Abstract
Neuronal cell adhesion molecule 2 (NCAM2) is a membrane protein with an important role in the morphological development of neurons. In the cortex and the hippocampus, NCAM2 is essential for proper neuronal differentiation, dendritic and axonal outgrowth and synapse formation. However, little is known about NCAM2 functional mechanisms and its interactive partners during brain development. Here we used mass spectrometry to study the molecular interactome of NCAM2 in the second postnatal week of the mouse cerebral cortex. We found that NCAM2 interacts with >100 proteins involved in numerous processes, including neuronal morphogenesis and synaptogenesis. We validated the most relevant interactors, including Neurofilaments (NEFs), Microtubule-associated protein 2 (MAP2), Calcium/calmodulin kinase II alpha (CaMKIIα), Actin and Nogo. An in silico analysis of the cytosolic tail of the NCAM2.1 isoform revealed specific phosphorylation site motifs with a putative affinity for some of these interactors. Our results expand the knowledge of NCAM2 interactome and confirm the key role of NCAM2 in cytoskeleton organization, neuronal morphogenesis and synaptogenesis. These findings are of interest in explaining the phenotypes observed in different pathologies with alterations in the NCAM2 gene.
Keywords: NCAM2, mass spectrometry, cytoskeleton, neuronal morphogenesis, MAP2, CaMKIIα, Neurofilaments, Nogo
1. Introduction
Neuronal differentiation, and the establishment of cell polarity and synaptic connections, are crucial events for the development of the brain [1,2]. These processes are tightly regulated by numerous factors, including cytoskeleton proteins, membrane receptors and elements of the extracellular matrix. Cell Adhesion Molecules (CAMs) are a family of membrane proteins with many functions that transduce extracellular and membrane-bound signals into cellular events, such as membrane remodeling, cytoskeletal rearrangement and dynamics, vesicular transport, gene expression and cell survival [3,4,5].
The immunoglobulin superfamily of cell adhesion molecules (IgSF CAM) includes more than 50 different members in mammals [6]. IgSF CAMs are characterized by an extracellular region with one or several immunoglobulin-like (Ig) domains, followed by fibronectin type III (Fn3) domains. Most IgSF CAMs present a single transmembrane domain with an intracellular tail, while other members are anchored to the cell membrane through a glycosylphosphatidylinositol (GPI) anchor [7]. Typically, the extracellular and the intracellular domains of IgSF CAMs interact with several proteins, ligands or modifiers, which determine their important roles during development [5,8,9].
The Neural Cell Adhesion Molecule (NCAM) family has two members, NCAM1 and NCAM2 [10,11]. Both proteins present a similar extracellular structure with five Ig domains and two Fn3 domains. NCAM1 has three different isoforms (180, 140 and 120 KDa), whereas NCAM2 has two isoforms: NCAM2.1 (with a transmembrane domain and a cytoplasmic tail) and NCAM2.2 (a shorter isoform with no transmembrane domain but a GPI-anchor motif) [12,13].
A large number of studies have investigated the functions of NCAM1, which play a fundamental role in both neural development and plasticity [8,14,15]. NCAM1 interacts with many extracellular ligands and adaptors, such as the Fibroblast Growth Factor Receptor (FGFR), Prion Protein (PRNP), Homer1, Tyrosine-protein kinase Fyn or Focal adhesion kinase 1 (FAK) [16,17,18,19,20]. By contrast, NCAM2 is less studied. NCAM2 is widely expressed in the Central Nervous System (CNS) during brain development. In the olfactory system NCAM2 is necessary for the formation and maintenance of dendritic and axonal compartments [21,22,23,24,25,26], and in synapse formation and maintenance [26,27,28]. Genetic studies suggest that the NCAM2 gene is implicated in the intellectual disability phenotype in Down syndrome and Autism Spectrum Disorders, as well as in other neurodevelopmental diseases [24,29,30,31,32]. In addition, NCAM2 has been involved in synaptic deficits in Alzheimer’s disease [28]. Although NCAM2 functions have begun to be clarified [25,26,28], the extracellular ligands and intracellular adaptors that interact with NCAM2 remain largely unknown. It has been recently shown that NCAM2 regulates neurite outgrowth through the kinase Src in the cerebral cortex [26,27], and that it mediates dendritic morphogenesis via the formation of molecular complexes with Microtubule-associated protein 2 (MAP2) and 14-3-3 family proteins [25].
To gain insight into NCAM2 functions, here we investigate the interactome of the NCAM2 protein during postnatal cortical development using proteomic and molecular approaches. We found more than 100 proteins that interact with NCAM2 using mass spectrometry. We further validated the more relevant interactions for cytoskeleton organization (CaMKIIα, NEFs, Actin and MAP2) by means of immunoprecipitation. To better characterize the NCAM2 interactome, our proteomic data were analyzed by bioinformatic tools; we detected significant enrichments in gene ontology terms and in cellular pathways linked to the cytoskeleton, as well as to other important neural functions. In addition, we identify putative phosphorylation sites of the NCAM2.1 cytosolic tail using in silico analysis. These data increase our knowledge about the interactome of the NCAM2 protein and open new perspectives for the study of NCAM2 functions.
2. Results
2.1. In Silico Analysis of the Cytoplasmic Domains of NCAM2.1
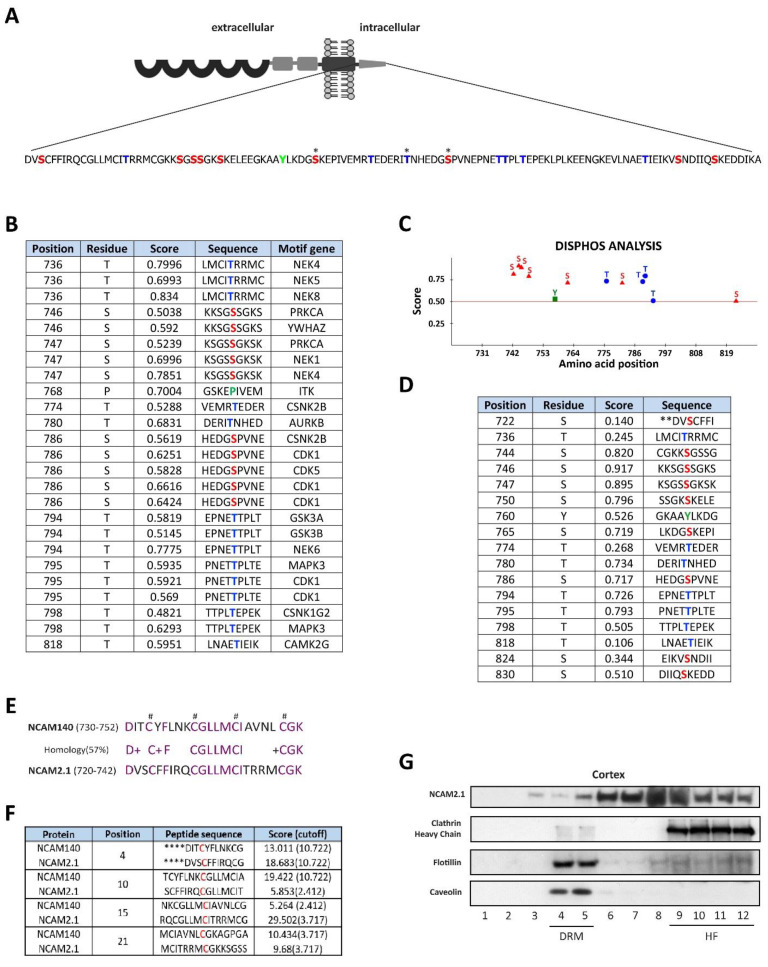
Previous studies showed the importance of the cytoplasmic domains of CAMs, such as NCAM1 (NCAM140 or NCAM180 isoforms). We thus performed a structural analysis of the amino acid sequence of the NCAM2.1 cytosolic domain (Figure 1A). The residues S765, T780 and S786 have already been described as phosphorylation sites [33]. We used different bioinformatic tools (Scansite 4.0 and Disphos 1.3 analysis, Koch Institute and MIT, Cambridge, MA, USA) to identify additional phosphorylatable residues. High scores for S746 and T818, which match the consensus sequences of 14-3-3ζ and CaMKIIγ, respectively, were detected with Scansite 4.0. Disphos analysis showed that approximately 70% (12/17) of the phosphorylatable residues have significant scores to be phosphorylated (serines 7/9, threonines 4/7 and tyrosine 1/1), as shown in Figure 1B–D. We also detected different motifs that match the consensus sequences of the kinases Cyclin dependent kinase 5 (CDK5), Protein kinase C alpha (PRKCA), Casein kinase II (CSNK2) and Glycogen synthase 3 (GSK3), using Scansite. These are kinases known to phosphorylate other CAMs, such as Cadherins, Epithelial cell adhesion molecule (EpCAM), Integrins or Neuronglia cell adhesion molecule (NgCAM) [34,35,36,37,38,39].
Figure 1.
In silico analysis of NCAM2.1 cytoplasmatic domain. (A) Schematic representation of phosphorylation sites in NCAM2.1 cytoplasmatic tail amino acid sequences. Serine (red), Tyrosine (green) and Threonine (blue) phosposites are represented. * phosphorylated sites previously described. (B) In silico analysis using Scansite 4.0 to identify motifs gene interactors (motif gene) that are likely to be phosphorylated by specific protein kinases or binding domains such as SH2 domains, 14-3-3 domains or PDZ. (C,D) Schematic representation and Table of phosphorylation sites of NCAM2 cytoplasmatic tail with their in silico predicted scores using Disphos. (E) Comparison of NCAM140 (730–752 amino acids) and NCAM2.1 (719–741 amino acids) sequences, NCAM1 has an intracellular region with palmitoylation modification sites that contain cysteine-residues which are critical for its localization in lipid rafts and also present in NCAM2.1 cytoplasmatic domain (#). (F) In silico analysis of NCAM2.1 and NCAM1 with their predicted scores using CSS-Palm software 4.0. NCAM2.1 contains the same potential palmitoylation sites similarly to NCAM1 with high scores (G) WB of NCAM2.1 from cortical extracts subjected to sucrose gradient. NCAM2.1 co-signals in lipid rafts (lanes 4–5, DRM, Detergent-resistant membrane, identified by Flotillin and Caveolin), but mainly outside lipids rafts (lanes 6–12, HF, High Fraction).
CAMs are known to have different roles depending on their membrane localization in lipids rafts [40,41,42,43], as in the case of NCAM140. Since the NCAM2 molecule is a paralog of NCAM1, we decided to compare the intracellular domains of NCAM140 and NCAM2.1. Although the cytoplasmic domain sequences of both proteins share an homology of 54% (65/120 amino acids, data not shown), the phosphorylation sites are not found in the homologous regions. Therefore, we focused on the comparison of the NCAM1 sequences responsible for lipid raft localization, corresponding to 730–752 amino acids [41], with their homologous regions in NCAM2.1 (Figure 1E). NCAM1 has an intracellular region with palmitoylation modification sites that contains cysteine-residues that are critical for NCAM1 targeting to lipid rafts. We observed the presence of four similar cysteine residues in the homologous region of NCAM2.1, suggesting that these conserved residues could be implicated in lipid raft localization (Figure 1E). Using CSS-Palm 4.0 software (Sun Yat-sen University, Guangzhou, China), we detected with high scores the same palmitoylation sites in NCAM2.1 and NCAM1 (Figure 1F). In order to confirm the NCAM2.1 localization in lipid rafts, we analyzed the expression of NCAM2.1 by Western blot (WB) in cortical extracts subjected to sucrose gradient (Figure 1G). We found that NCAM2.1 co-localized with lipid raft markers (lanes 4–5), but was predominantly expressed outside lipids rafts (lines 6–12).
2.2. NCAM2 Interactome in Postnatal Cerebral Cortex
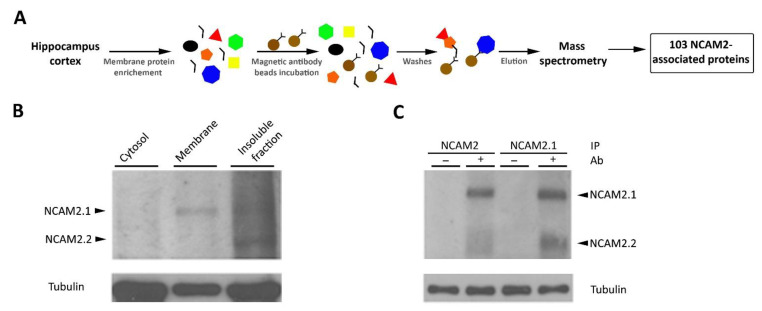
We performed protein immunoprecipitation and peptide detection as described [25] in Figure 2A. The membrane fraction was isolated from the cerebral cortex of postnatal P12–15 mice (Figure 2B) and NCAM2 was purified by immunoprecipitation with two different antibodies, one recognizing the cytoplasmic tail of NCAM2.1 (EB06991, Everest, Oxfordshire, UK), and the other recognizing the extracellular region of both NCAM2 isoforms (AF778, R&D Systems, Minneapolis, MN, USA). We used non-conjugated magnetic beads for control immunoprecipitations. Our data first confirmed the specific interaction of NCAM2.1 with NCAM2.2 as shown in Figure 2C. Immunoprecipitated proteins were then eluted and digested for mass spectrometry analysis. The resulting peptides were identified by LC-MS/MS to obtain putative protein partners (False Discovery Rate ≤ 0.01%) as represented in Figure 2A and Table S1.
Figure 2.
The interactome of NCAM2; the mass spectrometry approach. (A) Schematic representation of the mass spectrometry approach. Brain lysates from P10–12 mice were enriched in membrane proteins and incubated with magnetic beads, which were previously conjugated with an antibody against NCAM2.1 or against both NCAM2 isoforms. The eluted proteins were processed for mass spectrometry analysis. A list of 103 proteins that interact with NCAM2 was obtained with a False Discovery Rate ≤ 0.01%, Table 1. (B) WB detection of NCAM2.1 and NCAM2 proteins in the cytosolic, membrane and insoluble fractions of the brain lysates used for the mass spectrometry assay. NCAM2.1 isoform is detected in both fractions, the membrane and the insoluble fraction while NCAM2.2 is specially enriched in the insoluble fraction. Tubulin was used as a loading control. (C) WB detection of NCAM2 from samples of magnetic beads elution.
We detected 103 proteins that specifically interact with NCAM2 (Table S1) and 52 of them were identified with two or more different peptides (Table 1). Many of the peptides corresponded to microtubule, intermediate filaments or Actin cytoskeletal proteins (i.e., Actin, ACTB; Tubulin beta-4A, TUBB4A; Alpha-internexin, INA; Tubulin alpha-1A, TUBA1A; Tubulin alpha-1C, TUBA1C;Neurofilament light polypeptide, NEFL; Neurofilament medium polypeptide, NEFM; and Tubulin beta-6 chain, TUBB6) and to cytoskeleton-associated proteins (i.e., Microtubule-associated protein 2, MAP2; Microtubule-associated protein 1B, MAP1B; F-actin-capping protein beta, CAPZB; and F-actin-capping protein alpha-2, CAPZA2). Interestingly, the analysis also detected the interaction with motor proteins (i.e., Dynein light chain 1, DYNLL1; Myosin light polypeptide 6, MYL6; and Dynein light chain 2, DYNLL2), kinases and adapter proteins (i.e., CaMKII family proteins and 14-3-3 family proteins), calcium-binding proteins (i.e., CaMKII family proteins, Calumenin, CALU; Reticulocalbin-2, RCN2; Calmodulin, CALM1; and Hippocalcin-like protein 1, HPCAL1) and transcription activity regulators (i.e., Elongation factor 1-beta, EEF1B; Nuclease-sensitive element-binding protein 1, YBX1; Elongation factor 1-delta, EEF1D; and Eukaryotic translation initiation factor 3 H, EIF3H).
Table 1.
The interactome of NCAM2.
| Protein (Gene) | Ptd | Function |
|---|---|---|
| Microtubule-associated protein 2 (Map2) | 24 | actin binding, calmodulin binding, cytoskeletal regulatory, microtubule binding and protein kinase binding |
| Neurofilament light polypeptide (Nefl) | 20 | structural constituent of cytoskeleton andstructural constituent of postsynaptic intermediate filament cytoskeleton |
| Actin, cytoplasmic 1 (Actb) | 19 | structural constituent of cytoskeleton and structural constituent of postsynaptic actin cytoskeleton |
| Neurofilament medium polypeptide (Nefm) | 19 | microtubule binding and structural constituent of cytoskeleton |
| Tubulin beta-4A (Tubb4a) | 18 | structural constituent of cytoskeleton and GTPase activity |
| Alpha-internexin (Ina) | 17 | structural constituent of cytoskeleton, structural constituent of postsynaptic actin and intermediate filament cytoskeleton |
| Tubulin alpha-1A (Tuba1a) | 13 | structural constituent of cytoskeleton |
| Tubulin alpha-1C (Tuba1c) | 12 | structural constituent of cytoskeleton |
| Actin, alpha cardiac muscle 1 (Actc1) | 11 | structural constituent of cytoskeleton and myosin binding |
| Calcium/calmodulin-dependent protein kinase type II beta (Camk2b) | 11 | calmodulin-dependent protein kinase activity, protein serine/threonine kinase activity and structural constituent of postsynaptic actin cytoskeleton |
| Calcium/calmodulin-dependent protein kinase type II alpha(Camk2a) | 10 | calmodulin-dependent protein kinase activity, glutamate receptor binding and protein serine/threonine kinase activity |
| Microtubule-associated protein 1B (Map1b) | 9 | actin binding, cytoskeletal regulatory protein and binding |
| Beta-actin-like protein 2 (Actbl2) | 8 | structural constituent of postsynaptic actin cytoskeleton |
| F-actin-capping protein beta (Capzb) | 8 | actin binding and beta-tubulin binding |
| 14-3-3 protein zeta/delta (Ywhaz) | 8 | recognition of a phosphoserine or phosphothreonine motif |
| Tubulin beta-6 chain (Tubb6) | 7 | structural constituent of cytoskeleton |
| Calcium/calmodulin-dependent protein kinase type II delta (Camk2d) | 6 | calmodulin-dependent protein kinase activity and protein serine/threonine kinase activity |
| Calcium/calmodulin-dependent protein kinase type II gamma (Camk2g) | 6 | calmodulin-dependent protein kinase activity and protein serine/threonine kinase activity |
| Enhancer of rudimentary homolog (Erh) | 5 | Cell cycle |
| Thioredoxin-dependent peroxide reductase (Prdx3) | 5 | cysteine-type endopeptidase inhibitor activity involved in apoptotic process |
| Heat shock cognate 71 kDa protein (Hspa8) | 5 | protein quality control system (folding proteins and degredation), co-chaperone, signaling receptor binding and ubiquitin protein ligase binding |
| F-actin-capping protein alpha-2 (Capza2) | 4 | actin filament binding |
| Calumenin (Calu) | 4 | calcium ion binding and enzyme inhibitor activity |
| Heat shock factor-binding protein 1 (Hsbp1) | 3 | transcription corepressor activity |
| Granulins (Grn) | 3 | chaperone binding, cytokine activity, growth factor activity and RNA binding |
| Reticulocalbin-2 (Rcn2) | 3 | calcium ion binding |
| Elongation factor 1-beta (Eef1b) | 3 | guanyl-nucleotide exchange factor activity and translation elongation factor activity |
| 14-3-3 protein epsilon (Ywhae) | 3 | recognition of a phosphoserine or phosphothreonine motif |
| Hemoglobin beta-1 (Hbb-b1) | 3 | oxygen carrier activity |
| Nuclease-sensitive element-binding protein 1 (Ybx1) | 3 | DNA-binding transcription activator, transcription factor binding |
| Elongation factor 1-delta (Eef1d) | 3 | activating transcription factor bindingand translation elongation factor activity |
| Dynein light chain 1, cytoplasmic (Dynll1) | 2 | motor activity and scaffold protein binding |
| Myosin light polypeptide 6 (Myl6) | 2 | motor activity and actin-dependent ATPase activity |
| Barrier-to-autointegration factor (Banf1) | 2 | DNA binding and enzyme binding |
| Dynein light chain 2, cytoplasmic (Dynll2) | 2 | motor activity and scaffold protein binding |
| 60S acidic ribosomal protein P2 (Rplp2) | 2 | structural constituent of ribosome |
| Complement component 1 Q (C1qbp) | 2 | complement component C1q binding, mRNA binding and translation activator |
| Inter-alpha-trypsin inhibitor heavy chain H3 (Itih3) | 2 | binding protein between hyaluronan and other matrix protein |
| Reticulon-4 (Rtn4) | 2 | cadherin binding and neurite growth regulatory factor |
| Ig kappa chain V-III region PC 2413 (Kv3a5) | 1 | adaptive immune response |
| Ataxin-10 (Atxn10) | 1 | neuritogenesis |
| Protein LSM12 homolog (Lsm12) | 1 | interactor/competitor of ATXN2 |
| Eukaryotic translation initiation factor 3 H (Eif3h) | 1 | translation initiation factor activity |
| Annexin A2 (Anxa2) | 1 | calcium-dependent phospholipid and cytoskeletal protein binding |
| Calmodulin (Calm1) | 1 | calcium-dependent protein and protein kinase binding |
| FAST kinase domain-containing protein 2 (Fastkd2) | 1 | protein kinase activity |
| Haptoglobin (Hp) | 1 | hemoglobin binding |
| Hippocalcin-like protein 1 (Hpcal1) | 1 | calcium ion binding |
| Ras-related protein Rab-33A (Rab33a) | 1 | GTPase activity |
| Ubiquitin-protein ligase E3B (Ube3b) | 1 | ubiquitin conjugating enzyme activity |
Proteins identified by LC-MS/MS in the NCAM2-immunoprecipitated cerebral cortex of postnatal mice samples obtained with a False Discovery Rate ≤ 0.01% and detected with two or more peptides. The table shows the proteins, the number of different peptides (Ptd) found from each protein and the function of the proteins.
A total number of 16 proteins were detected with both NCAM2 antibodies, and 20 proteins were detected in both independent experiments (Table 1 and Table S2). Moreover, seven out of these 20 proteins were also detected with both antibodies (ACTB, TUBA1A, HSPA8, HSBP1, GRN and DYNLL1). Specifically, Actin (ACTB), Heat shock cognate 71 kDa protein (HSPA8) and Granulin (GRN) proteins were identified in all the replicates and with all antibodies, confirming the strong reliability of these detected interactions (Table 1 and Table S2). Additionally, mass spectrometry analysis showed different protein coverage among the detected NCAM2 interactors (Table 1 and Table S2).
2.3. NCAM2 Interacts with Cytoskeleton and Cytoskeleton-Associated Proteins
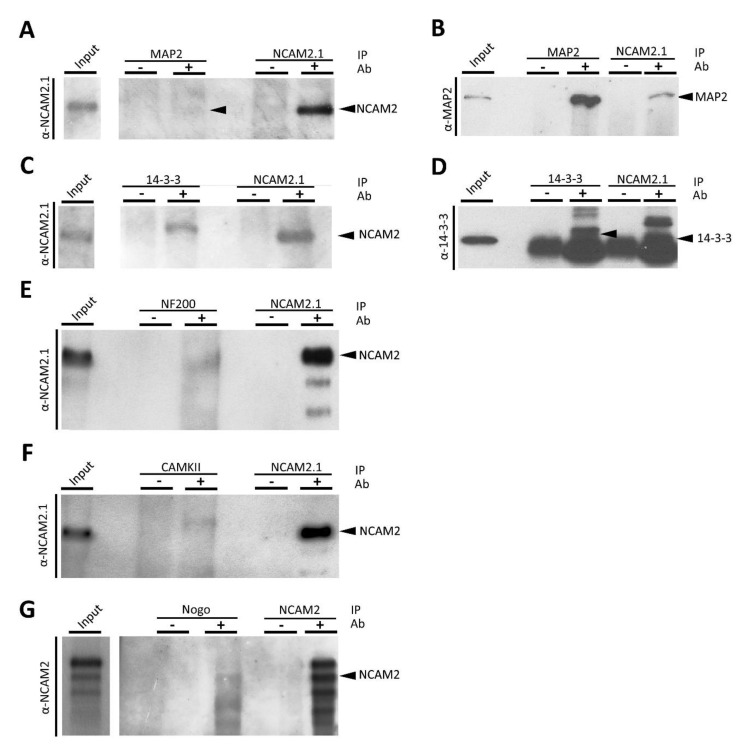
Mass spectrometry analysis identified some proteins with high robustness. These proteins are MAP2, Neurofilaments, and CaMKII. MAP2 is the protein with the largest number of peptides detected in the present analysis. We already described the interaction of MAP2 with NCAM2 (Figure 3A,B), as well as the interaction of NCAM2 with 14-3-3 family proteins (Figure 3C,D).
Figure 3.
NCAM2 interacts with MAP2, 14-3-3, NFs, CaMKII and Nogo. (A) WB detection of NCAM2.1 in different co-immunoprecipitation experiments with MAP2 (A), 14-3-3 (C), NEFs (E), CaMKII (F) or NEFs (G). Detection of MAP2 (B) or 14-3-3 (D) in different co-immunoprecipitation experiments with NCAM2.1 from P10–15 mouse cortex and hippocampal protein extracts. Immunoprecipitation using MAP2 (A), 14-3-3 (C), NEFs (E) or CaMKII (F) or Nogo (G) antibodies confirmed the presence of NCAM2.1 protein in the WBs after detection with NCAM2.1 or NCAM2 antibodies (G). Immunoprecipitation using NCAM2.1 antibodies confirmed the presence of MAP2 (B) and 14-3-3 (D).
We previously reported that NCAM2 regulates microtubule polymerization and stability [25]. The present data show that NCAM2 additionally interacts with different key cytoskeletal components. The cytoskeleton proteins ACTB, TUBA1A and ACTC1 were detected in different replicates. We confirmed the NCAM2-Actin interaction by immunoprecipitation, IP, using specific antibodies for NCAM2 (Figure S1). Besides the interaction with Actin filaments, our results detected the interaction of NCAM2 with CAPZA and CAPZB, Actin-interacting proteins forming a heterodimer that binds to the barbed-ends of Actin filaments, which block their polymerization and depolymerization [44,45]. CAPZ proteins play a relevant role in growth cone dynamics, dendritic spine development and synapse formation [46,47].
A large number of peptides corresponding to NEFL and NEFM were identified in the different experiments. Neurofilaments are important for the radial growth and the stability of axons and enable effective and high-velocity nerve conduction [48,49]. The NCAM2-NEFs interactions were validated by IP and WB (Figure 3E).
The CaMKII family of proteins was also identified with different peptides. CaMKIIα and CaMKIIβ play crucial roles in neuronal morphogenesis and plasticity [1,50,51]. Previous results showed the functional relation between NCAM2 and CaMKII, important for neurite branching and filopodia formation [26]. Here, our results show a direct interaction between these proteins, validated by IP and WB as shown in Figure 3F.
We detected Reticulon 4 (Nogo) with more than one peptide when the NCAM2.1 isoform was immunoprecipitated. The Nogo-NCAM2.1 interaction was additionally assessed by immunoprecipitation of Nogo and NCAM2 detection by WB (Figure 3G). Nogo proteins are important for neuron migration and neurite outgrowth and branching [52].
Finally, Heat shock cognate 71 kDa protein and Granulin were detected in all conditions. HSPA8 is a member of the chaperon family and participates together with HSC70 in protein folding and degradation, stress response and chaperone-mediated autophagy [53,54]. GRN is a secreted growth factor involved in different neurological functions.
2.4. Bioinformatic Analysis of the NCAM2 Interactome
A bioinformatic analysis was performed with the 52 proteins detected with two or more peptides using String 11.0 (Elixir, Hinxton, Cambridgeshire, UK). [55].
2.4.1. Bit Map
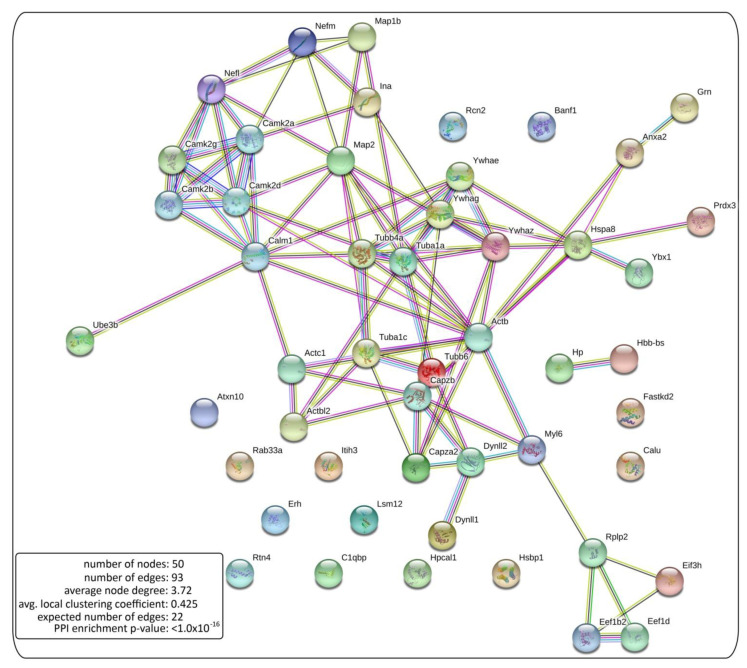
To visualize the proteomic results, we obtained a high quality graphic of NCAM2 interactions in bitmap format, showing a significant increased number of edges: 93 edges with our pull versus 22 expected in random condition (Figure 4). This significant increase shows that NCAM2 interacts with different protein complex previously described.
Figure 4.
NCAM2 protein interaction network. Bitmap representation of the NCAM2 interaction network. The network edges mean the evidence of interactions between the proteins of Table 1, proteins detected with two or more peptides in the mass spectrometry assay. Our bitmap shows a significant increased number of edges, 93 edges with our pull versus 22 expected in random condition.
2.4.2. Gene Ontology Terms
We next performed Gene Ontology (GO) and pathway enrichment analyses. We found a significant enrichment for Biological Process (Table 2 and Table S3); Molecular Function (Table 2 and Table S4); and Cellular Components GO terms (Table 2 and Table S5). Focusing on Biological Process GO terms, our results show that the partners of NCAM2 are significantly enriched in 118 biological process terms (Table 2 and Table S3), including cell differentiation and neuronal morphogenesis. Moreover, NCAM2 interactors are significantly enriched in GO terms linked to cytoskeleton, corroborating the above findings. We also found terms related to the organization and localization of plasma membrane proteins.
Table 2.
Gene Ontology (GO) terms enriched for Biological Process, Molecular Function and Cellular Components in the NCAM2 interactors.
| GOs Enrichment for Biological Process | ||
|---|---|---|
| GO | Term Description | Proteins |
| GO:0030030 | cell projection organization | Actb,Atxn10,Camk2a,Camk2b,Capzb,Dynll1,Dynll2,Map1b, Map2,Nefl,Nefm,Rtn4,Tubb4a |
| GO:0007010 | cytoskeleton organization | Actb,Actc1,Capzb,Ina,Map1b,Map2,Nefl,Nefm,Tuba1a, Tub1c,Tubb4a,Tubb6 |
| GO:0007017 | microtubule-based process | Dynll1,Dynll2,Map1b,Map2,Nefl,Nefm,Tuba1a,Tuba1c, Tubb4a,Tubb6 |
| GO:0060052 | neurofilament cytoskeleton organization | Ina,Nefl,Nefm |
| GO:0010769 | regulation of cell morphogenesis involved in differentiation | C1qbp,Camk2b,Map1b,Map2,Nefl,Nefm,Rtn4 |
| GO:0007399 | nervous system development | Actb,Atxn10,Camk2a,Camk2b,Camk2d,Camk2g,Capzb,Grn,Ina,Map1b,Map2,Nefl,Nefm,Rtn4,Ywhae,Ywhag |
| GO:0010970 | transport along microtubule | Dynll1,Dynll2,Map1b,Nefl,Nefm |
| GO:0031175 | neuron projection development | Actb,Atxn10,Camk2a,Capzb,Map1b,Map2,Nefl,Nefm,Rtn4 |
| GO:0048699 | generation of neurons | Actb,Atxn10,Camk2a,Camk2b,Capzb,Grn,Map1b,Map2, Nefl, Nefm,Rtn4,Ywhae,Ywhag |
| GO:0006414 | translational elongation | Eef1b2,Eef1d,Rplp2 |
| GO:0050770 | regulation of axonogenesis | Map1b,Map2,Nefl,Nefm,Rtn4 |
| GO:0061564 | axon development | Actb,Map1b,Map2,Nefl,Nefm,Rtn4 |
| GO:0045664 | regulation of neuron differentiation | Camk2b,Grn,Map1b,Map2,Nefl,Nefm,Rtn4,Ywhag |
| GO:0010975 | regulation of neuron projection development | Camk2b,Grn,Map1b,Map2,Nefl,Nefm,Rtn4 |
| GOs enrichment for Molecular Function | ||
| GO | Term Description | Proteins |
| GO:0005200 | structural constituent of cytoskeleton | Actb,Nefl,Tuba1a,Tuba1c,Tubb4a,Tubb6 |
| GO:0005198 | structural molecule activity | Actb,Ina,Myl6,Nefl,Nefm,Rplp2,Tuba1a,Tuba1c,Tubb4a, Tubb6 |
| GO:0004683 | calmodulin-dependent protein kinase activity | Camk2a,Camk2b,Camk2d,Camk2g |
| GO:0008092 | cytoskeletal protein binding | Actb,Actc1,Anxa2,Camk2d,Capza2,Capzb,Dynll1,Dynll2, Map1b,Map2,Ywhag |
| GO:0005519 | cytoskeletal regulatory protein binding | Map1b,Map2 |
| GO:0044325 | ion channel binding | Calm1,Camk2d,Ywhae,Ywhaz |
| GO:0003924 | GTPase activity | Rab33a,Tuba1a,Tuba1c,Tubb4a,Tubb6 |
| GO:0005509 | calcium ion binding | Anxa2,Calm1,Calu,Hpcal1,Myl6,Rcn2,Tubb4a |
| GO:0097110 | scaffold protein binding | Dynll1,Dynll2,Ywhae |
| GO:0003746 | translation elongation factor activity | Eef1b2,Eef1d |
| GOs enrichment for Cellular Component | ||
| GO | Term Description | Proteins |
| GO:0005856 | cytoskeleton | Actb,Actbl2,Actc1,Calm1,Camk2b,Capza2,Capzb,Dynll1, Dynll2,Hsbp1,Hspa8,Ina,Map1b,Map2,Myl6,Nefl,Nefm, Tuba1a,Tuba1c,Tubb4a,Tubb6,Ywhae |
| GO:0099513 | polymeric cytoskeletal fiber | Actc1,Capzb,Dynll1,Dynll2,Hspa8,Ina,Map1b,Map2,Nefl, Nefm, Tuba1a,Tuba1c,Tubb4a,Tubb6 |
| GO:0014069 | postsynaptic density | Actb,Camk2a,Camk2b,Camk2g,Hspa8,Map1b,Map2,Nefm, Rtn4,Ywhaz |
| GO:0043005 | neuron projection | Actb,Atxn10,Calm1,Camk2a,Camk2b,Camk2d,Camk2g, Capzb,Dynll1,Hspa8,Map1b,Map2,Nefl,Nefm,Rtn4,Tubb4a,Ybx1,Ywhae |
| GO:0030424 | axon | Actb,Calm1,Camk2a,Camk2d,Dynll1,Hspa8,Map1b,Map2, Nefl,Nefm,Rtn4,Tubb4a,Ywhae |
| GO:0098794 | postsynapse | Actb,Camk2a,Camk2b,Camk2g,Capzb,Hspa8,Map1b,Map2,Nefm,Rtn4,Ywhaz |
| GO:0005874 | microtubule | Dynll1,Dynll2,Hspa8,Map1b,Map2,Tuba1a,Tuba1c,Tubb4a,Tubb6 |
| GO:0045202 | synapse | Actb,Camk2a,Camk2b,Camk2d,Camk2g,Capzb,Grn,Hspa8,Map1b,Map2,Nefm,Rtn4,Ywhaz |
| GO:0005883 | neurofilament | Ina,Nefl,Nefm |
| GO:0015630 | microtubule cytoskeleton | Calm1,Camk2b,Dynll1,Dynll2,Hspa8,Map1b,Map2,Tuba1a, Tuba1c,Tubb4a,Tubb6,Ywhae |
| GO:0030426 | growth cone | Calm1,Map1b,Map2,Nefl,Rtn4,Ywhae |
| GO:0016529 | sarcoplasmic reticulum | Calu,Camk2b,Camk2d,Camk2g |
| GO:0030425 | dendrite | Atxn10,Camk2a,Camk2b,Capzb,Hspa8,Map1b,Map2,Rtn4, Ybx1 |
| GO:0150034 | distal axon | Calm1,Hspa8,Map1b,Map2,Nefl,Rtn4,Ywhae |
| GO:0015629 | actin cytoskeleton | Actb,Actc1,Capza2,Capzb,Dynll2,Map2,Myl6 |
| GO:0005853 | eukaryotic translation elongation factor 1 complex | Eef1b2,Eef1d |
| GO:0099524 | postsynaptic cytosol | Camk2a,Hspa8 |
| GO:0005882 | intermediate filament | Hspa8,Ina,Nefl,Nefm |
| GO:0008290 | F-actin capping protein complex | Capza2,Capzb |
| GO:0044294 | dendritic growth cone | Map2,Rtn4 |
| GO:0043194 | axon initial segment | Camk2d,Map2 |
List of the most relevant GOs involved in brain development, neuronal differentiation and synaptic formation. GOs enriched for Biological Process, Molecular Function and Cellular Components in NCAM2 interactome. The table shows the GO term identifier, the term description and the list of proteins detected in the mass spectrometry for each specific GO term.
Regarding Molecular Function, presented in Table 2 and Table S4, NCAM2-related proteins are associated with cytoskeleton functions including structural constituents of cytoskeleton (GO:0005200), cytoskeletal protein binding (GO:0008092), cytoskeletal regulatory protein binding (GO:0005519), scaffold protein binding (GO:0097110) and Actin binding (GO:0003779), as well as with translation processes: translation elongation factor activity (GO:0003746), translation factor activity and RNA binding (GO:0008135GO:0003723).
Furthermore, NCAM2 protein interactors are enriched in 78 significant cellular components, as shown in Table 2 and Table S5. Again, NCAM2 partners are classified as cytoskeleton cellular components, cellular components involved in neuronal morphogenesis and maintenance, and synaptogenesis and synaptic maintenance processes.
2.4.3. Pathways Analysis
The results of the Kyoto Encyclopedia of Genes and Genomes (KEGGS) pathways enrichment analysis (Table 3 and Table S6), and the Reactome pathway enrichment analysis (Table 3 and Table S7) are also displayed. Our data show an enrichment in 71 different pathways, including pathways related to calcium and neurotransmitter receptors, cell cycle regulation and to Rho GTPases (Table 3 and Table S7).
Table 3.
Kyoto Encyclopedia of Genes and Genomes (KEGGS) and The Reactome pathways enriched in NCAM2 interactome.
| KEGGs Enrichment | ||
|---|---|---|
| KEGG | Term Description | Proteins |
| mmu04722 | Neurotrophin signaling pathway | Calm1,Camk2a,Camk2b,Camk2d,Camk2g,Ywhae |
| mmu04720 | Long-term potentiation | Calm1,Camk2a,Camk2b,Camk2d,Camk2g |
| mmu04012 | ErbB signaling pathway | Camk2a,Camk2b,Camk2d,Camk2g |
| mmu04540 | Gap junction | Tuba1a,Tuba1c,Tubb4a,Tubb6 |
| mmu04020 | Calcium signaling pathway | Calm1,Camk2a,Camk2b,Camk2d,Camk2g |
| mmu04024 | cAMP signaling pathway | Calm1,Camk2a,Camk2b,Camk2d,Camk2g |
| mmu04360 | Axon guidance | Camk2a,Camk2b,Camk2d,Camk2g |
| Reactome pathways enriched | ||
| Reactome | Term Description | Proteins |
| MMU-195258 | RHO GTPase Effectors | Actb,Calm1,Dynll1,Dynll2,Myl6,Tuba1a,Tuba1c,Tubb4a, Tubb6,Ywhae,Ywhag,Ywhaz |
| MMU-442729 | CREB phosphorylation through the activation of CaMKII | Calm1,Camk2a,Camk2b,Camk2d,Camk2g,Nefl |
| MMU-442982 | Ras activation upon Ca2+ influx through NMDA receptor | Calm1,Camk2a,Camk2b,Camk2d,Camk2g,Nefl |
| MMU-199991 | Membrane Trafficking | Actb,Capza2,Capzb,Dynll1,Dynll2,Rab33a,Tuba1a,Tuba1c, Tubb4a,Tubb6,Ywhae,Ywhag,Ywhaz |
| MMU-438066 | Unblocking of NMDA receptors, glutamate binding and activation | Camk2a,Camk2b,Camk2d,Camk2g,Nefl |
| MMU-5576892 | Phase 0-rapid depolarization | Calm1,Camk2a,Camk2b,Camk2d,Camk2g |
| MMU-190828 | Gap junction trafficking | Actb,Tuba1a,Tuba1c,Tubb4a,Tubb6 |
| MMU-190840 | Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane | Tuba1a,Tuba1c,Tubb4a,Tubb6 |
| MMU-399719 | Trafficking of AMPA receptors | Camk2a,Camk2b,Camk2d,Camk2g |
| MMU-5673001 | RAF/MAP kinase cascade | Actb,Calm1,Camk2a,Camk2b,Camk2d,Camk2g,Nefl |
| MMU-1640170 | Cell Cycle | Dynll1,Dynll2,Tuba1a,Tuba1c,Tubb4a,Tubb6,Ywhae, Ywhag,Ywhaz |
| MMU-5620912 | Anchoring of the basal body to the plasma membrane | Dynll1,Tuba1a,Tubb4a,Ywhae,Ywhag |
List of the most relevant KEGGs and Reactome pathways enriched in NCAM2 interactome. The table presents the KEGGs or the Reactome pathways identifier, the term description and the list of proteins detected in the mass spectrometry for each specific item.
Taken together, our results suggest that NCAM2 plays a significant role in the organization of the cytoskeleton and it is involved in calcium signaling and membrane dynamics. These processes are essential for the proper neuronal morphogenesis, synapse formation and neuronal network maintenance.
3. Discussion
In the present study, we explored the interactome of NCAM2 and revealed a significant role of this protein in the organization and dynamics of the cytoskeleton. The mass spectrometry approach showed that NCAM2 interacts with key cytoskeleton components and with a large number of other intracellular proteins. These observations point to NCAM2 functions as crucial for different cytoskeleton-related functions, including neuronal differentiation and maintenance, and synaptogenesis. In addition, our data suggest that NCAM2 may act as a putative receptor for GRN. Our results reveal that NCAM2 interacts with more than 100 proteins in murine cortical samples at two weeks of postnatal development. Since 56% of the identified proteins with two or more peptides were also detected in different experimental conditions, we believe that the method employed here is robust and the resulting data consistent. Nonetheless, it is important to mention that the extraction protocol used in this assay led to an enrichment of the NCAM2.1 isoform compared with NCAM2.2. This is due to the fact that NCAM2.1 is present in the soluble fractions, while NCAM2.2 is more frequently found in lipid rafts (the insoluble fraction), which makes it more difficult to purify.
The results obtained in this work also contribute to a better characterization of NCAM2 protein functions. As previously described, NCAM2.1 bears a transmembrane domain and an intracellular cue, while NCAM2.2 lacks the transmembrane domain and binds to the membrane by a GPI anchor [21,23]. Those differences are important for the localization and possible functions of the isoforms. Our study suggests that the transmembrane isoform, NCAM2.1, could also be found in lipid rafts. The in silico analysis of the amino acid sequence of NCAM2.1 shows similarities in some cysteine residues with NCAM140. The cysteine residues adjacent to the transmembrane domain in NCAM140 are palmitoylation modification sites and are important for targeting the protein to lipid rafts [41,42,56]. Lipid raft localization is crucial for cellular signaling and for the interactions of cell adhesion molecules with other ligands, which, in turn, activate different pathways [41]. The localization of NCAM2.1 in lipid rafts, suggested by the analysis of the amino acidic sequence, was confirmed by the detection of this isoform in lipid rafts. Moreover, the in silico analysis of the NCAM2.1 sequence shows a high percentage of putative residues that are likely to be phosphorylated, some of them coincident with previously described phospho-sites, which could be relevant to better understand NCAM2 functions [33].
Recent studies have determined the importance of NCAM2 in neuronal polarization, cell morphogenesis and in the formation and maintenance of excitatory glutamatergic synapses [25,27,28]. NCAM2 has been reported to induce local calcium spikes through the activation of Src, and to regulate microtubule stability through the formation of a protein complex with MAP2 and 14-3-3 [25]. Here, we reveal the interaction of NCAM2 with a number of other proteins, including cytoskeleton components and cytoskeleton-associated proteins, kinases, translation factors, growth factors and other intracellular components. Regarding the interactions with the cytoskeleton, our data indicate that NCAM2 interacts with Actin and NEFs. The interactions of NCAM2 with Actin and NF200 were validated using immunoprecipitation. Consistent with these results, a reduction of NCAM2-altered Actin cytoskeleton produced an aberrant growth cone mobility in NCAM2-deficient neurons [25]. NCAM2 also interacts with proteins that modulate cytoskeleton function and dynamics, or motor proteins, including MAP2, MAP1B, CAPZA, CAPZB, DYNLL1 and DYNLL2. These proteins are necessary for processes, such as neuronal survival, growth cone extension, axon elongation, autophagy or synapses maintenance. The bioinformatic analysis confirmed the strong relationship between NCAM2 and the cytoskeleton by showing a significant enrichment in terms and pathways related to cytoskeleton organization and dynamics [57,58,59,60,61].
Alterations in cytoskeleton dynamics are found in some pathologies, such as Autism Spectrum Disorders (ASD) or other neurodevelopmental diseases, including lissencephaly [62,63,64]. For example, the dynamics of Actin polarization is impaired in cells from ASD patients [62]. Consistent with these observations, genetic analyses showed that deletions and single nucleotide polymorphism in NCAM2 gene are detected in ASD patients. Our results suggest that alterations of NCAM2 may cause destabilization or modification of the cytoskeleton, thereby affecting neurodevelopment.
In this study, we detected several NCAM2-interacting proteins that turn out to be linked to synaptogenesis and synaptic plasticity processes. The most relevant ones are CaMKII, HSPA8, MAP1B, CAPZ and elongation factors, such as EEF1B, EEF1D, EIF3H and EIF3F. NCAM2 is highly expressed in the adult brain and highly localized to synapses [28,65]. We detected a strong interaction of NCAM2 with CaMKII in our proteomic analysis. Previous studies have shown that NCAM2 can activate CaMKII [26]. CaMKII is a well-known regulator of neuronal differentiation, synaptogenesis and synaptic plasticity [66].
We detected an interaction of NCAM2 with HSPA8. HSPA8 is a member of the chaperons’ family and participates together with HSC70 in protein folding and degradation. HSC70 accumulates in presynaptic buttons and catalyzes the release of Clathrin from Clathrin-coated synaptic vesicles, an event that is necessary for the synaptic vesicle-recycling pathway [67,68]. Other key cytoskeleton proteins found to interact with NCAM2 are MAP1B and CAPZ. Both have an important role in synaptogenesis and synaptic plasticity [46,69]. CAPZ is a capping protein that stabilizes Actin fibers [70] located in the postsynaptic density [71]. Neuronal activity induces its accumulation in the spines, facilitating the remodeling of these postsynaptic structures [72]. The loss of a subunit from the CAPZ complex led to alterations in dendritic spine formation and to defects in the specification of the presynaptic and postsynaptic structures [73].
14-3-3 proteins are involved in the regulation of Cofilin phosphorylation and the stabilization of Actin filaments. The knock-out of 14-3-3 protein in murine models results in a reduction of the dendritic tree complexity and the number of spines. In addition, animals deficient in 14-3-3 proteins bear behavior problems linked to major susceptibility to develop schizophrenia [74,75,76].
Another novel finding from our mass spectrometry assay is the interaction of NCAM2 with local protein synthesis components, which is a crucial mechanism for synaptic plasticity and other neuronal functions [77]. We detected different proteins involved in translation, such as EEF1B, EEF1D, EIF3H and EIF3F. Overall, our data suggest that NCAM2 plays a key function in the process of synaptogenesis and in the maintenance of synapses and plasticity in adult stages. Amyloid-beta increases NCAM2 cleavage and reduces its function in synapses, which could explain the synaptic loss observed in early stages of Alzheimer’s disease [28].
Finally, GRN has been shown to participate in neurite outgrowth and branching, axon growth and synapses formation and maintenance. Furthermore, mutations in the GRN gene have been linked to frontotemporal dementia [78,79,80,81,82,83,84] and it has been proposed as a potential target for the treatment of those dementias. One of the proposed receptors for GRN is Sortilin 1 (SORT1) [85], but the induction of neurite outgrowth is not regulated by this receptor, which indicates that another receptor is involved in the process. Our proteomic results suggest that NCAM2 could act as a GRN receptor during neuronal differentiation.
In summary, the present study provides a detailed view of the interactome of NCAM2, and offers additional information about NCAM2 localization and structure. The observed interactions of NCAM2 with cytoskeleton proteins, growth factors and other intracellular components increase our knowledge about this cell adhesion molecule and contribute to explain its functions during brain development and synaptic plasticity. More analyses and studies will be necessary in order to understand the complexity of the NCAM2 interactome in other developmental periods or brain regions. The present work adds substantially to our understanding of NCAM2 and lays the groundwork for a better characterization of NCAM2 functions in the central nervous system during development and adult stages, as well as the implications of this protein in neuronal diseases.
4. Materials and Methods
All experimental procedures were carried out following the guidelines of the Committee for the Care of Research Animals of the University of Barcelona, in accordance with the directive of the Council of the European Community (2010/63 y 86/609/EEC) on animal experimentation. The experimental protocol was approved by the local University Committee (CEEA-UB, Comitè Ètic d’Experimentació Animal de la Universitat de Barcelona) and by the Catalan Government (Generalitat de Catalunya, Departament de Territori i Sostenibilitat).
4.1. Antibodies
The following commercial primary antibodies were: anti-14-3-3 (1657, SantaCruz, Dallas, TX, USA); anti-Actin (MAB1501, Chemicon International-Fischer Scientific, Waltham, MA, USA); anti-CaMKIIα (M1-048, ThermoFisher Scientific, Waltham, MA, USA); anti-Caveolin (ab2910, Abcam, Cambridge, UK); anti-Clathrin (610500, BD Biosciences, Franklin Lakes, NJ, USA), anti-Flotillin (610820, BD Biosciences, Franklin Lakes, NJ, USA), anti-MAP2 (M9942 clone HM-2, Sigma-Aldrich, Sant Louis, MO, USA); anti-NCAM2 (AF778, R&D Systems, Minneapolis, MN, USA)); anti-NCAM2.1 (EB06991, Everest, Oxfordshire, UK); anti-NF200 (N4142, Sigma-Aldrich, Sant Louis, MO, USA) and anti-Nogo (11027, Santa Cruz, Dallas, TX, USA).
4.2. Mass Spectrometry Assay
Protein immunoprecipitation and analysis were performed as described [25]. Briefly, hippocampus and cortex regions were dissected and homogenized in an isotonic buffer (Tris 10 mM a pH 7,4, KCl 10 mM, MgCl2 1.5 mM, EGTA 1 mM) with protease inhibitors (Complete, Roche, Basel, Switzerland), using a Polytron. The supernatant with the cytosolic fraction was discarded and the membrane fraction was homogenized in a lysis buffer (Hepes 50 mM pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, and 1% Triton X-100) in orbital agitation. Samples were centrifugated at 15,000 rpm for 15 min at 4 °C and the supernatant was selected.
For the mass spectrometry assay, magnetic beads (Dyneabeads Antibody Coupling Kit, Life Technologies, Carlsbad, CA, USA) were conjugated with an antibody against NCAM2 (AF778, R&D Systems, Minneapolis, MN, USA) or with an antibody against NCAM2.1 (EB06991, Everest, Oxfordshire, UK), according to the manufacturer’s instructions. The supernatant containing the membrane fraction previously obtained, was incubated with the conjugated magnetic beads o/n at 4 °C. After washing with the lysis buffer 3 times, proteins were eluted with 30 μL of a Urea buffer (urea 8 M, Tris 50 mM a pH 7.5, DTT 60 mM) during 15 min at room temperature. The samples were processed and analyzed at the Proteomic facility of PCB (Proteomics unit, Parc Cientific de Barcelona, Barcelona, Spain). Samples were digested with trypsin (2 μg, pH 8, 32.5 °C, o/n). The resulting peptides were separated by nanoUPLC (NanoAcquity, Waters, Milford, MA, USA) and detected with Orbitrap Velos (Thermo Fisher Scientific, Waltham, MA, USA). The detection was performed with a resolution of 60,000, a ratio 400 m/z and an acquisition of 300–1800 m/z. Protein lists were obtained with an FDR ≤ 0.01%. String 11.0 (Elixir, Hinxton, Cambridgeshire, UK) was used for the bioinformatics analysis. The most abundant detected protein was NCAM2 and it was not included in the list.
4.3. Immunoprecipitation for Western Blotting
The membrane fraction obtained as previously described, was incubated with 2 μg of the selected antibodies overnight (anti-NCAM2.1, anti-NCAM2, anti-MAP2, anti-14-3-3, anti-NF200, anti-CaMKIIα and anti-Actin). To precipitate the proteins, protein G-Sepharose beads (17-0618-01, GE Healthcare, Chicago, IL, USA) were added and samples were incubated for 2 h in orbital agitation. After washing with the lysis buffer, proteins were eluted with 20 μL of loading buffer (0.5 M Tris-HCl (pH 6.8), 2.15 M β-mercaptoethanol, 10% SDS, 30% glycerol, and 0.012% bromophenol blue) during 5 min at 95 °C and processed for Western blot. Samples were separated on 10% SDS-PAGE and transferred to nitrocellulose membranes (1620112; Bio-Rad, Hercules, CA, USA). Filters were blocked in a 5% dry milk-supplemented 0.1% Tween 20 PBS prior to immunoreaction and immunoblotted with antibodies against 14-3-3 (1:2000), Actin (1:5000), Map2 (1:1000), NCAM2 (1:500) and NCAM2.1 (1:1000). The membranes were incubated with HRP-labeled secondary antibodies (DAKO, Santa Clara, CA, USA) for 1 h at RT in TBST and developed with the ECL system (GE Healthcare, Chicago, IL, USA).
4.4. Sucrose Gradient for Lipid Raft Isolation
The cortex from CD1 mice were used for lipid raft isolation. Two cortices were homogenized in 3 mL of MES (2-morpholino ethanesulfonic acid)-buffered saline (34 mM, pH 6.5 and 0.15 mM NaCl) plus 1% Triton X-100 supplemented with Complete Protease Inhibitor Cocktail (11697498001; Basel, Switzerland). Sucrose was then added to achieve a final concentration of 40%. A 5–30% linear sucrose gradient was layered on top and centrifuged at 39,000 rpm for 16 h at 4 °C in a Beckman SwTi rotor. A total of 12 fractions, 1 mL each, were collected from the top and analyzed by Western blot, as previously described, using the following antibodies: NCAM2 (1:1000), Caveolin (1:5000), Flotillin (1:3000) and Clathrin (1:5000).
4.5. Bioinformatic Analysis
Genomes and proteomes were downloaded from the National Institutes of Health (NIH, Bethesda, MD, USA), Nation al Center for Biotechnology Information (NCBI, Bethesda, MD, USA) and UniProt database (Geneva, Switzerland). Proteins identified by two or more peptides obtained with FDR ≤0.01% were analyzed with String 11.0 [55]. The bit map was elaborated using basic settings. Network edges represent evidence interactions at medium confidence of the score (0.4). The whole genome of mice was assumed for the statistical background in the functional enrichment (Gene Ontology terms, Kyoto Encyclopedia of Genes and Genomes pathways and Reactome pathways). Genomes and proteomes were downloaded from the National Institutes of Health (NIH).
To identify NCAM2.1 phosphorylatable domains, the amino acid sequence of the NCAM2.1 cytoplasmatic domain (UniProtKB-O35136, NCAM2_MOUSE, 719–837 amino acids) was analyzed using ScanSite 4.0 at low stringency [86,87] and DISPHOS (DISorder-enhanced PHOSphorylation predictor) [88].
To compare the NCAM2.1 and NCAM140 cytoplasmic domains, amino acid sequences of NCAM2.1 (UniProtKB-O35136, NCAM2_MOUSE, 719–837 amino acids) and NCAM140 (UniProtKB-O35136, NCAM1_MOUSE, 730–810 and 1076–1115 amino acids) were analyzed with BLAST software (NIH) and CSS-Palm software 4.0.
4.6. Statistical Analysis
Statistical analysis was carried out using the GraphPad Prism 5 software (San Diego, CA, USA).
Acknowledgments
We would like to thank all members of the Soriano lab for experimental help and comments. We thank Alba del Valle Vilchez for excellent technical support.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22147404/s1.
Author Contributions
E.S., L.P., A.P. and A.O.-G. conceived and designed the study. A.P. and A.O.-G. performed most of the experiments and analyzed data. F.U. contributed to the design and performed immunoprecipitation experiments. E.d.O. and M.A.O. contributed to the design and performed the mass spectrometry experiments. M.H.-L. performed sucrose gradient for lipid raft isolation. M.B. contributed in resources and reviewed the different versions of the manuscript. A.P., A.O.-G. and E.S. wrote the manuscript and all authors read and corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Spanish MINECO (SAF2016-76340R and PID2019-106764RB-C21) and CIBERNED (ISCIII) to E.S., Spanish MECD (FPU14/02156, Excellence Unit María de Maeztu/Institute of Neurosciences to E.S. and L.P., and BES-2017-080570 to A.O.-G.) and a grant from Secretary of Universities and Research of the Department of Economy and Knowledge of the Generalitat de Catalunya to A.P.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University of Barcelona (OB36-21FAR, 4 May 2021).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Namba T., Funahashi Y., Nakamuta S., Xu C., Takano T., Kaibuchi K. Extracellular and intracellular signaling for neuronal polarity. Physiol. Rev. 2015;95:995–1024. doi: 10.1152/physrev.00025.2014. [DOI] [PubMed] [Google Scholar]
- 2.Takano T., Xu C., Funahashi Y., Namba T., Kaibuchi K. Neuronal polarization. Development. 2015;142:2088–2093. doi: 10.1242/dev.114454. [DOI] [PubMed] [Google Scholar]
- 3.Zinn K., Özkan E. Neural immunoglobulin superfamily interaction networks. Curr. Opin. Neurobiol. 2017;45:99–105. doi: 10.1016/j.conb.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Missaire M., Hindges R. The role of cell adhesion molecules in visual circuit formation: From neurite outgrowth to maps and synaptic specificity. Dev. Neurobiol. 2015;75:569–583. doi: 10.1002/dneu.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sytnyk V., Leshchyns’ka I., Schachner M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017;40:295–308. doi: 10.1016/j.tins.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Gu Z., Imai F., Kim I.J., Fujita H., Katayama K.I., Mori K., Yoshihara Y., Yoshida Y. Expression of the immunoglobulin superfamily cell adhesion molecules in the developing spinal cord and dorsal root ganglion. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro L., Love J., Colman D.R. Adhesion molecules in the nervous system: Structural insights into function and diversity. Annu. Rev. Neurosci. 2007;30:451–474. doi: 10.1146/annurev.neuro.29.051605.113034. [DOI] [PubMed] [Google Scholar]
- 8.Leshchyns’ka I., Sytnyk V. Reciprocal interactions between cell adhesion molecules of the immunoglobulin superfamily and the cytoskeleton in neurons. Front. Cell Dev. Biol. 2016;4:9. doi: 10.3389/fcell.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frei J.A., Stoeckli E.T. SynCAMs—From axon guidance to neurodevelopmental disorders. Mol. Cell. Neurosci. 2017;81:41–48. doi: 10.1016/j.mcn.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Makino T., McLysaght A. Ohnologs in the human genome are dosage balanced and frequently associated with disease. Proc. Natl. Acad. Sci. USA. 2010;107:9270–9274. doi: 10.1073/pnas.0914697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pébusque M.J., Coulier F., Birnbaum D., Pontarotti P. Ancient large-scale genome duplications: Phylogenetic and linkage analyses shed light on chordate genome evolution. Mol. Biol. Evol. 1998;15:1145–1159. doi: 10.1093/oxfordjournals.molbev.a026022. [DOI] [PubMed] [Google Scholar]
- 12.Alenius M., Bohm S. Identification of a novel neural cell adhesion molecule-related gene with a potential role in selective axonal projection. J. Biol. Chem. 1997;272:26083–26086. doi: 10.1074/jbc.272.42.26083. [DOI] [PubMed] [Google Scholar]
- 13.Yoshihara Y., Kawasaki M., Tamada A., Fujita H., Hayashi H., Kagamiyama H., Mori K. OCAM: A new member of the neural cell adhesion molecule family related to zone-to-zone projection of olfactory and vomeronasal axons. J. Neurosci. 1997;17:5830–5842. doi: 10.1523/JNEUROSCI.17-15-05830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jørgensen O.S., Bock E. Brain specific synaptosomal membrane proteins demonstrated by crossed immunoelectrophoresis. J. Neurochem. 1974;23:879–880. doi: 10.1111/j.1471-4159.1974.tb04419.x. [DOI] [PubMed] [Google Scholar]
- 15.Sheng L., Leshchyns’Ka I., Sytnyk V. Cell adhesion and intracellular calcium signaling in neurons. Cell Commun. Signal. 2013;11:94. doi: 10.1186/1478-811X-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen C., Berezin V., Bock E. Neural cell adhesion molecule differentially interacts with isoforms of the fibroblast growth factor receptor. Neuroreport. 2011;22:727–732. doi: 10.1097/WNR.0b013e3283491682. [DOI] [PubMed] [Google Scholar]
- 17.Ramser E.M., Buck F., Schachner M., Tilling T. Binding of αII spectrin to 14-3-3β is involved in NCAM-dependent neurite outgrowth. Mol. Cell. Neurosci. 2010;45:66–74. doi: 10.1016/j.mcn.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Li J., Zhang W., Yang H., Howrigan D.P., Wilkinson B., Souaiaia T., Evgrafov O.V., Genovese G., Clementel V.A., Tudor J.C., et al. Spatiotemporal profile of postsynaptic interactomes integrates components of complex brain disorders. Nat. Neurosci. 2017;20:1150–1161. doi: 10.1038/nn.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visser J.J., Cheng Y., Perry S.C., Chastain A.B., Parsa B., Masri S.S., Ray T.A., Kay J.N., Wojtowicz W.M. An extracellular biochemical screen reveals that FLRTs and Unc5s mediate neuronal subtype recognition in the retina. eLife. 2015;4 doi: 10.7554/eLife.08149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleene R., Mzoughi M., Joshi G., Kalus I., Bormann U., Schulze C., Xiao M.F., Dityatev A., Schachner M. NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments. J. Neurosci. 2010;30:10784–10798. doi: 10.1523/JNEUROSCI.0297-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alenius M., Bohm S. Differential function of RNCAM isoforms in precise target selection of olfactory sensory neurons. Development. 2003;130:917–927. doi: 10.1242/dev.00317. [DOI] [PubMed] [Google Scholar]
- 22.Von Campenhausen H., Yoshihara Y., Mori K. OCAM reveals segregated mitral/tufted cell pathways in developing accessory olfactory bulb. Neuroreport. 1997;8:2607–2612. doi: 10.1097/00001756-199707280-00037. [DOI] [PubMed] [Google Scholar]
- 23.Kulahin N., Walmod P.S. The neural cell adhesion molecule NCAM2/OCAM/RNCAM, a close relative to NCAM. Adv. Exp. Med. Biol. 2010;663:403–420. doi: 10.1007/978-1-4419-1170-4_25. [DOI] [PubMed] [Google Scholar]
- 24.Winther M., Berezin V., Walmod P.S. NCAM2/OCAM/RNCAM: Cell adhesion molecule with a role in neuronal compartmentalization. Int. J. Biochem. Cell Biol. 2012;44:441–446. doi: 10.1016/j.biocel.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Parcerisas A., Pujadas L., Ortega-Gascó A., Perelló-Amorós B., Viais R., Hino K., Figueiro-Silva J., La Torre A., Trullás R., Simó S., et al. NCAM2 Regulates Dendritic and Axonal Differentiation through the Cytoskeletal Proteins MAP2 and 14-3-3. Cereb. Cortex. 2020;30:3781–3799. doi: 10.1093/cercor/bhz342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng L., Leshchyns’Ka I., Sytnyk V. Neural cell adhesion molecule 2 promotes the formation of filopodia and neurite branching by inducing submembrane increases in Ca2+ levels. J. Neurosci. 2015;35:1739–1752. doi: 10.1523/JNEUROSCI.1714-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng L., Leshchyns’ka I., Sytnyk V. Neural Cell Adhesion Molecule 2 (NCAM2)-Induced c-Src-Dependent Propagation of Submembrane Ca 2+ Spikes Along Dendrites Inhibits Synapse Maturation. Cereb. Cortex. 2019;29:1439–1459. doi: 10.1093/cercor/bhy041. [DOI] [PubMed] [Google Scholar]
- 28.Leshchyns’Ka I., Liew H.T., Shepherd C., Halliday G.M., Stevens C.H., Ke Y.D., Ittner L.M., Sytnyk V. Aβ-dependent reduction of NCAM2-mediated synaptic adhesion contributes to synapse loss in Alzheimer’s disease. Nat. Commun. 2015;6 doi: 10.1038/ncomms9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scholz C., Steinemann D., Mälzer M., Roy M., Arslan-Kirchner M., Illig T., Schmidtke J., Stuhrmann M. NCAM2 deletion in a boy with macrocephaly and autism: Cause, association or predisposition? Eur. J. Med. Genet. 2016;59:493–498. doi: 10.1016/j.ejmg.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Molloy C.A., Keddache M., Martin L.J. Evidence for linkage on 21q and 7q in a subset of autism characterized by developmental regression. Mol. Psychiatry. 2005;10:741–746. doi: 10.1038/sj.mp.4001691. [DOI] [PubMed] [Google Scholar]
- 31.Hussman J.P., Chung R.H., Griswold A.J., Jaworski J.M., Salyakina D., Ma D., Konidari I., Whitehead P.L., Vance J.M., Martin E.R., et al. A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism. Mol. Autism. 2011;2:1. doi: 10.1186/2040-2392-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petit F., Plessis G., Decamp M., Cuisset J.M., Blyth M., Pendlebury M., Andrieux J. 21q21 deletion involving NCAM2: Report of 3 cases with neurodevelopmental disorders. Eur. J. Med. Genet. 2015;58:44–46. doi: 10.1016/j.ejmg.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Huttlin E.L., Jedrychowski M.P., Elias J.E., Goswami T., Rad R., Beausoleil S.A., Villén J., Haas W., Sowa M.E., Gygi S.P. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunz S., Ziegler U., Kunz B., Sonderegger P. Intracellular Signaling Is Changed after Clustering of the Neural Cell Adhesion Molecules Axonin-1 and NgCAM during Neurite Fasciculation. J. Cell Biol. 1996;135:253–267. doi: 10.1083/jcb.135.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith D. Cdk5 in neuroskeletal dynamics. NeuroSignals. 2003;12:239–251. doi: 10.1159/000074626. [DOI] [PubMed] [Google Scholar]
- 36.Lickert H., Bauer A., Kemler R., Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/β- catenin interaction and strengthens cell-cell adhesion. J. Biol. Chem. 2000;275:5090–5095. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- 37.Fogh B.S., Multhaupt H.A.B., Couchman J.R. Protein Kinase C, Focal Adhesions and the Regulation of Cell Migration. J. Histochem. Cytochem. 2014;62:172–184. doi: 10.1369/0022155413517701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon Y.T., Gupta A., Zhou Y., Nikolic M., Tsai L.H. Regulation of N-cadherin-mediated adhesion by the p35-Cdk5 kinase. Curr. Biol. 2000;10:363–372. doi: 10.1016/S0960-9822(00)00411-5. [DOI] [PubMed] [Google Scholar]
- 39.Cai X., Li M., Vrana J., Schaller M.D. Glycogen Synthase Kinase 3- and Extracellular Signal-Regulated Kinase-Dependent Phosphorylation of Paxillin Regulates Cytoskeletal Rearrangement. Mol. Cell. Biol. 2006;26:2857–2868. doi: 10.1128/MCB.26.7.2857-2868.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delling M., Wischmeyer E., Dityatev A., Sytnyk V., Veh R.W., Karschin A., Schachner M. The neural cell adhesion molecule regulates cell-surface delivery of G-protein-activated inwardly rectifying potassium channels via lipid rafts. J. Neurosci. 2002;22:7154–7164. doi: 10.1523/JNEUROSCI.22-16-07154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niethammer P., Delling M., Sytnyk V., Dityatev A., Fukami K., Schachner M. Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J. Cell Biol. 2002;157:521–532. doi: 10.1083/jcb.200109059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamiguchi H. The region-specific activities of lipid rafts during axon growth and guidance. J. Neurochem. 2006;98:330–335. doi: 10.1111/j.1471-4159.2006.03888.x. [DOI] [PubMed] [Google Scholar]
- 43.Hérincs Z., Corset V., Cahuzac N., Furne C., Castellani V., Hueber A.O., Mehlen P. DCC association with lipid rafts is required for netrin-1-mediated axon guidance. J. Cell Sci. 2005;118:1687–1692. doi: 10.1242/jcs.02296. [DOI] [PubMed] [Google Scholar]
- 44.Caldwell J.E., Heiss S.G., Mermall V., Cooper J.A. Effects of CapZ, an Actin Capping Protein of Muscle, on the Polymerization of Actin. Biochemistry. 1989;28:8506–8514. doi: 10.1021/bi00447a036. [DOI] [PubMed] [Google Scholar]
- 45.Wear M.A., Yamashita A., Kim K., Maéda Y., Cooper J.A. How capping protein binds the barbed end of the actin filament. Curr. Biol. 2003;13:1531–1537. doi: 10.1016/S0960-9822(03)00559-1. [DOI] [PubMed] [Google Scholar]
- 46.Davis D.A., Wilson M.H., Giraud J., Xie Z., Tseng H.C., England C., Herscovitz H., Tsai L.H., Delalle I. Capzb2 interacts with β-tubulin to regulate growth cone morphology and neurite outgrowth. PLoS Biol. 2009;7:e1000208. doi: 10.1371/journal.pbio.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinnar S.A., Antoku S., Saffin J.M., Cooper J.A., Halpain S. Capping protein is essential for cell migration in vivo and for filopodial morphology and dynamics. Mol. Biol. Cell. 2014;25:2152–2160. doi: 10.1091/mbc.e13-12-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan A., Rao M.V., Nixon R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 2017;9 doi: 10.1101/cshperspect.a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao M.V., Campbell J., Yuan A., Kumar A., Gotow T., Uchiyama Y., Nixon R.A. The neurofilament middle molecular mass subunit carboxyl-terminal tail domains is essential for the radial growth and cytoskeletal architecture of axons but not for regulating neurofilament transport rate. J. Cell Biol. 2003;163:1021–1031. doi: 10.1083/jcb.200308076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Y.C., Redmond L. Neuronal CaMKII acts as a structural kinase. Commun. Integr. Biol. 2009;2:40–41. doi: 10.4161/cib.2.1.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu G.Y., Cline H.T. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- 52.Schwab M.E. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 2010;11:799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- 53.Coyne A.N., Lorenzini I., Chou C.C., Torvund M., Rogers R.S., Starr A., Zaepfel B.L., Levy J., Johannesmeyer J., Schwartz J.C., et al. Post-transcriptional Inhibition of Hsc70-4/HSPA8 Expression Leads to Synaptic Vesicle Cycling Defects in Multiple Models of ALS. Cell Rep. 2017;21:110–125. doi: 10.1016/j.celrep.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu T., Daniels C.K., Cao S. Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol. Ther. 2012;136:354–374. doi: 10.1016/j.pharmthera.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L., Zhang X. Factors Regulating Neurogenesis in the Adult Dentate Gyrus. Hippocampus Plast. Funct. 2018 doi: 10.5772/intechopen.75631. [DOI] [Google Scholar]
- 57.Twelvetrees A.E.E., Pernigo S., Sanger A., Guedes-Dias P., Schiavo G., Steiner R.A.A., Dodding M.P.P., Holzbaur E.L.L.F. The Dynamic Localization of Cytoplasmic Dynein in Neurons Is Driven by Kinesin-1. Neuron. 2016;90:1000–1015. doi: 10.1016/j.neuron.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grabham P.W., Seale G.E., Bennecib M., Goldberg D.J., Vallee R.B. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. J. Neurosci. 2007;27:5823–5834. doi: 10.1523/JNEUROSCI.1135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roossien D.H., Lamoureux P., Miller K.E. Cytoplasmic dynein pushes the cytoskeletal meshwork forward during axonal elongation. J. Cell Sci. 2014;127:3593–3602. doi: 10.1242/jcs.152611. [DOI] [PubMed] [Google Scholar]
- 60.Maday S., Wallace K.E., Holzbaur E.L.F. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J. Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uchida A., Alami N.H., Brown A. Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol. Biol. Cell. 2009;20:4997–5006. doi: 10.1091/mbc.e09-04-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griesi-Oliveira K., Suzuki A.M., Alves A.Y., Mafra A.C.C.N., Yamamoto G.L., Ezquina S., Magalhães Y.T., Forti F.L., Sertie A.L., Zachi E.C., et al. Actin cytoskeleton dynamics in stem cells from autistic individuals. Sci. Rep. 2018;8:11138. doi: 10.1038/s41598-018-29309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moon H.M., Wynshaw-Boris A. Cytoskeleton in action: Lissencephaly, a neuronal migration disorder. Wiley Interdiscip. Rev. Dev. Biol. 2013;2:229–245. doi: 10.1002/wdev.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson R. Loss of microtubule-to-actin linkage disrupts cortical development. PLoS Biol. 2011;9:e1001175. doi: 10.1371/journal.pbio.1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ichinohe N., Yoshihara Y., Hashikawa T., Rockland K.S. Developmental study of dendritic bundles in layer 1 of the rat granular retrosplenial cortex with special reference to a cell adhesion molecule, OCAM. Eur. J. Neurosci. 2003;18:1764–1774. doi: 10.1046/j.1460-9568.2003.02900.x. [DOI] [PubMed] [Google Scholar]
- 66.Hell J.W. CaMKII: Claiming center stage in postsynaptic function and organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlossman D.M., Schmid S.L., Braell W.A., Rothman J.E. An enzyme that removes clathrin coats: Purification of an uncoating ATPase. J. Cell Biol. 1984;99:723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zinsmaier K.E., Bronk P. Molecular chaperones and the regulation of neurotransmitter exocytosis. Biochem. Pharmacol. 2001;62:1–11. doi: 10.1016/S0006-2952(01)00648-7. [DOI] [PubMed] [Google Scholar]
- 69.Tortosa E., Montenegro-Venegas C., Benoist M., Härtel S., González-Billault C., Esteban J.A., Avila J. Microtubule-associated protein 1B (MAP1B) is required for dendritic spine development and synaptic maturation. J. Biol. Chem. 2011;286:40638–40648. doi: 10.1074/jbc.M111.271320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edwards M., Zwolak A., Schafer D.A., Sept D., Dominguez R., Cooper J.A. Capping protein regulators fine-tune actin assembly dynamics. Nat. Rev. Mol. Cell Biol. 2014;15:677–689. doi: 10.1038/nrm3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshimura Y., Yamauchi Y., Shinkawa T., Taoka M., Donai H., Takahashi N., Isobe T., Yamauchi T. Molecular constituents of the postsynaptic density fraction revealed by proteomic analysis using multidimensional liquid chromatography-tandem mass spectrometry. J. Neurochem. 2004;88:759–768. doi: 10.1046/j.1471-4159.2003.02136.x. [DOI] [PubMed] [Google Scholar]
- 72.Kitanishi T., Sakai J., Kojima S., Saitoh Y., Inokuchi K., Fukaya M., Watanabe M., Matsuki N., Yamada M.K. Activity-dependent localization in spines of the F-actin capping protein CapZ screened in a rat model of dementia. Genes Cells. 2010;15:737–747. doi: 10.1111/j.1365-2443.2010.01411.x. [DOI] [PubMed] [Google Scholar]
- 73.Fan Y., Tang X., Vitriol E., Chen G., Zheng J.Q. Actin capping protein is required for dendritic spine development and synapse formation. J. Neurosci. 2011;31:10228–10233. doi: 10.1523/JNEUROSCI.0115-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikeda M., Hikita T., Taya S., Uraguchi-asaki J., Toyo-Oka K., Wynshaw-boris A., Ujike H., Inada T., Takao K., Miyakawa T.T., et al. Identification of YWHAE, a gene encoding 14-3-3epsilon, as a possible susceptibility gene for schizophrenia. Hum. Mol. Genet. 2008;17:3212–3222. doi: 10.1093/hmg/ddn217. [DOI] [PubMed] [Google Scholar]
- 75.Wachi T., Cornell B., Toyo-oka K. Complete ablation of the 14-3-3epsilon protein results in multiple defects in neuropsychiatric behaviors. Behav. Brain Res. 2017;319:31–36. doi: 10.1016/j.bbr.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Foote M., Qiao H., Graham K., Wu Y., Zhou Y. Inhibition of 14-3-3 Proteins Leads to Schizophrenia-Related Behavioral Phenotypes and Synaptic Defects in Mice. Biol. Psychiatry. 2015;78:386–395. doi: 10.1016/j.biopsych.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao Y., Portela M., Janikiewicz J., Doig J., Abbott C.M. Characterisation of translation elongation factor eEF1B subunit expression in mammalian cells and tissues and co-localisation with eEF1A2. PLoS ONE. 2014;9:e114117. doi: 10.1371/journal.pone.0114117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuang L., Hashimoto K., Huang E.J., Gentry M.S., Zhu H. Frontotemporal dementia non-sense mutation of progranulin rescued by aminoglycosides. Hum. Mol. Genet. 2020;29:624–634. doi: 10.1093/hmg/ddz280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galimberti D., Fumagalli G.G., Fenoglio C., Cioffi S.M.G., Arighi A., Serpente M., Borroni B., Padovani A., Tagliavini F., Masellis M., et al. Progranulin plasma levels predict the presence of GRN mutations in asymptomatic subjects and do not correlate with brain atrophy: Results from the GENFI study. Neurobiol. Aging. 2018;62:245.e9–245.e12. doi: 10.1016/j.neurobiolaging.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Premi E., Gazzina S., Bozzali M., Archetti S., Alberici A., Cercignani M., Bianchetti A., Gasparotti R., Turla M., Caltagirone C., et al. Cognitive Reserve in Granulin-Related Frontotemporal Dementia: From Preclinical to Clinical Stages. PLoS ONE. 2013;8:e74762. doi: 10.1371/journal.pone.0074762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roberson E.D., Filiano A.J., Martens L.H., Young A.H., Warmus B.A., Zhou P., Diaz-Ramirez G., Jiao J., Zhang Z., Huang E.J., et al. Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. Ann. Intern. Med. 2013;158:5352–5362. doi: 10.1523/JNEUROSCI.6103-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kao A.W., McKay A., Singh P.P., Brunet A., Huang E.J. Progranulin, lysosomal regulation and neurodegenerative disease. Nat. Rev. Neurosci. 2017;18:325–333. doi: 10.1038/nrn.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petkau T.L., Leavitt B.R. Progranulin in neurodegenerative disease. Trends Neurosci. 2014;37:388–398. doi: 10.1016/j.tins.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Petkau T.L., Neal S.J., Milnerwood A., Mew A., Hill A.M., Orban P., Gregg J., Lu G., Feldman H.H., Mackenzie I.R.A., et al. Synaptic dysfunction in progranulin-deficient mice. Neurobiol. Dis. 2012;45:711–722. doi: 10.1016/j.nbd.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 85.Gass J., Lee W.C., Cook C., Finch N., Stetler C., Jansen-West K., Lewis J., Link C.D., Rademakers R., Nykjær A., et al. Progranulin regulates neuronal outgrowth independent of Sortilin. Mol. Neurodegener. 2012;7:33. doi: 10.1186/1750-1326-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van de Kooij B., Creixell P., van Vlimmeren A., Joughin B.A., Miller C.J., Haider N., Simpson C.D., Linding R., Stambolic V., Turk B.E., et al. Comprehensive substrate specificity profiling of the human nek kinome reveals unexpected signaling outputs. eLife. 2019;8 doi: 10.7554/eLife.44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Obenauer J.C., Cantley L.C., Yaffe M.B. Scansite 2.0: Proteome-wide prediction of cell signalling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iakoucheva L.M., Radivojac P., Brown C.J., O’Connor T.R., Sikes J.G., Obradovic Z., Dunker A.K. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32:1037–1049. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.