Abstract
Background
To standardize the technical strategy for right upper lobe (RUL) segmentectomy, we previously developed simplified 3-dimensional (3D) anatomic models that classify the RUL anatomy into 14 patterns according to the branching pattern of bronchi and veins. We aimed to study the surgical outcome of RUL segmentectomy guided by these simplified anatomic models.
Methods
Patients were classified into the anatomic models, and the approach to the intersegmental veins was selected accordingly. The intersegmental vein and corresponding intersegmental plane were as follows: V1b (the apicoanterior plane), V2a (the apicoposterior plane), and V2c (the posteroanterior plane). Clinicopathologic characteristics and short- and long-term outcomes were analyzed retrospectively.
Results
Thirty-four consecutive patients who underwent thoracoscopic RUL segmentectomy guided by simplified anatomic models between January 2016 and December 2019 at Gunma University were analyzed. All the patients were classified into a model: anterior + central Iab type (47%), anterior + central Ib type (41%), anterior II type (12%), or central III type (0%). The standard approaches to intersegmental veins were an anterior approach for V1b, a posterobronchial approach for V2a, and an interlobar approach for V2c. The approach to intersegmental or intrasegmental veins was modified according to the anatomic model in 4 cases (12%). The median operative time, blood loss, and hospital stay were 222 minutes, 19 grams, and 7 days, respectively. Prolonged air leakage was observed in 1 patient.
Conclusions
Segmentectomy guided by simplified anatomic models promotes anatomic classification, development of a standardized approach for segmental vein identification, and acceptable outcomes, which can facilitate the implementation of RUL segmentectomy.
Key Words: segmentectomy, right upper lobe, 3D-CT, classification, anatomic model
Abbreviations and Acronyms: 3D, 3-dimensional; CT, computed tomography; RUL, right upper lobe
Graphical abstract
Right upper lobe (RUL) segmentectomy guided by a simplified anatomic model consisting of (1) a 2-step classification of the segmental anatomy into one of 14 anatomic models and (2) a standardized approach to intersegmental veins selected according to the anatomic model. This method led to the successful classification and completion of RUL segmentectomy in 34 consecutive patients.

Standardized approaches to intersegmental vein: anterior, interlobar, and posterobronchial.
Central Message.
Right upper lobe segmentectomy guided by anatomic models allows the classification of segmental anatomy, a standardized approach to the intersegmental veins, and successful completion of segmentectomy.
Perspective.
Right upper lobe (RUL) segmentectomy remains challenging, given the complex segmental anatomy and spatial relationship between the bronchus and vessels. RUL segmentectomy guided by anatomic models facilitates the precise classification of anatomy, development of a standardized approach to intersegmental veins, and successful completion of segmentectomy.
See Commentaries on pages 298 and 299.
Studies have shown that one-third of lung cancers develop in the right upper lobe (RUL).1 Owing to the increased rate at which small and early-stage lung cancers are being detected via computed tomography (CT) screening,2 the need for RUL segmentectomy has been rapidly increasing. However, techniques vary greatly, depending on the surgeon's experience or hospital's infrastructure.3 In addition, RUL segmentectomies are considered more complex compared with typical segmentectomies of the superior, basilar, lingular, or upper division, owing to greater technical difficulties and anatomic variations.4
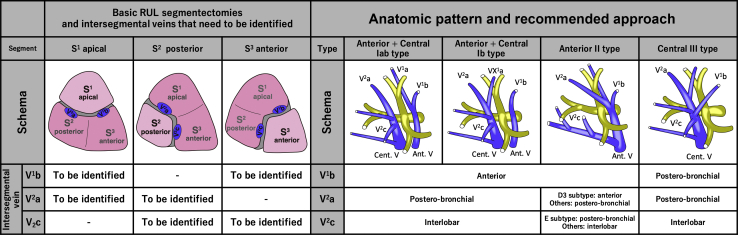
Successful segmentectomy requires intraoperative identification of intersegmental veins, which run through the intersegmental plane.5, 6, 7 However, identifying the intersegmental vein is often difficult owing to anatomic variations.8 The RUL consists of 3 intersegmental veins (Figure 1): V1b, running through the apicoanterior segmental plane (between S1 and S3); V2a, running through the apicoposterior segmental plane (between S1 and S2); and V2c, running through the posteroanterior segmental plane (between S2 and S3).
Figure 1.
Numerical naming style for pulmonary segments and veins of the right upper lobe. S1, S2, and S3 denote the apical, posterior, and anterior segments, respectively. The 3 intersegmental veins are designated V1b, running through the apicoanterior segmental plane (between S1 and S3); V2a, running through the apicoposterior segmental plane (between S1 and S2); and V2c, running through the posteroanterior segmental plane (between S2 and S3). V1a, V2b, and V3a are the intrasegmental veins running through the center of S1, S2, and S3, respectively.
We previously analyzed the right lung anatomic pattern using 3-dimensional (3D) CT images in >250 patients.9,10 Although several variants exist, RUL anatomy can be classified into 4 types based on the drainage pattern of the anterior and central pulmonary veins.9,11 In our clinical practice, we have been classifying lung anatomy based on these simplified anatomic models and use a methodological approach for identifying the intersegmental veins according to model subtype. In the present study, we aimed to investigate the surgical outcomes of RUL segmentectomy performed under this anatomic model classification and model-based approach to the intersegmental veins.
Methods
Patients and Variables
We performed a retrospective study of all patients who underwent RUL segmentectomy between January 2016 and December 2019 at Gunma University. The study was approved by the Institutional Review Board of Gunma University Hospital (protocol HS2019-279) and was conducted in accordance with the principles of the Declaration of Helsinki. The need for written informed consent from each patient was waived.
We included patients who had undergone thoracoscopic anatomic model-guided segmentectomy and excluded those who had undergone thoracotomy or additional use of CT-guided preoperative marking. The patients underwent preoperative imaging (CT scan, positron emission tomography/CT, and brain magnetic resonance imaging) for clinical staging and spirometry for functional evaluation. Charts were reviewed for age, sex, RUL anatomic model type, main location of lung lesion, comorbidities, preoperative diagnosis, consolidation/tumor ratio, pathological diagnosis, and surgical outcome. The surgical outcome encompassed operative time, bleeding, duration of chest tube placement, postoperative hospital stay, complications, and 90-day mortality. The main location of the tumor was identified using conventional CT and 3D-CT images, according to the spatial relationship with bronchi and intersegmental veins. Successful completion of segmentectomy was defined as follows: completion of segmentectomy as planned preoperatively using anatomic models and negative surgical margin at the time of final pathological diagnosis.
Selection Criteria for Segmentectomy
Sublobar resection (segmentectomy or wedge resection) was performed for patients considered to have noninvasive lung cancer (defined as a lung lesion ≤2.0 cm with a consolidation/tumor ratio ≤0.5),12,13 a metastatic lung lesion, or a lung lesion without a definite preoperative diagnosis. According to the criteria proposed by the guideline committee of the Japanese Association for Chest Surgery, sublobar resection was also performed for those who could not undergo standard lobectomy because of limited cardiopulmonary reserve.14 The choice of segmentectomy or wedge resection was based on the location and radiologic features of the lung nodule. In brief, wedge resection was performed for palpable nodules located at the surface of the lung, and segmentectomy was performed for impalpable nodules or those located deep in the lung parenchyma or close to the hilum. For cases of suspected malignancy, a resection that allowed for a surgical margin >2 cm, or larger than the tumor diameter for centrally-localized nodules, was planned. Bisegmentectomy or segmentectomy combined with subsegmentectomy was performed for lesions located at the border of a segment. Resected specimens were examined histopathologically and classified according to the World Health Organization's classification scheme. Staging was performed according to the TNM Classification of Malignant Tumors.
Pulmonary Segments and Veins in the RUL
In this study, we used a numerical naming system for pulmonary segments and veins (Figure 1). Accordingly, S1, S2, and S3 represent the apical, posterior, and anterior segment, respectively8; V1a, V2b, and V3a represent the intrasegmental vein running through the center of S1, S2, and S3, respectively; and V1b, V2a, and V2c represent the intersegmental vein running through the intersegmental plane between S1 and S3, S1 and S2, and S2 and S3, respectively.
3D Image Reconstruction and Classification Into a Simplified Anatomic Model
3D images were reconstructed from CT images obtained using a 64-channel multidetector row CT scanner (SOMATOM Definition Flash; Siemens Healthcare, Erlangen, Germany). A total of 35 mL of contrast agent was injected at 5 mL/s, immediately followed by the injection of 20 mL of saline. A solid image was constructed from 1.0-mm data slices of contrast-enhanced CT images using a volume-rendering software (Ziostation; Ziosoft, Tokyo, Japan), as described previously.9 Based on our previous analysis, the accuracy of the 3D-CT reconstruction of the bronchovascular structure is 98.7%.9 Each patient was classified into anatomic models by a 2-step process (Figures 2 and 3). The first step consisted of classification into 1 of the 4 major anatomic models based on the drainage pattern of the RUL's principal veins, the anterior and central veins.11 The second step included subclassification into 1 of the 14 subtypes, according to the drainage pattern of the intersegmental veins and the spatial relationship among segmental veins. V3 was not included in these models, because it is not an intersegmental vein and its drainage pattern is diverse (ie, drains more proximally than the anterior or central veins or drains into V4 and V5).
Figure 2.
Two-step classification of RUL anatomic models. Flowchart for the 2-step classification of anatomic models and example of classification (anterior + central Ib-A type). Step 1: Classification into 1 of the 3 major models. V1b and V2a are tracked down to identify the anterior and central vein. The anterior vein usually originates from V1b, runs through the anterior side of the RUL bronchus, and finally drains into the superior pulmonary vein from the mediastinal side. The central vein usually originates from V2a, runs through the central part of the RUL between B2 and B3, and finally drains into the superior pulmonary vein from the interlobar side. Step 1′: Classification into Iab or Ib type (for anterior + central I type). The drainage pattern of V1a is tracked down. If V1a drains into the anterior vein with V1b, the anatomy is classified as Iab type. If only V1b drains into the anterior vein, it is classified as Ib type. Step 2: Classification into subtypes. Each major anatomic model is further subclassified into 3 to 4 subtypes according to the drainage pattern of intersegmental veins V1b, V2a, and V2c. Central v., Central vein; anterior v., anterior vein; CT, computed tomography.
Figure 3.
Reference sheet for the 14 anatomic model subtypes. Anterior + central Iab, Ib type, and central III type were subclassified into subtypes A, B, and C, respectively, according to the drainage patterns of V2b and V2c. For the central III type, subtype A was named subtype A1 when V1b (VX1b) drained into V2a more peripherally than V2c and was named subtype A2 when V1b drained into V2a more centrally than V2c. Anterior II type was classified into subtypes D1, D2, D3, and E according to the drainage pattern of V2a (or VX2a, VXX2a), V2b (or VX2b), and V2c (or VX2c). Central v., Central vein; anterior v., anterior vein; CT, computed tomography.
This classification system focuses on the intersegmental veins for several reasons. First, the intersegmental veins are structures that physically define the boundaries of the segments, and thus they are used for preoperative planning to determine whether the surgical margin between the tumor and segmental border is sufficient or whether a (sub)segmentectomy is required. Second, the proximal part of the intersegmental plane created by other adjuncts, such as jet ventilation and/or injection of indocyanine green, is unclear at the hilum, close to the bronchus. Therefore, dissection of the intersegmental plane at the hilum is usually done along these intersegmental veins, especially when operating for small metastatic lung tumors located proximal to the hilum. Third, the peripheral parts of the intersegmental plane defined by other adjuncts are occasionally unclear. In such cases, additional guidance by the intersegmental veins is essential for determining the intersegmental plane. These features of the intersegmental veins are crucial during anatomic segmentectomy and cannot be compensated for by solely focusing on bronchus or arteries.
Surgical Procedure
RUL segmentectomy was planned according to the targeted segment and anatomic model subtype (Figures 3 and 4). In particular, we focused on the intersegmental veins and the approach for identifying them (Figure 4). The patient's anatomy was classified into one of the anatomic models, after which 3D-CT images were used to identify segmental arteries planned for resection and their spatial relationship with the bronchus and veins of the anatomic model. The anatomy was intraoperatively compared with the 3D-CT images to avoid misidentification of anatomic structures.
Figure 4.
Recommended approach to intersegmental veins according to the targeted segment and anatomic model. (Left) The 3 basic RUL segmentectomies and the intersegmental veins (blue-colored) that need to be identified: V1b and V2a for apical S1 segmentectomy, V2a and V2c for posterior S2 segmentectomy, and V1b and V2c for anterior S3 segmentectomy. (Right) The recommended approach to intersegmental veins according to the anatomic model. V1b can usually be identified by an anterior approach except for central III type, for which a posterobronchial approach is necessary. Similarly, V2a is usually identified by the posterobronchial approach, except for anterior II-D3 type, for which an anterior approach is necessary. In addition, V2c can usually be identified by an interlobar approach, except for anterior II-E type, for which a posterobronchial approach is necessary. RUL, Right upper lobe; Ant. V, anterior vein; Cent. V, central vein.
Our basic technique for segmentectomy has been described previously.6 The central portion of the intersegmental plane was dissected along the intersegmental veins in all cases. Intersegmental veins running through the surface of the lung parenchyma were identified from the anterior side of the hilum (anterior approach) or from the interlobar fissure (interlobar approach), whereas intersegmental veins running deep within the lung parenchyma were identified after resection of the targeted segmental bronchus (posterobronchial approach) (Figure 5). The standard approaches for V1b, V2a, and V2c were the anterior, posterobronchial, and interlobar approaches, respectively. Different approaches were used in patients with anatomic variants in their intersegmental veins.
Figure 5.
Three basic approaches to identifying intersegmental veins. Anterior approach (left column): identification of intersegmental vein (V1b) by dissecting along the anterior part of the hilum. Interlobar approach (central column): identification of intersegmental vein (V2c) by dissecting from the interlobar fissure along either the central vein or V2t. Posterobronchial approach (right column): identification of intersegmental vein (V2a) located posterior to the bronchus, usually after dissection of the targeted bronchus (B2). Cent v., Central vein; SPV, superior pulmonary vein; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; Trunks sup., trunks superior; AscA2, ascending A2.
The peripheral portion of the intersegmental plane was dissected along the inflation–deflation line created by selective jet ventilation of the targeted segmental bronchus and/or along the demarcation line created by the intravenous injection of indocyanine green after resection of the targeted pulmonary arteries. In selective jet ventilation, we introduced the bronchoscope into the target bronchus, fixed the bronchoscope by clamping the bronchus using forceps, and performed jet ventilation until complete inflation of the target segment. The intersegmental plane was resected either by electrocautery or stapling (Videos 1 and 2). Compared with stapling, dissection of the intersegmental plane by electrocautery alone could lead to air leakage when performed incorrectly along the intersegmental plane. Therefore, stapling was selected preoperatively for patients with emphysematous lung or interstitial lung disease, or intraoperatively when the demarcation line of the intersegmental plane was unclear.
Video 1.
Posterior S2 segmentectomy in a patient with anterior + central Ib-A type anatomy. First, the ascending A2 was dissected from the interlobar side, followed by dissection of the recurrent A2 at the hilum. Then the intersegmental vein V2c, defining the posteroanterior (S2-S3) plane, was identified by the interlobar approach. The intersegmental vein V2a, which defines the apicoposterior (S1-S2) plane, was identified by the posterobronchial approach after resection of the B2 bronchus. The peripheral part of the S2-S3 intersegmental plane was dissected by electrocautery along the demarcation line toward V2c. Similarly, the S1-S2 intersegmental plane was dissected by electrocautery toward V2a. The remaining central part of the intersegmental plane was dissected using a stapler. Video available at: https://www.jtcvs.org/article/S2666-2507(20)30393-X/fulltext.
Video 2.
Posterior S2 segmentectomy in an emphysematous patient with anterior II-E type anatomy. First, the ascending A2 was dissected from the interlobar side, followed by dissection of the recurrent A2 at the hilum. The intersegmental veins V2a (VX2a) and V2c (VX2c) were identified by the posterobronchial approach after resection of the B2 bronchus. The posteroanterior (S2-S3) plane was dissected with a stapler from the periphery toward VX2c. Similarly, the apicoposterior (S1-S2) plane was dissected with a stapler along VX2a. Video available at: https://www.jtcvs.org/article/S2666-2507(20)30393-X/fulltext.
Regarding lymph node dissection, sampling of the lymph nodes related to the resected segment was performed to determine the presence of malignancy in patients with lung cancer (LN 11s, 12u, or 13). LN 12u and 13 are usually dissected before encircling the targeted segmental bronchus. When a lymph node was positive for malignancy, the procedure was changed to lobectomy provided that the patient could tolerate it.
Statistical Analysis
Data are shown as median (interquartile range) for continuous variables and as number (%) for categorical variables. Statistical analysis was performed using SPSS version 24 (IBM, Armonk, NY).
Results
Thirty-nine patients underwent RUL segmentectomy at Gunma University between January 2016 and December 2019. Five of the patients were excluded from our analysis, including 1 patient who had undergone concurrent preoperative CT-guided marking, 3 patients because of an open approach, and 1 patient because of no anatomic model guidance owing to contrast agent allergy. A final cohort of 34 patients (87%) who underwent anatomic model-guided RUL segmentectomy were analyzed. As detailed in Table 1, this cohort included 23 males and 11 females, with a median patient age of 70 years (IQR, 66-76 years). Among the included patients, 74% (n = 25) had a pathological diagnosis of lung cancer, most of which were adenocarcinomas presenting as pure ground-glass nodules (n = 9) or part-solid nodules (n = 13). The remaining 3 patients with lung cancer with a solid appearance underwent segmentectomy owing to compromised cardiopulmonary function, which prevented them from undergoing lobectomy. The diagnosis was lung metastasis in 7 patients (21%), and granuloma in 2 patients (6%). The median tumor size was 1.6 cm for lung tumors (IQR, 1.4-2.2 cm), 1.8 cm for metastatic tumors (IQR, 1.4-2.3 cm), and 4.1 cm for granulomas (IQR, 3.7-4.6 cm). Complete pathological resection was confirmed in all patients. Regarding lymph node evaluation, 17 patients (50%) underwent sampling at 1 station (usually LN 12u or 13), 7 (21%) underwent sampling at more than 2 stations (LN 10, 11s, 12u, or 13), and 2 (6%) underwent mediastinal lymph node dissection (LN 2R and 4R). All dissected lymph nodes were negative for metastasis.
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| Age (y), median (IQR) | 70 (66-76) |
| Sex, n (%) | |
| Male | 23 (68) |
| Female | 11 (32) |
| Anatomic pattern, n (%) | |
| Anterior + central Iab type | 16 (47) |
| Anterior + central Ib type | 14 (41) |
| Anterior II type | 4 (12) |
| Central III type | 0 (0) |
| Main location of lesion, n (%) | |
| S1 | 9 (26) |
| S2 | 13 (38) |
| S3 | 12 (35) |
| Comorbidities, n (%) | |
| COPD | 12 (35) |
| Diabetes mellitus | 3 (9) |
| Cardiovascular disease | 4 (12) |
| Autoimmune disease | 1 (3) |
| Preoperative diagnosis, n (%) | |
| Lung cancer | 25 (74) |
| Metastasis | 7 (21) |
| Benign tumor | 2 (6) |
| Consolidation/tumor ratio (lung cancer), median (IQR) | 0.2 (0-0.4) |
| Pathological diagnosis, n (%) | |
| Lung cancer | 25 (74) |
| Adenocarcinoma | 22 (65) |
| Squamous cell carcinoma | 3 (9) |
| Metastasis | 7 (21) |
| Granuloma | 2 (6) |
IQR, Interquartile range; COPD, chronic obstructive pulmonary disease.
As shown in Table 2, the median operative time was 222 minutes (IQR, 202-242 minutes), and the median intraoperative blood loss was 19 g (IQR, 7-49 g). The median duration of chest tube insertion and postoperative hospital stay was 2 days (IQR, 2-3 days) and 7 days (IQR, 6-9 days), respectively. Prolonged postoperative air leak exceeding 7 days was noted in 1 patient, for which adhesion therapy was performed. The median duration of follow-up was 13 months (IQR, 5-19 months). Although none of the patients with lung cancer developed locoregional recurrence, 1 patient who underwent segmentectomy for compromised lung function died from brain metastasis. Among the 7 patients who underwent segmentectomy for metastatic lung lesions, 4 were undergoing treatment for new metastatic lesions at the time of this report.
Table 2.
Surgical results
| Characteristic | Value |
|---|---|
| Operative time (minutes), median (IQR) | 222 (202-242) |
| Bleeding (grams), median (IQR) | 19 (7-49) |
| Chest tube duration (d), median (IQR) | 2 (2-3) |
| Postoperative hospital stay (d), median (IQR) | 7 (6-9) |
| Complications, n (%) | |
| Prolonged air leak (>7 d) | 1 (3) |
| Elevated liver enzymes | 1 (3) |
| 90-d mortality, n (%) | 0 (0) |
IQR, Interquartile range.
All patients could be classified into 1 of the 4 anatomic models (Table 1): 16 patients (47%) with the anterior + central Iab type, 14 (41%) with the anterior + central Ib type, and 4 (12%) with the anterior II type. No patient had the central III type. Aberrant V2 was present in 1 patient with anterior + central Iab type, who had 2 V2a, one draining into the aberrant V2 and the other draining into the usual central vein. Tumors were located mainly at the S1 segment in 9 patients, the S2 segment in 13 patients, and the S3 segment in 12 patients (Tables 1 and 3). Single segmentectomy was the most common procedure (n = 26; 76%); bisegmentectomy was performed in 1 patient (3%), segmentectomy combined with subsegmentectomy was performed in 4 patients (12%), bisubsegmentectomy was performed in 3 patients (9%), and subsegmentectomy alone was performed in 1 patient (3%).
Table 3.
Details of anatomic model type and resected segments
| Main location of lesion | Anatomic model type (subtype) | Segmentectomy | Number |
|---|---|---|---|
| S1 (n = 9) | Iab (A/B/C) | S1 | 2 (1/1/0) |
| S1a + S2b | 1 (1/0/0) | ||
| S1a + S2 | 1 (0/0/1) | ||
| S1 + S2 | 1 (1/0/0) | ||
| Iab + aberrant V2 | S1 | 1 | |
| S1a + S2 | 1 | ||
| Ib (A/B/C) | S1 | 1 (0/0/1) | |
| II (E) | S1 | 1 | |
| S2 (n = 13) | Iab (A/B/C) | S2 | 3 (2/1/0) |
| S1a + S2 | 1 (1/0/0) | ||
| Ib (A/B/C) | S2 | 6 (4/1/1) | |
| S1a + S2 | 1 (1/0/0) | ||
| II (D1/D2/D3/E) | S2 | 1 (0/0/0/1) | |
| S2b + S3a | 1 (1/0/0/0) | ||
| S3 (n = 12) | Iab (A/B/C) | S3 | 4 (2/1/1) |
| S3b | 1 (1/0/0) | ||
| Ib (A/B/C) | S3 | 5 (3/1/1) | |
| II (D1/D2/D3/E) | S3 | 2 (1/0/1/0) |
The method of approach for identifying the intersegmental or intrasegmental veins was changed from a standard approach according to the anatomic subtype in 4 cases (12%). For example, V2c was usually identified by an interlobar approach, tracking along the central vein in a peripheral direction (Figure 6, A-C). However, in anterior II-E type (2.8%),11 V2c runs within the lung parenchyma and drains into the anterior vein. Therefore, in patients with anterior II-E type, V2c was identified through the posterobronchial approach after segmental bronchus resection (Figure 6, D-F).
Figure 6.
Different approaches for the V2c intersegmental vein according to anatomic model subtype during S2 segmentectomy. A to C, The V2c intersegmental vein, delineating the posteroanterior (S2-S3) plane, usually drains into the central vein after running along the interlobar fissure. Therefore, V2c was identified by tracking along the central vein in a peripheral direction for anterior + central Iab and Ib types and central III type, or along V2t for anterior II-D type (interlobar approach). D to F, For anterior II-E type, V2c (VX2c) ran within the lung parenchyma and finally drained into the anterior vein. Thus, it could not be observed from the interlobar fissure but was only identified after resection of the B2 segmental bronchus (posterobronchial approach). RLL, Right lower lobe; central v., central vein; RML, right middle lobe.
Discussion
In the present study, we evaluated the clinical outcome of anatomic model-guided RUL segmentectomy. Our findings can be summarized as follows: (1) all patients could be classified into 1 of the RUL anatomic models; (2) the approach for identifying intersegmental veins was simplified and standardized into 3 approaches: anterior, interlobar, and posterobronchial; (3) the appropriate approach for intersegmental veins was selected based on the anatomic model and differed from the standard approach in some anatomic models; and (4) the short- and long-term outcomes were acceptable.
Previous anatomic studies have extensively analyzed the lung anatomy to a subsegmental level.8 However, the sophisticated knowledge of RUL anatomy has not been efficiently implemented into the surgical field, especially in those related to segmentectomies. As reported previously, RUL anatomy can be classified as 1 of the 14 anatomic models in 93.8% of cases.11 In this study, there were no patients with central III type, likely because of the overall low incidence of this type (7%).11 Moreover, the incidence of lung lesions was almost equivalent for each segment (26% for S1, 38% for S2, and 35% for S3), suggesting that surgeons should be equally prepared for every type of RUL segmentectomy.
Theoretically, the method of approach for RUL intersegmental veins might differ from the standard approach in 6.3% to 10.5% of cases (Figure 4; Table 4). The incidence might be higher when the procedure involves a subsegmentectomy, which is affected by variations in both intersegmental and intrasegmental veins. As described in above, the approach for V2c differed in the anterior II-E type. Similarly, V2a usually can be identified using the posterobronchial approach; however, for anterior II-D3 type (3.5%),11 V2a ran on the mediastinal side at the surface of the lung parenchyma and was identified using the anterior approach. Furthermore, V1b usually can be identified using the anterior approach; however, for central III type (7%),11 V1b ran within the lung parenchyma and was identified using the posterobronchial approach.
Table 4.
Theoretical incidence of change in approach to intersegmental veins according to the anatomic model
| Target segment | Intersegmental vein | Standard approach | Change in approach according to subtype | Theoretical incidence (%) |
|---|---|---|---|---|
| S1 | V1b V2a |
Anterior Posterobronchial |
Posterobronchial approaching central III type (7%) Anterior approach in anterior II-D3 type (3.5%) |
10.5 |
| S2 | V2a V2c |
Posterobronchial Interlobar |
Anterior approach in anterior II-D3 type (3.5%) Posterobronchial approach in anterior-E type (2.8%) |
6.3 |
| S3 | V1b V2c |
Anterior Interlobar |
Posterobronchial approach in central III type (7%) Posterobronchial approach in anterior-E type (2.8%) |
9.8 |
Several limitations of this study are worth noting. First, it a retrospective analysis with a small sample size and has a selection bias. Therefore, we did not encounter all types of anatomic variations. Regarding minor but important variations of the bronchi, such as the defective B1 type,9 for which the B1a bronchus branches from B2 and the B1b bronchus branches from B3, our surgical strategy is applicable if it can be classified into 1 of the nearest analog subtypes. Second, patients with aberrant V2 (2.2%) might not have the usual intersegmental veins, V2a and/or V2c. In such a case, identification of the intersegmental plane should be guided not by intersegmental veins alone, but in combination with other adjuncts. Third, in our previous analysis,9,10 several patients had veins that crossed over segments with no usual intersegmental veins and could not be classified into our anatomic models. Although we did not encounter such patients during this study period, we would not recommend RUL anatomic segmentectomy for them, because they are likely to have atypical intersegmental borders and/or lobulation.
Conclusions
The present study demonstrates that converting the RUL anatomy into simplified anatomic models helps elucidate the spatial relationship between segmental bronchi and intersegmental veins, which is an essential component of 3D-CT–guided anatomic segmentectomy. Furthermore, classification into anatomic models aids the precise classification and development of a standardized approach to identifying intersegmental veins, even for cases with anatomic variation (Figure 7). We believe that the use of simplified anatomic models will allow for increased implementation of anatomic RUL segmentectomy.
Figure 7.
Right upper lobe (RUL) segmentectomy guided by a simplified anatomic model consisting of (1) a 2-step classification of the segmental anatomy into 1 of the 14 anatomic models and (2) a standardized approach to intersegmental veins selected according to the anatomic model. This method led to the successful classification and completion of RUL segmentectomy in 34 consecutive patients. Cent. V, Central vein; Ant. V, anterior vein.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
We thank Yasuhiro Fukushima, Junya Fukuda, and Hiroyuki Takei (Department of Radiology, Gunma University Hospital) for obtaining the 3D-CT images. We also thank Takeshi Araki (Gunma University Hospital) for his support in creating the illustrations used in this article.
Footnotes
Drs Nakazawa and Shimizu contributed equally to this work and should be considered co–first authors.
Supplementary Data
Posterior S2 segmentectomy in a patient with anterior + central Ib-A type anatomy. First, the ascending A2 was dissected from the interlobar side, followed by dissection of the recurrent A2 at the hilum. Then the intersegmental vein V2c, defining the posteroanterior (S2-S3) plane, was identified by the interlobar approach. The intersegmental vein V2a, which defines the apicoposterior (S1-S2) plane, was identified by the posterobronchial approach after resection of the B2 bronchus. The peripheral part of the S2-S3 intersegmental plane was dissected by electrocautery along the demarcation line toward V2c. Similarly, the S1-S2 intersegmental plane was dissected by electrocautery toward V2a. The remaining central part of the intersegmental plane was dissected using a stapler. Video available at: https://www.jtcvs.org/article/S2666-2507(20)30393-X/fulltext.
Posterior S2 segmentectomy in an emphysematous patient with anterior II-E type anatomy. First, the ascending A2 was dissected from the interlobar side, followed by dissection of the recurrent A2 at the hilum. The intersegmental veins V2a (VX2a) and V2c (VX2c) were identified by the posterobronchial approach after resection of the B2 bronchus. The posteroanterior (S2-S3) plane was dissected with a stapler from the periphery toward VX2c. Similarly, the apicoposterior (S1-S2) plane was dissected with a stapler along VX2a. Video available at: https://www.jtcvs.org/article/S2666-2507(20)30393-X/fulltext.
References
- 1.Okami J., Shintani Y., Okumura M., Ito H., Ohtsuka T., Toyooka S., et al. Demographics, safety and quality, and prognostic information in both the seventh and eighth editions of the TNM classification in 18,973 surgical cases of the Japanese joint committee of lung cancer registry database in 2010. J Thorac Oncol. 2019;14:212–222. doi: 10.1016/j.jtho.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 2.de Koning H.J., van der Aalst C.M., de Jong P.A., Scholten E.T., Nackaerts K., Heuvelmans M.A., et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 3.Andolfi M., Potenza R., Seguin-Givelet A., Gossot D. Identification of the intersegmental plane during thoracoscopic segmentectomy: state of the art. Interact Cardiovasc Thorac Surg. 2020;30:329–336. doi: 10.1093/icvts/ivz278. [DOI] [PubMed] [Google Scholar]
- 4.Bédat B., Abdelnour-Berchtold E., Krueger T., Perentes J.Y., Zellweger M., Triponez F., et al. Impact of complex segmentectomies by video-assisted thoracic surgery on peri-operative outcomes. J Thorac Dis. 2019;11:4109–4118. doi: 10.21037/jtd.2019.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu K., Nakano T., Kamiyoshihara M., Takeyoshi I. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg. 2012;15:194–196. doi: 10.1093/icvts/ivs202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakazawa S., Shimizu K., Mogi A., Kuwano H. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg. 2018;66:81–90. doi: 10.1007/s11748-017-0878-6. [DOI] [PubMed] [Google Scholar]
- 7.Ohtaki Y., Shimizu K. Anatomical thoracoscopic segmentectomy for lung cancer. Gen Thorac Cardiovasc Surg. 2014;62:586–593. doi: 10.1007/s11748-014-0409-7. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita H. Igaku-Shoin; New York: 1978. Roentgenologic Anatomy of the Lung. 389. [Google Scholar]
- 9.Nagashima T., Shimizu K., Ohtaki Y., Obayashi K., Kakegawa S., Nakazawa S., et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg. 2015;63:354–360. doi: 10.1007/s11748-015-0531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagashima T., Shimizu K., Ohtaki Y., Obayashi K., Nakazawa S., Mogi A., et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg. 2017;65:343–349. doi: 10.1007/s11748-017-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu K., Nagashima T., Ohtaki Y., Obayashi K., Nakazawa S., Kamiyoshihara M., et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg. 2016;64:604–611. doi: 10.1007/s11748-016-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asamura H., Hishida T., Suzuki K., Koike T., Nakamura K., Kusumoto M., et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan clinical oncology group 0201. J Thorac Cardiovasc Surg. 2013;146:24–30. doi: 10.1016/j.jtcvs.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K., Koike T., Asakawa T., Kusumoto M., Asamura H., Nagai K., et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan clinical oncology group 0201) J Thorac Oncol. 2011;6:751–756. doi: 10.1097/JTO.0b013e31821038ab. [DOI] [PubMed] [Google Scholar]
- 14.Sawabata N., Nagayasu T., Kadota Y., Goto T., Horio H., Mori T., et al. Risk assessment of lung resection for lung cancer according to pulmonary function: republication of systematic review and proposals by guideline committee of the Japanese Association for Chest Surgery 2014. Gen Thorac Cardiovasc Surg. 2015;63:14–21. doi: 10.1007/s11748-014-0475-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Posterior S2 segmentectomy in a patient with anterior + central Ib-A type anatomy. First, the ascending A2 was dissected from the interlobar side, followed by dissection of the recurrent A2 at the hilum. Then the intersegmental vein V2c, defining the posteroanterior (S2-S3) plane, was identified by the interlobar approach. The intersegmental vein V2a, which defines the apicoposterior (S1-S2) plane, was identified by the posterobronchial approach after resection of the B2 bronchus. The peripheral part of the S2-S3 intersegmental plane was dissected by electrocautery along the demarcation line toward V2c. Similarly, the S1-S2 intersegmental plane was dissected by electrocautery toward V2a. The remaining central part of the intersegmental plane was dissected using a stapler. Video available at: https://www.jtcvs.org/article/S2666-2507(20)30393-X/fulltext.
Posterior S2 segmentectomy in an emphysematous patient with anterior II-E type anatomy. First, the ascending A2 was dissected from the interlobar side, followed by dissection of the recurrent A2 at the hilum. The intersegmental veins V2a (VX2a) and V2c (VX2c) were identified by the posterobronchial approach after resection of the B2 bronchus. The posteroanterior (S2-S3) plane was dissected with a stapler from the periphery toward VX2c. Similarly, the apicoposterior (S1-S2) plane was dissected with a stapler along VX2a. Video available at: https://www.jtcvs.org/article/S2666-2507(20)30393-X/fulltext.