Abstract
Black pepper (Piper nigrum L.) is a prominent spice that is an indispensable ingredient in cuisine and traditional medicine. Phytophthora capsici, the causative agent of footrot disease, causes a drastic constraint in P. nigrum cultivation and productivity. To counterattack various biotic and abiotic stresses, plants employ a broad array of mechanisms that includes the accumulation of pathogenesis-related (PR) proteins. Through a genome-wide survey, eleven PR-1 genes that belong to a CAP superfamily protein with a caveolin-binding motif (CBM) and a CAP-derived peptide (CAPE) were identified from P. nigrum. Despite the critical functional domains, PnPR-1 homologs differ in their signal peptide motifs and core amino acid composition in the functional protein domains. The conserved motifs of PnPR-1 proteins were identified using MEME. Most of the PnPR-1 proteins were basic in nature. Secondary and 3D structure analyses of the PnPR-1 proteins were also predicted, which may be linked to a functional role in P. nigrum. The GO and KEGG functional annotations predicted their function in the defense responses of plant-pathogen interactions. Furthermore, a transcriptome-assisted FPKM analysis revealed PnPR-1 genes mapped to the P. nigrum-P. capsici interaction pathway. An altered expression pattern was detected for PnPR-1 transcripts among which a significant upregulation was noted for basic PnPR-1 genes such as CL10113.C1 and Unigene17664. The drastic variation in the transcript levels of CL10113.C1 was further validated through qRT-PCR and it showed a significant upregulation in infected leaf samples compared with the control. A subsequent analysis revealed the structural details, phylogenetic relationships, conserved sequence motifs and critical cis-regulatory elements of PnPR-1 genes. This is the first genome-wide study that identified the role of PR-1 genes during P. nigrum-P. capsici interactions. The detailed in silico experimental analysis revealed the vital role of PnPR-1 genes in regulating the first layer of defense towards a P. capsici infection in Panniyur-1 plants.
Keywords: footrot, black pepper, promoter, cap domain, plant immunity, cis-regulatory element, biotic stress
1. Introduction
Plant immunity involves multiple layers of defense responses. The first layer of defense is triggered by the detection of microbe-associated molecular patterns (MAMPs) through plant-, pathogen- or pattern-recognition receptors (PRRs), which activate PAMP-/pathogen-/pattern-triggered immunity (PTI) [1]. The defense machinery of plants has been forced to evolve continuously to combat a wide range of abiotic and biotic stress factors. These challenges activate an array of induced mechanisms such as a hypersensitive response (HR), which involves a series of events including the production of reactive oxygen species (ROS) and the synthesis of antimicrobial molecules and pathogenesis-related (PR) proteins. PR proteins induce programmed cell death, which inhibits the spread of infection contributing to a systemic acquired resistance (SAR) [2,3]. In the second layer of defense, pathogens suppress PTI by secreting the effector proteins that are later recognized by plant resistance (R) proteins leading to an effector-triggered immunity [1].
Salicylic acid and the subsequent activation of PR genes are necessary for the establishment of SAR in the distant regions of infections [4]. Arabidopsis mutants deficient in the non-expressor of pathogenesis-related 1 (NPR1) protein, a key SAR regulator, showed less PR gene expression, which, in turn, increased the susceptibility to pathogens. Various plant species overexpressing Arabidopsis NPR1 displayed an enhanced disease resistance to pathogens such as Rhizoctonia solani, Erwinia amylovora and Erysiphe necator [5,6,7]. To date, 17 families of PR proteins have been classified and characterized [8]. PR proteins are functionally diverse proteins that are inducible during a pathogen attack and are regulated by signaling compounds such as abscisic acid (ABA), ethylene (ET), jasmonic acid (JA) and salicylic acid (SA) [9,10].
PR-1a, the first member of the PR-1 family, was identified in Nicotiana tabacum plants infected with the tobacco mosaic virus. PR-1 proteins belong to the group of the most abundantly produced proteins during plant defense responses and they are ubiquitous across plant species. PR-1 proteins are involved in the cell wall thickening and thereby prevent the spread of the pathogens in the apoplast [11]. Apart from the biotic stresses, the role of PR-1 in abiotic stresses [12] was also reported. During infections, the overexpression of PR-1 proteins in the apoplast make them potential candidates for antimicrobial activity [13]. An enhanced tolerance to fungi [14], oomycetes [15] and bacterial infections [16] were demonstrated with an overexpression of PR-1 in the transgenic plants. Apart from their role in various biotic and abiotic stresses PR-1 and PR-1-like proteins have been reported to be involved in plant development, flowering and seed growth [17,18]. PR-1 proteins are widely reported across the plant kingdom. These are the members of the cysteine-rich secretory protein, antigen 5, and the pathogenesis-related 1 (CAP) protein superfamily. Stress signaling peptides such as CAPE-1 (CAP-derived peptide 1) are embedded within PR-1 proteins. A CAPE-1 peptide that comprised of the last 11 amino acids from the C-terminus of the PR-1 protein was reported from tomato plants subjected to wounding and methyl jasmonate treatment [19]. A caveolin-binding motif (CBM) in the CAP region, which is involved in sterol binding, is responsible for antimicrobial activity against oomycetes including Phytophthora and Pythium species, which require exogenous sterols for basic metabolism. Plants with an enhanced PR-1 expression are particularly well protected against oomycete pathogens [20]. The in-depth structural and biochemical analysis of PR-1 proteins can provide greater insights into their function during defense signaling in crop plants.
In the world of spices, black pepper (Piper nigrum L., family Piperaceae) is considered to be the king of spices due to its pungent constituent. It is a major additive in many ayurvedic medicinal preparations besides its use as a preservative, a pesticide and a spice. The major hindrance in the P. nigrum production is the destructive footrot or quick wilt disease caused by an oomycete, Phytophthora capsici [21]. In this work, to extend our knowledge on the defense mechanisms underlying the PR function in P. nigrum, we carried out a comprehensive genome-wide analysis and validation of PR-1 genes from P. nigrum. A transcriptome-assisted analysis and expression profiling revealed the differential expression of PR-1 genes during P. capsici infection in P. nigrum variety Panniyur-1.
2. Materials and Methods
2.1. Identification and Analysis of PR-1 Genes from the P. nigrum Genome
PR-1 genes of Arabidopsis thaliana were downloaded from the TAIR (The Arabidopsis Information Resource) database (https://www.arabidopsis.org/index.jsp, accessed on 7 January 2021) and a tblastn search against the P. nigrum genome assemblies was performed [22]. The coding sequences were translated and aligned by a multiple sequence alignment using the BioEdit Sequence Alignment Editor [23]. The nucleotide and protein sequence conservations of all of the PnPR-1 candidates were checked using Mega 7. The domain structure prediction was carried out using an NCBI-Conserved Domain Database (CDD) [24].
The molecular weight and pI of PnPR-1 proteins were estimated by the ExPASy ProtParam tool (https://web.expasy.org/protparam/, accessed on 15 January 2021). The potential signal peptide regions and the cleavage sites were also predicted using a SignalP 5.0 server (http://www.cbs.dtu.dk/cgi-bin, accessed on 15 January 2021). Subsequently, the conserved motifs of PnPR-1 proteins were predicted using MEME (Multiple Em for Motif Elicitation) (http://meme-suite.org/tools/meme, accessed on 20 January 2021) [25].
2.2. GO and KEGG Analysis
Gene ontology (GO) was classified into biological processes, cellular components and the molecular function. The PnPR-1 genes were analyzed for their role in GO using the PANNZER2 web server (http://ekhidna2.biocenter.helsinki.fi/sanspanz/, accessed on 25 January 2021) [26]. A KEGG (Kyoto Encyclopedia of Genes and Genomes) tool, BlastKOALA (KEGG Orthology and Links Annotation), a web server (https://www.kegg.jp/blastkoala/, accessed on 25 January 2021) [27], was used for the individual characterization of the gene functions.
2.3. Secondary and Tertiary Structure Prediction
The secondary structure prediction of the PnPR-1 proteins was predicted using the Self-Optimized Prediction Method with Alignment (SOPMA) server (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html, accessed on 27 January 2021). The predicted 3D structures were built using a Protein Homology/analogY Recognition Engine v2 (Phyre2) server (http://www.sbg.bio.ic.ac.uk/~phyre2/html/page.cgi?id=index, accessed on 27 January 2021) [28]. The CASTp (Computed Atlas of Surface Topography of proteins) tool (http://sts.bioe.uic.edu/castp/calculation.html, accessed on 27 January 2021) was used to predict the active site pockets and topology of the PnPR-1 protein structures [29].
2.4. Role of PnPR-1 in P. capsici-Infected P. nigrum
The control P. nigrum (uninfected) leaf transcriptome data (SRA050094) and the P. capsici-infected P. nigrum leaf transcriptome data (SRX853366) were reanalyzed and the final assembled data were used for the expression studies. The PnPR-1 sequences curated from the P. nigrum genome [22] were mapped to the transcriptome assembly files. The differential regulation of the obtained transcripts was checked using FPKM (fragments per kilobase of transcript per million mapped reads) values.
2.5. Plant-Pathogen Infections, Staining and RT-qPCR
Virulent, pure cultures of P. capsici and root cuttings of the P. nigrum cultivar Panniyur-1 were obtained from the College of Agriculture, Vellayani. P. capsici was subcultured every 15 days on potato dextrose agar (PDA) and stored at 28 °C. A 48 h old P. capsici culture in PDA was used in this study. P. capsici mycelial discs were used to infect the fully-expanded second leaf of the P. nigrum plant. The mock treatments were replaced with plain PDA discs. Mock-infected plants were used as a control. The infected leaf samples were collected at 6 h, 12 h and 24 h after infection. The mock samples were collected at 24 h. To detect and visualize the tissue damage in the leaf region after 24 h of pathogen infection, trypan blue staining was performed [30]. Three biological replicates were used for all of the studies.
At each timepoint, the leaf samples were collected and flash frozen in liquid nitrogen and stored at −80 °C until use. Total RNA was isolated from the collected leaf samples using a mirVana miRNA isolation kit (Invitrogen, Cat No: AM1560) according to the manufacturer’s instructions. The quality and the concentration of the RNA samples were checked by using a Colibri Microvolume Spectrometer. The RNA was reverse-transcribed into cDNA using a high-capacity cDNA synthesis kit (Applied Biosystems, Cat No: 4374966). RT-qPCR was carried out using the Applied Biosystems 7900 HT sequence detection system (ABI) using SYBR Green qPCR Master Mix (ABI). Each qPCR reaction was conducted in a 10 μL volume containing 1 μL of diluted cDNA (10 ng/μL), 5 μL of SYBR green and 5 pmol of forward (TCTTGTTGTTCGCAGCCCTAG) and reverse primer (GCGTAATTCCGTGCGTAGGT) with the following conditions: 40 cycles of 95 °C for 15 s DNA denaturation, annealing at 60 °C for 15 s and elongation at 72 °C for 30 s. The 5.8S RNA was used as an endogenous control [31]. The relative quantification was analyzed by the comparative CT method using the formula 2−∆∆CT and the standard deviation was represented as the error bar [32].
2.6. Prediction of cis-Acting Regulatory Elements in Promoter Regions
The genomic sequences of the 2.0 kbp upstream of the coding sequence (CDS) region of each PnPR-1 gene were extracted from the P. nigrum genome assembly. The promoter regions of each PR1 gene were scanned for the presence of functional motifs using Softberry (http://www.softberry.com/berry.phtml?topic=case_study_plants&no_menu=on, accessed on 30 January 2021) and New PLACE (https://www.dna.affrc.go.jp/PLACE/?action=newplace, accessed on 30 January 2021) [33].
3. Results
3.1. Genome-Wide Identification and Analysis of P. nigrum PR-1 Genes
Eleven potential PnPR-1 gene candidates were obtained from the genome-wide analysis in comparison with the A. thaliana PR genes. The number of exons in each PnPR-1 gene is one except in Pn2.357, which has two exons. The basic information of the PnPR-1 genes including the protein sequence length, isoelectric points and molecular weight are listed in Table 1. The length of the PnPR-1 proteins ranged from 127 to 357 amino acid residues with a molecular weight ranging from 14.38 to 38.49 kDa. The theoretical isoelectric point (pI) data categorized the majority of the PnPR-1 proteins into basic except Pn21.1032 and Pn36.35 that were acidic. The extreme acidic or basic properties can contribute to distinct functions of each PnPR-1 gene. Five gene loci for PnPR-1 were distributed in the P. nigrum genome scaffold 23. Furthermore, the signal peptide regions and cleavage sites were also identified in all of the PnPR-1 protein sequences except Pn11.1637 and Pn31.171 (Table 2). All of the PnPR-1 genes that were mapped to scaffold 23 had the same signal peptide cleavage positions in between the 24th and 25th amino acids.
Table 1.
Sequence characteristics and physio-chemical properties of the PR-1 proteins in Piper nigrum.
| Genome CDS Id | Genome Scaffold No. | Exon No. | Start | Stop | Strand | Protein Length (AA) | Molecular Weight (kDa) | Theoretical pI | |
|---|---|---|---|---|---|---|---|---|---|
| Pn2.460 | Pn23 | 1 | 1315655 | 1316146 | − | 155 | 17.09488 | 4.82 | Acidic |
| Pn2.459 | Pn23 | 1 | 1320928 | 1321419 | − | 192 | 21.91361 | 5.8 | |
| Pn2.433 | Pn23 | 1 | 1493563 | 1494054 | − | 168 | 18.35074 | 7.58 | Basic |
| Pn2.357 | Pn23 | 2 | 2226595 | 2229193 | − | 127 | 14.37881 | 7.62 | |
| Pn2.340 | Pn23 | 1 | 2402850 | 2403341 | − | 185 | 19.80756 | 9.1 | |
| Pn21.1032 | Pn3 | 1 | 3202437 | 3202904 | + | 163 | 17.85002 | 9.15 | |
| Pn31.171 | Pn15 | 1 | 26854949 | 26855332 | − | 163 | 17.87807 | 9.3 | |
| Pn36.35 | Pn25 | 1 | 5754975 | 5755553 | − | 163 | 17.88005 | 9.3 | |
| Pn11.1637 | Pn4 | 1 | 33322945 | 33323502 | − | 176 | 19.63337 | 9.37 | |
| Pn8.549 | Pn8 | 1 | 25779763 | 25780269 | − | 163 | 17.96421 | 9.44 | |
| Pn14.1312 | Pn14 | 1 | 5932334 | 5932864 | + | 357 | 38.49596 | 11.3 | |
Table 2.
Signal peptide region detected from the PnPR-1 proteins.
| Genome Id | Cleavage Site Position | Sequence Position | Probability | Protein Type | Signal Peptide (Sec/SPI) | Other |
|---|---|---|---|---|---|---|
| Pn21.1032 | 16 and 17 | CNA-QN | 0.9541 | Likelihood | 0.9981 | 0.0019 |
| Pn2.340 | 24 and 25 | AQA-QN | 0.8849 | Likelihood | 0.9984 | 0.0016 |
| Pn2.357 | 24 and 25 | AQA-QN | 0.8898 | Likelihood | 0.9986 | 0.0014 |
| Pn2.433 | 24 and 25 | AQA-QN | 0.8892 | Likelihood | 0.9987 | 0.0013 |
| Pn2.459 | 24 and 25 | AQA-QN | 0.8898 | Likelihood | 0.9986 | 0.0014 |
| Pn2.460 | 24 and 25 | AQA-QN | 0.8892 | Likelihood | 0.9987 | 0.0013 |
| Pn36.35 | 29 and 30 | ASS-SP | 0.6292 | Likelihood | 0.9813 | 0.0187 |
| Pn14.1312 | 31 and 32 | TNA-AL | 0.4399 | Likelihood | 0.7324 | 0.2676 |
| Pn8.549 | 30 and 31 | TLA-QN | 0.3454 | Likelihood | 0.5106 | 0.4894 |
| Pn31.171 | - | - | - | Likelihood | 0.0029 | 0.9971 |
| Pn11.1637 | - | - | - | Likelihood | 0.2691 | 0.7309 |
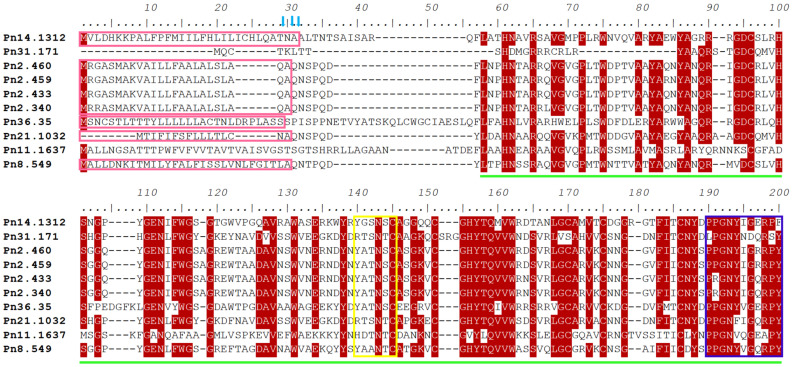
The multiple sequence alignment of all of the eleven PnPR-1 proteins revealed highly conserved CAP domain sequences, which further confirmed that these candidates belonged to the CAP superfamily. The CAP domain structure comprises of about 150 amino acids and also include a caveolin-binding motif (CBM) and a CAP-derived peptide (CAPE) motif (Figure 1). A comparison of the PnPR-1 sequences with various other monocot and dicot plants showed a distinct CAP domain with a CBM and a CAPE (Supplementary Figure S1) in P. nigrum. A SignalP analysis revealed about 24 amino acid long signal peptide regions (pink colored boxes) of PnPR-1 at the N terminal side. The predicted cleavage site is indicated by the arrowhead.
Figure 1.
Multiple sequence alignments of the PnPR-1 protein sequences. The pink colored rectangle boxes indicate the signal peptide regions; the cleavage sites predicted are indicated by the blue arrowheads. The green line indicates the CAP domain structure of ~150 bp. The yellow and blue color rectangles show the caveolin-binding motif (CBM) and CAP-derived peptide (CAPE), respectively.
3.2. Sequence Conservation of PnPR-1 Genes
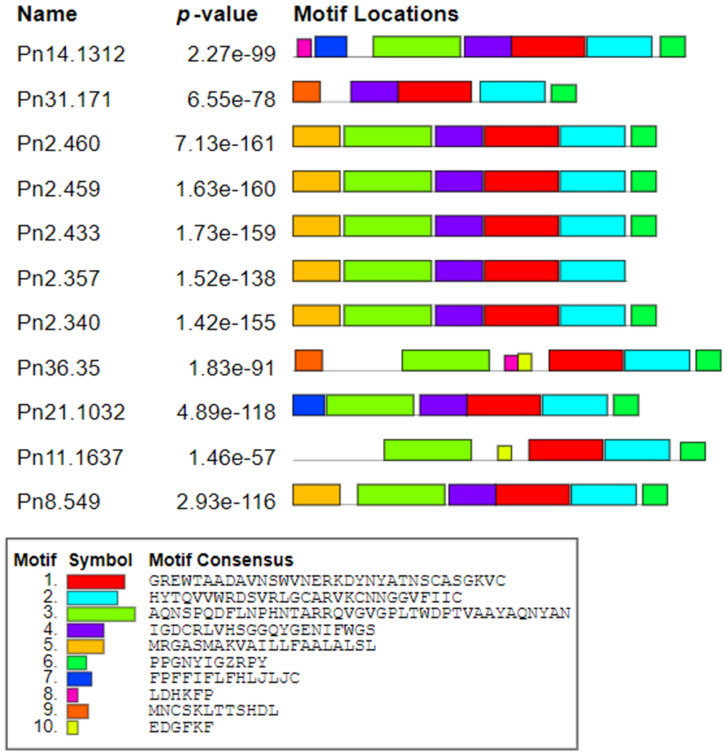
The subsequent phylogenetic analysis of all eleven PR-1 nucleotide and protein sequences through the maximum-likelihood method with 1000 bootstraps (Supplementary Figure S2A,B) revealed two main clusters leaving one outnumbered group (Pn21.1032). A total of ten conserved motifs were identified using the MEME server. Motifs 1 and 2 were conserved in all of the deduced PR-1 proteins whereas motif 3 and motif 6 were conserved in all PnPR-1 proteins except in Pn31.171 and Pn2.357, respectively. Motif 8, motif 9 and motif 10 were preserved in Pn36.35 along with Pn14.1312, Pn31.171 and Pn11.637, respectively (Figure 2).
Figure 2.
Conserved motifs identified from Piper nigrum PR-1 protein homologs.
A total of 10 conserved motifs were identified. Each color represents different motifs with consensus sequences.
3.3. GO and KEGG Pathway Analysis of PnPR-1 Genes
A gene ontology (GO) analysis yielded five biological processes, four molecular functions and four cellular components. Based on the GO enrichment analysis, most of the PR-1 genes had their role in defense responses and a response to a biotic stimulus in a biological function. In terms of the molecular function, it had protein kinase activity and adenyl nucleotide and purine ribonucleoside-binding activities. The cellular component showed its role in the extracellular region (Table 3). A KEGG pathways analysis categorized its role in the environmental information processing signal transduction pathways such as the MAPK signaling pathway (plant 04016), the plant hormone signal transduction (04075) and the plant-pathogen interaction (04626).
Table 3.
The gene ontology (GO) term distribution of PnPR-1 proteins.
| GO ID | GO Domain | Function Description |
|---|---|---|
| GO:0006952 | Biological process | Defense response |
| GO:0009607 | Biological process | Response to biotic stimulus |
| GO:0048544 | Biological process | Recognition of pollen |
| GO:0006468 | Biological process | Protein phosphorylation |
| GO:0010274 | Biological process | Hydrotropism |
| GO:0004672 | Molecular function | Protein kinase activity |
| GO:0030554 | Molecular function | Adenyl nucleotide binding |
| GO:0035639 | Molecular function | Purine ribonucleoside triphosphate binding |
| GO:0032555 | Molecular function | Purine ribonucleotide binding |
| GO:0005576 | Cellular component | Extracellular region |
| GO:0016020 | Cellular component | Membrane |
| GO:0031224 | Cellular component | Intrinsic component of membrane |
3.4. Secondary and 3D Structure of the PnPR-1 Protein
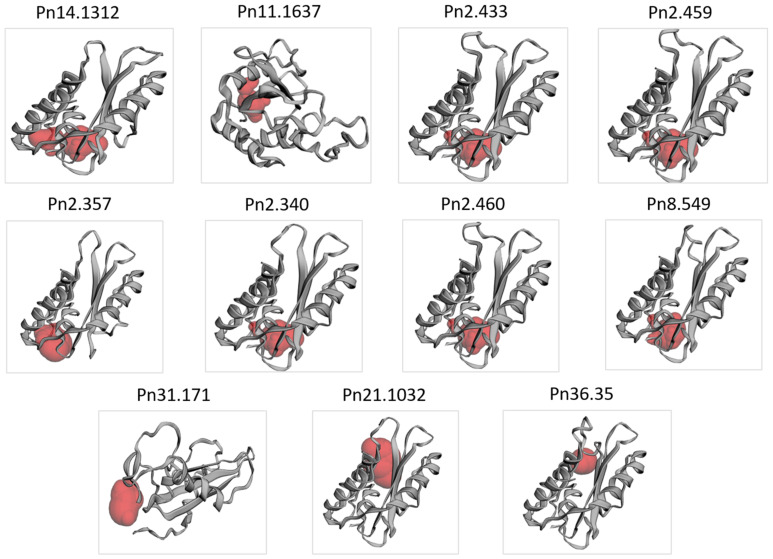
A varied percentage of α-helices (15.6–36.88%), extended strands (10.64–23.78%), β-turns (3.65–5.88%) and random coils (37.42–67.79%) were found in the Pn-PR1 proteins (Supplementary Figure S3). The relative proportion of the structural features differed among the PnPR-1 proteins. Pn31.171 and Pn2.357 showed less α-helix structures and more random coils compared with the other counterparts. Despite the 3D structural variations detected among the PnPR-1 proteins (Figure 3), the binding pockets critical for protein interaction were found in all of the eleven candidates. At the same time, the proportion of disordered regions of PR-1 proteins ranged from 4.5–28.1%.
Figure 3.
The predicted 3D structures of PnPR-1 proteins generated using the Phyre2 server and binding pockets identified by the CASTp 3.0 server (red color).
3.5. Cis-Regulatory Elements of the PnPR-1 Genes
The cis-elements were found to be distributed over the 2.0 kb upstream promoter region of the PnPR-1 genes (Figure 4) except in Pn2.357. The length of these cis-elements varied from 9 to 42 bp in the Softberry database whereas they were 4 to 24 bp in the New PLACE database. This was compared with the length of the cis-elements in A. thaliana (4–13 bp) and in O. sativa (4–10 bp) carried out by using the PlantCare program [34]. Among the ten PR-1 gene loci, the typical TGA binding site LS7, WBSI, G-box and C-motif were found in the promoter regions of Pn36.35. Meanwhile, the GT motif and Zc2A/T-2 were found in Pn2.340, Pn2.433 and Pn2.460. The hormone signaling elements such as ABI4 and GCC-box were present in Pn2.459, Pn2.340 and Pn2.433, respectively. The stress-responsive MYB was also detected in the promoter regions of the PnPR-1 genes such as Pn21.1032, Pn31.171 and Pn36.35. Among the 154 cis-elements detected from the New PLACE database, the CAAT box (CAAT), E-box (CANNTG) and DOFCOREZM (AAAG) regions were found to be widely distributed across the PnPR-1 promoter regions (Supplementary Figure S4).
Figure 4.
Analysis of the cis-acting elements of the PnRR-1 promoter regions (2 kb upstream from the CDS region) using Softberry software. Each element is represented with different colors.
3.6. Expression of PR-1 Genes during P. capsici Infection in P. nigrum
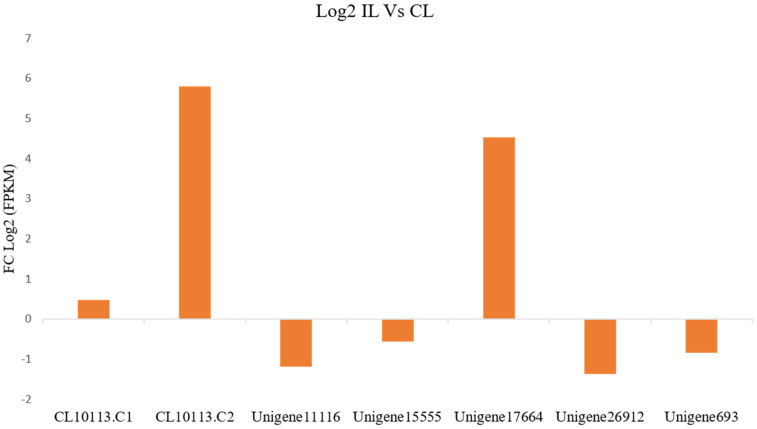
The assembled transcriptome of the RNA-seq data from the control (NCBI-SRA050094) and the P. capsici-infected P. nigrum (NCBI-SRX853366) plants revealed 60,437 transcripts. From the assembled data, seven transcripts of the PnPR-1 genes were mapped to the P. capsica-P. nigrum interaction pathway. The transcript lengths ranged from 391–1015 bps. A differential expression of these transcripts between the control leaf (CL) and the infected leaf (IL) was assessed from their corresponding FPKM values. CL10113.C1/2 and Unigene17664 were mapped to the Pn23 and Pn8 scaffolds, respectively, and were significantly upregulated (p-value < 0.01) in the IL compared with the CL. Meanwhile, Unigene11116, Unigene15555, Unigene26912 and Unigene693 were significantly downregulated (p-value < 0.01) in the IL compared with the CL (Figure 5).
Figure 5.
Expression analysis of the PnPR-1 transcripts in Phytophthora capsici-infected P. nigrum using FPKM (fragments per kilobase of transcript per million mapped reads) values. From the left, the PnPR-1 transcripts are designated as CL10113.C1, CL10113.C2, Unigene11116, Unigene15555, Unigene17664, Unigene26912 and Unigene693. Positive and negative numbers on the X-axis indicate the fold changes in the Log2 gene expression levels.
3.7. Trypan Blue Staining and Microscopic Detection of P. capsici Infection
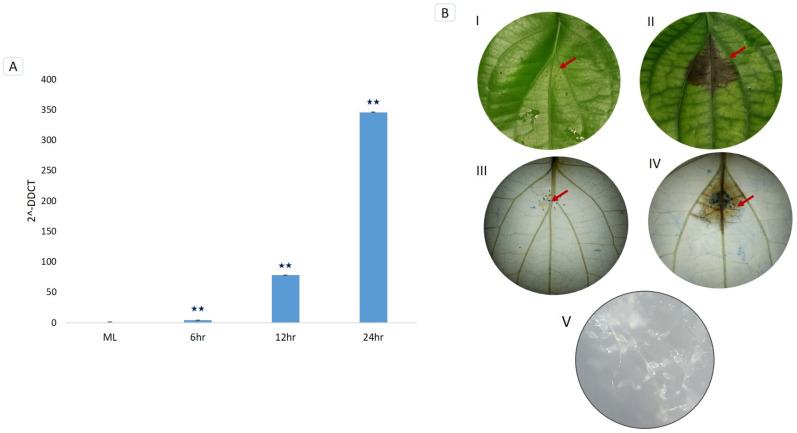
The development of necrotic lesions was detected on P. nigrum leaves after the P. capsici infection. As the lesion size progressed with the infection, the pathogen spores were profusely developed from the infected tissues. P. capsici-induced cell death on P. nigrum leaves was detected using trypan blue staining. The infected tissue was stained in blue whereas the viable cells were colorless (Figure 6B).
Figure 6.
(A) Temporal expression validation of CL10113.C2 PnPR-1 gene in Phytophthora capsici-infected Piper nigrum plants by RT-qPCR. ML (mock-infected leaf) 6 hpi (hours post-infection), 12 hpi and 24 hpi. Asterisks indicate significant changes (p-value < 0.001). (B) Mock and Phytophthora capsici-infected Piper nigrum leaves after 24 h of infection. (I) Mock-infected leaf. (II) Phytophthora capsici-infected Piper nigrum leaf. (III) Trypan blue-stained mock-infected leaf. (IV) Trypan blue-stained infected leaf. (V) Phytophthora capsici spores developed from the infected tissues. Red colored arrows indicate the infected regions.
3.8. RT-qPCR Validation of PnPR-1 Genes
We validated the relative expression of the remarkably upregulated PnPR-1 gene (CL10113.C2; Figure 5) using RT-qPCR from P. capsici-infected P. nigrum and mock control (ML) plants. The temporal expression at 6 h, 12 h and 24 h of P. capsici infections revealed a differential expression of the PnPR-1 (CL10113.C2) gene. A significant (>0.001) upregulation of the PnPR-1 gene (CL10113.C2) was observed in the leaves at 6 hpi, 12 hpi and 24 hpi compared with the mock control plants (Figure 6A).
4. Discussion
PR proteins are defense-related signaling molecules induced by phytopathogens that play a vital role in resisting the entry of the invading pathogen. PR proteins have been classified into many families based on their function, molecular weight, amino acid sequence and other properties [35]. In tobacco, PR proteins were initially classified as five major classes (PR1, PR2, PR3, PR4 and PR5) [36]. However, later studies in tobacco and the tomato have grouped them into 11 families [37]. The members belonging to the PR family can be either acidic or basic. Basic PR proteins are located intracellularly in the vacuole regions and are constitutively expressed to some extent and are also induced by stress signals whereas acidic types are produced extracellularly and only triggered by specific stress signals [38]. In our current study, both acidic and basic PR-1 proteins, which have a critical role during P. nigrum-Phytophthora interactions, were identified. The majority detected were basic in nature; this was the same as in the case of the PR1 proteins studied in S. lycopersicum during drought stress [12]. Contrasting to this, a greater number of acidic PR1 genes were also reported from rice, where among 12 PR1 rice protein candidates seven were acidic in nature [39]. In Glycine max during multiple biotic and abiotic stresses among 24 PR1 proteins, 15 were detected as acidic [40].
A KEGG orthology analysis revealed P. nigrum PR-1 genes mapped to the plant-pathogen interaction (04626), MAPK signaling pathway (04016) and plant hormone signal transduction (04075). The PR-1 family mainly possesses antifungal and anti-oomycete activities [35]. The overexpression of PR1 or similar proteins in various plants leads to an enhanced disease resistance to a wide variety of pathogens [39], especially the oomycetes [15]. The anti-oomycete properties of PR-1 proteins such as P14c and PR-1 were demonstrated against the sterol auxotroph pathogen Phytophthora brassicae [20].
As previously reported [13,20], P. nigrum PR-1 proteins possess the CAP tetrad, the CBM involved in sterol binding [41] and the CAPE involved in plant immune signaling [19]. The ability of the PR-1 family of proteins to bind sterols contributes towards their antimicrobial activity towards the Phytophthora species, a major plant pathogen belonging to the sterol auxotroph [20]. The CAPE-1 peptide has the consensus motif PxGNxxxxxPY, which is conserved between the monocots and dicots. A highly conserved and distinct similarity in the domains of the protein structure was observed in PnPR-1 proteins, which might account for a general strategy in responding to various biotic stresses as reported in other studies [42]. The role of the CAPE-1 peptide in the defense signaling was demonstrated in a previous study on the Pseudomonas syringae pv tomato (Pst) strain DC3000 interaction in tomatoes. A diverse set of defense-related genes was induced in the tomato plants pretreated with the CAPE-1 peptide. Furthermore, a non-canonical pathway other than PAMP-triggered immunity (PTI) signaling was suggested for the CAPE-1 mediated defense responses as the CAPE-1 did not induce the WRKY transcription factor 53 (WRKY53) [19]. The pathogen effector ToxA and Tox3 proteins from Parastagonospora nodorum were found to interact with wheat PR-1 proteins [43,44]. Promoters are the regulators of a gene at the transcriptional level [45]. Various computational methods are thoroughly being used for the identification of different cis-elements in the promoter region that are responsible for the regulation of genes [46]. A range of cis-elements was predicted from P. nigrum PR-1 genes, which were likely related to the regulation of the plant growth, development and response to various stresses. A high frequency of the CAAT box (CAAT), E-box (CANNTG) and DOFCOREZM (AAAG) regions was found in the PnPR-1 promoter regions. A high occurrence of the CAAT box was previously reported in A. thaliana PR proteins [34]. As previously reported, hormone-regulating sequence motifs [45] such as LS7, GCC-box, ABA-responsive elements (ABREs; also termed as G-box) [47] and ABI4 were detected from the upstream of certain PnPR-1 genes. LS7, which contains a TGA binding site, has been reported to be the key activator of PR-1 expression, NPR1 [48]. The ethylene-responsive factor binds to the GCC-box (ethylene-responsive element) and responds to various biotic and abiotic factors in Arabidopsis [49]. The ABA-insensitive-4 (ABI4) transcription factor was reported to be involved in ascorbate-dependent plant growth [50]. AC elements in the promoter region of the Leucoanthocyanidin reductase gene in the Proanthocyanidin pathway promoter harbors the binding site for MYB2 Myb-like transcription factors [51]. Subsequently, the WER-binding site (WBSI) and NonaLS are also reported in the PnPR-1 promoter region. WBSI was detected in the CAPRICE (CPC) promoter of Arabidopsis. WEREWOLF (WER) is a MYB protein and gene transcription activator during the specification of epidermal cell fates [52]. NonaLS (Nona-like sequence, GATCGGACG) is the positive cis-acting element of histone H1 genes in wheat, tomato and Arabidopsis [53]. The induction of both biotic- and abiotic-responsive cis-acting regulatory elements in PnPR-1 indicates that these genes play a key role in regulating resistance against P. capsici and other abiotic stresses in P. nigrum.
Even though PR-1 proteins belong to the group of abundant proteins expressed in the plant-pathogen interaction, all of the members of the family were not uniformly upregulated [13]. Likewise, a transcriptome-assisted analysis revealed a high upregulation of two basic PR-1 genes (pI > 7.35) such as CL10113.C1/2 and Unigene17664 in P. nigrum upon P. capsici infection. As PR-1 genes belong to the group of multigene families, they differ widely in their properties. PR-1 expression levels rise both transcriptionally and translationally upon pathogen infections [54,55]. Consistent with previous studies, the upregulation of PnPR-1 transcripts such as CL10113.C1, CL10113.C2 and Unigene17664 were detected during P. capsici infection in P. nigrum. The qRT-PCR expression studies also validated the drastically increased CL10113.C2 expression pattern at 24 hpi (Figure 6A). The significant upregulation of PR1 in P. nigrum-infected P. capsici showed similar expression patterns in the case of Brassica juncea and Erysiphecruciferarum pathogen interaction where the expression of PR1 was strongly upregulated [56]. This resembles the mechanism of PR genes being upregulated following pathogen infection, indicating that P. capsici actively works in manipulating the P. nigrum host defenses. In addition to the host defense response during pathogen infection, PR-1 proteins were also reported to have a role in abiotic stress stimuli [57,58,59].
The trypan blue staining clearly showed the necrotic region as the defense response of the host plant to the pathogen and its further effect to inhibit the growth of the pathogen to the surrounding regions (Figure 6B). This was, in turn, proved by the significant upregulation of the PnPR-1 genes at 24 h post-infection in the leaf samples using qPCR experiments. To date, only a few studies have been carried out on the role of PR proteins in P. nigrum or related Piperaceae species. The activity of PR protein chitinase, β-1,3-glucanase and their related enzymes were reported in P. capsici-infected P. nigrum plants [60,61,62]. The present study contributes a significant advancement in the understanding of the molecular function of PR-1 proteins in P. nigrum. PnPR-1 genes are found to have a key role in the early defense such as PTI towards P. capsici infection in Panniyur-1 plants. It may be possible that the key genes in the subsequent effector-triggered immunity act as critical players of the defense response in Panniyur-1 plants. Therefore, future studies on the identification of the potential P. capsici effectors coupled with PR-1 functional studies will ascertain the in-depth mechanisms of the defense signal amplification and anti-oomycete properties of these enigmatic proteins in P. nigrum.
5. Conclusions
The genome-wide survey identified eleven P. nigrum PR-1 gene homologs mapped to seven distinct genome scaffolds. A subsequent transcriptome analysis of P. capsici-infected P. nigrum plants showed the expression of PR-1 genes from all of the mapped loci. Our study revealed the differential regulation of PR-1 gene candidates in P. capsici-infected P. nigrum plants. A significant upregulation was detected for the transcripts of certain PnPR-1 genes such as CL10113.C2 and Unigene17664. A detailed in silico analysis revealed cis-regulatory elements such as phytohormone-responsive transcription activators in the promoter regions. The structural analysis revealed similar binding pockets in the predicted 3D structures of all PnPR-1 proteins except Pn31.171. The differential expression of certain PnPR-1 homologs revealed their crucial role during the early defense response in the P. capsici-P. nigrum interaction. Further in-depth functional studies on PnPR-1 genes, promoter cis-regulatory elements and the pathogen-specific effectors can provide the exact molecular mechanism of the susceptibility/tolerance of P. nigrum cultivars to Phytophthora infection, which, in turn, can contribute towards the novel disease protection strategies in P. nigrum plants.
Acknowledgments
K.D. acknowledges the Department of Biotechnology (DBT)—JRF and SRF and Commonwealth Scholarship Commission (CSC) for a split-site scholarship. S.A. greatly acknowledges the fellowship from the Council of Scientific and Industrial Research (CSIR)—JRF and SRF (S.A.). E.V.S. acknowledges financial support from DBT, New Delhi.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12071007/s1, Figure S1: The amino acid sequences from Piper nigrum, Nymphaea colorata, Elaeis guineensis, Cinnamomum micranthum, Gossypium tomentosum, Musa ABB, Amborella trichopoda and Phoenix dactylifera were aligned using Geneious bioinformatics software (https://www.geneious.com/, accessed on 28 January 2021) with default settings. The conserved domains are highlighted in different colors. The CAP domain structure with a caveolin-binding motif (CBM) and a CAP-derived peptide (CAPE) are shown in red and pink boxes; Figure S2: Phylogenetic analyses of Piper nigrum PR-1 nucleotide and protein sequences. The phylogenetic tree was constructed using MEGA 7.0. by the maximum-likelihood (ML) method with 1000 bootstrap replicates and default parameters. The PnPR-1 family genes were divided into two major groups, groups I and II; Figure S3: Secondary structure analyses of PnPR-1 proteins; Figure S4: Analysis of cis-acting elements in PnPR-1 promoters using the New PLACE online server. The number of elements in each gene is represented in data bars.
Author Contributions
D.K., A.S. and E.V.S. conceived the research plans and designed the experiments. D.K. and A.S. performed the experiments, analyzed the data and wrote the article, E.V.S. made critical revisions. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Department of Biotechnology (DBT), India. The funders have no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Control P. nigrum (uninfected) leaf transcriptome data and P. capsici-infected P. nigrum leaf transcriptome data can be accessed from NCBI SRA, accession numbers SRA050094 and SRX853366, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dangl J.L., Jones J.D.G. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 2.Van Baarlen P., Van Belkum A., Summerbell R.C., Crous P.W., Thomma B.P. Molecular mechanisms of pathogenicity: How do pathogenic microorganisms develop cross-kingdom host jumps? FEMS Microbiol. Rev. 2007;31:239–277. doi: 10.1111/j.1574-6976.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- 3.Chassot C., Nawrath C., Métraux J.-P. Cuticular defects lead to full immunity to a major plant pathogen. Plant J. 2007;49:972–980. doi: 10.1111/j.1365-313X.2006.03017.x. [DOI] [PubMed] [Google Scholar]
- 4.Boccardo N.A., Segretin M.E., Hernandez I., Mirkin F.G., Chacón O., Lopez Y., Borras-Hidalgo O., Bravo-Almonacid F.F. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-39568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Henanff G., Farine S., Kieffer-Mazet F., Miclot A.-S., Heitz T., Mestre P., Bertsch C., Chong J. Vitis vinifera VvNPR1.1 is the functional ortholog of AtNPR1 and its overexpression in grapevine triggers constitutive activation of PR genes and enhanced resistance to powdery mildew. Planta. 2011;234:405–417. doi: 10.1007/s00425-011-1412-1. [DOI] [PubMed] [Google Scholar]
- 6.Molla K.A., Karmakar S., Chanda P., Sarkar S.N., Datta S.K., Datta K. Tissue-specific expression of Arabidopsis NPR1 gene in rice for sheath blight resistance without compromising phenotypic cost. Plant Sci. 2016;250:105–114. doi: 10.1016/j.plantsci.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Malnoy M., Jin Q., Borejsza-Wysocka E.E., He S.Y., Aldwinckle H.S. Overexpression of the apple MpNPR1 gene confers increased disease resistance in Malus x domestica. Mol. Plant Microbe Interact. 2007;20:1568–1580. doi: 10.1094/MPMI-20-12-1568. [DOI] [PubMed] [Google Scholar]
- 8.Christensen A.B., Cho B.H.O., Næsby M., Gregersen P.L., Brandt J., Madriz-Ordeñana K., Collinge D.B., Thordal-Christensen H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant Pathol. 2002;3:135–144. doi: 10.1046/j.1364-3703.2002.00105.x. [DOI] [PubMed] [Google Scholar]
- 9.Loake G., Grant M. Salicylic acid in plant defence—The players and protagonists. Curr. Opin. Plant Biol. 2007;10:466–472. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Van Loon L.C., Rep M., Pieterse C.M.J. Significance of Inducible Defense-related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 11.Wang J.-E., Li D.-W., Zhang Y.-L., Zhao Q., He Y.-M., Gong Z.-H. Defence responses of pepper (Capsicum annuum L.) infected with incompatible and compatible strains of Phytophthora capsici. Eur. J. Plant Pathol. 2013;136:625–638. doi: 10.1007/s10658-013-0193-8. [DOI] [Google Scholar]
- 12.Akbudak M.A., Yildiz S., Filiz E. Pathogenesis related protein-1 (PR-1) genes in tomato (Solanum lycopersicum L.): Bioinformatics analyses and expression profiles in response to drought stress. Genomics. 2020;112:4089–4099. doi: 10.1016/j.ygeno.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Breen S., Williams S., Outram M., Kobe B., Solomon P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017;22:871–879. doi: 10.1016/j.tplants.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Kiba A., Nishihara M., Nakatsuka T., Yamamura S. Pathogenesis-related protein 1 homologue is an antifungal protein in Wasabia japonica leaves and confers resistance to Botrytis cinerea in transgenic tobacco. Plant Biotechnol. 2007;24:247–253. doi: 10.5511/plantbiotechnology.24.247. [DOI] [Google Scholar]
- 15.Sarowar S., Young J.K., Eui N.K., Ki D.K., Byung K.H., Islam R., Jeong S.S. Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep. 2005;24:216–224. doi: 10.1007/s00299-005-0928-x. [DOI] [PubMed] [Google Scholar]
- 16.Shin S.H., Pak J.-H., Kim M.J., Kim H.J., Oh J.S., Choi H.K., Jung-Hun P., Chung Y.S. An Acidic PATHOGENESIS-RELATED1 Gene of Oryza grandiglumis is Involved in Disease Resistance Response Against Bacterial Infection. Plant Pathol. J. 2014;30:208–214. doi: 10.5423/PPJ.NT.11.2013.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper B., Clarke J.D., Budworth P., Kreps J., Hutchison D., Park S., Guimil S., Dunn M., Luginbühl P., Ellero C., et al. A network of rice genes associated with stress response and seed development. Proc. Natl. Acad. Sci. USA. 2003;100:4945–4950. doi: 10.1073/pnas.0737574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotan T., Ori N., Fluhr R. Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell. 1989;1:881–887. doi: 10.1105/tpc.1.9.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.L., Lee C.Y., Cheng K.T., Chang W.H., Huang R.N., Nam H.G., Chen Y.R. Quantitative peptidomics study reveals that a wound-induced peptide from PR-1 regulates immune signaling in tomato. Plant Cell. 2014;26:4135–4148. doi: 10.1105/tpc.114.131185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamir J., Darwiche R., Hof P.V., Choudhary V., Stumpe M., Schneiter R., Mauch F. The sterol-binding activity of PATHOGENESIS-RELATED PROTEIN 1 reveals the mode of action of an antimicrobial protein. Plant J. 2017;89:502–509. doi: 10.1111/tpj.13398. [DOI] [PubMed] [Google Scholar]
- 21.Asha S., Sreekumar S., Soniya E.V. Unravelling the complexity of microRNA-mediated gene regulation in black pepper (Piper nigrum L.) using high-throughput small RNA profiling. Plant Cell Rep. 2016;35:53–63. doi: 10.1007/s00299-015-1866-x. [DOI] [PubMed] [Google Scholar]
- 22.Hu L., Xu Z., Wang M., Fan R., Yuan D., Wu B., Wu H., Qin X., Yan L., Tan L., et al. The chromosome-scale reference genome of black pepper provides insight into piperine biosynthesis. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-12607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall T.A. Nucleic Acids Symposium Series. Information Retrieval Ltd.; London, UK: 1999. BIOEDIT: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. [Google Scholar]
- 24.Marchler-Bauer A., Derbyshire M.K., Gonzales N.R., Lu S., Chitsaz F., Geer L.Y., Geer R.C., He J., Gwadz M., Hurwitz D.I., et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey T.L., Johnson J., Grant C.E., Noble W.S. The MEME Suite. Nucleic Acids Res. 2015;43:W39–W49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Törönen P., Medlar A., Holm L. PANNZER2: A rapid functional annotation web server. Nucleic Acids Res. 2018;46:W84–W88. doi: 10.1093/nar/gky350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanehisa M., Sato Y., Morishima K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016;428:726–731. doi: 10.1016/j.jmb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Kelley L.A., Mezulis S., Yates C.M., Wass M., Sternberg M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dundas J., Ouyang Z., Tseng J., Binkowski A., Turpaz Y., Liang J. CASTp: Computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006;34:W116–W118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández-Bautista N., Domínguez-Núñez J., Moreno M.M., Berrocal-Lobo M. Plant Tissue Trypan Blue Staining during Phytopathogen Infection. Bio-Protocol. 2016;6 doi: 10.21769/BioProtoc.2078. [DOI] [Google Scholar]
- 31.Asha S., Soniya E.V. Transfer RNA Derived Small RNAs Targeting Defense Responsive Genes Are Induced during Phytophthora capsici Infection in Black Pepper (Piper nigrum L.) Front. Plant Sci. 2016;7:1–16. doi: 10.3389/fpls.2016.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-DeltaDeltaC(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Higo K., Ugawa Y., Iwamoto M., Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur A., Pati P.K., Pati A.M., Nagpal A.K. In-silico analysis of cis-acting regulatory elements of pathogenesis-related proteins of Arabidopsis thaliana and Oryza sativa. PLoS ONE. 2017;12:e0184523. doi: 10.1371/journal.pone.0184523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrios G.N. Plant Pathology. 5th ed. Elsevier Academic Press; Amsterdam, The Netherlands: 2005. How plants defend themselves against pathogens. [Google Scholar]
- 36.Van Loon L.C., Gerritsen Y.A.M., Ritter C.E. Identification, purification, and characterization of pathogenesis-related proteins from virus-infected Samsun NN tobacco leaves. Plant Mol. Biol. 1987;9:593–609. doi: 10.1007/BF00020536. [DOI] [PubMed] [Google Scholar]
- 37.Van Loon L.C., Pierpoint W.S., Boller T., Conejero V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 1994;12:245–264. doi: 10.1007/BF02668748. [DOI] [Google Scholar]
- 38.Memelink J., Linthorst H.J.M., Schilperoort R.A., Hoge J.H.C. Tobacco genes encoding acidic and basic isoforms of pathogenesis-related proteins display different expression patterns. Plant Mol. Biol. 1990;14:119–126. doi: 10.1007/BF00018553. [DOI] [PubMed] [Google Scholar]
- 39.Mitsuhara I., Iwai T., Seo S., Yanagawa Y., Kawahigasi H., Hirose S., Ohkawa Y., Ohashi Y. Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180) Mol. Genet. Genom. 2008;279:415–427. doi: 10.1007/s00438-008-0322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida-Silva F., Venancio T.M. Pathogenesis-related protein 1 (PR-1) genes in soybean: Genome-wide identification, structural analysis and expression profiling under multiple biotic and abiotic stresses. bioRxiv. 2021 doi: 10.1101/2021.03.27.437342. [DOI] [PubMed] [Google Scholar]
- 41.Choudhary V., Darwiche R., Gfeller D., Zoete V., Michielin O., Schneiter R. The caveolin-binding motif of the pathogen-related yeast protein Pry1, a member of the CAP protein superfamily, is required for in vivo export of cholesteryl acetate. J. Lipid Res. 2014;55:883–894. doi: 10.1194/jlr.M047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lincoln J.E., Sanchez J.P., Zumstein K., Gilchrist D.G. Plant and animal PR1 family members inhibit programmed cell death and suppress bacterial pathogens in plant tissues. Mol. Plant Pathol. 2018;19:2111–2123. doi: 10.1111/mpp.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breen S., Williams S.J., Winterberg B., Kobe B., Solomon P.S. Wheat PR-1 proteins are targeted by necrotrophic pathogen effector proteins. Plant J. 2016;88:13–25. doi: 10.1111/tpj.13228. [DOI] [PubMed] [Google Scholar]
- 44.Lu S., Faris J., Sherwood R., Friesen T.L., Edwards M.C. A dimeric PR-1-type pathogenesis-related protein interacts with ToxA and potentially mediates ToxA-induced necrosis in sensitive wheat. Mol. Plant Pathol. 2014;15:650–663. doi: 10.1111/mpp.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danino Y.M., Even D., Ideses D., Juven-Gershon T. The core promoter: At the heart of gene expression. Biochim. Biophys. Acta BBA Bioenerg. 2015;1849:1116–1131. doi: 10.1016/j.bbagrm.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Kaur G., Pati P.K. Analysis of cis-acting regulatory elements of Respiratory burst oxidase homolog ( Rboh ) gene families in Arabidopsis and rice provides clues for their diverse functions. Comput. Biol. Chem. 2016;62:104–118. doi: 10.1016/j.compbiolchem.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Ross C., Shen Q.J. Computational Prediction and Experimental Verification of HVA1-like Abscisic Acid Responsive Promoters in Rice (Oryza sativa) Plant Mol. Biol. 2006;62:233–246. doi: 10.1007/s11103-006-9017-y. [DOI] [PubMed] [Google Scholar]
- 48.Kesarwani M., Yoo J., Dong X. Genetic Interactions of TGA Transcription Factors in the Regulation of Pathogenesis-Related Genes and Disease Resistance in Arabidopsis. Plant Physiol. 2007;144:336–346. doi: 10.1104/pp.106.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujimoto S.Y., Ohta M., Usui A., Shinshi H., Ohme-Takagi M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell. 2000;12:393–404. doi: 10.1105/tpc.12.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foyer C.H., Kerchev P.I., Hancock R.D. The ABA-INSENSITIVE-4 (ABI4) transcription factor links redox, hormone and sugar signaling pathways. Plant Signal. Behav. 2012;7:276–281. doi: 10.4161/psb.18770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akagi T., Ikegami A., Yonemori K. DkMyb2 wound-induced transcription factor of persimmon (Diospyros kaki Thunb.), contributes to proanthocyanidin regulation. Planta. 2010;232:1045–1059. doi: 10.1007/s00425-010-1241-7. [DOI] [PubMed] [Google Scholar]
- 52.Ryu K.H., Kang Y.H., Park Y.H., Hwang I., Schiefelbein J., Lee M.M. The WEREWOLF MYB protein directly regulates CAPRICE transcription during cell fate specification in the Arabidopsis root epidermis. Development. 2005;132:4765–4775. doi: 10.1242/dev.02055. [DOI] [PubMed] [Google Scholar]
- 53.Taoka K.-I., Ohtsubo N., Fujimoto Y., Mikami K., Meshi T., Iwabuchi M. The Modular Structure and Function of the Wheat H1 Promoter with S Phase-Specific Activity. Plant Cell Physiol. 1998;39:294–306. doi: 10.1093/oxfordjournals.pcp.a029370. [DOI] [PubMed] [Google Scholar]
- 54.Ali S., Mir Z.A., Bhat J.A., Tyagi A., Chandrashekar N., Yadav P., Rawat S., Sultana M., Grover A. Isolation and characterization of systemic acquired resistance marker gene PR1 and its promoter from Brassica juncea. 3 Biotech. 2018;8:10. doi: 10.1007/s13205-017-1027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tunsagool P., Jutidamrongphan W., Phaonakrop N., Jaresitthikunchai J., Roytrakul S., Leelasuphakul W. Insights into stress responses in mandarins triggered by Bacillus subtilis cyclic lipopeptides and exogenous plant hormones upon Penicillium digitatum infection. Plant Cell Rep. 2019;38:559–575. doi: 10.1007/s00299-019-02386-1. [DOI] [PubMed] [Google Scholar]
- 56.Ali S., Mir Z.A., Tyagi A., Bhat J.A., Chandrashekar N., Papolu P.K., Rawat S., Grover A. Identification and comparative analysis of Brassica juncea pathogenesis-related genes in response to hormonal, biotic and abiotic stresses. Acta Physiol. Plant. 2017;39:268. doi: 10.1007/s11738-017-2565-8. [DOI] [Google Scholar]
- 57.Seo P.J., Kim M.J., Park J.-Y., Kim S.-Y., Jeon J., Lee Y.-H., Kim J., Park C.-M. Cold activation of a plasma membrane-tethered NAC transcription factor induces a pathogen resistance response in Arabidopsis. Plant J. 2010;61:661–671. doi: 10.1111/j.1365-313X.2009.04091.x. [DOI] [PubMed] [Google Scholar]
- 58.Kothari K.S., Dansana P.K., Giri J., Tyagi A.K. Rice Stress Associated Protein 1 (OsSAP1) Interacts with Aminotransferase (OsAMTR1) and Pathogenesis-Related 1a Protein (OsSCP) and Regulates Abiotic Stress Responses. Front. Plant Sci. 2016;7:1057. doi: 10.3389/fpls.2016.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu W.-X., Zhang F.-C., Zhang W.-Z., Song L.-F., Wu W.-H., Chen Y.-F. Arabidopsis Di19 Functions as a Transcription Factor and Modulates PR1, PR2, and PR5 Expression in Response to Drought Stress. Mol. Plant. 2013;6:1487–1502. doi: 10.1093/mp/sst031. [DOI] [PubMed] [Google Scholar]
- 60.Nazeem P.A., Achuthan C.R., Babu T.D., Parab G.V., Girija D., Keshavachandran R., Samiyappan R. Expression of pathogenesis related proteins in black pepper (Piper nigrum L.) in relation to Phytophthora foot rot disease. J. Trop. Agric. 2008;46:45–51. [Google Scholar]
- 61.Trang Anh T., Bao Linh T., Vu Phong N., Lan Thanh Bien T., Thi Nha Tram T., Dinh Don L. Expression of Proteins Related to Phytophthora capsici Tolerance in Black Pepper (Piper nigrum L.) Int. J. Agric. Innov. Res. 2018;6:2319-1473. [Google Scholar]
- 62.Vijesh Kumar I.P., Johnson G.K., Anandaraj M. Real-Time Quantitative RT-PCR of Some Defense Inoculated with Phytophthora Capsici. Int. J. Agric. Sci. Res. 2016;6:69–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Control P. nigrum (uninfected) leaf transcriptome data and P. capsici-infected P. nigrum leaf transcriptome data can be accessed from NCBI SRA, accession numbers SRA050094 and SRX853366, respectively.