Abstract
High-risk coronary intervention involving the left main coronary artery represents an indication for mechanical circulatory support in hemodynamically unstable patients. Extracorporeal membrane oxygenation permits adequate hemodynamic stabilization and myocardial recovery from life-threatening pulmonary and cardiac failure. Our case report demonstrates the importance of choosing the correct method of hemodynamic support in different case scenarios. (Level of Difficulty: Advanced.)
Key Words: aortic valve stenosis, extracorporeal membrane oxygenation, heavily calcified coronary lesions, left main coronary artery, percutaneous coronary intervention, rotational atherectomy
Abbreviations and Acronyms: ECMO, extracorporeal membrane oxygenation; HCCL, heavily calcified coronary lesion; IABP, intra-aortic balloon pump; LAD, left anterior descending coronary artery; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; RCA, right coronary artery; RIM, ramus intermedius; TAVI, transcatheter aortic valve implantation
Graphical abstract

History of Presentation
A 74-year-old man was admitted to our department in the Coburg Clinic (Coburg, Germany) with shortness of breath and clinical features of cardiac decompensation New York Heart Association functional class III.
Learning Objectives
-
•
To make the right decision about the mechanical circulatory support needed in different case scenarios.
-
•
To understand the role of correct lesion preparation to ensure adequate results in treating highly calcified lesions.
Past Medical History
The patient’s medical history included severe ischemic cardiomyopathy, coronary artery bypass grafting 2 years previously (left internal mammary artery to the left anterior descending artery (LAD), venous bypass to the ramus intermedius [RIM], venous bypass to the right coronary artery [RCA]), and tricuspid valve reconstruction (Band Medtronic Simplici-T Anuplasty System 670, Medtronic, Minneapolis, Minnesota) 2 years previously (Table 1).
Table 1.
Patient’s Baseline Characteristics
| Sex | Male |
| Age, yrs | 74 |
| BMI, kg/m2 | 28.8 |
| Creatinine, mg/dl | 1.2 |
| GFR, ml/min | 63.15 |
| Important baseline diseases | Coronary: 3-vessel disease with a history of CABG (2018) Tricuspid valve reconstruction (2018) Severe secondary mitral valve insufficiency Chronic obstructive pulmonary disease Chronic renal disease Replacement of the abdominal aorta (2018, because of 68-mm aneurysm) Peripheral artery disease stage IIb |
| Cardiovascular risk factors | Arterial hypertension Type 2 diabetes mellitus Hyperlipidemia |
| EuroSCORE I, % | 69.69 |
| EuroSCORE II, % | 29.65 |
| STS score (risk of mortality), % | 26.96 |
| Left ventricle ejection fraction, % | 30 |
| Left ventricular end diastolic diameter, mm | 62 |
| Left atrium, mm | 47 |
| Right ventricle, mm | 40 |
| Right atrium, mm | 52 |
| Interventricular septum diameter, mm | 13 |
| Tricuspid annular plane systolic excursion, mm | 8 |
| Aortic valve opening area, cm2 | 0.9 |
BMI = body mass index; CABG = coronary artery bypass grafting; EuroSCORE = European System for Cardiac Operative Risk Evaluation; GRF = glomerular filtration rate; STS = The Society of Thoracic Surgeons.
Investigations
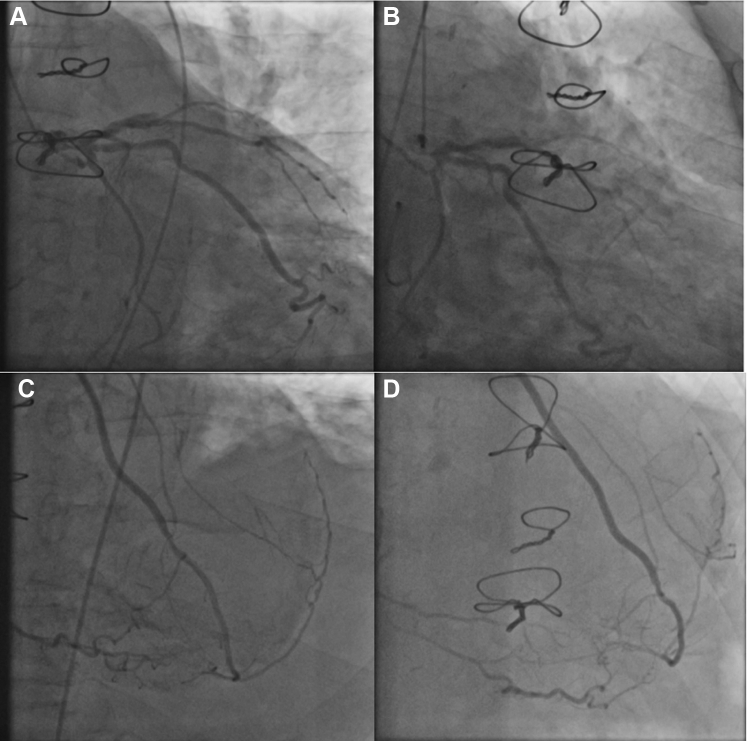
Transthoracic echocardiography revealed severe aortic valve stenosis (mean diastolic pressure 35 mm Hg), severe secondary mitral valve insufficiency, and severely compromised left ventricular systolic function (ejection fraction 30%), (Videos 1 and 2). Transesophageal echocardiography was performed to assess the aortic and mitral valve dysfunction more accurately. The aortic valve opening area was 0.90 cm2 (Video 3). Mitral insufficiency in this patient was caused by retraction of the posterior leaflet as a result of apical hyperkinesia (effective regurgitant orifice area 0.90 cm2) (Figures 1A to 1D). The patient then underwent coronary angiography, which revealed subtotal left main coronary artery (LMCA) stenosis, a blocked LAD in the medial segment, a patent left internal mammary artery to the LAD and retrograde to the RCA, a relatively large ramus diagonalis without bypass, proximal subtotal stenosis of the nondominant circumflex artery, and blocked venous bypasses to the RIM and RCA (Figures 2A to 2C). We confirmed that the bypasses were blocked by using coronary computer tomography. Cardiac magnetic resonance with late enhancement revealed a fixed anterior and septal posterolateral defect and vital myocardium in the inferior wall (Figure 3).
Figure 1.
Transesophageal Echocardiographic Findings
(A to C) Mitral insufficiency in transesophageal echocardiography. (D) Severe calcified aortic valve with opening area in the transesophageal echocardiography.
Figure 2.
Angiographic Findings
(A) (left anterior oblique view 1.0) and (B) (right anterior oblique view 30, caudal view 23) Heavily subtotal calcified stenosis of the left main coronary artery, blocked left anterior descending artery in the medial segment, ramus intermedius without absent flow from the blocked venous bypass, and large ramus diagonalis without bypass. (C) (left anterior oblique view 10) and (D) (right anterior oblique view 26, cranial view 30) Left internal mammary artery-bypass to the left anterior descending artery with retrograde flow to the right coronary artery.
Figure 3.

Cardiac Magnetic Resonance Showing a Fixed Anterior and Septal Posterolateral Defect and Vital Myocardium in the Inferior Wall
Management
Given the high risk of repeated operation (EuroSCORE II [European System for Cardiac Operative Risk Evaluation], 29.65%; Society of Thoracic Surgeons score, 26%), the patient was rejected for repeat coronary artery bypass grafting and for aortic and mitral valve replacement. Our team determined that percutaneous coronary intervention (PCI) would be a high-risk intervention in this patient because of his complex coronary status, aortic valve stenosis, and mitral insufficiency. The decision was made to use extracorporeal membrane oxygenation (ECMO) to provide hemodynamic support during the intervention. The intervention was performed using deep sedation with propofol. The common femoral vein was cannulated with a 23-F, 38-cm cannula, and the femoral artery was cannulated with a 15-F, 23-cm cannula. The circuit was connected to a third-generation centrifugal pump (Cardio-Help, Maquet, Rastatt, Germany). The average cardiopulmonary support was at 4.5 l/min. Because of the severe aortic valve stenosis, we decided to perform a valvuloplasty as a bridge to planned transcatheter aortic valve implantation (TAVI). This valvuloplasty was performed after initiating temporary pacing (under rapid pacing) with a 22 × 60 mm balloon (VACS II, Osypka, Rheinfelden, Germany) (Video 4). We then proceeded to the rotational atherectomy (Rotablator system, Boston Scientific, Natick, Massachusetts) of the LMCA. The procedure began with a 1.5-mm bur with a speed up to 180,000 rounds/min, following which the lesions were dilated with 3.5 × 15 mm and 4.0 × 20 mm noncompliant balloons at 20 to 24 atm (NC-Emerge, Boston Scientific) (Video 5). We succeeded after that to deliver a stent (Exposition S 3.5 to 4.5 × 22 mm, STENTYS, Paris, France) from the LMCA to the RIM and performed final apposition between the RIM and the LAD (Video 6). A final proximal optimization technique was carried out with a 6.0 × 8 mm NC-Emerge balloon in the LMCA. A very good angiographic result was shown (Figures 4A to 4F, Video 7). During the procedure, the patient was hemodynamically stable under ECMO support, which we removed directly after the procedure. No pre-procedural complications were registered. The patient was discharged home on day 5 after ECMO-assisted PCI and aortic valvuloplasty. The control echocardiogram revealed no pericardial effusion, and aortic valve function showed significant improvement, which is our definition of successful bridging to TAVI.
Figure 4.
Steps in Management
(A) Initial valvuloplasty of the aortic valve with a 22-mm balloon. (B) Rotational bur in the left main coronary artery. (C) Angiographic result after the rotablation in the left main coronary artery. (D) Angiographic result after stenting in the ramus intermedius. (E) Final apposition in the left anterior descending artery and ramus intermedius. (F) Final angiographic result in the ramus intermedius and left anterior descending artery.
Discussion
Heavily calcified coronary lesions (HCCLs) pose significant clinical challenges in the field of interventional cardiology and are associated with a high incidence of restenosis and targeted lesion revascularization (1). The degree of calcification and plaque structure occasionally prevents the balloon or stent from reaching the lesion. Adequate treatment of lesions before stent implantation remains an integral component of implantation of a coronary stent in patients with complex lesions, to enhance both immediate and long-term outcomes. Various tools and techniques have been developed in an effort to overcome the challenges raised by HCCLs. The use of rotational atherectomy for adequate lesion preparation and stent implantation is an effective and safe method for treating this type of lesion (2).
High-risk coronary intervention involving the LMCA represents an indication for mechanical circulatory support in hemodynamically unstable patients (3). ECMO permits adequate hemodynamic stabilization and myocardial recovery from life-threatening pulmonary and cardiac failure, and it may be a feasible alternative to the widely used intra-aortic balloon pump (IABP) (4).
Patients with severely compromised left ventricular systolic function who are undergoing high-risk PCI benefit from mechanical circulatory support (5). ECMO allows cardiopulmonary support for a long duration and provides adequate hemodynamic support independently of the patient’s native rhythm. It can partially or completely replace heart and lung function and thus increase the probability of recovery (6). Furthermore, as shown in the work of Thiele et al. (7), ECMO seems to be more effective than IABP for reversal of metabolic and hemodynamic disturbances. In our case, given the severe aortic valve stenosis and HCCLs in the LMCA, we assumed that the use of an Impella device (Abiomed, Danvers, Massachusetts) would not provide sufficient hemodynamic support. ECMO had shown very good results in extremely high-risk patients who were undergoing urgent PCI (8). Clinically, the status of our patient suggested the need for full hemodynamic support, which, as we suggest, could not be obtained using IABP or an Impella device. According to the latest guidelines on myocardial revascularization, patients with acute heart failure who are undergoing revascularization with potential recovery may benefit from ECMO support (9). Because lithotripsy was not available at our center on the day of the procedure, we decided to use the rotational atherectomy, which contributed to the procedure’s success. Our patient had severely compromised left ventricular systolic function (ejection fraction 30%) and a heavily calcified aortic valve with reduced function. In this case, the patient recovered completely and was discharged from our hospital 5 days after the intervention, with a scheduled appointment for a TAVI.
Follow-Up
The TAVI procedure was performed successfully 6 weeks after this intervention in one of our affiliated centers.
Conclusions
This report shows the importance of choosing the correct mechanical circulatory support system in different case scenarios. The full hemodynamic support attained in our case by using ECMO made a high-risk intervention smoother for the operator, as well as for the patient, who was discharged a few days after the intervention without any complications. Scores to determine the appropriate hemodynamic support depending on a patient’s risk factors are being currently developed, to help improve clinical decision making. Future prospective randomized trials may show the advantage of mechanical support.
Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transthoracic Echocardiography in the 2-Chamber View on Admission
Transthoracic Echocardiography in the 5-Chamber View on Admission
Transesophageal Echocardiography, Aortic Valve Opening Area
Aortic Valve Valvuloplasty
Begin With Rotablation of the Left Main Coronary Artery
Angiographic Result After Stenting of the Left Main Coronary Artery and the Ramus Intermedius
Angiographic Final Result
References
- 1.Moses J.W., Carlier S., Moussa I. Lesion preparation prior to stenting. Rev Cardiovasc Med. 2004;5(Suppl 2):S16–S21. [PubMed] [Google Scholar]
- 2.Benezet J., Díaz de la Llera L.S., Cubero J.M., Villa M., Fernández-Quero M., Sánchez-González A. Drug-eluting stents following rotational atherectomy for heavily calcified coronary lesions: long-term clinical outcomes. J Invasive Cardiol. 2011;23:28–32. [PubMed] [Google Scholar]
- 3.Ferrari M., Figulla H.R. Mechanische Herz-Kreislauf-Unterstützung in der Kardiologie [Circulatory assist devices in cardiology] Dtsch Med Wochenschr. 2005;130:652–656. doi: 10.1055/s-2005-865076. [DOI] [PubMed] [Google Scholar]
- 4.McArdle H., Bhandari M., Kovac J. Emergency coronary stenting of unprotected critical left main coronary artery stenosis in acute myocardial infarction and cardiogenic shock. Heart. 2003;89:e24. doi: 10.1136/heart.89.9.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitsis A.A., Visouli A. Mechanical circulatory support in the ICCU. Acute Card Care. 2009;11:204–215. doi: 10.1080/17482940903177028. [DOI] [PubMed] [Google Scholar]
- 6.Lafç G., Budak A.B., Yener A.Ü., Cicek O.F. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ. 2014;23:10–23. doi: 10.1016/j.hlc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Thiele H., Sick P., Boudriot E., Diederich K.W., Hambrecht R., Niebauer J., Schuler G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 8.Shi W.J., Zhang Y.X., Xu G.P. Extracorporeal Membrane Oxygenation-Assisted Percutaneous Coronary Intervention in Extremely High-Risk Patients. Chin Med J (Engl) 2018;131:1625–1627. doi: 10.4103/0366-6999.235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) European Association for Percutaneous Cardiovascular Interventions (EAPCI) Wijns W. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic Echocardiography in the 2-Chamber View on Admission
Transthoracic Echocardiography in the 5-Chamber View on Admission
Transesophageal Echocardiography, Aortic Valve Opening Area
Aortic Valve Valvuloplasty
Begin With Rotablation of the Left Main Coronary Artery
Angiographic Result After Stenting of the Left Main Coronary Artery and the Ramus Intermedius
Angiographic Final Result