Abstract
Corynebacterium striatum, a bacterium that is part of the normal skin microbiota, is also an opportunistic pathogen. In recent years, reports of infections and in-hospital and nosocomial outbreaks caused by antimicrobial multidrug-resistant C. striatum strains have been increasing worldwide. However, there are no studies about the genomic determinants related to antimicrobial resistance in C. striatum. This review updates global information related to antimicrobial resistance found in C. striatum and highlights the essential genomic aspects in its persistence and dissemination. The resistome of C. striatum comprises chromosomal and acquired elements. Resistance to fluoroquinolones and daptomycin are due to mutations in chromosomal genes. Conversely, resistance to macrolides, tetracyclines, phenicols, beta-lactams, and aminoglycosides are associated with mobile genomic elements such as plasmids and transposons. The presence and diversity of insertion sequences suggest an essential role in the expression of antimicrobial resistance genes (ARGs) in genomic rearrangements and their potential to transfer these elements to other pathogens. The present study underlines that the resistome of C. striatum is dynamic; it is in evident expansion and could be acting as a reservoir for ARGs.
Keywords: Corynebacterium striatum, multidrug resistance mechanisms, insertion sequences, transposons, Tn5432, MLS phenotype, resistome, emerging pathogen, pTP10 plasmid, antibiotic resistance genes
1. Introduction
A growing number of case reports have demonstrated the relevance of Corynebacterium spp. as etiologic agents of various infectious processes in both immunocompromised and immunocompetent patients. In addition to the etiologic agents of classic and zoonotic diphtheria, at least 50 diphtheria toxin (DT)-producing and non-DT-producing Corynebacterium species have been recognized as medical, veterinary, or biotechnologically relevant, including members of human microbiota [1,2,3]. Clinical implications in infected patients due to the expression of multidrug-resistance (MDR) profiles and virulence mechanisms have been increasingly observed for varied non-DT-producing Corynebacterium species, including Corynebacterium jeikeium, Corynebacterium urealyticum, Corynebacterium amycolatum, Corynebacterium pseudodiphtheriticum, Corynebacterium afermentans, and Corynebacterium striatum. Geographical variations in the frequency of non-DT-producing Corynebacterium spp. as an etiologic agent of a range of type infections have been reported [1,4,5,6]
In the past, C. striatum strains were described only as saprophytic microorganisms colonizing the skin and nasal mucosa of healthy individuals and unable of causing disease [7]. However, different studies have increasingly recognized C. striatum as the etiologic agent of various infections with signs and symptoms ranging from mild to severe to fatal outcomes, including bacteremia, endocarditis, meningitis, osteomyelitis, arthritis, corneal infection, and sinusitis [8,9]. Moreover, cases of infection of the respiratory tract, surgical wounds, skin lesions, or the eye, genital and urinary tract due to C. striatum strains have been also reported [10,11,12]. Previous studies verified nosocomial spread of MDR C. striatum strains, especially in patients of intensive care units and surgical wards making use of medical devices or with prolonged exposure to broad-spectrum antimicrobials [13,14,15]. The use of indwelling medical devices along with other clinical practices has been demonstrated to facilitate colonization, infection of skin lesions and/or mucosal surfaces with subsequent invasive dissemination by C. striatum strains [16,17,18,19,20,21]. Infections caused by C. striatum are also associated with sternal surgical wound wires [22]. Nosocomial infections and outbreaks caused by C. striatum are mainly found the respiratory tract, notably due to mechanical ventilation support as a risk factor in different countries [22,23,24,25,26]. During a nosocomial outbreak in Rio de Janeiro (RJ), Brazil, cases of ventilator-associated pneumonia and tracheobronchitis in addition to catheter-related sepsis caused by MDR C. striatum strains were verified [1]. Recently, a review study and analysis of clinical–epidemiological and microbiological features of a total of 218 reported research studies during a 44 year period (1976 to 2020) verified that MDR and multidrug susceptible (MDS) C. striatum strains were identified in 254 reported cases as nosocomial and community pathogens worldwide [25].
C. striatum strains expressing varied MDR profiles have been identified as the etiologic agent of various types of both nosocomial and non-healthcare associated infections reported in industrialized and developing countries [25,27]. Recently, resistance to antiseptic and disinfectants such as glutaraldehyde (GA) has also been reported for C. striatum [28]. Accordingly, medical surveillance programs should include control strategies to decrease potential risk factors of nosocomial infections and outbreaks due to C. striatum. Therefore, investigation of clinical, epidemiological, and virulence features is necessary, especially for preventing and disseminating C. striatum strains expressing MDR profiles among nosocomial patients worldwide. The emergence of MDR strains and heterogeneity of MDR profiles, in addition to the fact that different clones may be responsible for nosocomial outbreaks, have been problems of major concern [1,12,13,22,27,29].
In Brazil, the analysis by pulsed-field gel electrophoresis (PFGE) of C. striatum strains indicated the presence of four different PFGE types isolated from varied clinical specimens of patients in different hospital wards during a nosocomial outbreak: MDR C. striatum strains of PFGE-types I to II and non-MDR C. striatum strains of PFGE-types III to IV. MDR C. striatum strains expressing PFGE-type I and type II profiles were found predominant and mainly isolated from tracheal aspirates of patients undergoing endotracheal intubation procedures, while only two MDR C. striatum strains expressing PFGE-types I and II were isolated from blood samples [1]. However, a subsequent increase in the number of the bloodstream and catheter-related infections caused by C. striatum strains was verified in the Brazilian hospital. Further investigation revealed the permanence of MDR C. striatum strains PFGE-types I and II in nosocomial wards and predominance of PFGE- type I among patients with hematogenic infections and the presence of particular and genetically related PFGE-profiles. Therefore, virulence potential and invasive properties of C. striatum strains belonging to 10 particular or genetically related profiles expressing heterogenic MDR were documented by their ability to cause bloodstream and catheter-related infections within the nosocomial environment. Moreover, the high level of MDR associated with biofilm formation capacity observed for invasive C. striatum strains was also found as an issue of concern. Therefore, data suggested a relationship between biofilm formation ability, changes in genes, and increased resistance to drugs for C. striatum [12,27,28].
The pathogenicity potential of C. striatum strains due to a virulence mechanism of a multifactorial nature and the influence of environmental conditions has been investigated in different regions. Distinct studies verified the ability of biofilm formation as a significant factor affecting the clinical relevance of C. striatum. The ability of biofilm formation and survival on abiotic surfaces was confirmed for MDR and MDS C. striatum clinical isolates obtained from patients presenting varied types of infections [12,27,30,31].
Whole-genome sequencing (WGS) has been an essential tool for understanding molecular epidemiology, global transmission, and virulence mechanisms of pathogens. Although WGS has been used for C. striatum investigation of genomic features of MDR and non-MDR clinical isolates, further studies remain necessary to study strain-dependent genomic elements related to the acquisition of resistance to antimicrobial agents [16,32,33,34].
At the time of finishing this review, there were no studies on the antimicrobial resistance genes repertoire in C. striatum, possibly because the literature is scarce and related to specific countries. This review aims to compile bibliographic information and information derived from publicly available genomic data to understand the epidemiology of multidrug-resistant C. striatum and identify genomic determinants of this resistance. Thus, in this review, we describe the intrinsic and extrinsic factors related to the acquisition of antimicrobial resistance. Moreover, we detail the genomic context of these elements, the relationship between them and their impact on the conformation of the resistome in C. striatum. Finally, we discuss the role of mobile genetic elements in the acquisition, maintenance and dispersal of ARGs, as well as future challenges and actions related to the resistome of C. striatum.
2. Genome Organization of C. striatum
According to the genomes available in the NCBI Pathogens Database (https://www.ncbi.nlm.nih.gov/pathogens/), there are 131 genomes (accessed on 31 October 2020) of C. striatum available. Genomes include complete, draft, and raw data genomes from the USA, China, Brazil, Japan, and, more recently, genomes from Germany and Denmark. The genome length is ~2.8 Mb, and the GC content is around 59% [34]. Genomic data of C. striatum from NCBI shows three plasmids in C. striatum, pCs-Na-1, pCs-Na-2, and pTP10. The pTP10 plasmid has been reported in C. striatum M82B [35] and its presence in other genomes been studied [34]. The presence of viral genomes in the C. striatum has not been adequately studied, but recently a study identified a phage species from the Siphoviridae family in C. striatum [36]. Pangenomics studies have not yet been reported. More deposited genomes and genomic analyses are needed to better understand the genome structure and evolutionary dynamics of C. striatum.
3. C. striatum Resistome
The resistome is the total repertoire of genes that contribute to resistance in bacteria, including ARGs. Different antibiotic resistance mechanisms are described in isolates of clinical importance, which have been grouped into intrinsic and extrinsic mechanisms, referring to persistence and resistance mechanisms, respectively [37]. The intrinsic mechanisms are natural phenomena present in all the bacteria; they are generally obtained by regulating membrane permeability and non-specific efflux pumps. In addition, chromosomally encoded elements have been attributed to this phenotype [38]. Conversely, the extrinsic or generally acquired mechanisms are obtained via the horizontal transfer of mobile elements. These mechanisms include specific efflux pumps, antibiotic modifying enzymes, or modifying the antibiotic target using genes acquired by horizontal transfer [37]. In the genus Corynebacterium, several mechanisms of resistance to both intrinsic and extrinsic antimicrobials have been described. Resistance to fluoroquinolones and daptomycin in Corynebacterium spp. are examples of an intrinsic mechanism associated with point mutations in the gyrA and pgsA2 genes, respectively [39,40].
On the other hand, the presence of plasmids in Corynebacterium spp., although rare [41], is associated with the presence of ARGs. Examples of these plasmids in corynebacteria are: pJA144188 in C. resistens [42]; pNG2 in C. diphtheria [43]; and pTP10 in C. striatum. In C. striatum, resistance to fluoroquinolones and daptomycin has been described, which are intrinsic mechanisms. Resistance to other antimicrobials that have likely been acquired by horizontal gene transfer has also been described (Figure 1). Next, different resistance mechanisms in C. striatum are outlined.
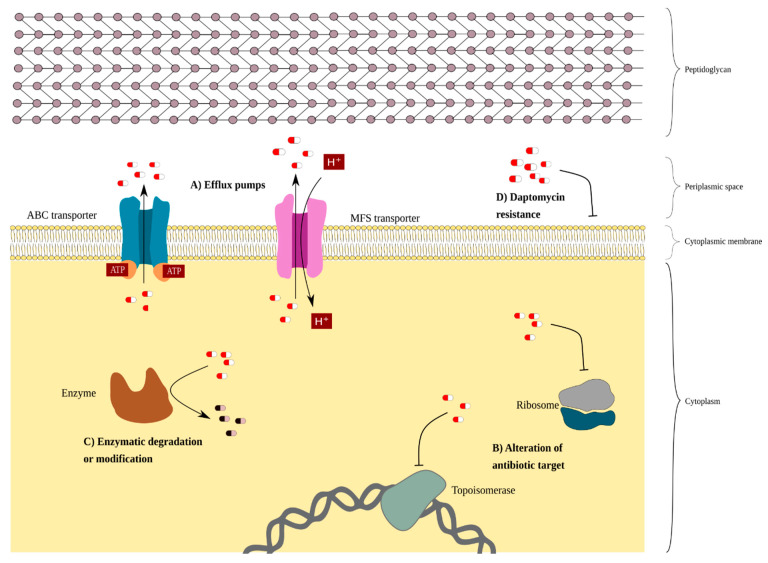
Figure 1.
A schematic representation of the drug-resistance mechanism of C. striatum. Antibiotics are represented as pill shapes in white and red. (A) The efflux pumps transport phenicols, tetracyclines, and beta-lactams out of the membrane cell. The blue complex protein represents an ABC transporter encoded by the tetA/B genes responsible for transporting tetracyclines and beta-lactams. The pink complex protein represents a specific MFS transporter for chloramphenicol. (B) Altering the target of the antibiotic is a form of resistance. Alteration of drug target due to mutations in gyrA prevent the binding of fluoroquinolones to topoisomerases. Resistance to macrolides is due to the modification of the target through the methylation of the 23S subunit of the ribosome, caused by the erm gene. Similarly, the tet(W) gene encoding a ribosomal protective protein, TET(W), confers resistance to tetracyclines. (C) Resistance to aminoglycosides is given by the enzymatic modification (phosphorylation or adenylation) of these antibiotics, preventing the binding to the ribosome. Beta-lactam resistance is due to B-lactamases encoded by the bla and ampC genes, which degrade beta-lactam antibiotics. (D) Daptomycin resistance is due to the mutation in the pgsA2 gene causing a phosphoglycerol (PG) deficiency in the membrane; this PG deficiency prevents the binding of daptomycin to the membrane.
3.1. Intrinsic Resistance in C. striatum
Fluoroquinolones are a group of synthetic broad-spectrum antimicrobials in Gram-negative and Gram-positive bacteria. Fluoroquinolones inhibit bacterial type II topoisomerases such as DNA gyrase and topoisomerase IV, which are crucial to unwinding and transcribing bacterial DNA [44]. Fluoroquinolone resistance mechanisms include the alteration of membrane permeability, efflux, inactivation by acetylation and, the most common mechanism, mutations at the site of action of the antibiotic in topoisomerases [37,38]. In C. striatum, several studies have described mutations in the quinolone resistance-determining regions (QRDR) in the gyrA gene [12,16,17,34,45,46,47]. Sierra and co-workers (2005) [46] reported mutations for positions 87 and 91 in the peptide sequence of GyrA. Later investigations performed by Ramos and co-workers (2018 and 2020) [34,45] showed a preference of C. striatum isolates to mutate from Ser87Val and less often from Asp91Asn or Asp91Ala. Alibi et al. (2017) [47] reported mutations of Ser87Phe and Asp91Ala or Asp91Gly, while Drogomirescu et al. (2020) [20] showed that C. striatum strains increased their resistance to fluoroquinolones when the mutation occurred from Ser87Phe and Asp91Ala. The change from Ser87 and Asp91 to non-polar amino acids suggests the –OH group from Ser87 and the –COOH group from Asp91 play an important role in binding the quinolone antibiotic. Interestingly, Nudel et al. (2018) [16] found new mutated positions in their C. striatum tested strains, i.e., Ser95Thr, Asp94Ala, Glu88Ala and Asp87Gly; however, they follow the same logic to switch polar for non-polar amino acids (except position 95), thus reducing their fluoroquinolone affinity.
Daptomycin is a calcium-dependent lipopeptide antibiotic produced by Streptomyces roseosporus. It is generally used to treat serious infections caused by Gram-positive bacteria. Daptomycin could act as a temporary ionophore in the membrane by forming a complex with phosphatidylglycerol (PG) and calcium ions, bringing about depolarization and a subsequent ion leakage. An ion leakage can have several consequences, including an osmotic imbalance and metabolite uptake deficiency. However, the bactericidal mechanisms of daptomycin are not yet entirely clear [48]. Initially, daptomycin resistance had been only reported in the genus of corynebacteria in C. jeikeium [6]; later isolates of daptomycin-resistant C. striatum were found [19,40,49,50,51,52]. Moreover, a recent study showed that several corynebacteria species could rapidly develop daptomycin non-susceptibility, including HLDR (high-level daptomycin resistance), after a short daptomycin exposure period [53].
The daptomycin-resistance mechanisms in C. striatum are due to the loss of mutation function in the pgsA2 gene. This gene encodes for phosphatidylglycerol synthase A, responsible for synthesizing PG from diphosphate diglyceride (CDP-DAG) in the PG synthesis pathway [40,51,54], which leads to a significant loss of PG in the membrane of C. striatum. A study showed that the loss of PG in the membrane could be lethal in Bacillus subtilis and therefore PG is vitally essential to membrane integrity [55]. Nevertheless, Goldner et al. (2018) [40] demonstrated that the HLDR phenotype in C. striatum was sufficient with the loss of pgsA2 gene function. Namely, the HDLR phenotype in C. striatum does not involve additional mutations or changes in the transcription levels of genes biosynthetically linked to pgsA2, which suggests that C. striatum is an even more persistent and adaptable bacterium than B. subtilis. The mutations found in pgsA2 can include conserved sites, active sites, substrate binding sites, and stop codon mutations [40,51]. Moreover, the HLDR phenotype has been easily produced in vitro and during treatment in patients [40].
Although no further gene mutations have been reported to date (in addition to gyrA and pgsA2) that confer a resistant phenotype, the rapid adaptation to daptomycin tolerance and growing resistance to fluoroquinolones demands that more studies and focused monitoring be performed on the emergence of the persistence of intrinsic mechanisms associated with antibiotic resistance in C. striatum.
3.2. Extrinsic Resistance Associated with Mobile Elements
In corynebacteria, different ARGs associated with mobile genetic elements, including plasmids, integrons (In), insertion sequences (IS), and transposons (Tn), have been reported [39,43,56,57]. In C. striatum, resistance is mainly determined by Tn, IS, and plasmids. Interestingly, a 38–66 kb region was found between the chromosomal genes dppD and cgrA/B in C. striatum. This region contains all the mobile elements with their associated ARGs, and this region is probably an access point to the chromosome for horizontal transfer vectors such as plasmids [16]. Of the three known plasmids in C. striatum (pCs-Na-1, pCs-Na-2 and pTP10), only pTP10 is known to confer resistance. The pTP10 plasmid is a multidrug resistance mosaic reported in 1983 by Kono et al. [58] on the C. striatum M82B strain initially poorly characterized as C. xerosis. The size of the pTP10 plasmid is 51,904 bp and contains 47 ORFs organized in eight regions, of which two are regulatory regions, and six are transporter regions of ARGs [35].
3.2.1. Resistance Due to Tn5432
The MLS phenotype (resistance to Macrolides, Lincosamides, Streptogramin) is a mechanism frequently observed in coagulase-negative staphylococci [59,60]. It is also widely seen in corynebacteria of clinical importance, such as Corynebacterium diphtheriae, Corynebacterium xerosis, C. jeikeium, C. pseudodiphtheriticum, C. amycolatum, C. urealyticum [61,62,63,64,65], and C. striatum. The MLS phenotype is associated with the erm(X) gene transported by transposon Tn5432 [35].
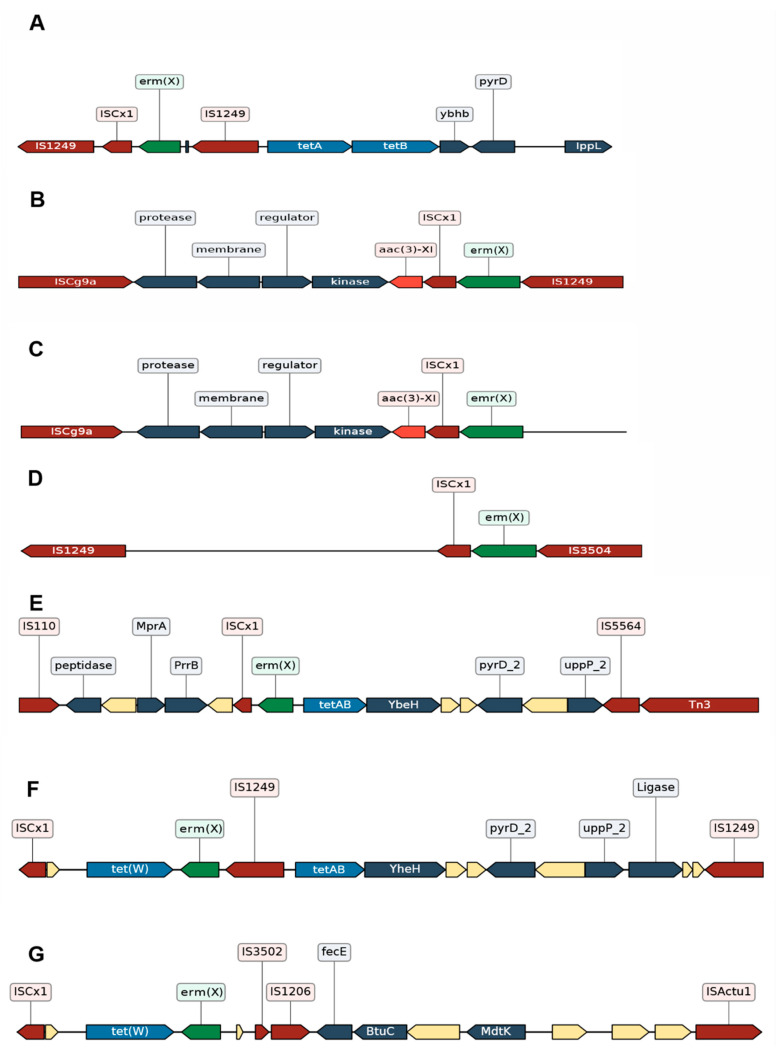
The transposon Tn5432 is the first region of the pTP10 plasmid, and it is a composite transposon. A composite transposon consists of two inverted repeats from two separate identical or related insertion sequences (IS) moving together as one unit and carrying the DNA between them (e.g., an ARG) [66]. Tn5432 is flanked by two identical insertion sequences (IS1249) and carries the erm(X) gene [35] (Figure 2A) responsible for resistance to lincosamides and macrolides such as clindamycin and erythromycin. There are different locations of the erm(X) gene and Tn5432; in both C. xerosis and C. diphtheriae, they are found in their plasmids, while in C. jeikeium and C. striatum, they are found in their chromosomes (53). Tn5432 carries ISCx1, which is interesting since ISCx1 can be evidence of a “genomic scar” of Tn5432 because it is a truncated IS element (due to a stop codon). We observed the presence of the ISCx1 element near the erm(X) gene in different studies conducted in the USA, Brazil, and China (Figure 2) [16,33,34]. Nevertheless, these studies report rearrangements for Tn5432. Nudel et al. (2018) [16] reported a genomic context similar to Tn5432 flanked by IS1249, ISCg9a-like, IS3504-like (Figure 2B–D), and IS407. Furthermore, in the Tn5432 context, they found the presence of the aac(3)-XI gene, responsible for resistance to aminoglycosides such as kanamycin, gentamicin, and streptomycin, among others. On the other hand, Wang et al. (2019) [33] reported a genomic context similar to Tn5432 (Figure 2E–G), but with rearrangements different from those presented by Nudel et al. (2018) [16]. Nevertheless, they have shown the ISCx1 and erm(X) together with the tet(W) gene and also the tetAB regulator, pyrD and ippL-like genes were present next to erm(X) and ISCx1 (Figure 2E,F). These genes correspond to the second region of the pTP10 plasmid reported by Tauch et al. (2000) [33], and a co-integration of this segment has been described together with Tn5432 in Corynebacterium glutamicum [35]. The presence of region II, the erm(X) gene, and ISCx1 in these studies suggests that in addition to resistance to lincosamides and macrolides, Tn5432 confers tetracycline resistance, which a past recombination event could have triggered. Eady et al. (2000) [62] evaluated the co-resistance of erythromycin and tetracyclines in different coryneform and Gram-positive cocci on the skin’s surface, and they found that most of the isolated bacteria, including C. striatum, presented resistance to tetracyclines as well as resistance to erythromycin. Interestingly, the region in Figure 2E comprising genes assigned as peptidase, hypothetical protein, mprA, and prrB is syntenic to the region from Figure 2B,C (genes assigned as protease, membrane, regulator and kinase). This observation further reinforces that they are in the same genomic context.
Figure 2.
Genomic context of Tn5432. Illustrated sequences from the genomic context of Tn5432 found in studies by Nudel et al., 2018; Tauch et al., 2000; Wang et al., 2019. The mobile elements are represented in red (IS and Tn), hypothetical proteins are depicted in the empty boxes, the genes directly unrelated to the resistance to antibiotics are in dark blue. (A) Tn5432 flanked by two IS1249 elements transports the erm(X) gene (in green) to its corresponding right, region II of the pTP10 plasmid of C. striatum that contains the tetA/tetB genes (in light blue). (B–D) Regions with the same genomic context as Tn5432 found in strains in Boston, USA [16], but in addition to the erm(X) gene, this region contains the aac(3)-XI gene (in orange). (E–G) Regions with the same genomic context as Tn5432 found in Beijing, China [33]. The erm(X) gene, the tetA/tetB genes, and the tet(W) gene can be seen. (E) Note that it is the same genomic context; if erm(X) is located in the center, on the right can be seen segment II of the pTP10 plasmid in the isolates from China, and on the left the same segment of the isolates from the USA although annotated as protease, membrane, regulator, and kinase. (B–D) correspond to the genes annotated as peptide, hypothetical protein (associated with membrane), MprA (a regulator) and PrrB (a kinase). The representation of the sequences was done with the DNA Features Viewer tool [68] by using as references the accession numbers AF024666.2 (A), GCA_002804085.1 (B), GCA_002803965.1 (C), SRR5120233 (D), GCA_015945985.1 (E), GCA_015946165.1 (F), GCA_015946545.1 (G).
According to the ISfinder database [67], IS1249, the insertion sequence flanking Tn5432, has been found in other genomes of the genus, such as C. urealyticum and C. jeikeium, and other bacteria such as Propionibacterium acnes and Bifidobacterium thermophilum. This scenario indicates that these elements are potential propagators of resistance at the interspecies level.
3.2.2. Acquired Resistance Genes for Chloramphenicol (cmx) and Aminoglycosides (strA and strB) Are in the Same Genomic Context
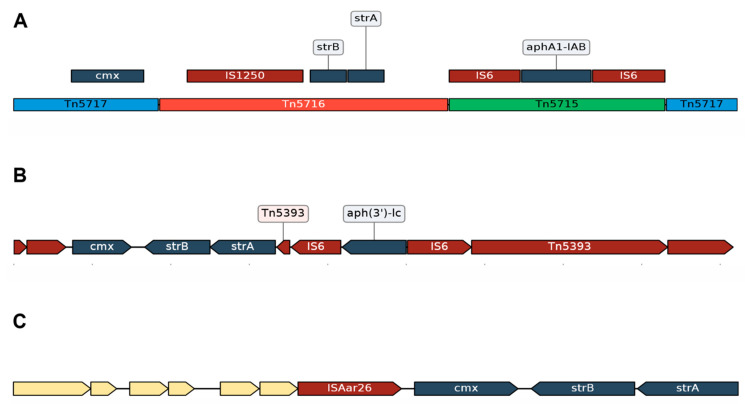
In C. striatum, the only mechanism of resistance to chloramphenicol is associated with the cmx gene, which codes for an efflux pump. On the other hand, strA and strB confer the resistance to the aminoglycoside streptomycin. Initially, the cmx, strA-strB, and aphA1-AB genes were carried by Tn5717, Tn5716, and Tn5715 (Figure 3A), respectively, in the pTP10 plasmid [35]. These transposons correspond to IV, V, and VI from this plasmid region. The organization of these regions is interesting, where Tn5717 is interrupted by Tn5716, which is interrupted by Tn5715. A similar ordering was found in the studies by Nudel et al., 2018 (Figure 3B) [16], in which the cmx, strB, and strA genes plus an element similar to Tn5715 composed of IS26 flanking the aph(3′)-lc gene can be seen together. Wang et al., 2019 [33], showed that the cmx, strB, and strA genes were found in the same genomic context transported by ISAar26 (Figure 3C) and were widely distributed in a cluster in their analyses. All this suggests that it is the same genomic context, and they were acquired together; for the rest, it has not been proven that these genes have been acquired independently. However, it is known that pTP10 transports Tn5564, which contains only the cmx gene and IS1513. Ramos et al. (2018) [34] assessed the presence of the pTP10 plasmid and demonstrated that a large part of the plasmid was found in the genome (C. striatum 2308), including Tn5564. Knowing that chloramphenicol resistance could be due to other intrinsic and extrinsic mechanisms, future studies to identify these will be needed for C. striatum.
Figure 3.
Genomic context of cmx, strA and strB genes. Antibiotic resistance genes are represented in dark blue, the mobile elements in dark red and the hypothetical proteins in the empty boxes. (A) Segment belonging to the pTP10 plasmid of C. striatum was documented by Tauch et al., 2000. The segment contains the transposons Tn5717 (transporting the cmx gene), Tn5716 (transporting the strB-strA genes) and Tn5715 (transporting the aphA1-IAB gene). (B) Segment belonging to the isolates from Boston, USA [16]. The region shows the cmx, strB-strA genes near a composite transposon (transporting the aph(3′)-lc gene) flanked by two IS26 similar to Tn5715; in addition, the absence of the IS1250 element can be observed. Similarly, the isolates from Beijing, China [33] (C) showed the cmx and strB-strA genes are close and without the IS1250 element, in the same sense as the isolates from the USA. The representation of the sequences was done with the DNA Features Viewer tool [68], considering the accession numbers AF024666.2 (A), GCA_002803965.1 (B), GCA_015946165.1 (C).
3.2.3. Different Genomic Contexts of the tet(W) Genes Suggests Possible Recombination Events
The tet(W) gene product eliminates the inhibitory effect of tetracycline on protein synthesis through the non-covalent modification of the ribosomes. The gene is not transported by pTP10, suggesting another recombination event beyond pTP10 (or this plasmid acquired this gene in later events). It has been reported that IS3504 carried the tet(W) gene in isolates from the USA [16], but it could also be transported by IS3503 and IS3502 [33]. According to the ISfinder database [67], both IS3503 and IS3502 are part of C. jeikeium, and it has been transported to its plasmids and to C. urealyticum. On the other hand, IS3504 was described in the pNG2 plasmid of C. diphtheria [35] and the pJA144188 plasmid of C. resistens [42]. The tet(W) gene was first described for corynebacteria in plasmid pJA144188 of C. resistens. The gene was described in a genomic context similar to how it presents in C. striatum, with a close IS3504 element, as the studies by Nudel et al. (2018) [16] described it, and close to the erm(X) gene as was found in the studies by Wang et al., 2019 [33].
The genomic contexts associated with transposons Tn5432, Tn5717, Tn5716, and Tn5715 suggest that pTP10 underwent past recombination events with the chromosome, constituting a portion in the chromosome open to new recombination events. New realignments of the transposons can explain this, the appearance of the tet(W) and aac(3)-XI genes, and the diversity of insertion sequences. Therefore, we propose that insertion sequences play a fundamental role in the continuous formation and evolution of the resistome of C. striatum.
3.2.4. Impact of the Insertion Sequences in the Resistome of C. striatum
Insertion sequences (IS) are small mobile elements; they typically contain a gene that codes for a transposase (tnp). IS elements are grouped according to the motives of the active site and according to their recombination mechanism. Traditionally, IS elements do not transport passenger genes, but they can move ARGs as part of a composite transposon, which is a region flanked by two copies of the same IS element (as is the case of the Tn5432; See Figure 2A) or a related one, which can be mobilized as a unit [66]. IS elements and Tn can be mobilized by themselves in the same DNA molecule or between different DNA molecules by recombination (conjugation, transduction, and transformation). C. striatum contains several IS elements (Table 1) related to the ARGs, grouped mainly in the IS3 and IS256 families. However, it also has elements from the IS6 and IS110 families. Regarding the origin of these elements, since most seem to be in Actinobacteria, it suggests that these elements are preserved in the phylum and the other species of the genus Corynebacterium (Table 1). Thus, we postulate that the diversity of IS elements present in C. striatum could be reflected in the nosocomial environment where it coexists with the rest of the microbiota. The IS3 family is one of the largest, being widely distributed in nature. The IS256 family is abundant in the vaginal metagenome, and the IS6 family is abundant in the skin metagenome [69,70]. The IS6 family only shows associations with aminoglycoside resistance genes in C. striatum (Table 1), although there are no patterns of families of IS elements with some types of antimicrobials [70]. Alternatively, unlike other IS elements that have a “cut-out-paste” or “copy-out-paste” mechanism, IS6/26 has a replication transposition mechanism or “copy-in”, which involves events of reciprocal recombination by resolvase (co-integrates), which leads to rearrangements of the genome [69,71,72].
Table 1.
Insertion sequences (IS) and composite transposons associated with resistance genes in C. striatum based on previous reports, ISfinder, and Tn registry web tools.
| IS 1 | Group 1 | IS Family 1 | Tn 2 | Determinant Associated 3 | Origin 4 |
|---|---|---|---|---|---|
| IS6110 | IS51 | IS3 | tet(W), blaB | Mycobacterium tuberculosis | |
| IS3502 | tet(W), erm(X), mdtK | Corynebacterium jeikeium | |||
| IS3501 | erm(X), tetA, tetB, tet(W), yheH | Corynebacterium jeikeium | |||
| IS3504 | erm(X), aac(3)-XI, cmx | Corynebacterium diphtheriae | |||
| IS1600 | aac(6′)-la | Shigella sonnei | |||
| IS1206 | tet(W). erm(X), mdtK | Corynebacterium glutamicum | |||
| IS407 | IS407 | erm(X), tet(W) | Burkhoderia cepacia | ||
| ISAar26 | IS3 | sul1 ermE, cmx, strB, strA | Arthobacter arilaitensis | ||
| IS3503 | IS1249 | IS256 | tet(W), blab | Corynebacterium jeikeium | |
| ISActu1 | tet(W), erm(X), mdtK | Actinomyces turicensis | |||
| IS1249 | Tn5432 | erm(X), tetA, tetB, tet(W), yheH | Corynebacterium striatum 5 | ||
| ISCre1 | aac(6′)-la | Corynebacterium reistens | |||
| IS1250 | ND | Tn5716 | strB, strA | Corynebacterium striatum | |
| IS6100 | ND | IS6 | aac(6′)-la, aadA1 | Mycobacterium fortuitum | |
| IS26 | IS6 | Tn5715 | aphA1-IAB, aph(3′)-I, strA, strB | Proteus vulgaris | |
| IS1628 | ND | aac(6′)-la | Corynebacterium glutamicum | ||
| IS110 | ND | IS110 | erm(X) tetAB, yheH | Streptomyces coelicolor | |
| ISCg9a | ND | erm(X), aac(3)-XI | Corynebacterium glutamicum | ||
| IS5564 | ND | IS481 | Tn5564 | cmxA, cml, erm(X) tetA/B, yheH | Corynebacterium striatum |
| IS1513 | ND | IS30 | Tn5564 | cmx, cml | Corynebacterium striatum |
1 See ISfinder (https://www-is.biotoul.fr/, accessed 15 November 2020) for details of IS. 2 See Tn registry (http://transposon.lstmed.ac.uk/, accessed 17 November 2020) for further details. 3 Information is available from references in the text as well as from other references [13,30,32]. Seen in the same genomic context, which may include the presence of other IS. 4 First organism where the element was described according to ISfinder. 5 May appear as C. xerosis in various records due to misidentification.
There are elements in the IS3 and IS256 families (apparently mostly in C. striatum) that can form hybrid promoters, which generate a promoter derived from two different sequences [73]. For the rest, it is known that a hybrid promoter can be more efficient than its parent promoters [74]; but, the hybrid promoters in some IS elements have been reviewed and seem to have a more random effect [73], being more or less efficient. Therefore, the resistance levels of C. striatum can be explained partly by the efficiency of the promoter of its IS elements. However, further research will be required on these elements in C. striatum.
In addition to the potential role of IS elements in the expression of ARGs, they may have a role in the grouping or concentration of genes through a selection process that could provide a common promoter [75]. The formation of operons and integrons would be advantageous to conserving the beneficial genes, in this case, ARGs, and would increase their intra- and intercellular mobility [70,75]. In other words, we postulate that the IS elements could contribute to the acquisition and maintenance of key genes for the adaptation and fitness of C. striatum, making it a potential reservoir for ARGs. This scenario could be explained due to the persistent exposure to antimicrobials, which select strains harboring ARGs and/or an improved association of IS with ARGs in C. striatum.
3.3. C. striatum Has Resistance Genes Probably Associated with Mobile Elements
C. striatum is resistant to several antimicrobials (Table 2), with penicillin being well known. In C. striatum, resistance to these beta-lactams has been associated with the ampC and bla genes which encode beta-lactam ring-modifying enzymes [47]. It has also been associated with non-specific efflux pumps encoded for the tetA/B genes [35]. In other corynebacteria such as C. jeikeium, C. urealyticum, and C. resistens, it has been noted that they harbor the bla gene, but they have not been associated with resistance to beta-lactams [47]. The blaB gene, which encodes for a beta-lactamase regulatory protein, has been noted in the studies by Wang et al. (2019) [33]; this suggests that C. striatum has the regulatory mechanism bla gene, needed for conferring beta-lactam resistance.
Table 2.
Genetic factors involved in drug resistance in Corynebacterium striatum.
| Chemical Class | Drug 1 | Mechanism of Action | Genetic Factor | Associated Function | Mechanism of Resistance | References |
|---|---|---|---|---|---|---|
| Fluoroquinolones | Levofloxacin Ciprofloxacin Moxifloxacin |
Inhibits DNA gyrase and DNA topoisomerase | gyrA | Negatively supercoils closed circular double-stranded DNA | Alteration of drug target due to mutation | [46] |
| Phenicols | Chloramphenicol | Inhibits protein elongation due to 23S ribosomal subunit binding | cmx | Encoding of a specific efflux protein of chloramphenicol | Transports chloramphenicol out of the membrane | [35] |
| Macrolides | Erythromycin Clindamycin |
Inhibits protein synthesis due to 50S ribosomal subunit binding |
erm(X)
ermB |
23S ribosomal RNA methyltransferase | Alteration of drug target due to methylation | [35,47] |
| Tetracyclines | Tetracycline Doxycycline |
Inhibits the initiation of translation by binding to 30S ribosomal subunit | tetA, tetB | Tetracycline efflux ABC transporter TetAB | Transports tetracyclines out of the membrane | [35] |
| tet(W) | Tetracycline resistance ribosomal protection protein | Binds to the ribosome and inhibits the binding of tetracyclines | [16] | |||
| Beta-Lactams | Penicillin Ampicillin Cefazolin Cefotiam Cefotaxime Meropenem Cefotaxime Imipenem Oxacillin Ceftriaxone |
Inhibits cell wall biosynthesis | bla | Beta-lactamase class A (serine hydrolase) | Alteration of the drug due to enzymatic modification | [20,33,47] |
|
tetA
tetB |
Tetracycline efflux ABC transporter TetAB | Transports beta-lactams out of the membrane | [35] | |||
| ampC | Beta-lactamase class C | Alteration of the drug due to enzymatic modification | [47] | |||
| Sulfonamides | Sulfamethoxazole -/trimethoprim |
Acts by blocking the synthesis of folic acid and inhibits growth | sul1 | Dihydropteroate synthase | Antibiotic target replacement | [33] |
| Trimethoprim | ND | ND | ND | |||
| Aminoglycosides | Tobramycin Amikacin Streptomycin |
Inhibits protein synthesis |
aph(3′)-Ic
aph(3”)-Ib (strA) aph(6)-Id (strB) |
O-phosphotransferases | Catalyzes ATP-dependent phosphorylation of hydroxyl group | [47,82] |
| Gentamicin Kanamycin |
aac(3)-XI | N-acetyltransferases | Catalyzes acetyl CoA-dependent adenylation of an amino group | [83] | ||
| Lipopeptides | Daptomycin | The aggregation of daptomycin alters the curvature of the membrane, which creates holes that leak ions | pgsA2 | Catalyzes the synthesis of phosphoglycerol (PG) | Inhibits membrane binding due to PG deficiency | [40] |
1 Drug: The drugs were not necessarily verified by the authors cited in the reference column.
The resistance to sulfonamides may be due to the sul genes found in the studies by Wang et al. (2019) [33]. The sul gene confers sulfamethoxazole resistance (Table 2). According to the NCBI pathogens database, resistance genes such as merA, which confers mercury resistance, are annotated. In addition, there is the qacEdelta1 gene, which confers resistance to quaternary ammonium compound biocides. Biocide resistance was reported and associated with biofilms by Souza et al. (2020) [28]. On the other hand, the sul gene and the qacEdelta1 gene are frequently seen in class 1 integrons. Integrons are considered scarce in Gram-positive bacteria. Nevertheless, in the genus Corynebacterium, class 1 integrons have already been documented in C. glutamicum, Corynebacterium ammoniagenes and Corynebacterium casei [76] and, recently, a class 1 integron was identified in clinical urine isolate characterized as Corynebacterium spp. [77]. Additionally, according to the INTEGRALL database [78], in the genus Corynebacterium, there are class 1 integrons in C. diphtheriae, C. amycolatum, and Corynebacterium efficiens. However, class 1 integrons have not been described in C. striatum. Therefore, to evaluate the emergence or presence of integrons in C. striatum, greater epidemiological monitoring will be required, with studies focused on identifying the integrase gene Int1 and its associated genes.
Fortunately, the glycopeptide vancomycin continues to show good results in the treatment of C. striatum. Although the vanW gene, which is associated with vancomycin resistance, was found in the genome of C. striatum 2308, this isolate proved to be susceptible to vancomycin [34]. However, vancomycin resistance in Corynebacterium aquaticum has been reported [39], and a case of infection due to vancomycin resistance in Corynebacterium spp. has also been documented [79].
Finally, based on searches of ARGs in databases like MEGARes [80] and ResFinder [81], we found several ARGs, indicating that the in silico resistome of C. striatum is larger (data not shown). This finding reflects the need to explore and characterize the resistome of C. striatum involving computational approaches of prediction methods and functional characterization.
4. Emergence of New C. striatum Clones
The studies compiled here indicate that C. striatum should not be underestimated, given that it is causing several types of infections. Nevertheless, despite the seeming virulence factors, recent studies revealed a mortal virulence of C. striatum in Caenorhabditis elegans [84,85], a nematode used as a simple animal model to study host–pathogen interactions [86]. Therefore, more attention must be paid to this pathogen in immunocompromised patients and the personnel assigned to this clinical picture.
The diversity of IS elements (Table 1) associated with ARGs in C. striatum highlights the plasticity of this bacterium in acquiring resistance genes and propagating as a persistent and pathogenic clone. Added to this, the ability to create biofilms in medical devices makes it a potential propagator of multidrug-resistant genomic elements. Thus, C. striatum may act as a potential reservoir to propagate resistance to other pathogenic bacteria.
Corynebacterium species can be detected in the clinical environment using biochemical techniques, commercial kits (such as API Coryne or Vitek 2) and recently MALDI-TOF (matrix-assisted laser desorption/ionization time-of-flight) [87]. Biochemical techniques do not distinguish among Corynebacterium species, so MALDI-TOF has become one of the most used clinical tools to detect pathogenic microorganisms, including C. striatum [3,87]. Whether or not molecular confirmation is needed at the species level is debatable; it has been recommended that the genus Corynebacterium spp. [88] should be identified at the species level. However, it is generally only necessary for drug treatment purposes to confirm if they belong to the genus Corynebacterium since the clinically relevant Corynebacterium species still show susceptibility to glycopeptides, such as vancomycin and linezolid [39,89,90].
Whether C. striatum could cause an epidemic is a question that has not yet been answered. Since it is part of the normal human microbiota, C. striatum has only primarily caused disease to date as an opportunistic pathogen. However, a recent phylogenetic study considering Chinese strains of C. striatum suggests that a lineage of C. striatum has been extended worldwide and could be a pandemic lineage [32]. Furthermore, due to the characteristics of its mobilome, the presence of persistent lineages, or the emergence of new ones, epidemiological monitoring of this microorganism and its potential related resistance factors are essential and urgent to be implemented.
5. Conclusions
In this review, based on the literature and searches in specialized databases, we have explored the antimicrobial resistance gene repertoire found in C. striatum. This resistome comprises chromosomal determinants and mainly acquired elements. Intrinsic resistance includes fluoroquinolone and daptomycin resistance via mutations on chromosomal genes. The increasing resistance and adaptation to daptomycin tolerance deserves special attention, which requires greater monitoring of its presence and the mechanisms that produce it. Many of the studies described here point out that a large part of the C. striatum resistance presents a genomic context similar to mobile elements such as insertion sequences and transposons mainly related to pTP10 plasmid.
The transposon Tn5432 stands out, which has been associated with the MLS phenotype and has insertion sequences along with different resistance genes. The ISs that compose this transposon have been identified in other bacteria and in isolates throughout the world. Although there are some similarities in the genomic context of ISs, we can observe some gene composition diversity and rearrangements in these regions. These findings suggest that insertion sequences are potential propagators of resistance at the interspecies level and play a fundamental role in the continuous formation and evolution of the resistome of C. striatum. Therefore, future challenges aim to systematically study the diversity of IS and its associated genes to understand the role of IS in the maintenance and dispersal of ARGs and its impact on the generation of lineages of concern of C striatum.
On the other hand, genes such as qacEdelta1, sul, bla and merA have not been associated with mobile genetic elements in C. striatum. However, considering their occurrence in other bacteria, they could be part of integrons or other genomic islands, making it necessary to carry out future studies focused on the genomic context of these genes. Moreover, although genes conferring resistance to vancomycin and linezolid have not been verified among nosocomial and community strains of C. striatum, the emergence of multidrug-resistant strains and acting as a reservoir for ARGs reveals a worrisome public health scenario. Therefore, in addition to the species-level confirmation, more significant efforts and attention should be dedicated to monitoring its occurrence and investigating its resistome.
This work uncovers the dynamics of antimicrobial resistance in C. striatum in the context of epidemiological factors, the ARGs repertoire, and its genomic localization. Furthermore, it is highlighted that the resistome of C. striatum is dynamic and in evident expansion. Consequently, it is emphasized that actions such as implementing genomic surveillance programs and studies on the mechanisms of antimicrobial resistance should be carried out. These measures would help to warn of the emergence of multidrug resistance lineages and prevent future public health problems.
Acknowledgments
We thank the Genomics and Bioinformatics Unit of BIOREN-UFRO and our colleagues, Monica Pavez, Ana Mutis, and Eulalia Sans, for their valuable comments regarding this review. This work was partially financed by the Dirección de Investigación, Universidad de La Frontera.
Author Contributions
B.L. performed the data curation, formal analysis, investigation, visualization and writing of the original draft; J.N.R., P.V.P.B., J.F.C.V. and C.S. performed the data curation and investigation; A.B., A.L.M.-G. and V.V.V. performed the data curation, investigation and writing—review and editing, M.A.M. performed the conceptualization of the study, investigation, supervision and writing—review & editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no specific grant from any funding agency.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baio P.V.P., Mota H.F., Freitas A.D., Gomes D.L.R., Ramos J.N., Sant’Anna L.O., Souza M.C., Camello T.C.F., Junior R.H., Vieira V.V., et al. Clonal multidrug-resistant Corynebacterium striatum within a nosocomial environment, Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2013;108:23–29. doi: 10.1590/S0074-02762013000100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parte A.C., Carbasse J.S., Meier-Kolthoff J.P., Reimer L.C., Göker M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020;70:5607–5612. doi: 10.1099/ijsem.0.004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zasada A.A., Mosiej E. Contemporary microbiology and identification of Corynebacteria spp. causing infections in human. Lett. Appl. Microbiol. 2018;66:472–483. doi: 10.1111/lam.12883. [DOI] [PubMed] [Google Scholar]
- 4.Camello T.C.F., Mattos-Guaraldi A.L., Formiga L.C.D., Marques E.A. Nondiphtherial Corynebacterium species isolated from clinical specimens of patients in a university hospital, Rio de Janeiro, Brazil. Braz. J. Microbiol. 2003;34:39–44. doi: 10.1590/S1517-83822003000100009. [DOI] [Google Scholar]
- 5.Renom F., Garau M., Rubi M., Ramis F., Galmes A., Soriano J.B. Nosocomial Outbreak of Corynebacterium striatum Infection in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Microbiol. 2007;45:2064–2067. doi: 10.1128/JCM.00152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoen C., Unzicker C., Stuhler G., Elias J., Einsele H., Grigoleit G.U., Abele-Horn M., Mielke S. Life-Threatening Infection Caused by Daptomycin-Resistant Corynebacterium jeikeium in a Neutropenic Patient. J. Clin. Microbiol. 2009;47:2328–2331. doi: 10.1128/JCM.00457-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyamoto M., Tsuboi R., Harada K., Cho O., Sugita T. Skin microbiome of patients with interdigital tinea pedis: Corynebacterium striatum is more abundant in the patients (published online ahead of print, 2021 Apr 8) J. Dermatol. 2021 doi: 10.1111/1346-8138.15877. [DOI] [PubMed] [Google Scholar]
- 8.Khan D., Shadi M., Mustafa A., Karam B., Munir A.B., Lafferty J., Glaser A., Mobarakai N. A Wolf in Sheep’s clothing; Case reports and literature review of Corynebacterium striatum endocarditis. IDCases. 2021;24:e01070. doi: 10.1016/j.idcr.2021.e01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanbhag S.S., Shih G., Bispo P.J.M., Chodosh J., Jacobs D.S., Saeed H.N. Diphtheroids as Corneal Pathogens in Chronic Ocular Surface Disease in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Cornea. 2021;40:774–779. doi: 10.1097/ICO.0000000000002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagadeeshan N., Jayaprakash S., Ramegowda R.T., Manjunath C.N., Lavanya V. An unusual case of Corynebacterium striatum endocarditis in a patient with congenital lymphedema and rheumatic heart disease. Indian Heart J. 2016;68:S271–S273. doi: 10.1016/j.ihj.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Carvalho R.V., Lima F.F.D.S., Santos C.S.D., de Souza M.C., da Silva R.S., de Mattos-Guaraldi A.L. Central venous catheter-related infections caused by Corynebacterium amycolatum and other multiresistant non-diphtherial corynebacteria in paediatric oncology patients. Braz. J. Infect. Dis. 2018;22:347–351. doi: 10.1016/j.bjid.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos J.N., Souza C., Faria Y.V., da Silva E.C., Veras J.F.C., Baio P.V.P., Seabra S.H., de Oliveira Moreira L., Júnior R.H., Mattos-Guaraldi A.L., et al. Bloodstream and catheter-related infections due to different clones of multidrug-resistant and biofilm producer Corynebacterium striatum. BMC Infect. Dis. 2019;19:672. doi: 10.1186/s12879-019-4294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otsuka Y., Ohkusu K., Kawamura Y., Baba S., Ezaki T., Kimura S. Emergence of multidrug-resistant Corynebacterium striatum as a nosocomial pathogen in long-term hospitalized patients with underlying diseases. Diagn. Microbiol. Infect. Dis. 2006;54:109–114. doi: 10.1016/j.diagmicrobio.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Severo C.B., Guazzelli L.S., Barra M.B., Hochhegger B., Severo L.C. Nódulos pulmonares múltiplos causados por Corynebacterium striatum numa paciente imunocompetente. Rev. Inst. Med. Trop. Sao Paulo. 2014;56:89–91. doi: 10.1590/S0036-46652014000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowstead T.T., Santiago S.M. Pleuropulmonary infection due to Corynebacterium striatum. Br. J. Dis. Chest. 1980;74:198–200. doi: 10.1016/0007-0971(80)90035-2. [DOI] [PubMed] [Google Scholar]
- 16.Nudel K., Zhao X., Basu S., Dong X., Hoffmann M., Feldgarden M., Allard M., Klompas M., Bry L. Genomics of Corynebacterium striatum, an emerging multidrug-resistant pathogen of immunocompromised patients. Clin. Microbiol. Infect. 2018;24:1016.e7–1016.e13. doi: 10.1016/j.cmi.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asgin N., Otlu B. Antimicrobial Resistance and Molecular Epidemiology of Corynebacterium striatum Isolated in a Tertiary Hospital in Turkey. Pathogens. 2020;9:136. doi: 10.3390/pathogens9020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalt F., Schulthess B., Sidler F., Herren S., Fucentese S.F., Zingg P.O., Berli M., Zinkernagel A.S., Zbinden R., Achermann Y. Corynebacterium Species Rarely Cause Orthopedic Infections. J. Clin. Microbiol. 2018;56:1–8. doi: 10.1128/JCM.01200-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mcmullen A.R., Anderson N., Wallace M.A., Shupe A., Burnham C.D. When Good Bugs Go Bad: Epidemiology and Antimicrobial Resistance Profiles of Pathogen. Antimicrob. Agents Chemother. 2017;61:e01111-17. doi: 10.1128/AAC.01111-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dragomirescu C.C., Lixandru B.E., Coldea I.L., Corneli O.N., Pana M., Palade A.M., Cristea V.C., Suciu I., Suciu G., Manolescu L.S.C., et al. Antimicrobial susceptibility testing for Corynebacterium species isolated from clinical samples in Romania. Antibiotics. 2020;9:31. doi: 10.3390/antibiotics9010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee P.P., Ferguson D.A., Sarubbi F.A. Corynebacterium striatum: An underappreciated community and nosocomial pathogen. J. Infect. 2005;50:338–343. doi: 10.1016/j.jinf.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Campanile F., Carretto E., Barbarini D., Grigis A., Falcone M., Goglio A., Venditti M., Stefani S. Clonal Multidrug-Resistant Corynebacterium striatum Strains, Italy. Emerg. Infect. Dis. 2009;15:75–78. doi: 10.3201/eid1501.080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez-Martínez L., Ortega M.C., Suárez A.I. Comparison of E-test with broth microdilution and disk diffusion for susceptibility testing of coryneform bacteria. J. Clin. Microbiol. 1995;33:1318–1321. doi: 10.1128/jcm.33.5.1318-1321.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandenburg A.H., van Belkum A., van Pelt C., Bruining H.A., Mouton J.W., Verbrugh H.A. Patient-to-patient spread of a single strain of Corynebacterium striatum causing infections in a surgical intensive care unit. J. Clin. Microbiol. 1996;34:2089–2094. doi: 10.1128/jcm.34.9.2089-2094.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva-Santana G., Silva C.M.F., Olivella J.G.B., Silva I.F., Fernandes L.M.O., Sued-Karam B.R., Santos C.S., Souza C., Mattos-Guaraldi A.L. Worldwide survey of Corynebacterium striatum increasingly associated with human invasive infections, nosocomial outbreak, and antimicrobial multidrug-resistance, 1976–2020. Arch. Microbiol. 2021 doi: 10.1007/s00203-021-02246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong K.Y., Chan Y.C., Wong C.Y. Corynebacterium striatum as an emerging pathogen. J. Hosp. Infect. 2010;76:371–372. doi: 10.1016/j.jhin.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 27.de Souza C., Faria Y.V., Sant’Anna L.D.O., Viana V.G., Seabra S.H., de Souza M.C., Vieira V.V., Júnior R.H., Moreira L.D.O., de Mattos-Guaraldi A.L. Biofilm production by multiresistant Corynebacterium striatum associated with nosocomial outbreak. Mem. Inst. Oswaldo Cruz. 2015;110:242–248. doi: 10.1590/0074-02760140373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza C., Mota H.F., Faria Y.V., Cabral F.D.O., de Oliveira D.R., Sant’Anna L.D.O., Nagao P.E., Santos C.D.S., Moreira L.O., Mattos-Guaraldi A.L. Resistance to Antiseptics and Disinfectants of Planktonic and Biofilm-Associated Forms of Corynebacterium striatum. Microb. Drug Resist. 2020;26:1546–1558. doi: 10.1089/mdr.2019.0124. [DOI] [PubMed] [Google Scholar]
- 29.Leonard R.B., Nowowiejski D.J., Warren J.J., Finn D.J., Coyle M.B. Molecular evidence of person-to-person transmission of a pigmented strain of Corynebacterium striatum in intensive care units. J. Clin. Microbiol. 1994;32:164–169. doi: 10.1128/jcm.32.1.164-169.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin L., Sakai Y., Bao R., Xie H., Masunaga K., Miura M., Hashimoto K., Tanamachi C., Hu B., Watanabe H. Characteristics of Multidrug-Resistant Corynebacterium spp. Isolated from Blood Cultures of Hospitalized Patients in Japan. Jpn. J. Infect. Dis. 2017;70:152–157. doi: 10.7883/yoken.JJID.2015.530. [DOI] [PubMed] [Google Scholar]
- 31.Kang S.J., Choi S.-M., Choi J.-A., Choi J.U., Oh T.-H., Kim S.E., Kim U.J., Won E.J., Jang H.-C., Park K.-H., et al. Factors affecting the clinical relevance of Corynebacterium striatum isolated from blood cultures. PLoS ONE. 2018;13:e0199454. doi: 10.1371/journal.pone.0199454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Zhou H., Du P., Lan R., Chen D., Dong A., Lin X., Qiu X., Xu S., Ji X., et al. Genomic epidemiology of Corynebacterium striatum from three regions of China: An emerging national nosocomial epidemic. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Zhou H., Chen D., Du P., Lan R., Qiu X., Hou X., Liu Z., Sun L., Xu S., et al. Whole-Genome Sequencing Reveals a Prolonged and Persistent Intrahospital Transmission of Corynebacterium striatum, an Emerging Multidrug-Resistant Pathogen. J. Clin. Microbiol. 2019;57:e00683-19. doi: 10.1128/JCM.00683-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos J.N., Rodrigues I.D.S., Baio P.V.P., Veras J.F.C., Ramos R.T.J., Pacheco L.G.C., Azevedo V.A., Júnior R.H., Marín M.A., de Mattos-Guaraldi A.L., et al. Genome sequence of a multidrug-resistant Corynebacterium striatum isolated from bloodstream infection from a nosocomial outbreak in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2018;113:e180051. doi: 10.1590/0074-02760180051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tauch A., Krieft S., Kalinowski J., Pühler A. The 51,409-bp R-plasmid pTP10 from the multiresistant clinical isolate Corynebacterium striatum M82B is composed of DNA segments initially identified in soil bacteria and in plant, animal, and human pathogens. Mol. Gen. Genet. 2000;263:1–11. doi: 10.1007/PL00008668. [DOI] [PubMed] [Google Scholar]
- 36.Zhao F., Ding T., Li M., Wang Y., Zhang X., Ren H., Tong Y. Complete genome analysis of a novel temperate bacteriophage induced from Corynebacterium striatum. Arch. Virol. 2019;164:2877–2880. doi: 10.1007/s00705-019-04370-2. [DOI] [PubMed] [Google Scholar]
- 37.Peterson E., Kaur P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018;9:2928. doi: 10.3389/fmicb.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox G., Wright G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013;303:287–292. doi: 10.1016/j.ijmm.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Olender A. Mechanisms of Antibiotic Resistance in Corynebacterium spp. Causing Infections in People. In: Pana M., editor. Antibiotic Resistant Bacteria—A Continuous Challenge in the New Millennium. Volume 15. InTech; Rijeka, Croatia: 2012. pp. 387–402. [Google Scholar]
- 40.Goldner N.K., Bulow C., Cho K., Wallace M., Hsu F.-F., Patti G.J., Burnham C.-A., Schlesinger P., Dantas G. Mechanism of High-Level Daptomycin Resistance in Corynebacterium striatum. mSphere. 2018;3:e00371-18. doi: 10.1128/mSphereDirect.00371-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira A., Oliveira L.C., Aburjaile F., Benevides L., Tiwari S., Jamal S.B., Silva A., Figueiredo H.C.P., Ghosh P., Portela R.W., et al. Insight of Genus Corynebacterium: Ascertaining the Role of Pathogenic and Non-pathogenic Species. Front. Microbiol. 2017;8:1937. doi: 10.3389/fmicb.2017.01937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schröder J., Maus I., Meyer K., Wördemann S., Blom J., Jaenicke S., Schneider J., Trost E., Tauch A. Complete genome sequence, lifestyle, and multi-drug resistance of the human pathogen Corynebacterium resistens DSM 45100 isolated from blood samples of a leukemia patient. BMC Genom. 2012;13:141. doi: 10.1186/1471-2164-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tauch A., Bischoff N., Brune I., Kalinowski J. Insights into the genetic organization of the Corynebacterium diphtheriae erythromycin resistance plasmid pNG2 deduced from its complete nucleotide sequence. Plasmid. 2003;49:63–74. doi: 10.1016/S0147-619X(02)00115-4. [DOI] [PubMed] [Google Scholar]
- 44.Redgrave L.S., Sutton S.B., Webber M.A., Piddock L.J.V. Fluoroquinolone resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014;22:438–445. doi: 10.1016/j.tim.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Ramos J.N., Valadão T.B., Baio P.V.P., Mattos-Guaraldi A.L., Vieira V.V. Novel mutations in the QRDR region gyrA gene in multidrug-resistance Corynebacterium spp. isolates from intravenous sites. Antonie van Leeuwenhoek. 2020;113:589–592. doi: 10.1007/s10482-019-01353-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sierra J.M., Martinez-Martinez L., Vázquez F., Giralt E., Vila J. Relationship between mutations in the gyrA gene and quinolone resistance in clinical isolates of Corynebacterium striatum and Corynebacterium amycolatum. Antimicrob. Agents Chemother. 2005;49:1714–1719. doi: 10.1128/AAC.49.5.1714-1719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alibi S., Ferjani A., Boukadida J., Cano M.E., Fernández-Martínez M., Martínez-Martínez L., Navas J. Occurrence of Corynebacterium striatum as an emerging antibiotic-resistant nosocomial pathogen in a Tunisian hospital. Sci. Rep. 2017;7:9704. doi: 10.1038/s41598-017-10081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H.W. DAPTOMYCIN, its membrane-active mechanism vs. that of other antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2020;1862:183395. doi: 10.1016/j.bbamem.2020.183395. [DOI] [PubMed] [Google Scholar]
- 49.Tran T.T., Jaijakul S., Lewis C.T., Diaz L., Panesso D., Kaplan H.B., Murray B.E., Wanger A., Arias C.A. Native Valve Endocarditis Caused by Corynebacterium striatum with Heterogeneous High-Level Daptomycin Resistance: Collateral Damage from Daptomycin Therapy? Antimicrob. Agents Chemother. 2012;56:3461–3464. doi: 10.1128/AAC.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.TeKippe E.M., Thomas B.S., Ewald G.A., Lawrence S.J., Burnham C.-A.D. Rapid emergence of daptomycin resistance in clinical isolates of Corynebacterium striatum… a cautionary tale. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:2199–2205. doi: 10.1007/s10096-014-2188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagiya H., Kimura K., Okuno H., Hamaguchi S., Morii D., Yoshida H., Mitsui T., Nishi I., Tomono K. Bacteremia due to high-level daptomycin-resistant Corynebacterium striatum: A case report with genetic investigation. J. Infect. Chemother. 2019;25:906–908. doi: 10.1016/j.jiac.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Werth B.J., Hahn W.O., Butler-Wu S.M., Rakita R.M. Emergence of High-Level Daptomycin Resistance in Corynebacterium striatum in Two Patients with Left Ventricular Assist Device Infections. Microb. Drug Resist. 2016;22:233–237. doi: 10.1089/mdr.2015.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell K.F., McElvania E., Wallace M.A., Droske L.E., Robertson A.E., Westblade L.F., Burnham C.-A.D. Evaluating the Rapid Emergence of Daptomycin Resistance in Corynebacterium: A Multi-Center Study. J. Clin. Microbiol. 2021;59:e02052-20. doi: 10.1128/JCM.02052-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hines K.M., Waalkes A., Penewit K., Holmes E.A., Salipante S.J., Werth B.J., Xu L. Characterization of the Mechanisms of Daptomycin Resistance among Gram-Positive Bacterial Pathogens by Multidimensional Lipidomics. mSphere. 2017;2:e00492-17. doi: 10.1128/mSphere.00492-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hachmann A.-B., Sevim E., Gaballa A., Popham D.L., Antelmann H., Helmann J.D. Reduction in Membrane Phosphatidylglycerol Content Leads to Daptomycin Resistance in Bacillus subtilis. Antimicrob. Agents Chemother. 2011;55:4326–4337. doi: 10.1128/AAC.01819-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hennart M., Panunzi L.G., Rodrigues C., Gaday Q., Baines S.L., Barros-Pinkelnig M., Carmi-Leroy A., Dazas M., Wehenkel A.M., Didelot X., et al. Population genomics and antimicrobial resistance in Corynebacterium diphtheriae. Genome Med. 2020;12:107. doi: 10.1186/s13073-020-00805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.NeÅ¡vera J., Hochmannová J., Pátek M. An integron of class 1 is present on the plasmid pCG4 from Gram-positive bacterium Corynebacterium glutamicum. FEMS Microbiol. Lett. 1998;169:391–395. doi: 10.1111/j.1574-6968.1998.tb13345.x. [DOI] [PubMed] [Google Scholar]
- 58.Kono M., Sasatsu M., Aoki T. R Plasmids in Corynebacterium xerosis Strains. Antimicrob. Agents Chemother. 1983;23:506–508. doi: 10.1128/AAC.23.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szemraj M., Kwaszewska A., Szewczyk E.M. New Gene Responsible for Resistance of Clinical Corynebacteria to Macrolide, Lincosamide and Streptogramin B. Pol. J. Microbiol. 2018;67:237–240. doi: 10.21307/pjm-2018-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szemraj M., Czekaj T., Kalisz J., Szewczyk E.M. Differences in distribution of MLS antibiotics resistance genes in clinical isolates of staphylococci belonging to species: S. epidermidis, S. hominis, S. haemolyticus, S. simulans and S. warneri. BMC Microbiol. 2019;19:124. doi: 10.1186/s12866-019-1496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coyle M.B., Minshew B.H., Bland J.A., Hsu P.C. Erythromycin and clindamycin resistance in Corynebacterium diphtheriae from skin lesions. Antimicrob. Agents Chemother. 1979;16:525–527. doi: 10.1128/AAC.16.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eady E.A., Coates P., Ross J.I., Ratyal A.H., Cove J.H. Antibiotic resistance patterns of aerobic coryneforms and furazolidone-resistant Gram-positive cocci from the skin surface of the human axilla and fourth toe cleft. J. Antimicrob. Chemother. 2000;46:205–213. doi: 10.1093/jac/46.2.205. [DOI] [PubMed] [Google Scholar]
- 63.Rosato A.E., Lee B.S., Nash K.A. Inducible Macrolide Resistance in Corynebacterium jeikeium. Antimicrob. Agents Chemother. 2001;45:1982–1989. doi: 10.1128/AAC.45.7.1982-1989.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guirao G.Y., Peris B.M., Martínez-Toldos M.C., González T.R., Guillén P.L.V., Hernández M.S. Implicación de los genes ermX en la resistencia a los macrólidos y la telitromicina de Corynebacterium jeikeium y Corynebacterium amycolatum. Rev. Española Quimioter. 2005;18:236–242. [PubMed] [Google Scholar]
- 65.Olender A., Niemcewicz M. Macrolide, Lincosamide, and Streptogramin B–Constitutive-Type Resistance in Corynebacterium pseudodiphtheriticum Isolated from Upper Respiratory Tract Specimens. Microb. Drug Resist. 2010;16:119–122. doi: 10.1089/mdr.2009.0122. [DOI] [PubMed] [Google Scholar]
- 66.Partridge S.R., Kwong S.M., Firth N., Jensen S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018;31:e00088-17. doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siguier P., Perochon J., Lestrade L., Mahillon J., Chandler M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zulkower V., Rosser S. DNA features viewer, a sequence annotations formatting and plotting library for python. bioRxiv. 2020 doi: 10.1093/bioinformatics/btaa213. [DOI] [PubMed] [Google Scholar]
- 69.Siguier P., Gourbeyre E., Varani A., Ton-Hoang B., Chandler M. Mobile DNA III. ASM Press; Washington, DC, USA: 2015. Everyman’s Guide to Bacterial Insertion Sequences; pp. 555–590. [DOI] [PubMed] [Google Scholar]
- 70.Razavi M., Kristiansson E., Flach C.-F., Larsson D.G.J. The Association between Insertion Sequences and Antibiotic Resistance Genes. mSphere. 2020;5:e00418-20. doi: 10.1128/mSphere.00418-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He S., Hickman A.B., Varani A.M., Siguier P., Chandler M., Dekker J.P., Dyda F. Insertion Sequence IS26 Reorganizes Plasmids in Clinically Isolated Multidrug-Resistant Bacteria by Replicative Transposition. MBio. 2015;6:e00762-15. doi: 10.1128/mBio.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harmer C.J., Hall R.M. An analysis of the IS6/IS26 family of insertion sequences: Is it a single family? Microb. Genomics. 2019;5 doi: 10.1099/mgen.0.000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vandecraen J., Chandler M., Aertsen A., Van Houdt R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit. Rev. Microbiol. 2017;43:709–730. doi: 10.1080/1040841X.2017.1303661. [DOI] [PubMed] [Google Scholar]
- 74.De Boer H.A., Comstock L.J., Vasser M. The tac promoter: A functional hybrid derived from the trp and lac promoters. Proc. Natl. Acad. Sci. USA. 1983;80:21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ballouz S., Francis A.R., Lan R., Tanaka M.M. Conditions for the Evolution of Gene Clusters in Bacterial Genomes. PLoS Comput. Biol. 2010;6:e1000672. doi: 10.1371/journal.pcbi.1000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deng Y., Bao X., Ji L., Chen L., Liu J., Miao J., Chen D., Bian H., Li Y., Yu G. Resistance integrons: Class 1, 2 and 3 integrons. Ann. Clin. Microbiol. Antimicrob. 2015;14:45. doi: 10.1186/s12941-015-0100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rocha D.J.P., Azevedo V., Brenig B., Silva A., Blom J., Ramos R.T., Aguiar E.R.G., Chapartegui-González I., Fernández-Martínez M., Martínez-Martínez L., et al. Whole-genome sequencing reveals misidentification of a multidrug-resistant urine clinical isolate as Corynebacterium urealyticum. J. Glob. Antimicrob. Resist. 2020;23:16–19. doi: 10.1016/j.jgar.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 78.Moura A., Soares M., Pereira C., Leitao N., Henriques I., Correia A. INTEGRALL: A database and search engine for integrons, integrases and gene cassettes. Bioinformatics. 2009;25:1096–1098. doi: 10.1093/bioinformatics/btp105. [DOI] [PubMed] [Google Scholar]
- 79.Barnass S., Holland K., Tabaqchali S. Vancomycin-resistant Corynebacterium species causing prosthetic valve endocarditis successfully treated with imipenem and ciprofloxacin. J. Infect. 1991;22:161–169. doi: 10.1016/0163-4453(91)91591-K. [DOI] [PubMed] [Google Scholar]
- 80.Doster E., Lakin S.M., Dean C.J., Wolfe C., Young J.G., Boucher C., Belk K.E., Noyes N.R., Morley P.S. MEGARes 2.0: A database for classification of antimicrobial drug, biocide and metal resistance determinants in metagenomic sequence data. Nucleic Acids Res. 2019;48:D561–D569. doi: 10.1093/nar/gkz1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bortolaia V., Kaas R.S., Ruppe E., Roberts M.C., Schwarz S., Cattoir V., Philippon A., Allesoe R.L., Rebelo A.R., Florensa A.F., et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Navas J., Fernández-Martínez M., Salas C., Cano M.E., Martínez-Martínez L. Susceptibility to Aminoglycosides and Distribution of aph and aac(3)-XI Genes among Corynebacterium striatum Clinical Isolates. PLoS ONE. 2016;11:e0167856. doi: 10.1371/journal.pone.0167856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galimand M., Fishovitz J., Lambert T., Barbe V., Zajicek J., Mobashery S., Courvalin P. AAC(3)-XI, a new aminoglycoside 3-N-acetyltransferase from Corynebacterium striatum. Antimicrob. Agents Chemother. 2015;59:5647–5653. doi: 10.1128/AAC.01203-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Souza C., Simpson-Louredo L., Mota H.F., Faria Y.V., Cabral F.D.O., Colodette S.D.S., Canellas M.E.F.C., Cucinelli A.D.E.S., de Luna M.D.G., Santos C.D.S., et al. Virulence potential of Corynebacterium striatum towards Caenorhabditis elegans. Antonie Van Leeuwenhoek. 2019;112:1331–1340. doi: 10.1007/s10482-019-01265-9. [DOI] [PubMed] [Google Scholar]
- 85.Alibi S., Ramos-Vivas J., Ben Selma W., Ben Mansour H., Boukadida J., Navas J. Virulence of clinically relevant multi-drug resistant Corynebacterium striatum strains and their ability to adhere to human epithelial cells and inert surfaces. Microb. Pathog. 2021;155:104887. doi: 10.1016/j.micpath.2021.104887. [DOI] [PubMed] [Google Scholar]
- 86.Kumar A., Baruah A., Tomioka M., Iino Y., Kalita M.C., Khan M. Caenorhabditis elegans: A model to understand host-microbe interactions. Cell. Mol. Life Sci. 2020;77:1229–1249. doi: 10.1007/s00018-019-03319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song S.A., Shin J.H. Microbiological Characteristics of Corynebacterium striatum, an Emerging Pathogen. Hanyang Med. Rev. 2018;38:93. doi: 10.7599/hmr.2018.38.2.93. [DOI] [Google Scholar]
- 88.Shariff M., Beri K. Corynebacterium striatum: An emerging respiratory pathogen. J. Infect. Dev. Ctries. 2018;12:581–586. doi: 10.3855/jidc.10406. [DOI] [PubMed] [Google Scholar]
- 89.Neemuchwala A., Soares D., Ravirajan V., Marchand-Austin A., Kus J.V., Patel S.N. In vitro antibiotic susceptibility pattern of non-diphtheriae Corynebacterium isolates in Ontario, Canada, from 2011 to 2016. Antimicrob. Agents Chemother. 2018;62:e01776-17. doi: 10.1128/AAC.01776-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abe M., Kimura M., Maruyama H., Watari T., Ogura S., Takagi S., Uchida N., Otsuka Y., Taniguchi S., Araoka H. Clinical characteristics and drug susceptibility patterns of Corynebacterium species in bacteremic patients with hematological disorders. Eur. J. Clin. Microbiol. Infect. Dis. 2021 doi: 10.1007/s10096-021-04257-8. [DOI] [PubMed] [Google Scholar]