Abstract
Three different zeolite nanocrystals (SAPO-34, PS-MFI and ETS-10) were incorporated into the polymer matrix (Matrimid® 5218) as polymer precursors, with the aim of fabricating mixed-matrix carbon molecular sieve membranes (CMSMs). These membranes are investigated for their potential for air separation process. Based on our gas permeation results, incorporating porous materials is feasible to improve O2 permeability, owing to the creation of additional porosities in the resulting mixed-matrix CMSMs. Owing to this, the performance of the CMSM with 30 wt% PS-MFI loading is able to surpass the upper bound limit. This study demonstrates the feasibility of zeolite nanocrystals in improving O2/N2 separation performance in CMSMs.
Keywords: zeolite, carbon molecular sieve membrane, air separation, Robeson upper bound, mixed-matrix
1. Introduction
Air, which mainly consists of oxygen (O2) and nitrogen (N2), is an important element for various industries and chemical processes. In general, fuel combustion is typically more advantageous if oxygen-enriched air (OEA) is utilized in comparison to atmospheric air, in order to increase the overall heating value and combustion efficiency [1,2,3]. Besides, high-purity oxygen is used in the several applications, including but not limited to, sewage treatment plants, medical industries, and indoor air quality (IAQ) management in production buildings [4]. Nitrogen-enriched air, on the other hand, is utilized mainly in food preservation to extend the expiry date of oxygen-sensitive food products as well as fire extinguishers used in the coal extraction process, considering its inert properties [5,6].
Conventionally, in industrial processes, the production of high-purity (>99.5 vol%) oxygen and nitrogen from air can be achieved by cryogenic distillation or pressure swing adsorption (PSA). Although these technologies have been present for at least 70 years with a daily production volume of 100 tonnes, high capital cost and large energy penalty challenge the need to consider an alternative process for air separation [7,8]. This attracted substantial research interest towards membrane-based separation. Today, membrane-based high-purity oxygen production is limited to only 25 tonnes per day [9,10], but its practical feasibility due to its simplicity in its operation, cost effectiveness and small plant footprint are competitive advantages over cryogenic distillation and PSA.
To date, membrane materials for the gas separation process are mainly dominated by polymeric materials due to their well-established large-scale synthesis together with the capability of adopting different configurations (spiral wound or hollow fiber). Nevertheless, polymeric membranes are well known for their trade-off limitation between permeability and selectivity as described by Robeson [11,12], given that gas transport in dense polymeric membranes follows the solution-diffusion mechanism [13]. Thus, current research efforts are mostly focused on overcoming this trade-off limitation. Interestingly, the development of carbonized polymeric precursors is a feasible approach in circumventing this limitation [14,15,16,17], through the creation of carbon molecular sieve membranes (CMSMs). In general, pyrolysis of polymer precursors under various treatment conditions gives large graphitic domains, which have been showcased to drastically improve gas permeability without sacrificing the mixed-gas selectivity considerably [18,19,20]. Nevertheless, the performance of CMSMs is heavily influenced by the selection of polymer matrix. For instance, CMSMs derived from highly permeable polymers demonstrate extraordinarily high gas separation performances, but the synthesis of these polymers is typically painstaking and laborious to ensure sufficiently large molecular weight for mechanical stability in the subsequent carbonization process [21,22,23].
Hence, in this work, we leverage a different approach, which is to tune the air separation performance by adopting mixed-matrix strategy into the fabrication of CMSMs. This approach involves the incorporation of zeolite nanocrystals as filler materials into the polymeric membrane, using Matrimid® 5218 polyimide as the precursor. Matrimid® 5218 is selected due to its commercial availability in comparison to PIM-1 and 6FDA-diamine polymers that possess high intrinsic gas permeability, which require extensive monomer purification [24]. Besides, Matrimid® has a high O2/N2 selectivity (c.a. 5.9) [25], such that a high performance can be realized simply by improving permeability without sacrificing the O2/N2 selectivity, which can be done by incorporating highly porous filler materials. In addition, research on mixed-matrix CMSMs for air separation process is scarce as compared to CO2-based separation process [15,21,26]. Herein, we chose three different types of zeolites nanocrystals having different pore sizes, namely Engelhardt Titanosilicate (ETS-10), silicalite-1 (pure silica MFI, PS-MFI) and silicoaluminophosphate (SAPO-34), as our filler materials. Based on our gas permeation results, PS-MFI at 30 wt% loading in carbon matrix of Matrimid® 5218 membrane was able to surpass the upper bound limit for O2/N2 separation.
2. Materials and Methods
2.1. Materials
Matrimid® 5218 polymer was obtained from Huntsman Corporation (Conroe, TX, USA). Titanium dioxide (TiO2, Aeroxide P25) was obtained from Jebsen and Jessen Ingredient (S) Pte Ltd. (Singapore). Aluminum isopropoxide (Al(i-C3H7O)3), phosphoric acid (85 wt% aqueous solution, H3PO4), sodium hydroxide (NaOH), tetraethyl orthosilicate (TEOS), tetraethylammonium hydroxide (35 wt% aqueous solution, TEAOH), colloidal silica (LUDOX TM-40, 40 wt% suspension in H2O, SiO2) were purchased from Sigma Aldrich (St. Louis, MO, USA). Tetrapropylammonium hydroxide (aqueous solution with 40 wt% TPAOH) was obtained from Alfa Aesar (Tewksbury, MA, USA). Chloroform and potassium fluoride (KF) were brought from VWR (Philadelphia, PA, USA). Distilled water, H2O was synthesized in-house.
2.2. Synthesis Procedure for SAPO-34, PS-MFI and ETS-10
SAPO-34 [27]: SAPO-34 was synthesized using a dry-gel conversion method. In this synthesis, TEOS and Al(i-C3H7O)3 were used as the aluminum and silicon source, respectively. In subsequent steps, Al(i-C3H7O)3, H3PO4, TEOS and TEAOH were added sequentially into H2O to give a solution mixture. During each process, the solution was allowed sufficient agitation (3 h) to ensure that the resulting gel remained homogeneous. The molar composition of the prepared gel was calculated as 1.00 Al2O3/0.30 SiO2/1.00 P2O5/1.00 TEAOH/52.00 H2O. The solvents (H2O and ethanol) were removed by heating at 80 °C, so as to form a dry gel. After the gel was sufficiently dried, H2O was added into the solution in order to achieve the desired ratio of 3:1 (H2O/Al2O3), which was then followed by a hydrothermal synthesis at non-agitated conditions (220 °C for 24 h). Centrifugation was used the remove any potential presence of any undesirable residual reactants that could be present in SAPO-34 crystals. This process was reported for at least three times with the use of distilled water, prior to drying at 110 °C overnight. The residual template (TEAOH) that was present in the dried SAPO-34 samples was removed via a calcination process in air at 500 °C for 6 h.
PS-MFI [28]: Pure silica MFI was developed as described below. TEOS was used as the silica source, as similar to SAPO-34. First, TEOS was added into a solution (that contained TEAOH) while maintaining an agitation process. The solution turned completely transparent after an additional 1 h of stirring. The solution was then allowed to agitate further for 24 h after H2O was added into the solution. The molar composition of the synthesized gel was determined as 1.00 TEOS/0.36 TPAOH/20.00 H2O. In the following step, PS-MFI crystals were obtained via a continuous agitation at 95 °C under a duration of 48 h. The precipitated crystals were washed with copious amount of H2O via a repetitive centrifugation process. The residual template (TPAOH) that was potentially present in the crystals was calcined in air at 550 °C for 8 h.
ETS-10 [29]: ETS-10 was synthesized based on the procedure as provided. Colloidal silica as well as TiO2 were used as the source of silicon and titanium, respectively. First, a suspension was created by dispersing TiO2 into H2O. The solution was agitated for 1 h after the addition of NaOH and KF, followed by an additional 6 h of stirring after colloidal silica was added into the mixture. At this stage, the molar composition of the solution was identified as 1.00 SiO2/0.30 Na2O/0.20 TiO2/0.15 KF/16.30 H2O. Subsequently, hydrothermal reaction was conducted at 215 °C for 72 h under static condition. The ETS-10 crystals were collected via a centrifugation process for at least three times using distilled water, before drying the samples at 60 °C overnight.
2.3. Development of Mixed-Matrix Carbon Molecular Sieve Membranes (CMSMs)
Polymeric precursor membranes were created in a flat sheet configuration using a solution-casting approach. As an illustration, to fabricate a polymeric membrane without the addition of zeolite nanocrystals, dissolution of Matrimid® 5218 into a chloroform solution was made to prepare a dope solution. For the case of mixed-matrix membranes (MMM), the dope solution was developed by first dispersing the zeolite fillers into the solution containing chloroform. Sonication horn (Qsonica, Q125, Newtown, CT, USA) was used to potentially minimize nanocrystals’ aggregation. Next, Matrimid® 5218 powder was added into the suspension. At least 24 h was set to stir the dope solution before casting the membranes. Using a casting knife, the membranes were casted onto glass plate. The glass plates were placed in a glove bag, where the environment was filled with chloroform vapor during the fabrication process to control solvent evaporation. The membranes were eventually placed in vacuum oven at 160 °C overnight. Horizontal tube furnace by Carbolite GERO (CTF 12/10/900) was used to carbonize the polymeric membranes to afford CMSMs. In this study, Argon (99.995%) that was purchased from Airliqude Singapore was utilized. The system was purged for the minimum duration of 1 h to displace any left-over oxygen that was present in the system. The carbonization process was conducted under the following profile: (1) 2 °C min−1 ramp (25 °C to 380 °C) for 0.5 h; (2) 0.5 °C min−1 ramp (380 °C to 550 °C) for 2 h. The CMSMs were cooled to ambient condition prior to sample retrieval.
2.4. Characterizations
NOVATouch LX2, a volumetric gas sorption analyzer, was used to assess the porosity properties of SAPO-34, PS-MFI and ETS-10 (Quantachrome, Boynton Beach, FL, USA). In a typical case, outgassing the zeolite samples was conducted at 250 °C for 24 h under high vacuum prior to the investigation of physisorption isotherm at 77 K (−169 °C), with the use of nitrogen (N2) as the adsorbate. On the other hand, pure component adsorption (O2 and N2, respectively) of SAPO-34, PS-MFI and ETS-10 was conducted at 35 °C, where the temperature was controlled using a water circulator. The samples were activated at the same condition as described above. The isosteric heat of adsorption (-Qst) of O2 and N2 of these porous materials was computed with the data from the isotherms measured at two temperatures (25 and 35 °C). This was calculated using the Clausius–Clapeyron equation, as described by Equation (1). q, T and P were described as adsorbed quantity (mmol g−1), absolute temperature (Kelvin) and pressure (bar), respectively. As the effect of temperature -Qst could be neglected in most circumstances [30], a well-defined mathematical solution could be obtained when Equation (2) (single-site Langmuir) was used. Fitting parameters for the adsorption isotherm at 25 and 35 °C [31,32] can be found in the Supplementary Information (Tables S1 and S2). b, P and qsat were described as Langmuir constant (bar−1), pressure (bar) and saturation loading (mmol g−1), respectively.
| (1) |
| (2) |
The pore size distribution of CMSM and mixed-matrix CMSMs were analyzed with the use of CO2 adsorption at 0 °C, measured by the volumetric gas sorption analyzer as mentioned in Section 2.4. Similarly, the carbonized membranes were degassed at 250 °C for 24 h before the measurement. Subsequently, the CO2 adsorption isotherm was modelled with a Dubinin–Radushkevich (DR) equation, as illustrated in Equation (3). In this expression, V, Vo, Eo and β were the amount of CO2 adsorbed (at specified relative pressure (P/Po) and temperature (T)), the micropore volume, characteristic adsorption energy and affinity coefficient (0.35 for CO2), respectively [33]. This process allowed the micropore volume, micropore size and micropore surface area to be computed. Such measurements were conducted as N2 physisorption at 77 K was unable to be determined in CMSMs as negligible N2 adsorption was typically observed [16]. This behavior potentially indicated the formation of ultramicropores during the membrane carbonization process, as reported elsewhere [34].
| (3) |
The verification of the crystallinity of the zeolite nanocrystals was performed with the use of powdered X-ray diffraction (PXRD). This measurement was conducted with the use of Bruker D2 phaser, which contained a diffractor with a CuKα radiation (1.5418 Å) (Billerica, MA, USA). Such analysis was conducted at an ambient condition. The measurement was conducted at 2θ range from 5° to 40°, using step size of 0.02°. Field-emission scanning electron microscope (FESEM) was performed to observe the particle morphologies. The acceleration voltage (Joel, JSM6701, Tokyo, Japan) was set as 5 kV. Image analysis tool (Nano Measure) was used to determine the particle size distribution of the zeolite nanocrystals, based on the FESEM images. The thermal stabilities of the zeolite nanocrystals were conducted using thermogravimetric/differential thermal analyzer (TG/DTA, TA Instrument, SDT Q600, New Castle, DE, USA). The ramping profile was set at 10 °C min−1 from 40 to 800 °C, with the use of nitrogen gas set at 0.1 L min−1.
2.5. O2 and N2 Permeation Analysis
Constant pressure-variable volume system by GTR Corporation was utilized to perform the O2 and N2 permeation test. Air (Purity of O2 and N2 of 99.8% and 99.995% at 21/79 vol ratio, Airliquide, Singapore) mixture and helium (99.995%, Airliquide, Singapore) were utilized in this setup for the measurement. First, the membrane was mounted onto the permeation cell, with the temperature of the measurement maintained at 35 °C. Membranes are subjected to the flow of air (test gas, at upstream) and helium (at downstream) in a continuous manner. Flow rates were controlled using mass flow controllers. The permeated gases were swept by helium gas into a gas chromatograph at a set time interval with the aid of a gas sampler. Once the amount of gas permeated did not demonstrate large fluctuation, the permeability and selectivity of the studied gases were calculated. At least three different samples were measured to confirm the reproducibility of the permeation results.
2.6. O2 and N2 Solubility-Diffusivity Analysis
The gas sorption analyzer as reported earlier in Section 2.4 was adopted to determine the gas adsorption of both O2 and N2, with the same activation condition. At the desired pressures (O2: 0.21 bar; N2: 0.79 bar), the solubility was calculated by Equation (4). The amount of gas adsorbed normalized by the mass of CMSMs, desired pressure as well as carbon membrane’s density were expressed as q, P and ρ, respectively. Analytical balance (Mettler Toledo, ME204) that was integrated with the accessories (density kit) was performed to obtain the density of the CMSMs. The diffusivity of the gas in CMSMs was then determined by dividing permeability with solubility.
| (4) |
2.7. Filler Enhancement Index, Findex
The effectiveness of a filler in mixed-matrix CMSMs can be calculated with the use of Filler Enhancement Index (Findex) [10]. This equation was described with the use of Equation (5). Pcomposite and Punfilled referred to O2 permeability while αcomposite and αunfilled referred to O2/N2 selectivity of mixed-matrix and pure polymeric CMSMs, respectively. η referred to the enhancement coefficient, which was obtained from the gradient of the O2/N2 upper bound defined in 2008 as described by Robeson, which is determined as 5.666 [12]. In this calculation, utilization of different zeolite nanocrystals in mixed-matrix CMSMs was computed to comprehend the effect by the zeolite fillers.
| (5) |
3. Results
3.1. Properties of Zeolite Nanocrystals
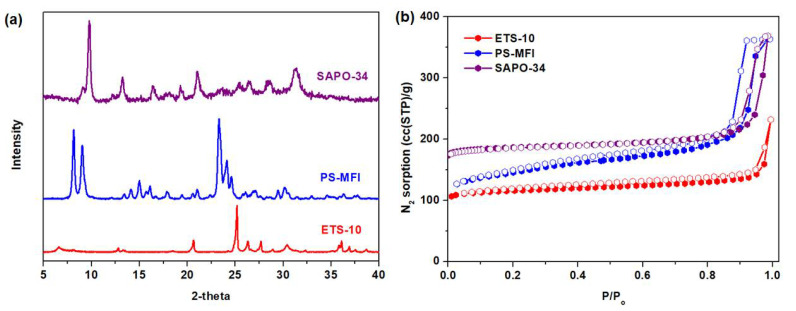
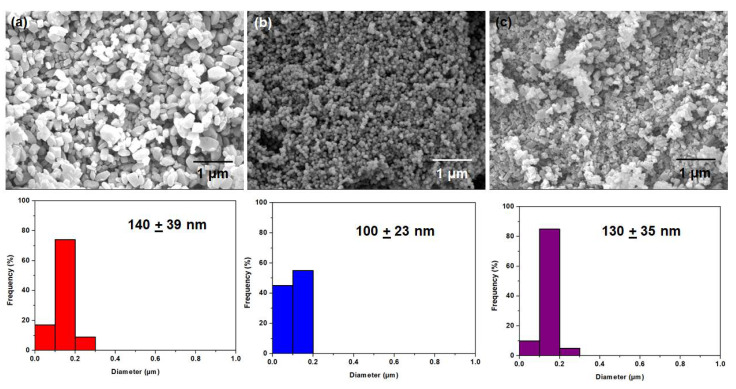
The crystallinity of zeolite nanocrystals that were developed in this work was verified via XRD diffraction (Figure 1a). The diffraction peaks are comparable with the literature data [35,36,37], indicating the successful synthesis of SAPO-34, PS-MFI and ETS-10. Subsequently, N2 physisorption at 77 K (Figure 1b) depicts a typical Type 1 isotherm across the studied zeolite nanocrystals. This is attributed to its large N2 sorption at low P/Po, indicating large micropore volumes (Table 1) [38]. Moreover, at P/Po > 0.9, the presence of unrestricted monolayer-multilayer adsorption can be seen based on the significant increase in N2 sorption at this region. Such behavior is often correlated to the plausible nanocrystal creation. This deduction is further corroborated by using visual inspection from FESEM. As illustrated in Figure 2, zeolites in nanocrystal forms were indeed successfully created. Essentially, it is recommended to utilize filler particles in nanocrystals forms in the formation of mixed-matrix membranes in order to increase the interfacial areas between the fillers and polymers. The particle size of our zeolites is estimated to be ranging between 100 and 200 nm.
Figure 1.
(a) PXRD and (b) N2 physisorption (77 K) for ETS-10, PS-MFI and SAPO-34.
Table 1.
Surface area and pore volumes of SAPO-34, PS-MFI and SAPO-34 (determined using N2 physisorption (77 K)).
| Sample | SBET
(m2 g−1) 1 |
SLANGMUIR
(m2 g−1) 1 |
SMICRO
(m2 g−1) 2 |
VMICRO
(cc g−1) 2 |
VTOTAL
(cc g−1) 3 |
|---|---|---|---|---|---|
| SAPO-34 | 631 | 819 | 592 | 0.272 | 0.572 |
| PS-MFI | 493 | 667 | 397 | 0.195 | 0.563 |
| ETS-10 | 359 | 518 | 319 | 0.163 | 0.358 |
1 Selection at P/Po from 0.05 to 0.2 is required to calculate BET and Langmuir surface area (SBET and SLANGMUIR). 2 Selection at P/Po from 0.4 to 0.6 is required to calculate micropore surface area (SMICRO) and micropore volume (VMICRO). 3 Selection at P/Po = 0.99 is required to calculate the total pore volume (VTOTAL).
Figure 2.
FESEM images of (a–c) ETS-10; PS-MFI and SAPO-34. Distribution of particle size was illustrated with the use of histogram.
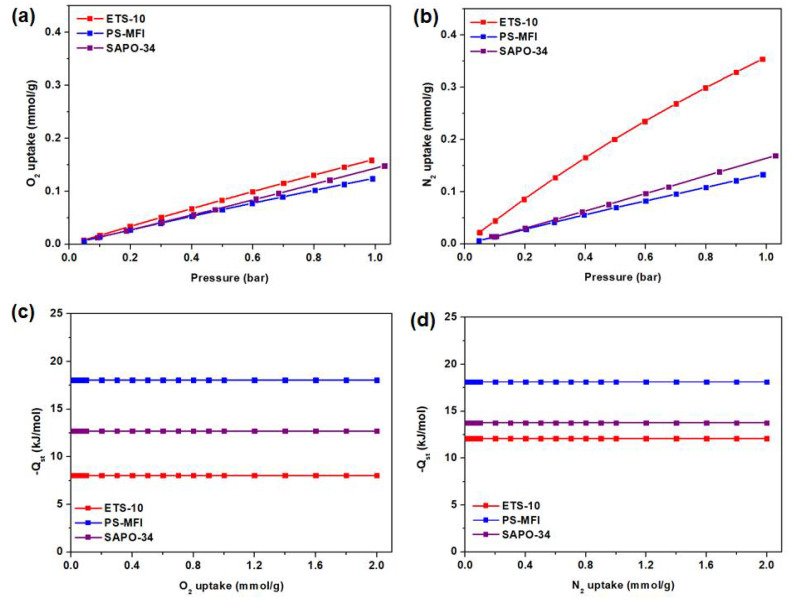
Subsequently, pure component gas adsorption isotherms of the zeolite nanocrystals were obtained under the pressure range from 0 to 1 bar (Figure 3a,b). The isotherm was performed at 35 °C. The -Qst calculations, on the other hand, which are typically calculated based on the measurement of at least two distinct temperatures, are calculated and summarized in Figure 3c,d. O2 and N2 isotherms at 25 °C are included in Figure S1. In general, linear gas adsorption isotherms were observed in both O2 and N2 isotherms, which suggest weak binding energy between adsorbate and adsorbent. This phenomenon is illustrated further from the analysis of -Qst, where active sites for O2 and N2 adsorption are considered homogeneous. Such behavior is not commonly observed for the case of CO2 adsorption where two distinctive steps (from higher to lower binding energy) can be seen [39,40]. Further comparison of the adsorption isotherms indicates that all studied zeolite nanocrystals demonstrate higher N2 adsorption as compared to O2, considering that the former possesses higher polarizability (17.6 × 10−25 cm3 vs. 15.4 × 10−25 cm3) [41]. Thus, we do not foresee these zeolites to achieve high O2/N2 sorption selectivity. Nevertheless, as compared to recent metal-organic frameworks (MOFs), which have been reported to achieve reasonable O2/N2 separation performance, these zeolites show stronger capacities for CMSMs fabrication given their better thermal stability and reversible O2 adsorption [42,43,44,45].
Figure 3.
(a,b) O2 and N2 adsorption at 35 °C; (c,d) isosteric heat of adsorption (-Qst) of O2 and N2 for zeolite nanocrystals.
3.2. Properties of Carbonized Membranes
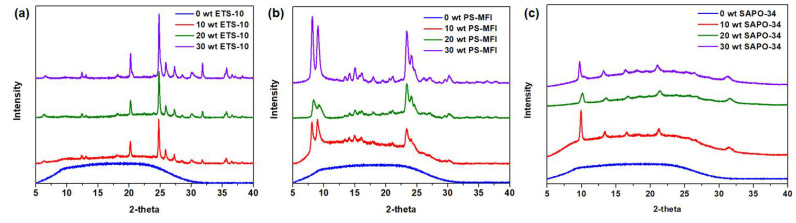
With the successful preparation of zeolite nanocrystals, mixed-matrix membrane precursors were developed next. First, thermal stabilities of zeolite nanocrystals were determined to verify that the carbonization temperature set in this work (550 °C) is appropriate. Based on the profile in Figure S2, a substantial weight loss was not observed across all samples. The decrease in weight at initial temperatures is attributed to the evaporation of residual solvents and/or water that are present in zeolite nanocrystals. Additionally, verification of the zeolites’ crystallinity after the carbonization process was also conducted using XRD on the mixed-matrix CMSMs, as shown in Figure 4. It can be seen that the characteristic peaks of zeolite nanocrystals (with reference to Figure 1a) remain, suggesting intact crystallinity and structural integrity of the zeolite nanocrystals.
Figure 4.
X-ray diffraction (XRD) pattern of carbonized membrane. (a–c) ETS-10; PS-MFI and SAPO-34 were added into the polymer matrix at 10, 20 and 30 wt% loading. Matrimid® 5218 is used as the polymer precursor in this work.
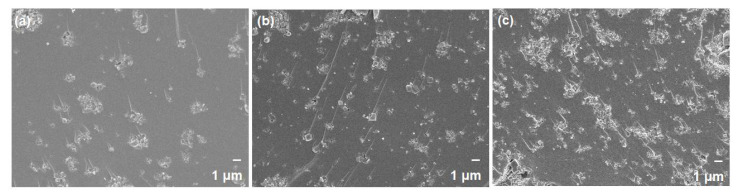
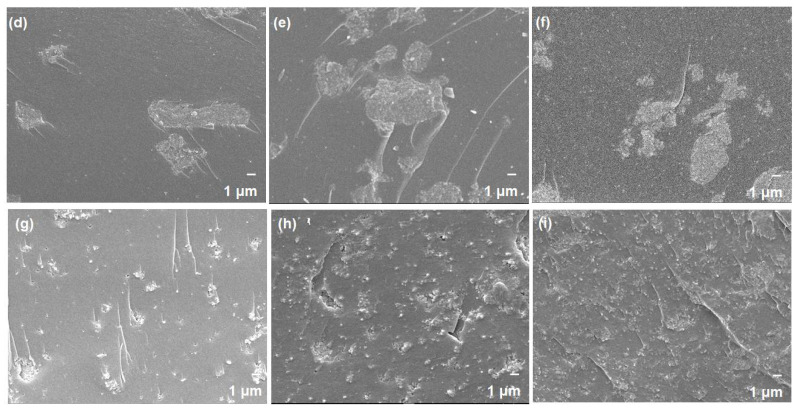
Cross-sectional morphologies of carbonized membrane with the addition of zeolite nanocrystals were imaged with the use of FESEM (Figure 5). It has been well-known that the addition of zeolites as porous fillers in mixed-matrix membranes often result in a sieve-in-a-cage morphology [46,47], considering its poor compatibility between the inorganic zeolites and polymer matrix. Nevertheless, defective voids are not visibly seen after membranes are carbonized. It is possibly due to the polymer chains undergoing thermal rearrangement during carbonization, which made it feasible to heal the interfacial defects that could be present in the mixed-matrix polymeric precursor membranes [14,15].
Figure 5.
FESEM images of mixed-matrix CMSMs for (a–c) ETS-10; (d–f) PS-MFI; (g–i) SAPO-34 at 10, 20 and 30 wt%, respectively, in carbonized Matrimid® 5218 membrane.
3.3. Gas Permeation Analysis of Carbonized Membranes
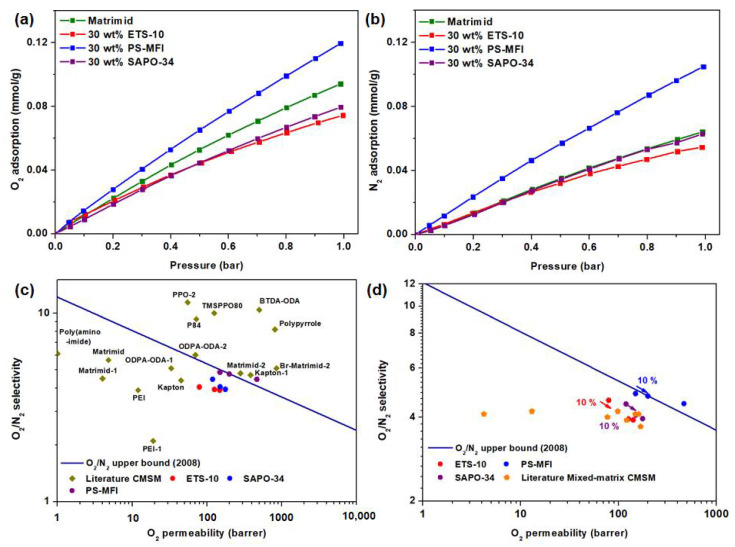
Measurements at 35 °C and 1 bar air feed were conducted to investigate the separation performance of the CMSMs. The results are summarized in Table 2. O2 permeability and O2/N2 selectivity of carbonized Matrimid® 5218 membrane is reported to be 5 barrer and 5.50, respectively, which correspond to a higher O2 permeability with a marginal dip in O2/N2 selectivity with reference to the dense Matrimid® 5218 polymeric precursor membrane [13]. The performance of carbonized Matrimid® 5218 membrane is comparable with the literature results [48]. Subsequently, gas permeation analyses with systematic loadings of zeolite nanocrystals (SAPO-34, PS-MFI and ETS-10) in carbonized membrane were conducted. Based on the gas permeation result, addition of zeolite nanocrystals demonstrated substantial enhancement in O2 permeability by 2720%, 9200% and 3420%, respectively, at 30 wt% loading, which is possibly attributed to the substantial increase in the micropore surface area and micropore volume, as described in Table S3 (the DR plots and CO2 adsorption at 0 °C are provided in Figure S3). For example, incorporation of 30 wt% PS-MFI in CMSMs could increase the micropore volume and micropore surface area by 26% and 24%, respectively (Table S3). Such a phenomenon is attributed to the pore size of the studied porous fillers (ETS-10: 6–8 Å; PS-MFI: 5 Å; SAPO-34: 3.8 Å) [49,50,51,52], which allows rapid transport of O2 (3.46 Å) [41] and N2 (3.64 Å) [41] molecules through the carbon matrix of the CMSMs. Besides, based on the O2/N2 selectivity of mixed-matrix CMSM, incorporation of ETS-10 suffers the highest dip in O2/N2 selectivity, considering its highest N2 adsorption as compared to other porous fillers.
Table 2.
O2/N2 permeation analysis of carbonized membrane. The 35 °C and 1 bar of air feed (O2/N2 = 21/79 vol/vol) was performed. Findex calculated from Equation (3) was added for comparison.
| CMSM | Thickness (μm) 1 | O2 Permeability (barrer) | O2/N2 Selectivity | Findex |
|---|---|---|---|---|
| Matrimid® 5218 | 208 ± 28 | 5 ± 1 | 5.50 ± 0.10 | - |
| 10 wt% ETS-10 | 135 ± 13 | 79 ± 4 | 4.60 ± 0.56 | 1.74 |
| 20 wt% ETS-10 | 251 ± 20 | 126 ± 23 | 3.94 ± 0.49 | 1.33 |
| 30 wt% ETS-10 | 248 ± 18 | 141 ± 14 | 3.91 ± 0.14 | 1.40 |
| 10 wt% PS-MFI | 138 ± 15 | 149 ± 1 | 4.86 ± 0.33 | 2.69 |
| 20 wt% PS-MFI | 140 ± 28 | 199 ± 12 | 4.76 ± 0.03 | 2.86 |
| 30 wt% PS-MFI | 157 ± 12 | 465 ± 5 | 4.46 ± 0.05 | 3.35 |
| 10 wt% SAPO-34 | 186 ± 38 | 119 ± 4 | 4.07 ± 0.10 | 1.46 |
| 20 wt% SAPO-34 | 184 ± 15 | 150 ± 2 | 4.46 ± 0.41 | 2.21 |
| 30 wt% SAPO-34 | 166 ± 2 | 176 ± 4 | 3.95 ± 0.22 | 1.68 |
1 The error bar on the thickness is reported as standard deviation.
Subsequently, in order to understand the O2/N2 separation performance of CMSMs as reported in Table 2, quantification of both solubility and diffusivity of O2 and N2 was conducted. Based on the isotherm profile as illustrated in Figure 6a,b, at the specified pressure of O2 (0.21 bar) and N2 (0.79 bar), the amounts of O2 and N2 adsorbed in CMSMs are comparable. Nevertheless, 30 wt% PS-MFI in CMSMs demonstrated a sharp increase in both O2 and N2 adsorption. Due to a higher N2 adsorption in comparison to O2 adsorption (Figure 3a,b), it is generally challenging for the studied membranes to demonstrate an improved solubility selectivity as compared to diffusion selectivity. The selectivity in this context is defined based on ratio between O2 and N2. As indicated in Table S3, mixed-matrix CMSMs showed increased N2 solubility as compared to that of pure CMSM. The improvement in diffusion selectivity is plausibly caused by the smaller average micropore size in the mixed-matrix CMSMs (Table S3), which is capable of improving size-dependent discrimination of N2 and O2.
Figure 6.
(a,b) Adsorption isotherms O2 and N2 of carbonized membrane. The measurement condition for this isotherm was set as 35 °C. (c,d) O2 permeabilities and O2/N2 selectivity of carbonized membrane that are reported in the literature are compared with the Robeson plot. Carbonized membrane in this work is included in the diagram for the ease of reference. Data points can be obtained from the supplementary information [15,21,26,48,53,54,55,56,57,58,59,60] (Tables S5 and S6).
3.4. Performance Benchmarking with Findex and Robeson Upper Bound
The performance of mixed-matrix CMSMs in this study was benchmarked with the O2/N2 upper bound as described by Robeson. Based on the results, the performance of our membranes surpasses the 2008 upper bound limit for O2/N2 separation as shown in Figure 6. Thus, our mixed-matrix strategy proves effective in improving the gas separation performance substantially with reference to pure Matrimid® CMSM. Although the performance of our CMSMs fall short to that of other pure CMSMs reported in the literature (Figure 6c), our strategy is much more straightforward as compared to the high-performance CMSMs developed using in-house synthesized polymers, which do not possess scalability potential. It is undeniable that the performance of our mixed-matrix CMSMs in this work is limited by the moderate decrease in O2/N2 selectivity. Nevertheless, the strong enhancement in O2 permeability reconciles this shortcoming. Furthermore, we studied the effectiveness of the zeolite nanocrystals using Findex. Findex is an empirical composite rating that was first initiated in 2018 to determine the particles’ effectiveness in mixed-matrix membrane [10]. Figure S4 and Table 2 summarize the calculated parameters. As a whole, PS-MFI at 20 and 30 wt% loading in CMSMs shows most promising performance for O2/N2 separation, with Findex values of 2.86 and 3.35, respectively. As previously defined [10], these values place PS-MFI filler under the “competent” category for mixed-matrix CMSMs (Figure 6d and Table S6).
4. Conclusions
In this work, mixed-matrix CMSMs, incorporating various zeolite nanocrystals, were demonstrated using Matrimid® 5218 as the polymer precursor. It can be observed that the addition of zeolite nanocrystals is feasible to elevate O2 permeability due to the presence of large micropore volumes on zeolite nanocrystals. Despite a slight decrease in O2/N2 selectivity, the O2/N2 Robeson upper bound can be successfully surpassed with the use of PS-MFI filler (at the loading of 20 and 30 wt%) in carbonized Matrimid® 5218 membranes. Calculation of Findex also demonstrates the feasibility of the studied mixed-matrix strategy to be competent for improving the O2/N2 separation performance. Future work that should be conducted is an investigation on the effect of zeolite nanocrystals on physical aging of CMSMs, which is an important consideration in practical applications. Besides, for application in industrial separation processes, the mixed-matrix CMSM should be fabricated into a thin-film composite membrane with an aid from porous supports to overcome its poor mechanical strength.
Acknowledgments
Not applicable.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/membranes11070489/s1, Figure S1: O2 and N2 adsorption of ETS-10, PS-MFI and SAPO-34 at 25 °C; Figure S2: TGA analysis of ETS-10, PS-MFI and SAPO-34; Figure S3: Dubinin–Radushkevich (DR) plot for CMSMs and mixed-matrix CMSMs: (a) Matrimid, (b) 30 wt% ETS-10, (c) 30 wt% PS-MFI, and (d) 30 wt% SAPO-34; (e) CO2 adsorption of CMSMs and mixed-matrix CMSMs at 0 °C. The saturation pressure of CO2 (denoted as Po) is set at 26,141 torr; Figure S4: Performance of mixed-matrix CMSM with Findex indicated in the figure as reference. The Findex value can be obtained from Table 2, based on the calculation from Equation (5); Table S1: Fitting parameters for O2 and N2 for ETS-10, PS-MFI and SAPO-34 at 25 °C; Table S2: Fitting parameters for O2 and N2 for ETS-10, PS-MFI and SAPO-34 at 35 °C; Table S3: Porosity properties of CMSM and mixed-matrix CMSM; Table S4: Solubility and diffusivity of O2 and N2 of CMSM and mixed-matrix CMSM at 35 °C under the feed pressure of 1 bar (0.21 bar for O2 and 0.79 bar for N2); Table S5: Performance of pure CMSMs that have been reported in the literature for O2/N2 separation; Table S6: Performance of mixed-matrix CMSMs that have been reported in the literature for O2/N2 separation.
Author Contributions
Original draft preparation, experimental and characterization: C.Y.C.; assistance in membrane characterization: K.G.; writing—review and editing: K.G., T.-H.B.; funding acquisition: T.-H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research is also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government MSIT (reference number: NRF-2021R1A2C3008570).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fernández-Barquín A., Casado-Coterillo C., Valencia S., Irabien A. Mixed matrix membranes for O2/N2 separation: The influence of temperature. Membranes. 2016;6:28. doi: 10.3390/membranes6020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoek E.M.V., Tarabara V.V. Encyclopedia of Membrane Science and Technology. Volume 3 Wiley Online Library; Hoboken, NJ, USA: 2013. [Google Scholar]
- 3.Chong K., Lai S., Thiam H., Teoh H., Heng S. Recent progress of oxygen/nitrogen separation using membrane technology. J. Eng. Sci. Technol. 2016;11:1016–1030. [Google Scholar]
- 4.Stafford T.M. Indoor air quality and academic performance. J. Environ. Econ. Manag. 2015;70:34–50. doi: 10.1016/j.jeem.2014.11.002. [DOI] [Google Scholar]
- 5.Bazzarelli F., Giorno L., Piacentini E. Encyclopedia of Membranes. Springer; Berlin/Heidelberg, Germany: 2016. [Google Scholar]
- 6.Samarasinghe S.A.S.C., Chuah C.Y., Karahan H.E., Sethunga G., Bae T.-H. Enhanced O2/N2 Separation of Mixed-Matrix Membrane Filled with Pluronic-Compatibilized Cobalt Phthalocyanine Particles. Membranes. 2020;10:75. doi: 10.3390/membranes10040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith A., Klosek J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process. Technol. 2001;70:115–134. doi: 10.1016/S0378-3820(01)00131-X. [DOI] [Google Scholar]
- 8.Chuah C.Y., Lee Y., Bae T.-H. Potential of adsorbents and membranes for SF6 capture and recovery: A review. Chem. Eng. J. 2020;404:126577. doi: 10.1016/j.cej.2020.126577. [DOI] [Google Scholar]
- 9.Murali R.S., Sankarshana T., Sridhar S. Air separation by polymer-based membrane technology. Sep. Purif. Rev. 2013;42:130–186. doi: 10.1080/15422119.2012.686000. [DOI] [Google Scholar]
- 10.Chuah C.Y., Goh K., Yang Y., Gong H., Li W., Karahan H.E., Guiver M.D., Wang R., Bae T.-H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018;118:8655–8769. doi: 10.1021/acs.chemrev.8b00091. [DOI] [PubMed] [Google Scholar]
- 11.Robeson L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991;62:165–185. doi: 10.1016/0376-7388(91)80060-J. [DOI] [Google Scholar]
- 12.Robeson L.M. The upper bound revisited. J. Membr. Sci. 2008;320:390–400. doi: 10.1016/j.memsci.2008.04.030. [DOI] [Google Scholar]
- 13.Samarasinghe S., Chuah C.Y., Li W., Sethunga G., Wang R., Bae T.-H. Incorporation of CoIII acetylacetonate and SNW-1 nanoparticles to tailor O2/N2 separation performance of mixed-matrix membrane. Sep. Purif. Technol. 2019;223:133–141. doi: 10.1016/j.seppur.2019.04.075. [DOI] [Google Scholar]
- 14.Li W., Goh K., Chuah C.Y., Bae T.-H. Mixed-matrix carbon molecular sieve membranes using hierarchical zeolite: A simple approach towards high CO2 permeability enhancements. J. Membr. Sci. 2019;588:117220. doi: 10.1016/j.memsci.2019.117220. [DOI] [Google Scholar]
- 15.Li W., Chuah C.Y., Bae T.-H. Hierarchical 5A Zeolite-Containing Carbon Molecular Sieve Membranes for O2/N2 Separation. Membr. J. 2020;30:260–268. doi: 10.14579/MEMBRANE_JOURNAL.2020.30.4.260. [DOI] [Google Scholar]
- 16.Chuah C.Y., Lee J., Bao Y., Song J., Bae T.-H. High-performance porous carbon-zeolite mixed-matrix membranes for CO2/N2 separation. J. Membr. Sci. 2021;622:119031. doi: 10.1016/j.memsci.2020.119031. [DOI] [Google Scholar]
- 17.Chuah C.Y., Lee J., Song J., Bae T.-H. Carbon Molecular Sieve Membranes Comprising Graphene Oxides and Porous Carbon for CO2/N2 Separation. Membranes. 2021;11:284. doi: 10.3390/membranes11040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail A.F., David L. A review on the latest development of carbon membranes for gas separation. J. Membr. Sci. 2001;193:1–18. doi: 10.1016/S0376-7388(01)00510-5. [DOI] [Google Scholar]
- 19.Salleh W.N.W., Ismail A.F., Matsuura T., Abdullah M.S. Precursor selection and process conditions in the preparation of carbon membrane for gas separation: A review. Sep. Purif. Rev. 2011;40:261–311. doi: 10.1080/15422119.2011.555648. [DOI] [Google Scholar]
- 20.Saufi S., Ismail A. Fabrication of carbon membranes for gas separation––A review. Carbon. 2004;42:241–259. doi: 10.1016/j.carbon.2003.10.022. [DOI] [Google Scholar]
- 21.Li W., Chuah C.Y., Kwon S., Goh K., Wang R., Na K., Bae T.-H. Nanosizing zeolite 5A fillers in mixed-matrix carbon molecular sieve membranes to improve gas separation performance. Chem. Eng. J. Adv. 2020;2:100016. doi: 10.1016/j.ceja.2020.100016. [DOI] [Google Scholar]
- 22.Gong H., Chuah C.Y., Yang Y., Bae T.-H. High performance composite membranes comprising Zn(pyrz)2(SiF6) nanocrystals for CO2/CH4 separation. J. Ind. Eng. Chem. 2018;60:279–285. doi: 10.1016/j.jiec.2017.11.014. [DOI] [Google Scholar]
- 23.Chuah C.Y., Samarasinghe S., Li W., Goh K., Bae T.-H. Leveraging nanocrystal HKUST-1 in mixed-matrix membranes for ethylene/ethane separation. Membranes. 2020;10:74. doi: 10.3390/membranes10040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y., Goh K., Wang R., Bae T.-H. High-performance nanocomposite membranes realized by efficient molecular sieving with CuBDC nanosheets. Chem. Commun. 2017;53:4254–4257. doi: 10.1039/C7CC00295E. [DOI] [PubMed] [Google Scholar]
- 25.Chuah C.Y., Bae T.-H. Incorporation of Cu3BTC2 nanocrystals to increase the permeability of polymeric membranes in O2/N2 separation. BMC Chem. Eng. 2019;1:2. doi: 10.1186/s42480-019-0002-z. [DOI] [Google Scholar]
- 26.Zhang B., Wu Y., Lu Y., Wang T., Jian X., Qiu J. Preparation and characterization of carbon and carbon/zeolite membranes from ODPA–ODA type polyetherimide. J. Membr. Sci. 2015;474:114–121. doi: 10.1016/j.memsci.2014.09.054. [DOI] [Google Scholar]
- 27.Li M., Wang Y., Bai L., Chang N., Nan G., Hu D., Zhang Y., Wei W. Solvent-free synthesis of SAPO-34 nanocrystals with reduced template consumption for methanol-to-olefins process. Appl. Catal. A Gen. 2017;531:203–211. doi: 10.1016/j.apcata.2016.11.005. [DOI] [Google Scholar]
- 28.Cheng C.-H., Bae T.-H., McCool B.A., Chance R.R., Nair S., Jones C.W. Functionalization of the internal surface of pure-silica MFI zeolite with aliphatic alcohols. J. Phys. Chem. C. 2008;112:3543–3551. doi: 10.1021/jp709867k. [DOI] [Google Scholar]
- 29.KerryáThomas J. Synthesis of microporous titanosilicates ETS-10 and ETS-4 using solid TiO2 as the source of titanium. Chem. Commun. 1996:1435–1436. doi: 10.1039/CC9960001433. [DOI] [Google Scholar]
- 30.Chuah C.Y., Yang Y., Bae T.-H. Hierarchically porous polymers containing triphenylamine for enhanced SF6 separation. Micropor. Mesopor. Mater. 2018;272:232–240. doi: 10.1016/j.micromeso.2018.06.039. [DOI] [Google Scholar]
- 31.Mason J.A., Sumida K., Herm Z.R., Krishna R., Long J.R. Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011;4:3030–3040. doi: 10.1039/c1ee01720a. [DOI] [Google Scholar]
- 32.Mathias P.M., Kumar R., Moyer J.D., Schork J.M., Srinivasan S.R., Auvil S.R., Talu O. Correlation of multicomponent gas adsorption by the dual-site Langmuir model. Application to nitrogen/oxygen adsorption on 5A-zeolite. Ind. Eng. Chem. Res. 1996;35:2477–2483. doi: 10.1021/ie950291y. [DOI] [Google Scholar]
- 33.Yang Y., Goh K., Chuah C.Y., Karahan H.E., Birer Ö., Bae T.-H. Sub-Ångström-level engineering of ultramicroporous carbons for enhanced sulfur hexafluoride capture. Carbon. 2019;155:56–64. doi: 10.1016/j.carbon.2019.08.034. [DOI] [Google Scholar]
- 34.Ma Y., Jue M.L., Zhang F., Mathias R., Jang H.Y., Lively R.P. Creation of well-defined “mid-sized” micropores in carbon molecular sieve membranes. Angew. Chem. 2019;131:13393–13399. doi: 10.1002/ange.201903105. [DOI] [PubMed] [Google Scholar]
- 35.Kim C., Cho H.S., Chang S., Cho S.J., Choi M. An ethylenediamine-grafted Y zeolite: A highly regenerable carbon dioxide adsorbent via temperature swing adsorption without urea formation. Energy Environ. Sci. 2016;9:1803–1811. doi: 10.1039/C6EE00601A. [DOI] [Google Scholar]
- 36.Dargahi M., Kazemian H., Soltanieh M., Hosseinpour M., Rohani S. High temperature synthesis of SAPO-34: Applying an L9 Taguchi orthogonal design to investigate the effects of experimental parameters. Powder Technol. 2012;217:223–230. doi: 10.1016/j.powtec.2011.10.030. [DOI] [Google Scholar]
- 37.Vinoth Kumar R., Pugazhenthi G. Removal of chromium from synthetic wastewater using MFI zeolite membrane supported on inexpensive tubular ceramic substrate. J. Water Reuse Desal. 2017;7:365–377. doi: 10.2166/wrd.2016.096. [DOI] [Google Scholar]
- 38.Thommes M., Kaneko K., Neimark A.V., Olivier J.P., Rodriguez-Reinoso F., Rouquerol J., Sing K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report) Pure Appl. Chem. 2015;87:1051–1069. doi: 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- 39.Yang Y., Chuah C.Y., Bae T.-H. Polyamine-appended porous organic polymers for efficient post-combustion CO2 capture. Chem. Eng. J. 2019;358:1227–1234. doi: 10.1016/j.cej.2018.10.122. [DOI] [Google Scholar]
- 40.Bae T.-H., Hudson M.R., Mason J.A., Queen W.L., Dutton J.J., Sumida K., Micklash K.J., Kaye S.S., Brown C.M., Long J.R. Evaluation of cation-exchanged zeolite adsorbents for post-combustion carbon dioxide capture. Energy Environ. Sci. 2013;6:128–138. doi: 10.1039/C2EE23337A. [DOI] [Google Scholar]
- 41.Li J.-R., Kuppler R.J., Zhou H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009;38:1477–1504. doi: 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- 42.Murray L.J., Dinca M., Yano J., Chavan S., Bordiga S., Brown C.M., Long J.R. Highly-selective and reversible O2 binding in Cr3(1,3,5-benzenetricarboxylate)2. J. Am. Chem. Soc. 2010;132:7856–7857. doi: 10.1021/ja1027925. [DOI] [PubMed] [Google Scholar]
- 43.Bloch E.D., Murray L.J., Queen W.L., Chavan S., Maximoff S.N., Bigi J.P., Krishna R., Peterson V.K., Grandjean F., Long G.J. Selective binding of O2 over N2 in a redox–active metal–organic framework with open iron (II) coordination sites. J. Am. Chem. Soc. 2011;133:14814–14822. doi: 10.1021/ja205976v. [DOI] [PubMed] [Google Scholar]
- 44.Jaffe A., Ziebel M.E., Halat D.M., Biggins N., Murphy R.A., Chakarawet K., Reimer J.A., Long J.R. Selective, High-Temperature O2 Adsorption in Chemically Reduced, Redox-Active Iron-Pyrazolate Metal–Organic Frameworks. J. Am. Chem. Soc. 2020;142:14627–14637. doi: 10.1021/jacs.0c06570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed D.A., Xiao D.J., Jiang H.Z., Chakarawet K., Oktawiec J., Long J.R. Biomimetic O2 adsorption in an iron metal–organic framework for air separation. Chem. Sci. 2020;11:1698–1702. doi: 10.1039/C9SC06047B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goh P., Ismail A., Sanip S., Ng B., Aziz M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011;81:243–264. doi: 10.1016/j.seppur.2011.07.042. [DOI] [Google Scholar]
- 47.Gong H., Lee S.S., Bae T.-H. Mixed-matrix membranes containing inorganically surface-modified 5A zeolite for enhanced CO2/CH4 separation. Micropor. Mesopor. Mater. 2017;237:82–89. doi: 10.1016/j.micromeso.2016.09.017. [DOI] [Google Scholar]
- 48.Fuertes A., Nevskaia D., Centeno T. Carbon composite membranes from Matrimid® and Kapton® polyimides for gas separation. Micropor. Mesopor. Mater. 1999;33:115–125. doi: 10.1016/S1387-1811(99)00129-8. [DOI] [Google Scholar]
- 49.Wang D., Tian P., Yang M., Xu S., Fan D., Su X., Yang Y., Wang C., Liu Z. Synthesis of SAPO-34 with alkanolamines as novel templates and their application for CO2 separation. Micropor. Mesopor. Mater. 2014;194:8–14. doi: 10.1016/j.micromeso.2014.03.028. [DOI] [Google Scholar]
- 50.Bao Z., Yu L., Dou T., Gong Y., Zhang Q., Ren Q., Lu X., Deng S. Adsorption equilibria of CO2, CH4, N2, O2, and Ar on high silica zeolites. J. Chem. Eng. Data. 2011;56:4017–4023. doi: 10.1021/je200394p. [DOI] [Google Scholar]
- 51.Datta S.J., Khumnoon C., Lee Z.H., Moon W.K., Docao S., Nguyen T.H., Hwang I.C., Moon D., Oleynikov P., Terasaki O. CO2 capture from humid flue gases and humid atmosphere using a microporous coppersilicate. Science. 2015;350:302–306. doi: 10.1126/science.aab1680. [DOI] [PubMed] [Google Scholar]
- 52.Chuah C.Y., Yu S., Na K., Bae T.-H. Enhanced SF6 recovery by hierarchically structured MFI zeolite. J. Ind. Eng. Chem. 2018;62:64–71. doi: 10.1016/j.jiec.2017.12.045. [DOI] [Google Scholar]
- 53.Kim Y.K., Park H.B., Lee Y.M. Carbon molecular sieve membranes derived from thermally labile polymer containing blend polymers and their gas separation properties. J. Membr. Sci. 2004;243:9–17. doi: 10.1016/j.memsci.2004.05.001. [DOI] [Google Scholar]
- 54.Suda H., Haraya K. Gas permeation through micropores of carbon molecular sieve membranes derived from Kapton polyimide. J. Phys. Chem. B. 1997;101:3988–3994. doi: 10.1021/jp963997u. [DOI] [Google Scholar]
- 55.Xiao Y., Dai Y., Chung T.-S., Guiver M.D. Effects of brominating Matrimid polyimide on the physical and gas transport properties of derived carbon membranes. Macromolecules. 2005;38:10042–10049. doi: 10.1021/ma051354j. [DOI] [Google Scholar]
- 56.Barsema J., Balster J., Jordan V., Van der Vegt N., Wessling M. Functionalized carbon molecular sieve membranes containing Ag-nanoclusters. J. Membr. Sci. 2003;219:47–57. doi: 10.1016/S0376-7388(03)00176-5. [DOI] [Google Scholar]
- 57.Itta A.K., Tseng H.-H., Wey M.-Y. Effect of dry/wet-phase inversion method on fabricating polyetherimide-derived CMS membrane for H2/N2 separation. Int. J. Hydrog. Energy. 2010;35:1650–1658. doi: 10.1016/j.ijhydene.2009.12.069. [DOI] [Google Scholar]
- 58.Rao P.S., Wey M.-Y., Tseng H.-H., Kumar I.A., Weng T.-H. A comparison of carbon/nanotube molecular sieve membranes with polymer blend carbon molecular sieve membranes for the gas permeation application. Micropor. Mesopor. Mater. 2008;113:499–510. doi: 10.1016/j.micromeso.2007.12.008. [DOI] [Google Scholar]
- 59.Kita H., Yoshino M., Tanaka K., Okamoto K.-I. Gas permselectivity of carbonized polypyrrolone membrane. Chem. Commun. 1997:1051–1052. doi: 10.1039/a700048k. [DOI] [Google Scholar]
- 60.Yoshimune M., Fujiwara I., Haraya K. Carbon molecular sieve membranes derived from trimethylsilyl substituted poly (phenylene oxide) for gas separation. Carbon. 2007;45:553–560. doi: 10.1016/j.carbon.2006.10.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.