Abstract
Aroma deterioration is one of the biggest problems in processing tea beverages. The aroma of tea infusion deteriorates fast during heat sterilization and the presence of ferrous ion (Fe2+) aggravates it. The underlying mechanism remains unveiled. In this study, Fe2+ was verified to deteriorate the aroma quality of green tea infusion with heat treatment. Catechins were necessary for Fe2+-mediated aroma deterioration. By enhancing the degradation of catechins, Fe2+ dramatically increased the production of hydrogen peroxide (H2O2). Fe2+ and H2O2 together exacerbated the aroma of green tea infusion with heat treatment. GC-MS analysis revealed that the presence of Fe2+ enhanced the loss of green/grassy volatiles and promoted the formation of new volatiles with diversified aroma characteristics, resulting in a dull scent of green tea infusion. Our results revealed how Fe2+ induced aroma deterioration of green tea infusion with heat treatment and could help guide tea producers in attenuating the aroma deterioration of tea infusion during processing.
Keywords: green tea infusion, aroma deterioration, ferrous ion, catechins, hydrogen peroxide
1. Introduction
Green tea has been traditionally and widely used as a beverage in China. Ready-to-drink tea is preferable in modern society because of its convenience. Compared with freshly-brewed tea, the aroma of ready-to-drink tea is less fresh, sometimes even dull, impairing its sensory quality. The aroma of tea infusion deteriorates fast during heat sterilization [1,2]. Green tea infusion, which contains a higher level of unoxidized catechins (Figure 1), tends to experience more severe aroma deterioration than black tea and dark tea infusions during heat treatment [3]. Aroma deterioration is positively correlated to the degradation of unoxidized catechins and the accumulation of hydrogen peroxide (H2O2) [4], suggesting catechins might be vital in the aroma deterioration. Nevertheless, there are other factors.
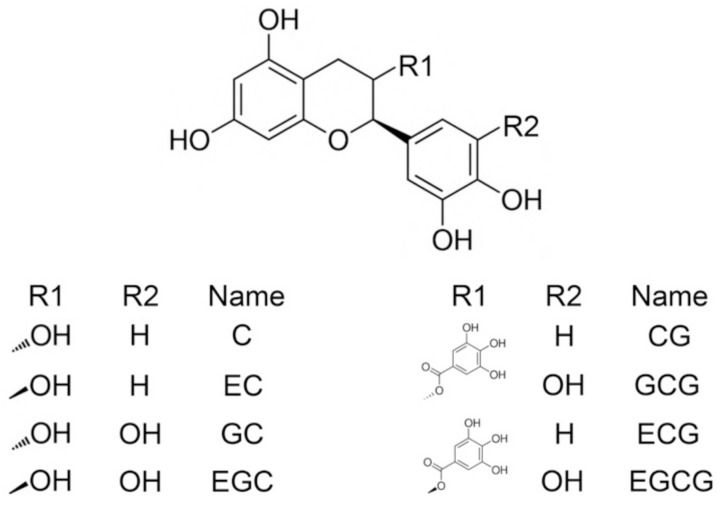
Figure 1.
Structural formula of catechins. C, EC, GC, EGC, CG, GCG, ECG, and EGCG are short for (+)-catechin, (−)-epicatechin, (−)-gallocatechin, (−)-epigallocatechin, (−)-catechin gallate, (−)-gallocatechin gallate, (−)-epicatechin gallate, and (−)-epigallocatechin gallate.
Many metal ions affect the aroma of tea infusion. Among common ions in tea infusion, calcium ion (Ca2+), ferrous ion (Fe2+), and zinc ion (Zn2+) have adverse effects on aroma [5,6]. In particular, Fe2+ deteriorates the aroma of tea infusion at concentrations as low as 0.1 ppm [6], implying aroma are very sensitive to Fe2+. However, the mechanism of Fe2+-mediated aroma deterioration remains unclear. To figure it out, the effects of Fe2+ on aroma quality, degradation of catechins, and generation of H2O2 in green tea infusion with heat treatment were assessed. The roles of catechins and H2O2 on Fe2+-mediated aroma deterioration were explored, respectively. The impacts of Fe2+ on volatile profiles were also evaluated. The results would deepen the knowledge of how Fe2+ deteriorated the aroma of tea infusion, and provide a new aspect for improving the aroma quality of ready-to-drink tea.
2. Results and Discussion
2.1. Fe2+ Deteriorates the Aroma Quality and Promotes the Degradation of Catechins in Green Tea Infusion with Heat Treatment
The untreated infusion had fresh green tea aroma (defined as “fresh”) (Table 1). Heat treatment of green tea infusion reduced the freshness of the aroma (defined as “slightly dull”). The presence of Fe2+ dose-dependently aggravated heat-induced aroma deterioration. It not only enhanced the reduction of the freshness, but also produced an off-aroma of the infusion (defined as “dull”).
Table 1.
Effect of Fe2+ on aroma quality of green tea infusion with heat treatment.
| Treatment | Description | Score |
|---|---|---|
| Untreated | Fresh | 8.7 ± 0.2 a |
| Heat treatment | Slightly dull | 7.0 ± 0.4 b |
| 2 μM FeCl2 + Heat treatment | Slightly dull | 6.5 ± 0.5 b |
| 4 μM FeCl2 + Heat treatment | Dull | 5.5 ± 0.4 c |
The description and score of infusions were given by a team of qualified panelists. A higher score indicates a better aroma acceptability. The same letter within each column indicates no significant difference (p > 0.05).
Aroma deterioration is associated with degradation of catechins [4]. Catechins are heat-sensitive. When incubated at 100 °C, epimerization was the predominant reaction of epi-form catechins whereas ester hydrolysis was the predominant reaction of GCG [7,8]. In this study, the concentration of total catechins was not significantly changed but the composition of catechins was changed after heat treatment (Table 2).
Table 2.
Effect of Fe2+ on the transformation of catechins in green tea infusion with heat treatment.
| Concentrations (μg/mL) | Untreated | Heat Treatment | 2 μM FeCl2 + Heat Treatment | 4 μM FeCl2 + Heat Treatment |
|---|---|---|---|---|
| GA | 4.41 ± 0.19 a | 12.6 ± 0.8 b | 13.0 ± 0.8 b | 13.4 ± 1.1 b |
| GC | 18.6 ± 0.1 a | 41.6 ± 1.3 b | 38.3 ± 0.6 c | 37.3 ± 0.1 d |
| EGC | 53.9 ± 3.6 a | 52.6 ± 2.7 a | 50.6 ± 3.3 a | 49.5 ± 2.0 a |
| C | 5.14 ± 0.18 a | 12.0 ± 0.8 b | 11.2 ± 0.1 b | 11.5 ± 0.2 b |
| EGCG | 818 ± 9 a | 582 ± 5 b | 568 ± 22 bc | 561 ± 5 c |
| EC | 45.5 ± 1.8 a | 33.2 ± 0.5 b | 33.0 ± 1.5 b | 32.9 ± 0.6 b |
| GCG | 5.07 ± 0.25 a | 219 ± 4 b | 200 ± 10 c | 197 ± 10 c |
| ECG | 93.3 ± 0.2 a | 95.7 ± 2.1 a | 93.7 ± 1.0 a | 92.9 ± 3.0 a |
| CG | 6.10 ± 0.11 a | 16.9 ± 0.2 b | 15.6 ± 1.0 bc | 15.5 ± 0.9 c |
| Total catechins | (1.05 ± 0.02) ×103 a | (1.05 ± 0.01) ×103 a | (1.01 ± 0.02) × 103 b | 998 ± 6 b |
| Non-gallated catechins | 123 ± 5 a | 139 ± 5 b | 133 ± 6 ab | 131 ± 3 a |
| Gallated catechins | 923 ± 9 a | 914 ± 7 a | 877 ± 10 b | 867 ± 9 b |
| Non-epi-catechins | 35.0 ± 0.2 a | 290 ± 6 b | 265 ± 11 c | 262 ± 11 c |
| Epi-catechins | (1.01 ± 0.01) ×103 a | 763 ± 6 b | 745 ± 26 bc | 737 ± 5 c |
Non-gallated catechins referred to GC, EGC, C, and EC, while gallated catechins referred to GCG, EGCG, CG, and ECG. Non-epi-catechins referred to GC, C, GCG, and CG, while epi-catechins referred to EGC, EC, EGCG, and ECG. The experiments were carried out in triplicate. The same letter within each row indicates no significant difference (p > 0.05).
The concentrations of two epi-form catechins, i.e., EGCG and EC, were decreased after heat treatment. Meanwhile, the concentrations of four non-epi-form catechins (GC, C, GCG, CG) and GA—a degradation product of catechins—were increased. The decrease of EGCG along with the increase of GCG indicated that the epimerization of EGCG took place under this condition. Notably, the loss of EGCG was higher than the increase of GCG. GC was increased though EGC was hardly changed. It suggested that part of GCG further degraded to GC. The change trends of EC and C were similar to that of EGCG and GCG, demonstrating the epimerization of EC also occurred. Taken together, heat treatment promoted the epimerization of EGCG and EC to their corresponding non-epi-forms, and triggered the production of non-gallated catechins. Compared with heated green tea infusion, levels of total catechins, EGCG, GCG, GC, and CG were decreased in heated green tea infusion with Fe2+ addition. It implied that Fe2+ predominantly promoted the degradation of catechins in green tea infusion with heat treatment.
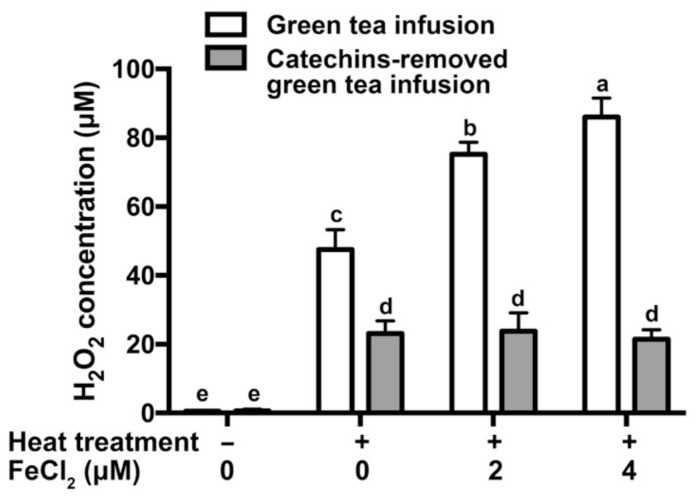
The degradation of catechins is coupled to the formation of H2O2 [9]. In a previous study, the concentrations of catechins in tea infusion were decreased and the concentrations of H2O2 were increased during storage [4]. A similar phenomenon was detected during heat treatment of tea infusion in this study (Figure 2). The addition of Fe2+ dose-dependently increased the levels of H2O2 in green tea infusion. In contrast, the levels of H2O2 in catechins-removed green tea infusion were not significantly elevated by the addition of Fe2+. It proved that H2O2 in heated green tea infusion was partially derived from catechins and Fe2+ promoted the generation of H2O2 dependently on catechins.
Figure 2.
Effect of Fe2+ on the accumulation of H2O2 in green tea infusion with heat treatment. The same letter within each column indicates no significant difference (p > 0.05).
2.2. Effect of Catechins and H2O2 on Fe2+-Mediated Aroma Deterioration in Green Tea Infusion with Heat Treatment
To investigate the roles of catechins on Fe2+-mediated aroma deterioration in green tea infusion with heat treatment, the effect of Fe2+ on aroma quality of catechins-removed green tea infusion was assessed. According to the results (Table 3), the effect of Fe2+ on aroma deterioration was diminished in catechins-removed green tea infusion, implying that catechins were necessary for Fe2+-mediated aroma deterioration.
Table 3.
Effect of Fe2+ on aroma quality of catechins-removed green tea infusion.
| Treatment | Score |
|---|---|
| Untreated | 8.2 ± 0.3 a |
| Heat treatment | 7.6 ± 0.4 ab |
| 2 μM FeCl2 + Heat treatment | 7.6 ± 0.4 ab |
| 4 μM FeCl2 + Heat treatment | 7.3 ± 0.5 b |
The same letter within each column indicates no significant difference (p > 0.05).
To further test whether catechins participated in Fe2+-mediated aroma deterioration via generating H2O2, the effect of Fe2+ on aroma quality of catechins-removed green tea infusion containing exogenous H2O2 was evaluated. The results (Table 4) revealed that exogenous H2O2 or Fe2+ alone hardly deteriorated the aroma of catechins-removed green tea infusion. However, the combination of exogenous H2O2 and Fe2+ effectively impaired the aroma. It suggested that H2O2 played an essential role in Fe2+-mediated aroma deterioration.
Table 4.
Effect of H2O2 on Fe2+-mediated aroma deterioration in catechins-removed green tea infusion.
| Treatment | Score |
|---|---|
| Untreated | 8.2 ± 0.3 a |
| Heat treatment | 7.5 ± 0.6 a |
| 100 μM H2O2 +Heat treatment | 7.5 ± 0.5 a |
| 4 μM FeCl2 + Heat treatment | 7.3 ± 0.6 a |
| 4 μM FeCl2 + 100 μM H2O2 +Heat treatment | 6.0 ± 0.4 b |
The same letter within each column indicates no significant difference (p > 0.05).
Previous studies have mentioned that Fe2+ can react with H2O2 to generate highly reactive radicals (e.g., hydroxyl radicals) [10]. Fe2+ can catalyze the reaction of H2O2 and superoxide ion to generate hydroxyl radicals and hydroxide ions [10]. Due to the massive production of reactive radicals, the two reactions play important roles in the degradation of various organic compounds, including hydrocarbons, alcohols, and organic acids [11]. Both H2O2 and superoxide ion are generated during the degradation of catechins [9]. Therefore, it is possible that Fe2+ also relies on these two reactions to induce aroma deterioration of green tea infusion. EGCG, the most abundant catechin in green tea infusion, was capable of enhancing the efficiency of Fe2+-mediated oxidative systems on the degradation of organic compounds, probably by accelerating the transformation of Fe3+ to Fe2+ [12]. This implied that catechins might not only participate in Fe2+-mediated aroma deterioration by generating H2O2, but also by promoting the regeneration of Fe2+. However, further experiments are needed to verify this hypothesis.
2.3. Effect of Fe2+ on the Volatile Profile of Green Tea Infusion with Heat Treatment
GC-MS analysis (Figure 3 and Table 5) revealed that 49, 54, and 61 volatiles were detected in unheated tea infusion, heated tea infusion, and heated tea infusion with Fe2+ addition, respectively. Among them, 42 volatiles were observed in all infusions. It indicated that the majority of volatile components in the three infusions were similar and the presence of Fe2+ promoted the formation of new volatiles.
Figure 3.
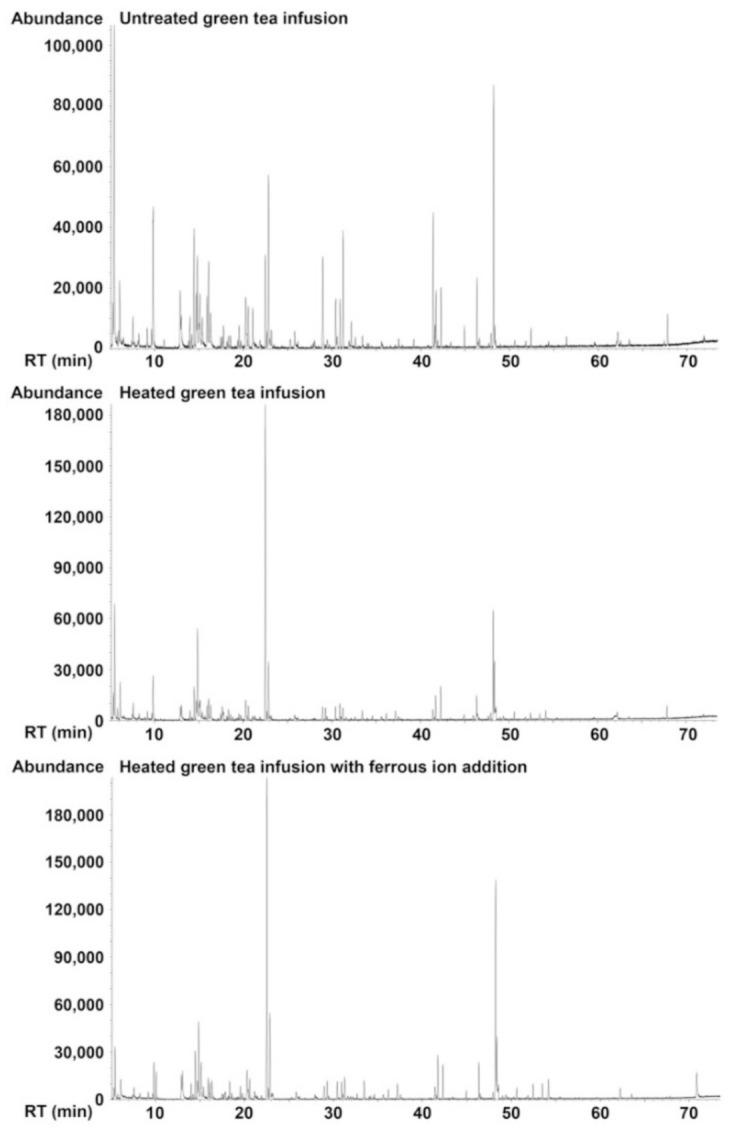
GC-MS total ion count chromatograms of green tea infusion with different treatments.
Table 5.
Effect of Fe2+ on the volatile profile of green tea infusion with heat treatment.
| RT | CAS No. | Molecular Formula | Name | Relative Abundance (%) | Aroma Properties | ||
|---|---|---|---|---|---|---|---|
| Unheated | Heat Treatment |
4 μM FeCl2 + Heat Treatment | |||||
| 5.310 | 141-79-7 | C6H10O | Mesityl oxide | 1.27 ± 0.05 a | 0.68 ± 0.05 b | <LOQ c | Pungent |
| 5.437 | 66-25-1 | C6H12O | Hexanal | 11.70 ± 0.92 a | 3.86 ± 0.53 b | 3.39 ± 0.13 b | Green, grassy |
| 5.764 | 617-92-5 | C6H9N | 1-Ethylpyrrole | 0.42 ± 0.05 a | 0.32 ± 0.01 b | 0.19 ± 0.05 c | |
| 5.944 | 123-86-4 | C6H12O2 | Butyl acetate | 0.49 ± 0.06 a | <LOQ b | <LOQ b | Fruity banana |
| 7.557 | 544-12-7 | C6H12O | Trans-3-hexen-1-ol | 1.06 ± 0.03 a | 0.71 ± 0.07 b | 0.58 ± 0.03 c | Green |
| 9.654 | 6728-31-0 | C7H12O | (Z)-4-Heptenal | 0.48 ± 0.03 a | 0.29 ± 0.05 b | 0.19 ± 0.05 c | Fatty, green, creamy |
| 9.818 | 111-71-7 | C7H14O | Heptanal | 3.67 ± 0.28 a | 1.98 ± 0.41 b | 2.00 ± 0.24 b | Fruity |
| 9.843 | 222866 | C8H9NO2 | Oxime-, methoxy-phenyl- | 1.56 ± 0.07 a | 0.99 ± 0.19 b | 1.31 ± 0.18 ab | |
| 12.882 | 57266-86-1 | C7H12O | (Z)-2-Heptenal | 2.17 ± 0.13 a | 1.41 ± 0.05 b | 1.49 ± 0.10 b | Oily |
| 12.998 | 100-52-7 | C7H6O | Benzaldehyde | 1.44 ± 0.08 a | 1.36 ± 0.02 a | 2.34 ± 0.22 b | Strong sharp almond aroma |
| 13.988 | 26456-76-8 | C9H18 | 3,5,5-Trimethyl-2-hexene | 1.10 ± 0.10 a | 0.81 ± 0.02 b | 0.83 ± 0.03 b | |
| 14.183 | 2918-13-0 | C7H12O | 1-Hepten-3-one | 0.44 ± 0.06 a | 0.28 ± 0.04 b | 0.33 ± 0.00 c | Metallic |
| 14.442 | 3391-86-4 | C8H16O | 1-Octen-3-ol | 4.55 ± 0.05 a | 2.86 ± 0.23 b | 2.72 ± 0.16 b | Mushroom aroma, earthy |
| 14.693 | 110-93-0 | C8H14O | 5-Hepten-2-one, 6-methyl- | 2.19 ± 0.14 a | 1.38 ± 0.08 b | 1.13 ± 0.08 c | Citrus and lemongrass aroma |
| 14.822 | 3050-69-9 | C8H14O2 | Vinyl hexanoate | 4.14 ± 0.65 a | 5.65 ± 0.47 b | 4.79 ± 0.27 a | Fruity, sweet |
| 15.366 | 13643-08-8 | C8H14 | 2,4-Octadiene | 1.05 ± 0.30 ab | 0.72 ± 0.07 a | 0.91 ± 0.11 b | |
| 15.903 | 124-13-0 | C8H16O | Octanal | 2.15 ± 0.01 a | 1.45 ± 0.08 b | 1.36 ± 0.00 c | Orange peel, fatty |
| 16.082 | 3681-71-8 | C8H14O2 | cis-3-Hexenyl acetate | 3.58 ± 0.15 a | 1.10 ± 0.12 b | 1.02 ± 0.06 b | Green, unripe banana |
| 16.297 | 4313-03-5 | C7H10O | (E,E)-2,4-Heptadienal | 1.81 ± 0.02 a | 1.37 ± 0.05 b | 1.41 ± 0.11 b | Fatty, green, oily |
| 17.473 | 5989-27-5 | C10H16 | D-Limonene | <LOQ a | <LOQ a | 0.43 ± 0.09 b | Citrus, orange |
| 17.633 | 411448 | C8H18O | 2-Ethyl-1-hexanol | 0.51 ± 0.15 a | <LOQ b | 0.22 ± 0.07 c | Citrus aroma |
| 17.764 | 100-51-6 | C7H8O | Benzyl alcohol | <LOQ a | <LOQ a | 0.58 ± 0.22 b | Floral, rose |
| 17.768 | 2408-37-9 | C9H16O | 2,6,6-Trimethylcyclohexanone | 0.92 ± 0.01 a | <LOQ b | <LOQ b | Pungent |
| 18.322 | 122-78-1 | C8H8O | Benzene acetaldehyde | 0.43 ± 0.02 a | 0.97 ± 0.06 b | 1.15 ± 0.13 b | Sweet floral, hyacinth aroma |
| 18.551 | 2167-14-8 | C7H9NO | 1H-Pyrrole-2-carboxaldehyde, 1-ethyl- | 0.44 ± 0.04 a | 0.33 ± 0.06 b | 0.37 ± 0.05 ab | Burnt, roasted, smoky |
| 19.223 | 65405-80-3 | C10H16O2 | (E,Z)-2-Butenoic acid, 3-hexenyl ester | <LOQ a | <LOQ a | 0.13 ± 0.01 b | Green vegetable |
| 19.377 | 78-59-1 | C9H14O | Isophorone | 0.27 ± 0.01 a | <LOQ b | 0.11 ± 0.00 c | Cooling woody |
| 19.515 | 2548-87-0 | C8H14O | (E)-2-Octenal | 0.79 ± 0.06 a | 0.68 ± 0.05 a | 0.74 ± 0.07 a | Fresh cucumber aroma |
| 19.731 | 98-86-2 | C8H8O | Acetophenone | 0.35 ± 0.02 a | 0.44 ± 0.06 b | 0.46 ± 0.01 b | Sweet, almond |
| 20.275 | 38284-27-4 | C8H12O | 3,5-Octadien-2-one | 2.08 ± 0.34 a | 1.91 ± 0.64 ab | 1.59 ± 0.01 b | Fruity, fatty, mushroom aroma |
| 20.556 | 111-87-5 | C8H18O | 1-Octanol | 1.72 ± 0.02 a | 1.22 ± 0.03 b | 1.38 ± 0.22 b | Waxy, orange |
| 21.889 | 30086-02-3 | C8H12O | (E,E)-3,5-Octadien-2-one | 0.25 ± 0.06 a | 0.23 ± 0.03 a | 0.25 ± 0.03 a | Green, grassy |
| 22.477 | 78-70-6 | C10H18O | Linalool | 3.45 ± 0.16 a | 19.79 ± 0.68 b | 19.99 ± 0.89 b | Floral |
| 22.673 | 29957-43-5 | C10H16O | 3,7-Dimethylocta-1,5,7-trien-3-ol | 0.68 ± 0.11 a | 0.64 ± 0.04 a | 0.63 ± 0.05 a | Mouldy |
| 22.836 | 124-19-6 | C9H18O | Nonanal | 6.57 ± 0.93 ab | 6.84 ± 0.18 a | 5.62 ± 0.24 b | Waxy, rosy, orange peel aroma |
| 22.991 | 60-12-8 | C8H10O | Phenylethyl Alcohol | 0.35 ± 0.00 a | 0.52 ± 0.04 b | 0.48 ± 0.01 b | Floral, rose |
| 28.922 | 119-36-8 | C8H8O3 | Methyl salicylate | <LOQ a | <LOQ a | 0.51 ± 0.13 b | Wintergreen mint |
| 28.960 | 53398-84-8 | C10H18O2 | Butanoic acid, 3-hexenyl ester, (E)- | 4.33 ± 0.06 a | 1.22 ± 0.10 b | 0.76 ± 0.10 c | |
| 29.321 | 98-55-5 | C10H18O | α-Terpineol | <LOQ a | 1.12 ± 0.06 b | 1.28 ± 0.00 c | Pine, lilac |
| 29.49 | 116-26-7 | C10H14O | Safranal | <LOQ a | <LOQ a | 0.23 ± 0.00 b | Fresh, herbal |
| 30.423 | 112-31-2 | C10H20O | Decanal | 1.47 ± 0.59 a | 2.95 ± 0.25 b | 1.21 ± 0.07 a | Citrus, sweet floral, waxy |
| 30.903 | 432-25-7 | C10H16O | β-Cyclocitral | 1.91 ± 0.01 a | 1.19 ± 0.08 b | 1.13 ± 0.00 b | Woody |
| 31.059 | 496-16-2 | C8H8O | 2,3-Dihydrobenzofuran | <LOQ a | 0.24 ± 0.01 b | 0.34 ± 0.03 c | Sweet, nut |
| 31.253 | 2177-77-7 | C7H14O2 | Methyl 2-, Methylpentanoate | 4.15 ± 0.66 a | 0.70 ± 0.14 b | 1.37 ± 0.02 c | Fruity |
| 32.183 | 35852-46-1 | C11H20O2 | n-Valeric acid cis-3-hexenyl ester | 1.06 ± 0.05 a | 0.20 ± 0.03 b | <LOQ c | Green fruity, unripe banana |
| 33.450 | 624-15-7 | C10H18O | 3,7-Dimethyl-2,6-octadienol | <LOQ a | 0.80 ± 0.11 b | 1.09 ± 0.06 c | |
| 35.575 | 120-72-9 | C8H7N | Indole | 0.28 ± 0.01 a | 0.34 ± 0.10 ab | 0.38 ± 0.06 b | Floral, fecal |
| 36.176 | 36431-72-8 | C13H22O | Theaspirane | <LOQ a | 0.84 ± 0.06 b | 0.58 ± 0.00 c | Woody, spicy |
| 37.193 | C13H23O | 2,6,10,10-Tetramethyl-1-oxaspiro[4.5]dec-7-ene | <LOQ a | 1.27 ± 0.02 b | 0.96 ± 0.05 c | ||
| 41.403 | 31501-11-8 | C12H22O2 | Hexanoic acid, 3-hexenyl ester, (Z)- | 5.08 ± 0.19 a | 1.27 ± 0.14 b | 0.79 ± 0.04 c | Fruity green, pear |
| 41.606 | 61444-38-0 | C12H20O2 | cis-3-Hexenyl cis-3-hexenoate | 0.87 ± 0.05 a | 0.27 ± 0.00 b | 0.19 ± 0.02 c | Green |
| 41.721 | 488-10-8 | C11H16O | Jasmone | 2.09 ± 0.00 a | 2.47 ± 0.19 b | 2.64 ± 0.01 c | Floral, Jasmin |
| 44.928 | 3879-26-3 | C13H22O | Nerylacetone | 0.58 ± 0.05 a | 0.94 ± 0.12 b | 0.47 ± 0.08 a | Woody |
| 46.344 | 79-77-6 | C13H20O | β-Ionone | 2.24 ± 0.02 a | 1.97 ± 0.16 b | 2.14 ± 0.14 ab | Floral, woody |
| 47.931 | 166273-38-7 | C19H30O3 | 2,4-Ditert-butylphenyl 5-hydroxypentanoate | 0.45 ± 0.05 a | 0.38 ± 0.07 a | 0.41 ± 0.05 a | |
| 48.219 | 500-66-3 | C11H16O2 | 1,3-Benzenediol, 5-pentyl- | 9.56 ± 0.04 a | 13.13 ± 1.13 b | 13.70 ± 0.21 c | Olive |
| 48.354 | 483-76-1 | C15H24 | (+)-δ-Cadinene | 0.79 ± 0.07 a | 3.00 ± 0.44 b | 4.01 ± 0.29 c | Herbal, woody |
| 48.469 | 483-77-2 | C15H22 | (−)-Calamenene | <LOQ a | <LOQ a | 0.73 ± 0.11 b | Herb spice |
| 48.545 | 41702-63-0 | C15H24 | Epizonarene | <LOQ a | <LOQ a | 0.69 ± 0.11 b | |
| 49.384 | 21391-99-1 | C15H20 | α-Calacorene | <LOQ a | <LOQ a | 0.32 ± 0.10 b | Woody |
| 50.619 | 142-50-7 | C15H26O | Nerolidol | <LOQ a | 0.92 ± 0.28 b | 0.51 ± 0.01 c | Floral |
| 52.427 | 77-53-2 | C15H26O | Cedrol | 0.52 ± 0.05 a | 0.81 ± 0.02 b | 0.88 ± 0.05 b | Cedarwood, woody, soft sweet |
| 53.501 | 16728-99-7 | C15H24 | Naphthalene, 1,2,3,4,4a,7-hexahydro-1,6-dimethyl-4-(1-methylethyl)- | <LOQ a | 0.89 ± 0.20 b | 0.92 ± 0.06 b | |
| 54.168 | 19912-67-5 | C15H26O | Epicubebol | <LOQ a | 1.23 ± 0.08 b | 1.34 ± 0.16 b | |
| 62.562 | 66408-55-7 | C17H28O | 5,9,13-Trimethyl-tetradeca-4,8,12-trienal | <LOQ a | 0.33 ± 0.11 b | <LOQ a | |
| 63.552 | 84-74-2 | C16H22O4 | Dibutyl phthalate | <LOQ a | <LOQ a | 0.30 ± 0.05 b | Faint odor |
| 67.845 | 57-10-3 | C16H32O2 | n-Hexadecanoic acid | 0.53 ± 0.13 a | <LOQ b | <LOQ b | Waxy, fatty |
| 71.894 | 83834-59-7 | C18H26O3 | 4-Methoxycinnamic acid 2-ethylhexyl ester | <LOQ a | 0.71 ± 0.11 b | <LOQ a | |
LOQ is short for the limit of quantification. The experiments were carried out in triplicate. The same letter within each row indicates no significant difference (p > 0.05).
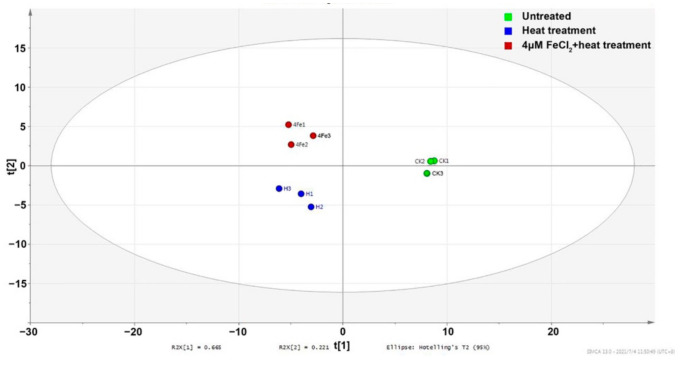
The volatile profiles of the three infusions were quite different. It was supported by the results of PCA. Points representing samples with different treatments in the PCA score plot were clearly separated (Figure 4). The types and relative abundance of alkenes, alcohols, and heterocycles were gradually increased in unheated tea infusion, heated tea infusion, and heated tea infusion with Fe2+ addition, respectively. Meanwhile the relative abundance of aldehydes, ketones, and esters were gradually decreased in the three infusions, respectively. When categorized by aroma characteristics, green/grassy volatiles were gradually decreased and woody volatiles were gradually increased in the three infusions, respectively.
Figure 4.
PCA score plot based on volatiles in tea infusions with different treatments.
A total of 28 volatiles were decreased after heat treatment. Eleven of them were further decreased in heated tea infusion with Fe2+ addition, most of which had green/grassy scents. Twenty-one volatiles were increased after heat treatment. Five of them were further increased in heated tea infusion with Fe2+ addition. They smelt sweet, fruity, or woody. Notably, there were nine volatiles only detected in the heated infusion with Fe2+ addition and their aroma characteristics were so different from each other, ranging from fruity, floral, minty, fresh, herbal, to woody. The loss of green/grassy volatiles might be associated with the less fresh aroma of green tea infusion. The gain of volatiles with too many different aroma characteristics might lead to an unpleasant complex scent. They together presented a dull scent in heated tea infusion with Fe2+ addition.
3. Materials and Methods
3.1. Reagents
Green tea was purchased from Jiangsu Xinpin Tea Co., Ltd (Changzhou, China). (−)-Epigallocatechin gallate (EGCG), (−)-gallocatechin gallate (GCG), (−)-epicatechin (EC), (+)-catechin (C), (−)-epicatechin gallate (ECG), (−)-catechin gallate (CG), (−)-epigallocatechin (EGC), (−)-gallocatechin (GC), and gallic acid (GA) were obtained from Sigma-Aldrich Co. LLC (Saint Louis, MO, USA). Polyvinylpolypyrrolidone (PVPP) and ferrous chloride (FeCl2) were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd (Shanghai, China). The 30% H2O2 was purchased from Sinopharm group Co., Ltd (Beijing, China).
3.2. Preparation of Green Tea Infusion
Green tea was brewed with deionized water (1:100, w/v) at 60 °C for 20 min and filtered through a 0.45 μm membrane to obtain tea infusion.
To assess the effects of Fe2+ on the aroma quality, degradation of catechins, and generation of H2O2 of green tea infusion with heat treatment, FeCl2 solution was added to green tea infusion to reach the final concentrations of 0, 2, and 4 μmol/L (μM). According to the requirement of the standards for drinking water quality (GB 5749-2006), the ferrous ion concentration in drinking water shall not exceed 5.36 μM. Therefore, the Fe2+ concentrations used for experiments in the study were defined as 2 and 4 μM.
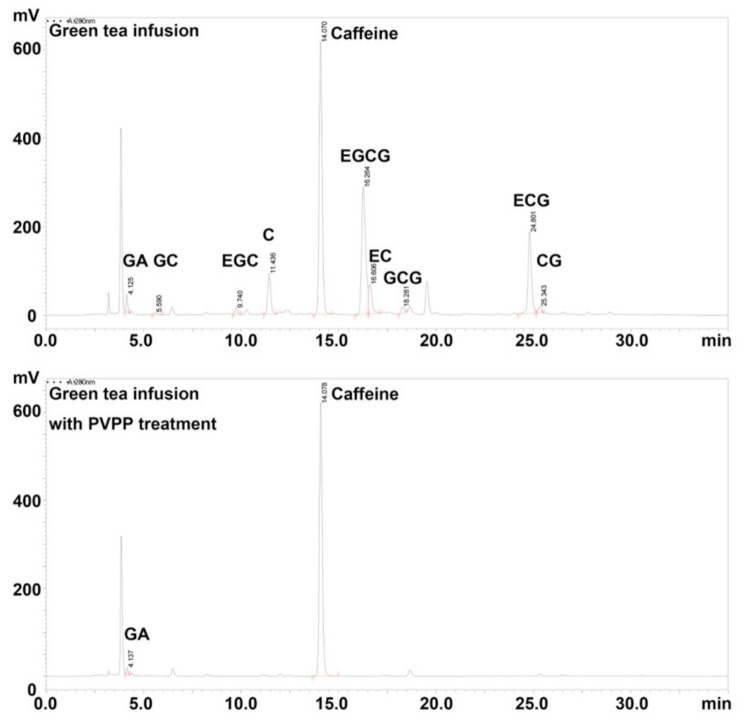
To investigate the effect of catechins on Fe2+-mediated aroma deterioration, green tea infusion was mixed with PVPP (5%, w/w) to remove catechins. After PVPP treatment, catechins in green tea infusion were no longer detectable using the HPLC method [13] (Figure 5). FeCl2 solution was added to the catechins-removed green tea infusion to reach the final concentrations of 0, 2, and 4 μM.
Figure 5.
HPLC spectrums of catechins in green tea infusion with and without olyvinylpolypyrrolidone (PVPP) treatment.
To investigate the effect of H2O2 on Fe2+-mediated aroma deterioration, H2O2 was added to the catechins-removed green tea infusion to reach the final concentration of 100 μM.
Infusions with different additions were incubated at 100 °C for 40 min and cooled to room temperature for further tests.
3.3. Sensory Evaluation of Green Tea Infusion
The aroma quality of tea infusion was assessed according to the national standard GB/T 21733-2008, and scored by a team of seven qualified panelists (three men and four women, 25–50 years old), all of whom achieved certificates for tea-quality evaluation from the Tea Scientific Society of China. A ten-point scale was used for scoring. The score 0 is for least acceptable and 10 is for most acceptable [14].
3.4. Determination of Catechins and GA in Green Tea Infusion
The concentrations of catechins and GA were measured by an established HPLC method [13].
The separation was performed on a Symmetry C18 column (5 μm, 4.6 mm × 250 mm, Waters, Milford, MA, USA) using a Shimadzu LC-20A system (Shimadzu, Tokyo, Japan) equipped with a UV detector set at 280 nm. The gradient separation was carried out using 2% acetic acid in water and acetonitrile as mobile phases A and B, with the flow rate at 1 mL/min for 30 min and the column temperature at 35 °C. Separation was conducted under the following conditions: 0–16 min, 6.5–15% B; 16–25 min, 15–25% B; and 25–30 min, and 25–6.5% B.
GA (3.54–56.7 mg/L), EGCG (3.23–51.7 mg/L), GCG (5.00–80.0 mg/L), EC (5.31–85.0 mg/L), C (5.31–85.0 mg/L), ECG (4.11–65.8 mg/L), CG (1.56–25.0 mg/L), EGC (5.21–83.3 mg/L), and GC (2.55–40.8 mg/L) were used as standards to prepare calibration curves.
3.5. Determination of H2O2 in Green Tea Infusion
The levels of H2O2 were determined using a Hydrogen Peroxide Assay Kit (Beyotime Biotechnology, Nantong, China) according to the manufacturer’s instructions. Briefly, 50 μL of the infusion was added to a 96-well plate, mixed with 100 μL of the hydrogen peroxide reaction reagent, stayed at room temperature for 30 min, and then the absorbance was read at 560 nm using the BioTek Synergy 2 multi-mode microplate reader (Winooski, VT, USA).
3.6. Analysis of Volatiles in Green Tea Infusion
Volatiles in tea infusion were determined by gas chromatography–mass spectrometry (GC-MS) as previously reported [15].
Thirty milliliters of the infusion containing ethyl caprate as the internal standard was added into a 50 mL glass vial. The glass vial was sealed and incubated at 60 °C. A SPME needle (Supelco, Bellefonte, PA, USA) was used to absorb volatiles for 60 min. Then the SPME needle was inserted into the injection port of GC and volatiles were desorbed at 250 °C for 5 min.
Volatiles were analyzed using an Agilent6890 gas chromatograph coupled with an Agilent HP 5973 mass selective detector (Agilent, Wilmington, DE, USA). The separation was performed on a DB-5MS capillary column (30 m × 250 μm × 0.25 μm). The GC condition was as follows: the GC inlet temperature of 250 °C, and the split ratio of 15:1, the carrier gas (high purity helium) flow of 1 mL/min. The separation was conducted under the following conditions: 0–2 min, 40 °C; 2–24.5 min, 40–85 °C; 24.5–26.5 min, 85 °C; 26.5–64.5 min, 85–180 °C; 64.5–66.5 min, 180 °C; 66.5–71.5 min, 180–230 °C; and 71.5–73.5 min, 230 °C.
For MS analysis, the temperature of the ion source was 230 °C, the voltage was 70 eV and the scan range was 40 to 400 m/z.
Tentative identification of volatiles was made by comparing the MS fragmentation patterns with data from the National Institute for Standards and Technology database (NIST 08, match percentage >80%). The relative abundance of each compound was calculated by comparing the peak area of each compound to the total peak area.
3.7. Statistical Analysis
Data are presented as the mean ± standard error of the mean (SEM). All experiments were carried out in triplicate. The principal component analysis (PCA) was performed using SIMCA-P 13.0 software (Umetric, Umea, Sweden). The results were analyzed with SPSS Version 18.0 for Windows using the one-way analysis of variance with 2-sided Dunnett’s post hoc test to determine overall differences between groups. p-values < 0.05 were considered to be statistically significant.
4. Conclusions
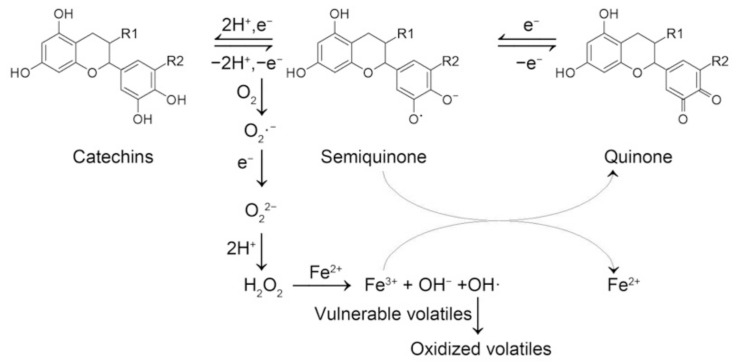
Fe2+ significantly exacerbated the aroma quality of green tea infusion with heat treatment. It promoted the degradation of catechins and the generation of H2O2. Catechins were prerequisites for Fe2+-mediated aroma deterioration. They were important sources of H2O2. H2O2 alone showed limited effects on aroma. Fe2+ and H2O2 worked together to induce aroma deterioration. Fe2+ significantly modified the volatile profiles of green tea infusion. Green/grassy volatiles were reduced and several volatiles with different aroma characteristics were increased or newly generated, leading to a dull scent. Based on the above results and previous references [12,16,17], a reaction scheme was proposed (Figure 6). It was deduced that under heat treatment, catechins could deprotonate and lose electrons to form semiquinone and quinone, which was reversible. The electrons reacted with dissolved oxygen and hydrogen ions, leading to the generation of H2O2. The presence of Fe2+ triggered the Fenton reaction and produced multiple free radicals, including hydroxyl radicals, which were regarded as the most active radicals and were capable of oxidizing vulnerable volatiles. Fe3+ produced during the process could be rapidly reduced to Fe2+ by EGCG, which promoted the transformation of semiquinone to quinone. Taken together, the presence of Fe2+ might promote the degradation of catechins to generate H2O2 and reacted with H2O2 to produce highly active radicals to deteriorate the aroma of green tea infusion. Our results partially revealed how Fe2+ induced aroma deterioration of green tea infusion with heat treatment and implied that elimination of Fe2+ in green tea infusion during processing might help to improve the sensory quality of ready-to-drink tea.
Figure 6.
Proposed reaction scheme for the effect of ferrous ion on heat-induced aroma deterioration of green tea infusion.
Author Contributions
Y.G., J.-F.Y., and Y.-Q.X. conceived and designed the experiments; Y.G., J.-X.C., F.W., G.-S.C., J.-F.Y., and Y.-Q.X. performed the experiments; Y.G. and J.-Q.W. analyzed the data; Y.G. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Zhejiang (LR17C160001), the Key Research and Development Program of Zhejiang (2019C02072), the Leading Talent Project of Zhejiang Ten Thousand Talents Plan (2018R52025), the Innovation Project for Chinese Academy of Agricultural Sciences, and the Scientific and Technological Program of Ningbo (202002N3020). The APC was funded by the Key Research and Development Program of Zhejiang (2019C02072).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hitoshi K., Tadakazu T. Deterioration mechanism for tea infusion aroma by retort pasteurization. Agric. Biol. Chem. 1990;54:2537–2542. [Google Scholar]
- 2.Lau H., Liu S.Q., Xu Y.Q., Lassabliere B., Sun J.C., Yu B. Characterising volatiles in tea (camellia sinensis). Part i: Comparison of headspace-solid phase microextraction and solvent assisted flavour evaporation. LWT Food Sci. Technol. 2018;94:178–189. doi: 10.1016/j.lwt.2018.04.058. [DOI] [Google Scholar]
- 3.Liu P.P., Xu Y.Q., Zou C., Gao Y., Wang F., Chen J.X., Yin J.F. Studies on the quality change of tea infusion beverages during sterilization and storage. J. Chin. Inst. Food Sci. Technol. 2018;18:202–210. [Google Scholar]
- 4.Dou H.L., Li C.M., Hao J.F., Hu W.F. Study on the change of the main biochemical components, representative aromatic compounds as well as their correlation of green tea beverages during storage. J. Tea Sci. 2008;28:181–188. [Google Scholar]
- 5.Jiang C.L. Research on the Quality of Tea Beverages Extracted and Blended by Different Water Property. Fujian Agriculture and Forestry University; Fuzhou, China: 2010. [Google Scholar]
- 6.Tu C.H., Zheng B., Ning H.M., Guo R.Y., Chen G.M., Chen X.Z. Effects of inorganic metal ions on tea infusion. Xiemen Sci. Technol. 2018;3:56–59. [Google Scholar]
- 7.Wang R., Zhou W., Jiang X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (egcg) in aqueous system over a wide temperature range. J. Agric. Food Chem. 2008;56:2694–2701. doi: 10.1021/jf0730338. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q.Q. The Stability of Gallocatechin Gallate under the Solution Condition. Northwest University; Chongqing, China: 2017. [Google Scholar]
- 9.Mochizuki M., Yamazaki S., Kano K., Ikeda T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta. 2002;1569:35–44. doi: 10.1016/S0304-4165(01)00230-6. [DOI] [PubMed] [Google Scholar]
- 10.Dziubla T., Butterfield D.A. Oxidative Stress and Biomaterials. Volume XIII. Elsevier; Amsterdam, The Netherlands: Academic Press; Boston, MA, USA: 2016. p. 389. [Google Scholar]
- 11.Stasicka Z. Transition metal complexes as solar photocatalysts in the environment: A short review of recent development. Adv. Inorg. Chem. 2011;63:291–343. [Google Scholar]
- 12.Bu L.J., Bi C., Shi Z., Zhou S.Q. Significant enhancement on ferrous/persulfate oxidation with epigallocatechin-3-gallate: Simultaneous chelating and reducing. Chem. Eng. J. 2017;321:642–650. doi: 10.1016/j.cej.2017.04.001. [DOI] [Google Scholar]
- 13.Zhang Y.N., Yin J.F., Chen J.X., Wang F., Du Q.Z., Jiang Y.W., Xu Y.Q. Improving the sweet aftertaste of green tea infusion with tannase. Food Chem. 2016;192:470–476. doi: 10.1016/j.foodchem.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 14.Alasalvar C., Topal B., Serpen A., Bahar B., Pelvan E., Gokmen V. Flavor characteristics of seven grades of black tea produced in turkey. J. Agric. Food Chem. 2012;60:6323–6332. doi: 10.1021/jf301498p. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y., Wang J.Q., Fu Y.Q., Yin J.F., Shi J., Xu Y.Q. Chemical composition, sensory properties and bioactivities of castanopsis lamontii buds and mature leaves. Food Chem. 2020;316:126370. doi: 10.1016/j.foodchem.2020.126370. [DOI] [PubMed] [Google Scholar]
- 16.Bugg T.D.H., Lin G. Solving the riddle of the intradiol and extradiol catechol dioxygenases: How do enzymes control hydroperoxide rearrangements? Chem. Commun. 2001;11:941–952. doi: 10.1039/b100484k. [DOI] [Google Scholar]
- 17.Shishido S., Miyano R., Nakashima T., Matsuo H., Iwatsuki M., Nakamura K., Kanno T., Egusa H., Niwano Y. A novel pathway for the photooxidation of catechin in relation to its prooxidative activity. Sci. Rep. 2018;8:12888. doi: 10.1038/s41598-018-31195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.