used.
Venn diagrams were created in JMP Pro 15.0.0 (SAS, Cary, NC, USA) using a Venn Diagram add-in. All other graphs were made in Prism (GraphPad, San Diego, CA, USA) version 8.4.3. Figure 1 and Figure 2 were created with BioRender.com (Science Suite, Inc., Toronto, ON, Canada).
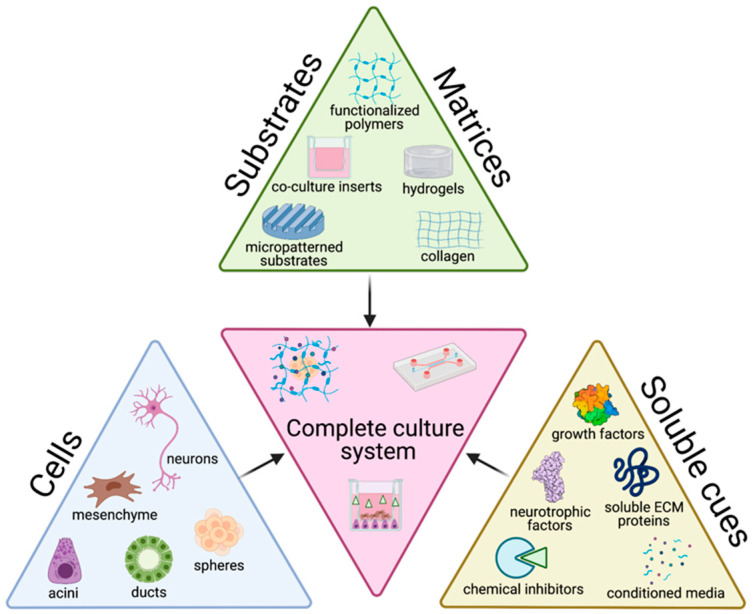
Figure 2.
Schematic of the tissue engineering triad as it relates to salivary gland cell culture strategies with examples of a complete culture system.
3. Trends in In Vitro Salivary Gland Tissue Culture
The tissue engineering paradigm is typically comprised of three main components: cells, matrix, and soluble cues. Each of these components alone, as well as the interconnectedness between these three branches have important implications on the success of culture methods. To accurately recapitulate the structure and function of native tissue, the choice of cell source and the biophysicochemical cues provided to cells are key factors in determining the success of the culture with broader impacts on experimental significance. Here, we review the cells, matrices/substrates, and soluble factors and highlight some of the combinatory approaches that have utilized multiple categories, summarized in Figure 2. We also discuss the most beneficial approaches and identify opportunities for further research.
3.1. Cells
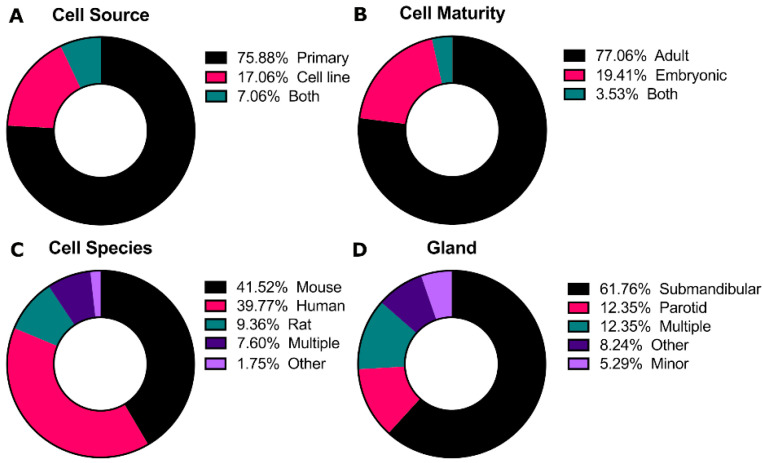
Cells are the central aspect of the tissue engineering paradigm. For the salivary glands, cell lines do not accurately represent all characteristics of normal salivary gland tissue [17] and many of these cell lines are contaminated with HeLa cells, including the most commonly use HSG cell line [18,19]. Additionally, pluripotent stem cell technology for the salivary gland is in its infancy [20]. Thus, the majority of studies evaluated use primary cells for culturing salivary gland cells (76%, Figure 3A). Cells are most commonly sourced from adult tissue (77%, Figure 3B) of either mouse or human origins (42% and 40%, respectively, Figure 3C). The submandibular gland is the most frequently used (62%, Figure 3D), likely due to the relatively large cell yield and superficial location of the submandibular gland in mice [21] and the fact that the submandibular gland is the most well-studied in mice [15,16,22,23,24,25].
Figure 3.
Categorization of the cells used in salivary gland culture in vitro. Distribution of the percentage frequency of the (A) cell source, (B) cell maturity, (C) cell species, and (D) gland type in the articles analyzed in this review.
Primary cultures from adult tissue typically start with dissociation of the entire gland, containing acinar, duct, and myoepithelial cells. While some groups dissociate glands into single cells [23,26], others have highlighted the importance of maintenance of partial tissue structure to retain cell-cell contacts to promote 3D morphology and polarization [27,28,29,30]. For embryonic cultures, tissues are commonly grown as explants on a membrane with an air-liquid interface [31,32,33], although they can also be dissociated similar to adult tissue. In some cases, cells are selected for specific subpopulations using flow cytometry and/or selective enhancement during in vitro culture for putative stem cell markers such as CD24 and CD29, Kit, or K5 [34,35,36,37]. These studies typically aim to develop a cell transplantation strategy for regenerating the glands.
Co-cultures of salivary gland cells with other cell types, such as mesenchyme and nerves, have been investigated to support secretory function. For example, mouse cortical neurons were shown to self-organize around salivary gland cells similar to native tissue [38]. A crucial next step will be to determine if the neurons promote acinar characteristics in this model. Vining et al., 2019 showed that salispheres only undergo branching morphogenesis when combined with mesenchyme and parasympathetic ganglion in the presence of neurturin-containing matrices [23]. In addition, co-cultured endothelial cells were required for proper salivary gland epithelial patterning in embryonic explants [39]. These studies highlight the complexity of salivary gland tissue engineering and the need to consider multiple cell types. Further development in this area will be beneficial for creating a hierarchical tissue structure, as well as continued development of iPSC models.
3.2. Matrices/Substrates
The extracellular matrix (ECM) is a network of proteins, glycosaminoglycans, and proteoglycans that fills the intracellular space and provides structural and adhesive motifs that can influence a wide variety of cell functions such as proliferation, differentiation, and migration [40]. In the salivary gland epithelium, the ECM and basement membrane consist of laminin, collagen I, collagen IV, and fibronectin with binding sites for β1, β4, α5, and α6 integrins, among others [41]. These ECM proteins and their integrins orchestrate events during salivary gland development and homeostasis including branching morphogenesis, cleft formation, apico-basal polarization, adhesion, growth, and migration, and can influence intracellular signaling.
Given their importance in affecting cell behavior, it is not surprising that matrix mimetics were commonly used for salivary gland in vitro studies over the past decade (28%, Figure 4A). Of these, Matrigel®, collagen, and other polymers (mainly natural polymers) were equally as common (Figure 4B). Some of these matrices include: chitosan that increases salisphere size and number without increasing the size of the lumens, which are hollow openings within the spheroid [25]; hyaluronic acid-catechol that increases branching proliferation [42]; Matrigel® that promotes 3D structure, amylase activity, tight junction formation and transepithelial resistance (TER) [43,44]; other laminin-containing matrices that can promote morphogenesis and Aqp5 expression in combination with FGF2 [22]; laminin hydrogels that induced branching morphogenesis and maintained epithelial progenitors [23]; matrix metalloproteinase (MMP)-degradable poly(ethylene glycol) (PEG) hydrogels that were found to promote polarized expression of zona occludins (ZO-1) and sodium potassium chloride channel 1 (Nkcc1) [15]; and human placenta basement membrane extract or fibronectin that were shown by transmission electron microscopy (TEM) to enhance tight junction formation [44].
Figure 4.
Categorization of the substrates used in salivary gland cell culture. Distribution of the percentage frequency of different (A) substrate categories and (B) substrates within the matrix mimetic category. Heat maps comparing the percentage frequency of different substrate categories by (C) cell source and (D) cell maturity for primary cells.
Poly(styrene) substrates (tissue culture plates, Petri dishes, etc.) were the second most common culture platform, which were predominantly used for salivary gland cell lines (Figure 4C). Membranes (Nucleopore filters, Transwells) and multiple substrates (culturing on a Matrigel® for some experiments and plastic for others) were more common for embryonic cells (Figure 4D). These polystyrene and coated substrates are standard, as many embryonic studies use explants and thus retain the structure of the developing gland without the need for extensive substrate engineering efforts.
Other substrates include: micropatterned PDMS-based craters with electrospun poly(lactic co glycolic acid) (PLGA) that were reported to increase the tight junction protein occuldin expression and expression of water channel Aqp5 [45]; poly(glycerol sebacate) (PGS)/PLGA core/shell nanofiber scaffolds that were reported to promote apical localization of tight junction proteins and tissue organization when combined with mesenchymal cells [46]; hanging drop cultures that were reported to produce microtissues mimicking tissue development [47]; and decellularized porcine gut matrix co-cultured with salivary gland cells and microvascular endothelial cells that were reported to promote amylase, claudin-1, and Aqp5 expression compared to 2D [48].
3.3. Soluble Cues
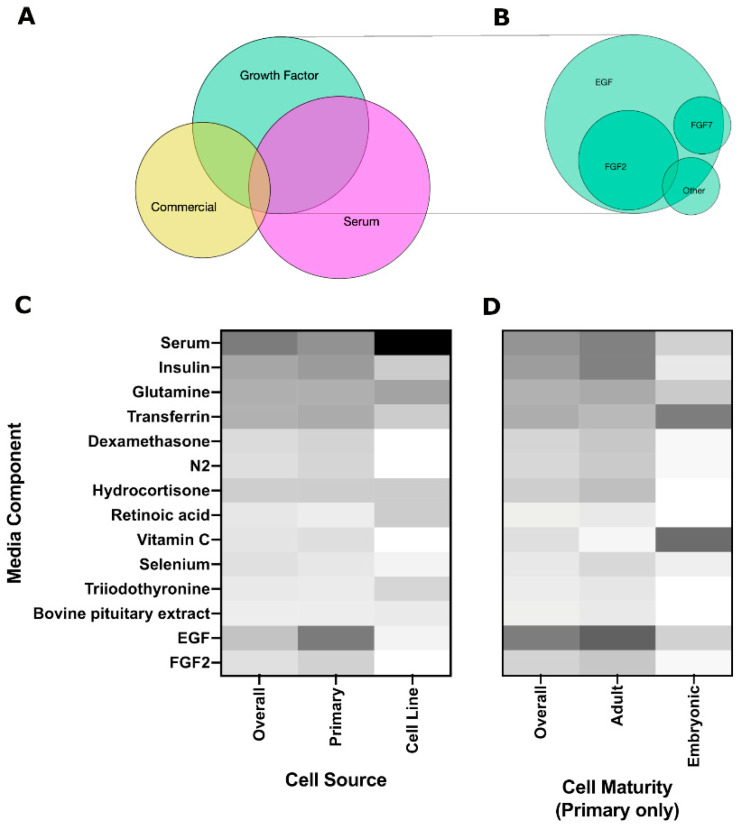
Soluble cues are typically provided to the cells through media supplementation. Salivary gland media commonly consists of Dulbecco’s Modified Eagle Medium (DMEM)/Ham’s F12 Nutrient Mixture with a variety of basic supplements to support cell growth in vitro, such as insulin, glutamine, transferrin, and antibiotics. Both serum-containing and growth factor-based media are common (Figure 5A), with EGF and FGF2 as the most common growth factors (Figure 5B). Use of commercial media, including HepatoSTIM, keratinocyte growth media (KGM), and bronchial epithelial growth medium (BGM), are becoming more common, although no salivary gland-specific media exists to date. There was significant overlap between different categories (ex. serum-containing media that was also supplemented with growth factors) as indicated by the Venn diagram in Figure 5A, suggesting that a variety of soluble cues are used to promote salivary gland cell growth, but specific studies contrasting individual benefits are lacking. Serum-containing media was more heavily used for culturing cell lines (Figure 5C), while Vitamin C (ascorbic acid) and transferrin were the main components of the media used for embryonic cells (Figure 5D), suggesting that cells may require a different optimized medium depending on cell source and cell maturity. A summary of some of the more promising soluble factors are provided in Table 2 and discussed further in the following sections.
Figure 5.
Categorization of the media type and percentage frequency of individual media components. Venn diagrams comparing the prevalence of different (A) media categories and (B) growth factors used in the growth factor-based media category. Heat maps comparing the percentage frequency of different media components by (C) cell source and (D) cell maturity for primary cells.
Table 2.
Soluble factors and matrices for improving cultured cell function.
| Soluble Factor | Cells | Substrate/Matrix | Outcomes | |
|---|---|---|---|---|
| Fibroblast growth factor 10 (FGF10) | Human submandibular | Matrigel® | Increased Mist1, AQP5, α-amylase and α-SMA, decreased K5, promoted budding with no effect on organoid size, increased carbachol-induced calcium release | [49] |
| Epidermal growth factor receptor (EGFR) inhibitor | Mouse E16 submandibular | Nuclepore filter | EGFR inhibitor AG1478 retained epithelial cells and AQP5 | [22] |
| Transforming growth factor β receptor I (TGFβRI) inhibitor | Mouse submandibular | Matrigel® | Treatment with TGFβ and TGFβR-I inhibitor SB525334 increased expression of amylase-1, Aqp5, ZO-1, Occuldin, Fgf7 and Fgf10 but not collagen type I when cultured on Matrigel and increased the size of acinar clusters | [50] |
| Epiregulin | Mouse submandibular | Collagen-coated culture dish | Increased cell proliferation and levels of EGFR ligands epiregulin, HB-EGF, amphiregulin and TGFα | [51] |
| Insulin-like growth factor I (IGF-I) | SMIE cell line (rat submandibular) | Collagen-coated Transwell | Treatment with 100 ng/mL IGF-I maintained cell number, cell viability, tight junction expression and localization and paracellular barrier function | [52] |
| Rho-associated protein kinase (ROCK) inhibitor Y-27632 | Mouse submandibular | Matrigel® | Enhanced growth (cell numbers), survival (Live/Dead), proliferation (EdU), motility (scratch assay), maintained α-amylase expression and induced C-Met expression | [53] |
| Neurotrophin 4 (NT-4) | Human parotid | Cell culture plate | Highest levels of intracellular and secreted α-amylase at 1 ng/mL NT-4 | [54] |
| Neurturin (NRTN) | Mouse or human submandibular with E13 mesenchyme and neuronal cells | Laminin-111 hydrogel | Initiated branching, innervation and self-aggregation of spheres | [23] |
| Neureglin 1 (NRG1) | Human parotid | Cell culture plate | Promoted branching and retention of acinar-like cells in submandibular | [54] |
| Wnt and R-spondin conditioned media | Mouse submandibular | Matrigel® | Increased population doubling and sphere-forming efficiency | [55] |
| Mesenchymal stem cell (MSC) conditioned media | Mouse submandibular | Matrigel® or laminin-1 | Increased acinar-like structure and when combined with laminin-111, increased AQP5 and K14 and decreased in α-SMA compared to fresh submandibular gland | [56] |
| p38 MAPK inhibitor/Src inhibitor | Rat parotid | Collagen-coated dish or cell culture insert | Reduced cell stress | [57] |
AQP5: aquaporin-5; α-SMA: α-smooth muscle actin; K5: keratin 5; K14: keratin 14; Nkcc1: sodium-potassium-chloride channel 1; ZO-1: zonula occludens 1; HB-EGF: heparin-binding EGF-like growth factor; TGF-α: transforming growth factor-α.
3.3.1. Growth Factors
Growth factors are important in salivary gland development, with FGFs contributing to branching morphogenesis and end bud formation [58,59], while EGF contributes to cell proliferation and differentiation along the ductal lineage [60]. EGF was added to the media in nearly all the studies using primary tissue, along with FGF2 in 25% of studies (Figure 5C). Other growth factors that are important to salivary gland development but are less commonly used include FGF7, which is reported to promote end bud formation, and FGF10, which enhances duct elongation [59]. Similarly, Miyajima et al., 2011 reported FGF7-induced bud expansion and FGF10-induced duct elongation, but no effect of EGF was observed on branching morphogenesis [24]. Additionally, FGF10 has been shown to increase Mist1, Aqp5, α-amylase, and α-SMA while decreasing K5 [49]. Insulin-like growth factor-1 (IGF-1) has been shown to support growth and maintenance of the paracellular barrier function (tight junction localization, TER, dextran permeability) at levels similar to supplementation with FBS, indicating that IGF-1 could be used to replace some of the functions of FBS in a serum-free media [52].
3.3.2. Chemical Inhibitors
ROCK Inhibitor
Rho-kinases (ROCKs) mediate important processes such as proliferation, motility, secretion, and cell shape [61]. In the salivary gland specifically, ROCKs play an important role in cleft formation and basement membrane positioning in epithelial tissue polarity [62,63]. In cell culture, ROCK inhibitors have been used to prevent dissociation-induced apoptosis and preserve stem cell populations [64,65] and thus have been used to promote cell survival in vitro in many different tissue types [64,66,67,68]. Additionally, it has also been shown that ROCK activation can lead to the formation of acinar-to-ductal metaplasia under chronic pancreatitis conditions [67], suggesting that ROCK inhibition may prevent undesirable cell plasticity.
ROCK inhibitor Y-27632 has been shown to enhance cell growth, survival, proliferation and amylase and c-Met expression in salivary gland cultures [53] as well as increase sphere forming percent of CD24hi/CD29hi cells [35]. However, the effect of ROCK inhibitor treatment may be dependent on other factors present in the culture media. Koslow et al. 2019 found an increase in sphere number, size, and proliferation and a decrease in cleaved caspase 3 in serum-free media; however, the increase in sphere size was not apparent in serum-containing media [69]. Additionally, it may promote different cell populations—Y-27632 in serum-free media increased Kit+ cells, while K5+ cells were increased in serum-containing media [69], suggesting it may promote different cell phenotypes altogether. Y-27632 has been used in culturing several other tissues (pancreas, prostate, lacrimal gland) to decrease cell stress [66,67,68], which may account for the increased growth in ROCK inhibitor-treated cultures. Conversely, it has also been shown to prevent salisphere formation [25] and cause cells to spread out and adhere to the surface instead of forming aggregates [25,53]. This may be a drawback to using Y-27632, since the increase in adhesion likely leads to a loss of 3-dimensional structure, organization, and, consequently, the secretory function of the salivary gland cells.
EGFR Inhibitors
The epidermal growth factor receptor (EGFR) modulates various processes, including proliferation, differentiation, and survival [70], and is involved in branching morphogenesis during salivary gland development [71]. However, aberrant EGFR signaling can lead to cancer and other diseases [70] and EGFR inhibition has been used as treatment for breast, lung, and colorectal cancer [67,68,69].
Several EGFR inhibitors have been used in salivary gland culture, including AG1478, PD198509, PD168393, and EKI-785. AG1478 retained epithelial cells and AQP5 [22], while PD198509 did not affect K5, K19, Kit, sphere diameter or sphere count [23] and PD168393 inhibited carbachol (CCh)-mediated morphogenesis and proliferation of K5+ and K19+ cells [60]. This suggests that EGFR inhibitors may be beneficial in promoting acinar cells/AQP5 expression, but not keratin-expressing duct cells. This is supported by the role of EGFR signaling in duct morphogenesis and differentiation and proliferation of the duct lineage [72] and by pancreas literature in which EKI-785 was used to prevent the transition of acinar cells into duct-like clusters in vitro [73]. This also highlights a key consideration in the selection of an optimized media—different goals may warrant different soluble cues. For example, researchers interested in isolating K5+ or Kit+ cells may benefit from having ROCK inhibitor in their media, but not EGFR inhibitor.
TGFβR Inhibitors
TGFβ has multiple roles in the salivary gland—it is important during morphogenesis [74], but it is also upregulated following stress, a major driver of fibrosis [75] and overexpression of TGF-β1 can lead to acinar loss [72,76]. Hence, it has been studied in salivary gland culture with different outcomes. The TGFβR inhibitor, RepSox, promoted cell growth, proliferation, expression of keratins 8, 14, and 19 and was selective for p63-expressing cells [77], while treatment with SB525334 increased acinar characteristics [50]. Since both inhibitors target TGFβR1, differences between these outcomes could be due to inhibitor potency, differences in culture conditions (tissue culture plate with commercial CnT-PR media [77] vs. growth factor-reduced Matrigel with DMEM/N2 media [50]), or difference in cell source (embryonic [77] vs. adult [50]).
3.3.3. Neurotrophic Factors
The salivary gland is highly innervated, and salivation is controlled by the autonomic nervous system [78]. Hence, adding neurotrophic factors to the culture media has been widely considered as an alternative to the complexity of co-culturing salivary gland cells with nerve cells. Results show that treatment with different nerve factors has positive effects on salivary gland cultures. Neurotrophin 4 (NT-4) increased levels of amylase [54], neurturin (NRTN) initiated branching and innervation [23], and neureglin 1 (NRG1) promoted branching and retention of acinar-like cells [79]. This is supported by a myriad of publications highlighting the importance of neurotrophic factors such as neurturin and glial cell-derived neurotrophic factor (GDNF) for development of the salivary gland [60,78,80,81,82,83].
3.3.4. Conditioned Media
Conditioned media from a variety of sources has also been shown to improve salivary gland cell culture. Wnt and R-spondin conditioned media increased long-term expansion of salivary gland stem cells in vitro, with increased population doubling and sphere-forming efficiency [55]. Mesenchymal stem cell (MSC)-conditioned media increased acinar-like structures and Aqp5 and K14 when combined with laminin-111 [56]. Fibroblast-conditioned media increased amylase protein levels [84] and amylase expression [54] but was dependent on the substrate the fibroblasts were grown on [54].
3.3.5. Soluble ECM Proteins
In addition to variations in the biomaterials used for the matrix, soluble ECM proteins have also been used to provide signaling cues to the salivary gland cells as media additives. For example, fibronectin induced branching and ductal elongation [85], which was enhanced with FGF7. Salivary gland ECM extract (s-Ecx) promoted a compact sphere structure and increased expression of keratins (K5, K7, K14, K19) and acinar markers such as Aqp5 and Muc-1 [86]. Chitosan has been shown to increase spheroid size and polarization [25], with the greatest effect from soluble chitosan.
4. Opportunities for Future Research
Some of the most promising approaches for salivary gland cell culture involve the combination of the tissue engineering triad—cells, matrices, and soluble cues. While certain substrates have been shown to promote growth and expression of acinar markers, matrices are more relevant to the in vivo environment and versatile, providing structure, signaling through integrins, allowing for entrapment of signaling molecules and modifications with matrix motifs. In particular, laminin-based biomaterials have shown promising results [22,23,35,55,79]; this is supported by the prevalence of laminin in the ECM and basement membrane of the salivary gland in vivo [41].
Despite the promising polarization supported by matrix mimetics, secretory function remains limited. This continued challenge points to the need for incorporating combinatory approaches that optimize the matrix along with the other arms of the tissue engineering paradigm. While a number of groups have tested one or two matrix and/or media conditions, a large scale, combined media, and matrix optimization has not been done. In addition, analysis of how these factors affect a wide variety of markers, both acinar and duct, would be beneficial for more widespread adoption across the field.
Increasing complexity of models by introducing mesenchyme and neurons will enable more representative tissue mimetics for fundamental biology studies. However, simplicity may be desired in other cases for increased convenience, lower costs, and ease of data interpretation. Thus, it would be beneficial to investigate the specific function provided by supportive tissues and whether media supplementation or matrix modification can produce the same results.
5. Conclusions
Traditional salivary gland tissue engineering approaches fail to provide the necessary conditions to promote secretory function. Researchers have addressed this issue by co-culturing with neural or mesenchymal tissue, investigating different biomaterials and supplementing culture media with a variety of soluble cues. A key consideration in determining the optimal conditions depends on the goal of the work, as some seek to increase acinar cell characteristics, while others are concerned with increasing expression of putative stem cell markers or mimicking branching morphogenesis. Specific soluble factors that have increased the acinar phenotype include FGF10, neurotrophic factors, and EGFR inhibitors. The use of matrix mimetics, such as Matrigel®, collagen, and functionalized synthetic polymers, rather than just inert substrates, provide increased opportunities to improve the acinar cell phenotype. Factors that may improve stem cell maintenance include ROCK inhibitors and Wnt/R-spondin conditioned media, with mixed results from TGFβR1 inhibitors and MSC-conditioned media.
Based on the current state of the field, this study has revealed there is room for further optimization to recapitulate the in vivo salivary gland using in vitro culture models. Studies are needed to simultaneously optimize the combination of soluble factors with matrix cues and it is intriguing to consider improvements that maybe gained by co-culturing salivary glands cells with supportive tissues, such as neural or mesenchymal tissue as described by Vining et al., 2019 and Hosseini et al., 2018. Other pioneering approaches could include the use of microfluidic devices or 3D printing technology to aid in increasing the complexity and relevance of cell culture models, such as those developed for other tissues [87,88,89,90,91].
Author Contributions
Conceptualization, L.R.P.; methodology, L.R.P.; investigation, L.R.P.; writing—original draft preparation, L.R.P.; writing—review and editing, L.R.P., L.A.D. and D.S.W.B.; visualization, L.R.P.; supervision, L.A.D. and D.S.W.B.; funding acquisition, L.A.D. and D.S.W.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Dental and Craniofacial Research (NIDCR) and National Center for Advancing Translational Sciences (NCATS) of the National Institute of Health, grant numbers UG3 DE027695, UH3 DE027695, and F31 DE029658.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Humphrey S.P., Williamson R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 2.Whelton H. Introduction: Anatomy and physiology of salivary glands. In: Dawes C., Edgar W.M., O’Mullane D.M., editors. Saliva and Oral Health. 4th ed. Stephen Hancock Limited; Oxford, UK: 2012. pp. 1–36. [Google Scholar]
- 3.Rocchi C., Emmerson E. Mouth-Watering Results: Clinical Need, Current Approaches, and Future Directions for Salivary Gland Regeneration. Trends Mol. Med. 2020;26:649–669. doi: 10.1016/j.molmed.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Varga G. Physiology of the salivary glands. Surgery (Oxf.) 2012;30:578–583. doi: 10.1016/j.mpsur.2012.09.010. [DOI] [Google Scholar]
- 5.Porcheri C., Mitsiadis T.A. Physiology, Pathology and Regeneration of Salivary Glands. Cells. 2019;8:976. doi: 10.3390/cells8090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escobar A., Aitken-Saavedra J.P. Xerostomia: An Update of Causes and Treatments. Salivary Gland. New Approaches Diagn. Treat. 2019:15–37. doi: 10.5772/intechopen.72307. [DOI] [Google Scholar]
- 7.Wolff A., Joshi R.K., Ekström J., Aframian D., Pedersen A.M.L., Proctor G., Narayana N., Villa A., Sia Y.W., Aliko A., et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs R&D. 2017;17:1–28. doi: 10.1007/s40268-016-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisbruch A. Reducing Xerostomia by IMRT: What May, and May Not, Be Achieved. J. Clin. Oncol. 2007;25:4863–4864. doi: 10.1200/JCO.2007.13.4874. [DOI] [PubMed] [Google Scholar]
- 9.Rades D., Fehlauer F., Bajrovic A., Mahlmann B., Richter E., Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother. Oncol. 2004;70:261–264. doi: 10.1016/j.radonc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Dijkema T., Terhaard C.H.J., Roesink J.M., Raaijmakers C.P.J., van den Keijbus P.A., Brand H.S., Veerman E.C. MUC5B levels in submandibular gland saliva of patients treated with radiotherapy for head-and-neck cancer: A pilot study. Radiat. Oncol. 2012;7:91. doi: 10.1186/1748-717X-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Eisbruch A. IMRT for head and neck cancer: Reducing xerostomia and dysphagia. J. Radiat. Res. 2016;57:i69–i75. doi: 10.1093/jrr/rrw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quissell D.O., Redman R.S., Mark M.R. Short-term primary culture of acinar-intercalated duct complexes from rat submandibular glands. Vitr. Cell. Dev. Biol. 1986;22:469–480. doi: 10.1007/BF02623448. [DOI] [PubMed] [Google Scholar]
- 13.Yeh C.-K., Mertz P.M., Oliver C., Baum B.J., Kousvelari E.E. Cellular characteristics of long-term cultured rat parotid acinar cells. Vitr. Cell. Dev. Biol. 1991;27:707–712. doi: 10.1007/BF02633215. [DOI] [PubMed] [Google Scholar]
- 14.Pin C., Rukstalis J.M., Johnson C., Konieczny S.F. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J. Cell Biol. 2001;155:519–530. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shubin A.D., Felong T.J., Schutrum B.E., Joe D.S., Ovitt C.E., Benoit D.S. Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater. 2017;50:437–449. doi: 10.1016/j.actbio.2016.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shubin A.D., Sharipol A., Felong T.J., Weng P.-L., Schutrum B.E., Joe D.S., Aure M.H., Benoit D.S., Ovitt C.E. Stress or injury induces cellular plasticity in salivary gland acinar cells. Cell Tissue Res. 2020;380:487–497. doi: 10.1007/s00441-019-03157-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson J., Manzella K., Baker O. Current cell models for bioengineering a salivary gland: A mini-review of emerging technologies. Oral Dis. 2012;19:236–244. doi: 10.1111/j.1601-0825.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capes-Davis A., Theodosopoulos G., Atkin I., Drexler H.G., Kohara A., MacLeod R.A., Masters J.R., Nakamura Y., Reid Y.A., Reddel R., et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int. J. Cancer. 2010;127:1–8. doi: 10.1002/ijc.25242. [DOI] [PubMed] [Google Scholar]
- 19.Lin L.-C., Elkashty O., Ramamoorthi M., Trinh N., Liu Y., Sunavala-Dossabhoy G., Pranzatelli T., Michael D.G., Chivasso C., Perret J., et al. Cross-contamination of the human salivary gland HSG cell line with HeLa cells: A STR analysis study. Oral Dis. 2018;24:1477–1483. doi: 10.1111/odi.12920. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka J., Ogawa M., Hojo H., Kawashima Y., Mabuchi Y., Hata K., Nakamura S., Yasuhara R., Takamatsu K., Irié T., et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 2018;9:1–13. doi: 10.1038/s41467-018-06469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruyama C.L., Monroe M., Hunt J., Buchmann L., Baker O.J. Comparing human and mouse salivary glands: A practice guide for salivary researchers. Oral Dis. 2019;25:403–415. doi: 10.1111/odi.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseini Z.F., Nelson D., Moskwa N., Sfakis L.M., Castracane J., Larsen M. FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids. J. Cell Sci. 2018;131 doi: 10.1242/jcs.208728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vining K., Lombaert I.M.A., Patel V.N., Kibbey S.E., Pradhan-Bhatt S., Witt R.L., Hoffman M.P. Neurturin-containing laminin matrices support innervated branching epithelium from adult epithelial salispheres. Biomaterials. 2019;216:119245. doi: 10.1016/j.biomaterials.2019.119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyajima H., Matsumoto T., Sakai T., Yamaguchi S., An S.H., Abe M., Wakisaka S., Lee K.Y., Egusa H., Imazato S. Hydrogel-based biomimetic environment for in vitro modulation of branching morphogenesis. Biomaterials. 2011;32:6754–6763. doi: 10.1016/j.biomaterials.2011.05.072. [DOI] [PubMed] [Google Scholar]
- 25.Lee H.-W., Hsiao Y.-C., Young T.-H., Yang T.-L. Maintenance of the spheroid organization and properties of glandular progenitor cells by fabricated chitosan based biomaterials. Biomater. Sci. 2018;6:1445–1456. doi: 10.1039/C7BM00559H. [DOI] [PubMed] [Google Scholar]
- 26.Pringle S., Maimets M., Van Der Zwaag M., Stokman M.A., Van Gosliga D., Zwart E., Witjes M., de Haan G., Van Os R., Coppes R.P. Human Salivary Gland Stem Cells Functionally Restore Radiation Damaged Salivary Glands. Stem Cells. 2016;34:640–652. doi: 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]
- 27.Lombaert I.M.A., Brunsting J.F., Wierenga P.K., Faber H., Stokman M.A., Kok T., Visser W.H., Kampinga H., de Haan G., Coppes R.P. Rescue of Salivary Gland Function after Stem Cell Transplantation in Irradiated Glands. PLoS ONE. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shubin A.D., Felong T.J., Graunke D., Ovitt C.E., Benoit D.S. Development of Poly(Ethylene Glycol) Hydrogels for Salivary Gland Tissue Engineering Applications. Tissue Eng. Part A. 2015;21:1733–1751. doi: 10.1089/ten.tea.2014.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo Y.J., Lilliu M.A., Abu Elghanam G., Nguyen T., Liu Y., Lee J.C., Presley J.F., Zeitouni A., El-Hakim M., Tran S.D. Cell culture of differentiated human salivary epithelial cells in a serum-free and scalable suspension system: The salivary functional units model. J. Tissue Eng. Regen. Med. 2019;13:1559–1570. doi: 10.1002/term.2908. [DOI] [PubMed] [Google Scholar]
- 30.Song Y., Uchida H., Sharipol A., Piraino L., Mereness J.A., Ingalls M.H., Rebhahn J., Newlands S.D., DeLouise L.A., Ovitt C.E., et al. Development of a functional salivary gland tissue chip with potential for high-content drug screening. Commun. Biol. 2021;4:1–15. doi: 10.1038/s42003-021-01876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsiao Y.-C., Chen C.-N., Chen Y.-T., Yang T.-L. Controlling branching structure formation of the salivary gland by the degree of chitosan deacetylation. Acta Biomater. 2013;9:8214–8223. doi: 10.1016/j.actbio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Yang T.-L., Hsiao Y.-C. Chitosan facilitates structure formation of the salivary gland by regulating the basement membrane components. Biomaterials. 2015;66:29–40. doi: 10.1016/j.biomaterials.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Kim J.M., Choi S., Lee S.W., Park K. Voltage-dependent Ca2+ channels promote branching morphogenesis of salivary glands by patterning differential growth. Sci. Rep. 2018;8:7566. doi: 10.1038/s41598-018-25957-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng J., van der Zwaag M., Stokman M.A., van Os R., Coppes R.P. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother. Oncol. 2009;92:466–471. doi: 10.1016/j.radonc.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Nanduri L.S., Baanstra M., Faber H., Rocchi C., Zwart E., de Haan G., van Os R., Coppes R.P. Purification and Ex Vivo Expansion of Fully Functional Salivary Gland Stem Cells. Stem Cell Rep. 2014;3:957–964. doi: 10.1016/j.stemcr.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel V.N., Lombaert I.M., Cowherd S.N., Shworak N.W., Xu Y., Liu J., Hoffman M.P. Hs3st3-Modified Heparan Sulfate Controls KIT+ Progenitor Expansion by Regulating 3-O-Sulfotransferases. Dev. Cell. 2014;29:662–673. doi: 10.1016/j.devcel.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasan P.P., Patel V.N., Liu S., Harrington D.A., Hoffman M.P., Jia X., Witt R.L., Farach-Carson M.C., Pradhan-Bhatt S. Primary Salivary Human Stem/Progenitor Cells Undergo Microenvironment-Driven Acinar-Like Differentiation in Hyaluronate Hydrogel Culture. Stem Cells Transl. Med. 2016;6:110–120. doi: 10.5966/sctm.2016-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker O.J., Sommakia S., Internationals O. Neurons Self-Organize Around Salivary Epithelial Cells in Novel Co-Culture Model. J. Stem Cell Regen. Biol. 2016;2:1–6. doi: 10.15436/2471-0598.16.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon H.R., Nelson D., DeSantis K.A., Morrissey J.M., Larsen M. Endothelial cell regulation of salivary gland epithelial patterning. Development. 2017;144:211–220. doi: 10.1242/dev.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teti A. Regulation of cellular functions by extracellular matrix. J. Am. Soc. Nephrol. 1992;2:S83. doi: 10.1681/ASN.V210s83. [DOI] [PubMed] [Google Scholar]
- 41.Sequeira S.J., Larsen M., Devine T. Extracellular Matrix and Growth Factors in Salivary Gland Development. Salivary Gland. 2010;14:48–77. doi: 10.1159/000313707. [DOI] [PubMed] [Google Scholar]
- 42.Lee S.-W., Ryu J.H., Do M.J., Namkoong E., Lee H., Park K. NiCHE Platform: Nature-Inspired Catechol-Conjugated Hyaluronic Acid Environment Platform for Salivary Gland Tissue Engineering. ACS Appl. Mater. Interfaces. 2020;12:4285–4294. doi: 10.1021/acsami.9b20546. [DOI] [PubMed] [Google Scholar]
- 43.Maria O.M., Zeitouni A., Gologan O., Tran S.D. Matrigel Improves Functional Properties of Primary Human Salivary Gland Cells. Tissue Eng. Part A. 2011;17:1229–1238. doi: 10.1089/ten.tea.2010.0297. [DOI] [PubMed] [Google Scholar]
- 44.Maria O.M., Liu Y., El-Hakim M., Zeitouni A., Tran S.D. The role of human fibronectin- or placenta basement membrane extract-based gels in favouring the formation of polarized salivary acinar-like structures. J. Tissue Eng. Regen. Med. 2017;11:2643–2657. doi: 10.1002/term.2164. [DOI] [PubMed] [Google Scholar]
- 45.Soscia D.A., Sequeira S.J., Schramm R.A., Jayarathanam K., Cantara S.I., Larsen M., Castracane J. Salivary gland cell differentiation and organization on micropatterned PLGA nanofiber craters. Biomaterials. 2013;34:6773–6784. doi: 10.1016/j.biomaterials.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sfakis L., Kamaldinov T., Khmaladze A., Hosseini Z.F., Nelson D.A., Larsen M., Castracane J. Mesenchymal Cells Affect Salivary Epithelial Cell Morphology on PGS/PLGA Core/Shell Nanofibers. Int. J. Mol. Sci. 2018;19:1031. doi: 10.3390/ijms19041031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bécavin T., Kökten T., Huck O., Messaddeq N., Lesot H., Deveaux E., Benkirane-Jessel N., Laetitia K., Kuchler-Bopp S. Well-organized spheroids as a new platform to examine cell interaction and behaviour during organ development. Cell Tissue Res. 2016;366:601–615. doi: 10.1007/s00441-016-2487-6. [DOI] [PubMed] [Google Scholar]
- 48.Burghartz M., Lennartz S., Schweinlin M., Hagen R., Kleinsasser N., Hackenberg S., Steußloff G., Scherzad A., Radeloff K., Ginzkey C., et al. Development of Human Salivary Gland-Like Tissue In Vitro. Tissue Eng. Part A. 2018;24:301–309. doi: 10.1089/ten.tea.2016.0466. [DOI] [PubMed] [Google Scholar]
- 49.Sui Y., Zhang S., Li Y., Zhang X., Hu W., Feng Y., Xiong J., Zhang Y., Wei S. Generation of functional salivary gland tissue from human submandibular gland stem/progenitor cells. Stem Cell Res. Ther. 2020;11:1–13. doi: 10.1186/s13287-020-01628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janebodin K., Buranaphatthana W., Ieronimakis N., Hays A.L., Reyes M. An In Vitro Culture System for Long-Term Expansion of Epithelial and Mesenchymal Salivary Gland Cells: Role of TGF-β1 in Salivary Gland Epithelial and Mesenchymal Differentiation. BioMed Res. Int. 2013;2013:1–20. doi: 10.1155/2013/815895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagai K., Arai H., Okudera M., Yamamura T., Oki H., Komiyama K. Epiregulin is critical for the acinar cell regeneration of the submandibular gland in a mouse duct ligation model. J. Oral Pathol. Med. 2013;43:378–387. doi: 10.1111/jop.12145. [DOI] [PubMed] [Google Scholar]
- 52.Mitsui R., Fujita-Yoshigaki J., Narita T., Matsuki-Fukushima M., Satoh K., Qi B., Guo M.-Y., Katsumata-Kato O., Sugiya H. Maintenance of paracellular barrier function by insulin-like growth factor-I in submandibular gland cells. Arch. Oral Biol. 2010;55:963–969. doi: 10.1016/j.archoralbio.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 53.Han C., An G.H., Woo D.-H., Kim J.-H., Park H.-K. Rho-associated kinase inhibitor enhances the culture condition of isolated mouse salivary gland cells in vitro. Tissue Cell. 2018;54:20–25. doi: 10.1016/j.tice.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Chou Y.-S., Young T.-H., Lou P.-J. Effects of biomaterial-derived fibroblast conditioned medium on the α-amylase expression of parotid gland acinar cells. Acta Biomater. 2015;27:214–223. doi: 10.1016/j.actbio.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 55.Maimets M., Rocchi C., Bron R., Pringle S., Kuipers J., Giepmans B.N., Vries R.G., Clevers H., de Haan G., van Os R., et al. Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Rep. 2016;6:150–162. doi: 10.1016/j.stemcr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maruyama C., Leigh N., Nelson J., McCall A., Mellas R., Lei P., Andreadis S., Baker O. Stem Cell–Soluble Signals Enhance Multilumen Formation in SMG Cell Clusters. J. Dent. Res. 2015;94:1610–1617. doi: 10.1177/0022034515600157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujita-Yoshigaki J. Claudins: Methods and Protocols. Humana Press; New York, NY, USA: 2011. Analysis of Changes in the Expression Pattern of Claudins Using Salivary Acinar Cells in Primary Culture; pp. 245–258. [DOI] [PubMed] [Google Scholar]
- 58.Lombaert I.M., Abrams S.R., Li L., Eswarakumar J., Sethi A.J., Witt R.L., Hoffman M.P. Combined KIT and FGFR2b Signaling Regulates Epithelial Progenitor Expansion during Organogenesis. Stem Cell Rep. 2013;1:604–619. doi: 10.1016/j.stemcr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinberg Z., Myers C., Heim V.M., Lathrop C.A., Rebustini I.T., Stewart J.S., Larsen M., Hoffman M.P. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223–1234. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- 60.Knox S.M., Lombaert I.M.A., Reed X., Vitale-Cross L., Gutkind J.S., Hoffman M.P. Parasympathetic Innervation Maintains Epithelial Progenitor Cells During Salivary Organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao J.K., Seto M., Noma K. Rho Kinase (ROCK) Inhibitors. J. Cardiovasc. Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daley W.P., Kohn J.M., Larsen M. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev. Dyn. 2011;240:2069–2083. doi: 10.1002/dvdy.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daley W.P., Gervais E.M., Centanni S.W., Gulfo K.M., Nelson D., Larsen M. ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development. 2012;139:411–422. doi: 10.1242/dev.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J.B., Nishikawa S., Nishikawa S.-I., Muguruma K., et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y., Tristan C.A., Chen L., Jovanovic V.M., Malley C., Chu P.-H., Ryu S., Deng T., Ormanoglu P., Tao D., et al. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat. Methods. 2021;18:528–541. doi: 10.1038/s41592-021-01126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L., Valdez J.M., Zhang B., Wei L., Chang J., Xin L. ROCK Inhibitor Y-27632 Suppresses Dissociation-Induced Apoptosis of Murine Prostate Stem/Progenitor Cells and Increases Their Cloning Efficiency. PLoS ONE. 2011;6:e18271. doi: 10.1371/journal.pone.0018271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tao X., Chen Q., Li N., Xiang H., Pan Y., Qu Y., Shang D., Go V.L.W., Xue J., Sun Y., et al. Serotonin-RhoA/ROCK axis promotes acinar-to-ductal metaplasia in caerulein-induced chronic pancreatitis. Biomed. Pharmacother. 2020;125:109999. doi: 10.1016/j.biopha.2020.109999. [DOI] [PubMed] [Google Scholar]
- 68.Xiao S., Zhang Y. Establishment of long-term serum-free culture for lacrimal gland stem cells aiming at lacrimal gland repair. Stem Cell Res. Ther. 2020;11:13–20. doi: 10.1186/s13287-019-1541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koslow M., O’Keefe K.J., Hosseini Z.F., Nelson D.A., Larsen M. ROCK inhibitor increases proacinar cells in adult salivary gland organoids. Stem Cell Res. 2019;41:101608. doi: 10.1016/j.scr.2019.101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J., Zeng F., Forrester S.J., Eguchi S., Zhang M.-Z., Harris R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016;96:1025–1069. doi: 10.1152/physrev.00030.2015. [DOI] [PubMed] [Google Scholar]
- 71.Gresik E.W., Kashimata M., Kadoya Y., Mathews R., Minami N., Yamashina S. Expression of epidermal growth factor receptor in fetal mouse submandibular gland detected by a biotinyltyramide-based catalyzed signal amplification method. J. Histochem. Cytochem. 1997;45:1651–1657. doi: 10.1177/002215549704501208. [DOI] [PubMed] [Google Scholar]
- 72.Mattingly A., Finley J.K., Knox S.M. Salivary gland development and disease. Wiley Interdiscip. Rev. Dev. Biol. 2015;4:573–590. doi: 10.1002/wdev.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Means A.L., Meszoely I.M., Suzuki K., Miyamoto Y., Rustgi A.K., Coffey R.J., Wright C.V.E., Stoffers D.A., Leach S.D. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 74.Jaskoll T., Melnick M. Embryonic Salivary Gland Branching Morphogenesis. In: Davies J.A., editor. Branching Morphogenesis. Landes Bioscience; Georgetown, TX, USA: 2007. pp. 160–175. [Google Scholar]
- 75.Woods L.T., Camden J.M., El-Sayed F.G., Khalafalla M.G., Petris M.J., Erb L., Weisman G.A. Increased Expression of TGF-β Signaling Components in a Mouse Model of Fibrosis Induced by Submandibular Gland Duct Ligation. PLoS ONE. 2015;10:e0123641. doi: 10.1371/journal.pone.0123641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hall E.B., Zheng C., Swaim W.D., Cho A., Nagineni C.N., Eckhaus M.A., Flanders K.C., Ambudkar I.S., Baum B.J., Kulkarni A.B. Conditional overexpression of TGF-β1 disrupts mouse salivary gland development and function. Lab. Investig. 2010;90:543–555. doi: 10.1038/labinvest.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki D., Pinto F., Senoo M. Inhibition of TGF-β signaling supports high proliferative potential of diverse p63+ mouse epithelial progenitor cells in vitro. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-06470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferreira J., Hoffman M.P. Interactions between developing nerves and salivary glands. Organogenesis. 2013;9:199–205. doi: 10.4161/org.25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakao A., Inaba T., Murakami-Sekimata A., Nogawa H. Morphogenesis and Mucus Production of Epithelial Tissues of Three Major Salivary Glands of Embryonic Mouse in 3D Culture. Zool. Sci. 2017;34:475. doi: 10.2108/zs160177. [DOI] [PubMed] [Google Scholar]
- 80.Knox S.M., Lombaert I.M.A., Haddox C., Abrams S.R., Cotrim A.P., Wilson A.J., Hoffman M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013;4:1–7. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nedvetsky P., Emmerson E., Finley J.K., Ettinger A., Cruz-Pacheco N., Prochazka J., Haddox C., Northrup E., Hodges C., Mostov K.E., et al. Parasympathetic Innervation Regulates Tubulogenesis in the Developing Salivary Gland. Dev. Cell. 2014;30:449–462. doi: 10.1016/j.devcel.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao N., Lin Y., Cao H., Sirjani D., Giaccia A.J., Koong A., Kong C.S., Diehn M., Le Q.-T. Neurotrophic factor GDNF promotes survival of salivary stem cells. J. Clin. Investig. 2014;124:3364–3377. doi: 10.1172/JCI74096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suzuki A., Ogata K., Iwata J. Cell signaling regulation in salivary gland development. Cell. Mol. Life Sci. 2021;78:3299–3315. doi: 10.1007/s00018-020-03741-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chou Y.-S., Lin Y.-C., Young T.-H., Lou P.-J. Effects of fibroblasts on the function of acinar cells from the same human parotid gland. Head Neck. 2016;38:E279–E286. doi: 10.1002/hed.23986. [DOI] [PubMed] [Google Scholar]
- 85.Farahat M., Kazi G.A.S., Taketa H., Hara E.S., Oshima M., Kuboki T., Matsumoto T. Fibronectin-induced ductal formation in salivary gland self-organization model. Dev. Dyn. 2019;248:813–825. doi: 10.1002/dvdy.78. [DOI] [PubMed] [Google Scholar]
- 86.Zhang S., Sui Y., Fu X., Feng Y., Luo Z., Zhang Y., Wei S. Specific complexes derived from extracellular matrix facilitate generation of structural and drug-responsive human salivary gland microtissues through maintenance stem cell homeostasis. J. Tissue Eng. Regen. Med. 2019;14:284–294. doi: 10.1002/term.2992. [DOI] [PubMed] [Google Scholar]
- 87.Campisi M., Shin Y., Osaki T., Hajal C., Chiono V., Kamm R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018;180:117–129. doi: 10.1016/j.biomaterials.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Czerniecki S.M., Cruz N.M., Harder J.L., Menon R., Annis J., Otto E.A., Gulieva R.E., Islas L.V., Kim Y.K., Tran L.M., et al. High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell. 2018;22:929–940. doi: 10.1016/j.stem.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sidar B., Jenkins B.R., Huang S., Spence J.R., Walk S.T., Wilking J.N. Long-term flow through human intestinal organoids with the gut organoid flow chip (GOFlowChip) Lab Chip. 2019;19:3552–3562. doi: 10.1039/C9LC00653B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chramiec A., Teles D., Yeager K., Marturano-Kruik A., Pak J., Chen T., Hao L., Wang M., Lock R., Tavakol D.N., et al. Integrated human organ-on-a-chip model for predictive studies of anti-tumor drug efficacy and cardiac safety. Lab Chip. 2020;20:4357–4372. doi: 10.1039/D0LC00424C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seiler K.M., Bajinting A., Alvarado D.M., Traore M.A., Binkley M.M., Goo W., Lanik W.E., Ou J., Ismail U., Iticovici M., et al. Patient-derived small intestinal myofibroblasts direct perfused, physiologically responsive capillary development in a microfluidic Gut-on-a-Chip Model. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-60672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.