Abstract
Integrase (IN) enzymes are found in all retroviruses and are crucial in the retroviral integration process. Many studies have revealed how exogenous IN enzymes, such as the human immunodeficiency virus (HIV) IN, contribute to altered cellular function. However, the same consideration has not been given to viral IN originating from symbionts within our own DNA. Endogenous retrovirus-K (ERVK) is pathologically associated with neurological and inflammatory diseases along with several cancers. The ERVK IN interactome is unknown, and the question of how conserved the ERVK IN protein–protein interaction motifs are as compared to other retroviral integrases is addressed in this paper. The ERVK IN protein sequence was analyzed using the Eukaryotic Linear Motif (ELM) database, and the results are compared to ELMs of other betaretroviral INs and similar eukaryotic INs. A list of putative ERVK IN cellular protein interactors was curated from the ELM list and submitted for STRING analysis to generate an ERVK IN interactome. KEGG analysis was used to identify key pathways potentially influenced by ERVK IN. It was determined that the ERVK IN potentially interacts with cellular proteins involved in the DNA damage response (DDR), cell cycle, immunity, inflammation, cell signaling, selective autophagy, and intracellular trafficking. The most prominent pathway identified was viral carcinogenesis, in addition to select cancers, neurological diseases, and diabetic complications. This potentiates the role of ERVK IN in these pathologies via protein–protein interactions facilitating alterations in key disease pathways.
Keywords: endogenous retrovirus, integrase, interactome, eukaryotic linear motif, DNA damage response, viral carcinogenesis, cancer, amyotrophic lateral sclerosis, diabetes, model organisms
1. Introduction
Viral proteins often usurp and alter cellular signaling pathways. For exogenous viruses, this tweaking of cellular function serves to enhance their replicative success through the modulation of pathways related to virion production, dissemination, cell survival, and immunity [1,2]. It is less clear in what manner ever-present viral symbionts such as endogenous retroviruses (ERVs) interact with the proteome of their hosts.
The genomes of eukaryotic organisms are widely populated with ERVs [3,4,5]. Endogenous retrovirus-K (ERVK/HERV-K) is a biomedically-relevant symbiont within the primate lineage [6,7]. Its expression has been associated with a variety of cancers [8], neurological conditions [9,10,11,12,13], autoimmune diseases [14], and infections [13,15]. A common thread that weaves through ERVK-associated disease is genomic instability. DNA damage and genomic alterations are hallmarks of many cancers [16], as well as in the neurons of patients with the motor neuron disease ALS [17,18]. One protein known to cause DNA damage during the retroviral life cycle is the integrase (IN) enzyme [19]. Recovery from IN-driven lesions is reliant on the host DNA damage response (DDR) [20,21].
We have previously shown that several ERVK insertions in the human genome have the potential to produce functional ERVK IN enzymes with the identical DDE active site motif found in human immunodeficiency virus (HIV) IN [22]. Based on homology modeling, we predict that the ERVK IN enzyme contains all the essential motifs and domain structures for retroviral IN function [22]. A recombinant ERVK-10 integrase enzyme also confirms that it has the potential for strand-transfer activity [23]. A remaining question is how ERVK IN interacts with cellular proteins and pathways, as has been shown for many other retroviral integrases [19,24,25,26].
Retroviral INs are involved in pre-integration complex (PIC) transport [27], viral genome integration into host DNA [19], and virion maturation [28]. Thus, retroviral integrase enzymes exhibit a diversity of cellular partners and have been shown to impact cell signaling and survival processes, including the DDR [19,29]. For example, retroviral IN often recruits viral proteins (reverse transcriptase, matrix, and capsid) and cellular factors (BANF1, HGMA1, LEDGF) to participate in the viral DNA integration process [30,31]. Moreover, successful viral DNA integration requires engagement of the host DDR proteins to repair residual single-stranded DNA gaps flanking the integration site [20,29,32]. In contrast, failed provirus insertion or unresolved lesions can lead to double-stranded DNA (dsDNA) breaks in the host genome [29]. The level of γH2AX foci is positively correlated with the number of double-stranded DNA breaks (DSB) in mammalian cells, and it is widely used as a quantitative biomarker of retrovirus-mediated DSBs [19,33,34]. This genomic damage is particularly hazardous to the cell, as DSB potentially lead to chromosomal rearrangements, cellular deregulation, and apoptosis [35,36]. Thus, as an intrinsic protective measure, select host proteins (RAD51, Kap1, TREX1, p21, HDAC10, TRIM33) are known antagonists of the retroviral integration process [31,37,38,39,40]. Many studies have identified direct protein binding partners and cellular complexes which interact with HIV integrase [41,42,43]; in contrast, the ERVK IN interactome remains unknown.
A complicating factor for the development of model systems to study the impact of ERVK proteins in vivo is that many other organisms contain ERVs with similarity to ERVK. Given the known cellular impacts of retroviral integrases, we hypothesized that a computational biology approach would identify potential cellular partners of ERVK IN and point toward its capacity to modulate cellular pathways. Additionally, a comparison with similar integrases in eukaryotic organism and model species may inform the future establishment of in vivo models for ERVK IN-driven pathology.
2. Materials and Methods
2.1. Database Curation
Integrases with sequence similarity to ERVK IN (based on ERVK-10 [22]; P10266.2) were identified using the National Centre for Biotechnology’s (NCBI) Protein–protein Basic Local Alignment Search Tool (BLASTp) within the non-redundant (nr), model organisms (mo), and transcriptome shotgun assembly proteins (tsa) databases [44]. Default algorithm parameters were used, with E-value cut-offs for each database as follows: E < 3.0 × 10−70 (nr), E < 0.01 (mo), E < 2.0 × 10−10 (tsa). Sequences were grouped based on phylogeny as informed by ICTV (International Committee on Taxonomy of Viruses; 2021, https://talk.ictvonline.org/ (accessed on 16 May 2021)) or OneZoom (OneZoom Tree of Life Explorer; version 3.4.1; Software for Technical Computation; United Kingdom, 2021, https://www.onezoom.org/ (accessed on 16 May 2021)) [45] and are listed in Table A1, Table A2, Table A3 and Table A4.
2.2. Protein Alignments and Eukaryotic Linear Motif Annotation
The ERVK IN protein sequence, as well as select representative integrases from exogenous Betaretroviruses (Figure 1) or endogenous retroviruses (Figure 2) were aligned using Geneious Prime (version 2021.0.3; Software for Technical Computation; San Diego, CA, USA; Auckland, New Zealand, 2021) software [46]. A global alignment with free end gaps using BLOSUM62 matrix was performed. Longer sequences were truncated to overlap with the ERVK IN reference sequence. Figures depict the sequence logo and integrase active sites, with HHCC and DDE regions highlighted based on Conserved Domains Database (CDD) annotation [47].
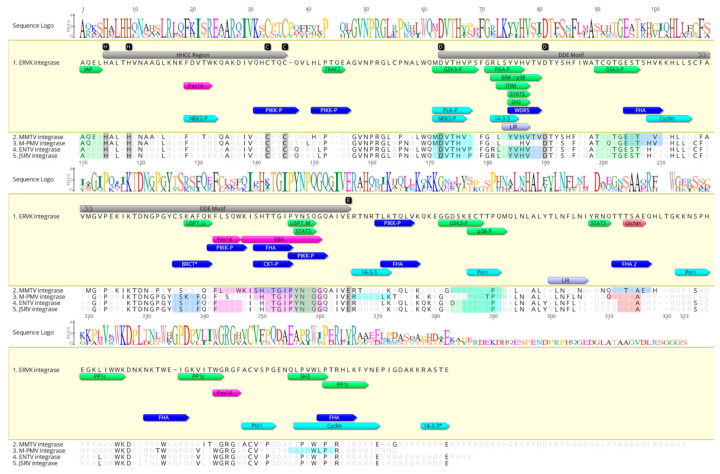
Figure 1.
ERVK integrase and exogenous betaretrovirus integrases share common eukaryotic linear motifs. In silico examination of the conserved and differential eukaryotic linear motifs (ELMs) within Endogenous retrovirus-K (ERVK) and similar betaretroviral integrases. A betaretroviral integrase consensus sequence was constructed using GenBank sequences as follows: Endogenous retrovirus-K (ERVK; P10266.2), Exogenous mouse mammary tumor virus (MMTV; AAF31469.1), Mason–Pfizer monkey virus 5 (M-PMV; BBG56792.1), Enzootic nasal tumor virus (ENTV; ANG58699.1) and Jaagsiekte sheep retrovirus (JSRV; NP_041186.1). The HHCC region and DDE active site motif (gray, with key aa. in black) was positioned based on Conserved Domains annotations. ELMs were grouped based on related pathways: DNA damage response (dark blue), cell cycle (cyan), cell signaling (green), cell trafficking (magenta), autophagy (mauve), and glycosylation (red). ELM abbreviations used include: 14-3-3 = 14-3-3 protein interaction site, BRCT = BRCA1 C-terminus domain interaction site, CK1-P = casein kinase 1 phosphorylation site, Cyclin = cyclin docking site, EBH = end binding homology domain interaction site, ERK/p38 = ERK1/2 and p38 MAP kinase docking site, FHA = Forkhead-associated domain interaction site, GlyNH = glycosaminoglycan attachment site, GSK3-P = GSK3 phosphorylation site, IAP = inhibitor of apoptosis protein interaction site, ITIM = immunoreceptor tyrosine-based inhibitory motif, LIR = site that interacts with Atg8 protein family members, NEK2-P = NEK2 phosphorylation site, p38-P = p38 phosphorylation site, Pex14 = peroxisomal membrane docking via Pex14, PIKK-P = PIKK family phosphorylation site, Pin1 = docking site for Pin1 via WW domain interaction, PKA-P = PKA phosphorylation site, PLK-P = polo-like kinase phosphorylation site, PP1c = protein phosphatase 1 catalytic subunit docking motif, SH2 = Src homology 2 domain interaction motif, SH3 = interaction site for non-canonical class I recognition specificity SH3 domains, STAT3 = STAT3 SH2 domain binding motif, STAT5 = STAT5 SH2 domain binding motif, TRAF2 = major TRAF2 binding consensus motif, USP7 = USP7 MATH (M) or UBL2 (U) domain interaction sites, WDR5 = interaction motif for WDR5 via WW domain interaction. Asterisks indicate ELMs unique to ERVK. Sequence alignment and annotation were performed using Geneious Prime software.
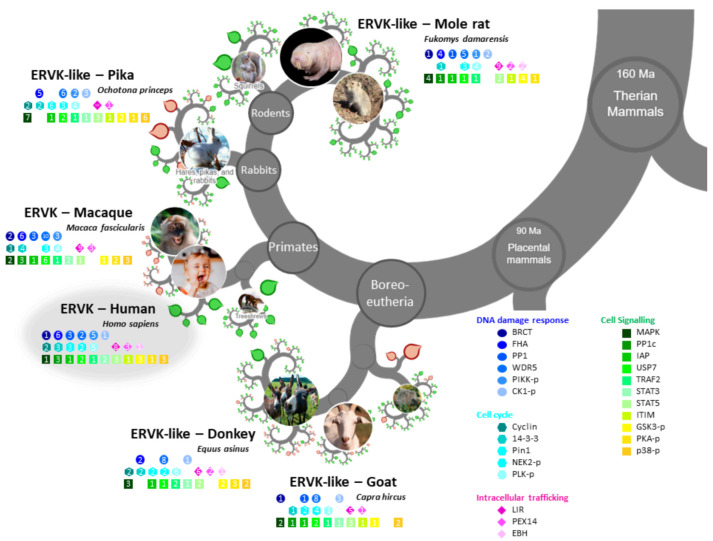
Figure 2.
ERVK integrase and similar endogenous integrases share eukaryotic linear motifs patterns. Modified OneZoom image illustrating the conservation of ELM motifs in integrases from eukaryotic organisms (Homo sapiens, Macaca fasicicularis, Fukomys damarensis, Ochotona princeps, Equis asinus, and Capra hircus). Motifs are color-grouped according to function; DDR (blue), cell cycle (cyan), cell signaling (green), and intracellular trafficking (magenta). The number in each colored shape refers to the number of motifs with the respective integrase enzyme.
Each aligned integrase sequence was submitted to the Eukaryotic Linear Motif (ELM; Software for Technical Computation; 2020, http://elm.eu.org/ (accessed on 16 May 2021)) resource [48]. A complete listing of ELMs identified in each integrase is presented in Table 1 and Table 2. ELMs unique to ERVK IN, as well as ELM sites exhibiting motif consensus above 70% with other integrases, were annotated in Figure 1 and Figure 2.
Table 1.
ELM motifs in integrases from ERVK and exogenous betaretroviruses.
| ELM Motif | ELM Accession | Alignment Notation | Integrase | Conservation | |||||
|---|---|---|---|---|---|---|---|---|---|
| ERVK | MMTV | M-PMV | ENTV | JSRV | |||||
| Cleavage and degradation |
CLV_C14_Caspase3-7 | ELME000321 | 1 | 0 | 0 | 0 | 2 | 0.4 | |
| CLV_PCSK_KEX2_1 | ELME000108 | 1 | 0 | 0 | 0 | 0 | 0.2 | ||
| CLV_NRD_NRD_1 | ELME000102 | 0 | 1 | 0 | 1 | 1 | 0.6 | ||
| CLV_PCSK_PC1ET2_1 | ELME000100 | 1 | 0 | 0 | 0 | 0 | 0.2 | ||
| CLV_PCSK_SKI1_1 | ELME000146 | 5 | 4 | 4 | 1 | 0 | 0.8 | ||
| DEG_APCC_DBOX_1 | ELME000231 | 0 | 0 | 0 | 1 | 0 | 0.2 | ||
| Docking | DOC_CKS1_1 | ELME000358 | 0 | 1 | 0 | 0 | 0 | 0.2 | |
| DOC_CYCLIN_RxL_1 | ELME000106 | Cyclin | 2 | 0 | 2 | 0 | 0 | 0.4 | |
| DOC_MAPK_gen_1 | ELME000233 | 0 | 2 | 0 | 1 | 1 | 0.6 | ||
| DOC_MAPK_MEF2A_6 | ELME000432 | ERK/p38 | 1 | 1 | 0 | 1 | 1 | 0.8 | |
| DOC_PP1_RVXF_1 | ELME000137 | PP1c | 3 | 1 | 1 | 1 | 1 | 1.0 | |
| DOC_PP2B_LxvP_1 | ELME000367 | 1 | 0 | 1 | 0 | 0 | 0.4 | ||
| DOC_PP4_FxxP_1 | ELME000477 | 0 | 1 | 0 | 1 | 1 | 0.6 | ||
| DOC_USP7_MATH_1 | ELME000239 | USP7_M | 1 | 3 | 0 | 2 | 2 | 0.8 | |
| DOC_USP7_UBL2_3 | ELME000394 | USP7_U | 1 | 0 | 1 | 0 | 0 | 0.4 | |
| DOC_WW_Pin1_4 | ELME000136 | Pin1 | 3 | 6 | 1 | 3 | 2 | 1.0 | |
| Ligand | LIG_14-3-3_CanoR_1 | ELME000417 | 14-3-3 | 2 | 1 | 1 | 2 | 2 | 1.0 |
| LIG_14-3-3_CterR_2 | ELME000418 | 14-3-3 * | 1 | 0 | 0 | 0 | 0 | 0.2 | |
| LIG_Actin_WH2_2 | ELME000313 | 0 | 1 | 0 | 1 | 1 | 0.6 | ||
| LIG_APCC_ABBA_1 | ELME000435 | 0 | 1 | 0 | 0 | 0 | 0.2 | ||
| LIG_BIR_II_1 | ELME000285 | IAP | 1 | 1 | 1 | 1 | 1 | 1.0 | |
| LIG_BIR_III_4 | ELME000293 | 0 | 0 | 0 | 1 | 0 | 0.2 | ||
| LIG_BRCT_BRCA1_1 | ELME000197 | BRCT | 1 | 0 | 1 | 1 | 1 | 0.8 | |
| LIG_BRCT_BRCA1_2 | ELME000198 | BRCT1 * | 1 | 0 | 0 | 0 | 0 | 0.2 | |
| LIG_CSL_BTD_1 | ELME000410 | 1 | 1 | 0 | 0 | 0 | 0.4 | ||
| LIG_EH1_1 | ELME000148 | 0 | 0 | 1 | 0 | 1 | 0.4 | ||
| LIG_eIF4E_1 | ELME000317 | 0 | 1 | 0 | 0 | 0 | 0.2 | ||
| LIG_FHA_1 | ELME000052 | FHA | 5 | 1 | 2 | 0 | 1 | 0.8 | |
| LIG_FHA_2 | ELME000220 | FHA 2 | 1 | 1 | 1 | 0 | 0 | 0.6 | |
| LIG_LIR_Apic_2 | ELME000369 | 0 | 3 | 2 | 1 | 1 | 0.8 | ||
| LIG_LIR_Gen_1 | ELME000368 | LIR | 2 | 0 | 0 | 0 | 0 | 0.2 | |
| LIG_MAD2 | ELME000167 | 0 | 0 | 0 | 1 | 0 | 0.2 | ||
| LIG_NRBOX | ELME000045 | 0 | 0 | 0 | 1 | 0 | 0.2 | ||
| LIG_LIR_Nem_3 | ELME000370 | 0 | 6 | 7 | 1 | 4 | 0.8 | ||
| LIG_Pex14_1 | ELME000080 | Pex14 | 1 | 0 | 0 | 0 | 0 | 0.2 | |
| LIG_Pex14_2 | ELME000328 | Pex14 | 2 | 2 | 1 | 1 | 1 | 1.0 | |
| LIG_PTB_Apo_2 | ELME000122 | 0 | 0 | 1 | 0 | 1 | 0.4 | ||
| LIG_PTB_Phospho_1 | ELME000095 | 0 | 0 | 0 | 0 | 1 | 0.2 | ||
| LIG_RPA_C_Fungi | ELME000382 | 0 | 0 | 1 | 1 | 1 | 0.6 | ||
| LIG_SH2_CRK | ELME000458 | 0 | 2 | 0 | 0 | 0 | 0.2 | ||
| LIG_SH2_NCK_1 | ELME000474 | 0 | 1 | 0 | 0 | 0 | 0.2 | ||
| LIG_SH2_PTP2 | ELME000083 | SH2 | 1 | 1 | 0 | 1 | 1 | 0.8 | |
| LIG_SH2_SRC | ELME000081 | SH2 | 1 | 1 | 1 | 1 | 1 | 1.0 | |
| LIG_SH2_STAP1 | ELME000465 | 2 | 1 | 0 | 0 | 0 | 0.4 | ||
| LIG_SH2_STAT3 | ELME000163 | STAT3 | 2 | 1 | 1 | 1 | 1 | 1.0 | |
| LIG_SH2_STAT5 | ELME000182 | STAT5 | 3 | 3 | 2 | 3 | 3 | 1.0 | |
| LIG_SH3_1 | ELME000005 | 0 | 1 | 0 | 0 | 0 | 0.2 | ||
| LIG_SH3_3 | ELME000155 | SH3 | 1 | 2 | 1 | 1 | 1 | 1.0 | |
| LIG_SH3_4 | ELME000156 | 0 | 0 | 0 | 1 | 0 | 0.2 | ||
| LIG_SxIP_EBH_1 | ELME000254 | EBH | 1 | 1 | 1 | 1 | 2 | 1.0 | |
| LIG_TRAF2_1 | ELME000117 | TRAF2 | 1 | 1 | 0 | 1 | 1 | 0.8 | |
| LIG_TYR_ITIM | ELME000020 | ITIM | 1 | 1 | 1 | 1 | 1 | 1.0 | |
| LIG_Vh1_VBS_1 | ELME000438 | 0 | 0 | 0 | 0 | 1 | 0.2 | ||
| LIG_WD40_WDR5_VDV_2 | ELME000365 | WDR5 | 2 | 10 | 3 | 8 | 9 | 1.0 | |
| LIG_WW_3 | ELME000135 | 0 | 1 | 0 | 0 | 0 | 0.2 | ||
| Modification | MOD_CDK_SPK_2 | ELME000429 | 0 | 0 | 1 | 0 | 0 | 0.2 | |
| MOD_CDK_SPxK_1 | ELME000153 | 0 | 1 | 0 | 0 | 0 | 0.2 | ||
| MOD_CK1_1 | ELME000063 | CK1-P | 1 | 3 | 2 | 4 | 4 | 1.0 | |
| MOD_CK2_1 | ELME000064 | 3 | 3 | 1 | 0 | 0 | 0.6 | ||
| MOD_Cter_Amidation | ELME000093 | 1 | 0 | 0 | 0 | 0 | 0.2 | ||
| MOD_GlcNHglycan | ELME000085 | GlyNH | 1 | 2 | 3 | 1 | 2 | 1.0 | |
| MOD_GSK3_1 | ELME000053 | GSK3-P | 3 | 4 | 2 | 1 | 2 | 1.0 | |
| MOD_NEK2_1 | ELME000336 | NEK2-P | 2 | 3 | 3 | 2 | 4 | 1.0 | |
| MOD_NEK2_2 | ELME000337 | 0 | 0 | 1 | 0 | 0 | 0.2 | ||
| MOD_N-GLC_1 | ELME000070 | 1 | 0 | 1 | 2 | 0 | 0.6 | ||
| MOD_PIKK_1 | ELME000202 | 5 | 0 | 1 | 0 | 0 | 0.4 | ||
| MOD_PKA_1 | ELME000008 | 0 | 1 | 0 | 0 | 0 | 0.2 | ||
| MOD_PKA_2 | ELME000062 | PKA-P | 1 | 0 | 1 | 2 | 2 | 0.8 | |
| MOD_Plk_1 | ELME000442 | PLK-P | 4 | 1 | 2 | 1 | 1 | 1.0 | |
| MOD_Plk_4 | ELME000444 | 1 | 0 | 1 | 0 | 0 | 0.4 | ||
| MOD_ProDKin_1 | ELME000159 | p38-P | 3 | 6 | 1 | 3 | 2 | 1.0 | |
| MOD_SUMO_rev_2 | ELME000393 | 1 | 1 | 0 | 0 | 0 | 0.4 | ||
| Target | TRG_ENDOCYTIC_2 | ELME000120 | 2 | 4 | 2 | 1 | 1 | 1.0 | |
| TRG_Pf-PMV_PEXEL_1 | ELME000462 | 1 | 2 | 1 | 1 | 1 | 1.0 | ||
GenBank accession numbers for betaretroviral integrase sequences are as follows: Endogenous retrovirus-K (ERVK; P10266.2), Exogenous mouse mammary tumor virus (MMTV; AAF31469.1), Mason–Pzifer monkey virus 5 (M-PMV; BBG56792.1), Enzootic nasal tumor virus (ENTV; ANG58699.1) and Jaagsiekte sheep retrovirus (JSRV; NP_041186.1). Asterisk indicates ERVK-specific ELM motif in Figure 1.
Table 2.
ELM motifs in ERVK integrase and similar endogenous integrases in eukaryotes.
| ELM Motif | ELM Accession | ERVK Integrase (Homo sapiens) |
ERVK-8 Pol protein-Like (Macaca fascicularis) |
Pol Protein (Chlorocebus sabaeus) |
Pol Protein (Fukomys darmarensis) |
Putative Protein (Ochonta princeps) |
ERVK-8 pol Protein-Like (Equus asinus) |
ERVK-18 pol Protein-Like (Capra hircus) |
Pol Protein (Ovis aries) |
Putative Protein (Zonotrichia albicollis) |
Putative Protein (Zosterops borbonicus) |
Integrase (Onchocerca flexuosa) |
Motif conservation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cleavage | CLV_C14_Caspase3-7 | ELME000321 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 |
| CLV_NRD_NRD_1 | ELME000102 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.2 | |
| CLV_PCSK_KEX2_1 | ELME000108 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.3 | |
| CLV_PCSK_PC1ET2_1 | ELME000100 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.3 | |
| CLV_PCSK_SKI1_1 | ELME000146 | 5 | 3 | 4 | 3 | 7 | 8 | 0 | 0 | 3 | 5 | 2 | 0.8 | |
| Degradation | DEG_APCC_DBOX_1 | ELME000231 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 |
| DEG_MDM2_SWIB_1 | ELME000184 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0.1 | |
| Docking | DOC_CKS1_1 | ELME000358 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 1 | 0.5 |
| DOC_CYCLIN_RxL_1 | ELME000106 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 0 | 3 | 3 | 1 | 0.7 | |
| DOC_MAPK_DCC_7 | ELME000433 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | |
| DOC_MAPK_gen_1 | ELME000233 | 0 | 1 | 0 | 3 | 5 | 2 | 1 | 1 | 2 | 4 | 1 | 0.8 | |
| DOC_MAPK_HePTP_8 | ELME000434 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | |
| DOC_MAPK_MEF2A_6 | ELME000432 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 3 | 4 | 3 | 0.8 | |
| DOC_PP1_RVXF_1 | ELME000137 | 3 | 3 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0.7 | |
| DOC_PP2A_B56_1 | ELME000425 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | |
| DOC_PP2B_LxvP_1 | ELME000367 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0.5 | |
| DOC_PP2B_PxIxI_1 | ELME000237 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | |
| DOC_PP4_FxxP_1 | ELME000477 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0.6 | |
| DOC_USP7_MATH_1 | ELME000239 | 1 | 3 | 0 | 1 | 1 | 1 | 2 | 2 | 3 | 1 | 3 | 0.9 | |
| DOC_USP7_MATH_2 | ELME000240 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | |
| DOC_USP7_UBL2_3 | ELME000394 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0.4 | |
| DOC_WW_Pin1_4 | ELME000136 | 3 | 0 | 1 | 0 | 6 | 2 | 2 | 2 | 3 | 3 | 1 | 0.8 | |
| Ligand | LIG_14-3-3_CanoR_1 | ELME000417 | 2 | 4 | 1 | 1 | 2 | 2 | 1 | 2 | 3 | 1 | 1 | 1.0 |
| LIG_14-3-3_CterR_2 | ELME000418 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | |
| LIG_Actin_WH2_2 | ELME000313 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0.5 | |
| LIG_APCC_ABBA_1 | ELME000435 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0.2 | |
| LIG_BIR_II_1 | ELME000285 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1.0 | |
| LIG_BIR_III_4 | ELME000293 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0.2 | |
| LIG_BRCT_BRCA1_1 | ELME000197 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0.5 | |
| LIG_BRCT_BRCA1_2 | ELME000198 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | |
| LIG_CSL_BTD_1 | ELME000410 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.5 | |
| LIG_deltaCOP1_diTrp_1 | ELME000459 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0.1 | |
| LIG_EH1_1 | ELME000148 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0.3 | |
| LIG_eIF4E_1 | ELME000317 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | |
| LIG_FHA_1 | ELME000052 | 5 | 5 | 5 | 3 | 2 | 0 | 0 | 1 | 1 | 1 | 4 | 0.8 | |
| LIG_FHA_2 | ELME000220 | 1 | 1 | 0 | 1 | 3 | 2 | 0 | 0 | 0 | 1 | 0 | 0.5 | |
| LIG_LIR_Apic_2 | ELME000369 | 0 | 3 | 3 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 0.8 | |
| LIG_LIR_Gen_1 | ELME000368 | 2 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 0.8 | |
| LIG_LIR_Nem_3 | ELME000370 | 6 | 5 | 7 | 6 | 7 | 3 | 4 | 4 | 1 | 3 | 6 | 1.0 | |
| LIG_LYPXL_S_1 | ELME000294 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | |
| LIG_PCNA_PIPBox_1 | ELME000140 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | |
| LIG_PCNA_yPIPBox_3 | ELME000482 | 0 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0.3 | |
| LIG_Pex14_1 | ELME000080 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0.4 | |
| LIG_Pex14_2 | ELME000328 | 2 | 3 | 1 | 2 | 0 | 2 | 1 | 1 | 1 | 2 | 1 | 0.9 | |
| LIG_PTB_Apo_2 | ELME000122 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0.5 | |
| LIG_PTB_Phospho_1 | ELME000095 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0.4 | |
| LIG_REV1ctd_RIR_1 | ELME000450 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0.1 | |
| LIG_RPA_C_Fungi | ELME000382 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0.3 | |
| LIG_SH2_CRK | ELME000458 | 0 | 1 | 0 | 1 | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0.5 | |
| LIG_SH2_GRB2like | ELME000084 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | |
| LIG_SH2_NCK_1 | ELME000474 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0.5 | |
| LIG_SH2_PTP2 | ELME000083 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0.7 | |
| LIG_SH2_SRC | ELME000081 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 0.8 | |
| LIG_SH2_STAP1 | ELME000465 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0.6 | |
| LIG_SH2_STAT3 | ELME000163 | 2 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0.7 | |
| LIG_SH2_STAT5 | ELME000182 | 3 | 1 | 3 | 2 | 3 | 2 | 3 | 3 | 3 | 2 | 5 | 1.0 | |
| LIG_SH3_3 | ELME000155 | 1 | 2 | 1 | 1 | 3 | 2 | 1 | 1 | 5 | 4 | 2 | 1.0 | |
| LIG_SUMO_SIM_par_1 | ELME000333 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0.4 | |
| LIG_SxIP_EBH_1 | ELME000254 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 0.6 | |
| LIG_TRAF2_1 | ELME000117 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0.6 | |
| LIG_TRAF6 | ELME000133 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0.1 | |
| LIG_TRFH_1 | ELME000249 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | |
| LIG_TYR_ITIM | ELME000020 | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0.7 | |
| LIG_UBA3_1 | ELME000395 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | |
| LIG_Vh1_VBS_1 | ELME000438 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0.4 | |
| LIG_WD40_WDR5_VDV_1 | ELME000364 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0.1 | |
| LIG_WD40_WDR5_VDV_2 | ELME000365 | 2 | 10 | 6 | 5 | 6 | 8 | 8 | 8 | 10 | 10 | 12 | 1.0 | |
| LIG_WW_3 | ELME000135 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | |
| Modification | MOD_CDK_SPK_2 | ELME000429 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.3 |
| MOD_CDK_SPxxK_3 | ELME000428 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0.4 | |
| MOD_CK1_1 | ELME000063 | 1 | 0 | 1 | 2 | 3 | 1 | 3 | 3 | 4 | 3 | 3 | 0.9 | |
| MOD_CK2_1 | ELME000064 | 3 | 2 | 1 | 4 | 3 | 2 | 0 | 0 | 0 | 1 | 0 | 0.6 | |
| MOD_CMANNOS | ELME000160 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | |
| MOD_Cter_Amidation | ELME000093 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | |
| MOD_GlcNHglycan | ELME000085 | 1 | 3 | 3 | 3 | 2 | 0 | 2 | 2 | 4 | 2 | 5 | 0.9 | |
| MOD_GSK3_1 | ELME000053 | 3 | 1 | 3 | 4 | 2 | 2 | 1 | 1 | 0 | 4 | 6 | 0.9 | |
| MOD_NEK2_1 | ELME000336 | 2 | 3 | 3 | 2 | 2 | 1 | 4 | 4 | 1 | 2 | 3 | 1.0 | |
| MOD_NEK2_2 | ELME000337 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0.5 | |
| MOD_N-GLC_1 | ELME000070 | 3 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0.6 | |
| MOD_N-GLC_2 | ELME000079 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0.3 | |
| MOD_PIKK_1 | ELME000202 | 5 | 3 | 2 | 1 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 0.6 | |
| MOD_PK_1 | ELME000065 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.3 | |
| MOD_PKA_1 | ELME000008 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0.4 | |
| MOD_PKA_2 | ELME000062 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 2 | 1 | 0 | 2 | 0.8 | |
| MOD_Plk_1 | ELME000442 | 4 | 2 | 1 | 2 | 2 | 4 | 1 | 1 | 2 | 3 | 2 | 1.0 | |
| MOD_Plk_4 | ELME000444 | 1 | 2 | 3 | 2 | 2 | 2 | 0 | 0 | 1 | 2 | 0 | 0.7 | |
| MOD_ProDKin_1 | ELME000159 | 3 | 3 | 1 | 0 | 6 | 2 | 2 | 2 | 3 | 3 | 1 | 0.9 | |
| MOD_SUMO_for_1 | ELME000002 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0.1 | |
| MOD_SUMO_rev_2 | ELME000393 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 | |
| Targeting | TRG_ENDOCYTIC_2 | ELME000120 | 2 | 1 | 3 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 1.0 |
| TRG_LysEnd_APsAcLL_1 | ELME000149 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.2 | |
| TRG_NLS_MonoExtC_3 | ELME000278 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0.1 | |
| TRG_Pf-PMV_PEXEL_1 | ELME000462 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1.0 | |
GenBank accession numbers for endogenous integrase sequences are as follows: Endogenous retrovirus-K (ERVK in Homo sapiens; P10266.2), ERVK-8 pol protein-like (Macaca fascicularis; XP_015309771.1), Pol protein (Chlorocebus sabaeus; KFO35018.1), Pol protein (Fukomys darmarensis; BBC20786.1), Putative protein (Ochonta princeps; XP_012786727.1), ERVK-8 pol protein-like (Equus asinus; XP_014715024.1), ERVK-18 pol protein-like (Capra hircus; XP_017905435.1), Pol protein (Ovis aries; ABV71120.1), Putative protein (Zonotrichia albicollis; TRZ15504.1), Putative protein (Zosterops borbonicus; XP_014125095.1), Integrase (Onchocerca flexuosa; OZC05619.1).
2.3. STRING Analysis and KEGG Pathways
To identify potential ERVK IN binding partners based on ELM motifs, the names of interacting proteins were curated from each ELM reference page. When only a general interaction domain for a given ELM was listed, it was further linked to the InterPro database to curate a list of human proteins containing the interaction domain. Based on the 48 ELMs identified in ERVK IN, a total of 213 putative human protein interaction partners were identified (Table A5). The list was submitted to STRING (String Consortium; version 11.0; Software for Technical Computation; 2020, https://string-db.org/, accessed on 16 May 2021) for network analysis. Full network analysis was performed using Experiment and Databases as active interaction sources. Nodes indicate submitted query proteins only, with edges indicating confidence lines with a minimum interaction score of 0.9 (highest confidence). Query proteins unlinked to the network were excluded from analysis. A payload list was used to color hub proteins based on cellular function. KEGG pathways associated with the network analysis (E value < 1.0 × 10−5) were presented in a heatmap using GraphPad Prism (version 9.1.1) software, and the full list of KEGG pathways is presented in Table A6.
3. Results
3.1. Characterization of Eukaryotic Linear Motifs in ERVK Integrase and Other Betaretroviral Integrases
To establish which exogenous and endogenous retroviruses contain integrase sequences most similar to ERVK IN, we performed BLASTp searches using the nr, mo, and tsa NCIB databases. As expected, exogenous Betaretroviruses were identified through BLASPp search, which included multiple hits for Mouse mammary tumor virus (MMTV), Mason–Pfizer monkey virus (M-PMV), Enzootic Nasal Tumor Virus (ENTV), and Jaagsiekte sheep retrovirus (JSRV) (Table A1).
Eukaryotic linear motif (ELM) analysis of a representative sequence from each genus was compared with ERVK IN and revealed the conservation of select protein motifs (Table 1, Figure 1). Apart from the conservation of the HHCC region and DDE active site motif, all betaretroviral integrases also contained many interaction motifs related to DRR, including Pin1 via [ST]P WW domain interaction motifs [49], PP1c docking motif for target dephosphorylation [50], and a S-X-X-S/T CK1 phosphorylation site [51]. All INs except for MMTV contained a low-affinity BRCA1 carboxy-terminal BRCT domain binding motif (CSKAF, aa. 126–132). Betaretroviral INs were also predicted to be phosphorylated by the cell cycle checkpoint kinases NEK2 [52] and PLK-1 [53], as well as interact with a canonical arginine-containing phospho-motif within cell cycle regulating 14-3-3 proteins [54]. Numerous cell signaling protein interactions were predicted including YXXQ motifs for the SH2 domain binding of STAT3 [55], additional SH2 binding motifs related to STAT5 [56] and SRC family kinases [57], SH3 binding motifs with non-canonical class I recognition specificity [58], an IAP-binding motif (aa. 1–4) for interaction with inhibitor of apoptosis proteins (IAP) [59], an ITIM motif [60], several GSK3 phosphorylation sites [61], and proline-directed ERK/p38 MAPK phosphorylation sites [62]. In addition, most betaretroviral IN enzymes contained features related to protein trafficking, such as a Wxxx[FY] motif (aa. 133–137 in ERVK, MMTV, ENTV, and JRSV) that binds Pex13 and Pex14 for peroxisomal import [63], a SxIP motif (aa. 137–150) that binds to EBH domains in end-binding proteins involved in microtubule transport [64], and a tyrosine-based YXXØ sorting signal (aa. 75–78) for interaction with the μ-subunit of adaptor protein complex [65] and a PEXEL-like motif [66]. The DDE region displayed the most consistent pattern of conserved ELMs among the betaretroviral INs. It is important to note that despite the similar complement of ELM motifs in betaretroviral integrases, many were positioned at sites differently than in ERVK IN. Additional ELMs and their motif frequencies in individual betaretroviral integrases are listed in Table 1.
3.2. Characterization of Eukaryotic Linear Motifs in ERVK Integrase and Other Endogenous Integrases
ERVK integrase-like sequences were found in boreoeutherians, including the Euarchontoglires (primates, rodents, and pikas), and Laurasiatherians (ungulates), along with other clades including Euteleostomi (birds) and Protostomes (worms, insects, and water fleas) (Table A2, Table A3 and Table A4). Results ranged from 26.43 to 83.77% identity and E values ranged from 0.001 to 2.0 × 10−127, demonstrating a high degree of similarity with ERVK IN.
The conservation of ELM motifs was apparent (Table 2, Figure 2), including DDR-related canonical 14-3-3 interaction motifs and WDR5 interaction, cell signaling associated with USP7 binding, IAP-binding motif, STAT5 binding motifs, SH3 protein interaction, as well as phosphorylation sites for CK proteins, GSK3, NEK2, polo-like kinases, and p38. Many LIR motifs for engaging Atg8 proteins during selective autophagy were also apparent. Finally, all IN displayed Pex14 binding motifs and potential to interact with the μ-subunit of the adaptor protein complex. Additional ELMs and their motif frequencies in individual endogenous ERVK-like INs are listed in Table 2.
ELMs within endogenous IN but not or rarely identified in ERVK IN were also noted. An Apicomplexa-specific variant of the canonical LIR motif that binds to Atg8 protein family members was present in all endogenous INs except for ERVK IN. In addition, WDR5 binding motifs were much more prevalent in endogenous INs (5–12 sites) other than ERVK IN (only two sites). ERVK IN contained a single MAPK docking site for ERK/p38, whereas other endogenous INs contained several other motifs for MAPK interaction. Lastly, only human and macaque ERVK INs displayed high-affinity BRCT domain interaction motifs.
3.3. Unlike Similar Enzymes, the ERVK Integrase Contains Distinct ELM Signatures
Two motifs in ERVK IN stand out as unique to this virus, while other signatures are enriched in ERVK IN as compared with similar integrases.
3.3.1. ERVK Integrase has a High-Affinity BRCA1 Binding Site
Among all the integrases examined, only ERVK IN in human and macaque harbored a high-affinity binding site for the BRCT domain of BRCA1 (aa. 125–131, CSKAFQK) (Table 1 and Table 2, Figure 1 and Figure 2).
3.3.2. ERVK Integrase C-Terminus Contains a 14-3-3 Binding RASTE Motif
Although 14-3-3 protein binding was predicted as conserved among ERVK-like integrases, only ERVK IN contained a C-terminal RASTE motif (aa. 276–280) mediating strong interaction with 14-3-3 proteins (Table 1, Figure 1). This suggests a putative ERVK IN interaction with 14-3-3 proteins through both canonical phospho-sites and a C-terminal phospho-site.
3.3.3. ERVK Integrase Is Likely Post-Translationally Sumoylated
Unlike all other INs examined, only ERVK and MMTV contain a C-terminal inverted version (D/ExKphi) of the canonical sumoylation motif [67]. Considering that sumoylation often causes re-localization of nuclear proteins, this modification may be related to ERVK IN nuclear positioning, association with chromatin, and ultimately successful integration of viral DNA [68,69].
3.3.4. ERVK Integrase Exhibits Enhanced Interaction Potential with DDR Proteins
Phospho-Ser/Thr binding domain proteins are key hub proteins in cell cycle regulation and DDR, and they include 14-3-3 proteins, WW domains, Polo-box domains, WD40 repeats, BRCA1 carboxy-terminal (BRCT) domains, and Forkhead-associated (FHA) domains [54], all of which are interacting domains of ELMs identified in ERVK IN (Table 1 and Table 2, Figure 1). Additionally, ERVK IN contained five (ST)Q motifs, which are potential phosphorylation sites for PIKK proteins, such as DDR-related proteins ATR, ATM, DNA-PK, and multi-functional protein mTOR [70]. As compared with exogenous betaretroviruses and endogenous ERVK-like integrases, ERVK IN displayed a greater number of DDR-related motifs: FHA domain protein interaction sites (6), PLK-1 phosphorylation sites (4), and PP1c docking motif for target dephosphorylation (3) [50]. In contrast to MMTV, ENTV, JSRV, and most other endogenous integrases, fewer WD40 repeat domain WDR5 interaction sites were found in ERVK IN (2 vs. 5–12 sites each). This suggests ERVK IN has potentially shifted away from WDR5 interaction in favor of BRCA1 (or BRCT domain) interaction as a means to interact with the DDR pathway [54,71].
3.3.5. ERVK Integrase Contains Canonical Selective Autophagy Motifs
Unlike any of the exogenous betaretroviruses, only ERVK IN and some endogenous integrases contained canonical LIR motifs (ELME000368) for binding Atg8 protein family members (Table 1 and Table 2, Figure 2). All endogenous INs contained nematode-specific LIR motifs (ELME000370). Additionally, most endogenous INs housed Apicomplexa-specific LIR motifs (ELME000369), whereas ERVK IN did not.
3.4. ERVK Interactome Reveals Association with a Diversity of Cellular Pathways
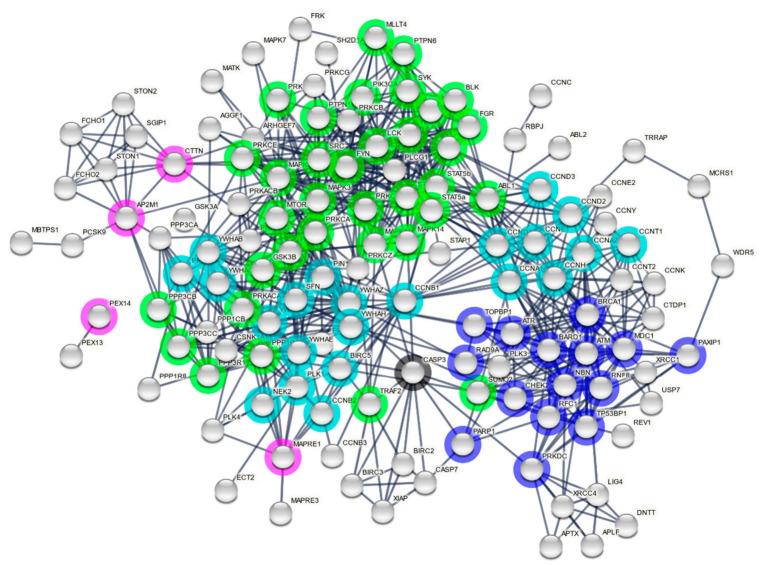
Based on ELMs identified in ERVK IN, a curated list of potential interacting proteins was generated and used to build an ERVK IN interactome network using STRING software (Figure 3). The ERVK IN network contained 189 nodes and 692 edges (expected number of edges 222), resulting in a significant PPI enrichment (p < 1.0 × 10−16). Only direct interactor query proteins are shown without links to second shell interactions. To illustrate key nodes and hub proteins, select network proteins were colored based on function related to DDR, cell cycle, apoptosis, cell signaling, or cellular transport. A complete list of the KEGG pathways significantly associated with the network is presented in Table A6.
Figure 3.
Predicted ERVK integrase interactome. Cellular proteins containing complementary interaction motifs for ELMs identified in Endogenous retrovirus-K (ERVK) integrase were listed as query proteins for STRING network analysis. Only query proteins with a minimum interaction score of 0.9 based on experiments and databases as interaction sources are indicated. Edges indicate both functional and physical protein associations. A payload list was generated to color nodes and hubs related to dominant pathways: DNA damage response (dark blue), cell cycle (cyan), apoptosis (black), cell signaling (green), and cell transport (magenta).
3.4.1. Many DNA Damage Response Proteins Are Potential ERVK Integrase Interactors
Gene ontology (GO) biological processes that were significantly enriched in the network included cellular response to DNA damage stimulus (p < 4.4 × 10−18), DNA repair (p < 4.24 × 10−12), DNA damage checkpoint (p < 4.89 × 10−12), double-strand break repair via non-homologous end joining (NHEJ) (p < 3.57 × 10−9), double-strand break repair (p < 1.06 × 10−8), and signal transduction in response to DNA damage (p < 2.12 × 10−8). Select BRCT domain containing proteins emerged as nodes with a higher-than-average degree of connections, including BRCA1, BARD1, NBN, MDC1, RCF1, TOPBP1, TP53BP1, and PAXIP1, while PARP1 and DRKDC (DNA-PK) appear to be hub proteins between DDR and apoptosis. The ERVK IN network also displayed four prominent DDR-related FHA proteins: CHEK2, NBN, MDC1, and RNF8.
3.4.2. ERVK Integrase Likely Modulates Cell Cycle Pathways
GO biological processes that were significantly enriched in the network included regulation of cell cycle (p < 1.45 × 10−33), cell division (p < 1.12 × 10−20), regulation of cyclin-dependent serine/threonine kinase activity (p < 6.86 × 10−20), mitotic cell cycle (p < 2.78 × 10−17), regulation of apoptotic process (p < 1.45 × 10−17), and cell cycle checkpoint (p < 6.72 × 10−12). Many cyclins and 14-3-3 proteins were identified in the network and are listed in Table A5. IAP-containing protein BIRC5 (also known as survivin) was also identified, which is suggestive of negative regulation of programmed cell death pathways [72]. PLK1 and NEK2 were also tied into the cell cycle framework and are both regulators of mitosis, in addition to displaying oncogenic properties [73,74].
3.4.3. Cell Signaling Pathways Associated with the ERVK Interactome
Among the potential signaling pathways often targeted by retroviruses, ERVK IN-associated cascades emerged as Forkhead box O (FoxO) signaling [75], p53 signaling [76], ErbB signaling [77], Wnt signaling [78], modulation of kinase activity [79,80], and multiple aspects of immune signaling [81] (Figure 4). Within these pathways, prominent immune-related signaling intermediates included STAT3 [55], STAT5 [56], and TRAF2 [82]. The SH2 and SH3 containing tyrosine-protein kinase ABL1 (Abelson murine leukemia viral oncogene homolog 1 [57]) appears to be a key hub protein linking DDR and downstream signaling cascades.
Figure 4.
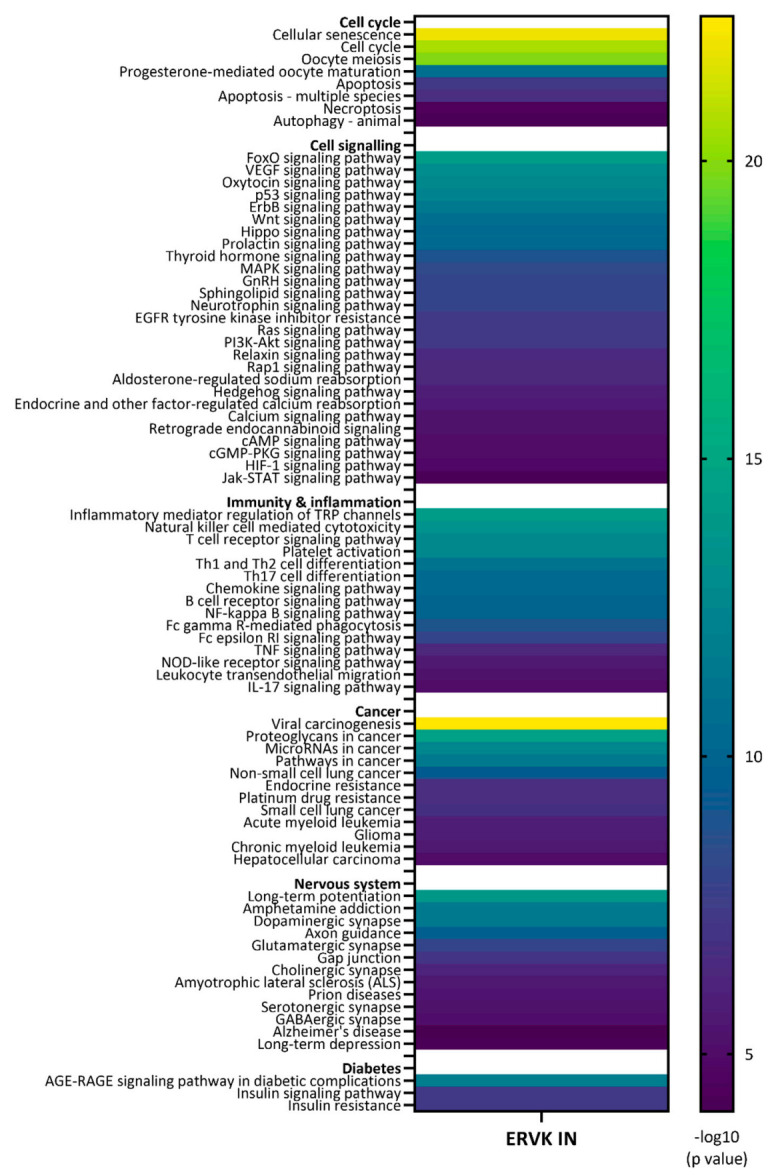
KEGG pathways associated with ERVK integrase interactome. Predicted interacting partners were curated based on ERVK IN ELM motifs and submitted to STRING network analysis software. Enriched KEGG pathways are reported along with significance scores (−log10 p value). ERVK IN is predicted to interact with cellular pathways involved in the cell cycle, cell signaling, immunity, and inflammation, as well as disease pathways associated with several cancers, the nervous system, and diabetes.
3.4.4. ERVK Integrase May Utilize Specific Cellular Transport Systems
The ERVK interactome contains proteins related to cellular transport. EB1 (MAPRE1) is an end-binding (EB) protein connected with both cell cycle and signaling pathways and is functionally associated with the regulation of microtubule dynamics [83]. Adaptor protein complex 2 associated proteins (AP2M1 and CTTN) were identified and indicate a role in cargo internalization via clathrin-mediated endocytosis and actin dynamics [65,84]. Lastly, ERVK IN may interact with Pex14 and Pex13 independently of the main network for peroxisome import [63]. While these pathways were likely important for the ancestral exogenous ERVK to transverse the cell and mediate infection, it remains unclear how endogenous IN may interact with these systems.
3.5. Diseases and Pathways Implicated in the ERVK Integrase Interactome
3.5.1. Cancers
Viral carcinogenesis was the top KEGG pathway identified in the ERVK IN network analysis (strength 1.22, E value 3.7 × 10−23), with 29 of 183 proteins represented (Figure 4). KEGG pathways for several known ERVK-associated cancers were also identified, including lung cancer [85], myeloid leukemia [86], and hepatocellular carcinoma [87] (Figure 4). Glioma was also identified, yet ERVK is downregulated in this condition [88]. Aligned with cellular transformation, proteins associated with cell cycle were also over-represented in the pathway analysis, which are specifically related to the cyclin docking site ELM (DOC_CYCLIN_RxL_1) and numerous FHA domain protein interaction sites (LIG_FHA_1 and LIG_FHA_2) in ERVK IN (Table 1 and Table 2, Figure 1 and Figure 2).
3.5.2. Neurological Disease
KEGG pathways for several ERVK-associated neurological conditions were identified, including ALS [9,12], Alzheimer’s disease [89], and prion disease [90] (Figure 4). Specifically, long-term potentiation and synaptic neurotransmitter release (dopaminergic, glutamatergic, cholinergic, serotonergic, and GABAergic) were associated with the ERVK IN interactome.
3.5.3. Diabetes
The role of ERVK in diabetes remains contentious [91,92,93,94]. However, network analysis suggests that the ERVK IN interactome is potentially linked to AGE-RAGE signaling in diabetic complications, insulin signaling, and insulin resistance (Figure 4).
4. Discussion
ERVK expression has been repeatedly associated with human disease states including cancer, neurological disease, and diabetes. By exploring the potential ERVK integrase interactome, we can postulate how this viral symbiont may contribute to disease pathogenesis via interaction with key proteins and pathways. Our analysis reveals that viral carcinogenesis and modulation of the DNA damage response are the most likely pathways to be pathologically associated with ERVK IN expression.
Retroviral integrase activity causes DNA lesions in the host genome as part of the proviral integration process [19]; therefore, interactions with DDR pathways are to be expected. Several DDR proteins have been shown to be essential for provirus suture into the host genome and maintenance of genome fidelity [19]. Yet, the impairment of select aspects of DDR has also been documented in exogenous retroviral infections, including HIV [95,96] and HTLV-1 [97,98]. This may be driven by the fact that NHEJ proteins also play an essential role in innate immune recognition of retroviral cytosolic ssDNA intermediates and dsDNA pre-integration complexes [98,99]. Thus, retroviruses must balance the benefits and drawbacks of DDR outcomes through the engagement and modulation of specific proteins.
BRCT domain, FHA domain, and 14-3-3 proteins work in concert during the DNA damage response (reviewed in [100]). Many of these DDR proteins are also cellular targets of retroviruses and oncogenic viruses [98,101,102,103,104]. BRCA1 BRCT domains recognize phosphopeptides based on a pSXXF motif, but XX residues and the surrounding amino acids also impact binding affinity and selectivity [105]. All the betaretroviral INs examined showed the capacity to interact with BRCT domains. However, only ERVK IN displayed a high affinity (S.F.K) BRCA1 BRCT domain binding site; the only other similar ELM structure is found in the DDR protein Fanconi anemia group J protein (FACJ/BACH1) [106]. It is also possible that dual anchoring onto the ERVK IN using both a BRCT domain and an FHA domain found in NBN or MDC1 may strengthen protein–protein interactions.
The utilization and evasion of 14-3-3 proteins are common among many viruses [104]. ERVK IN is unique in having a C-terminal RASTE motif, in addition to two other canonical arginine containing phospho-motifs recognized by 14-3-3 proteins. Given that an elevated expression of 14-3-3 proteins occurs in both cancers and neurodegenerative diseases [107,108], ERVK IN interaction with 14-3-3 protein members may be related to either modulation of the cell cycle and oncogenesis or regulation of protein aggregation, respectively. The deregulation of 14-3-3 and RAF kinase interaction can also lead to inappropriate downstream MAPK activity (associated with oncogenesis) [54,109] and may be an aspect to consider for the predicted ERVK IN network.
ABL1 appears to be a key hub protein linking DDR and downstream signaling cascades. Interestingly, DDR is known to be a rapid driver of ABL1 activation [110]. The ablation of ABL1 reduces retrovirus integration [111,112], while active ABL1 can turn on the HIV promoter independently of HIV Tat [113]. Putative interaction between ERVK IN and ABL1 may have been important for ERVK integration into germline cells, and it may additionally play a role in ERVK expression, specifically in neurodegenerative disease displaying enhanced ABL1 activity [114,115].
DDR is intimately tied to innate immune response, specifically NF-κB activation [116]. Considering ERVK’s dependence on NF-κB for driving its own expression [11], it is conceivable that ERVK IN plays a role in preparing the host cell for viral transcription. 14-3-3ϵ activity is key in driving ATM-TAK1-mediated NF-κB signaling during DDR [117,118]; thus, the predicted ERVK IN interaction with 14-3-3ϵ (YWHAE) may be a mechanism to favor viral transcription. The MAPK p38 was also predicted to both phosphorylate and bind ERVK IN. This association may be linked to p38′s regulation of inflammatory signaling, as well as its capacity to enhance the transcriptional activity of NF-κB p65 via modulation of the acetyltransferase activity of coactivator p300 [119]. Sustained NF-κB activity is linked to oncogenesis [116] and ties into the strongest ERVK IN-linked KEGG pathway: viral carcinogenesis. However, enhanced ERVK IN-associated NF-κB signaling may also fit with inflammatory and neurodegenerative conditions.
ERVK IN stability and protein turnover is likely linked to its cellular protein partnerships. In the case of HIV, binding select cellular proteins such as LEDGF/p75 and Ku70 prevents integrase proteosomal degradation [120,121]. Similarly, c-Jun N-terminal kinase (JNK) S57 phosphorylation of the core domain can make HIV IN a target for Pin1, thus enhancing its stability and activity [122,123]. In this study, Pin1′s WW domain was predicted to be an interactor based on three [ST]P motifs in the C-terminal portion of ERVK IN. This raises the possibility that similar to many other viral proteins [124], ERVK IN may be stabilized through Pin1 interaction. The functional significance of this interaction may underlie how elevated levels of ERVK IN are maintained and potentially drive pathology in select diseases, such as ALS and cancer.
Distinct from other exogenous betaretroviruses, only ERVK IN and some endogenous integrases contained canonical LIR motifs for binding Atg8 protein family members. Mammalian Atg8-like proteins include LC3 and GABARAP families, which mediate selective autophagy, as well as play essential roles in antiviral defense and innate immune signaling [125]. However, it is often observed that viruses subvert autophagy processes to avoid viral protein clearance and repurpose Atg8 proteins as well as autophagosomal membranes for viral replication [125,126]. Considering the perturbances of autophagy in neurodegenerative disease [127,128], the interaction between ERVK IN and Atg8 proteins warrants further investigation.
Consistent with genomic instability profiles in cancer [129], ALS [130], and Alzheimer’s disease [131], the ERVK interactome analysis identified each of these conditions as significant KEGG pathways. Despite differences in clinical presentation, the molecular underpinnings in cancer and neurodegenerative disease are remarkably similar and include alterations in DDR [129,130,132], 14-3-3 expression [133,134], p53 signaling [135,136], p38 signaling [137,138], and Wnt signaling [139,140]—which are all KEGG pathways enriched in the ERVK IN network. AGE-RAGE signaling was also identified as a potential pathway associated with the ERVK IN interactome. Not only is this pathway implicated in diabetic complications [141], but it also plays a role in nuclear response to DNA damage [142], carcinogenesis [143], and inflammatory neurodegenerative diseases [144]. Collectively, our results point to ERVK IN driving a pattern of pathology that, depending on cellular context, may lead to carcinogenesis, neurodegeneration, or contribute to diabetic complications. However, the engagement of DDR can also have beneficial impacts on lifespan extension, depending on tissue context and host genotype; thus, non-pathogenic effects of ERVK IN should also be considered [145,146].
Apart from the importance of putative ERVK IN interaction partners, it is also important to consider which cellular proteins were not associated with the ERVK IN interactome. One interaction that was not predicted was with LEDGF/p75, and indeed, this interactor is limited to partnership with lentiviral integrases [147,148]. Another set of DDR proteins commonly found to impact retroviral integration and replication is the DNA-PK complex [99,149]. HIV integrase directly interacts with Ku70 [120]; while ERVK IN was predicted to be phosphorylated by DNA-PKcs (PRKDC), it contained no ELMs to suggest direct interaction with Ku80 or Ku70. Another apparent difference is the use of EB proteins in microtubule trafficking for HIV and ERVK. ERVK IN contained an SxIP motif that binds EBH domains, whereas HIV capsid conversely has EB-like motifs that interact with SxIP motifs in plus-end tracking protein (+TIP) [150]. These genus-specific distinctions are likely to emerge as important considerations for therapeutic targeting strategies and imply that pharmaceuticals geared toward HIV infection may not consistently translate for use in ERVK-associated disease.
Another consideration that stems from this study is the choice and caveats of using animal models in ERVK research. A diversity of animals outside of the primate lineage are host to ERVK IN-like sequences, such as rodents, ungulates, fish, and insects. Drosophila, a common model organism, also contained ERVK IN-like elements in their genome, specifically LTR retrotransposons flea and Xanthias, as identified by FlyBase (Table A4). The transposable element Xanthias is known to be active in D. melanogaster [151,152], and it shares a degree of similarity with ERVK IN. The presence and activity of these ERVs is an important factor to consider when performing experiments.
It is shocking how little we understand of the impact endogenous viral symbionts have on cellular functioning. Herein, we have predicted that ERVK IN may participate in the modulation of cellular pathways such as DDR, cell cycle regulation, and kinase signaling cascades by way of select protein interaction motifs. The main caveat of in silico predictions is the requirement for experimental validation; while research into ERVK IN is currently underway, this study suggests there remains a myriad of disease-related betaretroviral integrase interactions to explore.
Acknowledgments
The authors would like to thank Samuel Narvey, Megan Rempel and Alex Vandenakker for their peer review of the manuscript drafts.
Appendix A
Table A1.
Exogenous viruses with similarity to ERVK integrase based on BLASTp search.
| Host | Species | Protein Name | Accession Number | Database | Percent Identity | E Value | Alignment |
|---|---|---|---|---|---|---|---|
| Retroviridae—Betaretrovirus | |||||||
| Mus Musculus | Mouse mammary tumor virus (MMTV) |
MMTV strain BR6 Integrase, partial | 5CZ2_A | nr | 56.46% | 3.00 × 10−71 | |
| Unnamed protein product | CAA25954.1 | nr | 52.36% | 2.00 × 10−70 | |||
| Chain A. Integrase (Core model of the MMTV Intasome) |
3JCA_A | nr | 51.50% | 2.00 × 10−82 | |||
| Pr160 | NP_056880.1 | nr | 51.11% | 7.00 × 10−76 | |||
| Pr160 gag pro pol precursor | AAA46542.1 | nr | 51.11% | 7.00 × 10−76 | |||
| p30DU-p13PR-RT-IN | NP_955564.1 | nr | 51.11% | 2.00 × 10−76 | |||
| Gag-Pro-Pol polyprotein | P11283.2 | nr | 51.11% | 3.00 × 10−75 | |||
| MMTV putative integrase | AAC24859.1 | nr | 50.37% | 5.00 × 10−81 | |||
| Gag-Pol-Pro polyprotein, partial | BAA03767.1 | nr | 49.65% | 1.00 × 10−77 | |||
| Exogenous MMTV Gag-Pro-Pol | AAF31469.1 | nr | 49.65% | 4.00 × 10−76 | • | ||
| Endogenous MTV1 Gag-Pro-Pol | AAF31464.1 | nr | 49.65% | 4.00 × 10−76 | |||
| Capra hircus | Enzootic Nasal Tumor Virus (ENTV) |
Pol protein, partial | AVG72436.1 | nr | 48.79% | 3.00 × 10−72 | |
| Pol protein, partial | AVG72437.1 | nr | 48.79% | 3.00 × 10−71 | |||
| Pol protein, partial | AVG72438.1 | nr | 48.79% | 7.00 × 10−71 | |||
| Pol protein, partial | AVG72441.1 | nr | 48.39% | 3.00 × 10−71 | |||
| Pol protein, partial | AVG72440.1 | nr | 48.39% | 4.00 × 10−71 | |||
| Pol protein, partial | AVG72435.1 | nr | 48.39% | 2.00 × 10−70 | |||
| Gag-Pro-Pol protein | QBP05340.1 | nr | 47.41% | 3.00 × 10−73 | |||
| Pol protein, partial | ANG58667.1 | nr | 47.41% | 2.00 × 10−72 | |||
| Pol protein | QEQ26602.1 | nr | 47.41% | 3.00 × 10−72 | |||
| Pol protein, partial | ANG58699.1 | nr | 47.41% | 2.00 × 10−71 | • | ||
| Pol protein, partial | ANG58695.1 | nr | 47.41% | 2.00 × 10−71 | |||
| Pol protein, partial | ANG58691.1 | nr | 47.41% | 2.00 × 10−71 | |||
| Pol protein, partial | ANG58679.1 | nr | 47.41% | 5.00 × 10−71 | |||
| Endogenous ENTV pol protein | QPG92760.1 | nr | 47.41% | 3.00 × 10−73 | |||
| Endogenous ENTV pol protein | QPG92768.1 | nr | 47.41% | 1.00 × 10−72 | |||
| Gag-Pro-Pol, partial | AOZ60515.1 | nr | 47.04% | 1.00 × 10−72 | |||
| Pol protein, partial | ANG58671.1 | nr | 47.04% | 1.00 × 10−71 | |||
| Pol protein, partial | ANG58683.1 | nr | 47.04% | 3.00 × 10−70 | |||
| Pol protein, partial | ANG58687.1 | nr | 47.04% | 2.00 × 10−71 | |||
| Pol protein, partial | ANG58663.1 | nr | 47.04% | 8.00 × 10−71 | |||
| Gag-Pro-Pol protein | ANC55859.1 | nr | 46.67% | 5.00 × 10−73 | |||
| Gag-Pro-Pol protein, partial | ADI50273.1 | nr | 46.67% | 4.00 × 10−72 | |||
| Gag-Pro-Pol protein, partial | AOZ60519.1 | nr | 46.67% | 7.00 × 10−72 | |||
| Pol protein, partial | ANG58675.1 | nr | 46.67% | 3.00 × 10−71 | |||
| Gag-Pro-Pol fusion, partial | NP_862833.2 | nr | 46.67% | 7.00 × 10−71 | |||
| Pol protein, partial | ANG58659.1 | nr | 46.10% | 1.00 × 10−72 | |||
| Macaca genus | Mason-Pfizer monkey virus (M-PMV) |
Simian AIDS retrovirus SRV-1-Pol polyprotein | GNLJSA | nr | 48.30% | 2.00 × 10−70 | |
| Simian retrovirus SRV-5-Pol polyprotein | BBG56792.1 | nr | 47.57% | 5.00 × 10−71 | • | ||
| Simian retrovirus SRV-Y-Pol protein, partial | BAM71050.1 | nr | 47.57% | 2.00 × 10−70 | |||
| Ovis Aries | Jaagsiekte sheep retrovirus | Reverse transcriptase, partial | CAA77117.1 | nr | 46.67% | 5.00 × 10−75 | |
| Reverse transcriptase, partial | CAA77113.1 | nr | 46.67% | 2.00 × 10−74 | |||
| Pol protein | NP_041186.1 | nr | 46.30% | 1.00 × 10−70 | • | ||
BLAST databases: nr denotes non-redundant protein database. Circle symbol: denotes integrase sequences used in protein alignment in Figure 1.
Table A2.
Boreoeutherian genomes with similarity to ERVK integrase based on BLASTp searches.
| Species | Protein Name | Accession Number | Database | Percent Identity | E Value | |
|---|---|---|---|---|---|---|
| Homo sapiens | Human Endogenous Retrovirus K (HERV-K/ ERVK) |
Pol/env ORF (bases 3878-8257) first start codon at 4172; Xxx; putative | AAA88033.1 | nr | 100.00% | 0 |
| Endogenous retrovirus group K member 10 Pol protein | P10266.2 | nr | 100.00% | 0 | ||
| Gag-Pro-Pol-Env protein | AAD51793.1 | nr | 100.00% | 0 | ||
| Pol protein, partial | CAA71417.1 | nr | 100.00% | 2.00 × 10−101 | ||
| Endogenous retrovirus group K member 11 Pol protein | Q9UQG0.2 | nr | 99.64% | 0 | ||
| Endogenous retrovirus group K member 7 Pol protein | P63135.1 | nr | 99.29% | 0 | ||
| Reverse transcriptase, partial | AGQ55918.1 | nr | 99.28% | 1.00 × 10−94 | ||
| Reverse transcriptase, partial | AGQ55922.1 | nr | 99.28% | 7.00 × 10−94 | ||
| Polymerase, partial | AAO27434.1 | nr | 99.27% | 0 | ||
| Gag-Pro-Pol protein | AAD51796.1 | nr | 99.17% | 5.00 × 10−161 | ||
| Endogenous retrovirus group K member 113 Pol protein | P63132.1 | nr | 98.21% | 0 | ||
| Polymerase, partial | AAK11553.1 | nr | 98.21% | 0 | ||
| Endogenous retrovirus group K member 6 Pol protein | Q9BXR3.2 | nr | 98.21% | 0 | ||
| Endogenous retrovirus group K member 8 Pol protein | P63133.1 | nr | 98.21% | 0 | ||
| Gag-Pro-Pol protein | AAD51797.1 | nr | 98.21% | 0 | ||
| Pol protein | CAA76885.1 | nr | 97.86% | 0 | ||
| Pol protein | CAA76879.1 | nr | 97.86% | 0 | ||
| Polymerase, partial | AAK11554.1 | nr | 97.86% | 0 | ||
| Reverse transcriptase, partial | AGQ55928.1 | nr | 97.84% | 6.00 × 10−93 | ||
| Reverse transcriptase, partial | AGQ55923.1 | nr | 97.84% | 8.00 × 10−93 | ||
| Reverse transcriptase, partial | AGQ55925.1 | nr | 97.83% | 2.00 × 10−92 | ||
| Reverse transcriptase, partial | AGQ55927.1 | nr | 97.83% | 3.00 × 10−92 | ||
| Reverse transcriptase, partial | AGQ55914.1 | nr | 97.76% | 6.00 × 10−89 | ||
| Pol protein | CAA76882.1 | nr | 97.50% | 0 | ||
| Endogenous retrovirus group K member 19 Pol protein | Q9WJR5.2 | nr | 97.50% | 0 | ||
| Endogenous retrovirus group K member 25 Pol protein | P63136.1 | nr | 97.50% | 0 | ||
| Pol protein | AAL60056.1 | nr | 97.30% | 1.00 × 10−152 | ||
| Reverse transcriptase, partial | AGQ55924.1 | nr | 97.12% | 2.00 × 10−92 | ||
| Reverse transcriptase, partial | AGQ55926.1 | nr | 97.10% | 9.00 × 10−92 | ||
| Reverse transcriptase, partial | AGQ55921.1 | nr | 97.10% | 9.00 × 10−92 | ||
| Reverse transcriptase, partial | AGQ55919.1 | nr | 97.10% | 1.00 × 10−91 | ||
| Pol/env protein, partial | AET81039.1 | nr | 96.97% | 1.00 × 10−81 | ||
| Reverse transcriptase, partial | AGQ55920.1 | nr | 96.38% | 2.00 × 10−91 | ||
| Pol protein | CAB56603.1 | nr | 90.13% | 2.00 × 10−132 | ||
| Endogenous retrovirus group K member 18 Pol protein | Q9QC07.2 | nr | 90.13% | 2.00 × 10−130 | ||
| hCG1808534 | EAW92672.1 | nr | 87.38% | 3.00 × 10−130 | ||
|
Macaca
fascicularis |
PREDICTED: endogenous retrovirus group K member 8 Pol protein-like | XP_015309771.1 | nr | 83.77% | 2.00 × 10−127 | |
|
Chlorocebus
sabaeus |
Pol protein, partial | BBC20786.1 | nr | 47.01% | 3.00 × 10−70 | |
|
Oryctolagus
cuniculus |
PREDICTED: endogenous retrovirus group K member 8 Pol protein-like | XP_017205812.1 | nr | 49.82% | 1.00 × 10−76 | |
|
Marmota
marmota |
PREDICTED: endogenous retrovirus group K member 11 Pol protein-like | XP_015349278.1 | nr | 47.96% | 4.00 × 10−71 | |
|
Ochotona
princeps |
Uncharacterized protein LOC105942652 | XP_012786727.1 | nr | 47.33% | 1.00 × 10−70 | |
|
Mus musculus (mouse) |
Agouti-signaling protein isoform X1 | XP_017174599.2 | mo | 46.93% | 6.00 × 10−70 | |
| Contactin-5 isoform X2 | XP_036010832.1 | mo | 44.11% | 3.00 × 10−63 | ||
| Contactin-5 isoform X1 | XP_036010831.1 | mo | 44.11% | 4.00 × 10−63 | ||
| Contactin-5 isoform X3 | XP_036010833.1 | mo | 44.11% | 4.00 × 10−63 | ||
| Protein NYNRIN-like isoform X1 | XP_036020530.1 | mo | 30.15% | 9.00 × 10−14 | ||
| Uncharacterized protein Gm39701 | XP_011237194.2 | mo | 30.15% | 9.00 × 10−14 | ||
| Uncharacterized protein LOC118567641, partial | XP_036010828.1 | mo | 29.71% | 2.00 × 10−12 | ||
|
Fukomys
damarensis |
Pol polyprotein | KFO35018.1 | nr | 45.14% | 1.00 × 10−71 | |
| Sus scrofa | TPA: uncharacterized protein | HCZ91355.1 | tsa | 61.54% | 2.00 × 10−65 | |
| TPA: uncharacterized protein | HDB33152.1 | tsa | 61.54% | 2.00 × 10−65 | ||
| Endogenous retrovirus group K member 11 Pol protein-like | HDB62800.1 | tsa | 60.66% | 2.00 × 10−17 | ||
| TPA: uncharacterized protein | HCZ89879.1 | tsa | 52.24% | 1.00 × 10−39 | ||
| Nuclear autoantigenic sperm protein isoform X4 | HDA97069.1 | tsa | 49.06% | 2.00 × 10−21 | ||
| Nuclear autoantigenic sperm protein isoform 2 | HCZ78574.1 | tsa | 48.86% | 5.00 × 10−15 | ||
| Nuclear autoantigenic sperm protein isoform 2 | HDB80991.1 | tsa | 48.42% | 7.00 × 10−17 | ||
| TPA: uncharacterized protein | HCZ87894.1 | tsa | 42.67% | 5.00 × 10−58 | ||
| TPA: uncharacterized protein | HDB20633.1 | tsa | 41.78% | 1.00 × 10−53 | ||
| TPA: uncharacterized protein | HDC25054.1 | tsa | 39.81% | 7.00 × 10−48 | ||
| TPA: uncharacterized protein | HDA81201.1 | tsa | 38.84% | 1.00 × 10−47 | ||
| Transmembrane protein 161B isoform X6-like | HDB13612.1 | tsa | 31.52% | 2.00 × 10−13 | ||
| TPA: uncharacterized protein | HDC69173.1 | tsa | 31.07% | 2.00 × 10−13 | ||
| TPA: uncharacterized protein | HDC69046.1 | tsa | 30.90% | 3.00 × 10−13 | ||
| Transmembrane protein 161B isoform X12-like | HDC79805.1 | tsa | 30.81% | 1.00 × 10−12 | ||
| Transmembrane protein 161B isoform X12-like | HDB85007.1 | tsa | 30.81% | 1.00 × 10−12 | ||
| Transmembrane protein 161B isoform X6-like | HDA96697.1 | tsa | 30.81% | 1.00 × 10−12 | ||
| Transmembrane protein 161B isoform X12-like | HDB79678.1 | tsa | 30.81% | 2.00 × 10−12 | ||
| Transmembrane protein 161B isoform X6 | HDB81123.1 | tsa | 30.77% | 1.00 × 10−11 | ||
| Transmembrane protein 161B isoform X6 | HDB82421.1 | tsa | 30.71% | 2.00 × 10−10 | ||
| TPA: uncharacterized protein | HDC70342.1 | tsa | 30.41% | 6.00 × 10−12 | ||
| TPA: uncharacterized protein | HDC70174.1 | tsa | 30.41% | 6.00 × 10−12 | ||
| TPA: uncharacterized protein | HDC69016.1 | tsa | 30.41% | 6.00 × 10−12 | ||
| putative protein-like | HDB95244.1 | tsa | 30.23% | 3.00 × 10−12 | ||
| TPA: uncharacterized protein | HDB49716.1 | tsa | 30.23% | 5.00 × 10−12 | ||
| TPA: uncharacterized protein | HDB49718.1 | tsa | 30.23% | 5.00 × 10−12 | ||
| TPA: uncharacterized protein | HDC70066.1 | tsa | 30.23% | 5.00 × 10−12 | ||
| TPA: uncharacterized protein | HDC69012.1 | tsa | 30.23% | 7.00 × 10−12 | ||
| TPA: uncharacterized protein | HDB50090.1 | tsa | 29.82% | 2.00 × 10−11 | ||
| TPA: uncharacterized protein | HDB54298.1 | tsa | 29.65% | 1.00 × 10−11 | ||
| Transmembrane protein 161B isoform X3-like | HDC79806.1 | tsa | 29.65% | 2.00 × 10−11 | ||
| Endogenous retrovirus group K member 25 Pol | HCZ79815.1 | tsa | 29.65% | 3.00 × 10−11 | ||
| Transmembrane protein 161B isoform X2-like | HDC79761.1 | tsa | 29.59% | 1.00 × 10−10 | ||
| Endogenous retrovirus group K member 25 Pol | HDA78544.1 | tsa | 29.24% | 4.00 × 10−11 | ||
| Endogenous retrovirus group K member 25 Pol | HDA79987.1 | tsa | 29.24% | 7.00 × 10−11 | ||
| TPA: uncharacterized protein | HDA79350.1 | tsa | 29.24% | 9.00 × 10−11 | ||
| Endogenous retrovirus group K member 25 Pol | HDA79988.1 | tsa | 29.24% | 9.00 × 10−11 | ||
| TPA: uncharacterized protein | HDA79349.1 | tsa | 29.24% | 1.00 × 10−10 | ||
| TPA: uncharacterized protein | HDC13198.1 | tsa | 29.24% | 1.00 × 10−10 | ||
| Transmembrane protein 161B isoform X6-like | HDB88647.1 | tsa | 29.07% | 3.00 × 10−11 | ||
| TPA: uncharacterized protein | HDA61679.1 | tsa | 29.07% | 5.00 × 10−11 | ||
| TPA: uncharacterized protein | HDB82700.1 | tsa | 29.07% | 1.00 × 10−10 | ||
| Equus asinus | PREDICTED: endogenous retrovirus group K member 8 Pol protein-like | XP_014715024.1 | nr | 56.46% | 3.00 × 10−95 | |
| Capra hircus | PREDICTED: LOW QUALITY PROTEIN: endogenous retrovirus group K member 18 Pol protein-like |
XP_017905435.1 | nr | 47.41% | 3.00 × 10−71 | |
| Ovis aries | Pol protein | ABV71120.1 | nr | 47.04% | 2.00 × 10−72 | |
| Pol protein | ABV71104.1 | nr | 46.67% | 4.00 × 10−71 | ||
| Pol protein | ABV71084.1 | nr | 46.67% | 4.00 × 10−71 | ||
| Pol protein | ABV71074.1 | nr | 46.67% | 4.00 × 10−71 | ||
| Pol protein | ABV71079.1 | nr | 46.67% | 4.00 × 10−71 | ||
| Pol protein | ABV71069.1 | nr | 46.67% | 4.00 × 10−71 | ||
| Pol protein (endogenous virus) | AST51848.1 | nr | 46.67% | 5.00 × 10−71 | ||
| Pol protein | ABV71094.1 | nr | 46.30% | 2.00 × 10−70 | ||
BLAST databases: nr = non-redundant protein database; mo = model organisms database; tsa = transcriptomics shotgun analysis non-redundant database. TPA = third party annotation.
Table A3.
Euteleostomi genomes with similarity to ERVK integrase based on BLASTp searches.
| Species | Protein Name | Accession Number | Data base | Percent Identity | E Value |
|---|---|---|---|---|---|
|
Micrurus
lemniscatus carvalhoi |
Hypothetical protein, partial | LAA32932.1 | tsa | 58.47% | 2.00 × 10−40 |
| Hypothetical protein, partial | LAA32929.1 | tsa | 57.63% | 6.00 × 10−40 | |
| Hypothetical protein, partial | LAA32939.1 | tsa | 48.29% | 4.00 × 10−54 | |
| Hypothetical protein, partial | LAA32941.1 | tsa | 44.00% | 1.00 × 10−26 | |
|
Zosterops
borbonicus |
Hypothetical protein HGM15179_011615 | TRZ15504.1 | nr | 46.97% | 3.00 × 10−71 |
|
Zonotrichia
albicollis |
Uncharacterized protein LOC106629581 | XP_014125095.1 | nr | 46.24% | 1.00 × 10−71 |
|
Micrurus
corallinus |
Hypothetical protein, partial | LAA64555.1 | tsa | 46.21% | 2.00 × 10−26 |
| Hypothetical protein, partial | LAA64556.1 | tsa | 45.08% | 2.00 × 10−21 | |
|
Micrurus
lemniscatus lemniscatus |
Hypothetical protein, partial | LAA89554.1 | tsa | 44.57% | 4.00 × 10−18 |
| Hypothetical protein, partial | LAA89545.1 | tsa | 43.75% | 1.00 × 10−23 | |
| Bird metagenome |
Gag-Pro-Pol polyprotein, partial | MBY11728.1 | tsa | 44.31% | 3.00 × 10−43 |
|
Gallirallus
okinawae |
Hypothetical protein, partial | LAC45429.1 | tsa | 43.96% | 3.00 × 10−57 |
|
Fundulus
heteroclitus |
Integrase core domain, partial | JAQ81073.1 | tsa | 35.05% | 2.00 × 10−11 |
|
Danio rerio (zebrafish) |
Uncharacterized protein LOC108190699 | XP_017212567.1 | mo | 33.64% | 4.00 × 10−12 |
| Uncharacterized protein K02A2.6-like | XP_021334762.1 | mo | 30.18% | 4.00 × 10−11 | |
| Uncharacterized protein K02A2.6-like | XP_017210639.2 | mo | 29.20% | 8.00 × 10−9 | |
| Uncharacterized protein LOC101886116 | XP_021327301.1 | mo | 28.47% | 1.00 × 10−5 | |
| Uncharacterized protein LOC110439859 | XP_021332670.1 | mo | 28.17% | 0.001 | |
| Uncharacterized protein K02A2.6-like | XP_003199161.1 | mo | 27.78% | 5.00 × 10−10 | |
| Uncharacterized protein K02A2.6-like | XP_002663225.3 | mo | 27.78% | 8.00 × 10−10 | |
| Uncharacterized protein LOC110438047 | XP_021323131.1 | mo | 26.43% | 0.006 | |
| Nothobranchius korthausae | Uncharacterized protein | SBQ67355.1 | tsa | 32.02% | 8.00 × 10−11 |
| Nothobranchius kadleci | Uncharacterized protein | SBP84572.1 | tsa | 32.00% | 8.00 × 10−11 |
| Nothobranchius furzeri | Uncharacterized protein | SBS54329.1 | tsa | 31.25% | 5.00 × 10−13 |
|
Nothobranchius
rachovii |
Uncharacterized protein | SBS11479.1 | tsa | 30.07% | 2.00 × 10−10 |
BLAST databases: nr = non-redundant protein database; mo = model organisms database; tsa = transcriptomics shotgun analysis non-redundant database.
Table A4.
Nephrozoa (non-boreoeutherian) genomes with similarity to ERVK integrase based on BLASTp searches.
| Species | Protein Name | Accession Number | Data base | Percent Identity | E Value |
|---|---|---|---|---|---|
| Onchocerca flexuosa | Integrase core domain protein | OZC05619.1 | nr | 45.68% | 6.00 × 10−73 |
| Ixodes ricinus | Putative tf2-11 polyprotein | MXV00662.1 | tsa | 35.81% | 2.00 × 10−10 |
| Putative tick transposon, partial | JAP73380.1 | tsa | 30.67% | 1.00 × 10−11 | |
| Putative tick transposon, partial | JAR90689.1 | tsa | 30.67% | 6.00 × 10−11 | |
| Putative bell all, partial | JAA73039.1 | tsa | 30.00% | 2.00 × 10−10 | |
| Putative gypsy11-i sp, partial | JAP69562.1 | tsa | 30.00% | 2.00 × 10−10 | |
| Putative tick transposon, partial | JAR92200.1 | tsa | 28.06% | 4.00 × 10−13 | |
| Putative transposon tf2-9 polyprotein | MXV00940.1 | tsa | 27.37% | 9.00 × 10−13 | |
| Littorina littorea | Transposon Ty3-G Gag-Pol polyprotein | MBX96210.1 | tsa | 35.54% | 4.00 × 10−12 |
| Transposon Ty3-I Gag-Pol polyprotein | MBX97975.1 | tsa | 30.00% | 4.00 × 10−14 | |
| Ixodes scapularis | Putative tick transposon, partial | MOY42200.1 | tsa | 34.29% | 4.00 × 10−12 |
| Putative tick transposon, partial | MOY42203.1 | tsa | 32.67% | 2.00 × 10−13 | |
| Putative tick transposon, partial | MOY42202.1 | tsa | 32.67% | 7.00 × 10−13 | |
| Putative gypsy-17 ga-i, partial | MOY42236.1 | tsa | 31.09% | 2.00 × 10−12 | |
| Hypothetical protein, partial | MOY42219.1 | tsa | 30.00% | 6.00 × 10−11 | |
| Putative gypsy11-i sp, partial | MOY42209.1 | tsa | 29.17% | 7.00 × 10−12 | |
| Putative gypsy21-i sp, partial | MOY42223.1 | tsa | 29.17% | 8.00 × 10−12 | |
| Putative tick transposon, partial | MOY42227.1 | tsa | 28.50% | 4.00 × 10−13 | |
| Putative tick transposon, partial | MOY42214.1 | tsa | 28.25% | 9.00 × 10−11 | |
| Putative gypsy-27 xt-i, partial | MOY35588.1 | tsa | 26.44% | 1.00 × 10−10 | |
| Lygus hesperus | Retrotransposable element Tf2 protein type 3, partial | JAQ12551.1 | tsa | 33.58% | 6.00 × 10−13 |
| Uncharacterized protein | JAG20083.1 | tsa | 33.58% | 6.00 × 10−13 | |
| Hypothetical protein, partial CM83_13537, partial | JAG31779.1 | tsa | 29.93% | 5.00 × 10−11 | |
| Uncharacterized protein, partial K02A2.6, partial | JAG32119.1 | tsa | 28.87% | 1.00 × 10−11 | |
| Uncharacterized protein, partial K02A2.6, partial | JAG38901.1 | tsa | 26.82% | 8.00 × 10−12 | |
| Amblyomma sculptum | Putative gypsy-7 adi-i, partial | JAU00762.1 | tsa | 32.88% | 1.00 × 10−12 |
| Putative tick transposon, partial | JAU00733.1 | tsa | 30.00% | 2.00 × 10−10 | |
| Putative tick transposon, partial | JAU00744.1 | tsa | 30.00% | 2.00 × 10−10 | |
|
Rhipicephalus
microplus |
Putative tick transposon | NIE47479.1 | tsa | 32.45% | 4.00 × 10−11 |
| Strongylocentrotus purpuratus | Integrase, catalytic core containing protein | MOS08875.1 | tsa | 32.19% | 2.00 × 10−16 |
| Integrase, catalytic core containing protein | MOS08729.1 | tsa | 32.03% | 7.00 × 10−13 | |
| Integrase, catalytic core containing protein | MOS14051.1 | tsa | 30.57% | 2.00 × 10−13 | |
| Reverse transcriptase domain-containing protein | MOS08491.1 | tsa | 30.57% | 2.00 × 10−13 | |
| Reverse transcriptase domain-containing protein | MOS11069.1 | tsa | 29.93% | 5.00 × 10−12 | |
| Integrase, catalytic core containing protein | MOS08728.1 | tsa | 29.76% | 2.00 × 10−10 | |
| Integrase, catalytic core containing protein | MOS08842.1 | tsa | 28.57% | 4.00 × 10−11 | |
| Integrase, catalytic core containing protein | MOS08745.1 | tsa | 24.12% | 2.00 × 10−10 | |
| Ornithodoros turicata | Putative transposon tf2-9 polyprotein | MBY06492.1 | tsa | 32.00% | 1.00 × 10−10 |
| Ornithodoros moubata | Esterase D, partial | JAW02195.1 | tsa | 29.86% | 2.00 × 10−10 |
| Photinus pyralis | Hypothetical protein | JAV53763.1 | tsa | 29.20% | 2.00 × 10−13 |
|
Lepeophtheirus
salmonis |
Uncharacterized protein K02A2.6like, partial | CDW28658.1 | tsa | 28.28% | 1.00 × 10−10 |
|
Drosophila melanogaster (Fruit fly) |
Unnamed protein product | CAA30503.1 | nr-DM | 28.87% | 0.004 |
| Sd02026p | AAK84933.1 | nr-DM | 28.57% | 3.00 × 10−9 | |
| Blastopia polyprotein | CAA81643.1 | nr-DM | 28.19% | 2.00 × 10−9 | |
| Polyprotein | ACI62137.1 | nr-DM | 28.00% | 2.00 × 10−4 |
BLAST databases: nr = non-redundant protein database; nr-DM = non-redundant protein database with Drosophila specified as species search constraint; tsa = transcriptomics shotgun analysis non-redundant database.
Table A5.
List of query proteins for STRING analysis based on ELM interaction motifs in ERVK integrase.
| Category | ELM | STRING Predicted Interactor (Gene Name) |
Network |
|---|---|---|---|
| Cleavage | CLV_C14_Caspase3-7 | CASP3 | • |
| CASP7 | • | ||
| CLV_PCSK_KEX2_1 CLV_PCSK_PC1ET2_1 CLV_PCSK_SKI1_1 |
PCSK1 | • | |
| PCSK2 | • | ||
| PCSK3 | • | ||
| PCSK4 | • | ||
| PCSK5 | • | ||
| PCSK6 | • | ||
| PCSK7 | • | ||
| PCSK8 | • | ||
| PCSK9 | • | ||
| Docking site | DOC_CYCLIN_RXL_1 | CCNA1 | • |
| CCNA2 | • | ||
| CCNB1 | • | ||
| CCNB2 | • | ||
| CCNB3 | • | ||
| CCNC | • | ||
| CCND1 | • | ||
| CCND2 | • | ||
| CCND3 | • | ||
| CCNE1 | • | ||
| CCNE2 | • | ||
| CCNF | • | ||
| CCNG1 | • | ||
| CCNG2 | • | ||
| CCNH | • | ||
| CCNI | • | ||
| CCNI2 | • | ||
| CCNJ | • | ||
| CCNJL | • | ||
| CCNK | • | ||
| CCNL1 | • | ||
| CCNL2 | • | ||
| CCNO | • | ||
| CCNP | • | ||
| CCNT1 | • | ||
| CCNT2 | • | ||
| CCNY | • | ||
| CCNYL1 | • | ||
| CNTD1 | • | ||
| DOC_MAPK_MEF2A_6 | MAPK1 | • | |
| MAPK3 | • | ||
| MAPK7 | • | ||
| MAPK11 | • | ||
| MAPK14 | • | ||
| DOC_PP1_RVXF_1 | PPP1CA | • | |
| PPP1CB | • | ||
| PPP1CC | • | ||
| PPP3CA | • | ||
| PPP3CB | • | ||
| PPP3CC | • | ||
| DOC_PP2B_LxvP_1 | PPP3R1 | • | |
| DOC_USP7_MATH_1 DOC_USP7_UBL2_3 |
USP7 | • | |
| DOC_WW_Pin1_4 | PCIF1 | • | |
| PIN1 | • | ||
| SENP6 | • | ||
| Ligand | LIG_14-3-3_CanoR_1 LIG_14-3-3_CterR_2 |
SFN | • |
| YWHAB | • | ||
| YWHAE | • | ||
| YWHAG | • | ||
| YWHAH | • | ||
| YWHAQ | • | ||
| YWHAZ | • | ||
| LIG_BIR_II_1 | BIRC2 | • | |
| BIRC3 | • | ||
| BIRC5 | • | ||
| BIRC6 | • | ||
| BIRC7 | • | ||
| NAIP | • | ||
| XIAP | • | ||
| LIG_BRCT_BRCA1_1 | BARD1 | • | |
| BRCA1 | • | ||
| CTDP1 | • | ||
| DNTT | • | ||
| ECT2 | • | ||
| LIG4 | • | ||
| MCPH1 | • | ||
| MDC1 | • | ||
| NBN | • | ||
| PARP1 | • | ||
| PARP4 | • | ||
| PAXIP1 | • | ||
| PES1 | • | ||
| RBPJ | • | ||
| REV1 | • | ||
| RFC1 | • | ||
| TOPBP1 | • | ||
| TP53BP1 | • | ||
| XRCC1 | • | ||
| LIG_FHA_1 LIG_FHA_2 |
AGGF1 | • | |
| APLF | • | ||
| APTX | • | ||
| CEP170 | • | ||
| CEP170B | • | ||
| CHEK2 | • | ||
| CHFR | • | ||
| FHAD1 | • | ||
| FOXK1 | • | ||
| FOXK2 | • | ||
| KIF13A | • | ||
| KIF13B | |||
| KIF14 | • | ||
| KIF16B | |||
| KIF1A | • | ||
| KIF1B | • | ||
| KIF1C | |||
| MCRS1 | • | ||
| MDC1 | • | ||
| MKI67 | • | ||
| MLLT4 | • | ||
| NBN | • | ||
| PHF12 | • | ||
| PPP1R8 | • | ||
| RNF8 | • | ||
| SLC4A1AP | • | ||
| SLMAP | • | ||
| SNIP1 | • | ||
| STARD9 | • | ||
| TCF19 | • | ||
| TIFA | • | ||
| TIFAB | • | ||
| LIG_CSL_BTD_1 | CHEK2 | • | |
| MRC1 | • | ||
| RAD9A | • | ||
| XRCC1 | • | ||
| XRCC4 | • | ||
| LIG_LIR_Gen_1 LIG_LIR_Nem_4 |
GABARAP | ||
| GABARAPL1 | |||
| GABARAPL2 | • | ||
| MAP1LC3A | |||
| MAP1LC3B | |||
| MAP1LC3B2 | • | ||
| MAP1LC3C | |||
| LIG_Pex14_1 | PEX13 | • | |
| PEX14 | • | ||
| LIG_SH2_PTP2 | PLCG1 | • | |
| PTPN11 | • | ||
| LIG_SH2_SRC | BLK | • | |
| FGR | • | ||
| FRK | • | ||
| FYN | • | ||
| HCK | • | ||
| LCK | • | ||
| LYN | • | ||
| SRC | • | ||
| YES1 | • | ||
| LIG_SH2_STAP1 | STAP1 | • | |
| LIG_TYR_ITIM | ABL1 | • | |
| ABL2 | • | ||
| FYN | • | ||
| LCK | • | ||
| MATK | • | ||
| PI3KCA | • | ||
| PLCG1 | • | ||
| SH2D1A | • | ||
| SHF | • | ||
| PTPN6 | • | ||
| PTPN11 | • | ||
| SRC | • | ||
| SYK | • | ||
| LIG_SH2_STAT3 | STAT3 | • | |
| LIG_SH2_STAT5 | STAT5A | • | |
| STAT5B | • | ||
| LIG_SH3_3 | ARHGEF7 | • | |
| CTTN | • | ||
| LIG_SxIP_EBH_1 | MAPRE1 | • | |
| MAPRE2 | • | ||
| MAPRE3 | • | ||
| LIG_TRAF2_1 | TRAF2 | • | |
| LIG_WD40_WDR5_VDV_2 | WDR5 | • | |
| Modification | MOD_CK1_1 | CSNK1A1 | • |
| MOD_CK2_1 | CSNK2A1 | • | |
| MOD_GSK3_1 | GSK3A | • | |
| GSK3B | • | ||
| MOD_N-GLC_1 | DDOST | • | |
| MOD_NEK2_1 | NEK2 | • | |
| MOD_PIKK_1 | ATM | • | |
| ATR | • | ||
| mTOR | • | ||
| PRKDC | • | ||
| SMG1 | • | ||
| TRRAP | • | ||
| MOD_PKA_2 | PAK1 | • | |
| PRKACA | • | ||
| PRKACB | • | ||
| PRKACG | • | ||
| PRKCA | • | ||
| PRKCB | • | ||
| PRKCE | • | ||
| PRKCG | • | ||
| PRKCH | • | ||
| PRKCI | • | ||
| PRKCQ | • | ||
| PRKCZ | • | ||
| MOD_Plk_1 MOD_Plk_4 |
PLK1 | • | |
| PLK2 | • | ||
| PLK3 | • | ||
| PLK4 | • | ||
| MOD_ProDKin_1 | MAPK11 | • | |
| MAPK12 | |||
| MAPK13 | |||
| MAPK14 | • | ||
| MOD_SUMO_rev_2 | SUMO2 | • | |
| Targeting | TRG_ENDOCYTIC_2 | AP1M1 | • |
| AP2M1 | • | ||
| AP3M1 | • | ||
| AP3M2 | |||
| AP4M1 | • | ||
| AP5M1 | |||
| ARCN1 | |||
| FCHO1 | • | ||
| FCHO2 | • | ||
| SGIP1 | • | ||
| STON1 | • | ||
| STON2 | • | ||
| TRG_Pf-PMV_PEXEL_1 | None | ||
Circle symbol: denotes protein depicted in network analysis in Figure 3.
Table A6.
Full list of KEGG pathways identified in the STRING analysis of the ERVK integrase interactome.
| KEGG Term ID | Term Description | Observed Gene Count | Background Gene Count | Strength | False Discovery Rate |
|---|---|---|---|---|---|
| hsa04218 | Cellular senescence | 28 | 156 | 1.27 | 2.30 × 10−23 |
| hsa05203 | Viral carcinogenesis | 29 | 183 | 1.21 | 3.47 × 10−23 |
| hsa04110 | Cell cycle | 25 | 123 | 1.32 | 2.38 × 10−22 |
| hsa04114 | Oocyte meiosis | 23 | 116 | 1.31 | 2.13 × 10−20 |
| hsa05169 | Epstein–Barr virus infection | 24 | 194 | 1.11 | 4.01 × 10−17 |
| hsa05205 | Proteoglycans in cancer | 23 | 195 | 1.08 | 4.79 × 10−16 |
| hsa04650 | Natural killer cell-mediated cytotoxicity | 19 | 124 | 1.2 | 3.96 × 10−15 |
| hsa04068 | FoxO signaling pathway | 19 | 130 | 1.18 | 7.58 × 10−15 |
| hsa04750 | Inflammatory mediator regulation of TRP channels | 17 | 92 | 1.28 | 8.12 × 10−15 |
| hsa05167 | Kaposi’s sarcoma-associated herpesvirus infection | 21 | 183 | 1.07 | 1.30 × 10−14 |
| hsa04720 | Long-term potentiation | 15 | 64 | 1.38 | 1.77 × 10−14 |
| hsa05161 | Hepatitis B | 19 | 142 | 1.14 | 2.19 × 10−14 |
| hsa05206 | MicroRNAs in cancer | 19 | 149 | 1.12 | 4.49 × 10−14 |
| hsa04370 | VEGF signaling pathway | 14 | 59 | 1.39 | 1.11 × 10−13 |
| hsa04660 | T cell receptor signaling pathway | 16 | 99 | 1.22 | 2.39 × 10−13 |
| hsa04012 | ErbB signaling pathway | 15 | 83 | 1.27 | 3.37 × 10−13 |
| hsa04611 | Platelet activation | 17 | 123 | 1.15 | 3.37 × 10−13 |
| hsa04921 | Oxytocin signaling pathway | 18 | 149 | 1.09 | 4.19 × 10−13 |
| hsa04115 | p53 signaling pathway | 14 | 68 | 1.33 | 4.53 × 10−13 |
| hsa05200 | Pathways in cancer | 29 | 515 | 0.76 | 6.20 × 10−13 |
| hsa05166 | HTLV-I infection | 21 | 250 | 0.94 | 1.82 × 10−12 |
| hsa04933 | AGE-RAGE signaling pathway in diabetic complications | 15 | 98 | 1.2 | 2.24 × 10−12 |
| hsa04510 | Focal adhesion | 19 | 197 | 1 | 2.57 × 10−12 |
| hsa04659 | Th17 cell differentiation | 15 | 102 | 1.18 | 3.46 × 10−12 |
| hsa05031 | Amphetamine addiction | 13 | 65 | 1.31 | 3.95 × 10−12 |
| hsa04728 | Dopaminergic synapse | 16 | 128 | 1.11 | 4.97 × 10−12 |
| hsa04658 | Th1 and Th2 cell differentiation | 14 | 88 | 1.21 | 7.29 × 10−12 |
| hsa05165 | Human papillomavirus infection | 22 | 317 | 0.85 | 1.27 × 10−11 |
| hsa04914 | Progesterone-mediated oocyte maturation | 14 | 94 | 1.19 | 1.52 × 10−11 |
| hsa04310 | Wnt signaling pathway | 16 | 143 | 1.06 | 2.02 × 10−11 |
| hsa04390 | Hippo signaling pathway | 16 | 152 | 1.04 | 4.56 × 10−11 |
| hsa04062 | Chemokine signaling pathway | 17 | 181 | 0.99 | 5.10 × 10−11 |
| hsa04917 | Prolactin signaling pathway | 12 | 69 | 1.25 | 1.03 × 10−10 |
| hsa04662 | B cell receptor signaling pathway | 12 | 71 | 1.24 | 1.35 × 10−10 |
| hsa04919 | Thyroid hormone signaling pathway | 14 | 115 | 1.1 | 1.47 × 10−10 |
| hsa04064 | NF-kappa B signaling pathway | 13 | 93 | 1.16 | 1.57 × 10−10 |
| hsa04270 | Vascular smooth muscle contraction | 14 | 119 | 1.08 | 2.11 × 10−10 |
| hsa04360 | Axon guidance | 16 | 173 | 0.98 | 2.24 × 10−10 |
| hsa05162 | Measles | 14 | 133 | 1.04 | 7.76 × 10−10 |
| hsa05223 | Non-small cell lung cancer | 11 | 66 | 1.23 | 9.08 × 10−10 |
| hsa04666 | Fc gamma R-mediated phagocytosis | 12 | 89 | 1.14 | 1.18 × 10−9 |
| hsa04380 | Osteoclast differentiation | 13 | 124 | 1.03 | 3.48 × 10−9 |
| hsa01521 | EGFR tyrosine kinase inhibitor resistance | 11 | 78 | 1.16 | 4.18 × 10−9 |
| hsa04010 | MAPK signaling pathway | 18 | 293 | 0.8 | 5.87 × 10−9 |
| hsa04931 | Insulin resistance | 12 | 107 | 1.06 | 7.37 × 10−9 |
| hsa04910 | Insulin signaling pathway | 13 | 134 | 1 | 7.61 × 10−9 |
| hsa04724 | Glutamatergic synapse | 12 | 112 | 1.04 | 1.14 × 10−8 |
| hsa04151 | PI3K-Akt signaling pathway | 19 | 348 | 0.75 | 1.17 × 10−8 |
| hsa04912 | GnRH signaling pathway | 11 | 88 | 1.11 | 1.17 × 10−8 |
| hsa04664 | Fc epsilon RI signaling pathway | 10 | 67 | 1.19 | 1.30 × 10−8 |
| hsa04071 | Sphingolipid signaling pathway | 12 | 116 | 1.03 | 1.51 × 10−8 |
| hsa04722 | Neurotrophin signaling pathway | 12 | 116 | 1.03 | 1.51 × 10−8 |
| hsa01522 | Endocrine resistance | 11 | 95 | 1.08 | 2.24 × 10−8 |
| hsa04014 | Ras signaling pathway | 15 | 228 | 0.83 | 5.08 × 10−8 |
| hsa04210 | Apoptosis | 12 | 135 | 0.96 | 6.74 × 10−8 |
| hsa05152 | Tuberculosis | 13 | 172 | 0.89 | 1.01 × 10−7 |
| hsa04540 | Gap junction | 10 | 87 | 1.07 | 1.11 × 10−7 |
| hsa05221 | Acute myeloid leukemia | 9 | 66 | 1.15 | 1.42 × 10−7 |
| hsa05214 | Glioma | 9 | 68 | 1.13 | 1.74 × 10−7 |
| hsa05222 | Small cell lung cancer | 10 | 92 | 1.05 | 1.74 × 10−7 |
| hsa01524 | Platinum drug resistance | 9 | 70 | 1.12 | 2.13 × 10−7 |
| hsa04215 | Apoptosis—multiple species | 7 | 31 | 1.37 | 2.13 × 10−7 |
| hsa04926 | Relaxin signaling pathway | 11 | 130 | 0.94 | 3.72 × 10−7 |
| hsa04015 | Rap1 signaling pathway | 13 | 203 | 0.82 | 5.46 × 10−7 |
| hsa04960 | Aldosterone-regulated sodium reabsorption | 7 | 37 | 1.29 | 5.77 × 10−7 |
| hsa04668 | TNF signaling pathway | 10 | 108 | 0.98 | 6.23 × 10−7 |
| hsa04261 | Adrenergic signaling in cardiomyocytes | 11 | 139 | 0.91 | 6.58 × 10−7 |
| hsa05145 | Toxoplasmosis | 10 | 109 | 0.98 | 6.58 × 10−7 |
| hsa04725 | Cholinergic synapse | 10 | 111 | 0.97 | 7.54 × 10−7 |
| hsa05120 | Epithelial cell signaling in Helicobacter pylori infection | 8 | 66 | 1.1 | 1.53 × 10−6 |
| hsa04340 | Hedgehog signaling pathway | 7 | 46 | 1.2 | 1.97 × 10−6 |
| hsa04961 | Endocrine and other factor-regulated calcium reabsorption | 7 | 47 | 1.19 | 2.22 × 10−6 |
| hsa04066 | HIF-1 signaling pathway | 9 | 98 | 0.98 | 2.44 × 10−6 |
| hsa04520 | Adherens junction | 8 | 71 | 1.06 | 2.44 × 10−6 |
| hsa04916 | Melanogenesis | 9 | 98 | 0.98 | 2.44 × 10−6 |
| hsa05225 | Hepatocellular carcinoma | 11 | 163 | 0.84 | 2.56 × 10−6 |
| hsa04621 | NOD-like receptor signaling pathway | 11 | 166 | 0.83 | 2.99 × 10−6 |
| hsa05014 | Amyotrophic lateral sclerosis (ALS) | 7 | 50 | 1.16 | 2.99 × 10−6 |
| hsa05220 | Chronic myeloid leukemia | 8 | 76 | 1.04 | 3.62 × 10−6 |
| hsa05020 | Prion diseases | 6 | 33 | 1.27 | 4.44 × 10−6 |
| hsa04020 | Calcium signaling pathway | 11 | 179 | 0.8 | 5.69 × 10−6 |
| hsa04670 | Leukocyte transendothelial migration | 9 | 112 | 0.92 | 6.16 × 10−6 |
| hsa04726 | Serotonergic synapse | 9 | 112 | 0.92 | 6.16 × 10−6 |
| hsa04723 | Retrograde endocannabinoid signaling | 10 | 148 | 0.84 | 7.20 × 10−6 |
| hsa04727 | GABAergic synapse | 8 | 88 | 0.97 | 9.28 × 10−6 |
| hsa04934 | Cushing’s syndrome | 10 | 153 | 0.83 | 9.30 × 10−6 |
| hsa04924 | Renin secretion | 7 | 63 | 1.06 | 1.09 × 10−5 |
| hsa04024 | cAMP signaling pathway | 11 | 195 | 0.76 | 1.14 × 10−5 |
| hsa04657 | IL-17 signaling pathway | 8 | 92 | 0.95 | 1.20 × 10−5 |
| hsa04022 | cGMP-PKG signaling pathway | 10 | 160 | 0.81 | 1.28 × 10−5 |
| hsa04140 | Autophagy—animal | 9 | 125 | 0.87 | 1.28 × 10−5 |
| hsa04630 | Jak-STAT signaling pathway | 10 | 160 | 0.81 | 1.28 × 10−5 |
| hsa04713 | Circadian entrainment | 8 | 93 | 0.95 | 1.28 × 10−5 |
| hsa05146 | Amoebiasis | 8 | 94 | 0.94 | 1.32 × 10−5 |
| hsa05215 | Prostate cancer | 8 | 97 | 0.93 | 1.62 × 10−5 |
| hsa05231 | Choline metabolism in cancer | 8 | 98 | 0.92 | 1.72 × 10−5 |
| hsa04371 | Apelin signaling pathway | 9 | 133 | 0.84 | 1.94 × 10−5 |
| hsa04930 | Type II diabetes mellitus | 6 | 46 | 1.13 | 2.04 × 10−5 |
| hsa03450 | Non-homologous end-joining | 4 | 13 | 1.5 | 3.50 × 10−5 |
| hsa04072 | Phospholipase D signaling pathway | 9 | 145 | 0.81 | 3.61 × 10−5 |
| hsa05226 | Gastric cancer | 9 | 147 | 0.8 | 3.96 × 10−5 |
| hsa05210 | Colorectal cancer | 7 | 85 | 0.93 | 5.70 × 10−5 |
| hsa04217 | Necroptosis | 9 | 155 | 0.78 | 5.77 × 10−5 |
| hsa04730 | Long-term depression | 6 | 60 | 1.01 | 7.67 × 10−5 |
| hsa04925 | Aldosterone synthesis and secretion | 7 | 93 | 0.89 | 9.48 × 10−5 |
| hsa04530 | Tight junction | 9 | 167 | 0.74 | 9.70 × 10−5 |
| hsa05131 | Shigellosis | 6 | 63 | 0.99 | 9.70 × 10−5 |
| hsa05010 | Alzheimer’s disease | 9 | 168 | 0.74 | 9.98 × 10−5 |
| hsa05164 | Influenza A | 9 | 168 | 0.74 | 9.98 × 10−5 |
| hsa05160 | Hepatitis C | 8 | 131 | 0.8 | 0.00011 |
| hsa03440 | Homologous recombination | 5 | 40 | 1.11 | 0.00012 |
| hsa04922 | Glucagon signaling pathway | 7 | 100 | 0.86 | 0.00014 |
| hsa05140 | Leishmaniasis | 6 | 70 | 0.95 | 0.00016 |
| hsa05168 | Herpes simplex infection | 9 | 181 | 0.71 | 0.00016 |
| hsa04971 | Gastric acid secretion | 6 | 72 | 0.93 | 0.00018 |
| hsa04918 | Thyroid hormone synthesis | 6 | 73 | 0.93 | 0.00019 |
| hsa05133 | Pertussis | 6 | 74 | 0.92 | 0.0002 |
| hsa05212 | Pancreatic cancer | 6 | 74 | 0.92 | 0.0002 |
| hsa05224 | Breast cancer | 8 | 147 | 0.75 | 0.00021 |
| hsa04150 | mTOR signaling pathway | 8 | 148 | 0.75 | 0.00022 |
| hsa05110 | Vibrio cholerae infection | 5 | 48 | 1.03 | 0.00025 |
| hsa04810 | Regulation of actin cytoskeleton | 9 | 205 | 0.66 | 0.00037 |
| hsa04911 | Insulin secretion | 6 | 84 | 0.87 | 0.00037 |
| hsa05130 | Pathogenic Escherichia coli infection | 5 | 53 | 0.99 | 0.00037 |
| hsa04970 | Salivary secretion | 6 | 86 | 0.86 | 0.00041 |
| hsa05416 | Viral myocarditis | 5 | 56 | 0.96 | 0.00046 |
| hsa05032 | Morphine addiction | 6 | 91 | 0.83 | 0.00053 |
| hsa05213 | Endometrial cancer | 5 | 58 | 0.95 | 0.00053 |
| hsa04915 | Estrogen signaling pathway | 7 | 133 | 0.73 | 0.00063 |
| hsa05418 | Fluid shear stress and atherosclerosis | 7 | 133 | 0.73 | 0.00063 |
| hsa04213 | Longevity regulating pathway—multiple species | 5 | 61 | 0.93 | 0.00064 |
| hsa04550 | Signaling pathways regulating pluripotency of stem cells | 7 | 138 | 0.72 | 0.00076 |
| hsa05211 | Renal cell carcinoma | 5 | 68 | 0.88 | 0.001 |
| hsa04920 | Adipocytokine signaling pathway | 5 | 69 | 0.87 | 0.0011 |
| hsa05219 | Bladder cancer | 4 | 41 | 1 | 0.0013 |
| hsa04211 | Longevity regulating pathway | 5 | 88 | 0.77 | 0.0029 |
| hsa04923 | Regulation of lipolysis in adipocytes | 4 | 53 | 0.89 | 0.0031 |
| hsa04120 | Ubiquitin mediated proteolysis | 6 | 134 | 0.66 | 0.0033 |
| hsa04070 | Phosphatidylinositol signaling system | 5 | 97 | 0.72 | 0.0043 |
| hsa05034 | Alcoholism | 6 | 142 | 0.64 | 0.0043 |
| hsa05142 | Chagas disease (American trypanosomiasis) | 5 | 101 | 0.71 | 0.005 |
| hsa04620 | Toll-like receptor signaling pathway | 5 | 102 | 0.7 | 0.0051 |
| hsa04932 | Non-alcoholic fatty liver disease (NAFLD) | 6 | 149 | 0.62 | 0.0053 |
| hsa05230 | Central carbon metabolism in cancer | 4 | 65 | 0.8 | 0.0059 |
| hsa03410 | Base excision repair | 3 | 33 | 0.97 | 0.0066 |
| hsa05143 | African trypanosomiasis | 3 | 34 | 0.96 | 0.0071 |
| hsa04622 | RIG-I-like receptor signaling pathway | 4 | 70 | 0.77 | 0.0075 |
| hsa05218 | Melanoma | 4 | 72 | 0.76 | 0.0081 |
| hsa05216 | Thyroid cancer | 3 | 37 | 0.92 | 0.0087 |
| hsa05202 | Transcriptional misregulation in cancer | 6 | 169 | 0.56 | 0.0091 |
| hsa04152 | AMPK signaling pathway | 5 | 120 | 0.63 | 0.0093 |
| hsa04962 | Vasopressin-regulated water reabsorption | 3 | 44 | 0.85 | 0.0132 |
| hsa05132 | Salmonella infection | 4 | 84 | 0.69 | 0.0132 |
| hsa03015 | mRNA surveillance pathway | 4 | 89 | 0.67 | 0.0157 |
| hsa04913 | Ovarian steroidogenesis | 3 | 49 | 0.8 | 0.0171 |
| hsa05030 | Cocaine addiction | 3 | 49 | 0.8 | 0.0171 |
| hsa03460 | Fanconi anemia pathway | 3 | 51 | 0.78 | 0.0187 |
| hsa05134 | Legionellosis | 3 | 54 | 0.76 | 0.0215 |
| hsa04137 | Mitophagy—animal | 3 | 63 | 0.69 | 0.0314 |
| hsa04714 | Thermogenesis | 6 | 228 | 0.43 | 0.0314 |
| hsa04927 | Cortisol synthesis and secretion | 3 | 63 | 0.69 | 0.0314 |
| hsa04976 | Bile secretion | 3 | 71 | 0.64 | 0.0413 |
| hsa04136 | Autophagy—other | 2 | 30 | 0.84 | 0.045 |
Author Contributions
Data curation, methodology, validation, investigation, and formal analysis by S.B., I.B. and R.N.D. Conceptualization, supervision, project administration, resources, visualization, and funding acquisition by R.N.D. Writing original draft preparation and review and editing by R.N.D. and I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded in part by the ALS Association (#477) and a Discovery grant from Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN-2016-05761). S.B. was funded by the University of Winnipeg Wiegand Biology Undergraduate Research Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
National Center for Biotechnology Information (NCBI; https://www.ncbi.nlm.nih.gov/, (accessed on 12 July 2021)) can be used to access sequences listed in the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Dedication
This study is dedicated to patients with ALS—we are working on it!
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Christiaansen A., Varga S.M., Spencer J.V. Viral manipulation of the host immune response. Curr. Opin. Immunol. 2015;36:54–60. doi: 10.1016/j.coi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLean J.E., Ruck A., Shirazian A., Pooyaei-Mehr F., Zakeri Z.F. Viral manipulation of cell death. Curr. Pharm. Des. 2008;14:198–220. doi: 10.2174/138161208783413329. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Etxebarria K., Sistiaga-Poveda M., Jugo B.M. Endogenous retroviruses in domestic animals. Curr. Genom. 2014;15:256–265. doi: 10.2174/1389202915666140520003503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X., Zhao H., Gong Z., Han G.Z. Endogenous retroviruses of non-avian/mammalian vertebrates illuminate diversity and deep history of retroviruses. PLoS Pathog. 2018;14:e1007072. doi: 10.1371/journal.ppat.1007072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson P.N., Carnegie P.R., Martin J., Davari Ejtehadi H., Hooley P., Roden D., Rowland-Jones S., Warren P., Astley J., Murray P.G. Demystified. Human endogenous retroviruses. Mol. Pathol. 2003;56:11–18. doi: 10.1136/mp.56.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer J., Meese E. Human endogenous retroviruses in the primate lineage and their influence on host genomes. Cytogenet. Genome Res. 2005;110:448–456. doi: 10.1159/000084977. [DOI] [PubMed] [Google Scholar]
- 7.Hohn O., Hanke K., Bannert N. HERV-K(HML-2), the Best Preserved Family of HERVs: Endogenization, Expression, and Implications in Health and Disease. Front. Oncol. 2013;3:246. doi: 10.3389/fonc.2013.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downey R.F., Sullivan F.J., Wang-Johanning F., Ambs S., Giles F.J., Glynn S.A. Human endogenous retrovirus K and cancer: Innocent bystander or tumorigenic accomplice? Int. J. Cancer. 2015;137:1249–1257. doi: 10.1002/ijc.29003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douville R., Liu J., Rothstein J., Nath A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann. Neurol. 2011;69:141–151. doi: 10.1002/ana.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manghera M., Ferguson-Parry J., Douville R.N. TDP-43 regulates endogenous retrovirus-K viral protein accumulation. Neurobiol. Dis. 2016;94:226–236. doi: 10.1016/j.nbd.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Manghera M., Ferguson-Parry J., Lin R., Douville R.N. NF-kappaB and IRF1 Induce Endogenous Retrovirus K Expression via Interferon-Stimulated Response Elements in Its 5′ Long Terminal Repeat. J. Virol. 2016;90:9338–9349. doi: 10.1128/JVI.01503-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arru G., Mameli G., Deiana G.A., Rassu A.L., Piredda R., Sechi E., Caggiu E., Bo M., Nako E., Urso D., et al. Humoral immunity response to human endogenous retroviruses K/W differentiates between amyotrophic lateral sclerosis and other neurological diseases. Eur. J. Neurol. 2018;25:e1076–e1084. doi: 10.1111/ene.13648. [DOI] [PubMed] [Google Scholar]
- 13.Romer C. Viruses and Endogenous Retroviruses as Roots for Neuroinflammation and Neurodegenerative Diseases. Front. Neurosci. 2021;15:648629. doi: 10.3389/fnins.2021.648629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenig M. HERVs, immunity, and autoimmunity: Understanding the connection. PeerJ. 2019;7:e6711. doi: 10.7717/peerj.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincendeau M., Gottesdorfer I., Schreml J.M., Wetie A.G., Mayer J., Greenwood A.D., Helfer M., Kramer S., Seifarth W., Hadian K., et al. Modulation of human endogenous retrovirus (HERV) transcription during persistent and de novo HIV-1 infection. Retrovirology. 2015;12:27. doi: 10.1186/s12977-015-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilie P.G., Tang C., Mills G.B., Yap T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019;16:81–104. doi: 10.1038/s41571-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penndorf D., Witte O.W., Kretz A. DNA plasticity and damage in amyotrophic lateral sclerosis. Neural Regen. Res. 2018;13:173–180. doi: 10.4103/1673-5374.226377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao F.B., Almeida S., Lopez-Gonzalez R. Dysregulated molecular pathways in amyotrophic lateral sclerosis-frontotemporal dementia spectrum disorder. EMBO J. 2017;36:2931–2950. doi: 10.15252/embj.201797568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessl J.J., Kutluay S.B., Townsend D., Rebensburg S., Slaughter A., Larue R.C., Shkriabai N., Bakouche N., Fuchs J.R., Bieniasz P.D., et al. HIV-1 Integrase Binds the Viral RNA Genome and Is Essential during Virion Morphogenesis. Cell. 2016;166:1257–1268.e1212. doi: 10.1016/j.cell.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesbats P., Engelman A.N., Cherepanov P. Retroviral DNA Integration. Chem. Rev. 2016;116:12730–12757. doi: 10.1021/acs.chemrev.6b00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel R., Kao G., Taganov K., Greger J.G., Favorova O., Merkel G., Yen T.J., Katz R.A., Skalka A.M. Evidence that the retroviral DNA integration process triggers an ATR-dependent DNA damage response. Proc. Natl. Acad. Sci. USA. 2003;100:4778–4783. doi: 10.1073/pnas.0730887100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skalka A.M., Katz R.A. Retroviral DNA integration and the DNA damage response. Cell Death Differ. 2005;12(Suppl. S1):971–978. doi: 10.1038/sj.cdd.4401573. [DOI] [PubMed] [Google Scholar]
- 23.Bray S., Turnbull M., Hebert S., Douville R.N. Insight into the ERVK Integrase—Propensity for DNA Damage. Front. Microbiol. 2016;7:1941. doi: 10.3389/fmicb.2016.01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitamura Y., Ayukawa T., Ishikawa T., Kanda T., Yoshiike K. Human endogenous retrovirus K10 encodes a functional integrase. J. Virol. 1996;70:3302–3306. doi: 10.1128/jvi.70.5.3302-3306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Maele B., Busschots K., Vandekerckhove L., Christ F., Debyser Z. Cellular co-factors of HIV-1 integration. Trends Biochem. Sci. 2006;31:98–105. doi: 10.1016/j.tibs.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Christ F., Debyser Z. The LEDGF/p75 integrase interaction, a novel target for anti-HIV therapy. Virology. 2013;435:102–109. doi: 10.1016/j.virol.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 27.Studamire B., Goff S.P. Interactions of host proteins with the murine leukemia virus integrase. Viruses. 2010;2:1110–1145. doi: 10.3390/v2051110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayappa K.D., Ao Z., Wang X., Mouland A.J., Shekhar S., Yang X., Yao X. Human immunodeficiency virus type 1 employs the cellular dynein light chain 1 protein for reverse transcription through interaction with its integrase protein. J. Virol. 2015;89:3497–3511. doi: 10.1128/JVI.03347-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan E.L., Hollingworth R., Grand R.J. Activation of the DNA Damage Response by RNA Viruses. Biomolecules. 2016;6:2. doi: 10.3390/biom6010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki Y., Craigie R. The road to chromatin—Nuclear entry of retroviruses. Nat. Rev. Microbiol. 2007;5:187–196. doi: 10.1038/nrmicro1579. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y., Chew M.L. Role of host-encoded proteins in restriction of retroviral integration. Front. Microbiol. 2012;3:227. doi: 10.3389/fmicb.2012.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniel R., Greger J.G., Katz R.A., Taganov K.D., Wu X., Kappes J.C., Skalka A.M. Evidence that stable retroviral transduction and cell survival following DNA integration depend on components of the nonhomologous end joining repair pathway. J. Virol. 2004;78:8573–8581. doi: 10.1128/JVI.78.16.8573-8581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turlure F., Devroe E., Silver P.A., Engelman A. Human cell proteins and human immunodeficiency virus DNA integration. Front. Biosci. 2004;9:3187–3208. doi: 10.2741/1472. [DOI] [PubMed] [Google Scholar]
- 34.Cooper A., Garcia M., Petrovas C., Yamamoto T., Koup R.A., Nabel G.J. HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature. 2013;498:376–379. doi: 10.1038/nature12274. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Mourad R., Ginalski K., Legube G., Cuvier O. Predicting double-strand DNA breaks using epigenome marks or DNA at kilobase resolution. Genome Biol. 2018;19:34. doi: 10.1186/s13059-018-1411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stetson D.B., Ko J.S., Heidmann T., Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J., Scadden D.T., Crumpacker C.S. Primitive hematopoietic cells resist HIV-1 infection via p21. J. Clin. Investig. 2007;117:473–481. doi: 10.1172/JCI28971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ran X., Ao Z., Olukitibi T., Yao X. Characterization of the Role of Host Cellular Factor Histone Deacetylase 10 during HIV-1 Replication. Viruses. 2019;12:28. doi: 10.3390/v12010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali H., Mano M., Braga L., Naseem A., Marini B., Vu D.M., Collesi C., Meroni G., Lusic M., Giacca M. Cellular TRIM33 restrains HIV-1 infection by targeting viral integrase for proteasomal degradation. Nat. Commun. 2019;10:926. doi: 10.1038/s41467-019-08810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulder L.C., Chakrabarti L.A., Muesing M.A. Interaction of HIV-1 integrase with DNA repair protein hRad18. J. Biol. Chem. 2002;277:27489–27493. doi: 10.1074/jbc.M203061200. [DOI] [PubMed] [Google Scholar]
- 42.Van Maele B., Debyser Z. HIV-1 integration: An interplay between HIV-1 integrase, cellular and viral proteins. AIDS Rev. 2005;7:26–43. [PubMed] [Google Scholar]
- 43.Anisenko A.N., Knyazhanskaya E.S., Zalevsky A.O., Agapkina J.Y., Sizov A.I., Zatsepin T.S., Gottikh M.B. Characterization of HIV-1 integrase interaction with human Ku70 protein and initial implications for drug targeting. Sci. Rep. 2017;7:5649. doi: 10.1038/s41598-017-05659-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.BLAST. [(accessed on 12 July 2021)]; Available online: https://www.ncbi.nlm.nih.gov/BLAST/
- 45.Rosindell J., Harmon L.J. OneZoom: A fractal explorer for the tree of life. PLoS Biol. 2012;10:e1001406. doi: 10.1371/journal.pbio.1001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu S., Wang J., Chitsaz F., Derbyshire M.K., Geer R.C., Gonzales N.R., Gwadz M., Hurwitz D.I., Marchler G.H., Song J.S., et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020;48:D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar M., Gouw M., Michael S., Sámano-Sánchez H., Pancsa R., Glavina J., Diakogianni A., Valverde J.A., Bukirova D., Čalyševa J., et al. ELM-the eukaryotic linear motif resource in 2020. Nucleic Acids Res. 2020;48:D296–D306. doi: 10.1093/nar/gkz1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee Y.M., Liou Y.C. Gears-In-Motion: The Interplay of WW and PPIase Domains in Pin1. Front. Oncol. 2018;8:469. doi: 10.3389/fonc.2018.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramos F., Villoria M.T., Alonso-Rodriguez E., Clemente-Blanco A. Role of protein phosphatases PP1, PP2A, PP4 and Cdc14 in the DNA damage response. Cell Stress. 2019;3:70–85. doi: 10.15698/cst2019.03.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knippschild U., Kruger M., Richter J., Xu P., Garcia-Reyes B., Peifer C., Halekotte J., Bakulev V., Bischof J. The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis. Front. Oncol. 2014;4:96. doi: 10.3389/fonc.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fry A.M., O’Regan L., Sabir S.R., Bayliss R. Cell cycle regulation by the NEK family of protein kinases. J. Cell Sci. 2012;125:4423–4433. doi: 10.1242/jcs.111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar S., Sharma G., Chakraborty C., Sharma A.R., Kim J. Regulatory functional territory of PLK-1 and their substrates beyond mitosis. Oncotarget. 2017;8:37942–37962. doi: 10.18632/oncotarget.16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reinhardt H.C., Yaffe M.B. Phospho-Ser/Thr-binding domains: Navigating the cell cycle and DNA damage response. Nat. Rev. Mol. Cell Biol. 2013;14:563–580. doi: 10.1038/nrm3640. [DOI] [PubMed] [Google Scholar]
- 55.Chang Z., Wang Y., Zhou X., Long J.E. STAT3 roles in viral infection: Antiviral or proviral? Future Virol. 2018;13:557–574. doi: 10.2217/fvl-2018-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rani A., Murphy J.J. STAT5 in Cancer and Immunity. J. Interferon Cytokine Res. 2016;36:226–237. doi: 10.1089/jir.2015.0054. [DOI] [PubMed] [Google Scholar]
- 57.Mahajan K., Mahajan N.P. Cross talk of tyrosine kinases with the DNA damage signaling pathways. Nucleic Acids Res. 2015;43:10588–10601. doi: 10.1093/nar/gkv1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teyra J., Huang H., Jain S., Guan X., Dong A., Liu Y., Tempel W., Min J., Tong Y., Kim P.M., et al. Comprehensive Analysis of the Human SH3 Domain Family Reveals a Wide Variety of Non-canonical Specificities. Structure. 2017;25:1598–1610.e3. doi: 10.1016/j.str.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 59.Lalaoui N., Vaux D.L. Recent advances in understanding inhibitor of apoptosis proteins. F1000Research. 2018;7 doi: 10.12688/f1000research.16439.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu H., Li L., Voss C., Wang F., Liu J., Li S.S. A Comprehensive Immunoreceptor Phosphotyrosine-based Signaling Network Revealed by Reciprocal Protein-Peptide Array Screening. Mol. Cell Proteom. 2015;14:1846–1858. doi: 10.1074/mcp.M115.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beurel E., Grieco S.F., Jope R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canovas B., Nebreda A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021;22:346–366. doi: 10.1038/s41580-020-00322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Covill-Cooke C., Toncheva V.S., Kittler J.T. Regulation of peroxisomal trafficking and distribution. Cell. Mol. Life Sci. 2021;78:1929–1941. doi: 10.1007/s00018-020-03687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar P., Wittmann T. + TIPs: SxIPping along microtubule ends. Trends Cell Biol. 2012;22:418–428. doi: 10.1016/j.tcb.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park S.Y., Guo X. Adaptor protein complexes and intracellular transport. Biosci. Rep. 2014;34 doi: 10.1042/BSR20140069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Egea P.F. Crossing the Vacuolar Rubicon: Structural Insights into Effector Protein Trafficking in Apicomplexan Parasites. Microorganisms. 2020;8:865. doi: 10.3390/microorganisms8060865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matic I., Schimmel J., Hendriks I.A., van Santen M.A., van de Rijke F., van Dam H., Gnad F., Mann M., Vertegaal A.C. Site-specific identification of SUMO-2 targets in cells reveals an inverted SUMOylation motif and a hydrophobic cluster SUMOylation motif. Mol. Cell. 2010;39:641–652. doi: 10.1016/j.molcel.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 68.Zamborlini A., Coiffic A., Beauclair G., Delelis O., Paris J., Koh Y., Magne F., Giron M.L., Tobaly-Tapiero J., Deprez E., et al. Impairment of human immunodeficiency virus type-1 integrase SUMOylation correlates with an early replication defect. J. Biol. Chem. 2011;286:21013–21022. doi: 10.1074/jbc.M110.189274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao X. SUMO-Mediated Regulation of Nuclear Functions and Signaling Processes. Mol. Cell. 2018;71:409–418. doi: 10.1016/j.molcel.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quek H., Lim Y.C., Lavin M.F., Roberts T.L. PIKKing a way to regulate inflammation. Immunol. Cell Biol. 2018;96:8–20. doi: 10.1111/imcb.1001. [DOI] [PubMed] [Google Scholar]
- 71.Penalosa-Ruiz G., Bousgouni V., Gerlach J.P., Waarlo S., van de Ven J.V., Veenstra T.E., Silva J.C.R., van Heeringen S.J., Bakal C., Mulder K.W., et al. WDR5, BRCA1, and BARD1 Co-regulate the DNA Damage Response and Modulate the Mesenchymal-to-Epithelial Transition during Early Reprogramming. Stem Cell Rep. 2019;12:743–756. doi: 10.1016/j.stemcr.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wheatley S.P., Altieri D.C. Survivin at a glance. J. Cell Sci. 2019;132 doi: 10.1242/jcs.223826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fang Y., Zhang X. Targeting NEK2 as a promising therapeutic approach for cancer treatment. Cell Cycle. 2016;15:895–907. doi: 10.1080/15384101.2016.1152430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Carcer G. The Mitotic Cancer Target Polo-Like Kinase 1: Oncogene or Tumor Suppressor? Genes. 2019;10:208. doi: 10.3390/genes10030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Grevenynghe J., Cubas R.A., DaFonseca S., Metcalf T., Tremblay C.L., Trautmann L., Sekaly R.P., Schatzle J., Haddad E.K. Foxo3a: An integrator of immune dysfunction during HIV infection. Cytokine Growth Factor Rev. 2012;23:215–221. doi: 10.1016/j.cytogfr.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aloni-Grinstein R., Charni-Natan M., Solomon H., Rotter V. p53 and the Viral Connection: Back into the Future (double dagger) Cancers. 2018;10:178. doi: 10.3390/cancers10060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ho J., Moyes D.L., Tavassoli M., Naglik J.R. The Role of ErbB Receptors in Infection. Trends Microbiol. 2017;25:942–952. doi: 10.1016/j.tim.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khanizadeh S., Hasanvand B., Esmaeil Lashgarian H., Almasian M., Goudarzi G. Interaction of viral oncogenic proteins with the Wnt signaling pathway. Iran. J. Basic Med. Sci. 2018;21:651–659. doi: 10.22038/IJBMS.2018.28903.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Martini W., Rahman R., Ojegba E., Jungwirth E., Macias J., Ackerly F., Fowler M., Cottrell J., Chu T., Chang S.L. Kinases: Understanding Their Role in HIV Infection. World J. AIDS. 2019;9:142–160. doi: 10.4236/wja.2019.93011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Medders K.E., Kaul M. Mitogen-activated protein kinase p38 in HIV infection and associated brain injury. J. Neuroimmune Pharmacol. 2011;6:202–215. doi: 10.1007/s11481-011-9260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saez-Cirion A., Manel N. Immune Responses to Retroviruses. Annu. Rev. Immunol. 2018;36:193–220. doi: 10.1146/annurev-immunol-051116-052155. [DOI] [PubMed] [Google Scholar]
- 82.Shi J.H., Sun S.C. Tumor Necrosis Factor Receptor-Associated Factor Regulation of Nuclear Factor kappaB and Mitogen-Activated Protein Kinase Pathways. Front. Immunol. 2018;9:1849. doi: 10.3389/fimmu.2018.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nehlig A., Molina A., Rodrigues-Ferreira S., Honore S., Nahmias C. Regulation of end-binding protein EB1 in the control of microtubule dynamics. Cell. Mol. Life Sci. 2017;74:2381–2393. doi: 10.1007/s00018-017-2476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao H., Orth J.D., Chen J., Weller S.G., Heuser J.E., McNiven M.A. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol. Cell. Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zare M., Mostafaei S., Ahmadi A., Azimzadeh Jamalkandi S., Abedini A., Esfahani-Monfared Z., Dorostkar R., Saadati M. Human endogenous retrovirus env genes: Potential blood biomarkers in lung cancer. Microb. Pathog. 2018;115:189–193. doi: 10.1016/j.micpath.2017.12.040. [DOI] [PubMed] [Google Scholar]
- 86.Bergallo M., Montanari P., Mareschi K., Merlino C., Berger M., Bini I., Dapra V., Galliano I., Fagioli F. Expression of the pol gene of human endogenous retroviruses HERV-K and -W in leukemia patients. Arch. Virol. 2017;162:3639–3644. doi: 10.1007/s00705-017-3526-7. [DOI] [PubMed] [Google Scholar]
- 87.Ma W., Hong Z., Liu H., Chen X., Ding L., Liu Z., Zhou F., Yuan Y. Human Endogenous Retroviruses-K (HML-2) Expression Is Correlated with Prognosis and Progress of Hepatocellular Carcinoma. BioMed Res. Int. 2016;2016:8201642. doi: 10.1155/2016/8201642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan Z., Yang Y., Zhang N., Soto C., Jiang X., An Z., Zheng W.J. Human Endogenous Retroviruses in Glioblastoma Multiforme. Microorganisms. 2021;9:764. doi: 10.3390/microorganisms9040764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dembny P., Newman A.G., Singh M., Hinz M., Szczepek M., Kruger C., Adalbert R., Dzaye O., Trimbuch T., Wallach T., et al. Human endogenous retrovirus HERV-K(HML-2) RNA causes neurodegeneration through Toll-like receptors. JCI Insight. 2020;5 doi: 10.1172/jci.insight.131093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeong B.H., Lee Y.J., Carp R.I., Kim Y.S. The prevalence of human endogenous retroviruses in cerebrospinal fluids from patients with sporadic Creutzfeldt-Jakob disease. J. Clin. Virol. 2010;47:136–142. doi: 10.1016/j.jcv.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 91.Tovo P.A., Rabbone I., Tinti D., Galliano I., Trada M., Dapra V., Cerutti F., Bergallo M. Enhanced expression of human endogenous retroviruses in new-onset type 1 diabetes: Potential pathogenetic and therapeutic implications. Autoimmunity. 2020;53:283–288. doi: 10.1080/08916934.2020.1777281. [DOI] [PubMed] [Google Scholar]
- 92.Mason M.J., Speake C., Gersuk V.H., Nguyen Q.A., O’Brien K.K., Odegard J.M., Buckner J.H., Greenbaum C.J., Chaussabel D., Nepom G.T. Low HERV-K(C4) copy number is associated with type 1 diabetes. Diabetes. 2014;63:1789–1795. doi: 10.2337/db13-1382. [DOI] [PubMed] [Google Scholar]
- 93.Dickerson F., Rubalcaba E., Viscidi R., Yang S., Stallings C., Sullens A., Origoni A., Leister F., Yolken R. Polymorphisms in human endogenous retrovirus K-18 and risk of type 2 diabetes in individuals with schizophrenia. Schizophr. Res. 2008;104:121–126. doi: 10.1016/j.schres.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 94.Cinek O., Kramna L., Odeh R., Alassaf A., Ibekwe M.A.U., Ahmadov G., Elmahi B.M.E., Mekki H., Lebl J., Abdullah M.A. Eukaryotic viruses in the fecal virome at the onset of type 1 diabetes: A study from four geographically distant African and Asian countries. Pediatr. Diabetes. 2021;22:558–566. doi: 10.1111/pedi.13207. [DOI] [PubMed] [Google Scholar]
- 95.Piekna-Przybylska D., Sharma G., Maggirwar S.B., Bambara R.A. Deficiency in DNA damage response, a new characteristic of cells infected with latent HIV-1. Cell Cycle. 2017;16:968–978. doi: 10.1080/15384101.2017.1312225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li D., Lopez A., Sandoval C., Nichols Doyle R., Fregoso O.I. HIV Vpr Modulates the Host DNA Damage Response at Two Independent Steps to Damage DNA and Repress Double-Strand DNA Break Repair. mBio. 2020;11 doi: 10.1128/mBio.00940-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhi H., Guo X., Ho Y.K., Pasupala N., Engstrom H.A.A., Semmes O.J., Giam C.Z. RNF8 Dysregulation and Down-regulation During HTLV-1 Infection Promote Genomic Instability in Adult T-Cell Leukemia. PLoS Pathog. 2020;16:e1008618. doi: 10.1371/journal.ppat.1008618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luftig M.A. Viruses and the DNA Damage Response: Activation and Antagonism. Annu. Rev. Virol. 2014;1:605–625. doi: 10.1146/annurev-virology-031413-085548. [DOI] [PubMed] [Google Scholar]
- 99.Hristova D.B., Lauer K.B., Ferguson B.J. Viral interactions with non-homologous end-joining: A game of hide-and-seek. J. Gen. Virol. 2020;101:1133–1144. doi: 10.1099/jgv.0.001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohammad D.H., Yaffe M.B. 14-3-3 proteins, FHA domains and BRCT domains in the DNA damage response. DNA Repair. 2009;8:1009–1017. doi: 10.1016/j.dnarep.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pancholi N.J., Price A.M., Weitzman M.D. Take your PIKK: Tumour viruses and DNA damage response pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zimmerman E.S., Chen J., Andersen J.L., Ardon O., Dehart J.L., Blackett J., Choudhary S.K., Camerini D., Nghiem P., Planelles V. Human immunodeficiency virus type 1 Vpr-mediated G2 arrest requires Rad17 and Hus1 and induces nuclear BRCA1 and gamma-H2AX focus formation. Mol. Cell. Biol. 2004;24:9286–9294. doi: 10.1128/MCB.24.21.9286-9294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shukrun M., Jabareen A., Abou-Kandil A., Chamias R., Aboud M., Huleihel M. HTLV-1 Tax oncoprotein inhibits the estrogen-induced-ER alpha-Mediated BRCA1 expression by interaction with CBP/p300 cofactors. PLoS ONE. 2014;9:e89390. doi: 10.1371/journal.pone.0089390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nathan K.G., Lal S.K. The Multifarious Role of 14-3-3 Family of Proteins in Viral Replication. Viruses. 2020;12:436. doi: 10.3390/v12040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu Q., Jubb H., Blundell T.L. Phosphopeptide interactions with BRCA1 BRCT domains: More than just a motif. Prog. Biophys. Mol. Biol. 2015;117:143–148. doi: 10.1016/j.pbiomolbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clapperton J.A., Manke I.A., Lowery D.M., Ho T., Haire L.F., Yaffe M.B., Smerdon S.J. Structure and mechanism of BRCA1 BRCT domain recognition of phosphorylated BACH1 with implications for cancer. Nat. Struct. Mol. Biol. 2004;11:512–518. doi: 10.1038/nsmb775. [DOI] [PubMed] [Google Scholar]
- 107.Fan X., Cui L., Zeng Y., Song W., Gaur U., Yang M. 14-3-3 Proteins Are on the Crossroads of Cancer, Aging, and Age-Related Neurodegenerative Disease. Int. J. Mol. Sci. 2019;20:3518. doi: 10.3390/ijms20143518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morales D., Skoulakis E.C., Acevedo S.F. 14-3-3s are potential biomarkers for HIV-related neurodegeneration. J. Neurovirol. 2012;18:341–353. doi: 10.1007/s13365-012-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roskoski R., Jr. RAF protein-serine/threonine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2010;399:313–317. doi: 10.1016/j.bbrc.2010.07.092. [DOI] [PubMed] [Google Scholar]
- 110.Tang J., Wang J.Y., Parker L.L. Detection of early Abl kinase activation after ionizing radiation by using a peptide biosensor. ChemBioChem Eur. J. Chem. Biol. 2012;13:665–673. doi: 10.1002/cbic.201100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCarthy S.D.S., Leontyev D., Nicoletti P., Binnington B., Kozlowski H.N., Ostrowski M., Cochrane A., Branch D.R., Wong R.W. Targeting ABL1 or ARG Tyrosine Kinases to Restrict HIV-1 Infection in Primary CD4+ T-Cells or in Humanized NSG Mice. J. Acquir. Immune Defic. Syndr. 2019;82:407–415. doi: 10.1097/QAI.0000000000002144. [DOI] [PubMed] [Google Scholar]
- 112.Kodama D., Tanaka M., Matsuzaki T., Izumo K., Nakano N., Matsuura E., Saito M., Nagai M., Horiuchi M., Utsunomiya A., et al. Inhibition of ABL1 tyrosine kinase reduces HTLV-1 proviral loads in peripheral blood mononuclear cells from patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. PLoS Negl. Trop. Dis. 2020;14:e0008361. doi: 10.1371/journal.pntd.0008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baskaran R., Escobar S.R., Wang J.Y. Nuclear c-Abl is a COOH-terminal repeated domain (CTD)-tyrosine (CTD)-tyrosine kinase-specific for the mammalian RNA polymerase II: Possible role in transcription elongation. Cell Growth Differ. 1999;10:387–396. [PubMed] [Google Scholar]
- 114.Schlatterer S.D., Acker C.M., Davies P. c-Abl in neurodegenerative disease. J. Mol. Neurosci. 2011;45:445–452. doi: 10.1007/s12031-011-9588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim B.W., Jeong Y.E., Wong M., Martin L.J. DNA damage accumulates and responses are engaged in human ALS brain and spinal motor neurons and DNA repair is activatable in iPSC-derived motor neurons with SOD1 mutations. Acta Neuropathol. Commun. 2020;8:7. doi: 10.1186/s40478-019-0874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang W., Mani A.M., Wu Z.H. DNA damage-induced nuclear factor-kappa B activation and its roles in cancer progression. J. Cancer Metastasis Treat. 2017;3:45–59. doi: 10.20517/2394-4722.2017.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zuo S., Xue Y., Tang S., Yao J., Du R., Yang P., Chen X. 14-3-3 epsilon dynamically interacts with key components of mitogen-activated protein kinase signal module for selective modulation of the TNF-alpha-induced time course-dependent NF-kappaB activity. J. Proteome Res. 2010;9:3465–3478. doi: 10.1021/pr9011377. [DOI] [PubMed] [Google Scholar]
- 118.Pennington K.L., Chan T.Y., Torres M.P., Andersen J.L. The dynamic and stress-adaptive signaling hub of 14-3-3: Emerging mechanisms of regulation and context-dependent protein-protein interactions. Oncogene. 2018;37:5587–5604. doi: 10.1038/s41388-018-0348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saha R.N., Jana M., Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J. Immunol. 2007;179:7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng Y., Ao Z., Wang B., Jayappa K.D., Yao X. Host protein Ku70 binds and protects HIV-1 integrase from proteasomal degradation and is required for HIV replication. J. Biol. Chem. 2011;286:17722–17735. doi: 10.1074/jbc.M110.184739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Llano M., Delgado S., Vanegas M., Poeschla E.M. Lens epithelium-derived growth factor/p75 prevents proteasomal degradation of HIV-1 integrase. J. Biol. Chem. 2004;279:55570–55577. doi: 10.1074/jbc.M408508200. [DOI] [PubMed] [Google Scholar]
- 122.Manganaro L., Lusic M., Gutierrez M.I., Cereseto A., Del Sal G., Giacca M. Concerted action of cellular JNK and Pin1 restricts HIV-1 genome integration to activated CD4+ T lymphocytes. Nat. Med. 2010;16:329–333. doi: 10.1038/nm.2102. [DOI] [PubMed] [Google Scholar]
- 123.Quy V.C., Carnevale V., Manganaro L., Lusic M., Rossetti G., Leone V., Fenollar-Ferrer C., Raugei S., Del Sal G., Giacca M., et al. HIV-1 integrase binding to its cellular partners: A perspective from computational biology. Curr. Pharm. Des. 2014;20:3412–3421. doi: 10.2174/13816128113199990631. [DOI] [PubMed] [Google Scholar]
- 124.Kojima Y., Ryo A. Pinning down viral proteins: A new prototype for virus-host cell interaction. Front. Microbiol. 2010;1:107. doi: 10.3389/fmicb.2010.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nieto-Torres J.L., Leidal A.M., Debnath J., Hansen M. Beyond Autophagy: The Expanding Roles of ATG8 Proteins. Trends Biochem. Sci. 2021 doi: 10.1016/j.tibs.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leymarie O., Lepont L., Berlioz-Torrent C. Canonical and Non-Canonical Autophagy in HIV-1 Replication Cycle. Viruses. 2017;9:270. doi: 10.3390/v9100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vicencio E., Beltran S., Labrador L., Manque P., Nassif M., Woehlbier U. Implications of Selective Autophagy Dysfunction for ALS Pathology. Cells. 2020;9:381. doi: 10.3390/cells9020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Watanabe Y., Taguchi K., Tanaka M. Ubiquitin, Autophagy and Neurodegenerative Diseases. Cells. 2020;9:2022. doi: 10.3390/cells9092022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Negrini S., Gorgoulis V.G., Halazonetis T.D. Genomic instability--an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 130.Sun Y., Curle A.J., Haider A.M., Balmus G. The role of DNA damage response in amyotrophic lateral sclerosis. Essays Biochem. 2020;64:847–861. doi: 10.1042/EBC20200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hou Y., Song H., Croteau D.L., Akbari M., Bohr V.A. Genome instability in Alzheimer disease. Mech. Ageing Dev. 2017;161:83–94. doi: 10.1016/j.mad.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Madabhushi R., Pan L., Tsai L.H. DNA damage and its links to neurodegeneration. Neuron. 2014;83:266–282. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Malaspina A., Kaushik N., de Belleroche J. A 14-3-3 mRNA is up-regulated in amyotrophic lateral sclerosis spinal cord. J. Neurochem. 2000;75:2511–2520. doi: 10.1046/j.1471-4159.2000.0752511.x. [DOI] [PubMed] [Google Scholar]
- 134.Hermeking H. The 14-3-3 cancer connection. Nat. Rev. Cancer. 2003;3:931–943. doi: 10.1038/nrc1230. [DOI] [PubMed] [Google Scholar]
- 135.Martin L.J. p53 is abnormally elevated and active in the CNS of patients with amyotrophic lateral sclerosis. Neurobiol. Dis. 2000;7:613–622. doi: 10.1006/nbdi.2000.0314. [DOI] [PubMed] [Google Scholar]
- 136.Ozaki T., Nakagawara A. Role of p53 in Cell Death and Human Cancers. Cancers. 2011;3:994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koul H.K., Pal M., Koul S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer. 2013;4:342–359. doi: 10.1177/1947601913507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo W., Vandoorne T., Steyaert J., Staats K.A., Van Den Bosch L. The multifaceted role of kinases in amyotrophic lateral sclerosis: Genetic, pathological and therapeutic implications. Brain. 2020;143:1651–1673. doi: 10.1093/brain/awaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jiang X., Guan Y., Zhao Z., Meng F., Wang X., Gao X., Liu J., Chen Y., Zhou F., Zhou S., et al. Potential Roles of the WNT Signaling Pathway in Amyotrophic Lateral Sclerosis. Cells. 2021;10:839. doi: 10.3390/cells10040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Menck K., Heinrichs S., Baden C., Bleckmann A. The WNT/ROR Pathway in Cancer: From Signaling to Therapeutic Intervention. Cells. 2021;10:142. doi: 10.3390/cells10010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Manigrasso M.B., Juranek J., Ramasamy R., Schmidt A.M. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol. Metab. 2014;25:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumar V., Fleming T., Terjung S., Gorzelanny C., Gebhardt C., Agrawal R., Mall M.A., Ranzinger J., Zeier M., Madhusudhan T., et al. Homeostatic nuclear RAGE-ATM interaction is essential for efficient DNA repair. Nucleic Acids Res. 2017;45:10595–10613. doi: 10.1093/nar/gkx705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abe R., Yamagishi S. AGE-RAGE system and carcinogenesis. Curr. Pharm. Des. 2008;14:940–945. doi: 10.2174/138161208784139765. [DOI] [PubMed] [Google Scholar]
- 144.Derk J., MacLean M., Juranek J., Schmidt A.M. The Receptor for Advanced Glycation Endproducts (RAGE) and Mediation of Inflammatory Neurodegeneration. J. Alzheimers Dis. Parkinsonism. 2018;8 doi: 10.4172/2161-0460.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shaposhnikov M., Proshkina E., Shilova L., Zhavoronkov A., Moskalev A. Lifespan and Stress Resistance in Drosophila with Overexpressed DNA Repair Genes. Sci. Rep. 2015;5:15299. doi: 10.1038/srep15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garinis G.A., van der Horst G.T., Vijg J., Hoeijmakers J.H. DNA damage and ageing: New-age ideas for an age-old problem. Nat. Cell Biol. 2008;10:1241–1247. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Engelman A.N. Multifaceted HIV integrase functionalities and therapeutic strategies for their inhibition. J. Biol. Chem. 2019;294:15137–15157. doi: 10.1074/jbc.REV119.006901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cherepanov P. LEDGF/p75 interacts with divergent lentiviral integrases and modulates their enzymatic activity in vitro. Nucleic Acids Res. 2007;35:113–124. doi: 10.1093/nar/gkl885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Anisenko A., Kan M., Shadrina O., Brattseva A., Gottikh M. Phosphorylation Targets of DNA-PK and Their Role in HIV-1 Replication. Cells. 2020;9:1907. doi: 10.3390/cells9081907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Santos da Silva E., Shanmugapriya S., Malikov V., Gu F., Delaney M.K., Naghavi M.H. HIV-1 capsids mimic a microtubule regulator to coordinate early stages of infection. EMBO J. 2020;39:e104870. doi: 10.15252/embj.2020104870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.De Oliveira D.S., Rosa M.T., Vieira C., Loreto E.L.S. Oxidative and radiation stress induces transposable element transcription in Drosophila melanogaster. J. Evol. Biol. 2021;34:628–638. doi: 10.1111/jeb.13762. [DOI] [PubMed] [Google Scholar]
- 152.Salces-Ortiz J., Vargas-Chavez C., Guio L., Rech G.E., Gonzalez J. Transposable elements contribute to the genomic response to insecticides in Drosophila melanogaster. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020;375:20190341. doi: 10.1098/rstb.2019.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
National Center for Biotechnology Information (NCBI; https://www.ncbi.nlm.nih.gov/, (accessed on 12 July 2021)) can be used to access sequences listed in the paper.