Key Points
Question
Do the incidence and risk of suicide among patients with head and neck cancer differ by rural vs urban or metropolitan residence status?
Findings
In this population-based cross-sectional study, the suicide mortality rate among patients with head and neck cancer was 59, 64, and 127 per 100 000 person-years among residents of metropolitan, urban, and rural counties, respectively. In competing-risk Fine-Gray proportional hazards models accounting for covariates, the suicide risk was nearly 2 times higher for residents of rural counties.
Meaning
This study suggests that suicide incidence is elevated in general among patients with head and neck cancer but is markedly higher for patients living in rural areas.
Abstract
Importance
Patients with head and neck cancer (HNC) are known to be at increased risk of suicide compared with the general population, but there has been insufficient research on whether this risk differs based on patients’ rural, urban, or metropolitan residence status.
Objective
To evaluate whether the risk of suicide among patients with HNC differs by rural vs urban or metropolitan residence status.
Design, Setting, and Participants
This cross-sectional study uses data from the Surveillance, Epidemiology, and End Results database on patients aged 18 to 74 years who received a diagnosis of HNC from January 1, 2000, to December 31, 2016. Statistical analysis was conducted from November 27, 2020, to June 3, 2021.
Exposures
Residence status, assessed using 2013 Rural Urban Continuum Codes.
Main Outcomes and Measures
Death due to suicide was assessed by International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes (U03, X60-X84, and Y87.0) and the cause of death recode (50220). Standardized mortality ratios (SMRs) of suicide, assessing the suicide risk among patients with HNC compared with the general population, were calculated. Suicide risk by residence status was compared using Fine-Gray proportional hazards regression models.
Results
Data from 134 510 patients with HNC (101 142 men [75.2%]; mean [SE] age, 57.7 [10.3] years) were analyzed, and 405 suicides were identified. Metropolitan residents composed 86.6% of the sample, urban residents composed 11.7%, and rural residents composed 1.7%. The mortality rate of suicide was 59.2 per 100 000 person-years in metropolitan counties, 64.0 per 100 000 person-years in urban counties, and 126.7 per 100 000 person-years in rural counties. Compared with the general population, the risk of suicide was markedly higher among patients with HNC in metropolitan (SMR, 2.78; 95% CI, 2.49-3.09), urban (SMR, 2.84; 95% CI, 2.13-3.71), and rural (SMR, 5.47; 95% CI, 3.06-9.02) areas. In Fine-Gray competing-risk analyses that adjusted for other covariates, there was no meaningful difference in suicide risk among urban vs metropolitan residents. However, compared with rural residents, residents of urban (subdistribution hazard ratio, 0.52; 95% CI, 0.29-0.94) and metropolitan counties (subdistribution hazard ratio, 0.55; 95% CI, 0.32-0.94) had greatly lower risk of suicide.
Conclusions and Relevance
The findings of this cross-sectional study suggest that suicide risk is elevated in general among patients with HNC but is significantly higher for patients residing in rural areas. Effective suicide prevention strategies in the population of patients with HNC need to account for rural health owing to the high risk of suicide among residents with HNC in rural areas.
This cross-sectional study uses data from the Surveillance, Epidemiology, and End Results database to evaluate whether the risk of suicide among patients with head and neck cancer differs by rural vs urban or metropolitan residence status.
Introduction
Suicide has been the eighth leading cause of death among male individuals and the 10th leading cause of death among male and female individuals combined since 2008.1,2 At the end of June 2020 (during the COVID-19 pandemic), 11% of adults in the United States reported that they had seriously considered suicide within the prior 30 days.3 Suicide mitigation is considered a national imperative; the Healthy People initiative and Zero Suicide campaign target between a 10% and 20% decrease in the suicide rate among the general population within a decade.4,5,6 Suicide has also been designated as 1 of 12 leading health indicators in the United States.7
Although suicide rates are high in the general population, the rate is significantly higher among cancer survivors and especially survivors of head and neck cancer (HNC). Suicide mortality among individuals with HNC is double that of individuals with cancer at other sites and about 4 times that of the general population.8 In addition, the suicide rate in the population with HNC has increased by 27% between 2000 and 2014,8 a significant amount given that there are currently half a million individuals in the United States outliving their HNC diagnosis.9,10
Studies have identified factors associated with suicide among patients with HNC, including anatomical subsite, race, sex, marital status, and human papillomavirus status.11,12,13 However, to our knowledge, little is known about how contextual factors such as rurality might be associated with suicide incidence and/or risk. About 1 in 5 cancer survivors resides in a rural area in the United States,14 and overall survival is lower among patients in rural areas.15 It is unknown whether area of residence increases the risk of suicide among patients with HNC. In the general population, evidence suggests that there are differential rates of suicide based on urban vs rural residential status.16,17,18,19 However, no similar investigation has been conducted in the population with HNC, to our knowledge. Such an investigation is imperative given the vast inequities of health care access and delivery that exist across rural communities,20 including access to cancer and mental health services.21
We hypothesized that significant differences exist in suicide incidence and risk among patients with HNC who reside in rural areas vs those in urban or metropolitan areas. To test our hypothesis, we calculated the suicide incidence among patients with HNC in rural vs urban or metropolitan areas and estimated differences in the risk of suicide among patients with HNC based on area of residence.
Methods
Study Sample
The Surveillance, Epidemiology, and End Results (SEER) Program database was queried for patients with HNC aged 18 to 74 years who received a diagnosis from January 1, 2000, to December 31, 2016. The Duke University Institutional Review Board determined that this study was exempt from review because it was based on publicly available, deidentified data and waived the need for informed patient consent because the study was a secondary analysis of publicly available data. Sites of HNC were defined as malignant neoplasms of the lip, oral cavity, pharynx, nasopharynx, sinuses, and larynx; these were later grouped as oropharynx and nonoropharynx. The primary outcome variable was the risk of suicide, based on International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes (U03, X60-X84, and Y87.0) and the cause of death recode (50220), and survival months, a measure of time from diagnosis until death or censoring (if alive past December 31, 2016). The primary measure was residential status based on the 2013 Rural-Urban Continuum Codes (RUCCs), defined as metropolitan (counties in metropolitan areas; RUCC designation 1-3), urban (counties with an urban population of ≥2500; RUCC designation 4-7), or rural (completely rural counties or <2500 urban population; RUCC designation 8-9). Covariates including age, race/ethnicity, sex, marital status, county-level income and educational level, cancer site, and cancer stage at diagnosis were also extracted. Cases with missing covariate information were excluded.
In addition, we collected information from the SEER*Stat program regarding suicide counts in the population with HNC compared with the expected suicide incidence based on the general population. The program estimates the rate of suicides per 100 000 person-years among cancer survivors and compares that rate with the general population, and this is called the standardized mortality ratio (SMR) of suicide. Standardized mortality ratios were calculated overall and by residential status, based on 2013 RUCCs. Analyses were restricted to cases with known rural, urban, or metropolitan residence (19 cases with 0 suicides had unknown or undefined residence). There was otherwise no missing information or exclusions in these analyses.
Statistical Analysis
Statistical analysis was conducted from November 27, 2020, to June 3, 2021. We used survival analysis methods suitable for competing risks because traditional survival analysis methods may overestimate the risk of an outcome in the presence of competing risks. In the setting of cancer-related suicide, death from another cause (particularly cancer) is a major competing risk. We used cumulative incidence analyses to plot the incidence of suicide over time. Fine-Gray proportional hazards regression models22 were used to estimate the association between residential status and suicide risk, with or without adjustment for covariates. Covariates included in the primary model were residence, age, race/ethnicity, sex, marital status, cancer site, and cancer stage at diagnosis. The proportional hazards assumption was examined using Schoenfeld residuals over time and using a test developed by Grambsch and Therneau.23 Covariates that failed the proportional hazards assumption (age, stage, and marital status) were used as strata. The final models had no significant deviations from the proportional hazards assumption. All P values were 2-sided, and we considered P < .05 statistically significant.
Sensitivity Analyses
County-level income and educational level covariates were not included in the primary model owing to suspected collinearity with residence, which was also based on county information. However, because there may be some important socioeconomic information that may be obtained at the county level, we performed sensitivity analyses including county-level income and educational level. Insurance status was not collected until 2007, but because it may be an important factor associated with suicide risk in the general population and a proxy for individual-level socioeconomic situation, we conducted sensitivity analyses with the inclusion of insurance status (defined as uninsured, Medicaid, and private or other insurance [which includes Medicare]) as a covariate, limited to the period from 2007 to 2016.
Results
A total of 147 810 individuals with HNC were identified. Owing to missing data, 13 300 cases were excluded, for a sample of 134 510 patients with HNC (101 142 men [75.2%]; mean [SE] age, 57.7 [10.3] years) (Table 1). Metropolitan residents comprised 86.6% of the sample, urban residents comprised 11.7%, and rural residents comprised 1.7%. Demographic information of included and excluded cases were similar with regard to suicide, residential status, age, race/ethnicity, sex, county-level income and educational level, and cancer site; there were relatively more unknown or unstaged cancers in the missing group, with subsequent relatively lower rates of other stages except localized disease at diagnosis (eTable 1 in the Supplement). There were 405 suicides among the cohort; those who died by suicide were mostly non-Hispanic White (90.1%) and male (93.1%). The median time from diagnosis to death by suicide in these patients was 26 months (range, 0-187 months). For all patients, the median follow-up time was 41.0 months (range, 0-203 months).
Table 1. Characteristics of the Study Population.
| Characteristic | Patients with head and neck cancer, No. (%) | ||
|---|---|---|---|
| Total (N = 134 510) | No suicide (n = 134 105) | Death from suicide (n = 405) | |
| Age, y | |||
| 18-39 | 6871 (5.1) | 6855 (5.1) | 16 (4.0) |
| 40-64 | 89 917 (66.8) | 89 641 (66.8) | 276 (68.1) |
| 65-74 | 37 722 (28.0) | 37 609 (28.0) | 113 (27.9) |
| Race/ethnicity | |||
| Non-Hispanic White | 98 444 (73.2) | 98 079 (73.1) | 365 (90.1) |
| Non-Hispanic othera | 9256 (6.9) | 9241 (6.9) | 15 (3.7) |
| Non-Hispanic Black | 15 952 (11.9) | 15 940 (11.9) | 12 (3.0) |
| Hispanic | 10 858 (8.1) | 10 845 (8.1) | 13 (3.2) |
| Sex | |||
| Male | 101 142 (75.2) | 100 765 (75.1) | 377 (93.1) |
| Female | 33 368 (24.8) | 33 340 (24.9) | 28 (6.9) |
| County-level income | |||
| High | 30 426 (22.6) | 30 348 (22.6) | 78 (19.3) |
| Mid-high | 33 868 (25.2) | 33 759 (25.2) | 109 (26.9) |
| Mid-low | 31 612 (23.5) | 31 519 (23.5) | 93 (23.0) |
| Low | 38 604 (28.7) | 38 479 (28.7) | 125 (30.9) |
| County-level educational level | |||
| High | 31 400 (23.3) | 31 305 (23.3) | 95 (23.5) |
| Mid-high | 31 231 (23.2) | 31 137 (23.2) | 94 (23.2) |
| Mid-low | 37 531 (27.9) | 37 429 (27.9) | 102 (25.2) |
| Low | 34 348 (25.5) | 34 234 (25.5) | 114 (28.1) |
| Residence | |||
| Metropolitan | 116 532 (86.6) | 116 190 (86.6) | 342 (84.4) |
| Urban | 15 710 (11.7) | 15 661 (11.7) | 49 (12.1) |
| Rural | 2268 (1.7) | 2254 (1.7) | 14 (3.5) |
| Cancer site | |||
| Nonoropharynx | 130 707 (97.2) | 130 320 (97.2) | 387 (95.6) |
| Oropharynx | 3803 (2.8) | 3785 (2.8) | 18 (4.4) |
| Stage at diagnosis | |||
| Distant | 12 953 (9.6) | 12 925 (9.6) | 28 (6.9) |
| Localized | 36 119 (26.9) | 36 004 (26.8) | 115 (28.4) |
| Regional | 28 159 (20.9) | 28 065 (20.9) | 94 (23.2) |
| Unknown or unstaged | 57 279 (42.6) | 57 111 (42.6) | 168 (41.5) |
| Marital status | |||
| Unmarried | 57 763 (42.9) | 57 573 (42.9) | 190 (46.9) |
| Married | 76 747 (57.1) | 76 532 (57.1) | 215 (53.1) |
Other race includes those who were Asian or Pacific Islander or American Indian or Alaska Native.
Suicide incidence differed greatly based on residence status. There were 59.2 suicides per 100 000 person-years in metropolitan counties, 64.0 suicides per 100 000 person-years in urban counties, and 126.7 suicides per 100 000 person-years in rural counties (Table 2). Compared with the general population, the risk of suicide for patients with HNC was significantly elevated, regardless of metropolitan (SMR, 2.78; 95% CI, 2.49-3.09), urban (SMR, 2.84; 95% CI, 2.13-3.71), or rural (SMR, 5.47; 95% CI, 3.06-9.02) residence status.
Table 2. Incidence and SMRs of Suicide by RUCC.
| Residential status by RUCC 2013 | No. of suicides | Incidence per 100 000 person-years | SMR (95% CI) |
|---|---|---|---|
| Metropolitan | |||
| All metropolitan counties | 347 | 59.2 | 2.78 (2.49-3.09) |
| Counties in metropolitan areas ≥1 million population | 218 | 55.3 | 2.62 (2.29-2.99) |
| Counties in metropolitan areas of 250 000 to 1 million population | 87 | 63.2 | 2.92 (2.34-3.61) |
| Counties in metropolitan areas of <250 000 population | 42 | 77.6 | 3.49 (2.51-4.71) |
| Nonmetropolitan | |||
| All nonmetropolitan counties | 69 | 71.7 | 3.17 (2.47-4.01) |
| Urban | |||
| All urban counties | 54 | 64.0 | 2.84 (2.13-3.71) |
| Urban population of ≥20 000 adjacent to a metropolitan area | 9 | 44.3 | 1.92 (0.88-3.64) |
| Urban population of ≥20 000 not adjacent to a metropolitan area | 9 | 57.0 | 2.66 (1.22-5.05) |
| Urban population of 2500 to 19 999, adjacent to a metropolitan area | 17 | 58.1 | 2.62 (1.52-4.19) |
| Urban population of 2500 to 19 999, not adjacent to a metropolitan area | 19 | 99.9 | 4.28 (2.58-6.68) |
| Rural | |||
| All rural counties | 15 | 126.7 | 5.47 (3.06-9.02) |
| Completely rural <2500 urban population, adjacent to a metropolitan area | 8 | 155.4 | 6.86 (2.96-13.52) |
| Completely rural <2500 urban population, not adjacent to metropolitan area | 7 | 104.5 | 4.44 (1.79-9.15) |
Abbreviations: RUCC, Rural-Urban Continuum Code; SMR, standardized mortality ratio.
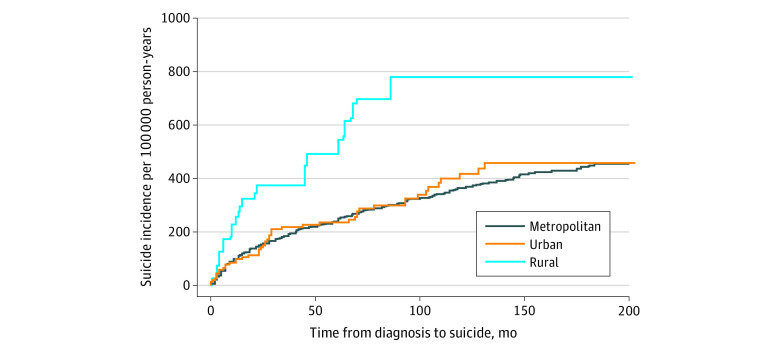
In cumulative incidence analyses, the incidence of suicide was also highest among rural patients with HNC (Figure). In unadjusted Fine-Gray models, relative to residents of rural counties, those in urban (hazard ratio [HR], 0.51; 95% CI, 0.28-0.92) and metropolitan counties (HR, 0.48; 95% CI, 0.28-0.82) had approximately half the risk of suicide. There was no difference between residents of urban and metropolitan counties (HR, 1.06; 95% CI, 0.78-1.42). Results were similar after accounting for covariates. Compared with residents of rural counties, residents of urban (subdistribution HR [sdHR], 0.52; 95% CI, 0.29-0.94) and metropolitan counties (sdHR, 0.55; 95% CI, 0.32-0.94) had approximately half the risk of suicide (Table 3).
Figure. Cumulative Incidence of Suicide by Residential Status.
Cumulative incidence curve shows increased incidence of suicide among patients with head and neck cancer in rural areas compared with those living in metropolitan and urban areas.
Table 3. Competing Risks Proportional Hazards Model Estimating Death by Suicide Among Patients With Head and Neck Cancer Based on Residence.
| Characteristic | sdHR (95% CI)a |
|---|---|
| Residence | |
| Rural | 1 [Reference] |
| Urban | 0.52 (0.29-0.94) |
| Metropolitan | 0.55 (0.32-0.94) |
| Race/ethnicity | |
| Non-Hispanic White | 1 [Reference] |
| Non-Hispanic Black | 0.19 (0.11-0.35) |
| Non-Hispanic otherb | 0.53 (0.31-0.89) |
| Hispanic | 0.36 (0.21-0.6) |
| Sex | |
| Male | 1 [Reference] |
| Female | 0.23 (0.15-0.33) |
| Cancer site | |
| Nonoropharynx | 1 [Reference] |
| Oropharynx | 1.63 (1.00-2.67) |
Abbreviation: sdHR, subdistribution hazard ratio.
The proportional hazards model was adjusted for age, race/ethnicity, sex, marital status, cancer site, and stage at diagnosis. Although not included as traditional covariates in the regression model, estimates were stratified by age, marital status, and stage at diagnosis (owing to nonproportional hazards in these variables). Note that the proportional hazards assumption was satisfied for this model.
Other race includes those who were Asian or Pacific Islander or American Indian or Alaska Natives.
In sensitivity analyses, the results were largely consistent (eTable 2 in the Supplement). When including county-level income and educational level in the regression model, there was a slight decrease in the effect size (relative to rural counties: sdHR for metropolitan counties, 0.62; 95% CI, 0.35-1.09; sdHR for urban counties, 0.55; 95% CI, 0.30-1.00). Limiting the sample to diagnoses from 2007 to 2016 and including insurance as a covariate, point estimates of suicide risk by residence status remained similar to the primary model, with expected decreases in precision due to the smaller sample size.
Discussion
The objective of this study was to estimate suicide incidence and risk among patients with HNC based on rural vs urban or metropolitan residential status. Previous studies have shown that there is higher HNC incidence and mortality among patients from rural areas vs urban areas.24,25 The general US population is aging, and so is the population of HNC survivors.26,27,28 A large proportion of older individuals live in rural areas,14 and the mean age of HNC diagnosis in the United States in the last 4 decades has been approximately 62.5 years.29 In the last 2 decades, the 45- to 64-year-old age group has seen stable to significantly increased rates of suicide in the general population.30 We therefore hypothesized that there are significant differences in suicide incidence and risk among patients with HNC in rural areas vs those in urban or metropolitan areas. Our findings support this hypothesis. We found that suicide incidence for patients with HNC was elevated across residential strata; however, it was markedly elevated for patients in rural areas. Understanding the underlying mechanisms underpinning these findings may contribute to mitigating suicide risks not just among patients with HNC and may also have broader implications across the cancer continuum.
Several studies have described an increased incidence of suicide among patients with HNC; however, the present study describes for the first time, to our knowledge, differences in suicide incidence based on rural vs urban or metropolitan residence. We report a 2-fold increased suicide incidence among patients with HNC in rural areas compared with those in urban or metropolitan areas. This increased suicide incidence among patients with HNC in rural areas may be associated with socioeconomic differences, based on extrapolations from the suicide literature in the general population.31,32 The potential association of socioeconomic status with suicide risk among patients with HNC could manifest in several ways. For example, patients with HNC in rural areas with lower socioeconomic status may have differential access to care.33 This differential access to HNC care due to rural vs urban residence is pervasive and has been reported even in countries with so-called universal health systems, such as Canada.34,35 In the United States, the complex nature of cancer care means that centralized or regionalized care is encouraged, and most of the high-volume facilities are typically academic centers in urban or metropolitan areas.36,37,38 However, not all patients with HNC are able to travel for care39; studies have shown that patients with lower socioeconomic status are significantly less likely to travel for care, and being able to travel 402.3 or more kilometers for cancer care is associated with better survival.40,41 Rural cancer survivors, on the other hand, are more likely to forgo care owing to cost or to face other types of hardships associated with receiving care.33,42 It might be that this lack of access to quality HNC care in rural areas is associated with hopelessness, described as an essential factor in the desire for hastened death.43,44,45
Closely associated with inadequate access to high-quality HNC care is the concomitant lack of access to mental health care and a disparate mental health care infrastructure in rural areas vs urban or metropolitan areas.46,47 Patients with HNC may have a higher rate of mental health conditions and/or substance use than the general population,48 yet many patients with cancer, HNC included, may not regularly seek professional mental health care, and while they may have overall higher rates of health care use, they are significantly more likely to have non–mental health care hospital visits.49 Thus, oncologists may be the last health care professionals to see patients with cancer before their suicide.45 This disparate availability of mental health care services in rural areas46,47 is concerning in the cancer context because patients with cancer in rural areas are more likely to report poor mental functioning, anxiety, distress, and depression,50 all of which are associated with suicide.45 Patients with HNC may prioritize survival, cure, or prevention of recurrence over mental wellness or psychosocial quality of life,51,52,53 but this prioritization might be exacerbated by both the shortage and inadequate integration of mental health services into cancer care.50,54 Further exploration of this dearth of mental health services in rural areas is critical in mitigating suicide risks among patients with HNC in these areas.
Although suicide incidence varied among patients with HNC based on rural vs urban or metropolitan residential status, the difference in suicide mortality in the context of the general population is significant. We report that, compared with the general population, rural residents with HNC have more than 5 times the suicide mortality rate. Previous studies in the general literature show that the suicide rate is higher in rural areas of the United States,55 and the present study corroborates these data in the population with HNC. Head and neck cancer has the second-highest suicide mortality rate of all cancers,8 and the factors associated with increased risk of suicide might be exacerbated in patients with HNC living in rural areas. For example, suicide risk is elevated among individuals with a history of alcoholism, substance abuse, and preexisting psychiatric illness, including depression. All of these comorbid factors are not only common among rural residents but are also increased among patients with HNC.48,56 In the presence of social isolation, which may be more acute in rural than in urban areas, the functional and esthetic dysfunction associated with HNC treatment could become difficult to manage and may increase patients’ emotional distress, depression, and perceived quality of life, and therefore the ability to cope with the disease might diminish to the extent that suicide becomes a means to end perceived pain.57
An additional concern that may be associated with the increased rate of suicide among patients with HNC in rural areas could be access to lethal means of suicide. In 2017, the percentage of households in rural areas of the United States that owned at least 1 firearm was more than double that of urban areas (46% vs 19%).58 Although not examined in the present study owing to lack of data, increased access to lethal means of suicide in rural areas has been highlighted as a primary factor associated with the increasing suicide rate in the general population.17,59 In the cancer survivor population specifically, one of the largest studies of cancer-associated suicide indicated that patients with cancer are 35% more likely to die by firearm injury compared with matched controls of individuals who also died by suicide but without any history of cancer.60 Future studies of suicide associated with HNC may be able to corroborate the association between access to lethal means of suicide and increased risk of suicide in this population.
Limitations and Strengths
There are important limitations to this study. First, our data are retrospective, and we are unable to make causal inferences about the association between rural status and suicide among patients with HNC. Second, we were unable to estimate individual-level socioeconomic characteristics that may be associated with rural living, including income and employment history. Third, our data do not provide information on other factors associated with suicide, including depression, history of alcohol use and/or substance abuse, or preexisting psychiatry comorbidity. Fourth, we did not have any data on means of suicide, and we were unable to make any correlation between population-level differences in access to lethal means associated with the population with HNC. Fifth, although the SEER database is highly validated and regarded as a premium-quality database, suicide as a cause of death is fraught with misclassification.
Notwithstanding these limitations, this study also has some strengths. It makes an important contribution to the literature, describing for the first time, to our knowledge, the differences in suicide incidence among patients with HNC who reside in rural, urban, and metropolitan areas, as well as differences between suicide rates in the population with HNC based on urban vs rural residence compared with the general population.
Clinical and Public Health Implications
One person dies by suicide in the United States every 11 minutes.61 However, the present study suggests that, compared with the general population, suicide incidence is 3 times more likely for patients with HNC who reside in urban or metropolitan areas and 5 times more likely for patients with HNC in rural areas. Without making causal inferences, these data warrant renewed focus on the multilevel factors underpinning the suicide burden of cancer survivors in general and those with HNC in particular. Otherwise, the well-intended national goals aimed at mitigating suicides may be unrealistic.
There are several questions raised by the present study that require further attention and broader transdisciplinary implications, at the very least, in epidemiology, implementation science, and health policy. First, mental health infrastructure in the rural United States needs more attention so that patients could have improved access to the help they need when receiving cancer care. Head and neck cancer is considered to be the most distressing of all cancers, and depending on the measure used, up to 1 in 2 patients with HNC may have a history of depression.62 Therefore, it is critical that mental health services are readily available for these patients, especially in rural areas where care is often decentralized. Second, there may be a need to develop a balanced, culturally appropriate approach to firearm ownership and access to lethal means of suicide, especially in high-risk areas such as rural areas where there is a higher proportion of firearm ownership. Third, the disfiguring nature of HNC is known to be associated with social isolation, deprivation, and lack of social support; however, social support is known to be associated with better overall, disease-specific, and suicide-specific mortality in the population of patients with HNC.13,63,64 There is a need to build social support into mainstream care for these patients to ameliorate the negative psychosocial quality of life associated with HNC care. Fourth, future suicide prevention policies should have a specific focus on rural health. Although reducing the suicide rate is a national imperative and is considered a leading health indicator in the United States, evidence shows that rural health has not been specifically targeted by the current national strategy for suicide prevention. However, the evidence from the general literature as well as this study of the cancer site with the second-highest suicide mortality rate suggests that it is critical to specifically consider rural health when designing action plans for suicide prevention.
Conclusions
Suicide incidences among patients with HNC differ considerably based on residential status. Patients with HNC who reside in rural areas are 50% more likely to die by suicide compared with those who reside in urban or metropolitan areas. In addition, patients with HNC who reside in rural areas have more than 5 times the suicide mortality rate of the general population. It is critical that the multilevel factors associated with suicide risk are comprehensively understood and that future suicide prevention strategies are developed with an increased focus on rural health, quality of life, and mental well-being of cancer survivors, including HNC survivors, who are at higher risk for death by suicide compared with the general population.
eTable 1. Characteristics of Missing Data
eTable 2. Sensitivity Analyses
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Leading causes of death—males—all races and origins—United States, 2017. Accessed February 15, 2021. https://www.cdc.gov/healthequity/lcod/men/2017/all-races-origins/index.htm
- 3.Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic—United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi: 10.15585/mmwr.mm6932a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Academies of Sciences, Engineering, and Medicine . Improving care to prevent suicide among people with serious mental illness: proceedings of a workshop. National Academies Press; 2019. [PubMed] [Google Scholar]
- 5.Gordon JA, Avenevoli S, Pearson JL. Suicide prevention research priorities in health care. JAMA Psychiatry. 2020;77(9):885-886. doi: 10.1001/jamapsychiatry.2020.1042 [DOI] [PubMed] [Google Scholar]
- 6.Office of Disease Prevention and Health Promotion . Mental health: suicides (MHMD-1). Accessed February 16, 2021. https://www.healthypeople.gov/2020/leading-health-indicators/2020-lhi-topics/Mental-Health/data#MHMD-1
- 7.Office of Disease Prevention and Health Promotion . Healthy People 2020: who’s leading the leading health indicators? Accessed February 16, 2021. https://www.healthypeople.gov/sites/default/files/mental%20health%20ppt..pdf
- 8.Osazuwa-Peters N, Simpson MC, Zhao L, et al. Suicide risk among cancer survivors: head and neck versus other cancers. Cancer. 2018;124(20):4072-4079. doi: 10.1002/cncr.31675 [DOI] [PubMed] [Google Scholar]
- 9.Support for People with Oral and Head and Neck Cancer (SPOHNC). About SPOHNC. Accessed February 16, 2021. https://www.spohnc.org/about-us/
- 10.Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66(3):203-239. doi: 10.3322/caac.21343 [DOI] [PubMed] [Google Scholar]
- 11.Kam D, Salib A, Gorgy G, et al. Incidence of suicide in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1075-1081. doi: 10.1001/jamaoto.2015.2480 [DOI] [PubMed] [Google Scholar]
- 12.Briscoe J, Webb JA. Scratching the surface of suicide in head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2016;142(6):610-610. doi: 10.1001/jamaoto.2016.0255 [DOI] [PubMed] [Google Scholar]
- 13.Osazuwa-Peters N, Arnold LD, Loux TM, Varvares MA, Schootman M. Factors associated with increased risk of suicide among survivors of head and neck cancer: a population-based analysis. Oral Oncol. 2018;81:29-34. doi: 10.1016/j.oraloncology.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 14.Weaver KE, Geiger AM, Lu L, Case LD. Rural-urban disparities in health status among US cancer survivors. Cancer. 2013;119(5):1050-1057. doi: 10.1002/cncr.27840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashibe M, Kirchhoff AC, Kepka D, et al. Disparities in cancer survival and incidence by metropolitan versus rural residence in Utah. Cancer Med. 2018;7(4):1490-1497. doi: 10.1002/cam4.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivey-Stephenson AZ, Crosby AE, Jack SPD, Haileyesus T, Kresnow-Sedacca MJ. Suicide trends among and within urbanization levels by sex, race/ethnicity, age group, and mechanism of death—United States, 2001–2015. MMWR Surveill Summ. 2017;66(18):1-16. doi: 10.15585/mmwr.ss6618a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steelesmith DL, Fontanella CA, Campo JV, Bridge JA, Warren KL, Root ED. Contextual factors associated with county-level suicide rates in the United States, 1999 to 2016. JAMA Netw Open. 2019;2(9):e1910936. doi: 10.1001/jamanetworkopen.2019.10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graves JM, Abshire DA, Mackelprang JL, Amiri S, Beck A. Association of rurality with availability of youth mental health facilities with suicide prevention services in the US. JAMA Netw Open. 2020;3(10):e2021471. doi: 10.1001/jamanetworkopen.2020.21471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Searles VB, Valley MA, Hedegaard H, Betz ME. Suicides in urban and rural counties in the United States, 2006-2008. Crisis. 2014;35(1):18-26. doi: 10.1027/0227-5910/a000224 [DOI] [PubMed] [Google Scholar]
- 20.Urban MJ, Wojcik C, Eggerstedt M, Jagasia AJ. Rural-urban disparities in otolaryngology: the state of Illinois. Laryngoscope. 2021;131(1):E70-E75. doi: 10.1002/lary.28652 [DOI] [PubMed] [Google Scholar]
- 21.Miller CE, Vasan RS. The southern rural health and mortality penalty: A review of regional health inequities in the United States. Soc Sci Med. 2021;268:113443. doi: 10.1016/j.socscimed.2020.113443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 23.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 24.Clarke JA, Despotis AM, Ramirez RJ, Zevallos JP, Mazul AL. Head and neck cancer survival disparities by race and rural-urban context. Cancer Epidemiol Biomarkers Prev. 2020;29(10):1955-1961. doi: 10.1158/1055-9965.EPI-20-0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahnd WE, James AS, Jenkins WD, et al. Rural-urban differences in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1265-1274. doi: 10.1158/1055-9965.EPI-17-0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massa ST, Cass LM, Challapalli S, et al. Demographic predictors of head and neck cancer survival differ in the elderly. Laryngoscope. 2019;129(1):146-153. doi: 10.1002/lary.27289 [DOI] [PubMed] [Google Scholar]
- 27.Zumsteg ZS, Cook-Wiens G, Yoshida E, et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol. 2016;2(12):1617-1623. doi: 10.1001/jamaoncol.2016.1804 [DOI] [PubMed] [Google Scholar]
- 28.Tota JE, Best AF, Zumsteg ZS, Gillison ML, Rosenberg PS, Chaturvedi AK. Evolution of the oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J Clin Oncol. 2019;37(18):1538-1546. doi: 10.1200/JCO.19.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cline BJ, Simpson MC, Gropler M, et al. Change in age at diagnosis of oropharyngeal cancer in the United States, 1975-2016. Cancers (Basel). 2020;12(11):E3191. doi: 10.3390/cancers12113191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedegaard H, Curtin SC, Warner M. Suicide mortality in the United States, 1999-2017. NCHS Data Brief. 2018;(330):1-8. Accessed July 13, 2021. https://www.cdc.gov/nchs/data/databriefs/db330-h.pdf [PubMed] [Google Scholar]
- 31.Denney JT, Rogers RG, Krueger PM, Wadsworth T. Adult suicide mortality in the United States: marital status, family size, socioeconomic status, and differences by sex. Soc Sci Q. 2009;90(5):1167. doi: 10.1111/j.1540-6237.2009.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crosby AE, Ortega L, Stevens MR; Centers for Disease Control and Prevention (CDC) . Suicides—United States, 2005-2009. MMWR Suppl. 2013;62(3):179-183. [PubMed] [Google Scholar]
- 33.Mackley HB, Teslova T, Camacho F, Short PF, Anderson RT. Does rurality influence treatment decisions in early stage laryngeal cancer? J Rural Health. 2014;30(4):406-411. doi: 10.1111/jrh.12069 [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Dziegielewski PT, Jean Nguyen TT, et al. The effects of geography on survival in patients with oral cavity squamous cell carcinoma. Oral Oncol. 2015;51(6):578-585. doi: 10.1016/j.oraloncology.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 35.Walker BB, Schuurman N, Auluck A, Lear SA, Rosin M. Socioeconomic disparities in head and neck cancer patients’ access to cancer treatment centers. Rural Remote Health. 2017;17(3):4210. doi: 10.22605/RRH4210 [DOI] [PubMed] [Google Scholar]
- 36.Eskander A, Merdad M, Irish JC, et al. Volume-outcome associations in head and neck cancer treatment: a systematic review and meta-analysis. Head Neck. 2014;36(12):1820-1834. doi: 10.1002/hed.23498 [DOI] [PubMed] [Google Scholar]
- 37.David JM, Ho AS, Luu M, et al. Treatment at high-volume facilities and academic centers is independently associated with improved survival in patients with locally advanced head and neck cancer. Cancer. 2017;123(20):3933-3942. doi: 10.1002/cncr.30843 [DOI] [PubMed] [Google Scholar]
- 38.Verma V, Allen PK, Simone CB II, Gay HA, Lin SH. Association of treatment at high-volume facilities with survival in patients receiving chemoradiotherapy for nasopharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2018;144(1):86-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massa ST, Liebendorfer AP, Zevallos JP, Mazul AL. Distance traveled to head and neck cancer provider: a measure of socioeconomic status and access. Otolaryngol Head Neck Surg. 2020;162(2):193-203. doi: 10.1177/0194599819892015 [DOI] [PubMed] [Google Scholar]
- 40.Gaubatz ME, Bukatko AR, Simpson MC, et al. Racial and socioeconomic disparities associated with 90-day mortality among patients with head and neck cancer in the United States. Oral Oncol. 2019;89:95-101. doi: 10.1016/j.oraloncology.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 41.Graboyes EM, Ellis MA, Li H, et al. Racial and ethnic disparities in travel for head and neck cancer treatment and the impact of travel distance on survival. Cancer. 2018;124(15):3181-3191. doi: 10.1002/cncr.31571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer NR, Geiger AM, Lu L, Case LD, Weaver KE. Impact of rural residence on forgoing healthcare after cancer because of cost. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1668-1676. doi: 10.1158/1055-9965.EPI-13-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911. doi: 10.1001/jama.284.22.2907 [DOI] [PubMed] [Google Scholar]
- 44.Walker J, Waters RA, Murray G, et al. Better off dead: suicidal thoughts in cancer patients. J Clin Oncol. 2008;26(29):4725-4730. doi: 10.1200/JCO.2007.11.8844 [DOI] [PubMed] [Google Scholar]
- 45.McFarland DC, Walsh L, Napolitano S, Morita J, Jaiswal R. Suicide in patients with cancer: identifying the risk factors. Oncology (Williston Park). 2019;33(6):221-226. [PubMed] [Google Scholar]
- 46.Cyr ME, Etchin AG, Guthrie BJ, Benneyan JC. Access to specialty healthcare in urban versus rural US populations: a systematic literature review. BMC Health Serv Res. 2019;19(1):974. doi: 10.1186/s12913-019-4815-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales DA, Barksdale CL, Beckel-Mitchener AC. A call to action to address rural mental health disparities. J Clin Transl Sci. 2020;4(5):463-467. doi: 10.1017/cts.2020.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, Ba D, Liu G, Leslie D, Zacharia BE, Goyal N. Association of head and neck cancer with mental health disorders in a large insurance claims database. JAMA Otolaryngol Head Neck Surg. 2019;145(4):339-344. doi: 10.1001/jamaoto.2018.4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mausbach BT, Irwin SA. Depression and healthcare service utilization in patients with cancer. Psychooncology. 2017;26(8):1133-1139. doi: 10.1002/pon.4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burris JL, Andrykowski M. Disparities in mental health between rural and nonrural cancer survivors: a preliminary study. Psychooncology. 2010;19(6):637-645. doi: 10.1002/pon.1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagedar NA, Kendell N, Christensen AJ, Thomsen TA, Gist M, Seaman AT. Head and neck cancer survivorship from the patient perspective. Head Neck. 2020;42(9):2431-2439. doi: 10.1002/hed.26265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tschiesner U, Sabariego C, Linseisen E, et al. Priorities of head and neck cancer patients: a patient survey based on the brief ICF core set for HNC. Eur Arch Otorhinolaryngol. 2013;270(12):3133-3142. doi: 10.1007/s00405-013-2446-8 [DOI] [PubMed] [Google Scholar]
- 53.Windon MJ, D’Souza G, Faraji F, et al. Priorities, concerns, and regret among patients with head and neck cancer. Cancer. 2019;125(8):1281-1289. doi: 10.1002/cncr.31920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of rural cancer care in the United States. Oncology (Williston Park). 2015;29(9):633-640. [PubMed] [Google Scholar]
- 55.Pettrone K, Curtin SC. Urban-rural differences in suicide rates, by sex and three leading methods: United States, 2000-2018. NCHS Data Brief. 2020;(373):1-8. [PubMed] [Google Scholar]
- 56.Rieke K, Schmid KK, Lydiatt W, Houfek J, Boilesen E, Watanabe-Galloway S. Depression and survival in head and neck cancer patients. Oral Oncol. 2017;65:76-82. doi: 10.1016/j.oraloncology.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shneidman ES. Suicide as psychache. J Nerv Ment Dis. 1993;181(3):145-147. doi: 10.1097/00005053-199303000-00001 [DOI] [PubMed] [Google Scholar]
- 58.Statista . Percentage of the population in the United States with at least one gun in the household in 2017, by proximity to urban centers. Accessed March 31, 2021. https://www.statista.com/statistics/625196/firearm-ownership-rate-by-proximity-to-urban-centers-us/
- 59.Nestadt PS, Triplett P, Fowler DR, Mojtabai R. Urban-rural differences in suicide in the state of Maryland: the role of firearms. Am J Public Health. 2017;107(10):1548-1553. doi: 10.2105/AJPH.2017.303865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massetti GM, Holland KM, Jack SPD, Ragan KR, Lunsford NB. Circumstances of suicide among individuals with a history of cancer. Psychooncology. 2018;27(7):1750-1756. doi: 10.1002/pon.4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention. Suicide prevention: fast facts. Accessed June 29, 2021. https://www.cdc.gov/suicide/facts/index.html
- 62.Rohde RL, Adjei Boakye E, Challapalli SD, et al. Prevalence and sociodemographic factors associated with depression among hospitalized patients with head and neck cancer—results from a national study. Psychooncology. 2018;27(12):2809-2814. doi: 10.1002/pon.4893 [DOI] [PubMed] [Google Scholar]
- 63.Simpson MC, Challapalli SD, Cass LM, et al. Impact of gender on the association between marital status and head and neck cancer outcomes. Oral Oncol. 2019;89:48-55. doi: 10.1016/j.oraloncology.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 64.Osazuwa-Peters N, Christopher KM, Cass LM, et al. What’s love got to do with it? marital status and survival of head and neck cancer. Eur J Cancer Care. 2019;28(4):e13022. doi: 10.1111/ecc.13022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Missing Data
eTable 2. Sensitivity Analyses