Abstract
The rapid emergence of drug-resistant bacteria is a major global health concern. Antimicrobial peptides (AMPs) and peptidomimetics have arisen as a new class of antibacterial agents in recent years in an attempt to overcome antibiotic resistance. A library of phenylglyoxamide-based small molecular peptidomimetics was synthesised by incorporating an N-alkylsulfonyl hydrophobic group with varying alkyl chain lengths and a hydrophilic cationic group into a glyoxamide core appended to phenyl ring systems. The quaternary ammonium iodide salts 16d and 17c showed excellent minimum inhibitory concentration (MIC) of 4 and 8 μM (2.9 and 5.6 μg/mL) against Staphylococcus aureus, respectively, while the guanidinium hydrochloride salt 34a showed an MIC of 16 μM (8.5 μg/mL) against Escherichia coli. Additionally, the quaternary ammonium iodide salt 17c inhibited 70% S. aureus biofilm formation at 16 μM. It also disrupted 44% of pre-established S. aureus biofilms at 32 μM and 28% of pre-established E. coli biofilms 64 μM, respectively. A cytoplasmic membrane permeability study indicated that the synthesised peptidomimetics acted via disruption and depolarisation of membranes. Moreover, the quaternary ammonium iodide salts 16d and 17c were non-toxic against human cells at their therapeutic dosages against S. aureus.
Keywords: antimicrobial peptide, peptidomimetics, terphenylglyoxamide, terphenyl, isatin, antibiofilm, membrane disruption
1. Introduction
Infections caused by multidrug-resistant bacteria pose a serious threat to humans and are a major health concern [1]. This multi-drug resistance arises from the selective survival pressure exerted by the mechanism of action of conventional antibiotics [2]. Bacteria resist antibiotics through various mechanisms, such as enzymatic degradation of antibiotics, reducing the permeability of the bacterial cell membrane, removal of antibiotics via efflux, and mutation or enzymatic alteration of drug binding sites [3,4,5].
Another survival strategy displayed by bacteria is the formation of biofilms. Biofilms are communities of bacteria encased in an extracellular polymeric matrix adhering to a surface [6,7,8]. Biofilm can form on both biotic surfaces, such as on the tooth and lung, as well as abiotic surfaces, such as catheters and shunts. Due to the protective barrier of the biofilm and the lower growth rate of bacteria inside biofilms, bacteria in biofilms are more resistant to antibiotics [6,8,9,10]. Bacteria in biofilms can be 10 to 1000 times more resistant to antibiotic treatment than in their planktonic form [10]. Biofilms are accountable for approximately 80% of chronic and recurrent bacterial infections [8,11,12]. As biofilms further complicate the treatment of bacterial infections, the ideal novel antibiotic should not only target planktonic bacteria, but also have the ability to disrupt bacterial biofilms.
In response to the rapid emergence of multidrug-resistant bacteria and the formation of bacterial biofilms, attention has been drawn to the development of antimicrobial peptides (AMPs) as a novel class of antibiotics [13,14]. AMPs are a diverse group of natural bioactive peptides found in living organisms [15,16,17] and they play a significant role in the human defence system against microbial infection [18,19]. They act against bacteria by predominantly disrupting the bacterial cell membrane [18,20]. Examples of AMPs include pexiganan (MSI-78), which has reached phase III clinical trials for the topical treatment of infected diabetic foot ulcers, while cathelicidin (LL-37) is in phase IIb clinical trials for the treatment of chronic leg ulcers [13,21]. As AMPs disrupt bacterial cell membranes via non-receptor interactions, the emergence of antibacterial resistance against AMPs is less likely than for conventional antibiotics [17,22,23]. Other than disrupting bacterial cell membranes, some AMPs are known to kill bacteria by inhibiting DNA, RNA and protein synthesis of bacteria [18]. While AMPs are active against bacteria, their high manufacturing cost from solid-phase peptide synthesis and poor pharmacokinetics, such as low bioavailability and high susceptibility to proteolytic degradation, have limited their use as antibacterial agents [18,21,24].
To address the limitations of AMPs, peptidomimetics have been developed [25,26]. These peptidomimetics include α-peptides [27,28], β-peptides [29,30,31], peptoids [28,32,33,34], N-acylated N-aminoethylpeptides [35,36,37] and oligoacyllysines [38,39]. Alternatively, efforts have also been made to develop small molecular antimicrobial peptidomimetics, such as arylamide foldamers [40,41,42], phenyleneethynylenes [43], anthranilamides [44], binaphthyls [45] and sofalcones [46]. Like natural AMPs, peptidomimetics also possess an amphiphilic structure with polar and non-polar groups. The cationic groups first interact with anionic lipids on the bacterial cell membrane, followed by insertion into the bacterial cell membrane with the aid of hydrophobic groups. This leads to the disruption and formation of pores on the bacterial cell membrane, causing cell death [18,47,48,49].
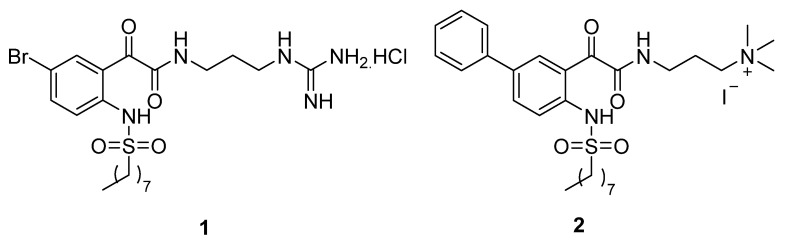
In our previous studies, we reported phenylglyoxamide- and biphenylglyoxamide-based small molecular antimicrobial peptidomimetics 1 and 2 (Figure 1) with high antibacterial activity against Staphylococcus aureus, with minimum inhibitory concentration (MIC) values of 12 and 16 μM, respectively [50,51]. Moreover, peptidomimetic 2 also showed a good MIC value of 32 μM against Escherichia coli. The hydrophobic octanesulfonyl group was found to be important for antibacterial activity, as potency was lost when the chain was shortened.
Figure 1.
Structures of phenylglyoxamide- and biphenylglyoxamide-based small molecular antimicrobial peptidomimetics 1 and 2.
Terphenyl derivatives possess antibacterial activities [52,53,54,55]; however, the effects of introducing a terphenyl moiety in place of the biphenyl moiety of the biphenylglyoxamide scaffold have not been explored. Additionally, lengthening the alkyl chain of the sulfonyl group has not been investigated either. In this study, we report the synthesis of new series of mono-, bi- and terphenylglyoxamide-based small molecular peptidomimetics with alkylsulfonyl groups of different alkyl chain lengths. The antibacterial activities of these synthesised peptidomimetics were evaluated against S. aureus, E. coli and Pseudomonas aeruginosa. The ability of the peptidomimetics to disrupt pre-established bacterial biofilms was also evaluated. The possible mechanism of action of the peptidomimetics was investigated by a membrane depolarisation assay, while their in vitro toxicity against human cells was also assessed to determine their specificity.
2. Results and Discussion
2.1. Synthesis of Polyphenylglyoxamide-Based Antimicrobial Peptidomimetics
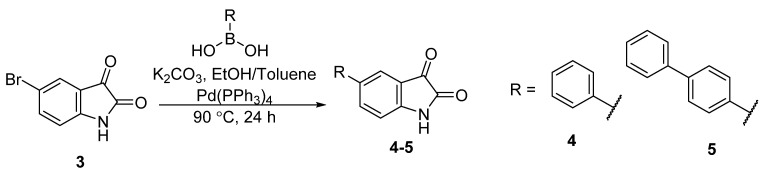
To incorporate the biphenyl or terphenyl group into the phenylglyoxamide backbone, 5-bromoisatin 3 was reacted with phenylboronic acid or 4-biphenylboronic acid with a catalytic amount of tetrakis(triphenylphosphine)palladium(0) via Suzuki–Miyaura cross-coupling reaction to give 5-phenylisatin 4 and 5-(biphenyl-4-yl)isatin 5 in good yields of 86% and 78%, respectively (Scheme 1) [51,56].
Scheme 1.
Synthesis of 5-phenylisatin 4 and 5-(biphenyl-4-yl)isatin 5.
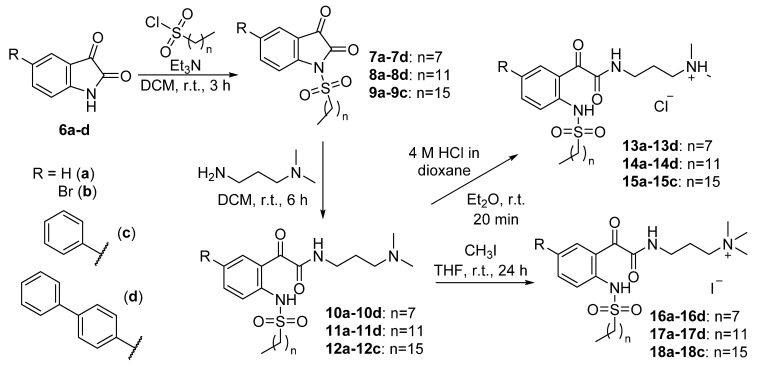
Alkylsulfonyl groups with different chain lengths were then installed into the isatin scaffold by following a previously reported procedure [50,51] to provide the hydrophobic group for the target peptidomimetics. Isatins 6a–6d were treated with triethylamine and various alkylsulfonyl chlorides in dichloromethane (DCM) at room temperature to afford N-sulfonylisatins 7a–7d (n = 7), 8a–8d (n = 11) and 9a–9c (n = 15) in 28–60% yield (Scheme 2). However, the reaction between 5-(biphenyl-4-yl)isatin 6d and 1-hexadecanesulfonyl chloride gave no product. It was initially suspected that the unsuccessful synthesis was due to the poor solubility of 5-(biphenyl-4-yl)isatin 6d and 1-hexadecanesulfonyl chloride in DCM; however, no reaction was observed, even when the sulfonylation reaction was then attempted in other solvents, such as tetrahydrofuran (THF), dimethylformamide (DMF) and toluene. Further attempts involved heating the reaction mixture at reflux in toluene and using sodium hydride or N,N-diisopropylethylamine (DIPEA) as the base, but none of the attempted conditions were successful in the synthesis of the desired product.
Scheme 2.
Synthesis of glyoxamides 10–12, tertiary ammonium chloride salts 13–15 and quaternary ammonium iodide salts 16–18.
The synthesised N-sulfonylisatins 7–9 were then ring-opened with 3-dimethylaminopropylamine in DCM to afford the corresponding glyoxamides 10–12 in excellent yields of 96–99%, except glyoxamides 10d and 11d which gave moderate yields of 61% and 53%, respectively. The lower yield of the latter compounds could be due to the low solubility of the starting material in DCM. The cationicity of the target molecule was then introduced by treating the glyoxamides 10–12 with 4 M HCl in dioxane to afford the corresponding tertiary ammonium chloride salts 13–15 in a moderate to good yield of 60–99%. Alternatively, a different cationic group can be installed by treating the glyoxamides 10–12 with iodomethane to afford the corresponding quaternary ammonium iodide salts 13–15 in yields of 42–91%.
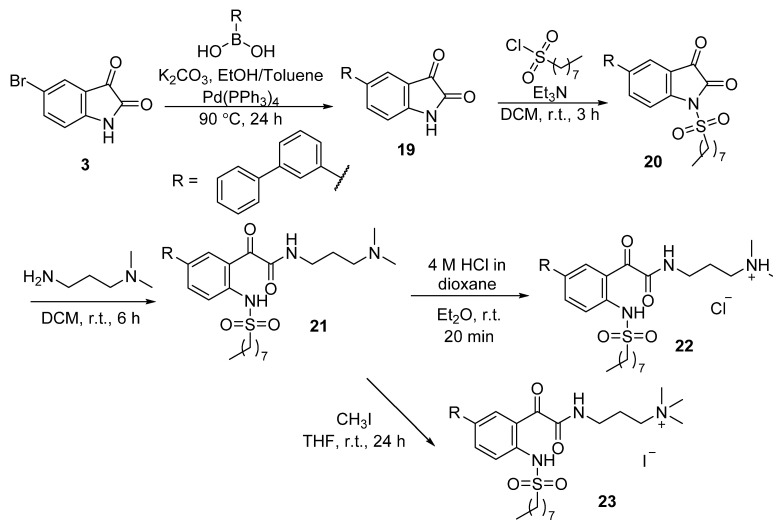
To explore whether changing the position of the terminal phenyl ring in the terphenyl system has any effect on antibacterial activity, ammonium salts 22–23 bearing a meta-terphenyl scaffold instead of a para-terphenyl scaffold were also synthesised following the above-mentioned synthetic route, with 3-biphenylboronic acid as the starting material in the initial Suzuki–Miyaura cross-coupling reaction (Scheme 3).
Scheme 3.
Synthesis of glyoxamide 21, tertiary ammonium chloride salt 22 and quaternary ammonium iodide salt 23 bearing a meta-terphenyl system.
As previous studies have reported that the introduction of a guanidine as the cationic group could increase the potency of peptidomimetics against Gram-positive S. aureus [50,57], the guanidinium hydrochloride salts 33–35 were also synthesised (Scheme 4). To achieve this, N-sulfonylisatins 7–9 were ring-opened with N-Boc-1,3-propanediamine to afford the Boc-protected glyoxamides 24–26 in excellent yields of 88–98%. The Boc-protecting group was then cleaved by treatment with 4 M HCl in dioxane to give the corresponding aminoglyoxamides 27–29 in yields of 60–95%. The guanidine moiety was then introduced to the molecule by reacting the aminoglyoxamides 27–29 with N,N′-di-Boc-1H-pyrazole-1-carboxamidine in the presence of triethylamine in acetonitrile (ACN) to give the Boc-protected guanidine glyoxamides 30–32 in yields of 51–87%. Finally, the Boc-protecting group was cleaved with trifluoroacetic acid in DCM, followed by anion exchange reaction with 4 M HCl in dioxane to give the guanidinium hydrochloride salts 33–35 in 48–97% yield.
Scheme 4.
Synthesis of guanidinium hydrochloride salts 33–35.
2.2. Antibacterial Activity
The synthesised peptidomimetics were tested in the minimal inhibitory concentration (MIC) assay for the determination of their antibacterial activities against Gram-positive S. aureus (SA38). In addition, selected peptidomimetics (16d, 17b, 17c, 18a, 34a, 34b), which showed high antibacterial activity against Gram-positive S. aureus, were also tested for their antibacterial activities against Gram-negative E. coli (K12) and P. aeruginosa (PAO1). In general, the tested peptidomimetics possess lower antibacterial activity against Gram-negative bacteria than Gram-positive S. aureus, especially P. aeruginosa, for which none of the tested peptidomimetics in this study showed any antibacterial activity at the highest concentration tested (250 μM). The MIC values of all tested peptidomimetics are shown in Table 1. There was no effect of the different growth media on MIC values, with the MIC of peptidomimetics 16d and 17c being 4 and 8 μM (2.9 and 5.6 μg/mL), respectively, in the trypticase soy broth (TSB), Mueller Hinton broth (MHB) or cationic adjusted MHB.
Table 1.
Minimum inhibitory concentration (MIC) of peptidomimetics against S. aureus, E. coli and P. aeruginosa and the calculated AlogP values of peptidomimetics.
| Compound | AlogP | MIC (μM/μg/mL) | |||
|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | |||
| Glyoxamide derivatives | 10a | 2.61 | 250/106 [50] | ND | ND |
| 10b | 3.38 | 94/47 [50] | ND | ND | |
| 10c | 4.26 | 24/12 [51] | ND | ND | |
| 10d | 5.91 | 8/4.6 | ND | ND | |
| 11a | 4.39 | 32/15 | ND | ND | |
| 11b | 5.16 | 32/18 | ND | ND | |
| 11c | 6.04 | 16/8.9 | ND | ND | |
| 11d | 7.69 | 250/158 | ND | ND | |
| 12a | 6.17 | 63/34 | ND | ND | |
| 12b | 6.94 | 250/154 | ND | ND | |
| 12c | 7.82 | >250/>153 | ND | ND | |
| 21 | 5.91 | 16/9.2 | ND | ND | |
| Tertiary ammonium chloride salts | 13a | −0.89 | >250/>116 [50] | ND | ND |
| 13b | −0.12 | 63/34 [50] | ND | ND | |
| 13c | 0.76 | 16/8.6 [51] | ND | ND | |
| 13d | 2.41 | 16/9.8 | ND | ND | |
| 14a | 0.89 | 16/8.3 | ND | ND | |
| 14b | 1.66 | 16/9.6 | ND | ND | |
| 14c | 2.54 | 16/9.5 | ND | ND | |
| 14d | 4.18 | 125/84 | ND | ND | |
| 15a | 2.67 | 63/36 | ND | ND | |
| 15b | 3.44 | 250/163 | ND | ND | |
| 15c | 4.32 | >250/>163 | ND | ND | |
| 22 | 2.41 | 16/9.8 | ND | ND | |
| Quaternary ammonium iodide salts | 16a | −1.55 | 250/142 [50] | ND | ND |
| 16b | −0.78 | 63/41 [50] | ND | ND | |
| 2, 16c | 0.10 | 16/10 [51] | 32/21 [51] | 125/80 [51] | |
| 16d | 1.75 | 4/2.9 | >250/>180 | >250/>180 | |
| 17a | 0.23 | 16/10 | ND | ND | |
| 17b | 1.00 | 8/5.6 | 32/22 | >250/>176 | |
| 17c | 1.88 | 8/5.6 | 32/22 | >250/>175 | |
| 17d | 3.52 | 63/48 | ND | ND | |
| 18a | 2.01 | 8/5.4 | >250 | >250 | |
| 18b | 2.78 | 125/95 | ND | ND | |
| 18c | 3.65 | 250/189 | ND | ND | |
| 23 | 1.75 | 8/5.8 | ND | ND | |
| Guanidinium hydrochloride salts | 33a | −1.46 | 47/22 [50] | ND | ND |
| 1, 33b | −0.69 | 12/6.7 [50] | ND | ND | |
| 33c | 0.19 | 8/4.4 [51] | 63/35 [51] | 63/35 [51] | |
| 33d | 1.84 | 16/10 | ND | ND | |
| 34a | 0.32 | 8/4.3 | 16/8.5 | >250/>133 | |
| 34b | 1.09 | 16/9.8 | >250/>153 | >250/>153 | |
| 34c | 1.97 | 32/19 | ND | ND | |
| 35a | 2.10 | 250/147 | ND | ND | |
| 35b | 2.87 | >250/>167 | ND | ND | |
| 35c | 3.75 | >250/>166 | ND | ND | |
ND = Not determined.
The incorporation of a biphenyl system in the glyoxamide scaffold enhanced the antibacterial activity of the peptidomimetics against both Gram-positive and Gram-negative bacteria [51]. To investigate the effect of lengthening the phenyl ring system on the antibacterial activity of the peptidomimetics, those bearing a meta- or para-terphenyl system were synthesised. Replacing the biphenyl system with a terphenyl system was generally beneficial for the antibacterial activity of the peptidomimetics against Gram-positive S. aureus. The previously reported quaternary ammonium iodide salt 16c bearing a biphenyl system and an octanesulfonyl group had an MIC value of 16 μM (10 μg/mL) [51]. When an additional phenyl ring was incorporated, the corresponding quaternary ammonium iodide salts 16d and 23 bearing a para-terphenyl or meta-terphenyl system, respectively, possessed MIC values of 4 and 8 μM (2.9 and 5.8 μg/mL) against S. aureus, suggesting that the terphenyl system was preferred over biphenyl. In terms of the position of the terminal phenyl ring in the terphenyl system, quaternary ammonium iodide salt 16d (MIC = 4 μM or 2.9 μg/mL) bearing a para-terphenyl system was slightly more potent than the corresponding quaternary ammonium iodide salt 23 (MIC = 8 μM or 5.8 μg/mL) bearing a meta-terphenyl system. Against Gram-negative E. coli and P. aeruginosa, while quaternary ammonium iodide salt 16c bearing a biphenyl system and an octanesulfonyl group possessed MIC values of 32 and 125 μM (21 and 80 μg/mL) against E. coli and P. aeruginosa, respectively, the antibacterial activity of the peptidomimetics was completely lost upon converting the biphenyl system to terphenyl as exhibited by the corresponding quaternary ammonium iodide salt 16d (MIC > 250 μM or >180 μg/mL). This suggested that the extra phenyl ring in the terphenyl system created steric hindrance, which may reduce the antibacterial activity of the peptidomimetics against Gram-negative bacteria.
As shortening the octanesulfonyl chain was previously reported to be detrimental to the antibacterial activity of the peptidomimetics [50,51] and lengthening the octanesulfonyl chain was not previously studied, peptidomimetics with dodecanesulfonyl and hexadecanesulfonyl groups were synthesised to assess the effect of longer chain lengths on antibacterial activity. Interestingly, the effect of lengthening the alkylsulfonyl chain depended on the size of the phenyl ring system. For the monophenyl system, the previously reported unsubstituted parent (16a) and 5-bromosubstituted quaternary ammonium iodide salts (16b) bearing an octanesulfonyl chain had MICs of 250 and 63 μM (142 and 41 μg/mL) against S. aureus, respectively. When the octanesulfonyl chain was lengthened to a dodecanesulfonyl chain, the MIC of the corresponding quaternary ammonium iodide salts 17a and 17b decreased to 16 and 8 μM (10 and 5.6 μg/mL) against S. aureus, respectively, indicating 16- and 8-fold increases in potency. A similar trend was also observed for quaternary ammonium iodide salts bearing a biphenyl system, although the increase in potency was only two-fold when comparing the quaternary ammonium iodide salt 16c (MIC = 16 μM or 10 μg/mL) bearing an octanesulfonyl chain with the corresponding dodecanesulfonyl compound 17c (MIC = 8 μM or 5.6 μg/mL). However, against Gram-negative E. coli, lengthening the octanesulfonyl chain to dodecanesulfonyl chain gave no improvement in antibacterial activity, as both 16c and 17c shared the same MIC value of 32 μM (10 and 5.6 μg/mL, respectively). Moreover, for the peptidomimetics bearing a terphenyl system, lengthening the octanesulfonyl chain to dodecanesulfonyl chain significantly decreased antibacterial activity against S. aureus, as the quaternary ammonium iodide salt 17d (MIC = 63 μM or 48 μg/mL) bearing a dodecanesulfonyl chain showed 16-fold lower potency than the corresponding octanesulfonyl compound 16d (MIC = 4 μM or 2.9 μg/mL). Further lengthening the alkylsulfonyl chain from dodecanesulfonyl to hexadecanesulfonyl was detrimental to the antibacterial activity of the peptidomimetics against S. aureus, as significant increases in MIC values were observed regardless of the phenyl ring system. One exception was the quaternary ammonium iodide salt 18a bearing an unsubstituted monophenyl system, where the lengthening of the alkylsulfonyl chain from dodecanesulfonyl (17a with MIC = 16 μM or 10 μg/mL) to hexadecanesulfonyl (18a with MIC = 8 μM or 5.4 μg/mL) showed a two-fold increase in antibacterial activity.
In terms of cationic group modification, previous studies have identified that guanidinium hydrochloride salts bearing an octanesulfonyl chain possesses superior antibacterial activity against S. aureus compared to their corresponding tertiary ammonium chloride salts, quaternary ammonium iodide salts or uncharged glyoxamide compounds [50,51]. However, this was not necessarily true in this study when the octanesulfonyl chain was lengthened to dodecansulfonyl and hexadecanesulfonyl. In fact, guanidinium hydrochloride salts bearing a dodecanesulfonyl or hexadecanesulfonyl chain showed lower antibacterial activity against S. aureus than their corresponding quaternary ammonium iodide salts. The only exception was the monophenyl guanidinium hydrochloride salt 34a (MIC = 8 μM or 4.3 μg/mL) bearing a dodecanesulfonyl chain, which showed slightly higher potency against S. aureus than its corresponding quaternary ammonium iodide salt 17a (MIC = 16 μM or 10 μg/mL). Against Gram-negative E. coli, a similar trend was observed, where the 5-bromosubstituted quaternary ammonium iodide salt 17b with a dodecanesulfonyl chain showed good antibacterial activity (MIC = 32 μM or 22 μg/mL), while the corresponding guanidinium hydrochloride salt 34b showed no antibacterial activity at the highest tested concentration (MIC > 250 μM or > 153 μg/mL).
Overall, the structure–activity relationship analysis for these peptidomimetics suggests that the optimal combination for Gram-positive antibacterial activity was a terphenyl system, an octanesulfonyl chain and a quaternary ammonium iodide cationic group. Compound 16d with a combination of these was the most potent compound (MIC = 4 μM or 2.9 μg/mL) against S. aureus in this study. Interestingly, there appeared to be an inverse relationship between the size of the phenyl system and the length of the alkylsulfonyl chain in favour of high antibacterial activity. Specifically, peptidomimetics bearing a bulky terphenyl system preferred a shorter octanesulfonyl chain while peptidomimetics bearing a non-bulky monophenyl system preferred a longer hexadecanesulfonyl chain for high antibacterial activity. In terms of the terminal cationic group, compounds with the terphenyl system or a long dodecanesulfonyl chain favoured the quaternary ammonium iodide salts for antibacterial activity against S. aureus, while octanesulfonyl compounds preferred the guanidinium hydrochloride salts.
2.3. Lipophilicity and Antibacterial Activity Correlation
The apparent inverse relationship between the size of the phenyl system and the length of the alkylsulfonyl chain for strong antibacterial activity suggested that the lipophilicity of the peptidomimetics might be an important factor driving the potency of the compounds. Hence, the logP values of all synthesised peptidomimetics were determined computationally.
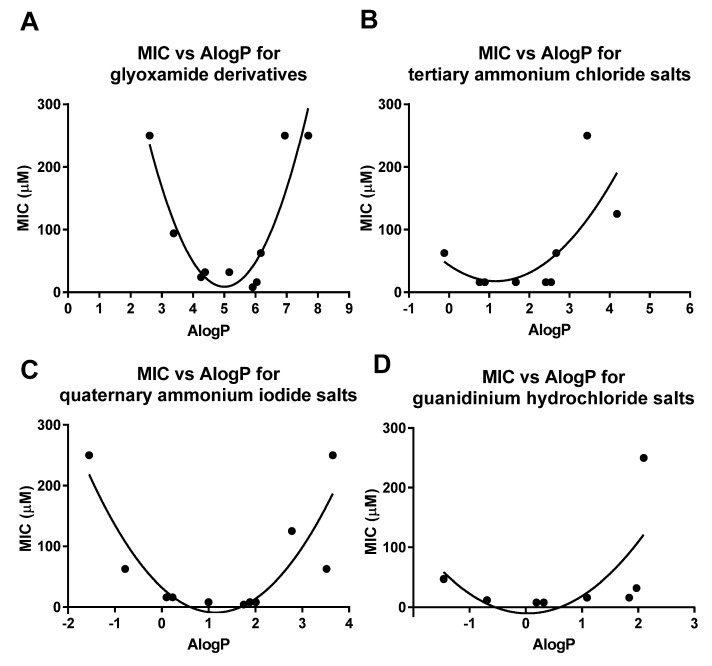
While there are different computational methods and approaches used to estimate logP values, the AlogP method under the empirical fragment-based approaches was used in this study, as empirical fragment-based approaches are highly efficient and the AlogP method was previously demonstrated to be one of the best performing methods for estimating logP (with mean absolute errors of 0.3 log unit or less), compared to other physics-based implicit and atomistic methods [58,59]. The AlogP method was developed by Ghose and Crippen in 1986 and was trained on nearly 900 molecular structures [60,61,62]. In this study, the AlogP values of all peptidomimetics were calculated, using MarvinSketch (Table 1) [63]. The MIC against S. aureus vs. AlogP plots for the glyoxamide derivatives, ammonium chloride salts, quaternary ammonium iodide salts and guanidinium hydrochloride salts are exhibited in Figure 2.
Figure 2.
MIC vs. AlogP plot for (A) glyoxamide derivatives; (B) tertiary ammonium chloride salts; (C) quaternary ammonium iodide salts; and (D) guanidinium hydrochloride salts.
The plots revealed that there is an optimum range of AlogP values for each series of peptidomimetics for high antibacterial activity. Peptidomimetics with AlogP value above or below the optimum range generally show lower antibacterial activity. For example, glyoxamide derivatives with AlogP values between 4.3 and 6.0 possessed high antibacterial activity with MIC values at or below 32 μM, while those outside of the optimum AlogP range exhibited MIC values at or above 63 μM. Similar trends were also observed for tertiary ammonium chloride salts, quaternary ammonium iodide salts and guanidinium hydrochloride salts, which exhibited optimal AlogP ranges of 0.8–2.5, 0.1–2.0 and −0.7–1.8, respectively. The elucidation of the optimal AlogP range for each series of peptidomimetics could be useful in the prediction of the antibacterial activity in the future development of glyoxamide-based antimicrobial peptidomimetics.
2.4. Biofilm Disruption
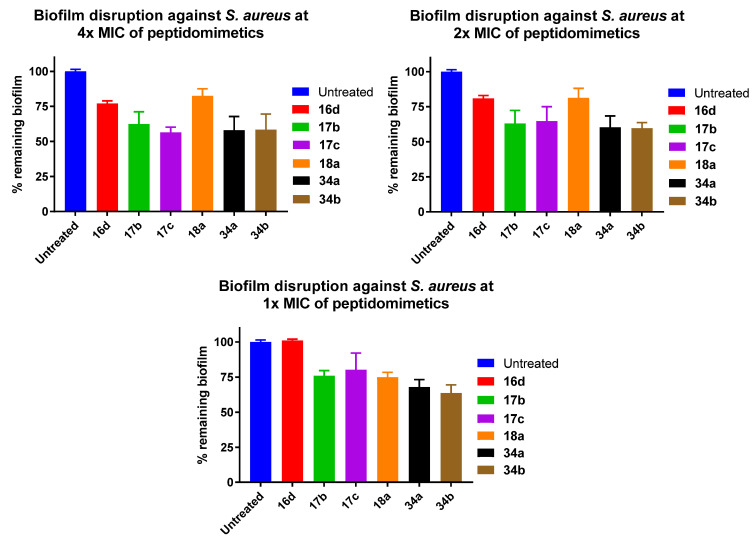
As bacterial biofilms are one of the major challenges in treating bacterial infection, the ability of the synthesised peptidomimetics to disrupt pre-established biofilms was investigated. In this study, selected potent peptidomimetics (16d, 17b, 17c, 18a, 34a, 34b) were tested against pre-established Gram-positive S. aureus biofilms at 1×, 2× and 4× MIC of the peptidomimetics, using the crystal violet assay (Figure 3). The ability of the tested peptidomimetics to disrupt pre-established biofilms generally increased with the concentration.
Figure 3.
Percentage remaining S. aureus biofilms following 24 h treatment with peptidomimetics at 4× (top left), 2× (top right) and 1× (bottom) MIC. Error bars represent the standard error of at least triplicates (n ≥ 3).
In general, guanidinium hydrochloride salts (34a, 34b) were more potent S. aureus biofilm disruptors than quaternary ammonium iodide salts (16d, 17b, 17c, 18a), as they were able to disrupt a higher amount of pre-established S. aureus biofilms at all tested concentrations. The only exception was quaternary ammonium iodide salt 17c, which displayed a slightly higher biofilm disruption at 4× MIC. At lower concentration (1× MIC), most peptidomimetics (17b, 17c, 18a, 34a, 34b) showed 20–36% S. aureus biofilm disruption, except for quaternary ammonium iodide salt 16d, bearing a terphenyl scaffold and octanesulfonyl group, which showed no antibiofilm activity at 1× MIC. Amongst the active peptidomimetics, the guanidinium hydrochloride salt 34b bearing a dodecanesulfonyl group was the most potent compound, disrupting 36% of pre-established S. aureus biofilms at 1× MIC. It was also one of the most active compounds at the highest tested concentration (4× MIC), possessing the same biofilm disruption ability as guanidinium hydrochloride salt 34b, disrupting 42% of pre-established S. aureus biofilms, slightly lower than quaternary ammonium iodide salt 17c, which managed to disrupt 44% of pre-established S. aureus biofilms. This was followed by quaternary ammonium iodide salt 17b, which disrupted 38% of pre-established S. aureus biofilms at 4× MIC. These compounds possessed similar antibiofilm activity as LL-37, which disrupted about 40% pre-established S. aureus biofilms at 4× MIC [64].
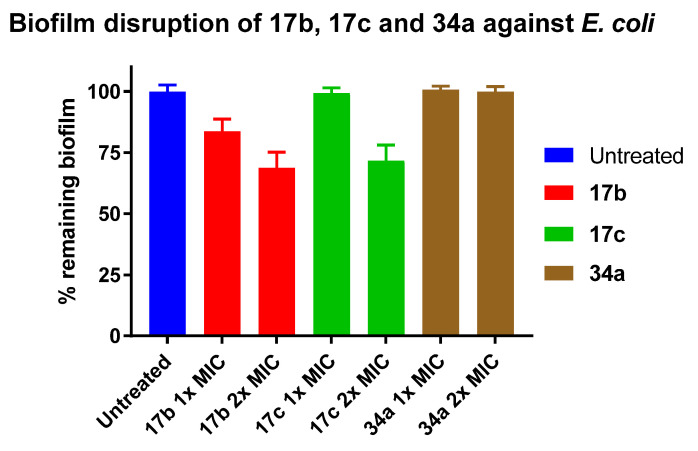
The biofilm disruption activities of the synthesised peptidomimetics 17b, 17c, and 34a, which possessed good antibacterial activity against E. coli in the MIC assay, were also investigated at 1× and 2× MIC against pre-established E. coli biofilms. All tested compounds showed lower biofilm disruption ability against Gram-negative E. coli than against Gram-positive S. aureus. Of the three tested compounds, the quaternary ammonium iodide salts 17b and 17c were able to disrupt 31% and 28% of the pre-established E. coli biofilms at 2× MIC (Figure 4). Although the guanidinium hydrochloride salt 34a was the most potent compound at inhibiting E. coli growth in the MIC assay and was one of the best S. aureus biofilm disruptors, it was unable to disrupt any pre-established E. coli biofilms. This suggests that it can only target E. coli cells in their planktonic form, but not those that are embedded in the polymeric matrices of biofilms. The observed discrepancy in the antibiofilm activity of these peptidomimetics against Gram-positive S. aureus and Gram-negative E. coli could be due to the differences in the composition of their biofilms [65,66,67].
Figure 4.
Percentage remaining E. coli biofilms following 24 h treatment with peptidomimetics 17b, 17c and 34a at 1× and 2× MIC. Error bars represent the standard error of triplicates (n = 3).
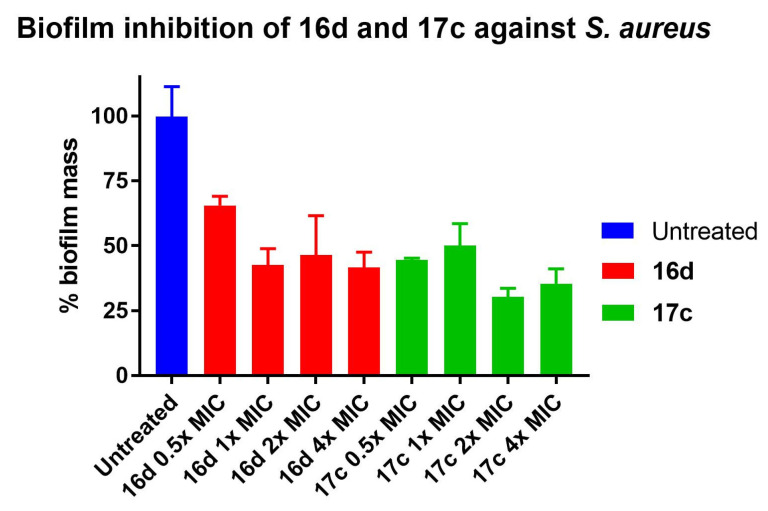
2.5. Biofilm Inhibition
In addition to biofilm disruption assay, selected potent peptidomimetics 16d and 17c were also tested for their ability to inhibit the formation of S. aureus biofilm at 0.5×, 1×, 2× and 4× MIC of the peptidomimetics (Figure 5). In this assay, S. aureus was incubated in the presence of different concentrations of the tested peptidomimetics under a static condition. After overnight incubation, planktonic bacteria were removed, and the remaining biofilms were quantified, using crystal violet staining.
Figure 5.
Inhibition of biofilm fomration of S. aureus by peptidomimetics 16d and 17c at 0.5×, 1×, 2× and 4× MIC. Error bars represent the standard error of triplicates (n = 3).
Both peptidomimetics 16d and 17c were effective in inhibiting S. aureus biofilm formation, as they were able to inhibit more than 50% of biofilm formation (except for peptidomimetics 16d at 0.5× of its MIC), with 17c showing a slightly higher inhibition than 16d, except at 1× MIC. Of the two peptidomimetics, maximum inhibition of biofilm formation was observed for peptidomimetic 17c at 2× and 4× of its MIC, inhibiting 70% and 65% of biofilm formation, respectively. At sub-MIC (0.5× MIC), 16d and 17c were able to inhibit 35% and 56% of biofilm formation, respectively.
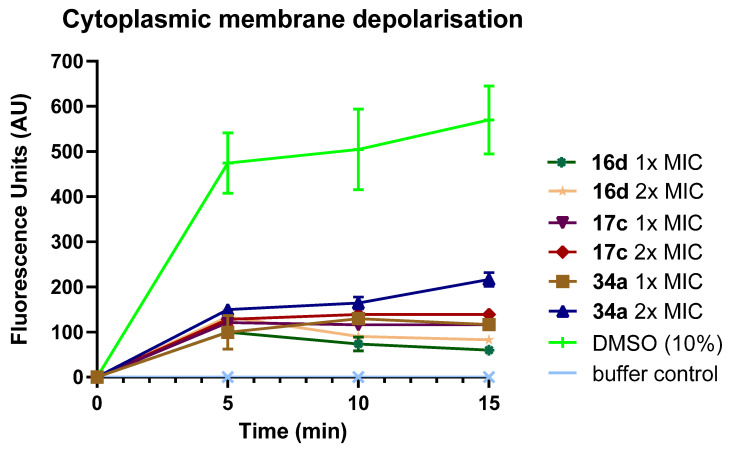
2.6. Cytoplasmic Membrane Permeability
The disruption effect of selected potent peptidomimetics (16d, 17c, 34a) on bacterial cytoplasmic membrane was investigated in a membrane dye release assay, using the membrane potential-sensitive dye 3,3′-dipropylthiadicarbocyanine iodide (diSC3-5). This dye readily partitions into and aggregates in bacterial cell membranes, quenching its own fluorescence. However, structural damage to the bacterial cell membrane causes a loss in the membrane potential, which results in the leakage of dye from the bacterial cell membrane into the medium and an increase in fluorescence intensity. By detecting changes in fluorescence intensity, the integrity of the bacterial cytoplasmic membrane can be monitored.
It was hypothesised that the synthesised peptidomimetics possess similar mechanisms of action as natural AMPs, which act via the electrostatic interactions between the negatively charged bacterial cell membrane and the cationic group of the peptidomimetics. As this disrupts the bacterial membrane and/or forms pores on the membrane, causing an alteration of the membrane potential, an increase in fluorescence intensity was expected upon the treatment of bacterial cells with the test compounds.
Overall, all three tested compounds disrupted the cytoplasmic membranes of S. aureus, as an increase in fluorescence intensity was observed within 5 min after the deployment of the test compounds. Moreover, all test compounds disrupted cytoplasmic membranes in a concentration-dependent manner, as evidenced by a higher increase in fluorescence intensity at the higher concentration (2× MIC) of the test compound than at the lower concentration (1× MIC) (Figure 6). Amongst the three compounds, guanidinium hydrochloride salt 34a was the most active compound at 2× MIC, as it induced the highest increase in fluorescence intensity at 15 min. However, at 1× MIC, there was no significant difference in the cytoplasmic membrane disruption ability between guanidinium hydrochloride salt 34a and the quaternary ammonium iodide salt 17c. The least active compound was the quaternary ammonium iodide salt 16d, as it showed the smallest increase in fluorescence intensity at 15 min. However, it is worth mentioning that while increasing the bacterial cytoplasmic membrane permeability can be a mechanism of action of the tested compounds, these compounds can also exert their antibacterial action through other mechanisms, such as interacting with DNA, RNA or nucleotides [68,69,70,71]; these potential mechanisms of action of AMP will be investigated in future studies.
Figure 6.
S. aureus cytoplasmic membrane depolarisation induced by peptidomimetics 16d, 17c and 34a at 1× MIC and 2× MIC. As a positive control, 10% DMSO was used. Error bars represent the standard error of triplicates (n = 3).
2.7. Cytotoxicity
The in vitro toxicity of selected potent peptidomimetics (10d, 13d, 16d, 17b, 17c, 18a, 22, 23, 33d, 34a, 34b) was assessed against MRC-5 normal human lung fibroblasts via the MTT assay. The IC50 values of the tested peptidomimetics were determined from a dose-response curve. TY-01 was used as the positive control in this assay with the IC50 value to be 14.2 μM (7.83 μg/mL) [51]. The therapeutic indices for each tested peptidomimetic against Gram-positive S. aureus and Gram-negative E. coli were calculated by dividing the IC50 value against human cells by the corresponding MIC value (Table 2). An antibacterial agent, which can be utilised clinically, should possess a high therapeutic index, as this indicates a high selectivity for bacterial cells over human cells.
Table 2.
IC50 value of peptidomimetics against MRC-5 normal human lung fibroblasts and their therapeutic indices with respect to S. aureus and E. coli.
| Compound | IC50 (μM/μg/mL) | Therapeutic Index | ||
|---|---|---|---|---|
| S. aureus | E. coli | |||
| Glyoxamide derivatives | 10d | 3.48/2.01 | 0.44 | N/A |
| Tertiary ammonium chloride salts | 13d | 3.76/2.31 | 0.24 | N/A |
| 22 | 3.46/2.13 | 0.22 | N/A | |
| Quaternary ammonium iodide salts | 16d | 53.7/38.6 | 15.4 | N/A |
| 17b | 18.3/12.9 | 2.29 | 0.57 | |
| 17c | 57.5/40.2 | 7.18 | 1.80 | |
| 18a | 52.4/35.6 | 6.54 | N/A | |
| 23 | 15.4/11.1 | 1.93 | N/A | |
| Guanidinium hydrochloride salts | 33d | 16.9/10.6 | 1.05 | N/A |
| 34a | 27.2/14.5 | 3.40 | 1.70 | |
| 34b | 21.1/14.8 | 1.32 | N/A | |
| Positive control | TY-01 | 14.2/7.83 | N/A | N/A |
N/A = Not applicable.
Without the terminal cationic group, the neutral glyoxamide 10d showed high toxicity with an IC50 of 3.48 μM (2.01 μg/mL) against human cells and a therapeutic index of 0.44 against S. aureus, indicating that it is more effective at targeting human cells rather than bacterial cells. Converting the tertiary amine group of 10d into the tertiary ammonium chloride salt 13d did not improve toxicity, as there was no significant difference in the IC50 value (~4 μM or ~2 μg/mL) between the two peptidomimetics. However, the corresponding quaternary ammonium iodide salt 16d showed a significant improvement in the toxicity, as the IC50 value against human cells rose to 53.7 μM (38.6 μg/mL), giving a therapeutic index of 15.4 against S. aureus. On the other hand, replacing the quaternary ammonium iodide group by a guanidinium hydrochloride group (33d) increased toxicity, as the IC50 value dropped to 16.9 μM (10.6 μg/mL), giving a therapeutic index of 1.05 against S. aureus. A similar trend was also observed for peptidomimetics bearing different phenyl systems and alkylsulfonyl chains, with quaternary ammonium iodide salts (17b, 17c, 18a) generally having higher therapeutic indices of 2.29–7.18 against S. aureus than guanidinium hydrochloride salts (33d, 34a, 34b) with therapeutic indices of 1.05–3.40. Against Gram-negative E. coli, the therapeutic indices of tested peptidomimetics (17b, 17c, 34a) were between 0.57 and 1.80. The lower therapeutic indices against E. coli compared to those of S. aureus were due to the higher MIC values of the peptidomimetics against E. coli.
In addition to assessing the cytotoxicity of the peptidomimetic on human lung fibroblasts, the potent peptidomimetics 16d and 17c were tested for their cytotoxicity against human red blood cells in a haemolysis assay. Both peptidomimetics gave relatively low haemolytic activities, with 16d and 17c showing only 11% and 16% haemolysis, respectively, at 32 μM. This concentration corresponded to 8× and 4× MIC of 16d and 17c, respectively, against S. aureus.
Overall, the relatively low cytotoxicity (against lung fibroblast cells and erythrocytes) of peptidomimetics suggests that they are relatively non-toxic to human cells and, therefore, the corresponding high therapeutic indices indicate that the peptidomimetics 16d, 17c, 18a might be suitable antibiotics to develop further for the treatment of S. aureus infection. However, the relatively low therapeutic indices against E. coli suggest that these peptidomimetics might be unsafe to be used as antibacterial agents against E. coli, clinically.
3. Materials and Methods
3.1. Synthesis of Analogues
3.1.1. General Information
All commercially available reagents and solvents were purchased from standard suppliers (Sigma Aldrich, Alfa-Aesar, Combi-Blocks and Oakwook Chemicals) and used without further purification. All reactions were performed under anhydrous condition with an anhydrous solvent obtained using PureSolv MD Solvent Purification System unless otherwise specified. The progress of the reactions was monitored by thin layer chromatography plates precoated with Merck silica gel 60 F254 and visualised using short and/or long wavelength of ultraviolet light. Flash chromatography was carried out using Grace Davisil LC60A silica.
Melting points of the compounds were measured using an SRS MPA100 OptiMelt melting point apparatus and are reported without correction. 1H and 13C NMR spectra were acquired in the specified solvents on a Bruker Avance III HD 400 or Bruker Avance III 600 Cryo spectrometer. Chemical shifts (δ) are in parts per million (ppm) internally referenced to the solvent nuclei. Multiplicities are assigned as singlet (s), broad singlet (bs), doublet (d), triplet (t), quartet (q), multiplet (m) or a combination of these (e.g., dd, dt, td), and coupling constants (J) are reported in Hertz (Hz). Infrared (IR) spectra were recorded using a Cary 630 FTIR spectrometer or NicoletTM iSTM 10 FTIR spectrometer fitted with a diamond attenuated total reflectance (ATR) sample interface. High-resolution mass spectrometry (HRMS) was performed, using a Thermo LTQ Orbitrap XL instrument.
3.1.2. Synthetic Procedures and Experimental Characterisation Data
5-Phenylindoline-2,3-dione (4)
The synthesis of the titled compound followed the procedure previously described [51] with slight modification. A 2 M potassium carbonate solution (7.7 mL, 15.4 mmol) was degassed for 30 min and then added into a suspension of 5-bromoisatin (1.72 g, 7.59 mmol) and phenylboronic acid (1.04 g, 8.35 mmol) in a degassed (for 30 min) 1:1 ethanol/toluene solution (30 mL). The dark brown solution mixture was further degassed for 30 min, followed by the addition of tetrakis(triphenylphosphine)palladium(0) (92 mg, 0.080 mmol). The resulting reaction mixture was heated at reflux under an atmosphere of nitrogen for 18 h. The brownish-black reaction mixture was concentrated in vacuo. Glacial acetic acid (30 mL) was added to the dark brown solid, and the resulting reaction mixture was heated at reflux for 20 min. The solution mixture was then allowed to cool to room temperature overnight. The red crystals formed was filtered and discarded. Water (50 mL) was then added to the filtrate. The organic layer was extracted thrice with dichloromethane (3 × 30 mL), washed with brine (50 mL), dried over sodium sulphate and concentrated in vacuo to give a red solid, which was purified by flash column chromatography on silica, using methanol/dichloromethane as the eluent to give the product as a red solid (1.14 g, 67%); mp 249.5–250.3 °C; 1H NMR (400 MHz, DMSO-d6): δ 11.12 (bs, 1H, NH), 7.91 (dd, J = 8.2, 2.0 Hz, 1H, ArH), 7.76 (d, J = 1.9 Hz, 1H, ArH), 7.67–7.63 (m, 2H, ArH), 7.48–7.43 (m. 2H, ArH), 7.39–7.33 (m, 1H, ArH), 7.01 (d, J = 8.2 Hz, 1H, ArH); MS (+ESI): m/z 246.12, [M+Na]+.
5-([1,1′-Biphenyl]-4-yl)indoline-2,3-dione (5)
The synthesis of the titled compound followed the procedure previously described [56]. A 2 M potassium carbonate solution (9.2 mL, 18.4 mmol) was degassed for 30 min, and then added into a suspension of 5-bromoisatin (2.06 g, 9.13 mmol) and 4-biphenylboronic acid (1.89 g, 9.54 mmol) in a degassed (for 30 min) 1:1 ethanol/toluene solution (40 mL). The dark brown solution mixture was further degassed for 30 min, followed by the addition of tetrakis(triphenylphosphine)palladium(0) (109 mg, 0.094 mmol). The resulting reaction mixture was heated at reflux under an atmosphere of nitrogen for 18 h. The dark brown reaction mixture was concentrated in vacuo. Glacial acetic acid (30 mL) was added to the dark brown solid, and the resulting reaction mixture was heated at reflux for 20 min. The dark red precipitate was collected by vacuum filtration to give the crude product, which was redissolved in dimethylformamide and filtered. The filtrate was then poured into 1:1 ice-water mixture and the red precipitate formed was collected by vacuum filtration to give the product as a red solid (2.28 g, 83%); mp 239.9–240.8 °C; 1H NMR (400 MHz, DMSO-d6): δ 11.14 (s, 1H, NH), 7.98 (dd, J = 8.2, 2.1 Hz, 1H, ArH), 7.84 (d, J = 2.0 Hz, 1H, ArH), 7.79–7.69 (m, 6H, ArH), 7.52–7.45 (m, 2H, ArH), 7.41–7.35 (m, 1H, ArH), 7.03 (d, J = 8.2 Hz, 1H, ArH); HRMS (+ESI): Found m/z 322.0839 [M+Na]+, C20H13NO2Na required 322.0838.
General synthetic procedure A for N-sulfonylisatins
Triethylamine (1.1 equivalents) was added slowly to a stirring solution of an appropriate isatin (1.0 equivalent) in dichloromethane (15 mL) at 0 °C in a nitrogen atmosphere. The reaction mixture was stirred at 0 °C for 20 min, followed by the dropwise addition of an appropriate 1-alkylsulfonyl chloride (1.0 equivalent) in dichloromethane (5 mL) with stirring. The reaction mixture was then stirred at room temperature for 3 h. After the completion of the reaction, the reaction mixture was concentrated in vacuo and washed with methanol to afford the product.
5-([1,1′-Biphenyl]-4-yl)-1-(octylsulfonyl)indoline-2,3-dione (7d)
The titled compound was synthesised from 5-([1,1′-biphenyl]-4-yl)indoline-2,3-dione 6d (0.44 g, 1.46 mmol), triethylamine (0.23 mL, 1.65 mmol) and 1-octanesulfonyl chloride (0.30 mL, 1.53 mmol) following general synthetic procedure A. After washing the crude with methanol, the precipitate was collected by vacuum filtration, and the filtrate was discarded. The residue was then washed with dichloromethane (5 mL) and filtered. The filtrate was concentrated in vacuo to afford the product as an orange solid (0.28 g, 40%); mp 173.5–173.9 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.14 (dd, J = 8.6, 2.0 Hz, 1H, ArH), 8.05 (d, J = 2.0 Hz, 1H, ArH), 7.87–7.70 (m, 7H, ArH), 7.50 (t, J = 7.8 Hz, 2H, ArH), 7.39 (t, J = 7.2 Hz, 1H, ArH), 3.67–3.60 (m, 2H, CH2), 1.87–1.76 (m, 2H, CH2), 1.44–1.15 (m, 10H, CH2), 0.84 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 178.7 (CO), 156.7 (CO), 146.2 (ArC), 139.7 (ArC), 139.4 (ArC), 136.9 (ArC), 136.4 (ArC), 135.7 (ArCH), 129.1 (ArCH), 127.7 (ArCH), 127.4 (ArCH), 127.1 (ArCH), 126.6 (ArCH), 122.3 (ArCH), 120.2 (ArC), 114.7 (ArCH), 53.7 (CH2), 31.1 (CH2), 28.4 (CH2), 28.4 (CH2), 27.3 (CH2), 22.2 (CH2), 22.0 (CH2), 13.9 (CH3); IR (ATR): νmax 3854, 3675, 2987, 2900, 1765, 1739, 1613, 1576, 1559, 1473, 1434, 1405, 1393, 1373, 1309, 1290, 1254, 1240, 1174, 1140, 1075, 1066, 1057, 1027, 944, 892, 851, 836, 766, 729, 719, 692 cm−1; HRMS (+ESI): Found m/z 498.1710 [M+Na]+, C28H29NO4SNa required 498.1710.
1-(Dodecylsulfonyl)indoline-2,3-dione (8a)
The titled compound was synthesised from isatin 6a (0.23 g, 1.54 mmol), triethylamine (0.24 mL, 1.72 mmol) and 1-dodecanesulfonyl chloride (0.42 g, 1.56 mmol) following general synthetic procedure A. The product was obtained as a yellow solid (0.30 g, 51%); mp 128.1–129.6 °C; 1H NMR (400 MHz, DMSO-d6): δ 7.78–7.68 (m, 3H, ArH), 7.36–7.28 (m, 1H, ArH), 3.64–3.56 (m, 2H, CH2), 1.83–1.72 (m, 2H, CH2), 1.41–1.14 (m, 18H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 178.8 (CO), 156.5 (CO), 146.9 (ArC), 137.9 (ArCH), 125.1 (ArCH), 125.0 (ArCH), 119.4 (ArC), 114.1 (ArCH), 53.7 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.1 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 2996, 2913. 2847, 1912, 1733, 1651, 1593, 1459, 1413, 1371, 1320, 1237, 1177, 1132, 1090, 1024, 933, 831, 784, 756 cm−1; HRMS (+ESI): Found m/z 380.1891 [M+H]+, C20H30NO4S required 380.1890.
5-Bromo-1-(dodecylsulfonyl)indoline-2,3-dione (8b)
The titled compound was synthesised from 5-bromoisatin 6b (0.35 g, 1.55 mmol), triethylamine (0.24 mL, 1.72 mmol) and 1-dodecanesulfonyl chloride (0.42 g, 1.58 mmol) following general synthetic procedure A. The product was obtained as a yellow solid (0.42 g, 60%); mp 141.9 °C; 1H NMR (400 MHz, DMSO-d6): δ 7.98–7.84 (m, 2H, ArH), 7.65 (d, J = 8.5 Hz, 1H, ArH), 3.65–3.54 (m, 2H, CH2), 1.85–1.68 (m, 2H, CH2), 1.44–1.10 (m, 18H, CH2), 0.92–0.78 (m, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 177.5 (CO), 156.0 (CO), 145.7 (ArC), 139.6 (ArCH), 127.1 (ArCH), 121.4 (ArC), 117.2 (ArC), 116.1 (ArCH), 53.7 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.0 (CH2), 14.0 (CH3); IR (ATR): νmax 3854, 3675, 3104, 3066, 2969, 2913, 2848, 2360, 1763, 1742, 1717, 1596, 1578, 1461, 1427, 1413, 1393, 1372, 1290, 1270, 1234, 1191, 1177, 1140, 1116, 1095, 1077, 1065, 1057, 1027, 939, 898, 845, 786, 722, 707, 696 cm−1; HRMS (+ESI): Found m/z 480.0816 [M+Na]+, C20H28BrNO4SNa required 480.0815.
1-(Dodecylsulfonyl)-5-phenylindoline-2,3-dione (8c)
The titled compound was synthesised from 5-phenylindoline-2,3-dione 6c (1.00 g, 4.48 mmol), triethylamine (0.70 mL, 5.02 mmol) and 1-dodecanesulfonyl chloride (1.21 g, 4.51 mmol) following general synthetic procedure A. The product was obtained as a yellow solid (0.94 g, 46%); mp 137.9–138.2 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.07 (dd, J = 8.6, 2.2 Hz, 1H, ArH), 7.98 (d, J = 1.9 Hz, 1H, ArH), 7.80 (d, J = 8.6 Hz, 1H, ArH), 7.75–7.70 (m, 2H, ArH), 7.52–7.46 (m. 2H, ArH), 7.43–7.38 (m, 1H, ArH), 3.67–3.58 (m, 2H, CH2), 1.86–1.74 (m, 2H, CH2), 1.42–1.16 (m, 18H, CH2), 0.84 (t, J = 6.9 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 178.7 (CO), 156.6 (CO), 146.1 (ArC), 138.0 (ArC), 137.0 (ArC), 135.8 (ArCH), 129.1 (ArCH), 128.0 (ArCH), 126.5 (ArCH), 122.4 (ArCH), 120.1 (ArC), 114.6 (ArCH), 53.7 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.2 (CH2), 22.1 (CH2), 13.9 (CH3); IR (ATR): νmax 3675, 2969, 2914, 2848, 2360, 1764, 1735, 1614, 1584, 1570, 1507, 1471, 1454, 1426, 1411, 1393, 1375, 1308, 1294, 1279, 1258, 1239, 1174, 1140, 1113, 1095, 1077, 1066, 1057, 1027, 944, 907, 852, 786, 763, 722, 704, 698, 650 cm−1; HRMS (+ESI): Found m/z 478.2024 [M+Na]+, C26H33NO4SNa required 478.2023.
5-([1,1′-Biphenyl]-4-yl)-1-(dodecylsulfonyl)indoline-2,3-dione (8d)
The titled compound was synthesised from 5-([1,1′-biphenyl]-4-yl)indoline-2,3-dione 6d (0.48 g, 1.59 mmol), triethylamine (0.25 mL, 1.79 mmol) and 1-dodecanesulfonyl chloride (0.43 g, 1.60 mmol) following general synthetic procedure A. After washing the crude with methanol, the precipitate was collected by vacuum filtration and the filtrate was discarded. The residue was then washed with dichloromethane (5 mL) and filtered. The filtrate was concentrated in vacuo to afford the product as an orange solid (0.47 g, 56%); mp 165.5–167.7 °C; 1H NMR (600 MHz, DMSO-d6): δ 8.14 (dd, J = 8.6, 2.1 Hz, 1H, ArH), 8.05 (d, J = 2.0 Hz, 1H, ArH), 7.87–7.76 (m, 5H, ArH), 7.76–7.71 (m, 2H, ArH), 7.50 (t, J = 7.8 Hz, 2H, ArH), 7.40 (t, J = 7.5 Hz, 1H, ArH), 3.66–3.61 (m, 2H, CH2), 1.85–1.78 (m, 2H, CH2), 1.41–1.34 (m, 2H, CH2), 1.29–1.14 (m, 16H, CH2), 0.83 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 178.7 (CO), 156.6 (CO), 146.1 (ArC), 139.7 (ArC), 139.4 (ArC), 136.9 (ArC), 136.4 (ArC), 135.7 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.3 (ArCH), 127.0 (ArCH), 126.6 (ArCH), 122.3 (ArCH), 120.2 (ArC), 114.6 (ArCH), 53.7 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.2 (CH2), 22.1 (CH2), 13.9 (CH3); IR (ATR): νmax 3032, 2914, 2848, 2679, 2343, 2107, 1738, 1613, 1577, 1471, 1373, 1289, 1237, 1174, 1114, 1023, 943, 851, 765, 719, 693 cm−1; HRMS (+ESI): Found m/z 554.2336 [M+Na]+, C32H37NO4SNa required 554.2336.
1-(Hexadecylsulfonyl)indoline-2,3-dione (9a)
The titled compound was synthesised from isatin 6a (0.31 g, 2.07 mmol), triethylamine (0.32 mL, 2.30 mmol) and 1-hexadecanesulfonyl chloride (0.68 g, 2.09 mmol) following general synthetic procedure A. The product was obtained as a yellow solid (0.36 g, 40%); mp 122.0–123.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 8.4 Hz, 1H, ArH), 7.78 (dd, J = 7.6, 0.9 Hz, 1H, ArH), 7.74–7.68 (m, 1H, ArH), 7.32 (td, J = 7.6, 0.6 Hz, 1H, ArH), 3.62–3.55 (m, 2H, CH2), 1.94–1.83 (m, 2H, CH2), 1.49–1.38 (m, 2H, CH2), 1.34–1.18 (m, 24H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 179.0 (CO), 156.7 (CO), 148.1 (ArC), 139.6 (ArCH), 126.3 (ArCH), 126.1 (ArCH), 118.8 (ArC), 115.4 (ArCH), 54.9 (CH2), 32.1 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.7 (CH2), 29.5 (CH2), 29.5 (CH2), 29.3 (CH2), 29.0 (CH2), 28.1 (CH2), 23.0 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 2914, 2846, 2669, 2343, 1912, 1733, 1651, 1596, 1459, 1413, 1372, 1320, 1237, 1177, 1132, 1090, 981, 934, 832, 786, 756, 723, 676 cm−1; HRMS (+ESI): Found m/z 436.2515 [M+H]+, C24H38NO4S required 436.2516.
5-Bromo-1-(hexadecylsulfonyl)indoline-2,3-dione (9b)
The titled compound was synthesised from 5-bromoisatin 6b (0.50 g, 2.19 mmol), triethylamine (0.34 mL, 2.44 mmol) and 1-hexadecanesulfonyl chloride (0.72 g, 2.20 mmol) following general synthetic procedure A. The product was obtained as a yellow solid (0.65 g, 58%); mp 142.4–144.0 °C; 1H NMR (600 MHz, DMSO-d6): δ 7.92–7.89 (m, 2H, ArH), 7.67–7.64 (m, 1H, ArH), 3.62–3.56 (m, 2H, CH2), 1.81–1.73 (m, 2H, CH2), 1.39–1.31 (m, 2H, CH2), 1.30–1.16 (m, 24H, CH2), 0.85 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 177.5 (CO), 156.0 (CO), 145.7 (ArC), 139.6 (ArCH), 127.1 (ArCH), 121.4 (ArC), 117.2 (ArC), 116.2 (ArCH), 53.8 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3473, 3067, 2998, 2914, 2846, 2670, 2116, 1741, 1595, 1460, 1372, 1290, 1234, 1176, 1115, 1016, 938, 845, 786, 721 cm−1; HRMS (+ESI): Found m/z 536.1433 [M+Na]+, C24H36BrNO4SNa required 536.1441.
1-(Hexadecylsulfonyl)-5-phenylindoline-2,3-dione (9c)
The titled compound was synthesised from 5-phenylindoline-2,3-dione 6c (0.81 g, 3.61 mmol), triethylamine (0.55 mL, 3.95 mmol) and 1-hexadecanesulfonyl chloride (1.17 g, 3.61 mmol) following general synthetic procedure A. The product was obtained as a yellow solid (0.53 g, 28%); mp 139.6–141.3 °C; 1H NMR (600 MHz, CDCl3): δ 8.00–7.96 (m, 2H, ArH), 7.92 (dd, J = 8.7, 1.9 Hz, 1H, ArH), 7.57–7.54 (m, 2H, ArH), 7.50–7.46 (m, 2H, ArH), 7.44–7.40 (m, 1H, ArH), 3.63–3.58 (m, 2H, CH2), 1.95–1.87 (m, 2H, CH2), 1.50–1.42 (m, 2H, CH2), 1.34–1.20 (m, 24H, CH2), 0.88 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, CDCl3): δ 179.2 (CO), 156.8 (CO), 147.1 (ArC), 139.6 (ArC), 138.3 (ArC), 138.0 (ArCH), 129.4 (ArCH), 128.6 (ArCH), 126.9 (ArCH), 124.4 (ArCH), 119.2 (ArC), 115.8 (ArCH), 55.0 (CH2), 32.1 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.3 (CH2), 29.0 (CH2), 28.2 (CH2), 23.0 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3055, 2914, 2847, 2343, 1892, 1736, 1614, 1584, 1505, 1469, 1426, 1375, 1308, 1237, 1175, 1114, 1040, 943, 853, 762, 700 cm−1; HRMS (+ESI): Found m/z 512.2812 [M+H]+, C30H42NO4S required 512.2829.
General synthetic procedure B for glyoxamide derivatives
3-Dimethylaminopropylamine (1.0 equivalent) was added to a stirring solution of N-sulfonylisatin (1.0 equivalent) in dichloromethane (5 mL) at 0 °C. The resulting reaction mixture was stirred at room temperature for 6 h. After the completion of the reaction, water was added to the reaction mixture, and the mixture was extracted into dichloromethane (3 × 25 mL), washed with brine, dried over anhydrous sodium sulfate and concentrated in vacuo to afford the product.
N-(3-(Dimethylamino)propyl)-2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamide (10d)
The titled compound was synthesised from 5-([1,1′-biphenyl]-4-yl)-1-(octylsulfonyl)indoline-2,3-dione 7d (0.10 g, 0.21 mmol) and 3-dimethylaminopropylamine (27 μL, 0.21 mmol) following general synthetic procedure B. The crude was purified by flash column chromatography on silica, using methanol/dichloromethane (1:9) as an eluent to afford the product as a yellow oil (75 mg, 61%); 1H NMR (600 MHz, CDCl3): δ 8.57 (d, J = 2.1 Hz, 1H, ArH), 8.44 (bs, 1H, ArH), 7.86 (dd, J = 8.7, 2.2 Hz, 1H, ArH), 7.82 (d, J = 8.7 Hz, 1H, ArH), 7.70–7.66 (m, 2H, ArH), 7.66–7.61 (m, 4H, ArH), 7.49–7.44 (m, 2H, ArH), 7.39–7.35 (m, 1H, ArH), 3.66–3.59 (m, 2H, CH2), 3.23–3.17 (m, 2H, CH2), 3.12–3.04 (m, 2H, CH2), 2.76 (s, 6H, CH3), 2.19–2.11 (m, 2H, CH2), 1.87–1.80 (m, 2H, CH2), 1.43–1.35 (m, 2H, CH2), 1.30–1.18 (m, 8H, CH2), 0.85 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, CDCl3): δ 191.7 (CO), 164.0 (CO), 140.8 (ArC), 140.5 (ArC), 137.9 (ArC), 135.7 (ArC), 134.7 (ArCH), 133.0 (ArCH), 129.0 (ArCH), 127.9 (ArCH), 127.7 (ArCH), 127.3 (ArCH), 127.2 (ArCH), 120.6 (ArC), 119.3 (ArCH), 55.9 (CH2), 52.9 (CH2), 43.5 (CH3), 36.9 (CH2), 31.8 (CH2), 29.1 (CH2), 29.1 (CH2), 28.3 (CH2), 24.4 (CH2), 23.5 (CH2), 22.7 (CH2), 14.2 (CH3); IR (ATR): νmax 3674, 3384, 2970, 2921, 1767, 1740, 1646, 1615, 1481, 1405, 1393, 1374, 1333, 1241, 1196, 1142, 1075, 1066, 1057, 1027, 915, 829, 765, 729, 696, 673 cm−1; HRMS (+ESI): Found m/z 578.3074 [M+H]+, C33H44N3O4S required 578.3047.
N-(3-(Dimethylamino)propyl)-2-(2-(dodecylsulfonamido)phenyl)-2-oxoacetamide (11a)
The titled compound was synthesised from 1-(dodecylsulfonyl)indoline-2,3-dione 8a (0.11 g, 0.28 mmol) and 3-dimethylaminopropylamine (36 μL, 0.28 mmol) following general synthetic procedure B. The product was obtained as a yellow oil (0.13 g, 98%); 1H NMR (400 MHz, CDCl3): δ 8.76 (bs, 1H, NH), 8.45 (dd, J = 8.1, 1.6 Hz, 1H, ArH), 7.77 (dd, J = 8.5, 0.9 Hz, 1H, ArH), 7.62–7.56 (m, 1H, ArH), 7.18–7.11 (m, 1H, ArH), 3.55–3.46 (m, 2H, CH2), 3.18–3.11 (m, 2H, CH2), 2.49 (t, J = 6.2 Hz, 2H, CH2), 2.28 (s, 6H, CH3), 1.85–1.71 (m, 4H, CH2), 1.40–1.16 (m, 18H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 192.1 (CO), 162.8 (CO), 142.0 (ArC), 136.5 (ArCH), 135.3 (ArCH), 122.6 (ArCH), 119.0 (ArC), 117.9 (ArCH), 59.0 (CH2), 52.6 (CH2), 45.4 (CH3), 40.1 (CH2), 32.0 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.2 (CH2), 25.3 (CH2), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3372, 3068, 2921, 2852, 2543, 2341, 2119, 1635, 1460, 1336, 1262, 1144, 1106, 1041, 941, 754, 719 cm−1; HRMS (+ESI): Found m/z 482.3047 [M+H]+, C25H44N3O4S required 482.3047.
2-(5-Bromo-2-(dodecylsulfonamido)phenyl)-N-(3-(dimethylamino)propyl)-2-oxoacetamide (11b)
The titled compound was synthesised from 5-bromo-1-(dodecylsulfonyl)indoline-2,3-dione 8b (0.15 g, 0.33 mmol) and 3-dimethylaminopropylamine (41 μL, 0.33 mmol) following general synthetic procedure B. The product was obtained as a yellow oil (0.18 g, 99%); 1H NMR (400 MHz, CDCl3): δ 8.95 (bs, 1H, NH), 7.66–7.53 (m, 1H, ArH), 7.71–7.64 (m, 2H, ArH), 3.51 (t, J = 6.1 Hz, 2H, CH2), 3.16–3.08 (m, 2H, CH2), 2.50 (t, J = 6.1 Hz, 2H, CH2), 2.29 (s, 6H, CH3), 1.83–1.72 (m, 4H, CH2), 1.41–1.17 (m, 18H, CH2), 0.87 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 190.8 (CO), 162.0 (CO), 140.9 (ArC), 139.1 (ArCH), 137.5 (ArCH), 120.7 (ArC), 119.9 (ArCH), 115.3 (ArC), 59.1 (CH2), 52.8 (CH2), 45.4 (CH3), 40.3 (CH2), 32.0 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.2 (CH2), 25.1 (CH2), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3674, 2970, 2921, 2361, 1653, 1464, 1405, 1393, 1334, 1259, 1270, 1148, 1075, 1066, 1027, 891, 800, 719, 653 cm−1; HRMS (+ESI): Found m/z 560.2153 [M+H]+, C25H43BrN3O4S required 560.2152.
N-(3-(Dimethylamino)propyl)-2-(4-(dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide (11c)
The titled compound was synthesised from 1-(dodecylsulfonyl)-5-phenylindoline-2,3-dione 8c (0.13 g, 0.28 mmol) and 3-dimethylaminopropylamine (35 μL, 0.28 mmol) following general synthetic procedure B. The product was obtained as a yellow oil (0.16 g, 99%); 1H NMR (400 MHz, CDCl3): δ 8.82 (bs, 1H, NH), 8.76–8.71 (m, 1H, ArH), 7.88–7.79 (m, 2H, ArH), 7.61–7.54 (m, 2H, ArH), 7.47–7.41 (m, 2H, ArH), 7.39–7.33 (m, 1H, ArH), 3.53 (t, J = 6.0 Hz, 2H, CH2), 3.22–3.14 (m, 2H, CH2), 2.50 (t, J = 6.1 Hz, 2H, CH2), 2.29 (s, 6H, CH3), 1.87–1.73 (m, 4H, CH2), 1.44–1.16 (m, 18H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 192.0 (CO), 162.7 (CO), 140.9 (ArC), 139.1 (ArC), 135.7 (ArC), 134.9 (ArCH), 133.7 (ArCH), 129.1 (ArCH), 127.9 (ArCH), 127.0 (ArCH), 119.6 (ArC), 118.5 (ArCH), 58.9 (CH2), 52.7 (CH2), 45.4 (CH3), 40.1 (CH2), 32.0 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.4 (CH2), 29.4 (CH2), 29.1 (CH2), 28.2 (CH2), 25.3 (CH2), 23.5 (CH2), 22.8 (CH2), 14.2 (CH3); IR (ATR): νmax 3675, 3344, 2970, 2920, 2853, 2759, 2362, 1661, 1632, 1582, 1511, 1487, 1466, 1394, 1344, 1261, 1225, 1201, 1140, 1076, 1066, 1056, 928, 893, 857, 801, 761, 722, 697, 684 cm−1; HRMS (+ESI): Found m/z 558.3361 [M+H]+, C31H48N3O4S required 558.3360.
N-(3-(Dimethylamino)propyl)-2-(4-(dodecylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamide (11d)
The titled compound was synthesised from 5-([1,1′-biphenyl]-4-yl)-1-(dodecylsulfonyl)indoline-2,3-dione 8d (0.17 g, 0.31 mmol) and 3-dimethylaminopropylamine (40 μL, 0.31 mmol) following general synthetic procedure B. The crude was purified by flash column chromatography on silica, using methanol/dichloromethane (1:9) as an eluent to afford the product as a yellow oil (0.11 g, 53%); 1H NMR (400 MHz, CDCl3): δ 8.64 (d, J = 1.9 Hz, 1H, ArH), 8.53 (bs, 1H, NH), 7.88–7.76 (m, 2H, ArH), 7.70–7.55 (m, 6H, ArH), 7.49–7.42 (m, 2H, ArH), 7.40–7.34 (m, 1H, ArH), 3.64–3.53 (m, 2H, CH2), 3.22–3.14 (m, 2H, CH2), 2.96–2.85 (m, 2H, CH2), 2.62 (s, 6H, CH3), 2.09–1.97 (m, 2H, CH2), 1.87–1.77 (m, 2H, CH2), 1.43–1.15 (m, 18H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 191.8 (CO), 163.6 (CO), 140.8 (ArC), 140.6 (ArC), 140.6 (ArC), 137.9 (ArC), 135.5 (ArC), 134.7 (ArCH), 133.1 (ArCH), 129.0 (ArCH), 127.8 (ArCH), 127.7 (ArCH), 127.3 (ArCH), 127.2 (ArCH), 120.5 (ArC), 119.2 (ArCH), 58.3 (CH2), 52.8 (CH2), 44.1 (CH3), 39.4 (CH2), 32.0 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.3 (CH2), 24.7 (CH2), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3341, 3190, 3030, 2921, 2851, 2527, 2342, 2111, 1911, 1610, 1481, 1390, 1333, 1276, 1196, 1141, 994, 919, 826, 764, 696 cm−1; HRMS (+ESI): Found m/z 634.3673 [M+H]+, C37H52N3O4S required 634.3673.
N-(3-(Dimethylamino)propyl)-2-(2-(hexadecylsulfonamido)phenyl)-2-oxoacetamide (12a)
The titled compound was synthesised from 1-(hexadecylsulfonyl)indoline-2,3-dione 9a (0.13 g, 0.29 mmol) and 3-dimethylaminopropylamine (36 μL, 0.29 mmol) following general synthetic procedure B. The product was obtained as a yellow sticky solid (0.15 g, 96%); 1H NMR (400 MHz, CDCl3): δ 8.74 (bs, 1H, NH), 8.45 (dd, J = 8.1, 1.5 Hz, 1H, ArH), 7.77 (dd, J = 8.5, 0.5 Hz, 1H, ArH), 7.62–7.56 (m, 1H, ArH), 7.18–7.10 (m, 1H, ArH), 3.51 (t, J = 5.9 Hz, 2H, CH2), 3.17–3.11 (m, 2H, CH2), 2.48 (t, J = 6.2 Hz, 2H, CH2), 2.27 (s, 6H, CH3), 1.83–1.71 (m, 4H, CH2), 1.40–1.16 (m, 26H, CH2), 0.87 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 192.1 (CO), 162.8 (CO), 142.0 (ArC), 136.5 (ArCH), 135.3 (ArCH), 122.6 (ArCH), 119.0 (ArC), 117.9 (ArCH), 58.9 (CH2), 52.6 (CH2), 45.4 (CH3), 40.0 (CH2), 32.0 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.2 (CH2), 25.3 (CH2), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3351, 3229, 3057, 2920, 2851, 2781, 2533, 2119, 1633, 1527, 1463, 1401, 1337, 1262, 1232, 1147, 1100, 1042, 984, 918, 755, 673 cm−1; HRMS (+ESI): Found m/z 538.3672 [M+H]+, C29H52N3O4S required 538.3673.
2-(5-Bromo-2-(hexadecylsulfonamido)phenyl)-N-(3-(dimethylamino)propyl)-2-oxoacetamide (12b)
The titled compound was synthesised from 5-bromo-1-(hexadecylsulfonyl)indoline-2,3-dione 9b (0.15 g, 0.30 mmol) and 3-dimethylaminopropylamine (38 μL, 0.30 mmol) following general synthetic procedure B. The product was obtained as a yellow oil (0.18 g, 98%); 1H NMR (400 MHz, CDCl3): δ 8.94 (bs, 1H, NH), 7.64–7.63 (m, 1H, ArH), 7.69–7.66 (m, 2H, ArH), 3.52 (t, J = 6.2 Hz, 2H, CH2), 3.16–3.08 (m, 2H, CH2), 2.52 (t, J = 6.1 Hz, 2H, CH2), 2.30 (s, 6H, CH3), 1.82–1.71 (m, 4H, CH2), 1.42–1.17 (m, 26H, CH2), 0.87 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 190.8 (CO), 162.1 (CO), 140.8 (ArC), 139.1 (ArCH), 137.4 (ArCH), 120.8 (ArC), 119.9 (ArCH), 115.3 (ArC), 58.9 (CH2), 52.8 (CH2), 45.3 (CH3), 40.2 (CH2), 32.1 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.2 (CH2), 25.1 (CH2), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 2922, 2852, 2361, 1682, 1575, 1465, 1334, 1285, 1148, 1100, 972, 868, 823, 720 cm−1; HRMS (+ESI): Found m/z 616.2779 [M+H]+, C29H51BrN3O4S required 616.2778.
N-(3-(Dimethylamino)propyl)-2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide (12c)
The titled compound was synthesised from 1-(hexadecylsulfonyl)-5-phenylindoline-2,3-dione 9c (0.11 g, 0.21 mmol) and 3-dimethylaminopropylamine (26 μL, 0.21 mmol) following general synthetic procedure B. The product was obtained as a yellow solid (0.12 g, 96%); mp 76.7–79.4 °C; 1H NMR (400 MHz, CDCl3): δ 8.74 (bs, 1H, NH), 8.71 (d, J = 1.7 Hz, 1H, ArH), 7.87–7.79 (m, 2H, ArH), 7.60–7.55 (m, 2H, ArH), 7.48–7.41 (m, 2H, ArH), 7.39–7.33 (m, 1H, ArH), 3.54 (t, J = 5.6 Hz, 2H, CH2), 3.21–3.14 (m, 2H, CH2), 2.59 (t, J = 6.1 Hz, 2H, CH2), 2.36 (s, 6H, CH3), 1.89–1.75 (m, 4H, CH2), 1.42–1.17 (m, 26H, CH2), 0.87 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 191.9 (CO), 162.9 (CO), 140.9 (ArC), 139.1 (ArC), 135.8 (ArC), 134.9 (ArCH), 133.6 (ArCH), 129.1 (ArCH), 127.9 (ArCH), 127.0 (ArCH), 119.7 (ArC), 118.6 (ArCH), 58.4 (CH2), 52.7 (CH2), 45.1 (CH3), 39.6 (CH2), 32.1 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.3 (CH2), 25.2 (CH2), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3346, 3181, 2918, 2850, 2758, 2342, 1631, 1581, 1511, 1486, 1395, 1344, 1264, 1200, 1138, 1098, 1034, 925, 892, 855, 822, 761, 684 cm−1; HRMS (+ESI): Found m/z 614.3986 [M+H]+, C35H56N3O4S required 614.3986.
General synthetic procedure C for tertiary ammonium chloride salts
4 M HCl in dioxane (5.0 equivalents) was added to a stirring solution of glyoxamide derivative (1.0 equivalent) in diethyl ether (5 mL). The resulting reaction mixture was stirred at room temperature for 20 min. After the completion of the reaction, the reaction solvent was removed under reduced pressure, and the residue was washed three times with diethyl ether and freeze-dried to afford the product.
N,N-Dimethyl-3-(2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamido)propan-1-aminium chloride (13d)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamide 10d (24 mg, 0.042 mmol) and 4 M HCl/dioxane (58 μL, 0.23 mmol) following general synthetic procedure C. The product was obtained as a yellow sticky solid (22 mg, 88%); 1H NMR (600 MHz, DMSO-d6): δ 10.28 (bs, 2H, NH), 9.04 (t, J = 6.0 Hz, 1H, NH), 8.09–8.03 (m, 2H, ArH), 7.83–7.79 (m, 2H, ArH), 7.78–7.75 (m, 2H, ArH), 7.75–7.71 (m, 2H, ArH), 7.66–7.61 (m, 1H, ArH), 7.52–7.47 (m, 2H, ArH), 7.42–7.37 (m, 1H, ArH), 3.26–3.21 (m, 2H, CH2), 3.13–3.08 (m, 2H, CH2), 2.74 (s, 6H, CH3), 3.12–3.04 (m, 2H, CH2), 1.99–1.91 (m, 2H, CH2), 1.72–1.64 (m, 2H, CH2), 1.37–1.30 (m, 2H, CH2), 1.27–1.15 (m, 8H, CH2), 0.83 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.1 (CO), 163.7 (CO), 139.6 (ArC), 139.4 (ArC), 138.0 (ArC), 137.2 (ArC), 135.3 (ArC), 132.7 (ArCH), 130.1 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.4 (ArCH), 127.0 (ArCH), 126.6 (ArCH), 125.8 (ArC), 122.4 (ArCH), 54.5 (CH2), 51.4 (CH2), 42.1 (CH3), 36.0 (CH2), 31.1 (CH2), 28.4 (CH2), 28.3 (CH2), 28.3 (CH2), 23.9 (CH2), 22.9 (CH2), 22.0 (CH2), 13.9 (CH3); IR (ATR): νmax 3366, 3030, 2925, 2854, 2697, 2360, 1647, 1527, 1481, 1395, 1332, 1272, 1196, 1142, 1076, 1006, 975, 917, 828, 764, 728, 696, 673 cm−1; HRMS (+ESI): Found m/z 578.3049 [M+H]+, C33H44N3O4S required 578.3047.
3-(2-(2-(Dodecylsulfonamido)phenyl)-2-oxoacetamido)-N,N-dimethylpropan-1-aminium chloride (14a)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(2-(dodecylsulfonamido) phenyl)-2-oxoacetamide 11a (30 mg, 0.062 mmol) and 4 M HCl/dioxane (0.10 mL, 0.40 mmol) following general synthetic procedure C. The product was obtained as a pale yellow sticky solid (25 mg, 76%); 1H NMR (600 MHz, DMSO-d6): δ 10.23 (bs, 2H, NH), 8.99 (t, J = 5.9 Hz, 1H, NH), 7.78 (dd, J = 7.8, 1.5 Hz, 1H, ArH), 7.72–7.68 (m, 1H, ArH), 7.53 (d, J = 8.2 Hz, 1H, ArH), 7.33–7.30 (m, 1H, ArH), 3.30 (q, J = 6.5 Hz, 2H, CH2), 3.23–3.19 (m, 2H, CH2), 3.11–3.06 (m, 2H, CH2), 2.74 (s, 6H, CH3), 1.96–1.89 (m, 2H, CH2), 1.68–1.60 (m, 2H, CH2), 1.35–1.15 (m, 18H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.9 (CO), 164.2 (CO), 139.1 (ArC), 135.3 (ArCH), 132.9 (ArCH), 124.1 (ArCH), 124.0 (ArC), 121.0 (ArCH), 54.5 (CH2), 51.3 (CH2), 42.1 (CH3), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.2 (CH2), 23.9 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3380, 3260, 3082, 2917, 2851, 2689, 2093, 1663, 1527, 1456, 1420, 1328, 1263, 1217, 1143, 1065, 989, 925, 869, 798, 774 cm−1; HRMS (+ESI): Found m/z 482.3046 [M+H]+, C25H44N3O4S required 482.3047.
3-(2-(5-Bromo-2-(dodecylsulfonamido)phenyl)-2-oxoacetamido)-N,N-dimethylpropan-1-aminium chloride (14b)
The titled compound was synthesised from 2-(5-bromo-2-(dodecylsulfonamido)phenyl)-N-(3-(dimethylamino)propyl)-2-oxoacetamide 11b (30 mg, 0.054 mmol) and 4 M HCl/dioxane (0.10 mL, 0.40 mmol) following general synthetic procedure C. The product was obtained as a yellow sticky solid (22 mg, 67%); 1H NMR (600 MHz, DMSO-d6): δ 10.10 (bs, 2H, NH), 8.96 (t, J = 6.0 Hz, 1H, NH), 7.88–7.83 (m, 2H, ArH), 7.43 (d, J = 8.6 Hz, 1H, ArH), 3.29 (q, J = 6.4 Hz, 2H, CH2), 3.17–3.05 (m, 4H, CH2), 2.74 (s, 6H, CH3), 1.94–1.88 (m, 2H, CH2), 1.66–1.59 (m, 2H, CH2), 1.34–1.16 (m, 18H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 190.0 (CO), 162.9 (CO), 137.2 (ArC), 136.8 (ArCH), 133.9 (ArCH), 129.1 (ArC), 124.9 (ArCH), 116.7 (ArC), 54.5 (CH2), 51.3 (CH2), 42.1 (CH3), 36.0 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 23.8 (CH2), 22.8 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3382, 2921, 2851, 2698, 2361, 1648, 1529, 1478, 1389, 1331, 1198, 1147, 1073, 975, 916, 824, 719, 655 cm−1; HRMS (+ESI): Found m/z 560.2150 [M+H]+, C25H43BrN3O4S required 560.2152.
3-(2-(4-(Dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)-N,N-dimethylpropan-1-aminium chloride (14c)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(4-(dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide 11c (30 mg, 0.054 mmol) and 4 M HCl/dioxane (0.10 mL, 0.40 mmol) following general synthetic procedure C. The product was obtained as a yellow sticky solid (19 mg, 60%); 1H NMR (600 MHz, DMSO-d6): δ 10.19 (bs, 2H, NH), 9.02 (t, J = 6.1 Hz, 1H, NH), 8.02–7.97 (m, 2H, ArH), 7.68–7.63 (m, 2H, ArH), 7.63–7.59 (m, 1H, ArH), 7.53–7.47 (m, 2H, ArH), 7.43–7.38 (m, 1H, ArH), 3.35–3.29 (m, 2H, CH2), 3.24–3.18 (m, 2H, CH2), 3.13–3.07 (m, 2H, CH2), 2.74 (s, 6H, CH3), 1.97–1.89 (m, 2H, CH2), 1.71–1.62 (m, 2H, CH2), 1.37–1.14 (m, 18H, CH2), 0.84 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.1 (CO), 163.7 (CO), 138.2 (ArC), 137.9 (ArC), 136.0 (ArC), 132.9 (ArCH), 130.2 (ArCH), 129.2 (ArCH), 127.9 (ArCH), 126.5 (ArCH), 125.8 (ArC), 122.4 (ArCH), 54.5 (CH2), 51.3 (CH2), 42.1 (CH3), 36.0 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.3 (CH2), 27.3 (CH2), 23.9 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3341, 2921, 2852, 2696, 2360, 1661, 1631, 1582, 1512, 1487, 1467, 1396, 1345, 1268, 1201, 1139, 1075, 1003, 973, 925, 889, 853, 826, 761, 721, 683 cm−1; HRMS (+ESI): Found m/z 558.3363 [M+H]+, C31H48N3O4S required 558.3360.
3-(2-(4-(Dodecylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamido)-N,N-dimethylpropan-1-aminium chloride (14d)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(4-(dodecylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamide 11d (37 mg, 0.058 mmol) and 4 M HCl/dioxane (0.10 mL, 0.40 mmol) following general synthetic procedure C. The product was obtained as a white sticky solid (19 mg, 48%); 1H NMR (600 MHz, DMSO-d6): δ 10.19 (bs, 2H, ArH), 9.03 (t, J = 5.9 Hz, 1H, NH), 8.09–8.03 (m, 2H, ArH), 7.83–7.79 (m, 2H, ArH), 7.78–7.75 (m, 2H, ArH), 7.75–7.70 (m, 2H, ArH), 7.65–7.61 (m, 1H, ArH), 7.52–7.47 (m, 2H, ArH), 7.42–7.37 (m, 1H, ArH), 3.36–3.30 (m, 2H, CH2), 3.25–3.21 (m, 2H, CH2), 3.13–3.07 (m, 2H, CH2), 2.74 (s, 6H, CH3), 1.98–1.90 (m, 2H, CH2), 1.71–1.63 (m, 2H, CH2), 1.37–1.30 (m, 2H, CH2), 1.27–1.15 (m, 16H, CH2), 0.82 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.1 (CO), 163.7 (CO), 139.6 (ArC), 139.4 (ArC), 138.0 (ArC), 137.2 (ArC), 135.4 (ArC), 132.7 (ArCH), 130.1 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.4 (ArCH), 127.0 (ArCH), 126.6 (ArCH), 125.8 (ArC), 122.4 (ArCH), 54.5 (CH2), 51.3 (CH2), 42.1 (CH3), 36.0 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 24.0 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3353, 3261, 3030, 2920, 2850, 2689, 2477, 2113, 1920, 1650, 1525, 1481, 1400, 1333, 1272, 1195, 1144, 1073, 1005, 974, 917, 826, 764, 719, 695 cm−1; HRMS (+ESI): Found m/z 634.3672 [M+H]+, C37H52N3O4S required 634.3673.
3-(2-(2-(Hexadecylsulfonamido)phenyl)-2-oxoacetamido)-N,N-dimethylpropan-1-aminium chloride (15a)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(2-(hexadecylsulfonamido) phenyl)-2-oxoacetamide 12a (30 mg, 0.056 mmol) and 4 M HCl/dioxane (0.10 mL, 0.40 mmol) following general synthetic procedure C. The product was obtained as a white sticky solid (32 mg, 99%); 1H NMR (600 MHz, DMSO-d6): δ 10.20 (bs, 2H, NH), 8.99 (t, J = 5.9 Hz, 1H, NH), 7.77 (dd, J = 7.9, 1.5 Hz, 1H, ArH), 7.72–7.67 (m, 1H, ArH), 7.53 (dd, J = 8.3, 0.7 Hz, 1H, ArH), 7.33–7.30 (m, 1H, ArH), 3.30 (q, J = 6.4 Hz, 2H, CH2), 3.23–3.18 (m, 2H, CH2), 3.10–3.05 (m, 2H, CH2), 2.74 (s, 6H, CH3), 1.96–1.88 (m, 2H, CH2), 1.68–1.60 (m, 2H, CH2), 1.35–1.14 (m, 26H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.9 (CO), 164.2 (CO), 139.1 (ArC), 135.3 (ArCH), 132.9 (ArCH), 124.1 (ArCH), 124.0 (ArC), 121.0 (ArCH), 54.5 (CH2), 51.3 (CH2), 42.2 (CH3), 35.9 (CH2), 31.3 (CH2), 29.1 (CH2), 29.1 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 23.9 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3312, 3297, 3033, 2916, 2848, 2692, 2479, 2108, 1649, 1573, 1534, 1491, 1465, 1399, 1333, 1211, 1147, 1067, 973, 922, 819, 757, 721, 673 cm−1; HRMS (+ESI): Found m/z 538.3672 [M+H]+, C29H52N3O4S required 538.3673.
3-(2-(5-Bromo-2-(hexadecylsulfonamido)phenyl)-2-oxoacetamido)-N,N-dimethylpropan-1-aminium chloride (15b)
The titled compound was synthesised from 2-(5-bromo-2-(hexadecylsulfonamido)phenyl)-N-(3-(dimethylamino)propyl)-2-oxoacetamide 12b (32 mg, 0.052 mmol) and 4 M HCl/dioxane (0.10 mL, 0.40 mmol) following general synthetic procedure C. The product was obtained as a pale yellow sticky solid (26 mg, 77%); 1H NMR (600 MHz, DMSO-d6): δ 10.28 (bs, 1H, NH), 10.11 (bs, 1H, NH), 8.97 (t, J = 6.2 Hz, 1H, NH), 7.89–7.82 (m, 2H, ArH), 7.44 (d, J = 8.8 Hz, 1H, ArH), 3.29 (q, J = 6.5 Hz, 2H, CH2), 3.16–3.06 (m, 4H, CH2), 2.74 (s, 6H, CH3), 1.95–1.87 (m, 2H, CH2), 1.68–1.58 (m, 2H, CH2), 1.35–1.15 (m, 26H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 190.0 (CO), 162.9 (CO), 137.3 (ArC), 136.8 (ArCH), 133.9 (ArCH), 129.0 (ArC), 124.9 (ArCH), 116.7 (ArC), 54.5 (CH2), 51.3 (CH2), 42.1 (CH3), 36.0 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 23.8 (CH2), 22.8 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3378, 3251, 3067, 2916, 2848, 2710, 2104, 1741, 1644, 1595, 1526, 1461, 1372, 1337, 1289, 1234, 1193, 1142, 975, 936, 843, 786, 720 cm−1; HRMS (+ESI): Found m/z 616.2778 [M+H]+, C29H51BrN3O4S required 616.2778.
3-(2-(4-(Hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)-N,N-dimethylpropan-1-aminium chloride (15c)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide 12c (31 mg, 0.051 mmol) and 4 M HCl/dioxane (0.10 mL, 0.40 mmol) following general synthetic procedure C. The product was obtained as a yellow sticky solid (26 mg, 79%); 1H NMR (600 MHz, DMSO-d6): δ 10.18 (bs, 2H, NH), 9.01 (t, J = 5.9 Hz, 1H, NH), 8.01–7.98 (m, 2H, ArH), 7.67–7.63 (m, 2H, ArH), 7.62–7.59 (m, 1H, ArH), 7.52–7.48 (m, 2H, ArH), 7.42–7.39 (m, 1H, ArH), 3.35–3.29 (m, 2H, CH2), 3.24–3.19 (m, 2H, CH2), 3.11–3.06 (m, 2H, CH2), 2.73 (s, 6H, CH3), 1.97–1.88 (m, 2H, CH2), 1.71–1.63 (m, 2H, CH2), 1.37–1.14 (m, 26H, CH2), 0.85 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.1 (CO), 163.7 (CO), 138.2 (ArC), 137.9 (ArC), 135.9 (ArC), 132.9 (ArCH), 130.2 (ArCH), 129.2 (ArCH), 127.9 (ArCH), 126.5 (ArCH), 125.8 (ArC), 122.3 (ArCH), 54.5 (CH2), 51.3 (CH2), 42.1 (CH3), 36.0 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 24.0 (CH2), 22.9 (CH2), 22.1 (CH2), 13.9 (CH3); IR (ATR): νmax 3345, 3186, 2918, 2850, 2643, 2477, 2110, 1631, 1581, 1511, 1486, 1396, 1345, 1268, 1201, 1139, 1088, 973, 926, 826, 761, 685 cm−1; HRMS (+ESI): Found m/z 614.3986 [M+H]+, C35H56N3O4S required 614.3986.
General synthetic procedure D for quaternary ammonium iodide salts
Iodomethane (2.5 equivalents) was added to a stirring solution of glyoxamide derivative (1.0 equivalent) in tetrahydrofuran (3 mL). The resulting reaction mixture was stirred at room temperature for 24 h. After the completion of the reaction, the reaction solvent was removed under reduced pressure, and the residue was washed three times with diethyl ether and freeze-dried to afford the product.
N,N,N-Trimethyl-3-(2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamido)propan-1-aminium iodide (16d)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamide 10d (21 mg, 0.036 mmol) and iodomethane (6.0 μL, 0.096 mmol) following general synthetic procedure D. The product was obtained as a yellow sticky solid (24 mg, 91%); 1H NMR (600 MHz, DMSO-d6): δ 10.11 (bs, 1H, ArH), 8.98 (bs, 1H, NH), 8.05 (d, J = 1.7 Hz, 2H, ArH), 7.82–7.75 (m, 4H, ArH), 7.75–7.71 (m, 2H, ArH), 7.62–7.57 (m, 1H, ArH), 7.52–7.47 (m, 2H, ArH), 7.42–7.37 (m, 1H, ArH), 3.41–3.29 (m, 4H, CH2), 3.19 (t, J = 7.8 Hz, 2H, CH2), 3.08 (s, 9H, CH3), 2.04–1.96 (m, 2H, CH2), 1.72–1.64 (m, 2H, CH2), 1.38–1.29 (m, 2H, CH2), 1.27–1.15 (m, 8H, CH2), 0.83 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 191.9 (CO), 163.7 (CO), 139.6 (ArC), 139.4 (ArC), 137.6 (ArC), 137.2 (ArC), 135.6 (ArC), 132.5 (ArCH), 129.9 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.4 (ArCH), 127.0 (ArCH), 126.9 (ArC), 126.6 (ArCH), 122.9 (ArCH), 63.5 (CH2), 52.3 (CH3), 51.2 (CH2), 35.9 (CH2), 31.1 (CH2), 28.4 (CH2), 28.3 (CH2), 27.3 (CH2), 22.9 (CH2), 22.6 (CH2), 22.0 (CH2), 13.9 (CH3); IR (ATR): νmax 3382, 2924, 2854, 2360, 1979, 1670, 1527, 1481, 1396, 1332, 1259, 1195, 1141, 1075, 1006, 914, 883, 829, 765, 729, 697, 673 cm−1; HRMS (+ESI): Found m/z 592.3206 [M]+, C34H46N3O4S required 592.3204.
3-(2-(2-(Dodecylsulfonamido)phenyl)-2-oxoacetamido)-N,N,N-trimethylpropan-1-aminium iodide (17a)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(2-(dodecylsulfonamido) phenyl)-2-oxoacetamide 11a (30 mg, 0.062 mmol) and iodomethane (10.0 μL, 0.16 mmol) following general synthetic procedure D. The product was obtained as a yellow sticky solid (25 mg, 64%); 1H NMR (600 MHz, DMSO-d6): δ 10.14 (bs, 1H, NH), 8.95 (t, J = 5.9 Hz, 1H, NH), 7.77 (dd, J = 7.9, 1.6 Hz, 1H, ArH), 7.73–7.68 (m, 1H, ArH), 7.50 (dd, J = 8.3, 0.7 Hz, 1H, ArH), 7.35–7.31 (m, 1H, ArH), 3.37–3.27 (m, 4H, CH2), 3.20–3.15 (m, 2H, CH2), 3.07 (s, 9H, CH3), 2.02–1.93 (m, 2H, CH2), 1.68–1.60 (m, 2H, CH2), 1.36–1.14 (m, 18H, CH2), 0.85 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.5 (CO), 164.1 (CO), 138.7 (ArC), 135.1 (ArCH), 132.6 (ArCH), 125.1 (ArC), 124.4 (ArCH), 121.6 (ArCH), 63.4 (CH2), 52.3 (CH3), 51.2 (CH2), 35.8 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.9 (CH2), 22.6 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3510, 3448, 3258, 3175, 3038, 2918, 2851, 1666, 1602, 1524, 1453, 1327, 1261, 1214, 1141, 1107, 984, 913, 883, 772 cm−1; HRMS (+ESI): Found m/z 496.3203 [M]+, C26H46N3O4S required 496.3204.
3-(2-(5-Bromo-2-(dodecylsulfonamido)phenyl)-2-oxoacetamido)-N,N,N-trimethylpropan-1-aminium iodide (17b)
The titled compound was synthesised from 2-(5-bromo-2-(dodecylsulfonamido)phenyl)-N-(3-(dimethylamino)propyl)-2-oxoacetamide 11b (34 mg, 0.061 mmol) and iodomethane (10.0 μL, 0.16 mmol) following general synthetic procedure D. The product was obtained as a yellow sticky solid (34 mg, 80%); 1H NMR (600 MHz, DMSO-d6): δ 10.00 (s, 1H, NH), 8.95 (t, J = 6.0 Hz, 1H, NH), 7.90–7.82 (m, 2H, ArH), 7.42–7.38 (m, 1H, ArH), 3.40–3.27 (m, 4H, CH2), 3.13–3.03 (m, 2H, CH2), 3.06 (s, 9H, CH3), 2.02–1.93 (m, 2H, CH2), 1.68–1.58 (m, 2H, CH2), 1.37–1.14 (m, 18H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 189.8 (CO), 162.8 (CO), 136.9 (ArC), 136.7 (ArCH), 133.9 (ArCH), 129.9 (ArC), 125.4 (ArCH), 117.1 (ArC), 63.5 (CH2), 52.3 (CH3), 51.2 (CH2), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.8 (CH2), 22.5 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3808, 3650, 3289, 2920, 2850, 2360, 1979, 1677, 1649, 1561, 1541, 1480, 1391, 1340, 1291, 1274, 1260, 1228, 1203, 1152, 1077, 967, 922, 871, 829, 721, 698, 655 cm−1; HRMS (+ESI): Found m/z 574.2318 [M]+, C26H45BrN3O4S required 574.2309.
3-(2-(4-(Dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)-N,N,N-trimethylpropan-1-aminium iodide (17c)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(4-(dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide 11c (34 mg, 0.061 mmol) and iodomethane (10.0 μL, 0.16 mmol) following general synthetic procedure D. The product was obtained as a yellow sticky solid (36 mg, 85%); 1H NMR (600 MHz, DMSO-d6): δ 10.10 (bs, 1H, NH), 8.98 (t, J = 6.0 Hz, 1H, NH), 8.04–7.94 (m, 2H, ArH), 7.68–7.64 (m, 2H, ArH), 7.60–7.56 (m, 1H, ArH), 7.52–7.46 (m, 2H, ArH), 7.43–7.38 (m, 1H, ArH), 3.41–3.28 (m, 4H, CH2), 3.23–3.14 (m, 2H, CH2), 3.07 (s, 9H, CH3), 2.04–1.93 (m, 2H, CH2), 1.71–1.61 (m, 2H, CH2), 1.39–1.14 (m, 18H, CH2), 0.84 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 191.8 (CO), 163.7 (CO), 138.3 (ArC), 137.5 (ArC), 136.3 (ArC), 132.7 (ArCH), 130.1 (ArCH), 129.2 (ArCH), 127.9 (ArCH), 126.8 (ArC), 126.5 (ArCH), 122.9 (ArCH), 63.5 (CH2), 52.3 (CH3), 51.2 (CH2), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.9 (CH2), 22.6 (CH2), 22.1 (CH2), 13.9 (CH3); IR (ATR): νmax 3410, 2922, 2852, 2359, 1979, 1670, 1648, 1582, 1509, 1483, 1395, 1330, 1264, 1195, 1140, 1075, 915, 841, 761, 698, 681 cm−1; HRMS (+ESI): Found m/z 572.3519 [M]+, C32H50N3O4S required 572.3517.
3-(2-(4-(Dodecylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamido)-N,N,N-trimethylpropan-1-aminium iodide (17d)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(4-(dodecylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamide 11d (32 mg, 0.051 mmol) and iodomethane (10.0 μL, 0.16 mmol) following general synthetic procedure D. The product was obtained as a white sticky solid (16 mg, 42%); 1H NMR (600 MHz, DMSO-d6): δ 10.12 (bs, 1H, ArH), 9.00 (t, J = 5.9 Hz, 1H, NH), 8.09–8.03 (m, 2H, ArH), 7.82–7.75 (m, 2H, ArH), 7.74–7.71 (m, 2H, ArH), 7.62–7.58 (m, 1H, ArH), 7.52–7.61 (m, 2H, ArH), 7.42–7.37 (m, 2H, ArH), 7.42–7.37 (m, 1H, ArH), 3.40–3.30 (m, 4H, CH2), 3.23–3.17 (m, 2H, CH2), 3.07 (s, 9H, CH3), 2.04–1.96 (m, 2H, CH2), 1.71–1.63 (m, 2H, CH2), 1.37–1.29 (m, 2H, CH2), 1.26–1.14 (m, 16H, CH2), 0.83 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 191.8 (CO), 163.7 (CO), 139.6 (ArC), 139.4 (ArC), 137.6 (ArC), 137.2 (ArC), 135.7 (ArC), 132.6 (ArCH), 130.0 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.4 (ArCH), 127.0 (ArCH), 126.8 (ArC), 126.6 (ArCH), 122.9 (ArCH), 63.5 (CH2), 52.3 (CH3), 51.2 (CH2), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.9 (CH2), 22.6 (CH2), 22.1 (CH2), 13.9 (CH3); IR (ATR): νmax 3751, 3376, 3264, 3029, 2920, 2850, 2723, 2485, 2295, 2103, 2075, 1650, 1524, 1480, 1398, 1333, 1272, 1194, 1141, 1073, 1005, 914, 827, 764, 718, 696 cm−1; HRMS (+ESI): Found m/z 648.3829 [M]+, C38H54N3O4S required 648.3830.
3-(2-(2-(Hexadecylsulfonamido)phenyl)-2-oxoacetamido)-N,N,N-trimethylpropan-1-aminium iodide (18a)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(2-(hexadecylsulfonamido) phenyl)-2-oxoacetamide 12a (30 mg, 0.056 mmol) and iodomethane (9.0 μL, 0.15 mmol) following general synthetic procedure D. The product was obtained as a white sticky solid (33 mg, 87%); 1H NMR (400 MHz, DMSO-d6): δ 10.13 (bs, 1H, NH), 8.95 (t, J = 5.8 Hz, 1H, NH), 7.76 (dd, J = 7.9, 1.3 Hz, 1H, ArH), 7.73–7.66 (m, 1H, ArH), 7.49 (d, J = 8.0 Hz, 1H, ArH), 7.33 (t, J = 7.5 Hz, 1H, ArH), 3.39–3.26 (m, 4H, CH2), 3.20–3.12 (m, 2H, CH2), 3.06 (s, 9H, CH3), 2.03–1.91 (m, 2H, CH2), 1.70–1.57 (m, 2H, CH2), 1.37–1.12 (m, 26H, CH2), 0.84 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.6 (CO), 164.2 (CO), 138.8 (ArC), 135.1 (ArCH), 132.7 (ArCH), 125.2 (ArC), 124.4 (ArCH), 121.6 (ArCH), 63.5 (CH2), 52.4 (CH3), 51.2 (CH2), 35.9 (CH2), 31.3 (CH2), 29.1 (CH2), 29.1 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 23.9 (CH2), 22.6 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3448, 3253, 3234, 3009, 2916, 2848, 2284, 1663, 1527, 1490, 1452, 1401, 1329, 1262, 1214, 1145, 1066, 919, 882, 800, 757, 721, 673 cm−1; HRMS (+ESI): Found m/z 552.3830 [M]+, C30H54N3O4S required 552.3830.
3-(2-(5-Bromo-2-(hexadecylsulfonamido)phenyl)-2-oxoacetamido)-N,N,N-trimethylpropan-1-aminium iodide (18b)
The titled compound was synthesised from 2-(5-bromo-2-(hexadecylsulfonamido)phenyl)-N-(3-(dimethylamino)propyl)-2-oxoacetamide 12b (38 mg, 0.062 mmol) and iodomethane (10.0 μL, 0.16 mmol) following general synthetic procedure D. The product was obtained as a yellow sticky solid (31 mg, 65%); 1H NMR (600 MHz, DMSO-d6): δ 10.00 (bs, 1H, NH), 8.94 (t, J = 5.9 Hz, 1H, NH), 7.92–7.82 (m, 2H, ArH), 7.40 (dd, J = 8.1, 0.9 Hz, 1H, ArH), 3.40–3.27 (m, 4H, CH2), 3.14–3.02 (m, 2H, CH2), 3.06 (s, 9H, CH3), 2.03–1.91 (m, 2H, CH2), 1.69–1.56 (m, 2H, CH2), 1.38–1.07 (m, 26H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 189.8 (CO), 162.8 (CO), 136.7 (ArCH), 134.9 (ArC), 133.8 (ArCH), 129.8 (ArC), 125.3 (ArCH), 117.0 (ArC), 63.5 (CH2), 52.3 (CH3), 51.2 (CH2), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.8 (CH2), 22.5 (CH2), 22.1 (CH2), 13.9 (CH3); IR (ATR): νmax 3410, 3223, 3014, 2919, 2851, 2087, 1896, 1651, 1526, 1477, 1390, 1334, 1197, 1144, 918, 821, 767, 717 cm−1; HRMS (+ESI): Found m/z 630.2933 [M]+, C30H53BrN3O4S required 630.2935.
3-(2-(4-(Hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)-N,N,N-trimethylpropan-1-aminium iodide (18c)
The titled compound was synthesised from N-(3-(dimethylamino)propyl)-2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide 12c (31 mg, 0.051 mmol) and iodomethane (10.0 μL, 0.16 mmol) following general synthetic procedure D. The product was obtained as a yellow sticky solid (21 mg, 53%); 1H NMR (600 MHz, DMSO-d6): δ 10.10 (bs, 1H, NH), 8.98 (bs, 1H, NH), 8.07–7.93 (m, 2H, ArH), 7.66 (dd, J = 8.5, 1.2 Hz, 2H, ArH), 7.60–7.55 (m, 1H, ArH), 7.52–7.47 (m, 2H, ArH), 7.40 (t, J = 7.4 Hz, 1H, ArH), 3.39–3.35 (m, 2H, CH2), 3.35–3.30 (m, 2H, CH2), 3.18 (t, J = 7.7 Hz, 2H, CH2), 3.07 (s, 9H, CH3), 2.02–1.95 (m, 2H, CH2), 1.70–1.63 (m, 2H, CH2), 1.37–1.14 (m, 26H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 191.8 (CO), 163.7 (CO), 138.3 (ArC), 137.5 (ArC), 136.3 (ArC), 132.7 (ArCH), 130.1 (ArCH), 129.1 (ArCH), 127.9 (ArCH), 126.8 (ArC), 126.5 (ArCH), 122.9 (ArCH), 63.5 (CH2), 52.3 (CH3), 51.2 (CH2), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.9 (CH2), 22.6 (CH2), 22.1 (CH2), 13.9 (CH3); IR (ATR): νmax 3317, 3189, 3009, 2919, 2851, 2299, 1907, 1632, 1511, 1486, 1395, 1343, 1266, 1200, 1139, 1088, 924, 848, 761, 683 cm−1; HRMS (+ESI): Found m/z 628.4142 [M]+, C36H58N3O4S required 628.4143.
5-([1,1′-Biphenyl]-3-yl)indoline-2,3-dione (19)
The synthesis of the titled compound followed the procedure previously described [56]. A 2 M potassium carbonate solution (12.3 mL, 24.6 mmol) was degassed for 30 min and then added into a suspension of 5-bromoisatin (2.71 g, 11.98 mmol) and 3-biphenylboronic acid (2.70 g, 13.64 mmol) in a degassed (for 30 min) 1:1 ethanol/toluene solution (30 mL). The dark brown solution mixture was further degassed for 30 min, followed by the addition of tetrakis(triphenylphosphine)palladium(0) (145 mg, 0.125 mmol). The resulting reaction mixture was heated at reflux under an atmosphere of nitrogen for 18 h. The dark brown reaction mixture was concentrated in vacuo. Glacial acetic acid (30 mL) was added to the dark brown solid, and the resulting reaction mixture was heated at reflux for 20 min, followed by hot filtration of the red reaction mixture. The resulting red solution mixture was allowed to cool to room temperature, and the red precipitate formed was collected by vacuum filtration to give the crude product, which was redissolved in dimethylformamide. The solution mixture was then poured into a 1:1 ice-water mixture, and the red precipitate formed was collected by vacuum filtration to give the product as a red solid (2.00 g, 56%); mp 233.0–234.7 °C; 1H NMR (400 MHz, DMSO-d6): δ 11.14 (bs, 1H, NH), 8.02 (dd, J = 8.2, 2.0 Hz, 1H, ArH), 7.94–7.87 (m, 2H, ArH), 7.81–7.75 (m, 2H, ArH), 7.68–7.62 (m, 2H, ArH), 7.58–7.45 (m, 3H, ArH), 7.42–7.36 (m, 1H, ArH), 7.02 (dd, J = 8.2, 0.4 Hz, 1H, ArH); HRMS (+ESI): Found m/z 300.1019 [M+H]+, C20H14NO2 required 300.1019.
5-([1,1′-Biphenyl]-3-yl)-1-(octylsulfonyl)indoline-2,3-dione (20)
Triethylamine (0.17 mL, 1.22 mmol) was added slowly to a stirring solution of 5-([1,1′-biphenyl]-3-yl)indoline-2,3-dione 19 (0.32 g, 1.06 mmol) in dichloromethane (20 mL) at 0 °C under nitrogen atmosphere. The reaction mixture was stirred at 0 °C for 20 min, followed by the dropwise addition of 1-octanesulfonyl chloride (0.21 mL, 1.07 mmol) with stirring. The reaction mixture was then stirred at room temperature for 3 h. After the completion of the reaction, the reaction mixture was concentrated in vacuo and washed with methanol to afford the product as a yellowish orange solid (0.27 g, 53%); mp 161.5–161.7 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.22–8.11 (m, 2H, ArH), 7.97 (s, 1H, ArH), 7.85–7.76 (m, 3H, ArH), 7.71 (t, J = 8.3 Hz, 2H, ArH), 7.58 (t, J = 8.0 Hz, 1H, ArH), 7.50 (t, J = 7.7 Hz, 2H, ArH), 7.40 (t, J = 7.3 Hz, 1H, ArH), 3.69–3.59 (m, 2H, CH2), 1.88–1.76 (m, 2H, CH2), 1.45–1.15 (m, 10H, CH2), 0.83 (t, J = 6.9 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 178.7 (CO), 156.6 (CO), 146.2 (ArC), 141.1 (ArC), 139.9 (ArC), 138.7 (ArC), 136.9 (ArC), 136.1 (ArCH), 129.7 (ArCH), 128.9 (ArCH), 127.7 (ArCH), 127.0 (ArCH), 126.4 (ArCH), 125.6 (ArCH), 125.0 (ArCH), 122.9 (ArCH), 120.1 (ArC), 114.5 (ArCH), 53.7 (CH2), 31.1 (CH2), 28.4 (CH2), 28.3 (CH2), 27.3 (CH2), 22.2 (CH2), 22.0 (CH2), 13.9 (CH3); IR (ATR): νmax 3854, 3675, 2970, 2922, 1779, 1744, 1616, 1589, 1496, 1471, 1441, 1404, 1394, 1368, 1298, 1232, 1161, 1140, 1075, 1066, 1057, 1027, 948, 891, 866, 840, 795, 776, 755, 725, 698, 654 cm−1; HRMS (+ESI): Found m/z 498.1710 [M+Na]+, C28H29NO4SNa required 498.1710.
N-(3-(Dimethylamino)propyl)-2-(4-(octylsulfonamido)-[1,1′:3′,1″-terphenyl]-3-yl)-2-oxoacetamide (21)
3-Dimethylaminopropylamine (39 μL, 0.31 mmol) was added to a stirring solution of 5-([1,1′-biphenyl]-3-yl)-1-(octylsulfonyl)indoline-2,3-dione 20 (0.15 g, 0.31 mmol) in dichloromethane (5 mL) at 0 °C. The resulting reaction mixture was stirred at room temperature for 3 h. After the completion of the reaction, water was added to the reaction mixture and the mixture was extracted into dichloromethane (3 × 25 mL), washed with brine, dried over anhydrous sodium sulfate and concentrated in vacuo to afford the product as a yellow oil (0.18 g, 99%); 1H NMR (400 MHz, CDCl3): δ 8.83 (bs, 1H, ArH), 8.77 (s, 1H, ArH), 7.90–7.85 (m, 2H, ArH), 7.77–7.73 (m, 1H, ArH), 7.66–7.43 (m, 7H, ArH), 7.38 (t, J = 7.3 Hz, 1H, ArH), 3.52 (t, J = 5.9 Hz, 2H, CH2), 3.23–3.14 (m, 2H, CH2), 2.50 (t, J = 6.2 Hz, 2H, CH2), 2.29 (s, 6H, CH3), 1.87–1.73 (m, 4H, CH2), 1.43–1.17 (m, 10H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 192.0 (CO), 162.6 (CO), 142.2 (ArC), 141.1 (ArC), 141.0 (ArC), 139.8 (ArC), 135.8 (ArC), 135.1 (ArCH), 133.8 (ArCH), 129.6 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.4 (ArCH), 126.8 (ArCH), 126.0 (ArCH), 125.9 (ArCH), 119.7 (ArC), 118.6 (ArCH), 58.9 (CH2), 52.7 (CH2), 45.4 (CH3), 40.1 (CH2), 31.8 (CH2), 29.1 (CH2), 29.0 (CH2), 28.2 (CH2), 25.3 (CH2), 23.5 (CH2), 22.7 (CH2), 14.2 (CH3); IR (ATR): νmax 3675, 2970, 2900, 1647, 1498, 1473, 1405, 1393, 1337, 1258, 1196, 1142, 1066, 1057, 891, 798, 757, 700 cm−1; HRMS (+ESI): Found m/z 578.3049 [M+H]+, C33H44N3O4S required 578.3047.
N,N-Dimethyl-3-(2-(4-(octylsulfonamido)-[1,1′:3′,1″-terphenyl]-3-yl)-2-oxoacetamido)propan-1-aminium chloride (22)
4 M HCl in dioxane (0.10 mL, 0.40 mmol) was added to a stirring solution of N-(3-(dimethylamino)propyl)-2-(4-(octylsulfonamido)-[1,1′:3′,1″-terphenyl]-3-yl)-2-oxoacetamide 21 (30 mg, 0.052 mmol) in diethyl ether (5 mL). The resulting reaction mixture was stirred at room temperature for 20 min. After the completion of the reaction, the reaction solvent was removed under reduced pressure, and the residue was washed three times with diethyl ether and freeze-dried to afford the product as a yellow sticky solid (25 mg, 67%); 1H NMR (600 MHz, DMSO-d6): δ 10.18 (bs, 2H, NH), 9.01 (t, J = 5.3 Hz, 1H, NH), 8.16–8.03 (m, 2H, ArH), 7.89 (s, 1H, ArH), 7.82–7.55 (m, 6H, ArH), 7.50 (t, J = 7.4 Hz, 2H, ArH), 7.41 (t, J = 7.0 Hz, 1H, ArH), 3.37–3.28 (m, 2H, CH2), 3.21 (t, J = 7.6 Hz, 2H, CH2), 3.09 (t, J = 7.6 Hz, 2H, CH2), 2.72 (s, 6H, CH3), 1.97–1.88 (m, 2H, CH2), 1.72–1.63 (m, 2H, CH2), 1.38–1.15 (m, 10H, CH2), 0.82 (t, J = 6.9 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 191.9 (CO), 163.6 (CO), 141.1 (ArC), 139.9 (ArC), 139.1 (ArC), 137.8 (ArC), 136.1 (ArC), 133.0 (ArCH), 130.4 (ArCH), 129.8 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.0 (ArCH), 126.4 (ArC), 126.3 (ArCH), 125.7 (ArCH), 125.0 (ArCH), 122.6 (ArCH), 54.5 (CH2), 51.3 (CH2), 42.1 (CH3), 36.0 (CH2), 31.1 (CH2), 28.4 (CH2), 28.3 (CH2), 27.3 (CH2), 23.9 (CH2), 22.9 (CH2), 22.0 (CH2), 13.9 (CH3); IR (ATR): νmax 3365, 2923, 2853, 2695, 2361, 1654, 1647, 1497, 1474, 1400, 1332, 1246, 1195, 1143, 1075, 975, 917, 890, 840, 797, 757, 720, 700, 680 cm−1; HRMS (+ESI): Found m/z 578.3049 [M+H]+, C33H44N3O4S required 578.3047.
N,N,N-Trimethyl-3-(2-(4-(octylsulfonamido)-[1,1′:3′,1″-terphenyl]-3-yl)-2-oxoacetamido)propan-1-aminium iodide (23)
Iodomethane (10.0 μL, 0.16 mmol) was added to a stirring solution of N-(3-(dimethylamino)propyl)-2-(4-(octylsulfonamido)-[1,1′:3′,1″-terphenyl]-3-yl)-2-oxoacetamide 21 (31 mg, 0.054 mmol) in tetrahydrofuran (3 mL). The resulting reaction mixture was stirred at room temperature for 24 h. After the completion of the reaction, the reaction solvent was removed under reduced pressure, and the residue was washed three times with diethyl ether and freeze-dried to afford the product as a yellow sticky solid (33 mg, 86%); 1H NMR (600 MHz, DMSO-d6): δ 10.09 (bs, 1H, ArH), 8.98 (t, J = 6.1 Hz, 1H, NH), 8.13–8.06 (m, 2H, ArH), 7.90 (t, J = 6.1 Hz, 1H, ArH), 7.78–7.63 (m, 4H, ArH), 7.62–7.56 (m, 2H, ArH), 7.52–7.47 (m, 2H, ArH), 7.43–7.39 (m, 1H, ArH), 3.39–3.29 (m, 4H, CH2), 3.18 (t, J = 8.0 Hz, 2H, CH2), 3.05 (s, 9H, CH3), 2.02–1.93 (m, 2H, CH2), 1.73–1.63 (m, 2H, CH2), 1.40–1.15 (m, 10H, CH2), 0.82 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 191.6 (CO), 163.6 (CO), 141.1 (ArC), 139.9 (ArC), 139.1 (ArC), 137.4 (ArC), 136.4 (ArC), 132.9 (ArCH), 130.2 (ArCH), 129.8 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.4 (ArC), 127.0 (ArCH), 126.4 (ArCH), 125.7 (ArCH), 125.0 (ArCH), 123.2 (ArCH), 63.5 (CH2), 52.3 (CH3), 51.2 (CH2), 35.9 (CH2), 31.1 (CH2), 28.4 (CH2), 28.3 (CH2), 27.3 (CH2), 22.9 (CH2), 22.6 (CH2), 22.0 (CH2), 13.9 (CH3); IR (ATR): νmax 3404, 2925, 2854, 2360, 1979, 1670, 1642, 1475, 1400, 1332, 1260, 1194, 1140, 1075, 916, 889, 842, 798, 758, 701, 680 cm−1; HRMS (+ESI): Found m/z 592.3206 [M]+, C34H46N3O4S required 592.3204.
General synthetic procedure E for Boc-protected glyoxamides
A solution of N-Boc-1,3-propanediamine (1.0 equivalent) in dichloromethane (3 mL) was added dropwise to a stirring solution of N-sulfonylisatin (1.0 equivalent) in dichloromethane (10 mL) at 0 °C. The resulting reaction mixture was stirred at room temperature for 6 h. After the completion of the reaction, water was added to the reaction mixture, and the mixture was extracted into dichloromethane (3 × 25 mL), washed with brine, dried over anhydrous sodium sulfate and concentrated in vacuo to afford the product.
tert-Butyl (3-(2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamido)propyl)carbamate (24d)
The titled compound was synthesised from 5-([1,1′-biphenyl]-4-yl)-1-(octylsulfonyl)indoline-2,3-dione 7d (0.35 g, 0.73 mmol) and N-Boc-1,3-propandiamine (0.13 g, 0.75 mmol) following general synthetic procedure E. The product was obtained as a brown solid (0.47 g, 98%); mp 129.4–129.6 °C; 1H NMR (400 MHz, CDCl3): δ 10.50 (s, 1H, NH), 8.81 (s, 1H, ArH), 7.91–7.84 (m, 2H, ArH), 7.77–7.59 (m, 7H, NH, ArH), 7.47 (t, J = 7.8 Hz, 2H, ArH), 7.37 (t, J = 7.1 Hz, 1H, ArH), 4.81 (bs, 1H, NH), 3.49 (q, J = 6.3 Hz, 2H, CH2), 3.30–3.15 (m, 4H, CH2), 1.88–1.71 (m, 4H, CH2), 1.45 (s, 9H, CH3), 1.49–1.16 (m, 10H, CH2), 0.86 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 191.5 (CO), 162.9 (CO), 156.9 (CO), 141.1 (ArC), 140.8 (ArC), 140.6 (ArC), 137.9 (ArC), 135.2 (ArC), 135.0 (ArCH), 133.5 (ArCH), 129.0 (ArCH), 127.9 (ArCH), 127.7 (ArCH), 127.3 (ArCH), 127.2 (ArCH), 119.4 (ArC), 118.6 (ArCH), 79.9 (C), 52.8 (CH2), 37.3 (CH2), 36.4 (CH2), 31.8 (CH2), 30.2 (CH2), 29.1 (CH2), 29.0 (CH2), 28.5 (CH3), 28.2 (CH2), 23.6 (CH2), 22.7 (CH2), 14.2 (CH3); IR (ATR): νmax 3350, 2924, 2869, 2360, 1685, 1666, 1641, 1521, 1481, 1449, 1387, 1364, 1341, 1267, 1245, 1213, 1195, 1154, 1070, 1039, 1006, 977, 912, 900, 883, 866, 830, 762, 716, 689, 673 cm−1; HRMS (+ESI): Found m/z 672.3082 [M+Na]+, C36H47N3O6SNa required 672.3078.
tert-Butyl (3-(2-(2-(dodecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamate (25a)
The titled compound was synthesised from 1-(dodecylsulfonyl)indoline-2,3-dione 8a (0.32 g, 0.84 mmol) and N-Boc-1,3-propandiamine (0.15 g, 0.84 mmol) following general synthetic procedure E. The product was obtained as a pale yellow solid (0.42 g, 89%); mp 77.2–80.5 °C; 1H NMR (400 MHz, CDCl3): δ 10.49 (s, 1H, NH), 8.44 (d, J = 7.9 Hz, 1H, ArH), 7.77 (d, J = 8.5 Hz, 1H, ArH), 7.68–7.55 (m, 2H, NH, ArH), 7.19–7.11 (m, 1H, ArH), 4.87 (bs, 1H, NH), 3.46 (q, J = 6.3 Hz, 2H, CH2), 3.28–3.11 (m, 4H, CH2), 1.85–1.69 (m, 4H, CH2), 1.44 (s, 9H, CH3), 1.41–1.15 (m, 18H, CH2), 0.87 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 191.7 (CO), 163.1 (CO), 156.9 (CO), 142.1 (ArC), 136.7 (ArCH), 135.3 (ArCH), 122.6 (ArCH), 118.8 (ArC), 117.9 (ArCH), 79.9 (C), 52.7 (CH2), 37.3 (CH2), 36.4 (CH2), 32.0 (CH2), 30.2 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.4 (CH2), 29.4 (CH2), 29.1 (CH2), 28.5 (CH3), 28.2 (CH2), 23.5 (CH2), 22.8 (CH2), 14.2 (CH3); IR (ATR): νmax 3363, 3238, 2918, 2850, 2297, 2106, 1684, 1637, 1602, 1571, 1523, 1449, 1398, 1334, 1247, 1220, 1152, 1077, 1039, 1005, 918, 817, 753, 671 cm−1; HRMS (+ESI): Found m/z 576.3078 [M+Na]+, C28H47N3O6SNa required 576.3078.
tert-Butyl (3-(2-(5-bromo-2-(dodecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamate (25b)
The titled compound was synthesised from 5-bromo-1-(dodecylsulfonyl)indoline-2,3-dione 8b (0.28 g, 0.60 mmol) and N-Boc-1,3-propandiamine (0.11 g, 0.60 mmol) following general synthetic procedure E. The product was obtained as a yellow solid (0.34 g, 89%); mp 98.0–98.2 °C; 1H NMR (400 MHz, CDCl3): δ 10.38 (s, 1H, NH), 8.66 (s, 1H, ArH), 7.76 (bs, 1H, NH), 7.71–7.66 (m, 2H, ArH), 4.80 (bs, 1H, NH), 3.46 (q, J = 6.4 Hz, 2H, CH2), 3.29–3.19 (m, 2H, CH2), 3.19–3.09 (m, 2H, CH2), 1.83–1.70 (m, 4H, CH2), 1.45 (s, 9H, CH3), 1.41–1.17 (m, 18H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 190.4 (CO), 162.2 (CO), 157.0 (CO), 141.0 (ArC), 139.3 (ArCH), 137.4 (ArCH), 120.4 (ArCH), 119.8 (ArC), 115.2 (ArC), 80.0 (C), 52.9 (CH2), 37.2 (CH2), 36.4 (CH2), 32.0 (CH2), 30.2 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.5 (CH3), 28.2 (CH2), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3382, 3329, 3173, 2918, 2850, 1683, 1664, 1630, 1560, 1519, 1479, 1452, 1392, 1366, 1339, 1315, 1283, 1249, 1198, 1170, 1147, 1066, 1039, 1012, 988, 916, 889, 860, 823, 776, 719, 708, 659 cm−1; HRMS (+ESI): Found m/z 654.2187 [M+Na]+, C28H46BrN3O6SNa required 654.2183.
tert-Butyl (3-(2-(4-(dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)propyl)carbamate (25c)
The titled compound was synthesised from 1-(dodecylsulfonyl)-5-phenylindoline-2,3-dione 8c (0.32 g, 0.71 mmol) and N-Boc-1,3-propandiamine (0.13 g, 0.71 mmol) following general synthetic procedure E. The product was obtained as a yellow solid (0.39 g, 88%); mp 120.8–121.0 °C; 1H NMR (400 MHz, CDCl3): δ 10.48 (s, 1H, NH), 8.75 (s, 1H, ArH), 7.89–7.79 (m, 2H, ArH), 7.71 (bs, 1H, NH), 7.60–7.53 (m, 2H, ArH), 7.48–7.41 (m, 2H, ArH), 7.39–7.33 (m, 1H, ArH), 4.81 (bs, 1H, NH), 3.48 (q, J = 6.3 Hz, 2H, CH2), 3.29–3.14 (m, 4H, CH2), 1.87–1.71 (m, 4H, CH2), 1.44 (s, 9H, CH3), 1.43–1.17 (m, 18H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 191.5 (CO), 162.9 (CO), 156.9 (CO), 141.1 (ArC), 139.1 (ArC), 135.7 (ArC), 135.1 (ArCH), 133.6 (ArCH), 129.2 (ArCH), 127.9 (ArCH), 126.9 (ArCH), 119.3 (ArC), 118.5 (ArCH), 79.9 (C), 52.8 (CH2), 37.3 (CH2), 36.4 (CH2), 32.0 (CH2), 30.2 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.4 (CH2), 29.4 (CH2), 29.1 (CH2), 28.5 (CH3), 28.2 (CH2), 23.5 (CH2), 22.8 (CH2), 14.2 (CH3); IR (ATR): νmax 3351, 3308, 2922, 2851, 1683, 1666, 1644, 1582, 1518, 1483, 1443, 1386, 1364, 1347, 1325, 1294, 1247, 1196, 1155, 1070, 1040, 1011, 977, 906, 895, 882, 864, 850, 804, 776, 758, 740, 716, 684 cm−1; HRMS (+ESI): Found m/z 652.3394 [M+Na]+, C34H51N3O6SNa required 652.3391.
tert-Butyl (3-(2-(2-(hexadecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamate (26a)
The titled compound was synthesised from 1-(hexadecylsulfonyl)indoline-2,3-dione 9a (0.21 g, 0.47 mmol) and N-Boc-1,3-propandiamine (85 mg, 0.47 mmol) following general synthetic procedure E. The product was obtained as a yellow solid (0.28 g, 97%); mp 86.7–90.1 °C; 1H NMR (400 MHz, CDCl3): δ 10.50 (s, 1H, NH), 8.45 (d, J = 7.9 Hz, 1H, ArH), 7.77 (d, J = 8.4 Hz, 1H, ArH), 7.68–7.56 (m, 2H, NH, ArH), 7.19–7.11 (m, 1H, ArH), 4.84 (bs, 1H, NH), 3.47 (q, J = 6.5 Hz, 2H, CH2), 3.29–3.11 (m, 4H, CH2), 1.85–1.69 (m, 4H, CH2), 1.44 (s, 9H, CH3), 1.40–1.16 (m, 26H, CH2), 0.87 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (150 MHz, CDCl3): δ 191.7 (CO), 163.1 (CO), 156.9 (CO), 142.1 (ArC), 136.7 (ArCH), 135.3 (ArCH), 122.6 (ArCH), 118.8 (ArC), 118.0 (ArCH), 79.9 (C), 52.7 (CH2), 37.3 (CH2), 36.4 (CH2), 32.1 (CH2), 30.2 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.5 (CH3), 28.2 (CH2), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3362, 3253, 2916, 2849, 2113, 1684, 1637, 1602, 1571, 1523, 1467, 1391, 1335, 1247, 1220, 1152, 1077, 1057, 1006, 918, 818, 753, 671 cm−1; HRMS (+ESI): Found m/z 632.3703 [M+Na]+, C32H55N3O6SNa required 632.3704.
tert-Butyl (3-(2-(5-bromo-2-(hexadecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamate (26b)
The titled compound was synthesised from 5-bromo-1-(hexadecylsulfonyl)indoline-2,3-dione 9b (0.33 g, 0.64 mmol) and N-Boc-1,3-propandiamine (0.12 g, 0.65 mmol) following general synthetic procedure E. The product was obtained as a yellow solid (0.41 g, 91%); mp 81.7–83.1 °C; 1H NMR (400 MHz, CDCl3): δ 10.38 (s, 1H, NH), 8.66 (s, 1H, ArH), 7.76 (bs, 1H, NH), 7.73–7.64 (m, 2H, NH, ArH), 4.80 (bs, 1H, NH), 3.46 (q, J = 6.5 Hz, 2H, CH2), 3.28–3.19 (m, 2H, CH2), 3.17–3.10 (m, 2H, CH2), 1.83–1.70 (m, 4H, CH2), 1.45 (s, 9H, CH3), 1.41–1.17 (m, 26H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 190.4 (CO), 162.2 (CO), 157.0 (CO), 141.0 (ArC), 139.3 (ArCH), 137.4 (ArCH), 120.4 (ArC), 119.8 (ArCH), 115.2 (ArC), 80.0 (C), 52.9 (CH2), 37.2 (CH2), 36.4 (CH2), 32.1 (CH2), 30.2 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.5 (CH3), 28.2 (CH2), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3370, 3184, 3113, 2918, 2850, 1685, 1635, 1519, 1479, 1389, 1335, 1248, 1145, 1010, 918, 826. 782, 709 cm−1; HRMS (+ESI): Found m/z 710.2810 [M+Na]+, C32H54BrN3O6SNa required 710.2809.
tert-Butyl (3-(2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)propyl)carbamate (26c)
The titled compound was synthesised from 1-(hexadecylsulfonyl)-5-phenylindoline-2,3-dione 9c (0.26 g, 0.51 mmol) and N-Boc-1,3-propandiamine (91 mg, 0.51 mmol) following general synthetic procedure E. The product was obtained as a yellow solid (0.32 g, 93%); mp 121.6–128.3 °C; 1H NMR (400 MHz, CDCl3): δ 10.48 (s, 1H, NH), 8.75 (s, 1H, ArH), 7.89–7.80 (m, 2H, ArH), 7.70 (bs, 1H, NH), 7.60–7.53 (m, 2H, ArH), 7.48–7.41 (m, 2H, ArH), 7.39–7.34 (m, 1H, ArH), 4.80 (bs, 1H, NH), 3.48 (q, J = 6.4 Hz, 2H, CH2), 3.29–3.14 (m, 4H, CH2), 1.87–1.70 (m, 4H, CH2), 1.44 (s, 9H, CH3), 1.43–1.17 (m, 26H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 191.5 (CO), 162.9 (CO), 156.9 (CO), 141.1 (ArC), 139.1 (ArC), 135.7 (ArC), 135.1 (ArCH), 133.7 (ArCH), 129.2 (ArCH), 127.9 (ArCH), 126.9 (ArCH), 119.3 (ArC), 118.5 (ArCH), 79.9 (C), 52.8 (CH2), 37.3 (CH2), 36.4 (CH2), 32.0 (CH2), 30.2 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.5 (CH3), 28.3 (CH2), 23.6 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3350, 3306, 2918, 2848, 2288, 2110, 1681, 1644, 1518, 1482, 1383, 1347, 1247, 1154, 1069, 1011, 975, 906, 864, 757, 684 cm−1; HRMS (+ESI): Found m/z 708.4018 [M+Na]+, C38H59N3O6SNa required 708.4017.
General synthetic procedure F for aminoglyoxamides
4 M HCl in dioxane (3 mL) was added to a stirring solution of Boc-protected glyoxamide (1.0 equivalent) in dichloromethane (10 mL) at 0 °C. The reaction mixture was stirred at room temperature for 6 h. After the completion of the reaction, the reaction solvent was removed under reduced pressure, and the residue was washed three times with diethyl ether and freeze-dried to afford the product.
N-(3-Aminopropyl)-2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamide hydrochloride (27d)
The titled compound was synthesised from tert-butyl (3-(2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamido)propyl)carbamate 24d (0.43 g, 0.67 mmol) following general synthetic procedure F. The product was obtained as a yellow solid (0.34 g, 88%); mp 165.1–165.4 °C; 1H NMR (400 MHz, DMSO-d6): δ 10.22 (s, 1H, NH), 9.07 (t, J = 5.9 Hz, 1H, NH), 8.12–8.04 (m, 2H, ArH), 8.00 (bs, 3H, NH), 7.82 (d, J = 8.3 Hz, 2H, ArH), 7.78–7.70 (m, 4H, ArH), 7.66 (d, J = 9.3 Hz, 1H, ArH), 7.50 (t, J = 7.7 Hz, 2H, ArH), 7.39 (t, J = 7.2 Hz, 1H, ArH), 3.39–3.31 (m, 2H, CH2), 3.31–3.22 (m, 2H, CH2), 2.93–2.81 (m, 2H, CH2), 1.92–1.79 (m, 2H, CH2), 1.74–1.62 (m, 2H, CH2), 1.40–1.13 (m, 10H, CH2), 0.82 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.4 (CO), 163.8 (CO), 139.6 (ArC), 139.4 (ArC), 138.3 (ArC), 137.1 (ArC), 135.0 (ArC), 133.0 (ArCH), 130.3 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.5 (ArCH), 126.9 (ArCH), 126.6 (ArCH), 124.5 (ArC), 121.7 (ArCH), 51.5 (CH2), 36.7 (CH2), 35.9 (CH2), 31.1 (CH2), 28.4 (CH2), 28.3 (CH2), 27.3 (CH2), 26.9 (CH2), 22.9 (CH2), 22.0 (CH2), 13.9 (CH3); IR (ATR): νmax 2924, 2854, 1719, 1648, 1497, 1396, 1332, 1255, 1195, 1139, 1051, 1006, 916, 826, 763, 717, 695, 673 cm−1; HRMS (+ESI): Found m/z 550.2736 [M+H]+, C31H40N3O4S required 550.2734.
N-(3-Aminopropyl)-2-(2-(dodecylsulfonamido)phenyl)-2-oxoacetamide hydrochloride (28a)
The titled compound was synthesised from tert-butyl (3-(2-(2-(dodecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamate 25a (0.38 g, 0.68 mmol) following general synthetic procedure F. The product was obtained as a pale yellow solid (0.29 g, 86%); mp 160.0–162.3 °C; 1H NMR (400 MHz, DMSO-d6): δ 10.27 (s, 1H, NH), 9.03 (t, J = 5.9 Hz, 1H, NH), 8.03 (bs, 3H, NH), 7.78 (dd, J = 7.9, 1.2 Hz, 1H, ArH), 7.73–7.67 (m, 1H, ArH), 7.56 (d, J = 8.0 Hz, 1H, ArH), 7.34–7.26 (m, 1H, ArH), 3.37–3.21 (m, 4H, CH2), 2.91–2.77 (m, 2H, CH2), 1.90–1.78 (m, 2H, CH2), 1.71–1.59 (m, 2H, CH2), 1.38–1.13 (m, 18H, CH2), 0.85 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 193.3 (CO), 164.3 (CO), 139.6 (ArC), 135.5 (ArCH), 133.2 (ArCH), 123.6 (ArCH), 122.5 (ArC), 120.2 (ArCH), 51.4 (CH2), 36.7 (CH2), 35.8 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 26.9 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3336, 3238, 2916, 2849, 2765, 2674, 2635, 2522, 2299, 2033, 1635, 1602, 1568, 1523, 1490, 1445, 1399, 1337, 1259, 1211, 1153, 1080, 958, 913, 849, 816, 753, 670 cm−1; HRMS (+ESI): Found m/z 454.2733 [M+H]+, C23H40N3O4S required 454.2734.
N-(3-Aminopropyl)-2-(5-bromo-2-(dodecylsulfonamido)phenyl)-2-oxoacetamide hydrochloride (28b)
The titled compound was synthesised from tert-butyl (3-(2-(5-bromo-2-(dodecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamate 25b (0.33 g, 0.52 mmol) following general synthetic procedure F. The product was obtained as a white solid (0.20 g, 66%); mp 73.7–74.2 °C; 1H NMR (400 MHz, DMSO-d6): δ 10.07 (s, 1H, NH), 8.98 (t, J = 5.9 Hz, 1H, NH), 7.92 (bs, 3H, NH), 7.90–7.82 (m, 2H, ArH), 7.46 (d, J = 8.6 Hz, 1H, ArH), 3.36–3.25 (m, 2H, CH2), 3.21–3.13 (m, 2H, CH2), 2.92–2.78 (m, 2H, CH2), 1.87–1.76 (m, 2H, CH2), 1.69–1.57 (m, 2H, CH2), 1.37–1.13 (m, 18H, CH2), 0.85 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 190.3 (CO), 162.9 (CO), 137.6 (ArC), 137.0 (ArCH), 134.2 (ArCH), 127.7 (ArC), 124.3 (ArCH), 116.2 (ArC), 51.4 (CH2), 36.7 (CH2), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 26.8 (CH2), 22.8 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3375, 2915, 2849, 2048, 1667, 1641, 1565, 1516, 1485, 1437, 1389, 1339, 1323, 1293, 1274, 1256, 1194, 1141, 1075, 1021, 992, 967, 923, 872, 819, 765, 722, 662 cm−1; HRMS (+ESI): Found m/z 532.1841 [M+H]+, C23H39BrN3O4S required 532.1839.
N-(3-Aminopropyl)-2-(4-(dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide hydrochloride (28c)
The titled compound was synthesised from tert-butyl (3-(2-(4-(dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)propyl)carbamate 25c (0.35 g, 0.55 mmol) following general synthetic procedure F. The product was obtained as a yellow solid (0.19 g, 60%); mp 118.0 °C; 1H NMR (600 MHz, DMSO-d6): δ 10.19 (s, 1H, NH), 9.03 (t, J = 6.0 Hz, 1H, NH), 8.03–7.98 (m, 2H, ArH), 7.91 (bs, 3H, NH), 7.66–7.60 (m, 3H, ArH), 7.53–7.48 (m, 2H, ArH), 7.43–7.39 (m, 1H, ArH), 3.40–3.28 (m, 2H, CH2), 3.27–3.22 (m, 2H, CH2), 2.90–2.82 (m, 2H, CH2), 1.88–1.80 (m, 2H, CH2), 1.71–1.62 (m, 2H, CH2), 1.37–1.29 (m, 2H, CH2), 1.28–1.12 (m, 16H, CH2), 0.84 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.4 (CO), 163.8 (CO), 138.3 (ArC), 138.2 (ArC), 135.6 (ArC), 133.2 (ArCH), 130.5 (ArCH), 129.2 (ArCH), 127.9 (ArCH), 126.4 (ArCH), 124.6 (ArC), 121.7 (ArCH), 51.4 (CH2), 36.7 (CH2), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 26.9 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3351, 3307, 2921, 2851, 1682, 1666, 1644, 1582, 1518, 1483, 1444, 1386, 1364, 1347, 1326, 1294, 1247, 1197, 1155, 1138, 1070, 1011, 978, 907, 895, 881, 864, 851, 830, 805, 758, 741, 716, 684 cm−1; HRMS (+ESI): Found m/z 530.3048 [M+H]+, C29H44N3O4S required 530.3047.
N-(3-Aminopropyl)-2-(2-(hexadecylsulfonamido)phenyl)-2-oxoacetamide hydrochloride (29a)
The titled compound was synthesised from tert-butyl (3-(2-(2-(hexadecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamate 26a (0.24 g, 0.39 mmol) following general synthetic procedure F. The product was obtained as a pale yellow solid (0.19 g, 86%); mp 157.3–160.0 °C; 1H NMR (400 MHz, DMSO-d6): δ 10.26 (s, 1H, NH), 9.02 (t, J = 5.9 Hz, 1H, NH), 7.95 (bs, 3H, NH), 7.77 (dd, J = 7.9, 1.3 Hz, 1H, ArH), 7.73–7.67 (m, 1H, ArH), 7.55 (d, J = 8.2 Hz, 1H, ArH), 7.30 (d, J = 7.3 Hz, 1H, ArH), 3.31 (q, J = 6.6 Hz, 2H, CH2), 3.28–3.21 (m, 2H, CH2), 2.90–2.79 (m, 2H, CH2), 1.87–1.77 (m, 2H, CH2), 1.70–1.59 (m, 2H, CH2), 1.37–1.13 (m, 26H, CH2), 0.85 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 193.3 (CO), 164.3 (CO), 139.5 (ArC), 135.5 (ArCH), 133.2 (ArCH), 123.7 (ArCH), 122.7 (ArC), 120.3 (ArCH), 51.4 (CH2), 36.7 (CH2), 35.8 (CH2), 31.3 (CH2), 29.1 (CH2), 29.1 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 26.9 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3336, 3237, 2915, 2848, 2780, 2673, 2636, 2521, 2322, 2033, 1634, 1602, 1569, 1524, 1491, 1446, 1399, 1338, 1259, 1211, 1154, 1080, 958, 913, 850, 818, 753, 739, 670 cm−1; HRMS (+ESI): Found m/z 510.3361 [M+H]+, C27H48N3O4S required 510.3360.
N-(3-Aminopropyl)-2-(5-bromo-2-(hexadecylsulfonamido)phenyl)-2-oxoacetamide hydrochloride (29b)
The titled compound was synthesised from tert-butyl (3-(2-(5-bromo-2-(hexadecylsulfonamido) phenyl)-2-oxoacetamido)propyl)carbamate 26b (0.36 g, 0.53 mmol) following general synthetic procedure F. The product was obtained as a yellow solid (0.31 g, 95%); mp 81.4–83.9 °C; 1H NMR (400 MHz, DMSO-d6): δ 10.07 (s, 1H, NH), 8.98 (t, J = 6.0 Hz, 1H, NH), 8.13–7.79 (m, 5H, NH, ArH), 7.45 (d, J = 8.7 Hz, 1H, ArH), 3.29 (q, J = 6.8 Hz, 2H, CH2), 3.21–3.13 (m, 2H, CH2), 2.91–2.79 (m, 2H, CH2), 1.87–1.76 (m, 2H, CH2), 1.68–1.57 (m, 2H, CH2), 1.36–1.13 (m, 26H, CH2), 0.85 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 190.3 (CO), 162.9 (CO), 137.6 (ArC), 137.0 (ArCH), 134.2 (ArCH), 127.7 (ArC), 124.3 (ArCH), 116.2 (ArC), 51.4 (CH2), 36.7 (CH2), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.3 (CH2), 27.3 (CH2), 26.8 (CH2), 22.8 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3379, 3174, 2916, 2848, 2342, 2051, 1920, 1642, 1525, 1479, 1384, 1337, 1197, 1146, 1074, 1017, 908, 823, 718 cm−1; HRMS (+ESI): Found m/z 588.2466 [M+H]+, C27H47BrN3O4S required 588.2465.
N-(3-Aminopropyl)-2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide hydrochloride (29c)
The titled compound was synthesised from tert-butyl (3-(2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)propyl)carbamate 26c (0.30 g, 0.43 mmol) following general synthetic procedure F. The product was obtained as a yellow solid (0.24 g, 89%); mp 81.9–84.1 °C; 1H NMR (400 MHz, DMSO-d6): δ 10.20 (s, 1H, NH), 9.05 (t, J = 6.0 Hz, 1H, NH), 8.08–7.90 (m, 5H, NH, ArH), 7.66–7.60 (m, 3H, ArH), 7.50 (t, J = 7.8 Hz, 2H, ArH), 7.43–7.37 (m, 1H, ArH), 3.40–3.29 (m, 2H, CH2), 3.29–3.21 (m, 2H, CH2), 2.92–2.80 (m, 2H, CH2), 1.89–1.79 (m, 2H, CH2), 1.72–1.61 (m, 2H, CH2), 1.39–1.12 (m, 26H, CH2), 0.84 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 192.4 (CO), 163.8 (CO), 138.3 (ArC), 138.2 (ArC), 135.6 (ArC), 133.1 (ArCH), 130.5 (ArCH), 129.2 (ArCH), 127.9 (ArCH), 126.4 (ArCH), 124.4 (ArC), 121.7 (ArCH), 51.5 (CH2), 36.7 (CH2), 35.9 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 26.9 (CH2), 22.9 (CH2), 22.1 (CH2), 13.9 (CH3); IR (ATR): νmax 3346, 3184, 2917, 2849, 2648, 2529, 2342, 2053, 1631, 1525, 1484, 1392, 1342, 1265, 1199, 1140, 1078, 1022, 922, 865, 840, 761, 682 cm−1; HRMS (+ESI): Found m/z 586.3673 [M+H]+, C33H52N3O4S required 586.3673.
General synthetic procedure G for Boc-protected guanidine glyoxamides
A solution of triethylamine (2.5 equivalents) in acetonitrile (3 mL) was added dropwise to a stirring solution of aminoglyoxamide (1.0 equivalent) and N,N′-di-Boc-1H-pyrazole-1-carboxamidine (1.3 equivalents) in acetonitrile (10 mL) at 0 °C under an atmosphere of nitrogen. The resulting reaction mixture was stirred at room temperature for 24 h. After the completion of the reaction, the reaction solvent was removed under reduced pressure. The resulting residue was purified by flash column chromatography on silica, using ethyl acetate/n-hexane (1:4) as an eluent to afford the product.
(E)-1-tert-Butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide (30d)
The titled compound was synthesised from N-(3-aminopropyl)-2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamide hydrochloride 27d (0.29 g, 0.49 mmol), N,N′-di-Boc-1H-pyrazole-1-carboxamidine (0.20 g, 0.64 mmol) and triethylamine (0.17 mL, 1.23 mmol) following general synthetic procedure G. The product was obtained as a yellow solid (0.27 g, 70%); mp 81.5–81.8 °C; 1H NMR (400 MHz, CDCl3): δ 11.47 (s, 1H, NH), 10.64 (s, 1H, NH), 8.68–8.60 (m, 2H, NH, ArH), 8.54 (bs, 1H, NH), 7.91–7.85 (m, 2H, ArH), 7.71–7.60 (m, 6H, ArH), 7.50–7.43 (m, 2H, ArH), 7.40–7.34 (m, 1H, ArH), 3.57 (q, J = 6.3 Hz, 2H, CH2), 3.47 (q, J = 6.3 Hz, 2H, CH2), 3.22–3.14 (m, 2H, CH2), 1.87–1.77 (m, 4H, CH2), 1.50 (s, 9H, CH3), 1.38 (s, 9H, CH3), 1.43–1.17 (m, 10H, CH2), 0.86 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 192.5 (CO), 163.7 (CO), 163.0 (CN), 157.4 (CO), 153.3 (CO), 141.2 (ArC), 140.8 (ArC), 140.6 (ArC), 138.0 (ArC), 135.1 (ArC), 134.8 (ArCH), 133.4 (ArCH), 129.0 (ArCH), 127.9 (ArCH), 127.7 (ArCH), 127.3 (ArCH), 127.2 (ArCH), 119.0 (ArC), 118.4 (ArCH), 83.8 (C), 79.8 (C), 52.8 (CH2), 37.3 (CH2), 35.9 (CH2), 31.8 (CH2), 30.1 (CH2), 29.1 (CH2), 29.0 (CH2), 28.4 (CH2), 28.3 (CH3), 28.2 (CH3), 23.6 (CH2), 22.7 (CH2), 14.2 (CH3); IR (ATR): νmax 3322, 2929, 1720, 1638, 1572, 1482, 1408, 1367, 1328, 1285, 1254, 1228, 1194, 1130, 1051, 1026, 1007, 979, 907, 857, 828, 808, 764, 697, 673 cm−1; HRMS (+ESI): Found m/z 792.4009 [M+H]+, C42H58N5O8S required 792.4001.
(E)-1-tert-Butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(2-(dodecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide (31a)
The titled compound was synthesised from N-(3-aminopropyl)-2-(2-(dodecylsulfonamido)phenyl)-2-oxoacetamide hydrochloride 28a (0.25 g, 0.50 mmol), N,N′-di-Boc-1H-pyrazole-1-carboxamidine (0.19 g, 0.63 mmol) and triethylamine (0.18 mL, 1.26 mmol) following general synthetic procedure G. The product was obtained as a yellow oil (0.31 g, 87%); 1H NMR (600 MHz, CDCl3): δ 11.46 (s, 1H, NH), 10.62 (s, 1H, NH), 8.65 (bs, 1H, NH), 8.55 (t, J = 6.4 Hz, 1H, NH), 8.24 (dd, J = 8.1, 1.5 Hz, 1H, ArH), 7.77 (dd, J = 8.4, 0.7 Hz, 1H, ArH), 8.60–8.56 (m, 1H, ArH), 7.16–7.12 (m, 1H, ArH), 3.67–3.56 (m, 2H, CH2), 3.46 (q, J = 6.3 Hz, 2H, CH2), 3.16–3.10 (m, 2H, CH2), 1.86–1.75 (m, 4H, CH2), 1.50 (s, 9H, CH3), 1.37 (s, 9H, CH3), 1.40–1.19 (m, 18H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, CDCl3): δ 192.8 (CO), 164.0 (CO), 162.3 (CN), 157.0 (CO), 153.2 (CO), 142.0 (ArC), 136.4 (ArCH), 135.1 (ArCH), 122.6 (ArCH), 118.8 (ArC), 118.0 (ArCH), 84.2 (C), 80.5 (C), 52.7 (CH2), 37.9 (CH2), 35.8 (CH2), 32.0 (CH2), 29.9 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.4 (CH2), 29.4 (CH2), 29.1 (CH2), 28.3 (CH2), 28.2 (CH3), 28.2 (CH3), 23.5 (CH2), 22.8 (CH2), 14.2 (CH3); IR (ATR): νmax 3327, 3194, 2926, 2855, 1722, 1640, 1574, 1491, 1452, 1410, 1328, 1283, 1219, 1131, 1050, 978, 913, 753, 671 cm−1; HRMS (+ESI): Found m/z 696.4000 [M+H]+, C34H58N5O8S required 696.4001.
(E)-1-tert-Butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(5-bromo-2-(dodecylsulfonamido) phenyl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide (31b)
The titled compound was synthesised from N-(3-aminopropyl)-2-(5-bromo-2-(dodecylsulfonamido) phenyl)-2-oxoacetamide hydrochloride 28b (0.17 g, 0.29 mmol), N,N′-di-Boc-1H-pyrazole-1-carboxamidine (0.13 g, 0.43 mmol) and triethylamine (0.10 mL, 0.73 mmol) following general synthetic procedure G. The product was obtained as a yellow oil (0.12 g, 55%); 1H NMR (400 MHz, CDCl3): δ 11.46 (s, 1H, NH), 10.51 (s, 1H, NH), 8.68 (t, J = 6.4 Hz, 1H, NH), 8.52 (t, J = 6.4 Hz, 1H, NH), 8.45 (d, J = 2.1 Hz, 1H, ArH), 7.71–7.64 (m, 2H, ArH), 3.54 (q, J = 6.4 Hz, 2H, CH2), 3.44 (q, J = 6.2 Hz, 2H, CH2), 3.15–3.07 (m, 2H, CH2), 1.83–1.72 (m, 4H, CH2), 1.49 (s, 9H, CH3), 1.37 (s, 9H, CH3), 1.41–1.17 (m, 18H, CH2), 0.86 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 191.4 (CO), 163.0 (CO), 157.4 (CO), 156.3 (CN), 153.3 (CO), 141.1 (ArC), 139.1 (ArCH), 137.3 (ArCH), 120.1 (ArC), 119.6 (ArCH), 115.1 (ArC), 83.8 (C), 79.8 (C), 52.9 (CH2), 37.2 (CH2), 35.9 (CH2), 32.0 (CH2), 30.0 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.4 (CH2), 29.3 (CH2), 29.1 (CH2), 28.2 (CH3), 28.2 (CH2), 28.2 (CH3), 23.5 (CH2), 22.8 (CH2), 14.2 (CH3); IR (ATR): νmax 3327, 2925, 2854, 2323, 1718, 1637, 1617, 1560, 1541, 1522, 1476, 1457, 1411, 1390, 1367, 1327, 1285, 1253, 1227, 1153, 1132, 1050, 1026, 979, 906, 808, 768, 712, 653 cm−1; HRMS (+ESI): Found m/z 774.3108 [M+H]+, C34H57BrN5O8S required 774.3106.
(E)-1-tert-Butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(4-(dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide (31c)
The titled compound was synthesised from N-(3-aminopropyl)-2-(4-(dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide hydrochloride 28c (0.15 g, 0.27 mmol), N,N′-di-Boc-1H-pyrazole-1-carboxamidine (0.17 g, 0.55 mmol) and triethylamine (92 μL, 0.66 mmol) following general synthetic procedure G. The product was obtained as a yellow solid (0.17 g, 83%); mp 63.6–63.8 °C; 1H NMR (600 MHz, CDCl3): δ 11.47 (s, 1H, NH), 10.62 (s, 1H, NH), 8.63 (t, J = 6.6 Hz, 1H, NH), 8.56 (d, J = 2.2 Hz, 1H, ArH), 8.52 (t, J = 6.2 Hz, 1H, NH), 7.86 (d, J = 8.7 Hz, 1H, ArH), 7.82 (dd, J = 8.7, 2.2 Hz, 1H, ArH), 7.58–7.55 (m, 2H, ArH), 7.46–7.42 (m, 2H, ArH), 7.38–7.34 (m, 1H, ArH), 3.55 (q, J = 6.4 Hz, 2H, CH2), 3.46 (q, J = 6.3 Hz, 2H, CH2), 3.19–3.15 (m, 2H, CH2), 1.84–1.77 (m, 4H, CH2), 1.49 (s, 9H, CH3), 1.37 (s, 9H, CH3), 1.41–1.34 (m, 2H, CH2), 1.31–1.19 (m, 16H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, CDCl3): δ 192.6 (CO), 163.7 (CO), 163.1 (CN), 157.4 (CO), 153.3 (CO), 141.2 (ArC), 139.1 (ArC), 135.6 (ArC), 135.0 (ArCH), 133.5 (ArCH), 129.1 (ArCH), 127.9 (ArCH), 126.9 (ArCH), 118.9 (ArC), 118.3 (ArCH), 83.7 (C), 79.8 (C), 52.8 (CH2), 37.2 (CH2), 35.8 (CH2), 32.0 (CH2), 30.1 (CH2), 29.7 (CH2), 29.7 (CH2), 29.6 (CH2), 29.4 (CH2), 29.4 (CH2), 29.1 (CH2), 28.3 (CH3), 28.2 (CH2), 28.2 (CH3), 23.6 (CH2), 22.8 (CH2), 14.2 (CH3); IR (ATR): νmax 3326, 2926, 2854, 1719, 1636, 1615, 1570, 1507, 1483, 1411, 1366, 1326, 1285, 1253, 1228, 1130, 1051, 1025, 979, 907, 854, 807, 760, 698, 681 cm−1; HRMS (+ESI): Found m/z 772.4320 [M+H]+, C40H62N5O8S required 772.4314.
(E)-1-tert-Butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(2-(hexadecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide (32a)
The titled compound was synthesised from N-(3-aminopropyl)-2-(2-(hexadecylsulfonamido)phenyl)-2-oxoacetamide hydrochloride 29a (0.16 g, 0.28 mmol), N,N′-di-Boc-1H-pyrazole-1-carboxamidine (0.11 g, 0.36 mmol) and triethylamine (0.10 mL, 0.72 mmol) following general synthetic procedure G. The product was obtained as a yellow oil (0.14 g, 67%); 1H NMR (400 MHz, CDCl3): δ 11.46 (s, 1H, NH), 10.63 (s, 1H, NH), 8.58 (t, J = 6.4 Hz, 1H, NH), 8.52 (t, J = 6.3 Hz, 1H, NH), 8.26 (dd, J = 8.0, 1.5 Hz, 1H, ArH), 7.77 (dd, J = 8.4, 0.7 Hz, 1H, ArH), 7.62–7.55 (m, 1H, ArH), 7.17–7.11 (m, 1H, ArH), 3.55 (q, J = 6.6 Hz, 2H, CH2), 3.44 (q, J = 6.1 Hz, 2H, CH2), 3.17–3.10 (m, 2H, CH2), 1.84–1.73 (m, 4H, CH2), 1.49 (s, 9H, CH3), 1.35 (s, 9H, CH3), 1.39–1.18 (m, 26H, CH2), 0.87 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 192.8 (CO), 163.9 (CO), 163.1 (CN), 157.4 (CO), 153.4 (CO), 142.2 (ArC), 136.5 (ArCH), 135.2 (ArCH), 122.5 (ArCH), 118.5 (ArC), 117.8 (ArCH), 83.7 (C), 79.7 (C), 52.7 (CH2), 37.2 (CH2), 35.8 (CH2), 32.1 (CH2), 30.1 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.3 (CH2), 28.2 (CH3), 28.2 (CH3), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3327, 3195, 2923, 2853, 2340, 1723, 1640, 1614, 1574, 1491, 1452, 1409, 1330, 1283, 1221, 1133, 1050, 914, 753, 672 cm−1; HRMS (+ESI): Found m/z 752.4626 [M+H]+, C38H66N5O8S required 752.4627.
(E)-1-tert-Butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(5-bromo-2-(hexadecylsulfonamido) phenyl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide (32b)
The titled compound was synthesised from N-(3-aminopropyl)-2-(5-bromo-2-(hexadecylsulfonamido) phenyl)-2-oxoacetamide hydrochloride 29b (0.24 g, 0.38 mmol), N,N′-di-Boc-1H-pyrazole-1-carboxamidine (0.14 g, 0.46 mmol) and triethylamine (0.13 mL, 0.95 mmol) following general synthetic procedure G. The product was obtained as a yellow oil (0.16 g, 51%); 1H NMR (600 MHz, CDCl3): δ 11.47 (s, 1H, NH), 10.52 (s, 1H, NH), 8.69 (t, J = 6.4 Hz, 1H, NH), 8.53 (t, J = 6.4 Hz, 1H, NH), 8.46 (d, J = 2.2 Hz, 1H, ArH), 7.71–7.65 (m, 2H, ArH), 3.54 (q, J = 6.4 Hz, 2H, CH2), 3.44 (q, J = 6.2 Hz, 2H, CH2), 3.15–3.08 (m, 2H, CH2), 1.82–1.73 (m, 4H, CH2), 1.50 (s, 9H, CH3), 1.37 (s, 9H, CH3), 1.40–1.32 (m, 2H, CH2), 1.31–1.19 (m, 24H, CH2), 0.87 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, CDCl3): δ 191.4 (CO), 163.0 (CO), 163.0 (CN), 157.5 (CO), 153.3 (CO), 141.1 (ArC), 139.2 (ArCH), 137.3 (ArCH), 120.0 (ArC), 119.6 (ArCH), 115.1 (ArC), 83.8 (C), 79.8 (C), 52.9 (CH2), 37.2 (CH2), 35.9 (CH2), 32.1 (CH2), 30.0 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.3 (CH3), 28.2 (CH2), 28.2 (CH3), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3326, 2924, 2854, 2097, 1721, 1642, 1569, 1477, 1413, 1327, 1285, 1227, 1131, 1050, 979, 906, 808, 767, 713 cm−1; HRMS (+ESI): Found m/z 830.3731 [M+H]+, C36H65BrN5O8S required 830.3732.
(E)-1-tert-Butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide (32c)
The titled compound was synthesised from N-(3-aminopropyl)-2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide hydrochloride 29c (0.20 g, 0.31 mmol), N,N′-di-Boc-1H-pyrazole-1-carboxamidine (0.12 g, 0.39 mmol) and triethylamine (0.10 mL, 0.79 mmol) following general synthetic procedure G. The product was obtained as a yellow oil (0.17 g, 66%); 1H NMR (600 MHz, CDCl3): δ 11.47 (s, 1H, NH), 10.62 (s, 1H, NH), 8.62 (t, J = 6.3 Hz, 1H, NH), 8.60 (bs, 1H, NH), 8.55 (d, J = 2.1 Hz, 1H, ArH), 7.86 (d, J = 8.7 Hz, 1H, ArH), 7.82 (dd, J = 8.7, 2.2 Hz, 1H, ArH), 7.58–7.55 (m, 2H, ArH), 7.46–7.42 (m, 2H, ArH), 7.38–7.34 (m, 1H, ArH), 3.64–3.54 (m, 2H, CH2), 3.46 (q, J = 6.4 Hz, 2H, CH2), 3.19–3.15 (m, 2H, CH2), 1.85–1.78 (m, 4H, CH2), 1.50 (s, 9H, CH3), 1.38 (s, 9H, CH3), 1.41–1.33 (m, 2H, CH2), 1.32–1.20 (m, 24H, CH2), 0.87 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, CDCl3): δ 192.6 (CO), 163.8 (CO), 163.8 (CN), 157.2 (CO), 153.3 (CO), 141.1 (ArC), 139.1 (ArC), 135.7 (ArC), 134.9 (ArCH), 133.5 (ArCH), 129.1 (ArCH), 127.9 (ArCH), 127.0 (ArCH), 119.2 (ArC), 118.4 (ArCH), 84.0 (C), 80.3 (C), 52.8 (CH2), 37.6 (CH2), 35.9 (CH2), 32.1 (CH2), 30.0 (CH2), 29.8 (CH2), 29.8 (CH2), 29.8 (CH2), 29.7 (CH2), 29.6 (CH2), 29.5 (CH2), 29.4 (CH2), 29.1 (CH2), 28.3 (CH2), 28.2 (CH3), 28.2 (CH3), 23.5 (CH2), 22.8 (CH2), 14.3 (CH3); IR (ATR): νmax 3326, 3194, 2922, 2852, 2321, 2090, 1720, 1638, 1575, 1482, 1410, 1326, 1283, 1130, 1050, 978, 907, 854, 806, 759, 697 cm−1; HRMS (+ESI): Found m/z 828.4939 [M+H]+, C44H70N5O8S required 828.4940.
General synthetic procedure H for guanidinium hydrochloride salts
Trifluoroacetic acid (2 mL) was added to a stirring solution of Boc-protected guanidine glyoxamide (1.0 equivalent) in dichloromethane (2 mL). The resulting reaction mixture was stirred at room temperature for 3 h. After the completion of the reaction, the reaction solvent was removed under reduced pressure and the residue was washed thrice with diethyl ether. To the residue redissolved in dichloromethane (2 mL) was added 4 M HCl in dioxane (2 mL), and the resulting reaction mixture was stirred at room temperature for 30 min. After the completion of the reaction, the reaction solvent was removed under reduced pressure. The residue was washed thrice with diethyl ether and freeze-dried to afford the product.
N-(3-Guanidinopropyl)-2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamide hydrochloride (33d)
The titled compound was synthesised from (E)-1-tert-butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(4-(octylsulfonamido)-[1,1′:4′,1″-terphenyl]-3-yl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide 30d (0.15 g, 0.19 mmol) following general synthetic procedure H. The product was obtained as a yellow solid (58 mg, 48%); mp 49.6–50.1 °C; 1H NMR (600 MHz, DMSO-d6): δ 10.22 (s, 1H, NH), 9.03 (t, J = 5.8 Hz, 1H, NH), 8.12–8.02 (m, 2H, ArH), 7.81 (d, J = 8.5 Hz, 2H, ArH), 7.76–7.69 (m, 5H, NH, ArH), 7.66 (d, J = 8.7 Hz, 1H, ArH), 7.57–6.83 (m, 7H, NH, ArH), 3.32 (q, J = 6.7 Hz, 2H, CH2), 3.29–3.25 (m, 2H, CH2), 3.21 (q, J = 6.7 Hz, 2H, CH2), 1.79–1.72 (m, 2H, CH2), 1.72–1.65 (m, 2H, CH2), 1.38–1.31 (m, 2H, CH2), 1.27–1.16 (m, 8H, CH2), 0.82 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.5 (CO), 163.8 (CO), 156.4 (CN), 139.6 (ArC), 139.4 (ArC), 138.4 (ArC), 137.2 (ArC), 135.0 (ArC), 133.0 (ArCH), 130.4 (ArCH), 129.0 (ArCH), 127.7 (ArCH), 127.4 (ArCH), 126.9 (ArCH), 126.6 (ArCH), 124.4 (ArC), 121.7 (ArCH), 51.5 (CH2), 38.4 (CH2), 36.1 (CH2), 31.1 (CH2), 28.4 (CH2), 28.4 (CH2), 28.3 (CH2), 27.3 (CH2), 22.9 (CH2), 22.0 (CH2), 13.9 (CH3); IR (ATR): νmax 3808, 3273, 3162, 2924, 2854, 2359, 1650, 1529, 1497, 1482, 1400, 1333, 1272, 1196, 1142, 1076, 1006, 917, 827, 764, 719, 695, 674 cm−1; HRMS (+ESI): Found m/z 592.2956 [M+H]+, C32H42N5O4S required 592.2952.
2-(2-(Dodecylsulfonamido)phenyl)-N-(3-guanidinopropyl)-2-oxoacetamide hydrochloride (34a)
The titled compound was synthesised from (E)-1-tert-butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(2-(dodecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide 31a (0.11 g, 0.16 mmol) following general synthetic procedure H. The product was obtained as a pale yellow solid (71 g, 82%); mp 70.0–72.8 °C; 1H NMR (600 MHz, DMSO-d6): δ 10.27 (s, 1H, NH), 8.98 (t, J = 5.7 Hz, 1H, NH), 7.78 (dd, J = 7.9, 1.5 Hz, 1H, ArH), 7.74 (t, J = 5.8 Hz, 1H, NH), 7.72–7.68 (m, 1H, ArH), 7.63–6.78 (m, 6H, NH, ArH), 3.31–3.23 (m, 4H, CH2), 3.18 (q, J = 6.7 Hz, 2H, CH2), 1.76–1.69 (m, 2H, CH2), 1.68–1.61 (m, 2H, CH2), 1.35–1.15 (m, 18H, CH2), 0.85 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 193.4 (CO), 164.3 (CO), 157.0 (CN), 139.6 (ArC), 135.5 (ArCH), 133.2 (ArCH), 123.6 (ArCH), 122.4 (ArC), 120.1 (ArCH), 51.4 (CH2), 38.4 (CH2), 36.0 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 28.3 (CH2), 27.2 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3329, 3249, 3157, 2917, 2850, 1635, 1572, 1528, 1492, 1451, 1398, 1337, 1224, 1149, 1065, 912, 822, 750, 673 cm−1; HRMS (+ESI): Found m/z 496.2951 [M+H]+, C24H42N5O4S required 496.2952.
2-(5-Bromo-2-(dodecylsulfonamido)phenyl)-N-(3-guanidinopropyl)-2-oxoacetamide hydrochloride (34b)
The titled compound was synthesised from (E)-1-tert-butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(5-bromo-2-(dodecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide 31b (71 mg, 0.092 mmol) following general synthetic procedure H. The product was obtained as a white solid (47 mg, 84%); mp 50.0–50.1 °C; 1H NMR (600 MHz, DMSO-d6): δ 10.08 (s, 1H, NH), 8.94 (bs, 1H, NH), 7.93–7.79 (m, 2H, ArH), 7.70–6.72 (m, 6H, NH, ArH), 3.26 (q, J = 6.8 Hz, 2H, CH2), 3.21–3.14 (m, 4H, CH2), 1.76–1.68 (m, 2H, CH2), 1.67–1.59 (m, 2H, CH2), 1.34–1.15 (m, 18H, CH2), 0.85 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 190.4 (CO), 163.0 (CO), 156.9 (CN), 137.6 (ArC), 137.0 (ArCH), 134.2 (ArCH), 127.6 (ArC), 124.2 (ArCH), 116.2 (ArC), 51.4 (CH2), 38.3 (CH2), 36.1 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 28.3 (CH2), 27.3 (CH2), 22.8 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3171, 2919, 2851, 2360, 1642, 1562, 1530, 1479, 1385, 1337, 1293, 1223, 1198, 1147, 907, 823, 710, 654 cm−1; HRMS (+ESI): Found m/z 574.2061 [M+H]+, C24H41BrN5O4S required 574.2057.
2-(4-(Dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-N-(3-guanidinopropyl)-2-oxoacetamide hydrochloride (34c)
The titled compound was synthesised from (E)-1-tert-butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(4-(dodecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide 31c (82 mg, 0.11 mmol) following general synthetic procedure H. The product was obtained as a yellow solid (41 mg, 63%); mp 73.2–73.5 °C; 1H NMR (600 MHz, DMSO-d6): δ 10.21 (s, 1H, NH), 9.02 (bs, 1H, NH), 8.03–7.98 (m, 2H, ArH), 7.71 (bs, 1H, NH), 7.66–7.60 (m, 3H, ArH), 7.56–6.78 (m, 7H, NH, ArH), 3.30 (q, J = 6.7 Hz, 2H, CH2), 3.28–3.23 (m, 2H, CH2), 2.22–2.15 (m, 2H, CH2), 1.78–1.71 (m, 2H, CH2), 1.70–1.63 (m, 2H, CH2), 1.36–1.30 (m, 2H, CH2), 1.28–1.14 (m, 16H, CH2), 0.84 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 192.6 (CO), 163.8 (CO), 157.0 (CN), 138.3 (ArC), 138.2 (ArC), 135.5 (ArC), 133.2 (ArCH), 130.5 (ArCH), 129.2 (ArCH), 127.9 (ArCH), 126.4 (ArCH), 124.3 (ArC), 121.6 (ArCH), 51.4 (CH2), 38.4 (CH2), 36.1 (CH2), 31.3 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 28.4 (CH2), 27.3 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3365, 3167, 2919, 2850, 2359, 1767, 1736, 1676, 1626, 1582, 1527, 1512, 1487, 1467, 1396, 1376, 1347, 1309, 1266, 1214, 1200, 1175, 1139, 1094, 925, 910, 853, 824, 780, 759, 720, 683, 651 cm−1; HRMS (+ESI): Found m/z 572.3267 [M+H]+, C30H46N5O4S required 572.3265.
N-(3-Guanidinopropyl)-2-(2-(hexadecylsulfonamido)phenyl)-2-oxoacetamide hydrochloride (35a)
The titled compound was synthesised from (E)-1-tert-butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(2-(hexadecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide 32a (61 mg, 0.081 mmol) following general synthetic procedure H. The product was obtained as a white sticky solid (31 mg, 65%); 1H NMR (600 MHz, DMSO-d6): δ 10.27 (s, 1H, NH), 8.97 (t, J = 5.8 Hz, 1H, NH), 7.78 (dd, J = 7.9, 1.5 Hz, 1H, ArH), 7.74–7.68 (m, 2H, NH, ArH), 7.62–6.78 (m, 6H, NH, ArH), 3.31–3.23 (m, 4H, CH2), 3.18 (q, J = 6.7 Hz, 2H, CH2), 1.77–1.70 (m, 2H, CH2), 1.68–1.61 (m, 2H, CH2), 1.36–1.15 (m, 26H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (150 MHz, DMSO-d6): δ 193.4 (CO), 164.3 (CO), 156.9 (CN), 139.6 (ArC), 135.5 (ArCH), 133.2 (ArCH), 123.6 (ArCH), 122.5 (ArC), 120.1 (ArCH), 51.4 (CH2), 38.4 (CH2), 36.0 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 29.0 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 28.3 (CH2), 27.3 (CH2), 22.9 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3343, 3245, 3173, 2915, 2849, 2290, 2106, 1626, 1573, 1543, 1493, 1453, 1398, 1339, 1219, 1150, 1070, 913, 885, 821, 750, 675 cm−1; HRMS (+ESI): Found m/z 552.3578 [M+H]+, C28H50N5O4S required 552.3578.
2-(5-Bromo-2-(hexadecylsulfonamido)phenyl)-N-(3-guanidinopropyl)-2-oxoacetamide hydrochloride (35b)
The titled compound was synthesised from (E)-1-tert-butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(5-bromo-2-(hexadecylsulfonamido)phenyl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide 32b (85 mg, 0.10 mmol) following general synthetic procedure H. The product was obtained as a yellow solid (66 mg, 97%); mp 64.3–66.6 °C; 1H NMR (400 MHz, DMSO-d6): δ 10.08 (s, 1H, NH), 8.95 (t, J = 5.8 Hz, 1H, NH), 7.90–7.82 (m, 2H, ArH), 7.66 (bs, 1H, NH), 7.57–6.81 (m, 5H, NH, ArH), 3.31–3.11 (m, 6H, CH2), 1.79–1.57 (m, 4H, CH2), 1.39–1.13 (m, 26H, CH2), 0.85 (t, J = 7.1 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 190.4 (CO), 163.0 (CO), 156.9 (CN), 137.7 (ArC), 137.0 (ArCH), 134.2 (ArCH), 127.6 (ArC), 124.2 (ArCH), 116.2 (ArC), 51.4 (CH2), 38.3 (CH2), 36.1 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 28.3 (CH2), 27.3 (CH2), 22.8 (CH2), 22.1 (CH2), 14.0 (CH3); IR (ATR): νmax 3342, 3165, 2918, 2850, 2342, 2104, 1642, 1529, 1478, 1385, 1337, 1197, 1148, 905, 822, 718 cm−1; HRMS (+ESI): Found m/z 630.2683 [M+H]+, C28H49BrN5O4S required 630.2683.
N-(3-Guanidinopropyl)-2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamide hydrochloride (35c)
The titled compound was synthesised from (E)-1-tert-butyl-N-(N′-((tert-butyloxidanyl)carbonyl)-N-(3-(2-(4-(hexadecylsulfonamido)-[1,1′-biphenyl]-3-yl)-2-oxoacetamido)propyl)carbamimidoyl)-1-oxidanecarboxamide 32c (75 mg, 0.091 mmol) following general synthetic procedure H. The product was obtained as a yellow solid (42 mg, 71%); mp 74.5–77.1 °C; 1H NMR (400 MHz, DMSO-d6): δ 10.21 (s, 1H, NH), 9.01 (t, J = 5.8 Hz, 1H, NH), 8.03–7.96 (m, 2H, ArH), 7.71 (t, J = 5.9 Hz, 1H, NH), 7.67–7.60 (m, 3H, ArH), 7.55–6.78 (m, 7H, NH, ArH), 3.35–3.16 (m, 6H, CH2), 1.80–1.61 (m, 4H, CH2), 1.38–1.13 (m, 26H, CH2), 0.84 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ 192.6 (CO), 163.8 (CO), 156.9 (CN), 138.3 (ArC), 138.2 (ArC), 135.5 (ArC), 133.2 (ArCH), 130.5 (ArCH), 129.2 (ArCH), 127.9 (ArCH), 126.4 (ArCH), 124.3 (ArC), 121.6 (ArCH), 51.4 (CH2), 38.4 (CH2), 36.1 (CH2), 31.3 (CH2), 29.0 (CH2), 29.0 (CH2), 28.9 (CH2), 28.7 (CH2), 28.7 (CH2), 28.4 (CH2), 27.3 (CH2), 22.8 (CH2), 22.1 (CH2), 13.9 (CH3); IR (ATR): νmax 3365, 3164, 2917, 2849, 2343, 2084, 1625, 1525, 1486, 1395, 1345, 1266, 1200, 1138, 924, 851, 759, 720, 682 cm−1; HRMS (+ESI): Found m/z 628.3891 [M+H]+, C34H54N5O4S required 628.3891.
3.2. Minimum Inhibitory Concentration (MIC)
A MIC assay was conducted to evaluate the antimicrobial activity of the synthesised peptidomimetics. In brief, a single colony of bacteria (S. aureus, E. coli and P. aeruginosa) was cultured in trypticase soy broth (TSB; Oxoid, Basingstoke, U.K.) and Muller Hinton broth (MHB; Oxoid, Basingstoke, UK) at 37 °C with shaking at 120 rpm for 18–24 h. The resulting bacterial culture was washed twice with the media with centrifugation after each wash. The bacterial solution was then diluted with fresh media to a turbidity of OD660 = 0.1 in TSB (equivalent to 108 colony forming unit (CFU)/mL of bacteria), followed by diluting to 106 CFU/mL in media. A total of 100 µL of the bacterial solution was added to wells of a 96-well polystyrene plate (Costar; Sigma-Aldrich, St. Louis, MO, USA) containing 100 µL serially diluted peptidomimetics, with final concentrations ranging from 1–250 µM. The final concentration of DMSO was at or below 1.0%, and wells with 1.0% DMSO was used as the solvent control. Wells with only media were set as blank, while wells with bacteria and media but no compounds were used as a negative control. Plates wrapped with parafilm were then incubated at 37 °C with shaking at 120 rpm for 18–24 h, and the OD value at 660 nm was measured, using a FLUOstar Omega (BMG Labtech) microplate reader. The MIC value of each compound was determined as the lowest concentration that completely inhibited the growth of bacteria. Each experiment was performed in triplicate and was repeated in three independent experiments.
3.3. Biofilm Disruption
Bacterial cultures (S. aureus and E. coli) were cultured in MHB at 37 °C with shaking at 120 rpm for 24 h. The resulting bacterial culture was washed twice with MHB with centrifugation after each wash. The bacterial solution was then diluted with fresh MHB to a turbidity of OD660 = 0.1 in MHB (equivalent to 108 colony forming unit (CFU)/mL of bacteria), followed by diluting to 106 CFU/mL in MHB. A total of 200 µL of the bacterial solution was dispensed to wells in a flat-bottom 96-well plate (Costar). The biofilm was then grown in the plate at 37 °C for 24 h. After incubation, loosely bound cells were washed away with 1× phosphate-buffered saline (PBS, pH 7.4). The biofilms were then supplemented with different concentrations of peptidomimetics dissolved in DMSO and incubated at 37 °C with shaking at 120 rpm for a further 24 h. Biofilms adhered on the plate substratum were quantified, using crystal violet staining as described previously [72,73]. The experiment was performed in triplicate.
3.4. Biofilm Inhibition
S. aureus was cultured in Muller Hinton broth (MHB; Oxoid) at 37 °C with shaking at 120 rpm for 24 h. The resulting bacterial culture was washed twice with MHB with centrifugation after each wash. The bacterial solution was then diluted with fresh MHB to a turbidity of OD660 = 0.1 in MHB (equivalent to 108 colony forming unit (CFU)/mL of bacteria), followed by diluting to 106 CFU/mL in MHB. A total of 100 µL of the bacterial solution was added to wells of a 96-well plate (Costar; Sigma-Aldrich, St. Louis, MO, USA) containing 100 µL serially diluted peptidomimetics. After incubation at 37 °C for 24 h, loosely bound cells were washed away with 1× phosphate-buffered saline (PBS, pH 7.4). Biofilms adhered on the plate substratum were quantified, using crystal violet staining as described previously [72,73]. Untreated bacteria were used as a negative control, where the percentage of biofilm mass reflected 100% biofilm growth. The experiment was performed in triplicate.
3.5. Cytoplasmic Membrane Permeability Assay
Membrane potential sensitive dye diSC3–5 (3,3′-dipropylthiadicarbocyanine iodide), which penetrates inside bacterial cells depending on the membrane potential gradient of the cytoplasmic membrane, was used to determine the bacterial cytoplasmic membrane permeability following the procedure previously described [71,74] with slight modification. Bacteria (S. aureus) were incubated in MHB with shaking at 37 °C for 16 h. After incubation, the bacteria were washed with 5 mM HEPES, supplemented with 20 mM glucose at pH 7.2 and diluted in the same buffer to an OD600 0.05–0.06 (equivalent to a final concentration of 107 CFU/mL bacteria). The diSC3–5 dye was added at 4 μM to the bacterial suspension. The suspensions were incubated at room temperature in darkness for 1 h for maximum dye take-up by the bacterial cells. A total of 100 mM KCl was then added to balance the K+ outside and inside the bacterial cell to prevent the release or further uptake of the dye. Then, 100 μL of bacterial suspension was added to a 96-well microtiter plate containing 100 µL of serially diluted peptidomimetics. The final concentration of DMSO was at or below 1.0%, and wells with 1.0% DMSO were used as a solvent control. 10% DMSO was used as a positive control, while wells with only dye and bacterial cells were set as the negative control. Fluorescence due to the release of dye was measured with a FLUOstar Omega (BMG Labtech) microplate reader at 5 min intervals at an excitation wavelength of 621 nm and an emission wavelength of 670 nm.
3.6. Toxicity Assays
The toxicity of the peptidomimetics against normal human lung fibroblasts MRC-5 cells was measured, using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma Aldrich, St. Louis, MO, USA) colorimetric assay. The cells were cultured in minimal essential medium (MEM, Invitrogen) containing 10% foetal bovine serum (FBS). The cells were maintained at 37 °C in 5% CO2 as an adherent monolayer and was passaged upon reaching confluence by standard cell culture techniques. MRC-5 cells were seeded at 5000 cells/well in 96-well plates to ensure full confluence (quiescence) and left overnight to adhere to the plate wells. The adherent cells were treated with 1–500 μM of compounds by dissolving in DMSO and serially diluting with media. TY-01 was used as the positive control. The final concentration of DMSO was 0.5% (v/v). After the 72 h drug incubation, 20 mL stock MTT solution (5 mg/mL) was added, and the cells were incubated for another 3.5–4 h. The MTT solution containing media was carefully aspirated without displacing the purple crystals from the bottom, and 80 mL acid-isopropanol was added to all wells and mixed slowly by shaking with an orbital shaker to dissolve the dark blue crystals. The metabolic activity was detected by spectrophotometric analysis by assessing the read absorbance (590/620 nm) on the EnSight plate reader (PerkinElmer, Waltham, MA, USA), and cell viability was expressed as a percentage of the untreated control cells. The determination of IC50 values was performed using GraphPad Prism 7 (GraphPad, San Diego, CA, USA). Each experiment was performed in triplicate and was repeated in three independent experiments.
A total of 20 mL of human blood collected in EDTA coated tubes was transferred into a 50 mL centrifuge tube and centrifuged at 500× g (700) for 5 min. The supernatant plasma was discarded and an equal volume of 1× PBS was added. The washing was repeated five times with cells mixed by inversion. The washed pallet was then resuspended in 1:10 with PBS to yield an RBC concentration of ~5 × 108 RBC/mL. The peptidomimetics were serially diluted with PBS, with final concentrations ranging from 8 to 500 µM. Milli-Q water and 1× PBS were used as positive and negative controls. The cells were then incubated at 37 °C for 3 h. Following incubation, the suspension was centrifuged at 1100× g for 5 min. The final optical density was measured at 540 nm. The percentage of haemolysis was calculated, using the following equation.
4. Conclusions
A library of glyoxamide-based small molecular peptidomimetics was synthesised. These peptidomimetics contained different phenyl ring systems appended to the glyoxamide group core, various N-alkylsulfonyl chains as the hydrophobic group, and either a tertiary ammonium hydrochloride, quaternary ammonium iodide or guanidinium hydrochloride cationic group. In the MIC assay, quaternary ammonium iodide salts 16d, 17b, 17c, 18a and guanidinium hydrochloride salt 34a showed excellent antibacterial activity against Gram-positive S. aureus. Moreover, quaternary ammonium iodide salts 17b–17c and guanidinium hydrochloride salt 34a also showed good antibacterial activity against Gram-negative E. coli. The SAR studies suggested an inverse relationship between the bulkiness of the phenyl system and the length of the alkylsulfonyl chain for the high antibacterial activity of peptidomimetics. This observation was also supported by analysis of AlogP values, which suggested that there is an optimum AlogP range for a peptidomimetic to show high antibacterial activity. The more potent peptidomimetics 17c, 34a, 34b and 17b managed to disrupt 44%, 42%, 42% and 38%, respectively, of pre-established S. aureus biofilms at 4× MIC. Additionally, peptidomimetics 17b and 17c were also able to disrupt 31% and 28%, respectively, of the pre-established E. coli biofilms at 2× MIC. Moreover, peptidomimetic 17c also demonstrated its ability to inhibit 70% and 65% S. aureus biofilm formation at 2× and 4× of its MIC, respectively. Cytoplasmic membrane permeability studies suggested that the disruption and depolarisation of the bacterial cell membrane could be a possible mechanism of action of the peptidomimetics. Furthermore, in vitro toxicity assays against human MRC-5 fibroblast cells and human red blood cells demonstrated the relatively low cytotoxicity of quaternary ammonium iodide salts 16d, 17c and 18a. Overall, the quaternary ammonium iodide salt 17c possesses good antibacterial activity against both Gram-positive and Gram-negative planktonic bacteria and their biofilms, and is identified to be a new lead in this study.
Acknowledgments
We thank the BMSF and NMR facilities at UNSW Mark Wainwright Analytical Centre for the structural determination of the synthesised compounds. T.T.Y. is thankful to UNSW Sydney for the University International Postgraduate Award.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22147344/s1, 1H and 13C NMR spectra of the synthesised compounds.
Author Contributions
N.K., D.S.B. and M.D.P.W. directed this project. The synthesis and spectroscopic characterization of compounds, MIC assays and biofilm disruption assay was conducted by T.T.Y. Computational studies were conducted by T.T.Y. and J.H. The cytoplasmic membrane depolarisation assay was conducted by T.T.Y., R.K. and M.Y. The cytotoxicity assay was conducted by M.M.H. The haemolysis assay was conducted by M.S. The manuscript was prepared by T.T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Discovery Project from Australian Research Council grant (DP 180100845).
Institutional Review Board Statement
Ethical review and approval were waived for this study as the person performing the research on the human red blood cells decided to collect samples themselves of their own blood. In such cases there is no other party to be protected from unethical behavior and so review was not needed.
Informed Consent Statement
Informed consent has been obtained from the person who contributed the red blood cells.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies J., Davies D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010;74:417. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munita J.M., Arias C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016;4:2. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen F., Starosta A.L., Arenz S., Sohmen D., Donhofer A., Wilson D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014;395:559–575. doi: 10.1515/hsz-2013-0292. [DOI] [PubMed] [Google Scholar]
- 5.Douafer H., Andrieu V., Phanstiel O., Brunel J.M. Antibiotic Adjuvants: Make Antibiotics Great Again! J. Med. Chem. 2019;62:8665–8681. doi: 10.1021/acs.jmedchem.8b01781. [DOI] [PubMed] [Google Scholar]
- 6.Verderosa A.D., Totsika M., Fairfull-Smith K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019;7:824. doi: 10.3389/fchem.2019.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flemming H.-C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 8.Sharma D., Misba L., Khan A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019;8:76. doi: 10.1186/s13756-019-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mah T.F.C., O’Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 10.Davies D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 11.Sadekuzzaman M., Yang S., Mizan M.F.R., Ha S.D. Current and Recent Advanced Strategies for Combating Biofilms. Compr. Rev. Food Sci. Food Saf. 2015;14:491–509. doi: 10.1111/1541-4337.12144. [DOI] [Google Scholar]
- 12.Lamret F., Colin M., Mongaret C., Gangloff S.C., Reffuveille F. Antibiotic Tolerance of Staphylococcus aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies. Antibiotics. 2020;9:547. doi: 10.3390/antibiotics9090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang H.-K., Kim C., Seo C.H., Park Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017;55:1–12. doi: 10.1007/s12275-017-6452-1. [DOI] [PubMed] [Google Scholar]
- 14.Mishra B., Reiling S., Zarena D., Wang G. Host defense antimicrobial peptides as antibiotics: Design and application strategies. Curr. Opin. Chem. Biol. 2017;38:87–96. doi: 10.1016/j.cbpa.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magana M., Pushpanathan M., Santos A.L., Leanse L., Fernandez M., Ioannidis A., Giulianotti M.A., Apidianakis Y., Bradfute S., Ferguson A.L., et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020;20:e216–e230. doi: 10.1016/S1473-3099(20)30327-3. [DOI] [PubMed] [Google Scholar]
- 16.Fox J.L. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013;31:379–382. doi: 10.1038/nbt.2572. [DOI] [PubMed] [Google Scholar]
- 17.Mahlapuu M., Håkansson J., Ringstad L., Björn C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016;6:1–12. doi: 10.3389/fcimb.2016.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahar A.A., Ren D. Antimicrobial Peptides. Pharmaceuticals. 2013;6:1543–1575. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostaff M.J., Stange E.F., Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med. 2013;5:1465–1483. doi: 10.1002/emmm.201201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel S., Akhtar N. Antimicrobial peptides (AMPs): The quintessential ‘offense and defense’ molecules are more than antimicrobials. Biomed. Pharmacother. 2017;95:1276–1283. doi: 10.1016/j.biopha.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Koo H.B., Seo J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019;111:e24122. doi: 10.1002/pep2.24122. [DOI] [Google Scholar]
- 22.Ghosh C., Sarkar P., Issa R., Haldar J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019;27:323–338. doi: 10.1016/j.tim.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Lei J., Sun L., Huang S., Zhu C., Li P., He J., Mackey V., Coy D.H., He Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019;11:3919–3931. [PMC free article] [PubMed] [Google Scholar]
- 24.Hancock R.E.W., Sahl H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh C., Manjunath G.B., Akkapeddi P., Yarlagadda V., Hoque J., Uppu D.S.S.M., Konai M.M., Haldar J. Small Molecular Antibacterial Peptoid Mimics: The Simpler the Better! J. Med. Chem. 2014;57:1428–1436. doi: 10.1021/jm401680a. [DOI] [PubMed] [Google Scholar]
- 26.Engler A.C., Wiradharma N., Ong Z.Y., Coady D.J., Hedrick J.L., Yang Y.-Y. Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today. 2012;7:201–222. doi: 10.1016/j.nantod.2012.04.003. [DOI] [Google Scholar]
- 27.Haug B.E., Stensen W., Kalaaji M., Rekdal Ø., Svendsen J.S. Synthetic Antimicrobial Peptidomimetics with Therapeutic Potential. J. Med. Chem. 2008;51:4306–4314. doi: 10.1021/jm701600a. [DOI] [PubMed] [Google Scholar]
- 28.Molchanova N., Wang H., Hansen P.R., Høiby N., Nielsen H.M., Franzyk H. Antimicrobial Activity of α-Peptide/β-Peptoid Lysine-Based Peptidomimetics Against Colistin-Resistant Pseudomonas aeruginosa Isolated From Cystic Fibrosis Patients. Front. Microbiol. 2019;10:275. doi: 10.3389/fmicb.2019.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson A.J., Pomerantz W.C., Neilsen K.J., Gellman S.H., Palecek S.P. Effect of Sequence and Structural Properties on 14-Helical β-Peptide Activity against Candida albicans Planktonic Cells and Biofilms. ACS Chem. Biol. 2009;4:567–579. doi: 10.1021/cb900093r. [DOI] [PubMed] [Google Scholar]
- 30.Epand R.F., Raguse L., Gellman S.H., Epand R.M. Antimicrobial 14-Helical β-Peptides: Potent Bilayer Disrupting Agents. Biochemistry. 2004;43:9527–9535. doi: 10.1021/bi049414l. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson A.J., Flessner R.M., Gellman S.H., Lynn D.M., Palecek S.P. Polyelectrolyte Multilayers Fabricated from Antifungal β-Peptides: Design of Surfaces that Exhibit Antifungal Activity Against Candida albicans. Biomacromolecules. 2010;11:2321–2328. doi: 10.1021/bm100424s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chongsiriwatana N.P., Patch J.A., Czyzewski A.M., Dohm M.T., Ivankin A., Gidalevitz D., Zuckermann R.N., Barron A.E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. USA. 2008;105:2794. doi: 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chongsiriwatana N.P., Miller T.M., Wetzler M., Vakulenko S., Karlsson A.J., Palecek S.P., Mobashery S., Barron A.E. Short Alkylated Peptoid Mimics of Antimicrobial Lipopeptides. Antimicrob. Agents Chemother. 2011;55:417. doi: 10.1128/AAC.01080-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patch J.A., Barron A.E. Helical Peptoid Mimics of Magainin-2 Amide. J. Am. Chem. Soc. 2003;125:12092–12093. doi: 10.1021/ja037320d. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Smith C., Wu H., Padhee S., Manoj N., Cardiello J., Qiao Q., Cao C., Yin H., Cai J. Lipidated Cyclic γ-AApeptides Display Both Antimicrobial and Anti-inflammatory Activity. ACS Chem. Biol. 2014;9:211–217. doi: 10.1021/cb4006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padhee S., Hu Y., Niu Y., Bai G., Wu H., Costanza F., West L., Harrington L., Shaw L.N., Cao C., et al. Non-hemolytic α-AApeptides as antimicrobial peptidomimetics. Chem. Commun. 2011;47:9729–9731. doi: 10.1039/c1cc13684d. [DOI] [PubMed] [Google Scholar]
- 37.Niu Y., Padhee S., Wu H., Bai G., Qiao Q., Hu Y., Harrington L., Burda W.N., Shaw L.N., Cao C., et al. Lipo-γ-AApeptides as a New Class of Potent and Broad-Spectrum Antimicrobial Agents. J. Med. Chem. 2012;55:4003–4009. doi: 10.1021/jm300274p. [DOI] [PubMed] [Google Scholar]
- 38.Sarig H., Rotem S., Ziserman L., Danino D., Mor A. Impact of Self-Assembly Properties on Antibacterial Activity of Short Acyl-Lysine Oligomers. Antimicrob. Agents Chemother. 2008;52:4308. doi: 10.1128/AAC.00656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaknoon F., Goldberg K., Sarig H., Epand R.F., Epand R.M., Mor A. Antibacterial Properties of an Oligo-Acyl-Lysyl Hexamer Targeting Gram-Negative Species. Antimicrob. Agents Chemother. 2012;56:4827. doi: 10.1128/AAC.00511-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi S., Isaacs A., Clements D., Liu D., Kim H., Scott R.W., Winkler J.D., DeGrado W.F. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc. Natl. Acad. Sci. USA. 2009;106:6968–6973. doi: 10.1073/pnas.0811818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang H., Doerksen R.J., Jones T.V., Klein M.L., Tew G.N. Biomimetic Facially Amphiphilic Antibacterial Oligomers with Conformationally Stiff Backbones. Chem. Biol. 2006;13:427–435. doi: 10.1016/j.chembiol.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Liu D., Choi S., Chen B., Doerksen R.J., Clements D.J., Winkler J.D., Klein M.L., DeGrado W.F. Nontoxic Membrane-Active Antimicrobial Arylamide Oligomers. Angew. Chem. Int. Ed. 2004;43:1158–1162. doi: 10.1002/anie.200352791. [DOI] [PubMed] [Google Scholar]
- 43.Ishitsuka Y., Arnt L., Majewski J., Frey S., Ratajczek M., Kjaer K., Tew G.N., Lee K.Y.C. Amphiphilic Poly(phenyleneethynylene)s Can Mimic Antimicrobial Peptide Membrane Disordering Effect by Membrane Insertion. J. Am. Chem. Soc. 2006;128:13123–13129. doi: 10.1021/ja061186q. [DOI] [PubMed] [Google Scholar]
- 44.Kuppusamy R., Yasir M., Yee E., Willcox M., Black D.S., Kumar N. Guanidine functionalized anthranilamides as effective antibacterials with biofilm disruption activity. Org. Biomol. Chem. 2018;16:5871–5888. doi: 10.1039/c8ob01699b. [DOI] [PubMed] [Google Scholar]
- 45.Wales S.M., Hammer K.A., Somphol K., Kemker I., Schröder D.C., Tague A.J., Brkic Z., King A.M., Lyras D., Riley T.V., et al. Synthesis and antimicrobial activity of binaphthyl-based, functionalized oxazole and thiazole peptidomimetics. Org. Biomol. Chem. 2015;13:10813–10824. doi: 10.1039/C5OB01638J. [DOI] [PubMed] [Google Scholar]
- 46.Lin S., Chen Y., Li H., Liu J., Liu S. Design, synthesis, and evaluation of amphiphilic sofalcone derivatives as potent Gram-positive antibacterial agents. Eur. J. Med. Chem. 2020;202:112596. doi: 10.1016/j.ejmech.2020.112596. [DOI] [PubMed] [Google Scholar]
- 47.Thapa R.K., Diep D.B., Tønnesen H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020;103:52–67. doi: 10.1016/j.actbio.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Rios A.C., Moutinho C.G., Pinto F.C., Del Fiol F.S., Jozala A., Chaud M.V., Vila M.M.D.C., Teixeira J.A., Balcão V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016;191:51–80. doi: 10.1016/j.micres.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Patrulea V., Borchard G., Jordan O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics. 2020;12:840. doi: 10.3390/pharmaceutics12090840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu T.T., Nizalapur S., Ho K.K.K., Yee E., Berry T., Cranfield C.G., Willcox M., Black D.S., Kumar N. Design, Synthesis and Biological Evaluation of N-Sulfonylphenyl glyoxamide-Based Antimicrobial Peptide Mimics as Novel Antimicrobial Agents. ChemistrySelect. 2017;2:3452–3461. doi: 10.1002/slct.201700336. [DOI] [Google Scholar]
- 51.Yu T.T., Kuppusamy R., Yasir M., Hassan M.M., Alghalayini A., Gadde S., Deplazes E., Cranfield C., Willcox M.D.P., Black D.S., et al. Design, Synthesis and Biological Evaluation of Biphenylglyoxamide-Based Small Molecular Antimicrobial Peptide Mimics as Antibacterial Agents. Int. J. Mol. Sci. 2020;21:6789. doi: 10.3390/ijms21186789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian S.-Z., Pu X., Luo G., Zhao L.-X., Xu L.-H., Li W.-J., Luo Y. Isolation and Characterization of New p-Terphenyls with Antifungal, Antibacterial, and Antioxidant Activities from Halophilic Actinomycete Nocardiopsis gilva YIM 90087. J. Agric. Food Chem. 2013;61:3006–3012. doi: 10.1021/jf400718w. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X.-Q., Mou X.-F., Mao N., Hao J.-J., Liu M., Zheng J.-Y., Wang C.-Y., Gu Y.-C., Shao C.-L. Design, semisynthesis, α-glucosidase inhibitory, cytotoxic, and antibacterial activities of p-terphenyl derivatives. Eur. J. Med. Chem. 2018;146:232–244. doi: 10.1016/j.ejmech.2018.01.057. [DOI] [PubMed] [Google Scholar]
- 54.Yan W., Li S.-J., Guo Z.-K., Zhang W.-J., Wei W., Tan R.-X., Jiao R.-H. New p-terphenyls from the endophytic fungus Aspergillus sp. YXf3. Bioorg. Med. Chem. Lett. 2017;27:51–54. doi: 10.1016/j.bmcl.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 55.Samshuddin S., Narayana B., Sarojini B.K., Shetty D.N., Suchetha Kumari N. Synthesis, Characterization, and Biological Evaluation of Some New Functionalized Terphenyl Derivatives. Int. J. Med. Chem. 2012;2012:530392. doi: 10.1155/2012/530392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu T.T., Bhadbhade M., Kuppusamy R., Black D.S., Kumar N. A facile synthesis of meta- and para-terphenylglyoxamide-based peptidomimetics. Tetrahedron. Lett. 2020;61:152560. [Google Scholar]
- 57.Nizalapur S., Kimyon O., Yee E., Ho K., Berry T., Manefield M., Cranfield C.G., Willcox M., Black D.S., Kumar N. Amphipathic guanidine-embedded glyoxamide-based peptidomimetics as novel antibacterial agents and biofilm disruptors. Org. Biomol. Chem. 2017;15:2033–2051. doi: 10.1039/C7OB00053G. [DOI] [PubMed] [Google Scholar]
- 58.Kundi V., Ho J. Predicting Octanol–Water Partition Coefficients: Are Quantum Mechanical Implicit Solvent Models Better than Empirical Fragment-Based Methods? J. Phys. Chem. B. 2019;123:6810–6822. doi: 10.1021/acs.jpcb.9b04061. [DOI] [PubMed] [Google Scholar]
- 59.Wu Y.M., Salas Y.L., Leung Y.C., Hunter L., Ho J. Predicting Octanol–Water Partition Coefficients of Fluorinated Drug-Like Molecules: A Combined Experimental and Theoretical Study. Aust. J. Chem. 2020;73:677–685. doi: 10.1071/CH19648. [DOI] [Google Scholar]
- 60.Ghose A.K., Crippen G.M. Atomic Physicochemical Parameters for Three-Dimensional Structure-Directed Quantitative Structure-Activity Relationships I. Partition Coefficients as a Measure of Hydrophobicity. J. Comput. Chem. 1986;7:565–577. doi: 10.1021/ci00053a005. [DOI] [PubMed] [Google Scholar]
- 61.Klopman G., Li J.-Y., Wang S., Dimayuga M. Computer Automated log P Calculations Based on an Extended Group Contribution Approach. J. Chem. Inf. Comput. Sci. 1994;34:752–781. doi: 10.1021/ci00020a009. [DOI] [Google Scholar]
- 62.Viswanadhan V.N., Ghose A.K., Revankar G.R., Robins R.K. Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J. Chem. Inf. Comput. Sci. 1989;29:163–172. [Google Scholar]
- 63.MarvinSketch 19.25.0. ChemAxon; Boston, MA, USA: 2018. [Google Scholar]
- 64.Mohamed M.F., Abdelkhalek A., Seleem M.N. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016;6:29707. doi: 10.1038/srep29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otto M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boles B.R., Horswill A.R. Staphylococcal biofilm disassembly. Trends Microbiol. 2011;19:449–455. doi: 10.1016/j.tim.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma G., Sharma S., Sharma P., Chandola D., Dang S., Gupta S., Gabrani R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016;121:309–319. doi: 10.1111/jam.13078. [DOI] [PubMed] [Google Scholar]
- 68.Le C.-F., Fang C.-M., Sekaran S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017;61:e02340-16. doi: 10.1128/AAC.02340-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raheem N., Straus S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019;10:2866. doi: 10.3389/fmicb.2019.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yasir M., Dutta D., Willcox M.D.P. Comparative mode of action of the antimicrobial peptide melimine and its derivative Mel4 against Pseudomonas aeruginosa. Sci. Rep. 2019;9:7063. doi: 10.1038/s41598-019-42440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yasir M., Dutta D., Willcox M.D.P. Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS ONE. 2019;14:e0215703. doi: 10.1371/journal.pone.0215703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Toole G.A. Microtiter Dish Biofilm Formation Assay. JoVE. 2011;47:e2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yasir M., Dutta D., Willcox M.D.P. Activity of Antimicrobial Peptides and Ciprofloxacin against Pseudomonas aeruginosa Biofilms. Molecules. 2020;25:3843. doi: 10.3390/molecules25173843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu M., Maier E., Benz R., Hancock R.E.W. Mechanism of Interaction of Different Classes of Cationic Antimicrobial Peptides with Planar Bilayers and with the Cytoplasmic Membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and Supplementary Materials.