Abstract
The lung extracellular matrix (ECM) plays a key role in the normal architecture of the lung, from embryonic lung development to mechanical stability and elastic recoil of the breathing adult lung. The lung ECM can modulate the biophysical environment of cells through ECM stiffness, porosity, topography and insolubility. In a reciprocal interaction, lung ECM dynamics result from the synthesis, degradation and organization of ECM components by the surrounding structural and immune cells. Repeated lung injury and repair can trigger a vicious cycle of aberrant ECM protein deposition, accompanied by elevated ECM stiffness, which has a lasting effect on cell and tissue function. The processes governing the resolution of injury repair are regulated by several pathways; however, in chronic lung diseases such as asthma, chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary disease (IPF) these processes are compromised, resulting in impaired cell function and ECM remodeling. Current estimates show that more than 60% of the human coding transcripts are regulated by miRNAs. miRNAs are small non-coding RNAs that regulate gene expressions and modulate cellular functions. This review is focused on the current knowledge of miRNAs in regulating ECM synthesis, degradation and topography by cells and their dysregulation in asthma, COPD and IPF.
Keywords: extracellular matrix, lung, miRNA, asthma, chronic obstructive pulmonary disease, idiopathic pulmonary disease
1. Introduction
The lung extracellular matrix (ECM) plays a key role in the normal architecture of the lung, from embryonic lung development, alveolarization at birth, to mechanical stability and elastic recoil of the breathing adult lung [1,2]. The lung ECM provides both biochemical and biophysical cues, which direct cellular functions and differentiation. The lung ECM can also alter the biochemical environment of surrounding cells, by storing and sequestering growth factors and cytokines to regulate spatially and temporally their bioavailability. The lung ECM can modulate the biophysical environment of cells through ECM stiffness, porosity, topography (spatial arrangement and orientation) and insolubility. Lung ECM molecules connect to cells via integrins, syndecans and other receptors to influence cell signalling, migration and proliferation. In response, the lung ECM is remodelled by cells, whereby its components are deposited, degraded or modified in a reciprocal relationship [3,4]. The objective of this review is to evaluate the current knowledge on the regulation of the lung ECM in health and disease by cells through the epigenetic regulation by miRNAs.
2. The Lung Extracellular Matrix
The “core matrisome” of the lung ECM comprises over 300 proteins [5], and each has distinct physical and biochemical properties that dictate its function. The lung matrisome is organized into two main structural subtypes: (1) basement membranes that provide the anchorage site for epithelia, endothelia, muscle, fat and peripheral nerves; and (2) the interstitial matrix that connects the structural cells within the lung. The predominant components of the basement membrane include collagen IV, collagen V, laminins (which are also the most abundant non-collagenous component), chondroitin sulphate proteoglycans (perlecan, agrin and dystroglycan), entactin, fibronectin, fibulin I and fibulin II [6,7]. The interstitial matrix comprises largely a meshwork of elastin, collagen I, collagen III, fibronectin, vitronectin, tenascin, versican and decorin [8,9]. The matrisome network of protein–protein and protein–proteoglycan interactions form the supramolecular assemblies of collagen and elastic fibers that shape the structural scaffold of the ECM within the lung. The arrangement of this meshwork allows for the non-linear stress–strain behaviour within the lung, with elastin providing viscoelasticity and collagen tensile strength.
3. How Cells Modify the Lung Extracellular Matrix
The lung ECM functions as a physical barrier, an anchorage site and a migration highway for cells within the organ. The ECM dynamics within each cellular niche are tightly regulated to ensure normal development, physiology and homeostasis of organ systems. Alterations in the lung ECM environment result from the synthesis, degradation or altered organization of ECM components by surrounding structural and immune cells. This is achieved by redundancy in the mechanisms used to modulate the expression and function of the ECM and ECM-modifying enzymes. When such control mechanisms are corrupted, ECM dynamics become deregulated, leading to various congenital defects or disease.
3.1. Synthesis of ECM by Cells
There is a dynamic biophysical and biochemical reciprocal interaction between cells and their surrounding ECM microenvironment. Variations in cell phenotype determine the assembly and composition of ECM proteins, resulting in different tissue morphologies and functions. To initiate ECM production, cells relay signals via the membrane and cytoskeleton by recruiting factors such as cytokines, adhesion molecules and growth factors to activate gene transcription. The synthesis and turnover expression rates of different ECM proteins within a specific tissue vary. For example, in the normal lung, collagen synthesis is estimated to be 10% per day, while that of other non-collagenous proteins is up to 35% per day [10,11]. Interestingly, the rate of ECM synthesis in a specific tissue may also vary as a result of age-related changes. Mays and colleagues showed that lung collagen synthesis decreased from 13% per day in a one-month-old mouse to 0.97% per day in two-year-old mice [12].
Mesenchymal cells are the dominant producers of ECM proteins [13,14], with fibroblasts being the largest synthesizer of the fibrillar ECM components such as collagen, laminins and fibronectin [15]. Basement membrane proteins such as collagen IV and laminins are synthesized by epithelial and endothelial cells. Other specialized cells involved in the synthesis of ECM protein include smooth muscle cells (versican), vascular smooth muscle cells and endothelial cells (Emilin-1 and 2). Inflammatory cells including monocytes and macrophages are also able to synthesize proteoglycans such as perlecan, serglycin, syndecans, amyloid precursor-like protein-2 and glypican-1 in response to anti-inflammatory signals [16]. In a physiological state, once ECM protein expression turnover is achieved, the activated cells return to a quiescent state to prevent the exaggerated synthesis of ECM proteins [17,18].
3.2. Degradation of ECM by Cells
ECM degradation is an essential process for tissue homeostasis and can be detrimental in tissue remodeling. The most significant enzymes in ECM remodelling are metalloproteinases [19], which include two main families of metalloproteinases, matrix metalloproteinases (MMP) and A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) families.
MMPs are grouped according to their modular domain structures. Presently, they are classified into eight groups: minimal domain (MMP-7, MMP-26), simple hemopexin domain (MMP-1, MMP-3, MMP-8, MMP-10, MMP-12, MMP-13, MMP-18, MMP-19, MMP-20, MMP-22, MMP-27), gelatin-binding (MMP-2, MMP-9), membrane-type (MMP-14, MMP-15, MMP-16, MMP-24), furin-activated secreted (MMP-11, MMP-28), glycophosphatidyl inositol-anchoring domain (MMP-17, MMP-25), vitronectin-like insert linker-less (MMP-23) and cysteine/proline-rich interleukin-1 receptor-like domain MMPs (MMP-23) [20]. All MMPs contain a catalytic domain and an auto-inhibitory prodomain, which houses an active zinc-binding site. The unique catalytic domains of each MMP dictate the substrate recognition and cleavage site. For example, both MMP-2 and MMP-9 have a high affinity towards collagen and other ECM proteins including elastin, fibronectin and vitronectin, whereas MMP-3 and MMP-10 have a higher affinity towards proteoglycans, laminins and fibronectin [19,21]. The auto-inhibitory prodomains can be activated through direct cleavage by other endoproteinases, allosteric conformation or modification by reactive oxygen species and non-physiological agents [22]. Once activated, MMPs can mediate ECM protein degradation by directly cleaving the ECM substrate precursor proteins. Additionally, MMPs can modify the activities of signalling molecules such as cell surface receptors, adhesion molecules, chemokines, growth factors and proteinase inhibitors within the ECM, enabling modification of intercellular junctions and cell–ECM adhesions [23]. For example, MMP-9 can alter epithelial migration through transforming growth factor (TGF)-β activation from an inactive fibronectin-TGF-β complex [24]. Alternatively, MMPs can modulate signalling molecules by generating cleavage fragments with cell-binding properties, from the cleaved substrate. For example, tumstatin, a cleaved fragment of the alpha-subunit of collagen IV by MMP-9, has proapoptotic properties and also serves as an angiostatic peptide [25]. Another example is TGF-β; in its latent state it is bound to the fibrillin protein latency-associated peptide (LAP), a substrate of MMP-2, MMP-9, MMP-13 and MMP-14. Cleavage of this complex by these MMPs releases the TGF-β and increases its availability for signalling [22].
Similarly, ADAMTS proteinases have a prodomain amongst several other domains including the disintegrin and metalloproteinase domains. ADAMTS (except ADAMTS-2) have a high affinity towards degrading proteoglycans; hence, they are often called proteoglycanases. For example, the proteoglycan aggrecan is targeted for degradation by ADAMTS-4 and ADAMTS-5 [26]. Serine proteases (elastase and cathepsin G) and cysteine proteases (cathepsins B and K) are also ECM-degrading enzymes secreted outside the cell to digest the ECM [27]. Serine proteases play various roles in ECM degradation and target essentially all the ECM components. As an example, Albrengues and colleagues demonstrated that laminin-111 is sequentially cleaved by neutrophil elastase and MMP-9 [28]. Cathepsin K has potent aggrecan degrading activity that further potentiates degradation of collagen I and collagen II [29].
Dysregulation of ECM protein degradation can have significant effects on the host tissue architecture. Therefore, proteases are controlled by their endogenous activators and inhibitors. MMPs and ADAMTS are tightly controlled through their transcription, activation and inactivation by tissue inhibitor of metalloproteinases (TIMPs 1, 2, 3 and 4). TIMPs have different binding affinities, for example, TIMP-3 has a high affinity towards inhibiting ADAMTS-4 and ADAMTS-5 [30], while TIMP-1 is more potent for MMP-3 and MMP-7(22). Other non-specific protease inhibitors such as alpha-2 macroglobulin can also inhibit MMPs [22], while plasminogen activator inhibitor-1 and alpha-2 antiplasmin target serine proteases [31].
3.3. Alterations in ECM Topography by Cells
The cross-linking of ECM proteins via covalent and noncovalent modifications greatly influences the organization and topography of the ECM environment, which in turn has fundamental effects on cell behavior [32,33,34]. Proteomic studies in mouse models have shown that during the various stages of lung development and physiological processes, the ECM undergoes large changes in its topography and composition [35]. In addition, the composition of the matrix network and architectural changes in the ECM in varying physiological states has also been documented [36]. Interstitial collagens can undergo several posttranslational modifications, including covalent and noncovalent cross-linking. The extent of collagen–elastin intermolecular crosslinking is largely determined by the expression and activation of the family of lysyl oxidases (LOX) and lysyl hydroxylases. For example, up-regulation of collagen cross-linking, due to excess LOX activity, leads to an increase in the matrix stiffness and thus tensile strength of tissues, which can profoundly influence cellular behaviours [15,37]. Inhibition of LOX has also been shown to decrease fibril collagen thickness and subsequent tissue stiffening in IPF [38]. Levental and colleagues showed that collagen crosslinking can modulate breast tissue fibrosis and stiffness, leading to enhanced growth factor signalling and breast malignancies [37].
In the pulmonary vasculature, Emlinin-1 and -2 (elastin microfibril interface-located proteins) are incorporated into elastin and fibrillin microfibrils and have been shown to be important extracellular regulators of global TGF- β, BNP (bone morphogenic protein) and Wnt signaling pathways [39]. Alteration in the expression of Emilin-1 and -2 results in disruption of cell adhesion, apoptosis, migration and proliferation and destabilization of microfibrils, which subsequently alters the ECM architecture and tissue integrity [39,40,41,42,43,44].
Cells can also manipulate ECM tomography through traction of ECM proteins by binding of cell receptors and anchorage with the cell cytoskeleton. Such traction force interferes with the cross-linking bonds between ECM proteins and the cell’s actin cytoskeleton, leading to modification of cellular signalling and gene expression. For example, stretching of fibronectin by cellular traction force increases its binding force to integrin receptors, other fibronectin dimers and collagen. This, in turn, increases the size, density and rigidity of fibronectin fibres and causes pleiotropic changes in cell growth, differentiation and migration [45], often influencing the progression of fibrosis in cells.
In summary, ECM synthesis, degradation and topography are controlled at multiple levels including transcriptional and posttranslational regulation. For the remainder of this review, we will assess the role of epigenetic regulation through the expression and release of miRNAs by cells and their influence on the lung ECM microenvironment in health and disease.
4. The Role of miRNAs in Modifying the ECM Microenvironment
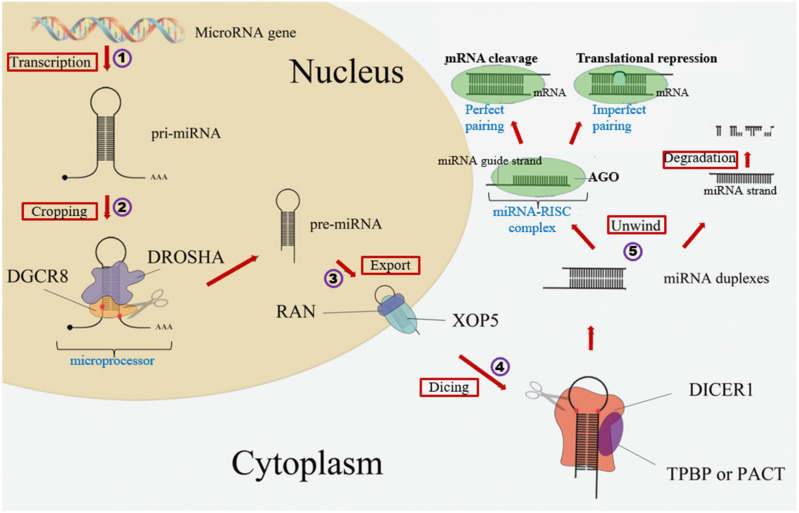
Current estimates show that more than 60% of the human coding transcripts are regulated by miRNAs [46]. Rodrigues et al. (2004) and Griffiths-Jones et al. (2006) have listed 2588 annotated miRNAs in the human genome [47,48]. MiRNAs are short, non-coding RNAs that are approximately 22 nucleotides long. As shown in Figure 1, miRNAs are primarily generated via (1) transcription by RNA polymerase enzyme, after which they undergo (2) cropping to precursor miRNAs (pre-miRNA) by the microprocessor complex of DROSHA (ribonuclease III Drosha and DGCR8 (DiGeorge Syndrome Critical Region 8)). The precursor miRNAs are then (3) exported by the nuclear protein exportin-5 (XPO5) from the cell nucleus into the cytoplasm where they are further processed to mature miRNA following (4) dicing by RNase III endonuclease Dicer. The functional strand of the mature miRNA is then (5) loaded into the risk inducing silencing complex (RISC) comprising the argonaute (AGO) family of proteins to mediate various functions [49].
Figure 1.
miRNA biogenesis. RNA genes are (1) transcribed to primary miRNA (pri-miRNA) by RNA polymerase enzyme II and then later (2) cropped by the microprocessor (DROSHA-DGCR8) to precursor miRNA (pre-miRNA). (3) Export of these pre-miRNA from the nucleus into the cytoplasm by Exportin-5 (XPO5). (4) Dicing by DICER and then (5) processing into mature miRNA and loaded on the RNA-inducing silencing complex (RISC) to mediate various functions. This figure is adapted with permission from He J. et al, PeerJ, 2016 (https://doi.org/10.7717/peerj.2706). (Accessed on 10 May 2021) DGCR8—DiGeorge syndrome critical region 8, MicroRNA—miRNA, DROSHA—Ribonuclease III, RAN—Ras-related nuclear protein, XOP5—Exportin 5, DICER1—Riboendonuclease, TPBP—Transactivation response RNA binding protein, PACT—Protein activator, AGO—Argonaute protein, mRNA—messenger RNA.
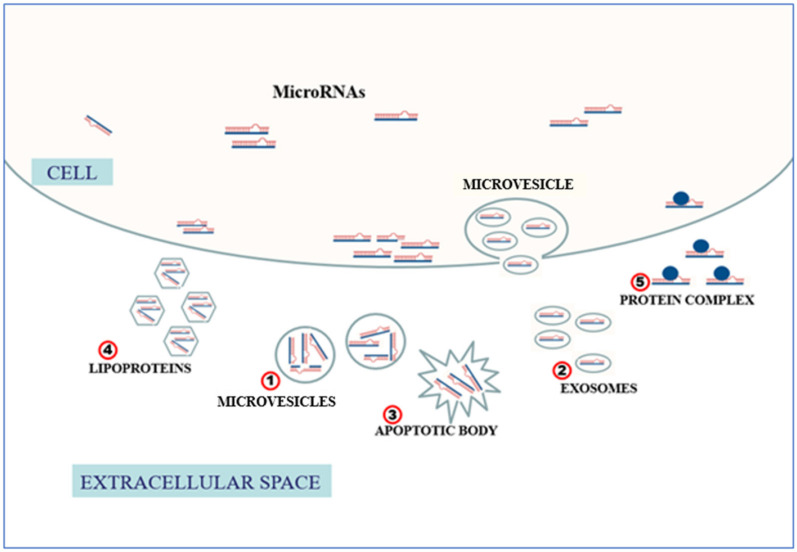
The classic mechanism of miRNA-mediated gene regulation involves binding of the miRNA seed sequence to the 3’UTR of mRNA and, to a lesser extent, the 5’UTR of the mRNA, gene promoters or coding sequences [50]. Based on the level of complementarity binding with the interacting sites, they trigger transcript degradation or inhibition of translation. Until recently, it was believed that miRNAs function within their cell of origin. However, there is now strong evidence for miRNAs in the extracellular microenvironment [51,52,53,54,55] and their involvement in cell–cell communication [56,57]. Recent studies have shown that miRNAs are present in the extracellular environment, including different biological fluids such as bronchial lavage and breast milk [53]. As shown in Figure 2, the release of miRNA into the extracellular compartment is mediated by (1) enwrapping into microvesicles, (2) selective incorporation into exosomes (3) or apoptotic bodies, and (4) coupling with high-density lipoproteins or (5) protein complexes such as AGO to enable them to function as intercellular signalling molecules [58,59,60,61].
Figure 2.
Release of miRNA to the extracellular compartment. Mature miRNAs are transported out of the cell by (1) enwrapping into microvesicles, (2) selective incorporation into exosomes, (3) apoptotic bodies, (4) coupling with high-density lipoproteins and (5) protein complexes such as AGO to be released into the extracellular environment or other cells.
It is now understood that some miRNAs are exclusively associated with microvesicles such as let-7a, and other miRNAs act independently of vesicles such as miR-122 [62]. The stability of AGO proteins, and the remarkable degree of impermeability of microvesicles and exosomes to RNases, means long-term stability of miRNAs in vesicles. The miRNA pathway, through extracellular release thus possess a critical mechanism of regulating the ECM environment [63].
5. ECM Regulation by miRNAs
MiRNAs are involved in a number of cellular processes that regulate the synthesis of ECM molecules in various organs and model systems. On 14 May 2021, we searched the scientific literature in PubMed (with no date or language restrictions) for the following search terms “each ECM protein” and “miRNA”. As shown in Table 1, for 19 ECM proteins expressed within the lung, all have been shown in one or more model system to be regulated by the classic regulatory mechanism exerted by miRNAs, by direct interaction with their cognate binding mRNA. Specifically, we found 35 miRNAs that have been shown to regulate ECM protein translation through binding the 3’UTR. For example, down-regulation of miR-206 modulates in human lung adenocarcinoma epithelial cell line (A549’s) increase in the translation of fibronectin 1 through direct binding to the 3’UTR of the fibronectin 1 gene [64].
Table 1.
Regulation extracellular matrix remodeling by miRNAs.
| ECM-Related Molecule | miRNA | Direction of miRNA Change | Direction of ECM Molecule Change | Mechanism | Cell Type | Disease/Develop-Mental PROCESS | Reference |
|---|---|---|---|---|---|---|---|
| ECM Proteins | |||||||
| Collagen I | miR-205 | ↑ | ↓ | binds to the ∆Np63 | cardiomyocytes | myocardial fibrosis | Castoldi et al., 2011 |
| Collagen I | miR-133a, miR-29b | ↑ | ↓ | bind to COL1A1 3’UTR | Hepatic stellate cells | liver fibrosis | Sekiya et al., 2011 |
| Collagen I | miR-29b | ↑ | ↓ | Targets COL1A1, COL1A2, ITGB1, and PDGFR-B | Hepatocellular carcinoma cells | hepatocellular carcinoma | Ji et al., 2010 |
| Collagen I | Let-7g | ↑ | ↓ | Let-7g binds to COLIA1 3’UTR | Hepatocellular carcinoma cells | hepatocellular carcinoma | Ji et al., 2011 |
| Collagen I | miR-21, miR-29a |
↑ (μιΡ21), ↓ (μPNA-29α) |
↓ (μιΡ21), ↓ (μPNA-29α) |
targets TGF-β signalling pathway | dermal fibroblasts | systemic sclerosis | Jafarinejad-Farsangi et al., 2019 |
| Collagen I | miR-152 | ↑ | ↓ | binds to 3’UTR of ITGA5 | human dermal fibroblasts | Ageing | Mancini et al., 2012 |
| Collagen III | miR-29 | ↓ | ↑ | - | leiomyoma and myometrial cells | uterine fibroids | Marsh et al., 2016 |
| Collagen IV | miR-29 | ↑ | ↓ | binds to 3’UTR | Ageing | Takahashi et al., 2012 | |
| Collagen V | miR-185, miR-186 | ↓ | ↑ | binds 3’UTR of COL5A1 | human alveolar epithelial cells | Idiopathic pulmonary fibrosis | Lei et al., 2016 |
| Fibronectin type III domain containing 3B | miR-143 | ↑ | ↓ | binds to FNDC3B 3’UTR | hepatocytes | hepatocellular carcinoma | Zhang et al., 2009 |
| Fibronectin | miR-146a | ↓ | ↑ | binds to fibronectin 3’UTR | renal endothelial cells | Chronic diabetes | Feng et al., 2011 |
| Fibronectin | miR-377 | ↑ | ↑ | taregts p21-activated kinase and superoxide dismutase expression | mesangial cells | Diabetic nephropathy | Wang et al., 2008 |
| Fibronectin I | miR-206 | ↓ | ↑ | binds 3’UTR of fibronectin 1 | human lung adenocarcinoma epithelial cells | bronchopulmonary dysplasia | Zhang et al., 2013 |
| Fibronectin | miR-17 | ↑ | ↓ | binds 3’UTR of fibronectin | endothelial cells | Health | Shan et al., 2009 |
| Laminin and integrin | miR-29s | ↑ | ↓ | binds 3’UTR of LAMC2 and ITGA6 | head and neck squamous carcinoma cells | Cancer | Kinoshita et al., 2013 |
| Laminin-322 | miR-218 | ↓ | ↑ | binds to 3’UTR of LAMB3 | head and neck squamous carcinoma cells | Cancer | Kinoshita et al., 2012 |
| SPARC, Collagen I, Collagen IV, laminin | miR-29 | ↑ | ↓ | Targets TGF-β2/SMAD3 signalling | human TM cells | Glaucoma | Villarreal et al., 2011 |
| Collagen XVI | miR-181a | ↑ | ↓ | binds to 3’UTR of COL16A1 | human dermal fibroblasts | Ageing | Mancini et al., 2012 |
| Laminin γ1 (LAMC1) | miR-205 | ↓ | ↑ | binds 3’UTR of LAMC1 | triple-negative breast cancer cells | breast cancer | Piovan et al., 2012 |
| Laminin γ1 (LAMC1) | miR-124a | ↓ | ↑ | binds 3’UTR of LAMC1 | Glioblastoma cells | Glioblastoma (GBM) | Fowler et al., 2011 |
| laminin β2 (LAMB2) | Let-7b | ↑ | ↓ | down-regulates transcription factor HMGA | differentiating podocytes | Diabetic nephropathy | Schaeffer et al., 2012 |
| Perlecan/Heparin sulfate proteoglycan 2 | miR-663 | ↑ | ↓ | binds 3’UTR of HSPG2 | chemoresistant breast tumour cells | breast cancer | Hu et al., 2013 |
| Syndecan-1 | miR-10b | ↑ | ↓ | binds 3’UTR of syndecan 1 | breast cancer cells | breast cancer | Ibrahim et al., 2012 |
| Glypican-3 and fibronectin-1 | miR-96 | ↑ | ↓ | binds 3’UTR of glypican-3 and fibronectin-1 | hepatocytes | hepatocellular carcinoma and hepatoblastoma | Jalvy-Delvaille et al., 2011 |
| Nephronectin | miR-378 | ↑ | ↑ | binds 3’UTR of nephronectin | osteoblasts | bone development | Kahai et al., 2009 |
| Nephronectin | miR-23a, miR-101a, miR-296-5p, miR-328, miR-340-3p, miR-425 | ↑ | ↑ | binds 3’UTR of nephronectin | osteoblasts | Bone transplant/repair | Lee et al., 2011 |
| Versican | miR-138 | ↑ | ↓ | binds to CSPG2 3’UTR | cardiomyocytes | disrupted cardio-morphogenesis | Morton et al., 2008 |
| Versican | miR-143 | ↓ | ↓ | binds to 3’UTR of versican | cardiomyocytes | Cardiovascular diseases | Wang et al., 2010 |
| Aggrecan | miR-181a | ↑ | ↓ | targets CCN1 and ACAN signalling | human chondrocytes | cartilage metabolism | Sumiyoshi et al., 2010, Sumiyoshi et al., 2013 |
| Glypican-3 | miR-1291, miR-1271 | ↓ | ↑ | binds to 3’UTR of GPC3 and IRE1α | hepatocytes | hepatocellular carcinoma | Maurel et al., 2012, Maurel et al., 2013 |
| Glypican-3 | miR-219-5p | ↓ | ↑ | binds to 3’UTR of GPC3 | hepatocytes | hepatocellular carcinoma | Huang et al., 2012 |
| Glypican-3 | miR-520c-3p | ↓ | ↑ | binds to 3’UTR of GPC3 | hepatocytes | hepatocellular carcinoma | Miao et al., 2014 |
| Glypican-4 | miR-125a | ↑ | ↓ | binds to 3’UTR of glypican-4 | hepatocytes | cell proliferation | Feng et al., 2012 |
| ECM enzymes | |||||||
| MMP-1 | miR-203 | ↑ | ↑ | - | synovial fibroblasts | rheumatoid arthritis | Stanczyk et al., 2011 |
| MMP-2 | miR-29b | ↑ | ↓ | binds 3’UTR of COL1A1, COL3A1, COL4A1, and MBP-1 | prostate cancer cells | prostate cancer | Steele et al., 2010 |
| MMP-2, MMP-9 | miR-145 | ↑ | ↓ | binds KLF5 3’UTR | airway smooth muscle cells | Asthma | Liu et al., 2015 |
| MMP-2, MMP-9 | miR-206 | ↓ | ↑ | Targets Rho-cdc42/myosin signalling | Epithelial breast cancer cell line (MDA-MB-231) | Breast cancer | Liu et al., 2018 |
| MMP-2, MMP-9 | miR-340 | ↓ | ↑ | binds to 3’UTR of C’Met | breast cancer cells | Breast cancer | Wu et al., 2011 |
| MMP-3 | miR-155 | ↑ | ↓ | targets LPS signalling, TNF-receptor superfamily-interacting serine-threonine kinase 1 and IkB kinase activity | synovial fibroblasts | rheumatoid arthritis | Stanczyk et al., 2008 |
| MMP-9 | miR-218 | ↑ | ↑ | binds to MMP-9 3’UTR | Rat osteoclasts | Periodontitis | Guo et al., 2018 |
| MMP-9 | MiR-373 | ↑ | ↑ | binds to the 3’UTR of mTOR and SIRT1 | Human fibrosarcoma cells | cancer | Liu P. and Wilson M., 2011 |
| MMP-9 | miR-340 | ↓ | ↑ | binds to MMP-9 3’UTR | retinal ganglion cells | Glaucoma | Surgucheva et al., 2010 |
| MMP-9 | miR-212, miR-132 | ↑ | ↓ | binds to MMP-9 3’UTR | miR-212/132−/− mice | mammary gland development | Ucar et al., 2010 |
| MMP-13 | miR-27b | ↓ | ↑ | binds to MMP-13 3’UTR | chondrocytes | osteoarthritis | Akhtar et al., 2011 |
| MMP-13 | miR-143 | ↓ | ↑ | - | osteosarcoma cells | osteosarcoma | Osaki et al., 2011 |
| MMP-14 | let-7 | ↓ | ↑ | Targets TGF-β1/ERK signalling | pancreatic cancer cells | pancreatic ductal adenocarcinoma | Dangi-Garimella et al., 2011 |
| MMP-16 | miR-146b | ↑ | ↓ | binds to MMP-16 3’UTR | glioblastoma cells | glioblastoma | Xia et al., 2009 |
| MMP-16 | miR-155 | ↑ | ↓ | binds to MMP-16 3’UTR | cardiomyocyte progenitor cells | Transplantation therapy | Liu et al., 2012 |
| TIMP-1 | miR-29a | ↑ | ↓ | binds to TAB1 3’UTR | dermal fibroblasts | systemic sclerosis | Ciechomska et al., 2014 |
| RECK | miR-21 | ↑ | ↓ | binds to 3’UTR of RECK | glioma cells | glioblastoma | Gabriely et al., 2008 |
| TIMP-3 | miR-181b | ↑ | ↓ | Targets miR-191b | Hepatocellular carcinoma cells | Hepatic cancer | Wang et al., 2010 |
| TIMP-3 | miR-221, miR-222 | ↑ | ↓ | bind to 3’UTR of TIMP3 | Non-small cell lung cancer and hepatocarcinoma cells | small cell lung cancer and hepatocellular carcinoma | Garofalo et al., 2010 |
| TIMP-3 | miR-181a | ↑ | ↓ | - | osteosarcoma cells | osteosarcoma | Jianwei et al., 2013 |
| Neutrophil elastase(HNE), MUC5ac, EGFR | miR-146a | ↓ | ↑ | binds to EGFR 3’UTR | human bronchial epithelial cells | Mucus hypersecretion | Zhong et al., 2011 |
| Heparanase (HPSE) | miR-30 | ↑ | ↓ | Targets TGF-β1 and IL-6 signalling | human melanoma cells | melanoma | Liu et al., 2013 |
| Heparanase (HPSE) | miR-1258 | ↑ | ↓ | binds to HPSE 3’UTR | BMBC cells | brain metastatic breast cancer | Zhang et al., 2011 |
| Heparanase (HPSE) | miR-1252-5p | ↓ | ↑ | binds to HPSE 3’UTR | Multiple myeloma cells | Multiple myeloma | Rodrigues et al., 2021 |
| Cytokines and growth factors | |||||||
| IL-6 | miR-203 | ↑ | ↑ | - | synovial fibroblasts | rheumatoid arthritis | Stanczyk et al., 2011 |
| Keratinocyte growth factor | miR-155 | ↑ | ↓ | binds to 3’UTR of KGF | lung fibroblasts | IPF | Pottier et al., 2009 |
| TGF-β1 | miR-26a | ↑ | ↓ | binds to 3’UTR of HMGA2 | Lung epithelial cells | IPF | Liang et al., 2014 |
| TGF-β | miR-200 | ↓ | ↑ | Targets TGF-β/bone morphogenetic protein signalling | Triple-negative breast cancer cell | Breast cancer | Truong et al., 2014 |
| TGF- β | miR-29b | ↓ | ↑ | binds to 3’UTR of HSP47 | Skin fibroblast | Wound healing | Zhu Y. et al, 2016. |
| TGFBR2 | miR-153 | ↓ | ↓ | binds to TGFBR2 3’UTR | lung fibroblasts | IPF | Liang et al., 2015 |
| TGFBR2 | miR-145 | ↑ | ↓ | binds to 3’UTR of TGFBR2 | Aortic smooth muscle cell | Vascular diseases | Zhao et al., 2016 |
| TGFBR1, CTGF, COL1A1 | miR-133a | ↑ | ↓ | binds to 3’UTR of TGFBR1, CTGF and COL1A1 | human lung fibroblasts | IPF | Wei et al., 2019 |
| VEGF | miR-503 | ↓ | ↑ | miR-503 binds to VEGF 3’ UTR | human lung fibroblasts | COPD | Ikari et al., 2017 |
| VEGF-A | miR-126 | ↓ | ↑ | miR-126 binds to VEGF-A 3’ UTR | Oral squamous cell carcinoma cell | oral cancer | Sasahira et al., 2012 |
| VEGFA/CCL2, FOXO3a | miR-1, miR-31, miR-206 | ↓ | ↓ | bind to 3’UTR of CCL2/VEGFA and FOXO3a | cancer-associated lung fibroblasts | lung cancer | Shen et al., 2016 |
| FOXO3a | miR-96 | ↓ | ↑ | binds to FoxO3a 3’UTR | human lung fibroblasts | IPF | Nho et al., 2014 |
| FGF2 | miR-195 | ↓ | ↑ | binds to FGF2 3’UTR | prostatic cancer cells | prostate cancer | Liu et al., 2015 |
| Smad7 | miR-21 | ↑ | ↑ | binds to Smad7 3’UTR | myofibroblasts | IPF | Liu et al., 2010 |
| SLIT2/Robo1 | miR-203 | ↓ | ↑ | binds to Robo1 3’UTR | mouse mammary gland cells | breast tumour | Le et al., 2016 |
| Topography | |||||||
| Collagen | miR-203 | ↓ | ↑ | targets SLIT2/Robo1 signalling | Mammalian epithelial cell | Breast tumour | Le et al., 2016 |
| LOX | miR-19b | ↓ | ↑ | targets CTGF | myocardium | Aortic stenosis | Beaumont J. et al., 2017 |
| LOXL2 | miR-29a, miR-29b, miR-29c | ↓ | ↑ | MiR-29 family directly target LOXL2 | Renal cell carcinoma | Renal cell carcinoma | Nishikawa R. et al., 2015 |
| LOXL2, PLOD2 | miR-26a, miR-26b | ↓ | ↑ | bind to 3’UTR of LOXL2 and PLOD2 | renal cell carcinoma cells | renal cell carcinoma | Kurozumi et al., 2016 |
| LOX, LOX1, elastin and collagen | miR-145 | ↓ | ↑ | targets notch signalling | Vascular muscle cells | Angiotensin II-induced fibrosis | Zhao N. et al., 2015 |
A shown in Table 1, we found 41 miRNAs that have been shown to indirectly regulate the ECM by targeting ECM transcription factors including transcriptional activators and repressors. For example, in skin fibroblasts, TGF-β1/smad-induced collagen 1 synthesis is enhanced due to up-regulation of heat shock protein 47 (HSP47), a chaperon protein associated with collagen 1 synthesis and down-regulation of miR-29b expression [65]. Suppression of HSP90 by over-expressing miR-628-3p via its 3’UTR and miR-27 via Akt signaling in A549 lung cancer cells and esophageal squamous cell carcinoma, respectively, has been shown to modulate collagen deposition [66,67,68]. The relationship between ECM proteins and miRNA is not always unidirectional, as numerous downstream signalling pathways can also be affected. For example, in breast cancer cells, miR-10b binds with the 3’UTR of the epithelial transmembrane proteoglycan syndecan-1, leading to down-regulated expression. However, the down-regulation of syndecan-1 by miR-10b results in the increased expression of fibronectin and laminin via the focal adhesion kinase and Rho-GTPase pathways [69].
MiRNAs are also able to influence ECM expression through protein degradation. To date, 23 miRNAs have been shown to affect ECM composition through the regulation of proteases and anti-proteinases. For example, the expressions of MMP-9, MMP-16 and TIMP-3 associated with collagen homeostasis, cell migration and invasion are modulated by miR-373, miR-146b and miR-21, respectively [70,71,72].
Various cytokines and growth factors such as TGF-β and epidermal growth factors play important roles in the modulation of ECM assembly, and most of these functions are regulated by miRNAs. Fifteen miRNAs have been shown to influence ECM expression through growth factors and cytokines expression. Specifically, MiR-1, miR-31 and miR-206 have been shown to regulate lung fibroblast cells, a key player in the synthesis, deposition and remodelling of ECM through the vascular endothelial growth factor (VEGF), chemokine (c-c motif) ligand (CCL)-2 and FoxO3a signalling pathways [73]. Lastly, ECM density and tensional homeostasis has been shown to be regulated by miR-203 via the SLIT2/Robo1 receptor signalling pathway [74]. In mammary epithelial cells, repressed miR-203 expression enhances SLIT2/Robo1 signalling and the ability of mammary epithelial cell cytoskeleton to pull on collagen fiber [74].
6. The ECM in Lung Remodeling and Disease
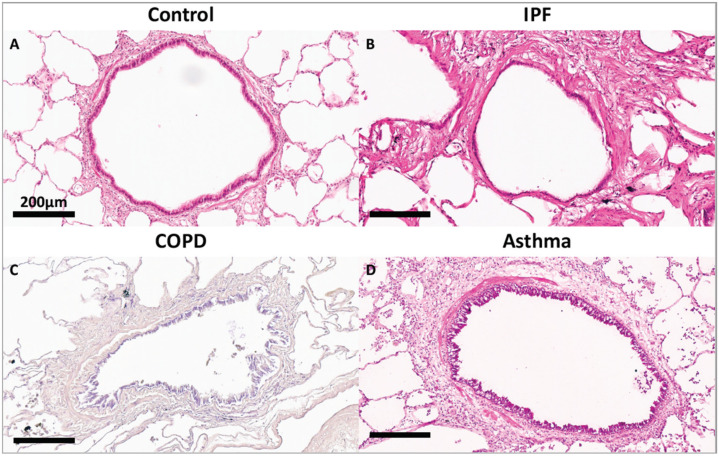
To maintain homeostasis, the lung ECM undergoes remodelling, a process whereby old or damaged ECM proteins undergo a series of proteolytic events and are replaced by newly synthesized proteins. Lung ECM remodeling is modulated by the synthesis rate of new ECM molecules and the surfeit of proteases released by specialized mesenchymal cells, predominantly fibroblasts and, to a lesser extent, airway smooth muscle, immune cells and epithelial cells of the lungs [4,75]. The remodelling process is directed by the interaction between the different cell types, cytokines, growth factors and enzymes within the lung ECM. The levels and ratios of ECM proteins during the repair response must be maintained to avoid disrupting the lung ECM characteristics, including tensile strength and elastic recoil. Upon lung injury, activation of the coagulation process initiates damage control and a provisional matrix (fibronectin), which is then followed by the development of an acute inflammatory response that recruits polymorphonuclear leukocytes (PMNs) and macrophages to protect against pathogens and remove debris from dead and dying cells. These important early steps prepare the site for activation of local fibroblasts, migrating myofibroblasts and recruited fibrocytes that remodel collagenous and noncollagenous tissues into scar tissue. Following a single injury, inflammatory cells usually leave the site before the resident fibroblasts and migrating myofibroblasts, and fibrocytes are activated to drive the tissue repair process. Over time, the provisional ECM is degraded via cell-mediated or proteolytic pathways and replaced with a restorative ECM consisting mainly of collagen I and collagen III, before these cells then undergo apoptosis [76]. In sharp contrast, repetitive injury is associated with the persistence of inflammatory immune cell infiltration during the remodelling of the ECM, which increases the potential for the development of an abnormal repair process creating further injury with abnormal scar formation, remodelling or permanent destruction of damaged tissue. Repeated lung injury and repair can trigger a vicious cycle of aberrant ECM protein deposition, accompanied by elevated ECM stiffness, further resulting in changes in cell phenotype and function, and this has a lasting effect on tissue function and ultimately disease progression [77]. Although the processes governing the resolution of injury repair are regulated by several pathways, in chronic fibrotic lung diseases the processes are compromised, thus resulting in impaired fibroblast proliferation, apoptosis and aberrant ECM remodelling [3,76]. Disruption of this balance changes the dynamics of the lung ECM with characteristics such as stiffness and elastance, as seen in asthma, chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) [78,79,80]. Compared to the normal lung ECM (0.5–5 kPa), studies have shown that a stiff ECM (15–100 kPA) [81,82,83,84] can exacerbate TGF-β activation leading to enhanced pro-fibrotic signalling. Disruption of homeostasis within the lung ECM can also lead to the dysregulated synthesis of ECM proteins, such as fibrillar collagen I, by activated fibroblasts, leading to tissue fibrosis regardless of the inflammatory response [85]. The interplay between several diverse factors (cell types, genes, cytokines, growth factors, enzymes and epigenetics) is essential for the normal repair and remodeling processes within the lung [86]. Studies have shown that deviations in the posttranslational modification of ECM proteins, including enzymatic cross-linking, glycation and oxidation, impact the tensile strength, biomechanics and cell–ECM interactions and are contributors to lung diseases [87,88]. As shown in Figure 3, compared to a terminal bronchiole (last generation of conducting airways within the lung) from a donor control, there is extensive fibrosis surrounding the terminal bronchiole airway walls in the lungs from patients with IPF, COPD and asthma. Recent studies have provided evidence for the epigenetic role of miRNAs in the pathogenesis of lung diseases. For this review, only the regulatory effects of miRNA on ECM expression, degradation and topography in lung diseases, specifically, asthma, COPD and IPF, will be considered.
Figure 3.
Representative image of a terminal bronchiole stained with hematoxylin and eosin from (A) donor control lung, (B) a lung from an end-stage idiopathic pulmonary fibrosis patient, (C) a lung from a patient with mild chronic obstructive pulmonary disease. (D) Terminal bronchiole stained with Masson’s trichome from a lung from a patient with asthma.
6.1. Asthma
Asthma is characterized by chronic airway inflammation, bronchial hyperresponsiveness and airway remodeling. The deposition of ECM is a prominent feature of lung remodelling in patients with asthma irrespective of age, disease severity or steroid use [89,90,91]. ECM remodelling in asthma involves the airway epithelial basement membrane, laminar propria, bronchial and pulmonary vasculature [89,92,93]. The compositions of the airway smooth muscle (ASM) ECM, epithelial basement membrane and interstitial ECM have also been shown to be altered in asthma, with a predominant deposition of fibronectin, collagen I and III [94]. Most recently, the topography of fibrillar collagen fibers has also been assessed and shown to be more disorganized and fragmented in the airway laminar propria and pulmonary vasculature [89,95]. Several miRNAs have been reported to be differentially expressed in asthma and have primarily focused on the regulation of inflammation [96,97,98]. However, recent emerging studies using various samples including serum, ASM, bronchial fibroblasts and airway epithelial cells have shown miRNAs that are differentially expressed in asthma pathology and contribute to ECM remodelling.
In addition to its role in bronchoconstriction, ASM plays an important interactive role with structural cells and inflammatory mediators contributing to inflammation and ECM remodeling in asthma [99]. In ASM cells from asthmatic patients, a number of miRNAs are downregulated compared to control subjects, leading to elevated ECM expression. The expression of miR-19 is decreased in ASM cells from asthmatic patients and induces elevated expression of collagen I, fibronectin and arginine methyltransferase activity through the ERK1/MAPK signalling pathway [100]. miR-204-5p has also been shown to be down-regulated in ASM cells from asthmatic patients and promotes the expressions of fibronectin and collagen III via the Six1 gene (a TGF-β1 inducible gene) [101]. Cheng and colleagues reported that miR-143-3p is significantly reduced in ASM cells of asthmatic patients compared to the controls [102]. They showed that miR-143-3p directly targets the nuclear factor of activated T-cells 1 (NFATc1) to promote collagen 1 and fibronectin expressions, leading to elevated ASM cell proliferation and up-regulation of CDK4 and cyclin D1 expressions. Lastly, Li and colleagues showed that miR-378 is elevated in ASM cells from asthmatic patients and, via MAPK and calcium signalling, can up-regulate collagen I and fibronectin expression [103].
With regards to inflammation and ASM, Liu and colleagues investigated the role of miR-145 in ASM cells treated with IL-1β, TNF-α and IFN-γ to mimic airway inflammation, and they found that miR-145 was significantly elevated and led to increased collagen I and myosin heavy chain expression through negative regulation of the transcription factor kruppel-like factor 4 (KLF4) protein and downstream activation of MMP-2 and MMP-9 [104]. Kuhn and colleagues showed that inhibition of miR-25 in IL-1β, TNF-α and IFN-γ-stimulated ASM cells, had a greater than two-fold down regulatory effect on collagen XI expression, and to a lesser extent the expressions of collagen (V and XV), fibronectin, MMP-9 and integrin (αm and β2), by stimulating KLF4 expression [105]. Growth factors such as TGF-β1 have also been shown to modulate miRNA expression in ASM. TGF-β1 has been shown to elevate miR-181a expression in ASM, leading to the overexpression of collagen I and fibronectin, via the Akt signalling pathway [106]. Lastly, miR-142 has been shown to be overexpressed in ASM cells derived from an asthma rat model and inhibits TGF-β expression via epidermal growth factor receptor (EGFR) signalling [107], leading to decreased collagen I and collagen III expressions. In support of this finding, the expression level of miR-142-3p was shown to be up-regulated in the sputum of asthmatic patients and correlates with sputum neutrophil cell counts [108].
In vivo models of allergic inflammation and asthma have shown that miR-485-3p is up-regulated in ASM cells derived from both pediatric and adult murine models of asthma [109]. miR-485-3p was shown to be involved in mediating airway remodelling by decreasing sprout-related EVH1 domain-containing protein (spred)-2 expression to promote growth factor-mediated Ras/ERK activation [109]. The overexpression of MiR-21-5p in an immunoglobulin E (IgE)-induced mouse model of allergic inflammation was found to increase the expressions of collagen I and fibronectin through inhibition of two signalling pathways: PTEN and PI3K/mTOR [110]. Furthermore, a recent study by Pan and colleagues using an ovalbumin-induced chronic murine model showed that the PI3K/Akt signalling pathway is regulated by increased miR-221 expression in ASM cells and leads to increased expressions of collagen I and collagen III [111].
Regarding other cell types within the lung, Zhang and colleagues showed that miR-221 expression is up-regulated in bronchial epithelial cells from asthmatic patients, and that overexpression of miR-221 significantly down-regulates sirtuin1 (SIRT1) expression in BEAS2B cells [112], which is known to regulate collagen I alpha-2 expression [113]. Yu and colleagues showed that TGF-β1-treated human bronchial fibroblasts have elevated miR-21, which leads to the up-regulation of collagen I alpha-1, fibronectin-1 and alpha-smooth muscle actin expressions, via negatively regulating SMAD7, leading to activated TGF-β1-SMAD signalling [114]. Lastly, treatment of the A549, BEAS-2B and H1299 cell lines with a miR-3162-3p mimic was shown to decrease β-catenin activation, leading to attenuation of TGF-β1-induced collagen I alpha-1 and fibronectin expressions [115]. In conclusion, there is strong evidence that alterations of miRNA signalling in asthma may lead to reorganization of the ECM particularly surrounding ASM. Further work is required to understand the contribution of miRNAs to ECM deposition in asthma, particularly in fibroblasts, epithelial and endothelial cells.
6.2. Chronic Obstructive Pulmonary Disease
COPD is characterized by chronic lung inflammation and irreversible airflow limitation. COPD is caused by the chronic inhalation of cigarette smoke or other harmful particles, which cause pulmonary injury, leading to chronic airway inflammation and tissue remodelling. The major histopathological changes observed within the lung include chronic bronchitis, small airway disease and emphysema. Recent studies have shown that small airway disease precedes emphysematous tissue destruction, suggesting a temporal pattern of ECM deposition and destruction in the disease progression [116]. Using a large cross-sectional study, Hogg and colleagues showed that the progression of COPD from GOLD (Global Initiative for Obstructive Lung Disease) stage 0 to GOLD stage 4 is strongly associated with thickening of the airway wall and each of its compartments by remodelling of the ECM, which affects airway wall elasticity, thickness and resistance [117,118]. In addition, loss of elastic recoil from the destruction of the ECM within the parenchyma (emphysematous tissue destruction) is a well-described feature of COPD [119], with several studies showing decreased protein expression and volume fraction of elastin in both the conducting airways and the parenchyma of patients with mild, moderate or severe COPD [120,121].
With regards to miRNAs, several studies have also shown changes in the epigenetics of the COPD lung and implicated miRNAs as an important player in COPD pathology [122,123,124,125]. When looking at ECM remodelling in COPD lung tissue, miR-15b has been shown to be significantly increased in lung tissue from patients with mild, moderate or very severe COPD, with the highest expression in very severe cases [125]. The same study also showed that in the epithelial BEAS2B cell line, TGF-β treatment leads to increased expression of miR-15b, which negatively regulates decorin expression via SMAD7 and SMURF2. This finding correlates with the results obtained by Noordhoek and colleagues, who showed decreased expression of decorin in fibroblasts obtained from the lung tissue of patients with mild or severe COPD [126]. Dang and colleagues (2019) have also reported a decreased expression of miR-145-5p in the lung tissue of smokers with mild to moderate COPD compared to non-smokers [127]. Furthermore, the authors showed that human bronchial epithelial cells (HBECs) when exposed to cigarette smoke extract (CSE), down-regulate miR-145-5p expression, leading to increased Kruppel-like factor subfamily-5 (KLF5) expression level. Abe and colleagues (2016) have previously reported that KLF5 is up-regulated in fibroblasts and endothelial cells from ex-smokers with COPD, which is correlated with the severity of airflow limitation in patients with COPD [128]. The authors also showed that silencing of KLF5 suppressed oxidative/nitrosative stress-related responses within lung fibroblasts, leading to decreased collagen, MMP-2 and MMP-9 expression. Lastly, Du and colleagues (2017) found decreased expression levels of the miR-181c in parenchymal tissue of patients with very severe COPD and CSE-exposed HBECs [129]. The miR-181c was shown to negatively regulate the transcription factor CCN1 (Cellular Communication Network Factor 1) to induce TGF-β-mediated fibronectin and collagen up-regulation via SMAD protein phosphorylation [129,130].
In terms of fibroblasts, which are an important producer of ECM within the lamina propria, Ong and colleagues showed that parenchymal lung fibroblasts of COPD patients overexpress miR-455-3p and miR-21-3p, compared to controls, when stimulated by TGF-β, resulting in the induction of fibronectin and collagen I through the TGF-β and wnt (wingless and Int-1) signalling pathways [131].
In terms of in vivo models, Chi et al. (2019) and Tang et al. (2019) in a rat model of COPD showed decreased expression of miR-29 [132,133]. Using rat airway epithelial cells from the model, they found that the decrease in miR-29 expression was found to correlate with the increased expression level of collagen III alpha-1 and collagen IV alpha 1 [134]. In summary, a number of studies have demonstrated miRNAs regulating ECM synthesis, primarily collagen and fibronectin, by epithelial cells and fibroblasts in patients with COPD in mild to very severe disease.
6.3. Idiopathic Pulmonary Fibrosis
IPF is the most common and progressive type of idiopathic interstitial pneumonia that is unresponsive to treatment, leading to a median survival of 3–5 years [135,136,137,138]. The pathology of IPF is characterized by heterogeneous interstitial fibrosis, honeycomb cysts and fibrotic foci associated with excessive deposition of ECM proteins resulting in aberrant matrix metalloproteinases, connective tissues, morphogens and impaired signalling of growth factors [139,140,141,142,143]. The current hypothesis for tissue destruction in IPF involves continuous damage and senescence of the alveolar epithelium, leading to the destruction of the basement membrane and activation of myofibroblasts [144]. As the disease progresses, the composition of the ECM has been shown to vary, with an accumulation of versican in the onset of IPF, whereas collagen I and collagen III accumulation is observed in both the early and late stages of IPF [145]. The severity of fibrosis in IPF has also been shown to correlate with the number of elastic fibers, with a higher elastic fiber score being related to worse disease outcomes [146]. The diseased ECM is a critical linchpin in IPF, and it serves as a causal link for alteration in cell gene expression patterns at the translational level [3]. Several studies have implicated the role of miRNAs in IPF pathology [139,147,148].
Recently, Guiot and colleagues (2020) showed that the expression levels of miR-142-3p are elevated in sputum and plasma exosomes of IPF patients and positively correlate with sputum macrophage number [149]. This group further showed that miR-142-3p represses TGFβ-R1 in both A549 and a fetal lung fibroblast cell line (MRC5), resulting in decreased collagen I alpha-1 and collagen III alpha-1 expression. In bronchoalveolar lavage cells of IPF patients, miR-29a and miR-185 are down-regulated, leading to activation of TGF-β and PTEN signalling and elevated collagen I alpha-1 expression [150]. Within the IPF lung, the expression level of miR-200 is decreased and was shown to correlate with collagen I alpha-1 and fibronectin expression. However, Yang and colleagues showed that the miR-200 expression level is higher in airway epithelial cells compared to lung fibroblasts in mice with experimental pulmonary fibrosis [151]. Specifically, in the alveoli epithelium, IPF patients compared to donor controls have decreased expression of let-7d. Pandit V. et al (2010) showed reduced expression of let-7d, which led to overexpression of its direct target HMGA2 (high-motility group AT-hook-2), resulting in the over-expression of mesenchymal markers, decreased expression of epithelial markers and increased collagen deposition in alveolar epithelial cells [152].
In vivo models using bleomycin are commonly used to understand inflammation and ECM remodeling in IPF. In the lung of bleomycin-treated mice, miR-26 and miR-326 are down-regulated, leading to increased collagen synthesis via TGF-β1/Smad signalling [153,154]. Down-regulation of miR-15a expression in bleomycin-treated mice has also been shown to increase the expressions of collagen I alpha-1, collagen II alpha-1, fibronectin 1, alpha-SMA and connective tissue growth factor (CTGF) via the yes-associated protein (YAP)1/twist/TEAD dependent pathway [155]. In Lung fibroblasts derived from bleomycin-induced mice compared to controls, there is down-regulation of miR-29 expression [156]. Xiao and colleagues (2012) further showed that miR-29 is negatively regulated by the TGF-β/Smad pathway, altering ECM remodelling by promoting the expression of fibronectin, collagen I and collagen III [156]. In agreement with this finding, Cushing and colleagues (2011) showed in the fetal lung fibroblast cell line (IMR-90) that knock-down of endogenous miR-29 leads to upregulated expression of collagen types (I, III, IV, V, XV) and laminin (alpha-1,3, beta-1) via TGF-β1 and possibly NF-kB signalling pathways [134]. Lung myofibroblasts isolated from bleomycin-treated mice have elevated miR-21 levels, which correlated with fibronectin lung expression. miR-21 was shown to stimulate Smad7 and PTEN/ERK signalling, and treatment of myofibroblast cells with miR-21 antisense probes prevented the enhanced deposition and synthesis of collagen and fibronectin [157]. In bleomycin-treated pleural mesothelial cells (PMC), miR-18a-5p expression is decreased, which is a direct target of TGF-β receptor II [158]. Decreased miR-18a-5p led to over-expression of alpha-SMA, collagen I and vimentin while decreasing E-cadherin and cytokeratin 8 expressions [158]. In summary, a number of studies have demonstrated miRNAs regulating ECM synthesis, primarily collagen, through TGF-β signalling fibroblasts in patients with IPF.
7. Potential for miRNA Therapeutics in Lung Disease
Compared to disease conditions with a single genetic link, we now understand that complex lung diseases such as asthma, COPD and IPF involve multiple genes and pathways. As miRNAs can target hundreds and potentially thousands of genes, miRNAs may prove therapeutically advantageous to regulate entire biological pathways that are pathogenically altered in complex diseases. It should be noted that the pleiotropic nature of miRNAs does warrant concern for off-target effects; however, steroids commonly used in multiple inflammatory diseases are effective due to their pleiotropic effects and provide further support for miRNAs as future therapeutics.
As miRNAs are ubiquitously expressed throughout the body, they can readily be measured from peripheral blood, tissue biopsies, saliva, urine, cerebrospinal fluid (CSF) and other biological samples [53,159,160]. Due to this accessibility, a growing number of studies have shown that one or subsets of miRNAs can be used as biomarkers to indicate disease pathology, staging and progression with high sensitivity and specificity [161,162,163,164,165,166,167,168,169,170,171]. To date, several miRNA biomarker clinical studies have been completed in the clinicaltrials.gov database for a range of conditions including breast cancer, coronary heart disease, diabetes, epilepsy, influenza and stroke.
In terms of use as therapeutic interventions, the first small-interfering RNA (siRNA) drug trial in humans was conducted in 2004. Fifteen years later, the drug Patisiran was granted FDA approval in 2018 for treatment of polyneuropathy caused by hereditary transthyretin-mediated (hATTR) amyloidosis. While there are no current FDA-approved miRNA therapeutics for medical intervention, several miRNA drug candidates have been entered into the clinicaltrials.gov database as phase 1 or 2 clinical trials for a range of health conditions including cancer, hepatitis C, heart abnormalities, kidney disease and pathologic fibrosis. Relevant to this review, there is a current phase 2 clinical trial of Remlarsen (mir-29) from Mirage Therapeutics, which is focused on decreasing the expression of collagen and other proteins involved in scar formation over a 1 year follow up [172]. This study will be of great interest to understand the potential of miRNAs as therapeutics to modify the pathogenic remodelling of the ECM in the skin and other organs such as the lung.
In terms of administration, current miRNA trials are focused on injection or intravenous administration to mimic (over-express transcript) or silence (repress transcript) mRNA. For cancer treatments, injection into the tumour site has been shown to enhance target specificity, efficacy and minimise side effects [173,174]. Another recent application of miRNAs is focused on improving drug resistance. A number of studies have shown that manipulating the expression of specific miRNAs can alter drug sensitivity. For example, many of the standard therapies for breast cancer, such as doxorubicin, cisplatin, and Taxol, are all associated with deregulated miRNAs, which may modulate resistance to the drug [170]. The altered expression of miRNAs has also been associated with drug resistance in the treatment of conditions such as epilepsy [163], multidrug-resistant (MDR) tuberculosis [175] and insulin sensitivity [176,177]. Emerging evidence suggests that the ATP binding cassette (ABC) transporter family of proteins that is involved in drug resistance is regulated by miRNAs, and this provides a potential mechanism for the use of miRNAs in drug resistance [178].
8. Conclusions
The initiation and progression of chronic lung diseases are modulated by complex environmental and epigenetic factors. In the initiation and progression of these diseases, there is an intricate interaction between cells and the ECM through various molecules and signalling pathways, amongst which epigenetic regulation by miRNAs has emerged as an important player. We have outlined in this review the current knowledge on miRNAs regulating ECM synthesis, degradation and topography by cells and their dysregulation in asthma, COPD and IPF. The accumulating evidence of differences in the expression profiles of miRNAs in lung diseases, and their correlation with lung function measures and ECM gene targets, highlights the potential of miRNAs as biomarkers for diagnosis, staging and future therapeutic drugs in chronic lung disease.
Acknowledgments
We acknowledge Dragos Vasilescu from the Centre for Heart Lung Innovation for providing the haemotoxolin and eosin images of a small airway from a patient with idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease.
Author Contributions
Conceptualization: K.U. and T.-L.H. Writing and original draft preparation: K.U., A.H. and T.-L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by a Canadian Institutes of Health Research (CIHR) operating grant MOP 130504. K.U. was supported by a Providence Airway Center and Michael Smith Foundation Health Research (MSFHR) PhD Fellowship. T.-L.H. is supported by a Tier I Canada Research Chair in Lung Pathobiology and Therapeutics.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rozario T., DeSimone D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattazzo F., Urciuolo A., Bonaldo P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker M.W., Rossi D., Peterson M., Smith K., Sikström K., White E., Connett J.E., Henke C.A., Larsson O., Bitterman P. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J. Clin. Investig. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y., Horowitz J.C., Naba A., Ambalavanan N., Atabai K., Balestrini J., Bitterman P.B., Corley R.A., Ding B.-S., Engler A.J., et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018;73:77–104. doi: 10.1016/j.matbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hynes R.O., Naba A. Overview of the Matrisome--An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2011;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halfter W., Oertle P., Monnier C., Camenzind L., Reyes-Lua M., Hu H., Candiello J., Labilloy A., Balasubramani M., Henrich P.B., et al. New concepts in basement membrane biology. FEBS J. 2015;282:4466–4479. doi: 10.1111/febs.13495. [DOI] [PubMed] [Google Scholar]
- 7.LeBleu V.S., Macdonald B., Kalluri R. Structure and Function of Basement Membranes. Exp. Biol. Med. 2007;232:1121–1129. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G., Striker L.J., Hudson L.D., Striker G.E. Extracellular matrix in normal and fibrotic human lungs. Am. Rev. Respir. Dis. 1985;131:281–289. doi: 10.1164/arrd.1985.131.2.281. [DOI] [PubMed] [Google Scholar]
- 9.Faffe D.S., Zin W.A. Lung Parenchymal Mechanics in Health and Disease. Physiol. Rev. 2009;89:759–775. doi: 10.1152/physrev.00019.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stolz D., Leeming D.J., Kristensen J.H.E., Karsdal M.A., Boersma W., Louis R., Milenkovic B., Kostikas K., Blasi F., Aerts J., et al. Systemic Biomarkers of Collagen and Elastin Turnover Are Associated with Clinically Relevant Outcomes in COPD. Chest. 2017;151:47–59. doi: 10.1016/j.chest.2016.08.1440. [DOI] [PubMed] [Google Scholar]
- 11.Laurent G.J. Rates of collagen synthesis in lung, skin and muscle obtained in vivo by a simplified method using [3H]proline. Biochem. J. 1982;206:535–544. doi: 10.1042/bj2060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mays P.K., McAnulty R.J., Laurent G.J. Age-related Changes in Lung Collagen Metabolism: A Role for Degradation in Regulating Lung Collagen Production. Am. Rev. Respir. Dis. 1989;140:410–416. doi: 10.1164/ajrccm/140.2.410. [DOI] [PubMed] [Google Scholar]
- 13.Hung C., Linn G., Chow Y.-H., Kobayashi A., Mittelsteadt K., Altemeier W., Gharib S.A., Schnapp L.M., Duffield J.S. Role of Lung Pericytes and Resident Fibroblasts in the Pathogenesis of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2013;188:820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan T.E., Cowper S., Wu S.-P., Bockenstedt L.K., Bucala R. Circulating fibrocytes: Collagen-secreting cells of the peripheral blood. Int. J. Biochem. Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. Pt 24J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang M.Y., Chan C.K., Braun K.R., Green P.S., O’Brien K.D., Chait A., Day A.J., Wight T.N. Monocyte-to-macrophage differentiation: Synthesis and secretion of a complex extracellular matrix. J. Biol. Chem. 2012;287:14122–14135. doi: 10.1074/jbc.M111.324988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiore A.P.Z.P., Ribeiro P.D.F., Bruni-Cardoso A. Sleeping Beauty and the Microenvironment Enchantment: Microenvironmental Regulation of the Proliferation-Quiescence Decision in Normal Tissues and in Cancer Development. Front. Cell Dev. Biol. 2018;6 doi: 10.3389/fcell.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh E.J., Remillard M.Y., Legesse-Miller A., Johnson E.L., Lemons J.M.S., Chapman T.R., Forman J.J., Kojima M., Silberman E.S., Coller H.A. A microRNA network regulates proliferative timing and extracellular matrix synthesis during cellular quiescence in fibroblasts. Genome Biol. 2012;13:R121. doi: 10.1186/gb-2012-13-12-r121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawston T.E., Young D.A. Proteinases involved in matrix turnover during cartilage and bone breakdown. Cell Tissue Res. 2009;339:221–235. doi: 10.1007/s00441-009-0887-6. [DOI] [PubMed] [Google Scholar]
- 20.Sternlicht M.D., Werb Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alaseem A., Alhazzani K., Dondapati P., Alobid S., Bishayee A., Rathinavelu A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2019;56:100–115. doi: 10.1016/j.semcancer.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Loffek S., Schilling O., Franzke C.-W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- 23.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohan R., Chintala S.K., Jung J.C., Villar W.V.L., McCabe F., Russo L.A., Lee Y., McCarthy B.E., Wollenberg K.R., Jester J.V., et al. Matrix Metalloproteinase Gelatinase B (MMP-9) Coordinates and Effects Epithelial Regeneration. J. Biol. Chem. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- 25.Suhr F. Extracellular matrix, proteases and physical exercise. Dtsch. Z. Sportmed. 2019;2019:97–104. doi: 10.5960/dzsm.2019.367. [DOI] [Google Scholar]
- 26.Song R.-H., Tortorella M.D., Malfait A.-M., Alston J.T., Yang Z., Arner E.C., Griggs D.W. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 27.Green K.A., Lund L.R. ECM degrading proteases and tissue remodelling in the mammary gland. BioEssays. 2005;27:894–903. doi: 10.1002/bies.20281. [DOI] [PubMed] [Google Scholar]
- 28.Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E., Upadhyay P., Uyeminami D.L., Pommier A., Küttner V., et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou W.-S., Li Z., Gordon R.E., Chan K., Klein M.J., Levy R., Keysser M., Keyszer G., Brömme D. Cathepsin K Is a Critical Protease in Synovial Fibroblast-Mediated Collagen Degradation. Am. J. Pathol. 2001;159:2167–2177. doi: 10.1016/S0002-9440(10)63068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashiwagi M., Tortorella M., Nagase H., Brew K. TIMP-3 Is a Potent Inhibitor of Aggrecanase 1 (ADAM-TS4) and Aggrecanase 2 (ADAM-TS5) J. Biol. Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 31.Mutch N.J., Thomas L., Moore N.R., Lisiak K.M., Booth N.A. TAFIa, PAI-1 and α2-antiplasmin: Complementary roles in regulating lysis of thrombi and plasma clots. J. Thromb. Haemost. 2007;5:812–817. doi: 10.1111/j.1538-7836.2007.02430.x. [DOI] [PubMed] [Google Scholar]
- 32.Lopez J.I., Mouw J.K., Weaver V.M. Biomechanical regulation of cell orientation and fate. Oncogene. 2008;27:6981–6993. doi: 10.1038/onc.2008.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engler A., Humbert P., Wehrle-Haller B., Weaver V.M. Multiscale Modeling of Form and Function. Science. 2009;324:208–212. doi: 10.1126/science.1170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egeblad M., Rasch M.G., Weaver V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiller H.B., Fernandez I.E., Burgstaller G., Schaab C., Scheltema R., Schwarzmayr T., Strom T.M., Eickelberg O., Mann M. Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol. Syst. Biol. 2015;11:819. doi: 10.15252/msb.20156123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decaris M.L., Gatmaitan M., FlorCruz S., Luo F., Blisnick T., Holmes W.E., Hellerstein M.K., Turner S.M., Emson C.L., Subota I., et al. Proteomic Analysis of Altered Extracellular Matrix Turnover in Bleomycin-induced Pulmonary Fibrosis. Mol. Cell. Proteom. 2014;13:1741–1752. doi: 10.1074/mcp.M113.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levental K., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J., Fong S.F., Csiszar K., Giaccia A., Weninger W., et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tjin G., White E.S., Faiz A., Sicard D., Tschumperlin D.J., Mahar A., Kable E.P.W., Burgess J.K. Lysyl oxidases regulate fibrillar collagen remodelling in idiopathic pulmonary fibrosis. Dis. Model. Mech. 2017;10:1301–1312. doi: 10.1242/dmm.030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiavinato A., Keene D.R., Wohl A.P., Corallo D., Colombatti A., Wagener R., Paulsson M., Bonaldo P., Sengle G. Targeting of EMILIN-1 and EMILIN-2 to Fibrillin Microfibrils Facilitates their Incorporation into the Extracellular Matrix. J. Investig. Dermatol. 2016;136:1150–1160. doi: 10.1016/j.jid.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Schiavinato A., Keene D.R., Imhof T., Doliana R., Sasaki T., Sengle G. Fibulin-4 deposition requires EMILIN-1 in the extracellular matrix of osteoblasts. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-05835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munjal C., Opoka A.M., Osinska H., James J.F., Bressan G.M., Hinton R.B. TGF-β mediates early angiogenesis and latent fibrosis in an Emilin1-deficient mouse model of aortic valve disease. Dis. Model. Mech. 2014;7:987–996. doi: 10.1242/dmm.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danussi C., Petrucco A., Wassermann B., Pivetta E., Modica T.M.E., Belluz L.D.B., Colombatti A., Spessotto P. EMILIN1-α4/α9 integrin interaction inhibits dermal fibroblast and keratinocyte proliferation. J. Cell Biol. 2011;195:131–145. doi: 10.1083/jcb.201008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spessotto P., Cervi M., Mucignat M.T., Mungiguerra G., Sartoretto I., Doliana R., Colombatti A. β1 Integrin-dependent Cell Adhesion to EMILIN-1 Is Mediated by the gC1q Domain. J. Biol. Chem. 2003;278:6160–6167. doi: 10.1074/jbc.M208322200. [DOI] [PubMed] [Google Scholar]
- 44.Spessotto P., Bulla R., Danussi C., Radillo O., Cervi M., Monami G., Bossi F., Tedesco F., Doliana R., Colombatti A. EMILIN1 represents a major stromal element determining human trophoblast invasion of the uterine wall. J. Cell Sci. 2006;119:4574–4584. doi: 10.1242/jcs.03232. [DOI] [PubMed] [Google Scholar]
- 45.Friedland J.C., Lee M.H., Boettiger D. Mechanically activated integrin switch controls α5β 1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 46.Friedman R., Farh K.K.-H., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez A., Griffiths-Jones S., Ashurst J.L., Bradley A. Identification of Mammalian microRNA Host Genes and Transcription Units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffiths-Jones S., Grocock R.J., Van Dongen S., Bateman A., Enright A. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 50.Sunkar R., Zhu J.K. Novel and stress regulated microRNAs and other small RNAs from Arabidopsis w inside box sign. Plant Cell. 2004;16:2001–2019. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chim S., Shing T.K.F., Hung E.C.W., Leung T.-Y., Lau T.K., Chiu R.W.K., Lo Y.M.D. Detection and Characterization of Placental MicroRNAs in Maternal Plasma. Clin. Chem. 2008;54:482–490. doi: 10.1373/clinchem.2007.097972. [DOI] [PubMed] [Google Scholar]
- 53.Weber J.A., Baxter D.H., Zhang S., Huang D.Y., Huang K.-H., Lee M.-J., Galas D.J., Wang K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guduric-Fuchs J., O’Connor A., Camp B., O’Neill C.L., Medina R.J., Simpson D.A. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genom. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li C.C.Y., Eaton S., Young P.E., Lee M., Shuttleworth R., Humphreys D., Grau G., Combes V., Bebawy M., Gong J., et al. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol. 2013;10:1333–1344. doi: 10.4161/rna.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makarova J.A., Shkurnikov M.U., Wicklein D., Lange T., Samatov T.R., Turchinovich A.A., Tonevitsky A.G. Intracellular and extracellular microRNA: An update on localization and biological role. Prog. Histochem. Cytochem. 2016;51:33–49. doi: 10.1016/j.proghi.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Squadrito M.L., Baer C., Burdet F., Maderna C., Gilfillan G., Lyle R., Ibberson M., De Palma M. Endogenous RNAs Modulate MicroRNA Sorting to Exosomes and Transfer to Acceptor Cells. Cell Rep. 2014;8:1432–1446. doi: 10.1016/j.celrep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 58.Curtale G., Rubino M., Locati M. MicroRNAs as Molecular Switches in Macrophage Activation. Front. Immunol. 2019;10:799. doi: 10.3389/fimmu.2019.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turchinovich A., Weiz L., Burwinkel B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem. Sci. 2012;37:460–465. doi: 10.1016/j.tibs.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Vickers K.C., Palmisano B.T., Shoucri B.M., Shamburek R.D., Remaley A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zernecke A., Bidzhekov K., Noels H., Shagdarsuren E., Gan L., Denecke B., Hristov M., Köppel T., Jahantigh M.N., Lutgens E., et al. Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12-Dependent Vascular Protection. Sci. Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 62.Arroyo J.D., Chevillet J., Kroh E.M., Ruf I.K., Pritchard C.C., Gibson D.F., Mitchell P., Bennett C.F., Pogosova-Agadjanyan E.L., Stirewalt D.L., et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turchinovich A., Weiz L., Langheinz A., Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X., Xu J., Wang J., Gortner L., Zhang S., Wei X., Song J., Zhang Y., Li Q., Feng Z. Reduction of MicroRNA-206 Contributes to the Development of Bronchopulmonary Dysplasia through Up-Regulation of Fibronectin 1. PLoS ONE. 2013;8:e74750. doi: 10.1371/journal.pone.0074750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Y., Li Z., Wang Y., Li L., Wang D., Zhang W., Liu L., Jiang H., Yang J., Cheng J. Overexpression of miR-29b reduces collagen biosynthesis by inhibiting heat shock protein 47 during skin wound healing. Transl. Res. 2016;178:38–53.e6. doi: 10.1016/j.trsl.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Pan J., Jiang F., Zhou J., Wu D., Sheng Z., Li M. HSP90: A Novel Target Gene of miRNA-628-3p in A549 Cells. BioMed Res. Int. 2018;2018:1–10. doi: 10.1155/2018/4149707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X., An D., Liu X., Wang X., Li B. MicroRNA-27a downregulates the expression of Hsp90 and enhances the radiosensitivity in esophageal squamous cell carcinoma. OncoTargets Ther. 2019;12:5967–5977. doi: 10.2147/OTT.S197456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solopov P., Biancatelli R.M.L.C., Marinova M., Dimitropoulou C., Catravas J.D. The HSP90 Inhibitor, AUY-922, Ameliorates the Development of Nitrogen Mustard-Induced Pulmonary Fibrosis and Lung Dysfunction in Mice. Int. J. Mol. Sci. 2020;21:4740. doi: 10.3390/ijms21134740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ibrahim S.A., Yip G.W., Stock C., Pan J.-W., Neubauer C., Poeter M., Pupjalis D., Koo C.Y., Kelsch R., Schüle R., et al. Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase- and E-cadherin-dependent mechanism. Int. J. Cancer. 2012;131:E884–E896. doi: 10.1002/ijc.27629. [DOI] [PubMed] [Google Scholar]
- 70.Liu P., Wilson M.J. miR-520c and miR-373 upregulate MMP9 expression by targeting mTOR and SIRT1, and activate the Ras/Raf/MEK/Erk signaling pathway and NF-κB factor in human fibrosarcoma cells. J. Cell. Physiol. 2012;227:867–876. doi: 10.1002/jcp.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia H., Qi Y., Ng S.S., Chen X., Li D., Chen S., Ge R., Jiang S., Li G., Chen Y., et al. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009;1269:158–165. doi: 10.1016/j.brainres.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 72.Gabriely G., Wurdinger T., Kesari S., Esau C.C., Burchard J., Linsley P.S., Krichevsky A.M. MicroRNA 21 Promotes Glioma Invasion by Targeting Matrix Metalloproteinase Regulators. Mol. Cell. Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen H., Yu X., Yang F., Zhang Z., Shen J., Sun J., Choksi S., Jitkaew S., Shu Y. Reprogramming of Normal Fibroblasts into Cancer-Associated Fibroblasts by miRNAs-Mediated CCL2/VEGFA Signaling. PLoS Genet. 2016;12:e1006244. doi: 10.1371/journal.pgen.1006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le L.T.-N., Cazares O., Mouw J.K., Chatterjee S., Macias H., Moran A., Ramos J., Keely P.J., Weaver V.M., Hinck L. Loss of miR-203 regulates cell shape and matrix adhesion through ROBO1/Rac/FAK in response to stiffness. J. Cell Biol. 2016;212:707–719. doi: 10.1083/jcb.201507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandsma C., Berge M.V.D., Hackett T., Brusselle G., Timens W. Recent advances in chronic obstructive pulmonary disease pathogenesis: From disease mechanisms to precision medicine. J. Pathol. 2020;250:624–635. doi: 10.1002/path.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Friedman S.L., Sheppard D., Duffield J.S., Violette S. Therapy for Fibrotic Diseases: Nearing the Starting Line. Sci. Transl. Med. 2013;5:167sr1. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 77.Burgstaller G., Oehrle B., Gerckens M., White E.S., Schiller H.B., Eickelberg O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017;50:1601805. doi: 10.1183/13993003.01805-2016. [DOI] [PubMed] [Google Scholar]
- 78.Polio S.R., Stasiak S.E., Jamieson R.R., Balestrini J.L., Krishnan R., Parameswaran H. Extracellular matrix stiffness regulates human airway smooth muscle contraction by altering the cell-cell coupling. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-45716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKleroy W., Lee T.-H., Atabai K. Always cleave up your mess: Targeting collagen degradation to treat tissue fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2013;304:L709–L721. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White E. Lung Extracellular Matrix and Fibroblast Function. Ann. Am. Thorac. Soc. 2015;12:S30–S33. doi: 10.1513/AnnalsATS.201406-240MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Southern B.D., Grove L.M., Rahaman S.O., Abraham S., Scheraga R., Niese K.A., Sun H., Herzog E.L., Liu F., Tschumperlin D.J., et al. Matrix-driven Myosin II Mediates the Pro-fibrotic Fibroblast Phenotype. J. Biol. Chem. 2016;291:6083–6095. doi: 10.1074/jbc.M115.712380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hinz B. Mechanical aspects of lung fibrosis: A spotlight on the myofibroblast. Proc. Am. Thorac. Soc. 2012;9:137–147. doi: 10.1513/pats.201202-017AW. [DOI] [PubMed] [Google Scholar]
- 83.Booth A.J., Hadley R., Cornett A.M., Dreffs A.A., Matthes S.A., Tsui J.L., Weiss K., Horowitz J.C., Fiore V.F., Barker T.H., et al. Acellular Normal and Fibrotic Human Lung Matrices as a Culture System forIn VitroInvestigation. Am. J. Respir. Crit. Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu F., Mih J.D., Shea B.S., Kho A.T., Sharif A.S., Tager A.M., Tschumperlin D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karsdal M.A., Nielsen M.J., Sand J.M., Henriksen K., Genovese F., Bay-Jensen A.-C., Smith V., Adamkewicz J.I., Christiansen C., Leeming D.J. Extracellular Matrix Remodeling: The Common Denominator in Connective Tissue DiseasesPossibilities for Evaluation and Current Understanding of the Matrix as More Than a Passive Architecture, but a Key Player in Tissue Failure. ASSAY Drug Dev. Technol. 2013;11:70–92. doi: 10.1089/adt.2012.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cox T.R., Erler J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Erler J.T., Weaver V.M. Three-dimensional context regulation of metastasis. Clin. Exp. Metastasis. 2008;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng D.T., Kim D.K., Cockayne D.A., Belousov A., Bitter H., Cho M.H., Duvoix A., Edwards L., Lomas D.A., Miller B.E., et al. Systemic Soluble Receptor for Advanced Glycation Endproducts Is a Biomarker of Emphysema and Associated with AGER Genetic Variants in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013;188:948–957. doi: 10.1164/rccm.201302-0247OC. [DOI] [PubMed] [Google Scholar]
- 89.Mostaço-Guidolin L., Osei E., Ullah J., Hajimohammadi S., Fouadi M., Li X., Li V., Shaheen F., Yang C.X., Chu F., et al. Defective Fibrillar Collagen Organization by Fibroblasts Contributes to Airway Remodeling in Asthma. Am. J. Respir. Crit. Care Med. 2019;200:431–443. doi: 10.1164/rccm.201810-1855OC. [DOI] [PubMed] [Google Scholar]
- 90.Roche W., Williams J., Beasley R., Holgate S. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;333:520–524. doi: 10.1016/S0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 91.Dunnill M.S. The pathology of asthma, with special reference to changes in the bronchial mucosa. J. Clin. Pathol. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carroll N., Elliot J., Morton A., James A. The Structure of Large and Small Airways in Nonfatal and Fatal Asthma. Am. Rev. Respir. Dis. 1993;147:405–410. doi: 10.1164/ajrccm/147.2.405. [DOI] [PubMed] [Google Scholar]
- 93.James A.L., Paré P.D., Hogg J.C. The Mechanics of Airway Narrowing in Asthma. Am. Rev. Respir. Dis. 1989;139:242–246. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- 94.Mostaco-Guidolin L.B., Loube J., Barlow A., Osei E.T., Vasilescu D.M., Hsieh A., Fouadi M., Young C., Scott A.L., Mitzner W., et al. Second harmonic generation imaging of collagen scaffolds within the alveolar ducts of healthy and emphysematous mouse lungs. Histochem. Cell Biol. 2021;155:279–289. doi: 10.1007/s00418-020-01959-6. [DOI] [PubMed] [Google Scholar]
- 95.Cañas J.A., Rodrigo-Muñoz J.M., Sastre B., Gil-Martinez M., Redondo N., Del Pozo V. MicroRNAs as Potential Regulators of Immune Response Networks in Asthma and Chronic Obstructive Pulmonary Disease. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.608666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mousavi S.R., Ahmadi A., Jamalkandi S.A., Salimian J. Involvement of microRNAs in physiological and pathological processes in asthma. J. Cell. Physiol. 2019;234:21547–21559. doi: 10.1002/jcp.28781. [DOI] [PubMed] [Google Scholar]
- 97.Szymczak I., Wieczfinska J., Pawliczak R. Molecular Background of miRNA Role in Asthma and COPD: An Updated Insight. BioMed Res. Int. 2016;2016:1–10. doi: 10.1155/2016/7802521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Araujo B.B., Dolhnikoff M., da Silva L.F.F., Elliot J., Lindeman J.H.N., Ferreira D., Mulder A., Gomes H.A.P., Fernezlian S.M., James A., et al. Extracellular matrix components and regulators in the airway smooth muscle in asthma. Eur. Respir. J. 2008;32:61–69. doi: 10.1183/09031936.00147807. [DOI] [PubMed] [Google Scholar]
- 99.Sun Q., Liu L., Wang H., Mandal J., Khan P., Hostettler K., Stolz D., Tamm M., Molino A., Lardinois D., et al. Constitutive high expression of protein arginine methyltransferase 1 in asthmatic airway smooth muscle cells is caused by reduced microRNA-19a expression and leads to enhanced remodeling. J. Allergy Clin. Immunol. 2017;140:510–524.e3. doi: 10.1016/j.jaci.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 100.Yang Z., Qu Z., Yi M., Lv Z., Wang Y., Shan Y., Ran N., Liu X. MiR-204-5p Inhibits Transforming Growth Factor-β1-Induced Proliferation and Extracellular Matrix Production of Airway Smooth Muscle Cells by Regulating Six1 in Asthma. Int. Arch. Allergy Immunol. 2020;181:239–248. doi: 10.1159/000505064. [DOI] [PubMed] [Google Scholar]
- 101.Cheng W., Yan K., Xie L.-Y., Chen F., Yu H.-C., Huang Y.-X., Dang C.-X. MiR-143-3p controls TGF-β1-induced cell proliferation and extracellular matrix production in airway smooth muscle via negative regulation of the nuclear factor of activated T cells 1. Mol. Immunol. 2016;78:133–139. doi: 10.1016/j.molimm.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Li P., Lang X., Xia S. Elevated expression of microRNA-378 in children with asthma aggravates airway remodeling by promoting the proliferation and apoptosis resistance of airway smooth muscle cells. Exp. Ther. Med. 2018;17:1529–1536. doi: 10.3892/etm.2018.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Y., Sun X., Wu Y., Fang P., Shi H., Xu J., Li M. Effects of miRNA-145 on airway smooth muscle cells function. Mol. Cell. Biochem. 2015;409:135–143. doi: 10.1007/s11010-015-2519-7. [DOI] [PubMed] [Google Scholar]
- 104.Kuhn A.R., Schlauch K., Lao R., Halayko A.J., Gerthoffer W.T., Singer C.A. MicroRNA expression in human airway smooth muscle cells: Role of miR-25 in regulation of airway smooth muscle phenotype. Am. J. Respir. Cell Mol. Biol. 2010;42:506–513. doi: 10.1165/rcmb.2009-0123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lv X., Li Y., Gong Q., Jiang Z. TGF-β1 induces airway smooth muscle cell proliferation and remodeling in asthmatic mice by up-regulating miR-181a and suppressing PTEN. Int. J. Clin. Exp. Pathol. 2019;12:173–181. [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J., Wang H.-S., Su Z.-B. MicroRNA-142 Inhibits Proliferation and Promotes Apoptosis in Airway Smooth Muscle Cells During Airway Remodeling in Asthmatic Rats via the Inhibition of TGF-β -Dependent EGFR Signaling Pathway. Cell. Physiol. Biochem. 2018;47:1682–1695. doi: 10.1159/000490986. [DOI] [PubMed] [Google Scholar]
- 107.Maes T., Cobos F.A., Schleich F., Sorbello V., Henket M., De Preter K., Bracke K., Conickx G., Mesnil C., Vandesompele J., et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J. Allergy Clin. Immunol. 2016;137:1433–1446. doi: 10.1016/j.jaci.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 108.Liu F., Qin H.-B., Xu B., Zhou H., Zhao D.-Y. Profiling of miRNAs in pediatric asthma: Upregulation of miRNA-221 and miRNA-485-3p. Mol. Med. Rep. 2012;6:1178–1182. doi: 10.3892/mmr.2012.1030. [DOI] [PubMed] [Google Scholar]
- 109.Fang L., Wang X., Sun Q., Papakonstantinou E., S’Ng C., Tamm M., Stolz D., Roth M. IgE Downregulates PTEN through MicroRNA-21-5p and Stimulates Airway Smooth Muscle Cell Remodeling. Int. J. Mol. Sci. 2019;20:875. doi: 10.3390/ijms20040875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pan J., Yang Q., Zhou Y., Deng H., Zhu Y., Zhao D., Liu F. MicroRNA-221 Modulates Airway Remodeling via the PI3K/AKT Pathway in OVA-Induced Chronic Murine Asthma. Front. Cell Dev. Biol. 2020;8:495. doi: 10.3389/fcell.2020.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang H., Song Y., Rong W., Fan L., Cai Y., Qu Q., Gao Y., Zhao H. miR-221 participates in the airway epithelial cells injury in asthma via targeting SIRT1. Exp. Lung Res. 2018;44:272–279. doi: 10.1080/01902148.2018.1533051. [DOI] [PubMed] [Google Scholar]
- 112.Xia J., Wu X., Yang Y., Zhao Y., Fang M., Xie W., Wang H., Xu Y. SIRT1 deacetylates RFX5 and antagonizes repression of collagen type I (COL1A2) transcription in smooth muscle cells. Biochem. Biophys. Res. Commun. 2012;428:264–270. doi: 10.1016/j.bbrc.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 113.Yu Z.-W., Xu Y.-Q., Zhang X.-J., Pan J.-R., Xiang H.-X., Gu X.-H., Ji S.-B., Qian J. Mutual regulation between miR-21 and the TGFβ/Smad signaling pathway in human bronchial fibroblasts promotes airway remodeling. J. Asthma. 2018;56:341–349. doi: 10.1080/02770903.2018.1455859. [DOI] [PubMed] [Google Scholar]
- 114.Baarsma H., Menzen M.H., Halayko A.J., Meurs H., Kerstjens H.A.M., Gosens R. β-Catenin signaling is required for TGF-β1-induced extracellular matrix production by airway smooth muscle cells. Am. J. Physiol. Cell. Mol. Physiol. 2011;301:L956–L965. doi: 10.1152/ajplung.00123.2011. [DOI] [PubMed] [Google Scholar]
- 115.Koo H.-K., Vasilescu D.M., Booth S., Hsieh A., Katsamenis O., Fishbane N., Elliott W.M., Kirby M., Lackie P., Sinclair I., et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: A cross-sectional study. Lancet Respir. Med. 2018;6:591–602. doi: 10.1016/S2213-2600(18)30196-6. [DOI] [PubMed] [Google Scholar]
- 116.Hogg J.C., Timens W. The Pathology of Chronic Obstructive Pulmonary Disease. Annu. Rev. Pathol. Mech. Dis. 2009;4:435–459. doi: 10.1146/annurev.pathol.4.110807.092145. [DOI] [PubMed] [Google Scholar]
- 117.Agustí A., Hogg J.C. Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2019;381:1248–1256. doi: 10.1056/NEJMra1900475. [DOI] [PubMed] [Google Scholar]
- 118.Williams M.H. Chronic Obstructive Pulmonary Disease [Internet] N. Y. State J. Med. 1979;79:919–921. [PubMed] [Google Scholar]
- 119.Black P.N., Ching P.S.T., Beaumont B., Ranasinghe S., Taylor G., Merrilees M.J. Changes in elastic fibres in the small airways and alveoli in COPD. Eur. Respir. J. 2008;31:998–1004. doi: 10.1183/09031936.00017207. [DOI] [PubMed] [Google Scholar]
- 120.Merrilees M.J., Ching P.S., Beaumont B., Hinek A., Wight T.N., Black P.N. Changes in elastin, elastin binding protein and versican in alveoli in chronic obstructive pulmonary disease. Respir. Res. 2008;9:41. doi: 10.1186/1465-9921-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tan B.W., Sim W.L., Cheong J.K., Kuan W.S., Tran T., Lim H.F. MicroRNAs in chronic airway diseases: Clinical correlation and translational applications. Pharmacol. Res. 2020;160:105045. doi: 10.1016/j.phrs.2020.105045. [DOI] [PubMed] [Google Scholar]
- 122.Huang X., Zhu Z., Guo X., Kong X. The roles of microRNAs in the pathogenesis of chronic obstructive pulmonary disease. Int. Immunopharmacol. 2019;67:335–347. doi: 10.1016/j.intimp.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 123.Osei E.T., Florez-Sampedro L., Timens W., Postma D.S., Heijink I.H., Brandsma C.A. Unravelling the complexity of COPD by microRNAs: It’s a small world after all. Eur. Respir. J. 2015;46:807–818. doi: 10.1183/13993003.02139-2014. [DOI] [PubMed] [Google Scholar]
- 124.Ezzie M.E., Crawford M., Cho J.-H., Orellana R., Zhang S., Gelinas R., Batte K., Yu L., Nuovo G., Galas D., et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2011;67:122–131. doi: 10.1136/thoraxjnl-2011-200089. [DOI] [PubMed] [Google Scholar]
- 125.Noordhoek J.A., Postma D.S., Chong L.L., Menkema L., Kauffman H.F., Timens W., Van Straaten J.F.M., Van Der Geld Y.M. Different Modulation of Decorin Production by Lung Fibroblasts from Patients with Mild and Severe Emphysema. COPD J. Chronic Obstr. Pulm. Dis. 2005;2:17–25. doi: 10.1081/COPD-200050678. [DOI] [PubMed] [Google Scholar]
- 126.Dang X., Yang L., Guo J., Hu H., Li F., Liu Y., Pang Y. miR-145-5p is associated with smoke-related chronic obstructive pulmonary disease via targeting KLF5. Chem. Interact. 2019;300:82–90. doi: 10.1016/j.cbi.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 127.Abe K., Sugiura H., Hashimoto Y., Ichikawa T., Koarai A., Yamada M., Numakura T., Onodera K., Tanaka R., Sato K., et al. Possible role of Krüppel-like factor 5 in the remodeling of small airways and pulmonary vessels in chronic obstructive pulmonary disease. Respir. Res. 2016;17:7. doi: 10.1186/s12931-016-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Du Y., Ding Y., Chen X., Mei Z., Ding H., Wu Y., Jie Z. MicroRNA-181c inhibits cigarette smoke–induced chronic obstructive pulmonary disease by regulating CCN1 expression. Respir. Res. 2017;18:1–8. doi: 10.1186/s12931-017-0639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holbourn K.P., Acharya K.R., Perbal B. The CCN family of proteins: Structure–function relationships. Trends Biochem. Sci. 2008;33:461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ong J., Timens W., Rajendran V., Algra A., Spira A., Lenburg M.E., Campbell J.D., Berge M.V.D., Postma D.S., Berg A.V.D., et al. Identification of transforming growth factor-beta-regulated microRNAs and the microRNA-targetomes in primary lung fibroblasts. PLoS ONE. 2017;12:e0183815. doi: 10.1371/journal.pone.0183815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chi Y., Di Q., Han G., Li M., Sun B. Mir-29b mediates the regulation of Nrf2 on airway epithelial remodeling and Th1/Th2 differentiation in COPD rats. Saudi J. Biol. Sci. 2019;26:1915–1921. doi: 10.1016/j.sjbs.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tang K., Zhao J., Xie J., Wang J. Decreased miR-29b expression is associated with airway inflammation in chronic obstructive pulmonary disease. Am. J. Physiol. Cell. Mol. Physiol. 2019;316:L621–L629. doi: 10.1152/ajplung.00436.2018. [DOI] [PubMed] [Google Scholar]
- 133.Cushing L., Kuang P.P., Qian J., Shao F., Wu J., Little F., Thannickal V.J., Cardoso W.V., Lü J. miR-29 Is a Major Regulator of Genes Associated with Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.King T.E., Bradford W.Z., Castro-Bernardini S., Fagan E.A., Glaspole I., Glassberg M.K., Gorina E., Hopkins P.M., Kardatzke D., Lancaster L., et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 135.Richeldi L., Du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., Cottin V., Flaherty K.R., Hansell D.M., Inoue Y., et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 136.Hughes G., Toellner H., Morris H., Leonard C., Chaudhuri N. Real World Experiences: Pirfenidone and Nintedanib are Effective and Well Tolerated Treatments for Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2016;5:78. doi: 10.3390/jcm5090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Behr J., Cottin V., Danoff S.K., Morell F., et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 138.Takagi K., Yamakuchi M., Hashiguchi T., Inoue H. The Role of miRNAs in Idiopathic Pulmonary Fibrosis. Interstitial Lung Dis. 2019;2:5–22. doi: 10.5772/intechopen.82771. [DOI] [Google Scholar]
- 139.Jones M.G., Fabre A., Schneider P., Cinetto F., Sgalla G., Mavrogordato M., Jogai S., Alzetani A., Marshall B., O’Reilly K.M., et al. Three-dimensional characterization of fibroblast foci in idiopathic pulmonary fibrosis. JCI Insight. 2016;1 doi: 10.1172/jci.insight.86375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.King T.E., Pardo A., Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 141.Liebow A.A. Definition and Classification of Interstitial Pneumonias in Human Pathology1. Clin. Exerc. Test. 2015;8:1–33. doi: 10.1159/000398285. [DOI] [Google Scholar]
- 142.Wolters P.J., Collard H.R., Jones K.D. Pathogenesis of Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Le Saux C.J., Chapman H.A. Idiopathic Pulmonary Fibrosis: Cell Death and Inflammation Revisited. Am. J. Respir. Cell Mol. Biol. 2018;59:137–138. doi: 10.1165/rcmb.2018-0083ED. [DOI] [PubMed] [Google Scholar]
- 144.Burgess J.K., Mauad T., Tjin G., Karlsson J.C., Westergren-Thorsson G. The extracellular matrix–The under-recognized element in lung disease? J. Pathol. 2016;240:397–409. doi: 10.1002/path.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Enomoto N., Suda T., Kono M., Kaida Y., Hashimoto D., Fujisawa T., Nakamura Y., Inui N., Imokawa S., Chida K. Amounts of Elastic Fibers Predict Prognosis of Idiopathic Pulmonary Fibrosis. Respir. Med. 2013;107:1608–1616. doi: 10.1016/j.rmed.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 146.Zhang H., Song M., Guo J., Ma J., Qiu M., Yang Z. The function of non-coding RNAs in idiopathic pulmonary fibrosis. Open Med. 2021;16:481–490. doi: 10.1515/med-2021-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rajasekaran S., Rajaguru P., Gandhi P.S.S. MicroRNAs as potential targets for progressive pulmonary fibrosis. Front. Pharmacol. 2015;6:254. doi: 10.3389/fphar.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guiot J., Cambier M., Boeckx A., Henket M., Nivelles O., Gester F., Louis E., Malaise M., Dequiedt F., Louis R., et al. Macrophage-derived exosomes attenuate fibrosis in airway epithelial cells through delivery of antifibrotic miR-142-3p. Thorax. 2020;75:870–881. doi: 10.1136/thoraxjnl-2019-214077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bibaki E., Tsitoura E., Vasarmidi E., Margaritopoulos G., Trachalaki A., Koutoulaki C., Georgopoulou T., Spandidos D., Tzanakis N., Antoniou K.M. miR-185 and miR-29a are similarly expressed in the bronchoalveolar lavage cells in IPF and lung cancer but common targets DNMT1 and COL1A1 show disease specific patterns. Mol. Med. Rep. 2018;17:7105–7112. doi: 10.3892/mmr.2018.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang S., Banerjee S., de Freitas A., Sanders Y.Y., Ding Q., Matalon S., Thannickal V.J., Abraham E., Liu G. Participation of miR-200 in Pulmonary Fibrosis. Am. J. Pathol. 2012;180:484–493. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pandit K.V., Corcoran D., Yousef H., Yarlagadda M., Tzouvelekis A., Gibson K.F., Konishi K., Yousem S.A., Singh M., Handley D., et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liang H., Xu C., Pan Z., Zhang Y., Xu Z., Chen Y., Li T., Li X., Liu Y., Huangfu L., et al. The Antifibrotic Effects and Mechanisms of MicroRNA-26a Action in Idiopathic Pulmonary Fibrosis. Mol. Ther. 2014;22:1122–1133. doi: 10.1038/mt.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Das S., Kumar M., Negi V., Pattnaik B., Prakash Y.S., Agrawal A., Ghosh B. MicroRNA-326 Regulates Profibrotic Functions of Transforming Growth Factor-β in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2014;50:882–892. doi: 10.1165/rcmb.2013-0195OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen Y., Zhao X., Sun J., Su W., Zhang L., Li Y., Liu Y., Zhang L., Lu Y., Shan H., et al. YAP1/Twist promotes fibroblast activation and lung fibrosis that conferred by miR-15a loss in IPF. Cell Death Differ. 2019;26:1832–1844. doi: 10.1038/s41418-018-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xiao J., Meng X.-M., Huang X.R., Chung A.C., Feng Y.-L., Hui D., Yu C.-M., Sung J.J.Y., Lan H.Y. miR-29 Inhibits Bleomycin-induced Pulmonary Fibrosis in Mice. Mol. Ther. 2012;20:1251–1260. doi: 10.1038/mt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu G., Friggeri A., Yang Y., Milosevic J., Ding Q., Thannickal V.J., Kaminski N., Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Q., Ye H., Xiang F., Song L.-J., Zhou L.-L., Cai P.-C., Zhang J.-C., Yu F., Shi H.-Z., Su Y., et al. miR-18a-5p Inhibits Sub-pleural Pulmonary Fibrosis by Targeting TGF-β Receptor II. Mol. Ther. 2017;25:728–738. doi: 10.1016/j.ymthe.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gallo A., Tandon M., Alevizos I., Illei G.G. The Majority of MicroRNAs Detectable in Serum and Saliva Is Concentrated in Exosomes. PLoS ONE. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun X.-Y., Lu J., Zhang L., Song H.-T., Zhao L., Fan H.-M., Zhong A.-F., Niu W., Guo Z.-M., Dai Y.-H., et al. Aberrant microRNA expression in peripheral plasma and mononuclear cells as specific blood-based biomarkers in schizophrenia patients. J. Clin. Neurosci. 2015;22:570–574. doi: 10.1016/j.jocn.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 160.Kocerha J., Kouri N., Baker M., Finch N., DeJesus-Hernandez M., Gonzalez J., Chidamparam K., Josephs K.A., Boeve B.F., Graff-Radford N.R., et al. Altered microRNA expression in frontotemporal lobar degeneration with TDP-43 pathology caused by progranulin mutations. BMC Genom. 2011;12:527. doi: 10.1186/1471-2164-12-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang R., Li N., Zhang Y., Ran Y., Pu J. Circulating MicroRNAs are Promising Novel Biomarkers of Acute Myocardial Infarction. Intern. Med. 2011;50:1789–1795. doi: 10.2169/internalmedicine.50.5129. [DOI] [PubMed] [Google Scholar]
- 162.Wang J., Tan L., Tan L., Tian Y., Ma J., Tan C.-C., Wang H.-F., Liu Y., Tan M.-S., Jiang T., et al. Circulating microRNAs are promising novel biomarkers for drug-resistant epilepsy. Sci. Rep. 2015;5:10201. doi: 10.1038/srep10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weir D.W., Sturrock A., Leavitt B.R. Development of biomarkers for Huntington’s disease. Lancet Neurol. 2011;10:573–590. doi: 10.1016/S1474-4422(11)70070-9. [DOI] [PubMed] [Google Scholar]
- 164.Elfimova N., Schlattjan M., Sowa J.-P., Dienes H.P., Canbay A., Odenthal M. Circulating microRNAs: Promising candidates serving as novel biomarkers of acute hepatitis. Front. Physiol. 2012;3:476. doi: 10.3389/fphys.2012.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li Y.-J., Xu M., Gao Z.-H., Wang Y.-Q., Yue Z., Zhang Y.-X., Li X.-X., Zhang C., Xie S.-Y., Wang P.-Y. Alterations of Serum Levels of BDNF-Related miRNAs in Patients with Depression. PLoS ONE. 2013;8:e63648. doi: 10.1371/journal.pone.0063648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Recchioni R., Marcheselli F., Olivieri F., Ricci S., Procopio A.D., Antonicelli R. Conventional and novel diagnostic biomarkers of acute myocardial infarction: A promising role for circulating microRNAs. Biomarkers. 2013;18:547–558. doi: 10.3109/1354750X.2013.833294. [DOI] [PubMed] [Google Scholar]
- 167.Scott K.A., Hoban A.E., Clarke G., Moloney G.M., Dinan T.G., Cryan J.F. Thinking small: Towards microRNA-based therapeutics for anxiety disorders. Expert Opin. Investig. Drugs. 2015;24:529–542. doi: 10.1517/13543784.2014.997873. [DOI] [PubMed] [Google Scholar]
- 168.Biswas S. MicroRNAs as Therapeutic Agents: The Future of the Battle Against Cancer. Curr. Top. Med. Chem. 2019;18:2544–2554. doi: 10.2174/1568026619666181120121830. [DOI] [PubMed] [Google Scholar]
- 169.Hu W.Z., Tan C.L., He Y.J., Zhang G.Q., Xu Y., Tang J.H. Functional miRNAs in breast cancer drug resistance. Onco. Targets Ther. 2018;11:1529–1541. doi: 10.2147/OTT.S152462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hanna J., Hossain G.S., Kocerha J. The potential for microRNA therapeutics and clinical research. Front. Genet. 2019;10:478. doi: 10.3389/fgene.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Efficacy, Safety, and Tolerability of Remlarsen (MRG-201) Following Intradermal Injection in Subjects with a History of Keloids—Full Text View—ClinicalTrials.gov. [(accessed on 24 June 2021)];2020 Available online: https://clinicaltrials.gov/ct2/show/NCT03601052.
- 172.Mercatelli N., Coppola V., Bonci D., Miele F., Costantini A., Guadagnoli M., Bonanno E., Muto G., Frajese G.V., De Maria R., et al. The Inhibition of the Highly Expressed Mir-221 and Mir-222 Impairs the Growth of Prostate Carcinoma Xenografts in Mice. PLoS ONE. 2008;3:e4029. doi: 10.1371/journal.pone.0004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen Y., Gao D.Y., Huang L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. [(accessed on 24 June 2021)];Adv. Drug Deliv. Rev. 2015 81:128–141. doi: 10.1016/j.addr.2014.05.009. Available online: /pmc/articles/PMC5009470/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wagh V., Urhekar A., Modi D. Levels of microRNA miR-16 and miR-155 are altered in serum of patients with tuberculosis and associate with responses to therapy. Tuberculosis. 2017;102:24–30. doi: 10.1016/j.tube.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 175.Gupta S., Singhal N.K., Ganesh S., Sandhir S.G.A.R. Extending Arms of Insulin Resistance from Diabetes to Alzheimer’s Disease: Identification of Potential Therapeutic Targets. CNS Neurol. Disord. Drug Targets. 2019;18:172–184. doi: 10.2174/1871527317666181114163515. [DOI] [PubMed] [Google Scholar]
- 176.Montastier E., Beuzelin D., Martins F., Mir L., Marqués M.-A., Thalamas C., Iacovoni J., Langin D., Viguerie N. Niacin induces miR-502-3p expression which impairs insulin sensitivity in human adipocytes. Int. J. Obes. 2018;43:1485–1490. doi: 10.1038/s41366-018-0260-5. [DOI] [PubMed] [Google Scholar]
- 177.Xie Y., Shao Y., Deng X., Wang M., Chen Y. MicroRNA-298 Reverses Multidrug Resistance to Antiepileptic Drugs by Suppressing MDR1/P-gp Expression in vitro. Front. Neurosci. 2018;12:602. doi: 10.3389/fnins.2018.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.He J., Zhao J., Zhu W., Qi D., Wang L., Sun J., Wang B., Ma X., Dai Q., Yu X. MicroRNA biogenesis pathway genes polymorphisms and cancer risk: A systematic review and meta-analysis. PeerJ. 2016;4:e2706. doi: 10.7717/peerj.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.