Abstract
Intramyocardial dissection is a rare complication of myocardial infarction, trauma, and percutaneous intervention. It is usually caused by hemorrhagic dissection among the spiral myocardial fibers. We hereby report the case of a patient with left ventricular intramyocardial dissection who presented with acute decompensated heart failure. (Level of Difficulty: Advanced.)
Key Words: acute heart failure, cardiac magnetic resonance, contrast agent, dissection, myocardial infarction
Abbreviations and Acronyms: EF, ejection fraction; IDH, intramyocardial dissecting hematoma; IHD, ischemic heart disease; IVS, interventricular septum; LV, left ventricle; MI, myocardial infarction; MR, magnetic resonance; NYHA, New York Heart Association
Graphical abstract
A 45-year-old man, an active tobacco smoker with a known history of diabetes mellitus and hypertension, presented with a recent onset of New York Heart Association (NYHA) functional class IV heart failure. He had sudden-onset chest discomfort 1 month before, which resolved spontaneously within a day.
Learning Objectives
-
•
To recognize that intramyocardial dissecting hematoma is a rare complication of myocardial infarction.
-
•
To understand that delayed presentation can mimic left-ventricular clot on transthoracic echocardiogram.
-
•
To comprehend that the distinction from intracavitary thrombus relies on the clear identification of the endocardial layer surrounding the neoformation and its systolic expansion.
-
•
To realize that management of IDH depends on multiple factors including age of the patient, hemodynamic stability, size of hematoma, presence of ventricular septal defect, LV function, and pericardial effusion.
Physical Examination
The patient had tachycardia (pulse rate of 120 beats/min) with low-volume pulse, blood pressure of 80/60 mm Hg, elevated jugular venous pressure (prominent "a" and "v" waves), anasarca, S3 gallop, and hepatomegaly. There was no significant medical history relevant to the current presentation.
Differential Diagnosis
In view of his history of chest pain 1 month earlier, we kept the possibility of an acute coronary syndrome, now presenting with decompensated heart failure, as our first differential diagnosis. Another possible differential diagnosis could have been a longstanding ischemic cardiomyopathy with severe biventricular dysfunction.
Investigations
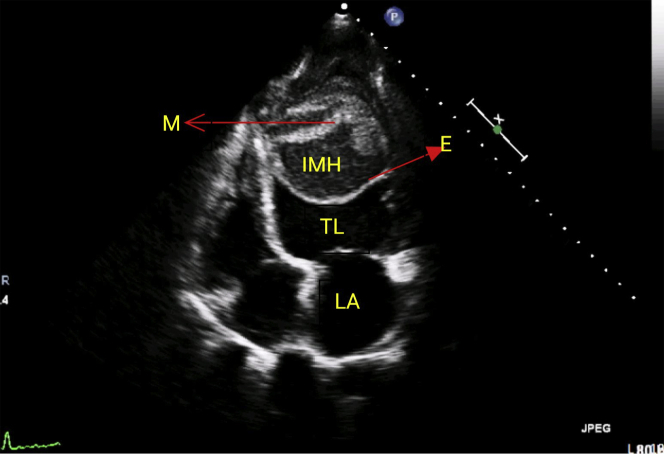
The electrocardiogram (ECG) showed sinus rhythm with right bundle branch block and low- voltage QRS complexes in limb leads and T inversion in V1 to V6 (Figure 1).Two-dimensional echocardiography showed left-ventricular (LV) dysfunction (ejection fraction [EF] ∼25%) with akinetic interventricular septum (IVS). There was a large intramyocardial dissecting hematoma (IDH) within the LV cavity, with a mobile endocardial flap (Figures 2, 3, 4, and 5, Videos 1, 2, 3, 4, 5, and 6). The 3-dimensional echocardiogram delineated the mobile endocardial flap and dissected myocardial flap clearly (Figure 6). The contrast (Definity, Lantheus Medical Imaging, North Bellerica, Massachusetts) echo opacified the true LV cavity, with no leak of contrast into the neocavity (Figure 7, Video 7). Cine magnetic resonance (MR) sequences confirmed the diagnosis of intramyocardial dissection with multiple layers of hematoma and thrombus (Figures 8 and 9, Videos 8 and 9).
Figure 1.
12-Lead Electrocardiogram Showing Sinus Rhythm With Right Bundle Branch Block and T-Wave Inversion in V1 to V6
Figure 2.
Parasternal Long-Axis View Showing Intramyocardial Hematoma
E = endocardial flap; IMH = intramyocardial hematoma; LA = left atrium; TL = true left-ventricular cavity.
Figure 3.
Modified Short-Axis View Showing Multiple Layers of Hematoma
Abbreviations as in Figure 2.
Figure 4.
Apical 4-Chamber View Showing the Hematoma With Dissected Myocardium
M = myocardial flap; other abbreviations as in Figure 2.
Figure 5.
Color Doppler in Apical 4-Chamber View Confirming No Flow Into the Neocavity
Figure 6.
3-Dimensional Echo Showing the Mobile Endocardial Flap With Intramyocardial Dissecting Hematoma
Figure 7.
Contrast Echo Differentiating the True Left Ventricular Cavity From the Neocavity
Abbreviations as in Figure 2.
Figure 8.
Cardiac Magnetic Resonance Demonstrating Intramyocardial Dissecting Hematoma With Thrombus
Abbreviations as in Figure 2.
Figure 9.
Late Gadolinium Enhancement Magnetic Resonance Showing the Extensive Myocardial Scar
LGE = late gadolinium enhancement; other abbreviations as in Figures 2 and 8.
Management
Our final diagnosis was LV dissection with intramyocardial hematoma and severe LV dysfunction with acute decompensated heart failure. After initial stabilization with inotropes and diuretics, the heart team was consulted. Owing to the complexity of dissection and a prohibitive risk for surgery (EuroSCORE II 65.8%), hematoma evacuation and ventricular wall repair would have been challenging, and therefore the patient was kept on conservative management. He was discharged 2 weeks later on diuretics, ramipril, atorvastatin, and a single antiplatelet (aspirin).
Discussion
IDH is a rare complication of myocardial infarction (MI), chest trauma, and percutaneous intervention. It can develop in the LV free wall, the right ventricle, or the IVS (1). Formation of IDH may result from rupture of intramyocardial vessels into the interstitial space, decreased tensile strength of the infarcted area, and acute increase of coronary capillary perfusion pressure (2).
IDH consists of a cavity filled with blood, the outer wall of which is the myocardium and pericardium, and the inner wall, facing the ventricular cavity, is part of the myocardium and endocardium. Differential diagnosis includes pseudoaneurysm, intracavitary thrombus, or prominent ventricular trabeculations. Establishing the integrity of epicardium differentiates IDH from pseudoaneurysm, as pseudoaneurysm comprises a complete rupture of the myocardial wall contained by the pericardium. The distinction from intracavitary thrombus relies on the clear identification of the endocardial layer surrounding the neoformation and its systolic expansion. A completely irregular shape of the ventricular wall with flow within it is the hallmark of prominent trabeculations (3).
In our patient, the most likely cause of LV dissection was MI, as he had multiple risk factors (diabetes, hypertension, and smoking) for ischemic heart disease (IHD). The finding of akinetic anterior wall segments on echo and cardiac MR confirmed our diagnosis of IHD.
In more than 90% of cases, rupture occurs after the first MI and has a strong correlation with single-vessel disease, reflecting lack of collateral circulation (4). Risk factors include anterior-wall infarct, large transmural infarction, age ≥60 years, hypertension, female sex, single-vessel disease, and absence of previous cardiac events (5).
Echocardiographic features of IDH include the formation of 1 or more neocavitations within the tissue with an echolucent center; a thinned and mobile endomyocardial border surrounding the cavitary defect; ventricular myocardium identified in the regions outside of the cystic areas; andchanges in the echogenicity of the neocavitation, suggesting blood content (6).
The management of IDH depends on multiple factors including age of the patient, hemodynamic stability, size of the hematoma, presence of ventricular septal defect, LV function, and pericardial effusion. IDH limited to the apex has a high probability of spontaneous reabsorption, and an initial conservative approach may be reasonable (6). Those patients with expansion of dissection on serial echocardiogram; ventricular septal defect; and with compromised hemodynamics, and low EF in anterior wall MI should undergo surgery (7). There have been case reports of surgical management that involved median sternotomy, cardiopulmonary bypass, and clot evacuation with subsequent ventricular wall repair; however, such procedures were done in patients who were not in cardiogenic shock (8). In our case, although the patient presented in acute decompensated heart failure with severe LV dysfunction, he had significant hemodynamic improvement with conservative management, and, also, such an extensive dissecting hematoma was beyond repair.
In a case series by Leitman et al. (7) published in 2018, 42 cases of IDH have been diagnosed and published thus far. In this series, in-hospital mortality was 23%, and the strongest independent risk factors for mortality were low EF (<35%), age ≥60 years, and anterior-wall MI. Late presentation (>24 h after onset of symptoms) was also associated with increased mortality.
Follow-Up
At present, at 6 months of follow-up, the patient is symptomatically better (NYHA functional class II), with no further extension of the dissecting hematoma.
Conclusions
IDH is a rare complication of MI and a diagnostic challenge. Management is based on individual clinical and imaging parameters. Low EF, age ≥60 years, anterior-wall MI, cardiogenic shock, and late diagnosis are predictors of in-hospital mortality. Conservative management is the usual approach in cases with small hematomas localized to the apex, whereas cases with hemodynamic instability and with expanding hematomas may need surgical repair.
Author Disclosures
This work was funded by a research grant from Sri Jayadeva Institute of Cardiovascular Sciences & Research, Bengaluru, Karnataka, India. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Dr. K.H. Srinivas and Dr. Prabhavathi for their guidance and Dr. C.N. Manjunath for funding this paper with the institute’s research grant.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
References
- 1.Dias V., Cabral S., Gomes C. Intramyocardial dissecting hematoma: a rare complication of acute myocardial infarction. Eur J Echocardiogr. 2009;10:585–587. doi: 10.1093/ejechocard/jep027. [DOI] [PubMed] [Google Scholar]
- 2.Slepian R., Salemi A., Min J., Skubas N. A hypoechoic, intramyocardial space: echocardiographic characteristics of an intramyocardial dissecting hematoma. Int Anesth Res Soc. 2007;6:1564–1566. doi: 10.1213/01.ane.0000287251.23400.df. [DOI] [PubMed] [Google Scholar]
- 3.Nakata A., Hirota S., Tsuji H., Takazakura E. Interventricular septal dissection in a patient with an old myocardial infarction. Intern Med. 1996;35:33–35. doi: 10.2169/internalmedicine.35.33. [DOI] [PubMed] [Google Scholar]
- 4.Mann J.M., Roberts W.C. Rupture of the left ventricular free wall during acute myocardial infarction: analysis of 138 necropsy patients and comparison with 50 necropsy patients with acute myocardial infarction without rupture. Am J Cardiol. 1988;62:847–859. doi: 10.1016/0002-9149(88)90881-8. [DOI] [PubMed] [Google Scholar]
- 5.Mishra P.K., Pathi V., Murday A. Post myocardial infarction left ventricular free wall rupture. Interact Cardiovasc Thorac Surg. 2007;6:39–42. doi: 10.1510/icvts.2006.138511. [DOI] [PubMed] [Google Scholar]
- 6.Vargas-Barron J., Roldan F., Romero-Cardenas A. Dissecting intramyocardial hematoma: clinical presentation, pathophysiology, outcomes and delineation by echocardiography. Echocardiography. 2009;3:254–261. doi: 10.1111/j.1540-8175.2008.00804.x. [DOI] [PubMed] [Google Scholar]
- 7.Leitman M., Tyomkin V., Sternik L., Copel L., Goitein O., Vered Z. Intramyocardial dissecting hematoma: two case reports and a meta-analysis of the literature. Echocardiography. 2018;35:1–7. doi: 10.1111/echo.13796. [DOI] [PubMed] [Google Scholar]
- 8.Vinayak N.B., Ajay M.N., Yash L., Anil G.T. Intramyocardial dissecting hematoma. Circulation. 1998;97:2470–2472. doi: 10.1161/01.cir.97.24.2470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.