Abstract
Fertility preservation is an emerging discipline, which is of substantial clinical value in the care of young patients with cancer. Chemotherapy and radiation may induce ovarian damage in prepubertal girls and young women. Although many studies have explored the mechanisms implicated in ovarian toxicity during cancer treatment, its molecular pathophysiology is not fully understood. Chemotherapy may accelerate follicular apoptosis and follicle reservoir utilization and damage the ovarian stroma via multiple molecular reactions. Oxidative stress and the radiosensitivity of oocytes are the main causes of gonadal damage after radiation treatment. Fertility preservation options can be differentiated by patient age, desire for conception, treatment regimen, socioeconomic status, and treatment duration. This review will help highlight the importance of multidisciplinary oncofertility strategies for providing high-quality care to young female cancer patients.
Keywords: chemotherapy, radiotherapy, gonadotoxicity, fertility preservation, embryo cryopreservation, oocyte cryopreservation, ovarian tissue cryopreservation, oocyte in vitro maturation, ovarian suppression, oncofertility
1. Introduction
It is estimated that more than 9.2 million women were newly diagnosed with cancer worldwide in 2020 [1]. Furthermore, there were 89,500 new cancer cases and 9270 cancer deaths in adolescents and young adults (AYAs) aged 15–39 years in the United States [2]. The survival of cancer patients has significantly improved due to recent advances in cancer treatment [3,4]. However, oncologic therapies can affect ovarian function in young women [5,6,7,8]. The exhaustion of ovarian follicle reservoirs may lead to not only loss of fertility but also premature ovarian failure, which could result in poor quality of life in young female cancer survivors [9,10,11]. Recently, fertility preservation (FP) has become an emerging discipline with significant clinical value in the care of AYA cancer patients [12,13,14], and many organizations have provided recommendations for FP during cancer treatment [15,16,17,18,19].
Chemotherapy has toxic effects on the ovaries and causes the loss of the primordial follicle (PF) reserve [20]. Endocrine therapy can increase the risk of infertility in patients with hormone receptor-positive malignancies [21]. In the case of abdominal or pelvic cancers, treatments including radiotherapy or surgery may alter future fertility because of direct gonadal damage [22,23]. Many studies have explored the mechanisms implicated in ovarian toxicity during cancer treatment; however, the underlying molecular pathophysiology is not fully understood [24,25,26,27,28].
This article will review the mechanisms of cancer therapy-induced ovarian dysfunction and explore the future perspectives for preventing infertility in AYAs with cancer.
2. Regulation of the Ovarian Follicular Reserve
In the ovary, there is a finite number of PFs that continue to decline until menopause [29]. A single layer of granulosa cells (GCs) surrounds an immature oocyte to form each PF, which together constitute the “ovarian reserve”, which refers to the total population of PFs [30]. In mammals, the ovarian reserve is established early in life and then declines regularly throughout the reproductive years. In the human ovary, 85% of the potential oocytes are lost before birth [30]. PFs usually remain quiescent for years, and this quiescence is maintained by several molecules, including PTEN, the TSC1–TSC2 complex, Foxo3A, P27, anti-Müllerian hormone (AMH), and FoxL2 [31]. Most PFs then undergo atresia, but a minority of follicles reach the pre-ovulatory stage. The activation of dormant follicles is mediated by the upregulation of the PI3K/PTEN/Akt pathway [32,33,34,35]. The survival of PFs is regulated by PDK1 signaling or RPS6 [32]. Several studies have reported that the ovarian reserve is regulated by autophagy [36,37]. Bcl-2 and its associated proteins, such as BAX, play critical roles in the survival or apoptosis of PFs [32]. Therefore, a combination of follicular activation and multiple inhibitory/activator molecules is necessary to preserve the ovarian reserve, and any disturbance in these mechanisms may induce a premature loss of ovarian function [38].
3. Cancer Treatment-Induced Ovarian Damage
3.1. Mechanism of Chemotherapy-Induced Ovarian Damage
Chemotherapy can adversely affect the ovarian reserve [39,40,41,42,43,44,45]. Table 1 lists known gonadotoxic chemotherapy agents [29,45,46,47]. Chemotherapy-induced amenorrhea may be transient, and menstruation may recur after treatment completion. The oocytes and GCs are vulnerable to chemotherapeutic agents. Each agent may have a different mechanism of action on cancer cells, resulting in the cessation of the cell cycle.
Table 1.
Ovarian damage risk with chemotherapeutic agents and their respective mechanisms of action.
| Class | Agents | Type of Cancer | Mechanisms of Action | Damage Risk |
|---|---|---|---|---|
| Alkylating agents | Cyclophosphamide Ifosfamide Nitrosureas Chlorambucil Melphalan Busulphan Mechlorethamine |
Leukemia, breast cancer, lung cancer, ovarian cancer, prostate cancer, lymphoma, myeloma, sarcoma, Hodgkin’s disease |
Interference with cell division via intra-strand/inter-strand cross-linking of DNA. Induction of a reduction in mitochondrial transmembrane potential. Inhibition of the accumulation of cytochrome c in the cytosol. Induction of DSBs in oocytes and GCs. | High |
| Vinka alkaloids | Vinblastine Vincristine |
Testicular cancer, lymphoma, Hodgkin’s disease, breast cancer, germ cell tumors, lung cancer, sarcoma, neuroblastoma |
Inhibition of tubulin from forming into microtubules. Not gonadotoxic. | Low |
| Antimetabolites | Cytarabine Methotrexate 5-fluorouracil |
Leukemia, breast cancer, ovarian cancer, gastrointestinal cancer |
Inhibition of purine, pyrimidine becoming incorporated into DNA during S phase of cell cycle. Inhibition of RNA synthesis. Not gonadotoxic. | Low |
| Platinum agents | Cisplatin Carboplatin Oxaliplatin |
Bladder cancer, colorectal cancer, head and neck cancer, lung cancer, ovarian cancer, testicular cancer |
DNA damage by formation of inter-strand/intra-strand DNA adducts which interfere with cellular transcription and replication. Accumulation of abl and TAp63-alpha protein in the oocyte leading to oocyte death. | Moderate |
| Anthracycline antibiotics | Daunorubicin Bleomycin Doxorubicin |
Lymphoma, leukemia, breast cancer, sarcoma |
Intercalation with DNA and prevention of its replication and transcription via the inhibition of topoisomerase II. Upregulation of P53 protein which induces apoptosis in the presence of high levels of DNA damage. DNA DSBs leading to activation of ATM, which initiates apoptosis. GCs are usually targeted due to their mitotically and metabolically active characteristics. | Low/moderate |
| Others | Procarbazine | Hodgkin’s disease, brain cancer |
Inhibition of DNA methylation, and RNA and protein synthesis. | High |
3.1.1. PF Loss via DNA Alteration, Follicular Atresia, and Apoptosis
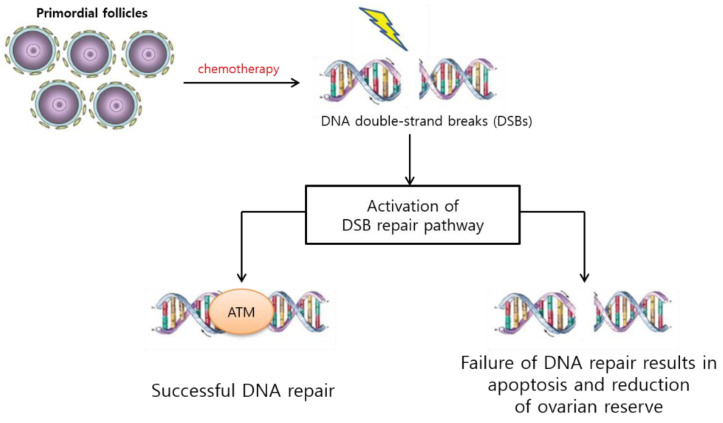
Chemotherapeutic agents can induce double-stranded breaks (DSBs) in DNA. If a DSB is repaired successfully, the cell survives via ataxia–telangiectasia mutated (ATM)-mediated DNA damage repair pathways. However, if the repair pathways fail, DNA damage can result in cellular apoptosis [48] (Figure 1). Apoptosis in the ovary has been demonstrated in growing follicles and has been shown to originate in proliferating GCs [49]. Within mature oocytes, the P63 protein activates BAX and BAK proteins, which can be transmitted by the activation of Tap73, a P53-upregulated modulator of apoptosis, and phorbol-12-myristate-13-acetate-induced protein 1 [50]. Many studies have demonstrated these mechanisms in vitro and in vivo [51,52,53,54,55]. Notably, even chemotherapeutic agents with low gonadotoxicity can alter the development of growing follicles because mitotic cells are especially sensitive to chemotherapeutic agents [51,55].
Figure 1.
Chemotherapy-induced DNA double-stranded breaks (DSBs). If the DSB is repaired successfully, the cell survives via ataxia–telangiectasia mutated (ATM)-mediated DNA damage repair pathways. However, DNA damage can result in cellular apoptosis and a reduction in the ovarian reserve if the repair pathway fails.
3.1.2. Follicle Loss via Activation and “Burnout”
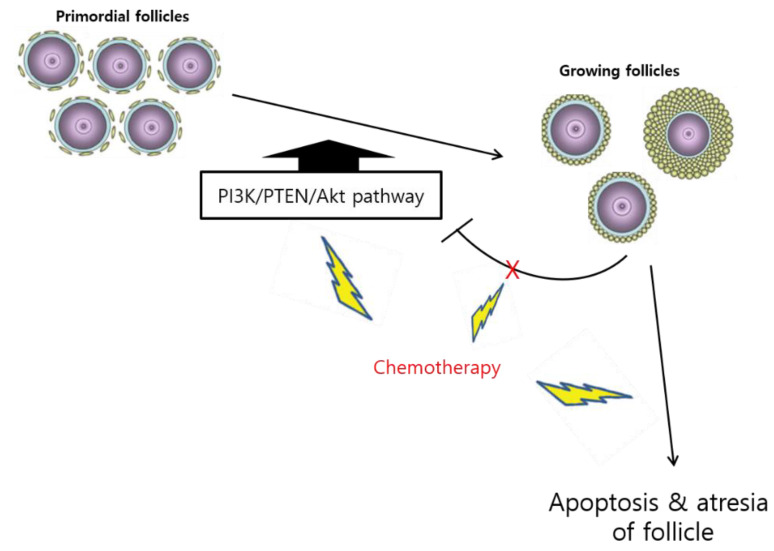
The PI3K/PTEN/Akt pathway terminates follicle dormancy, directly influences the oocytes and pre-GCs of PFs, and indirectly destroys large follicles [24]. The destruction of follicles induces the impairment of AMH and reduces the suppression of the PF pool, which is followed by the activation of PFs in an attempt to compensate for the decrease in the number of growing follicles [56]; this phenomenon is called the “burnout effect” [57,58] (Figure 2). In a previous study, exposure to 3-methylcholanthrene, a carcinogen and ovotoxicant, resulted in PF activation and depletion [59], implying that the burnout effect triggers the growth of dormant follicles. However, this induction of dormant follicle growth is affected by the upregulation of the PI3K/PTEN/Akt pathway and substantial follicular apoptosis, which results in a reduction in AMH secretion. This could be caused by a direct effect on oocytes and pre-GCs [56,60,61]. Reduced AMH levels and enhanced follicular recruitment and atresia in patients with ovarian endometriosis may have the same effect [62]. However, the mechanism by which chemotherapeutic agents induce the activation of this pathway is still under investigation.
Figure 2.
“Burnout” theory of ovarian follicle reserve. The chemotherapy-induced PI3K/PTEN/Akt pathway activates the destruction of follicles, followed by an impairment of AMH and the loss of the suppression of the primordial follicle (PF) pool. Consequently, the PFs are activated to compensate for the decrease in the number of growing follicles.
3.1.3. Stromal and Microvascular Damage
The ovarian stroma can be indirectly damaged by chemotherapeutic agents [29,63]. For example, the administration of doxorubicin results in a significant reduction in ovarian blood supply and small vessel spasms in the ovary [64]. Another study reported that chemotherapy led to stromal fibrosis and ovarian vascular abnormalities [65]. These findings imply that damage to blood vessels and focal fibrosis of the ovarian cortex could be another mechanism of chemotherapy-induced ovarian dysfunction [66]. Additionally, in patients previously exposed to chemotherapy, the ovaries show thickening and hyalinization of cortical stromal vessels caused by the disorganization of blood vessels in the ovarian cortex and cortical fibrosis [67]. The production of sex steroids decreases because of damage to endocrine ovarian function after exposure to chemotherapeutic agents [68]. This is also supported by a recent study that showed an inverse correlation between ovarian vascular density and PF apoptosis [69], thus suggesting an indirect mechanism by which chemotherapy-induced ovarian vascular injury reduces the number of PFs.
3.2. Radiation-Induced Ovarian Damage
Ionizing radiation to the abdominopelvic region has deleterious effects on gonadal function at all ages [70]. For example, cervical and rectal cancers usually require pelvic irradiation, and craniospinal radiotherapy is performed in cases of central nervous system malignancy. In some patients with Hodgkin’s disease, pelvic lymph nodes require irradiation, and total body irradiation may be necessary prior to bone marrow transplantation.
The resulting damage depends on the dose and field of irradiation and the age of the patient. Women who received radiation treatment outside the pelvis had a low risk of ovarian dysfunction [71]. In the prepubertal period, the ovaries are relatively resistant to gonadotoxicity [72].
3.2.1. Radiosensitivity of Oocytes
Dividing GCs appear to be the main target of radiation-related gonadotoxicity. Prominent cell death has been observed within a few hours of irradiation [73]. Oocytes are highly radiosensitive because the estimated dose at which half of the follicles are lost in humans (LD50) is <2 Gy [74]. A single oocyte is highly radiosensitive to a D0 of 0.12 Gy (reciprocal of the slope of the exponential region of a survival curve). This sensitivity is affected by age; women younger than 40 years of age are less sensitive, requiring 20 Gy to experience permanent damage, whereas older women require only 6 Gy [75]. The radiosensitivity of oocytes differs according to their growth phase. A quiescent PF is usually more radio-resistant than a large maturing follicle [74]. Radiotherapy-induced ovarian damage also occurs in the stroma with vascular damage, resulting in tissue atrophy and fibrosis [73]. In general, a combination of multiple factors determines the extent of radiosensitivity, including age, the use of combination therapy, and radiation dose [76].
3.2.2. Linear Energy Transfer
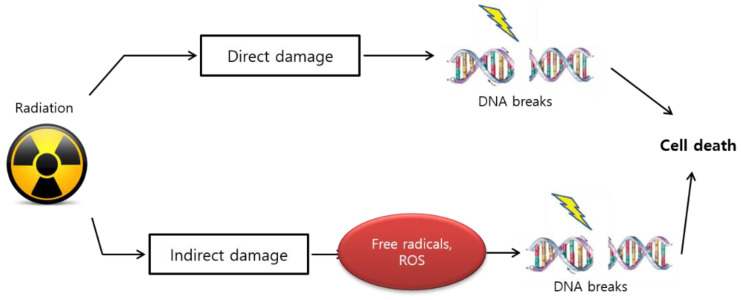
The biological effect of radiation treatment is also affected by linear energy transfer (LET) in tumors [77]. LET radiation induces anticancer effects by depositing physical energy or radiation into malignant cells, which results in stable free radicals and induces cellular damage because of the direct ionization of the cellular macromolecules, such as DNA, RNA, lipids, and proteins [78]. High LET radiation results in gonadal DNA damage that causes multiple lesions within the helical turns of the DNA, which is referred to as “direct” damage (Figure 3).
Figure 3.
Biological effect of radiation via linear energy transfer (LET) in tumors. High LET radiation results in “direct” gonadal DNA damage that incorporates multiple lesions within the helical turns of the DNA molecule. Conversely, the increase in reactive oxygen species (ROS) induces rapid primordial follicle loss via “indirect” damage.
3.2.3. Oxidative Stress Resulting in DNA Damage
Radiotherapy also leads to the generation of reactive oxygen species (ROS) in cancer cells as a result of water radiolysis, inducing oxidative stress and the diminution of the antioxidant defense mechanisms, which could also affect healthy normal tissues, including the ovaries [79]. Thus, the imbalance between the free radicals and the oxidative radicals may play a role in the etiology of radiotherapy-induced gonadotoxicity [80]. Increased ROS levels induce rapid PF loss and female infertility via DNA damage, which is termed as “indirect” damage [81] (Figure 3). ROS can induce apoptosis, leading to oxidative stress exerted on cellular macromolecules, ultimately activating the intrinsic mitochondrial pathway of apoptosis via p53 activation, which results in cytochrome c release and caspase activation [82,83]. Activated caspases cleave DNA damage repair enzymes to block cellular DNA repair and enhance apoptosis [84]. Another study identified increased activity of the MAPK signaling pathway in irradiation-induced GC apoptosis [85]. Human oocytes express DNA repair genes, but their role in the repair of radiation-induced genomic damage is unclear [86]. As ionizing radiation generates free radicals that induce DNA damage, compounds that scavenge free radicals may be useful for protection against radiation damage [87].
4. Detection of Ovarian Damage
4.1. Clinical Considerations
Older age is an important risk factor for ovarian damage compared to younger age. A significantly higher incidence of amenorrhea after chemotherapy was observed in female patients with breast cancer aged >40 years than in younger women [88]. Another study confirmed similar results [89]. A cross-sectional analysis of data from a prospective cohort study demonstrated that the ovarian reserve was impaired in a dose-dependent manner in patients exposed to cancer therapies, including chemotherapy or pelvic radiotherapy [90].
4.2. Biochemical Markers for Ovarian Reserve
4.2.1. AMH
AMH is produced by GCs from ovarian follicles, which are mainly small antral follicles. Typically, its level rapidly decreases with age and becomes undetectable during menopause [91]. Accordingly, AMH, at a low level, exhibits high sensitivity and specificity for chemotherapy-exposed women with poor ovarian reserve [92]. AMH levels before or after cancer treatment provide information regarding the diversity of gonadal damage according to the type of the chemotherapeutic agent and help predict long-term ovarian function [93]. Several studies have shown that there is an AMH level threshold that predicts the risk of amenorrhea after chemotherapy [94,95,96].
However, the level of AMH does not always correlate with the quality of oocytes because it only reflects the quantity of oocytes [97]. Additionally, AMH concentration could be altered by the handling of the blood sample or the assay method used to measure AMH levels [98].
4.2.2. Basal Follicle-Stimulating Hormone and Estradiol
Follicle-stimulating hormone (FSH) concentrations usually change throughout the menstrual cycle and can be best measured during the early follicular phase. Basal FSH, which is the FSH level during the early follicular phase, is widely used to determine ovarian reserve. FSH levels >10 IU/L on menstrual cycle day 2 or 3 may indicate a decreased ovarian reserve. Elevated FSH levels in young women with amenorrhea suggest premature ovarian insufficiency.
After chemotherapy, FSH levels usually increase due to follicular depletion. However, basal FSH is not always a valuable marker of ovarian reserve in patients who have undergone cancer treatment. For example, if women have regular menstrual cycles, FSH levels may show normal values, even though the ovarian reserve decreases after treatment [89,99]. In such instances, that is, when the FSH levels are within the normal range, estradiol concentration in the early follicular phase may provide additional information [100].
4.2.3. Inhibin-B
Inhibin-B is secreted by the GCs of antral follicles and it regulates FSH levels via a negative feedback reaction. Inhibin-B is usually exhibited at low levels in women with a decreased ovarian reserve [101]. However, it is not a reliable marker of the ovarian reserve because its levels vary widely during menstrual cycles [102].
4.3. Ultrasonographic Markers
During the early follicular phase, transvaginal ultrasound can be used to count antral follicles measuring 2–10 mm in both ovaries [103]. A low AFC may be related to a diminished response to ovarian stimulation. Furthermore, a few studies have demonstrated that a low AFC could be a marker for the risk of developing amenorrhea after cancer treatment [94,96]. However, the estimation of ovarian volume using ultrasound provides limited clinical utility as an ovarian reserve marker.
5. Prevention and Management of Ovarian Damage
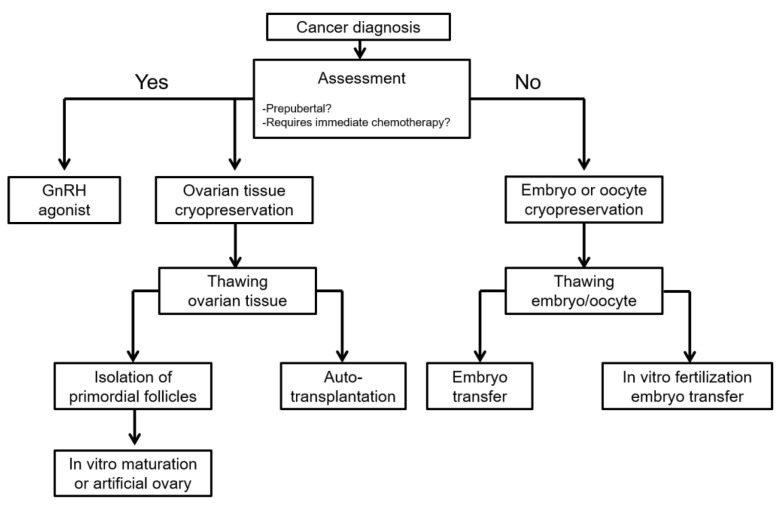
FP options could be differentiated by patient age, desire for conception, treatment regimen, socioeconomic status, and treatment duration (Figure 4) [45]. Such options include the use of hormonal medications for ovarian suppression, various methods for protecting the ovaries during radiotherapy, cryopreservation, in vitro oocyte maturation, artificial ovaries, and stem cell technologies.
Figure 4.
Fertility preservation options for female cancer patients. This could be differentiated by individual situations. Adapted and reprinted, with permission, from Cho, 2020 [45].
5.1. Prevention of Chemotherapy-Induced Ovarian Damage
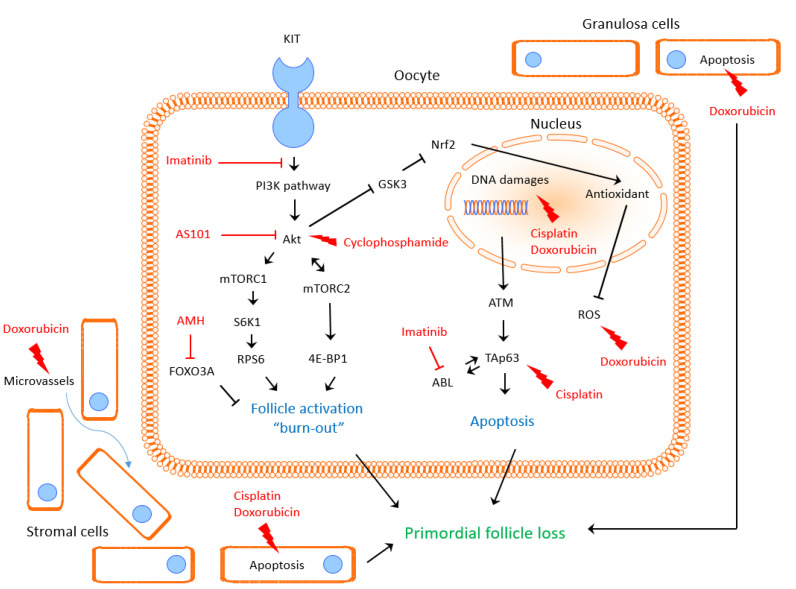
With conventional chemotherapy regimens, cytotoxicity-associated ovarian insufficiency involves PF pool depletion by apoptosis or hyperactivation mechanisms, notably mediated by the ABL/TAp63 and PI3K/Akt/mTOR pathways (Figure 5).
Figure 5.
Impact of cytotoxic agents and protective molecules on pathways involved in primordial follicle pool loss.
5.1.1. Gonadotropin-Releasing Hormone (GnRH) Agonists
GnRH agonists are hormonal medications that modulate gonadotropins and sex hormones [104]. Ovarian suppression via the administration of a GnRH agonist before or during chemotherapy may have protective effects on the ovaries by downregulating the secretion of FSH and pituitary luteinizing hormone [105]. Previous studies have shown that ovarian suppression with GnRH agonists during chemotherapy protects ovarian function in AYAs treated for lymphoma, breast cancer, and other diseases [106,107,108]. The American Society of Clinical Oncology guidelines state that there are conflicting recommendations regarding GnRH agonists and other means of ovarian suppression for FP [15].
GnRH analogs have two possible mechanisms of action [109,110,111]. The first involves decreasing the sensitivity of the PFs entering the growing pool to gonadotoxicity by the administration of a GnRH agonist. The second constitutes the direct anti-apoptotic effect of GnRH agonists on ovarian germline stem cells. Several studies have demonstrated that the use of GnRH agonists reduces chemotherapy-induced ovarian damage; however, the mechanism underlying the protective effect on ovaries remains unclear [112,113,114,115,116,117]. Therefore, the use of GnRH agonists is considered suitable for AYAs with cancers for whom other established FP options are not suitable—such as cryopreservation—to reduce chemotherapy-induced ovarian insufficiency. The use of GnRH agonists, in combination with other modalities, including oocyte or embryo freezing, may be a good option [118]. GnRH agonists usually do not have a protective effect against radiation treatment-induced gonadotoxicity [119,120].
5.1.2. AS101
AS101 is a non-toxic immunomodulator that acts on the PI3K/PTEN/Akt pathway [121]. AS101 is known to inhibit Akt activation in mouse multiple myeloma cell lines [122]. An in vivo study demonstrated that AS101 was effective in reducing apoptosis in the GCs of growing follicles by regulating the PI3K/PTEN/Akt pathway [123]. Moreover, AS101 does not interfere with the therapeutic effect of chemotherapy and in fact shows synergistic antitumor activity [124,125,126,127]. According to an in vivo study using cyclophosphamide-treated mice, cyclophosphamide induced an increase in early growing follicles and an increased ratio of growing/dormant follicles was observed histologically. This suggests that cyclophosphamide-induced primordial follicle loss is not due to apoptosis, but the activation of primordial follicles to undergo recruitment and growth. AS101 cotreatment reduces cyclophosphamide-induced activation of PTEN/PI3K/Akt pathway proteins in the ovaries. AS101 was also found to reduce follicle loss and improve AMH concentration after cyclophosphamide treatment. In addition to preventing follicle loss and maintaining fertility, AS101 also has the added benefit of improving the responsiveness of breast cancer cells to cyclophosphamide chemotherapy [122].
5.1.3. Anti-Müllerian Hormone (AMH)
AMH is produced in GCs of growing follicles and has a suppressive effect on follicle activation [128,129,130]. AMH inhibits PF recruitment and growth, and the loss of AMH results in the depletion of the PF pool. A previous study confirmed that the initiation of primordial follicle growth was inhibited when human ovarian cortical tissue was cultured with recombinant AMH [127]. The combination of recombinant AMH with the cyclophosphamide metabolite in an ex vivo culture system maintained a high number of PFs in the ovaries [24]. Moreover, when recombinant AMH was administered together in a model of CY-treated pubertal mice, FOXO3A phosphorylation, the main factor for PMF activation, was significantly reduced. The pPS6K level was decreased in the mice treated with AMH compared to the mice treated with only CY, suggesting that AMH prevents PF activation through the mTOR pathway [58]. AMH shows minimal side effects because it is an endogenous hormone with activity limited to the ovaries.
5.1.4. Imatinib
Imatinib, a tyrosine kinase inhibitor used in cancer treatment, selectively inhibits the ABL kinase domain of the bcr-abl oncogenic protein present in patients with chronic myelogenous leukemia [131]. It is also known to inhibit cKIT and platelet-derived growth factor receptor tyrosine kinases in gastrointestinal stromal tumors [132,133]. As PF depletion by apoptosis or activation mechanisms are mainly mediated by the ABL/TAp63 and KIT/PI3K pathways, respectively, it can be hypothesized that imatinib might be able to prevent ovarian dysfunction caused by these pathways [134]. Previous studies have demonstrated that c-Abl helps maintain genomic integrity by managing DNA breaks [135,136]. Many studies have investigated the protective effects of imatinib, but conflicting results have been reported [137,138,139]. In PFs of postnatal day 5 mice, cisplatin induced c-Abl nuclear accumulation, increased TAp63 levels, and eventually caused cell apoptosis. Treatment with the c-Abl kinase inhibitor, imatinib, rescued cisplatin-induced depletion of the follicle reserve. The average number of pups and pregnancy rates were also higher in mice transplanted with ovarian tissue treated with combined cisplatin and imatinib [130]. However, in a similar mouse model study, the number of PFs and the average number of pups were not significantly higher in the group treated with imatinib in addition to cisplatin [133]. This conflicting result is thought to be due to the different types of cisplatin used in each study, and thus the toxicity to PFs at the same dose was different.
5.1.5. Sphingosine-1-Phosphate
Sphingosine-1-phosphate (S1P), a naturally occurring sphingolipid, inhibits the ceramide-promoted apoptotic pathway [140]. It increases vascularity and angiogenesis, and reduces PF apoptosis [69]. In mice xenotransplanted with human ovarian cortical tissue, the co-administration of S1P with cyclophosphamide and doxorubicin was associated with a lower rate of apoptosis. The authors analyzed the expression of activated caspase 3 using immunohistochemistry to evaluate the activation of apoptotic cell death pathways. The expression of activated caspase 3 in human ovarian tissue cotreated with S1P was significantly lower than that in the group treated with cyclophosphamide or doxorubicin alone [141]. It also showed a protective effect in mice treated with dacarbazine [142]. However, another study found no reduction in chemotherapy-induced follicle loss in S1P-treated rats [143].
5.1.6. Granulocyte Colony-Stimulating Factor
Granulocyte colony-stimulating factor (G-CSF) treatment significantly decreases the loss of PFs induced by chemotherapeutic agents, prevents damage to vascular structures, and reduces DNA damage in oocytes or growing follicles. In 6-week-old mice treated with high-dose alkylating chemotherapy (cyclophosphamide and busulfan), G-CSF with or without stem cell factor maintained PF numbers. G-CSF treatment with or without stem cell factor treatment decreases chemotherapy-induced gamma H2AX phosphorylation in early growing follicles, which is the earliest cellular response to DNA damage. G-CSF coadministration also increases microvessel density, as assessed by immunofluorescent staining for PECAM1/CD31 in vascular endothelial cells after chemotherapy treatment [69,144]. It also shows an anti-apoptotic effect; however, there is a concern that G-CSF may induce follicle loss associated with focal ischemia, fibrosis, and infarcts of blood vessels [69,145].
5.2. Prevention of Ovarian Damage before and during Radiation Treatment
5.2.1. Identifying the Location of the Ovaries
Technical improvements in radiotherapy have maximized target coverage while sparing normal organs using highly conformal dose distributions [73]. Identifying the location of the ovaries during treatment is essential for determining the dose administered to the ovaries during radiotherapy. In one study, ovaries were identified in 75% of the patients via an abdominal computed tomography (CT) scan [146]. However, the position of the ovary is affected by the contents of the bladder. Variations in the ovary position can result in their displacement to a high-dose irradiation field [147]. Therefore, daily on-board CT imaging may be used to map the ovary position before radiation treatment. Three-dimensional imaging provides relevant information for use in adaptive radiotherapy. Additionally, patient education regarding bladder filling before treatment would help not only to maintain an accurate radiation field for effective treatment but also to improve the protection of the ovaries [73,148].
5.2.2. Alternative Techniques for Craniospinal Radiotherapy
Craniospinal irradiation can induce gonadal damage [149,150]. Traditionally, craniospinal irradiation has been conducted using the standard posterior–anterior inferior spinal field. However, opposed lateral fields for the inferior spine help to reduce the radiation dose around the ovaries [151]. In other studies, proton therapy for craniospinal irradiation generated with a posterior–anterior proton beam stopping just after the thecal sac showed a reduced ovarian dose without an unexpected exit dose [152,153,154].
5.2.3. Ovarian Transposition
In certain situations, the ovaries may be located proximally to the lesion targeted by the high-dose radiation. The transposition of ovaries out of the fields, which is a procedure called oophoropexy, may help to protect the ovaries against radiation-induced damage [155,156,157], and it has been recommended for all AYAs requiring radiation [158,159]. Ovarian transposition has been recommended for patients with advanced cervical cancer who require radiotherapy, but only a quarter of patients younger than 40 years have undergone oophoropexy before treatment [160,161,162,163]. Laparoscopic techniques can be used to minimize surgical morbidity [164,165]. Ovaries are usually fixed to the anterolateral abdominal wall. During the procedure, the ovarian vessels should be carefully mobilized to avoid damage. Metallic clips are usually applied at the base of the transposed ovary [159,162]. As there is a risk of gonadal failure after pelvic irradiation following ovarian transposition, concurrent ovarian tissue cryopreservation during surgery is strongly recommended [166]. External beam radiation could be the main risk factor for ovarian failure even after transposition, and combined chemotherapy may aggravate the risk [74,162,167]. Ovarian transposition should be offered to all women who have a high possibility of radiation exposure near the ovaries. However, it should not be offered to women with poor ovarian reserve, with a high risk of ovarian metastasis, or who are scheduled for chemotherapy only.
5.3. Cryopreservation
5.3.1. Embryo Cryopreservation
Although ovarian damage can be reduced using the above-mentioned surgical or imaging-guided methods or via the administration of protective agents before chemotherapy, embryo cryopreservation is the most well-established method for preserving fertility [168]. Embryo freezing should initially be considered for FP treatment if there is adequate time for ovarian stimulation, and a partner or donor sperm is available [169]. This technique is safe and effective for patients undergoing assisted reproductive procedures [170,171]. Slow freezing and vitrification methods offer excellent results and are widely used [172]. Several studies have suggested that embryo vitrification and thawing methods are better than slow freezing and thawing methods in terms of pregnancy and live birth rates [173,174,175]. Many institutions prefer vitrification as a simpler and less expensive alternative to slow freezing, which requires the use of controlled-rate freezers and large quantities of liquid nitrogen.
Embryo cryopreservation requires ovarian stimulation; therefore, this option is not adequate for prepubertal girls. Additionally, embryo cryopreservation may not be appropriate for women who do not have a partner or do not want to use donor sperm. Furthermore, this technique may entail the risk of losing reproductive autonomy and present possible issues with the ownership of stored embryos. In patients with hormone-dependent breast cancer, aromatase inhibitors can be used as alternatives to ovarian stimulation [176]. If there is insufficient time for FP procedures before cancer treatment, random-start ovarian stimulation would be appropriate [177,178,179]. In studies comparing the results of in vitro fertilization and embryo cryopreservation in patients with cancer and those without cancer, contradictory results were observed in terms of fertilization and live birth rates [178,180,181,182].
5.3.2. Oocyte Cryopreservation
Oocyte cryopreservation is another widely used method, which is considered a standard technique for FP in patients with cancer [183]. The introduction of vitrification into assisted reproductive techniques has resulted in oocyte cryopreservation outcomes similar to those obtained with fresh oocytes [184,185]. In 2013, the American Society for Reproductive Medicine (ASRM) approved oocyte cryopreservation for FP based on the results of four clinical trials [186,187,188,189]. This technique has similar disadvantages to those of embryo cryopreservation because it involves ovarian stimulation, which makes it unsuitable for prepubertal girls. However, it can be utilized for women who are single or do not want sperm donation. Vitrification was more effective than slow freezing in avoiding crystallization because the former reduced cellular damage and chilling injury during the freezing process [189,190]. Accordingly, the National Institute for Health and Care Excellence guidelines recommend vitrification instead of controlled-rate freezing for oocyte cryopreservation, given the availability of the necessary equipment and expertise [191].
As oocyte freezing involves the removal of cumulus cells before cryopreservation, it can induce changes in the zona pellucida, which may affect the fertilization rates of conventional insemination. Therefore, ASRM recommends intracytoplasmic sperm injection for frozen oocytes as the preferred procedure [168,192].
The combination of oocyte cryopreservation and ovarian tissue cryopreservation can enhance the results of the FP procedure [193]. However, the cryopreservation of ovarian tissue concomitant with oocyte retrieval is ineffective; thus, it is not recommended after ovarian stimulation with human menopausal gonadotropin or recombinant FSH followed by human chorionic gonadotropin [194,195].
5.3.3. Ovarian Tissue Cryopreservation and Transplantation
Ovarian tissue cryopreservation is generally the only option for FP in children or AYAs with cancer who need immediate treatment and do not have enough time for ovarian stimulation and other procedures. Using this technique, a large number of oocytes, including PFs, can be preserved, and hormonal function of the ovary can be protected to improve the quality of life of the young patients [196].
Ovarian tissues are excised via biopsy, partial oophorectomy, or total oophorectomy, and are frozen for preservation before the initiation of cancer treatment [197]. To date, slow freezing has been established as the preferred method for ovarian tissue cryopreservation over vitrification [198]. However, several recent studies have shown promising results after vitrification [199,200,201]. A review of 60 cases showed that ovarian activity was restored in 92.9% of the cases after the transplantation of the ovarian tissue that was cryopreserved using the slow-freezing method [195]. After ovarian tissue transplantation, recovery of ovarian function has been reported, along with successful live births [183].
Oocyte cryopreservation is not suitable for patients with ovarian or hematologic malignancies because of the possible contamination of the ovarian tissue with malignant cells, as shown in several studies [202,203]. Nonetheless, ovarian tissue cryopreservation may be considered after an initial dose of chemotherapy to reduce the risk of malignant cell contamination, despite possible partial ovarian damage [204].
Recently, a study evaluated the changes in telomere length and senescence markers during ovarian tissue cryopreservation. The mean telomere length was significantly decreased after cryopreservation, and Western blot analysis indicated that senescence markers were affected by cryopreservation [205]. The possibility of irreversible DNA changes, such as the shortening of telomere length and alterations in senescence markers, should be considered when using this technique.
6. Future Perspectives on FP
6.1. Whole Ovarian Transplantation
Whole ovarian transplantation has the benefit of immediate revascularization following blood vessel anastomosis, thereby reducing ischemic injury; however, potential injury due to hypothermic damage to blood vessels and difficulties in dispersing a sufficient amount of cryoprotective agent make it challenging in clinical practice [206,207]. Successful whole ovarian cryopreservation and transplantation have been reported in animal studies, and whether vitrification or slow freezing is preferable for cryopreservation is still under debate [208,209,210,211]. Before whole ovarian transplantation can be established in clinical use, there may be several challenges to its adoption, including proficient and diligent use of surgical techniques and identifying optimal protocols for cryopreservation and thawing. To date, there has been no experimental model to explore techniques for the cryopreservation and retransplantation of human ovaries. Table 2 shows several studies that evaluated the feasibility of the xenotransplantation of human ovaries into animals for study purposes.
Table 2.
Experiments which evaluated the feasibility of human ovarian xenotransplantation into animals.
| Evaluation | Animal | Materials | Transplantation Site | Publication |
|---|---|---|---|---|
| Oocyte maturation after xenotransplantation | SCID mice | Ovarian tissue | Back muscle, kidney capsule | Soleimani et al. [212] |
| SCID mice | Ovarian tissue | Kidney capsule | Gook et al. [213,214] | |
| SCID mice | Ovarian tissue | Neck muscle | Lotz et al. [215] | |
| SCID mice | Ovarian tissue | Subcutaneous space | Kim et al. [216] | |
| Follicular development | SCID mice | Ovarian tissue | Intraperitoneum | Ayuandari et al. [217] |
| NOG mice | Ovarian cortex | Back skin, kidney capsule, ovarian bursa | Terada et al. [218] | |
| SCID mice | Clot containing preantral follicle | Ovarian bursa | Dolmans et al. [219] | |
| SCID/hpg mice | Ovarian tissue | Kidney capsule | Oktay et al. [220] | |
| Swiss nu/nu mice | Ovarian tissue | Intraperitoneum | David et al. [221] | |
| NOD/SCID mice | Ovarian tissue | Subcutaneous space | Weissman et al. [222] | |
| NMRI nu/nu | Ovarian tissue | Intraperitoneum, subcutaneous space, ovarian bursa, thigh muscle | Dath et al. [223] | |
| Swiss nu/nu mice | Clot containing preantral follicle | Intraperitoneum | Paulini et al. [224] | |
| SCID mice | Ovarian tissue | Intraperitoneum | Amorim et al. [225] | |
| NMRI nu/nu | Ovarian tissue | Thigh muscle | Jafarabadi et al. [226] | |
| NOD/SCID mice | Ovarian cortex | Subcutaneous space | Campos-Junior et al. [227] | |
| Optimization of grafting protocols | NMRI nu/nu | Ovarian tissue | Intraperitoneum | Van Eyck et al. [228] |
| SCID mice | Ovarian cortex | Back muscle | Soleimani et al. [69] | |
| B6cg nude mice | Ovarian tissue | Back skin, thigh muscle | Hormozi et al. [229] | |
| NOD/SCID mice | Ovarian cortex | Kidney capsule, subcutaneous space | Hernandez-Fonseca et al. [230] | |
| SCID mice | Ovarian tissue | Neck muscle | Maltaris et al. [231] | |
| Balb/C nu/nu | Ovarian tissue | Back muscle | Friedman et al. [232] | |
| SCID mice | Ovarian stroma | Subcutaneous space | Fu et al. [233] | |
| NMRI nu/nu | Ovarian tissue | Intraperitoneum | Van Langendonckt et al. [234] | |
| New Zealand rabbits | Ovarian tissue | Back muscle | Wang et al. [235] | |
| Balb/C nu/nu | Ovarian tissue | Ovarian bursa, subcutaneous space | Ruan et al. [236] | |
| Reimplantation of malignant cells | SCID mice | Ovarian cortex | Intraperitoneum | Luyckx et al. [237] |
| NOD/SCID mice | Ovarian tissue | Subcutaneous space | Kim et al. [238] | |
| NMRI nu/nu | Ovarian tissue | Subcutaneous space | Greve et al. [239] | |
| SCID mice | Ovarian tissue | Neck muscle | Lotz et al. [240] | |
| SCID mice | Ovarian tissue | Intraperitoneum | Dolmans et al. [241] |
NMRI: National Medical Research Institute; NOD: non-obese diabetic/severe combined immunodeficient; NOG: non-obese diabetic/severe combined immunodeficient/γcnull; SCID: severe combined immunodeficient; SCID/hpg: severe combined immunodeficient/hypogonadism.
6.2. In Vitro Maturation
In vitro maturation (IVM) is usually used in patients with polycystic ovarian syndrome (PCOS). This requires immature oocyte retrieval and cryopreservation either at an immature stage or at a post-IVM mature state [242]. This can also be used in patients with cancer who lack adequate time for ovarian stimulation or prepubertal girls who need immediate treatment. Although many researchers have attempted to improve outcomes by combining IVM and vitrification, only a few live births have been reported after IVM procedures in patients with cancer [243,244,245]. Further technical improvements may allow the use of this technology for FP in the near future.
6.3. Artificial Ovaries
The transplantation of ovarian tissue with a scaffold accelerates tissue vascularization via the release of multiple bioactive materials [246,247]. Recently, this technique has been most commonly used to enable the transplantation of various substances or accessory cells, including stem cells, together with ovarian tissues [248,249,250]. Artificial ovaries can be useful for developing mature oocytes via multiple processes, including in vitro culture of oocytes, isolated follicles, and ovarian tissue [251,252]. In animal studies, this approach restored endocrine function, enabling in vivo follicle development and successful pregnancy; however, there have been no successful reports in humans [252,253]. Improvements in techniques and addressing genetic safety concerns are warranted for more consistent results in human applications.
6.4. Ovarian Stem Cells
Stem cells are being investigated for use in FP. One study reported the successful detection and isolation of ovarian stem cells in animals and humans [254,255]. Additionally, egg-producing stem cells isolated from ovaries differentiate into young oocytes [254]. This technique may provide another option for prepubertal children with cancer and women for whom conventional FP methods are not suitable. However, it is not commonly applied in clinical practice because of insufficient evidence in human-assisted reproduction, the scarcity of ovarian stem cells, and the ethical issues related to the use of oocytes and embryos [256]. Further studies are required to implement these approaches in clinical practice.
7. Conclusions
Improving our knowledge regarding the molecular mechanisms involved in cancer therapy-induced ovarian damage can lead to the development of treatments to limit follicular damage. Some of the molecular mechanisms involved in the protective effects of various agents are unclear. Although several studies have assessed the effect of disease and treatment on human ovaries, few studies have been conducted because of ethical concerns [257,258]. However, understanding the molecular etiology of treatment-induced ovarian dysfunction can not only aid in identifying targets to prevent and reduce gonadal damage during cancer treatment, but also increase the number of options for FP.
Gonadotoxic cancer treatments induce iatrogenic premature ovarian insufficiency and loss of fertility in prepubertal girls and AYAs with cancer. An individualized strategy including established and experimental techniques should be provided based on patient age, marital status, economic status, chemotherapy regimen, cancer type, staging upon diagnosis, and available time for the FP process to prevent loss of ovarian function and fertility.
Novel therapies are of great interest, as they may limit follicular loss and protect the ovaries before or during cancer treatment. These therapies could be utilized in combination with standard FP techniques, or they may be used alone in the future. These strategies can assist young women who are not eligible for traditional FP methods because of their age or limited time before the initiation of disease treatment. Effective multidisciplinary oncofertility strategies involving a highly skilled and experienced team composed of medical oncologists, gynecologists, reproductive biologists, surgeons, patient care coordinators, psychologists, and research scientists should be carefully considered for each patient to provide high-quality care.
Author Contributions
Writing—original draft preparation, S.K. and S.-W.K.; writing—review and editing, S.-W.K. and S.L.; conceptualization and visualization, S.L.; supervision, intellectual content, and paper coordination, S.-J.H., H.-T.P., J.-Y.S. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea Grant by the Korean Government (grant number NRF-2016R1C1B3015250).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., Benaoudia M.F., Keegan T.H., Hipp H.S., Jemal A., Siegel R.L. Cancer statistics for adolescents and young adults. CA Cancer J. Clin. 2020;70:443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 3.Birch J.M., Marsden H.B., Jones P.H., Pearson D., Blair V. Improvements in survival from childhood cancer: Results of a population based survey over 30 years. Br. Med. J. (Clin. Res. Ed.) 1988;296:1372–1376. doi: 10.1136/bmj.296.6633.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wingo P.A., Ries L.A., Parker S.L., Heath C.W., Jr. Long-term cancer patient survival in the United States. Cancer Epidemiol. Biomark. Prev. 1998;7:271–282. [PubMed] [Google Scholar]
- 5.Sklar C.A., Mertens A.C., Mitby P., Whitton J., Stovall M., Kasper C., Mulder J., Green D., Nicholson H.S., Yasui Y., et al. Premature menopause in survivors of childhood cancer: A report from the childhood cancer survivor study. J. Natl. Cancer Inst. 2006;98:890–896. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 6.Wallace W.H.B., Thomson A.B., Saran F., Kelsey T.W. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:738–744. doi: 10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 7.Vriens I.J.H., de Bie A.J.R., Aarts M.J.B., de Boer M., van Hellemond I.E.G., Roijen J.H.E., van Golde R.J.T., Voogd A.C., Heijnen V.C.G.T. The correlation of age with chemotherapy-induced ovarian function failure in breast cancer patients. Oncotarget. 2017;8:11372–11379. doi: 10.18632/oncotarget.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemaitilly W., Li Z., Krasin M.J., Brooke R.J., Wilson C.L., Green D.M., Klosky J.L., Barnes N., Clark K.L., Farr J.B., et al. Premature Ovarian Insufficiency in Childhood Cancer Survivors: A Report From the St. Jude Lifetime Cohort. J. Clin. Endocrinol. Metab. 2017;102:2242–2250. doi: 10.1210/jc.2016-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganz P.A., Greendale G.A., Petersen L., Kahn B., Bower J.E. Breast cancer in younger women: Reproductive and late health effects of treatment. J. Clin. Oncol. 2003;21:4184–4193. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 10.Muka T., Williams C.O., Kunutsor S., Laven J.S.E., Fauser B.C.J.M., Chowdhury R., Kavousi M., Franco O.H. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016;1:767–776. doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- 11.Wu X., Cai H., Kallianpur A., Li H., Yang G., Gao J., Xiang Y.B., Ji B.T., Tang Y., Zheng W., et al. Impact of premature ovarian failure on mortality and morbidity among Chinese women. PLoS ONE. 2014;9:e89597. doi: 10.1371/journal.pone.0089597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S., Heytens E., Moy F., Ozkavukcu S., Oktay K. Determinants of access to fertility preservation in women with breast cancer. Fertil. Steril. 2011;95:1932–1936. doi: 10.1016/j.fertnstert.2011.01.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S., Ozkavukcu S., Heytens E., Moy F., Oktay K. Value of early referral to fertility preservation in young women with breast cancer. J. Clin. Oncol. 2010;28:4683–4686. doi: 10.1200/JCO.2010.30.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J., Oktay K., Gracia C., Lee S., Morse C., Mersereau J.E. Which patients pursue fertility preservation treatments? A multicenter analysis of the predictors of fertility preservation in women with breast cancer. Fertil. Steril. 2012;97:671–676. doi: 10.1016/j.fertnstert.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oktay K., Harvey B.E., Partridge A.H., Quinn G.P., Reinecke J., Taylor H.S., Wallace W.H., Wang E.T., Loren A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36:1994–2001. doi: 10.1200/JCO.2018.78.1914. [DOI] [PubMed] [Google Scholar]
- 16.Lee S., Kim S.K., Hwang K.J., Kim T., Kim S.H. Fertility preservation for patients with gynecologic malignancies: The Korean Society for Fertility Preservation clinical guidelines. Clin. Exp. Reprod. Med. 2017;44:175–180. doi: 10.5653/cerm.2017.44.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell S.B., Woodard T.L. An update on fertility preservation strategies for women with cancer. Gynecol. Oncol. 2020;156:3–5. doi: 10.1016/j.ygyno.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Lambertini M., Peccatori F.A., Demeestere I., Amant F., Wyns C., Stukenborg J.-B., Paluch-Shimon S., Halaska M.J., Uzan C., Meissner J., et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines(dagger) Ann. Oncol. 2020;31:1664–1678. doi: 10.1016/j.annonc.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Coccia P.F., Pappo A.S., Beaupin L., Borges V.F., Borinstein S.C., Chugh R., Dinner S., Folbrecht J., Frazier A.L., Goldsby R., et al. Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2018;16:66–97. doi: 10.6004/jnccn.2018.0001. [DOI] [PubMed] [Google Scholar]
- 20.Letourneau J.M., Ebbel E.E., Katz P.P., Oktay K.H., McCulloch C.E., Ai W.Z., Chien A.J., Melisko M.E., Cedars M.I., Rosen M.P. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118:1933–1939. doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies C., Pan H., Godwin J., Gray R., Arriagada R., Raina V., Abraham M., Alencar V.H.M., Badran A., Bonfill X., et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stupart D., Win A.K., Jenkins M., Winship I.M. Female fertility and colorectal cancer. Int. J. Colorectal. Dis. 2008;23:735–743. doi: 10.1007/s00384-008-0483-3. [DOI] [PubMed] [Google Scholar]
- 23.Zapardiel I., Cruz M., Diestro M.D., Requena A., Velasco J.A.G. Assisted reproductive techniques after fertility-sparing treatments in gynaecological cancers. Hum. Reprod. Update. 2016;22:281–305. doi: 10.1093/humupd/dmv066. [DOI] [PubMed] [Google Scholar]
- 24.Roness H., Kashi O., Meirow D. Prevention of chemotherapy-induced ovarian damage. Fertil. Steril. 2016;105:20–29. doi: 10.1016/j.fertnstert.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 25.Sonigo C., Beau I., Binart N., Grynberg M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int. J. Mol. Sci. 2019;20:5342. doi: 10.3390/ijms20215342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szymanska K.J., Tan X., Oktay K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020;26:553–566. doi: 10.1093/molehr/gaaa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosgrove C.M., Salani R. Ovarian effects of radiation and cytotoxic chemotherapy damage. Best Pract. Res. Clin. Obstet. Gynaecol. 2019;55:37–48. doi: 10.1016/j.bpobgyn.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Marci R., Mallozzi M., di Benedetto L., Schimberni M., Mossa S., Soave I., Palomba S., Caserta D. Radiations and female fertility. Reprod. Biol. Endocrinol. 2018;16:112. doi: 10.1186/s12958-018-0432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedoschi G., Navarro P.A., Oktay K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016;12:2333–2344. doi: 10.2217/fon-2016-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace W.H., Kelsey T.W. Human ovarian reserve from conception to the menopause. PLoS ONE. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy P., Zheng W., Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol. Metab. 2010;21:96–103. doi: 10.1016/j.tem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Adhikari D., Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 2009;30:438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin M., Kinnell H.L., Anderson R.A., Telfer E.E. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol. Hum. Reprod. 2014;20:736–744. doi: 10.1093/molehr/gau037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J., Kawamura K., Cheng Y., Liu S., Klein C., Liu S., Duan E., Hsueh A.J.W. Activation of dormant ovarian follicles to generate mature eggs. Proc. Natl. Acad. Sci. USA. 2010;107:10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsueh A.J.W., Kawamura K., Cheng Y., Fauser B.C.J.M. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015;36:1–24. doi: 10.1210/er.2014-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gawriluk T.R., Hale A.N., Flaws J.A., Dillon C.P., Green D.R., Rucker E.B., 3rd Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction. 2011;141:759–765. doi: 10.1530/REP-10-0489. [DOI] [PubMed] [Google Scholar]
- 37.Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 38.De Vos M., Devroey P., Fauser B.C. Primary ovarian insufficiency. Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 39.Chapman R.M. Effect of cytotoxic therapy on sexuality and gonadal function. Semin. Oncol. 1982;9:84–94. [PubMed] [Google Scholar]
- 40.Miller J.J., 3rd, Williams G.F., Leissring J.C. Multiple late complications of therapy with cyclophosphamide, including ovarian destruction. Am. J. Med. 1971;50:530–535. doi: 10.1016/0002-9343(71)90341-X. [DOI] [PubMed] [Google Scholar]
- 41.Rose D.P., Davis T.E. Ovarian function in patients receiving adjuvant chemotherapy for breast cancer. Lancet. 1977;1:1174–1176. doi: 10.1016/S0140-6736(77)92716-7. [DOI] [PubMed] [Google Scholar]
- 42.Shalet S.M. Effects of cancer chemotherapy on gonadal function of patients. Cancer Treat. Rev. 1980;7:141–152. doi: 10.1016/S0305-7372(80)80028-4. [DOI] [PubMed] [Google Scholar]
- 43.Koyama H., Wada T., Nishizawa Y., Iwanaga T., Aoki Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1977;39:1403–1409. doi: 10.1002/1097-0142(197704)39:4<1403::AID-CNCR2820390408>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 44.Andrieu J.M., Ochoa-Molina M.E. Menstrual cycle, pregnancies and offspring before and after MOPP therapy for Hodgkin’s disease. Cancer. 1983;52:435–438. doi: 10.1002/1097-0142(19830801)52:3<435::AID-CNCR2820520308>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 45.Cho H.W., Lee S., Min K.J., Hong J.H., Song J.Y., Lee J.K., Lee N.W., Kim T. Advances in the Treatment and Prevention of Chemotherapy-Induced Ovarian Toxicity. Int. J. Mol. Sci. 2020;21:7792. doi: 10.3390/ijms21207792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomson A.B., Critchley H.O., Wallace W.H. Fertility and progeny. Eur. J. Cancer. 2002;38 doi: 10.1016/S0959-8049(02)00093-X. [DOI] [PubMed] [Google Scholar]
- 47.Morgan S., Anderson R.A., Gourley C., Wallace W.H., Spears N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update. 2012;18:525–535. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 48.Winship A.L., Stringer J.M., Liew S.H., Hutt K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum. Reprod. Update. 2018;24:119–134. doi: 10.1093/humupd/dmy002. [DOI] [PubMed] [Google Scholar]
- 49.Utsunomiya T., Tanaka T., Utsunomiya H., Umesaki N. A novel molecular mechanism for anticancer drug-induced ovarian failure: Irinotecan HCl, an anticancer topoisomerase I inhibitor, induces specific FasL expression in granulosa cells of large ovarian follicles to enhance follicular apoptosis. Int. J. Oncol. 2008;32:991–1000. doi: 10.3892/ijo.32.5.991. [DOI] [PubMed] [Google Scholar]
- 50.Kerr J.B., Hutt K.J., Michalak E.M., Cook M., Vandenberg C.J., Liew S.H., Bouillet P., Mills A., Scott C.L., Findlay J.K., et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol. Cell. 2012;48:343–352. doi: 10.1016/j.molcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oktem O., Oktay K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007;67:10159–10162. doi: 10.1158/0008-5472.CAN-07-2042. [DOI] [PubMed] [Google Scholar]
- 52.Petrillo S.K., Desmeules P., Truong T.Q., Devine P.J. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol. Appl. Pharmacol. 2011;253:94–102. doi: 10.1016/j.taap.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Ganesan S., Keating A.F. The ovarian DNA damage repair response is induced prior to phosphoramide mustard-induced follicle depletion, and ataxia telangiectasia mutated inhibition prevents PM-induced follicle depletion. Toxicol. Appl. Pharmacol. 2016;292:65–74. doi: 10.1016/j.taap.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen Q.N., Zerafa N., Liew S.H., Findlay J.K., Hickey M., Hutt K.J. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol. Hum. Reprod. 2019;25:433–444. doi: 10.1093/molehr/gaz020. [DOI] [PubMed] [Google Scholar]
- 55.Soleimani R., Heytens E., Darzynkiewicz Z., Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging. 2011;3:782–793. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roness H., Gavish Z., Cohen Y., Meirow D. Ovarian follicle burnout: A universal phenomenon? Cell Cycle. 2013;12:3245–3246. doi: 10.4161/cc.26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X.Y., Xia H.X., Guan H.Y., Li B., Zhang W. Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter? Int. J. Mol. Sci. 2016;17:836. doi: 10.3390/ijms17060836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonigo C., Beau I., Grynberg M., Binart N. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J. 2019;33:1278–1287. doi: 10.1096/fj.201801089R. [DOI] [PubMed] [Google Scholar]
- 59.Sobinoff A.P., Nixon B., Roman S.D., McLaughlin E.A. Staying alive: PI3K pathway promotes primordial follicle activation and survival in response to 3MC-induced ovotoxicity. Toxicol. Sci. 2012;128:258–271. doi: 10.1093/toxsci/kfs137. [DOI] [PubMed] [Google Scholar]
- 60.Chang E.M., Lim E., Yoon S., Jeong K., Bae S., Lee D.R., Yoon T.K., Choi Y., Lee W.S. Cisplatin Induces Overactivation of the Dormant Primordial Follicle through PTEN/AKT/FOXO3a Pathway which Leads to Loss of Ovarian Reserve in Mice. PLoS ONE. 2015;10:e0144245. doi: 10.1371/journal.pone.0144245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jang H., Na Y., Hong K., Lee S., Moon S., Cho M., Park M., Lee O.H., Chang E.M., Lee D.R. Synergistic effect of melatonin and ghrelin in preventing cisplatin-induced ovarian damage via regulation of FOXO3a phosphorylation and binding to the p27(Kip1) promoter in primordial follicles. J. Pineal. Res. 2017:63. doi: 10.1111/jpi.12432. [DOI] [PubMed] [Google Scholar]
- 62.Kitajima M., Dolmans M.M., Donnez O., Masuzaki H., Soares M., Donnez J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014;101:1031–1037. doi: 10.1016/j.fertnstert.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 63.Marcello M.F., Nuciforo G., Romeo R., di Dino G., Russo I., Russo A., Palumbo G., Schilirò G. Structural and ultrastructural study of the ovary in childhood leukemia after successful treatment. Cancer. 1990;66:2099–2104. doi: 10.1002/1097-0142(19901115)66:10<2099::AID-CNCR2820661010>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 64.Doll D.C., Ringenberg Q.S., Yarbro J.W. Vascular toxicity associated with antineoplastic agents. J. Clin. Oncol. 1986;4:1405–1417. doi: 10.1200/JCO.1986.4.9.1405. [DOI] [PubMed] [Google Scholar]
- 65.Nicosia S.V., Matus-Ridley M., Meadows A.T. Gonadal effects of cancer therapy in girls. Cancer. 1985;55:2364–2372. doi: 10.1002/1097-0142(19850515)55:10<2364::AID-CNCR2820551011>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 66.Aharon I.B., Meizner I., Granot T., Uri S., Hasky N., Rizel S., Yerushalmi R., Sulkes A., Stemmer S.M. Chemotherapy-induced ovarian failure as a prototype for acute vascular toxicity. Oncologist. 2012;17:1386–1393. doi: 10.1634/theoncologist.2012-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meirow D., Dor J., Kaufman B., Shrim A., Rabinovici J., Schiff E., Raanani H., Levron J., Fridman E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum. Reprod. 2007;22:1626–1633. doi: 10.1093/humrep/dem027. [DOI] [PubMed] [Google Scholar]
- 68.Oktay K., Oktem O., Reh A., Vahdat L. Measuring the impact of chemotherapy on fertility in women with breast cancer. J. Clin. Oncol. 2006;24:4044–4046. doi: 10.1200/JCO.2006.06.9823. [DOI] [PubMed] [Google Scholar]
- 69.Soleimani R., Heytens E., Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS ONE. 2011;6:e19475. doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howell S., Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol. Metab. Clin. N. Am. 1998;27:927–943. doi: 10.1016/S0889-8529(05)70048-7. [DOI] [PubMed] [Google Scholar]
- 71.Madsen B.L., Giudice L., Donaldson S.S. Radiation-induced premature menopause: A misconception. Int. J. Radiat. Oncol. Biol. Phys. 1995;32:1461–1464. doi: 10.1016/0360-3016(95)00025-T. [DOI] [PubMed] [Google Scholar]
- 72.Beerendonk C.C.M., Braat D.D.M. Present and future options for the preservation of fertility in female adolescents with cancer. Endocr. Dev. 2005;8:166–175. doi: 10.1159/000084101. [DOI] [PubMed] [Google Scholar]
- 73.Stroud J.S., Mutch D., Rader J., Powell M., Thaker P.H., Grigsby P.W. Effects of cancer treatment on ovarian function. Fertil. Steril. 2009;92:417–427. doi: 10.1016/j.fertnstert.2008.07.1714. [DOI] [PubMed] [Google Scholar]
- 74.Wallace W.H., Thomson A.B., Kelsey T.W. The radiosensitivity of the human oocyte. Hum. Reprod. 2003;18:117–121. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- 75.Lushbaugh C.C., Casarett G.W. The effects of gonadal irradiation in clinical radiation therapy: A review. Cancer. 1976;37(Suppl. 2):1111–1125. doi: 10.1002/1097-0142(197602)37:2+<1111::AID-CNCR2820370821>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 76.Wo J.Y., Viswanathan A.N. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009;73:1304–1312. doi: 10.1016/j.ijrobp.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall E.J. Cancer caused by x-rays—A random event? Lancet Oncol. 2007;8:369–370. doi: 10.1016/S1470-2045(07)70113-4. [DOI] [PubMed] [Google Scholar]
- 78.Maier P., Hartmann L., Wenz F., Herskind C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016;17:102. doi: 10.3390/ijms17010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alhumaidha K.A., Saleh D.O., el Fattah M.A.A., el Eraky W.I., Moawad H. Cardiorenal protective effect of taurine against cyclophosphamide-induced toxicity in albino rats. Can. J. Physiol. Pharmacol. 2016;94:131–139. doi: 10.1139/cjpp-2015-0138. [DOI] [PubMed] [Google Scholar]
- 80.Kumar M., Pathak D., Venkatesh S., Kriplani A., Ammini A.C., Dada R. Chromosomal abnormalities & oxidative stress in women with premature ovarian failure (POF) Indian J. Med. Res. 2012;135:92–97. doi: 10.4103/0971-5916.93430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Devine P.J., Perreault S.D., Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012;86:27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pala Ş., Atilgan R., Kuloğlu T., Kara M., Başpinar M., Can B., Artaş G. Protective effects of vitamin C and vitamin E against hysterosalpingography-induced epithelial degeneration and proliferation in rat endometrium. Drug Des. Devel. Ther. 2016;10:4079–4089. doi: 10.2147/DDDT.S117207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nambiar D., Rajamani P., Singh R.P. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat. Res. 2011;728:139–157. doi: 10.1016/j.mrrev.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Kim B.M., Hong Y., Lee S., Liu P., Lim J.H., Lee Y.H., Lee T.H., Chang K.T., Hong Y. Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy. Int. J. Mol. Sci. 2015;16:26880–26913. doi: 10.3390/ijms161125991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mantawy E.M., Said R.S., Abdel-Aziz A.K. Mechanistic approach of the inhibitory effect of chrysin on inflammatory and apoptotic events implicated in radiation-induced premature ovarian failure: Emphasis on TGF-beta/MAPKs signaling pathway. Biomed. Pharmacother. 2019;109:293–303. doi: 10.1016/j.biopha.2018.10.092. [DOI] [PubMed] [Google Scholar]
- 86.Jaroudi S., Kakourou G., Cawood S., Doshi A., Ranieri D.M., Serhal P., Harper J.C., SenGupta S.B. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum. Reprod. 2009;24:2649–2655. doi: 10.1093/humrep/dep224. [DOI] [PubMed] [Google Scholar]
- 87.Duncan F.E., Kimler B.F., Briley S.M. Combating radiation therapy-induced damage to the ovarian environment. Future Oncol. 2016;12:1687–1690. doi: 10.2217/fon-2016-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petrek J.A., Naughton M.J., Case L.D., Paskett E.D., Naftalis E.Z., Singletary S.E., Sukumvanich P. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J. Clin. Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 89.Anderson R.A., Wallace W.H. Antimullerian hormone, the assessment of the ovarian reserve, and the reproductive outcome of the young patient with cancer. Fertil. Steril. 2013;99:1469–1475. doi: 10.1016/j.fertnstert.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 90.Gracia C.R., Sammel M.D., Freeman E., Prewitt M., Carlson C., Ray A., Vance A., Ginsberg J.P. Impact of cancer therapies on ovarian reserve. Fertil. Steril. 2012;97:134–140.e1. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kevenaar M.E., Meerasahib M.F., Kramer P., Born B.M.N.V., de Jong F.H., Groome N.P., Themmen A.P.N., Visser J.A. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147:3228–3234. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- 92.Peñarrubia J., Fábregues F., Manau D., Creus M., Casals G., Casamitjana R., Carmona F., Vanrell J.A., Balasch J. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist—Gonadotropin treatment. Hum. Reprod. 2005;20:915–922. doi: 10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- 93.Peigne M., Decanter C. Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: A systematic review. Reprod. Biol. Endocrinol. 2014;12:26. doi: 10.1186/1477-7827-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Avila Â.M., Biolchi V., Capp E., Corleta H.V. Age, anti-mullerian hormone, antral follicles count to predict amenorrhea or oligomenorrhea after chemotherapy with cyclophosphamide. J. Ovarian. Res. 2015;8:82. doi: 10.1186/s13048-015-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anders C., Marcom P.K., Peterson B., Gu L., Unruhe S., Welch R., Lyons P., Behera M., Copland S., Kimmick G., et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Investig. 2008;26:286–295. doi: 10.1080/07357900701829777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderson R.A., Cameron D.A. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J. Clin. Endocrinol. Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 97.Toner J.P., Seifer D.B. Why we may abandon basal follicle-stimulating hormone testing: A sea change in determining ovarian reserve using antimullerian hormone. Fertil. Steril. 2013;99:1825–1830. doi: 10.1016/j.fertnstert.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 98.Nelson S.M. Biomarkers of ovarian response: Current and future applications. Fertil. Steril. 2013;99:963–969. doi: 10.1016/j.fertnstert.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 99.Jung M., Shin H.J., Rha S.Y., Jeung H.C., Hong S., Moon Y.W., Kim H.S., Oh K.J., Yang W.I., Roh J.K., et al. The clinical outcome of chemotherapy-induced amenorrhea in premenopausal young patients with breast cancer with long-term follow-up. Ann. Surg. Oncol. 2010;17:3259–3268. doi: 10.1245/s10434-010-1172-3. [DOI] [PubMed] [Google Scholar]
- 100.Broekmans F.J., Kwee J., Hendriks D.J., Mol B.W., Lambalk C.B. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum. Reprod. Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 101.Knauff E.A.H., Eijkemans M.J.C., Lambalk C.B., Booij M.J.t.K., Hoek A., Beerendonk C.C.M., Laven J.S.E., Goverde A.J., Broekmans F.J.M., Themmen A.P.N. Anti-Mullerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. J. Clin. Endocrinol. Metab. 2009;94:786–792. doi: 10.1210/jc.2008-1818. [DOI] [PubMed] [Google Scholar]
- 102.Muttukrishna S., McGarrigle H., Wakim R., Khadum I., Ranieri D.M., Serhal P. Antral follicle count, anti-mullerian hormone and inhibin B: Predictors of ovarian response in assisted reproductive technology? BJOG. 2005;112:1384–1390. doi: 10.1111/j.1471-0528.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 103.Frattarelli J.L., Levi A.J., Miller B.T., Segars J.H. A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil. Steril. 2003;80:350–355. doi: 10.1016/S0015-0282(03)00664-2. [DOI] [PubMed] [Google Scholar]
- 104.Magon N. Gonadotropin releasing hormone agonists: Expanding vistas. Indian J. Endocrinol. Metab. 2011;15:261–267. doi: 10.4103/2230-8210.85575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blumenfeld Z., Dann E. GnRH agonist for the prevention of chemotherap.y-induced ovarian failure in lymphoma. J. Clin. Oncol. 2013;31:3721. doi: 10.1200/JCO.2012.47.8222. [DOI] [PubMed] [Google Scholar]
- 106.Blumenfeld Z., Avivi I., Linn S., Epelbaum R., Shahar M.B., Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum. Reprod. 1996;11:1620–1626. doi: 10.1093/oxfordjournals.humrep.a019457. [DOI] [PubMed] [Google Scholar]
- 107.Pacheco B.P., Ribas J.M.M., Milone G., Fernández I., Kvicala R., Mila T., di Noto A., Ortiz O.C., Pavlovsky S. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: A preliminary report. Gynecol. Oncol. 2001;81:391–397. doi: 10.1006/gyno.2001.6181. [DOI] [PubMed] [Google Scholar]
- 108.Recchia F., Sica G., de Filippis S., Saggio G., Rosselli M., Rea S. Goserelin as ovarian protection in the adjuvant treatment of premenopausal breast cancer: A phase II pilot study. Anticancer Drugs. 2002;13:417–424. doi: 10.1097/00001813-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 109.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–1054. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 110.Blumenfeld Z., Eckman A. Preservation of fertility and ovarian function and minimization of chemotherapy-induced gonadotoxicity in young women by GnRH-a. J. Natl. Cancer Inst. Monogr. 2005;2005:40–43. doi: 10.1093/jncimonographs/lgi015. [DOI] [PubMed] [Google Scholar]
- 111.Lambertini M., Horicks F., del Mastro L., Partridge A.H., Demeestere I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: From biological evidence to clinical application. Cancer Treat. Rev. 2019;72:65–77. doi: 10.1016/j.ctrv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 112.Blumenfeld Z. Fertility preservation and GnRHa for chemotherapy: Debate. Cancer Manag. Res. 2014;6:313–315. doi: 10.2147/CMAR.S66600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oktay K., Sönmezer M., Oktem O., Fox K., Emons G., Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12:1055–1066. doi: 10.1634/theoncologist.12-9-1055. [DOI] [PubMed] [Google Scholar]
- 114.Bedaiwy M.A., Abou-Setta A.M., Desai N., Hurd W., Starks D., El-Nashar S.A., al Inany H.G., Falcone T. Gonadotropin-releasing hormone analog cotreatment for preservation of ovarian function during gonadotoxic chemotherapy: A systematic review and meta-analysis. Fertil. Steril. 2011;95:906–914. doi: 10.1016/j.fertnstert.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 115.Chen H., Xiao L., Li J., Cui L., Huang W. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database Syst. Rev. 2019;3:CD008018. doi: 10.1002/14651858.CD008018.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clowse M.E.B., Behera M.A., Anders C.K., Copland S., Coffman C.J., Leppert P.C., Bastian L.A. Ovarian preservation by GnRH agonists during chemotherapy: A meta-analysis. J. Womens Health. 2009;18:311–319. doi: 10.1089/jwh.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Del Mastro L., Boni L., Michelotti A., Gamucci T., Olmeo N., Gori S., Giordano M., Garrone O., Pronzato P., Bighin C., et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: A randomized trial. JAMA. 2011;306:269–276. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 118.De Pedro M., Otero B., Martin B. Fertility preservation and breast cancer: A review. Ecancermedicalscience. 2015;9:503. doi: 10.3332/ecancer.2015.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peccatori F.A., Azim H.A., Jr., Orecchia R., Hoekstra H.J., Pavlidis N., Kesic V., Pentheroudakis G., ESMO Guidelines Working Group Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24(Suppl. 6):vi160–vi170. doi: 10.1093/annonc/mdt199. [DOI] [PubMed] [Google Scholar]
- 120.Woodruff T.K. The Oncofertility Consortium—Addressing fertility in young people with cancer. Nat. Rev. Clin. Oncol. 2010;7:466–475. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Makarovsky D., Kalechman Y., Sonino T., Freidkin I., Teitz S., Albeck M., Weil M., Geffen-Aricha R., Yadid G., Sredni B. Tellurium compound AS101 induces PC12 differentiation and rescues the neurons from apoptotic death. Ann. N. Y. Acad. Sci. 2003;1010:659–666. doi: 10.1196/annals.1299.120. [DOI] [PubMed] [Google Scholar]
- 122.Hayun M., Naor Y., Weil M., Albeck M., Peled A., Don J., Haran-Ghera N., Sredni B. The immunomodulator AS101 induces growth arrest and apoptosis in multiple myeloma: Association with the Akt/survivin pathway. Biochem. Pharmacol. 2006;72:1423–1431. doi: 10.1016/j.bcp.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 123.Philosoph L.K., Roness H., Carmely A., Fishel-Bartal M., Ligumsky H., Paglin S., Wolf I., Kanety H., Sredni B., Meirow D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013;5:185ra62. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 124.Kalechman Y., Shani A., Barkai I.S., Albeck M., Sredni B. The protective role of ammonium trichloro(dioxoethylene-O,O’)tellurate in combination with several cytotoxic drugs acting by different mechanisms of action. Cancer Res. 1993;53:5962–5969. [PubMed] [Google Scholar]
- 125.Kalechman Y., Barkai I.S., Albeck M., Sredni B. Protection of bone marrow stromal cells from the toxic effects of cyclophosphamide in vivo and of ASTA-Z 7557 and etoposide in vitro by ammonium trichloro(dioxyethylene-O-O’)tellurate (AS101) Cancer Res. 1993;53:1838–1844. [PubMed] [Google Scholar]
- 126.Sredni B., Albeck M., Kazimirsky G., Shalit F. The immunomodulator AS101 administered orally as a chemoprotective and radioprotective agent. Int. J. Immunopharmacol. 1992;14:613–619. doi: 10.1016/0192-0561(92)90122-2. [DOI] [PubMed] [Google Scholar]
- 127.Kalechman Y., Albeck M., Oron M., Sobelman D., Gurwith M., Horwith G., Kirsch T., Maida B., Sehgal S.N., Sredni B. Protective and restorative role of AS101 in combination with chemotherapy. Cancer Res. 1991;51:1499–1503. [PubMed] [Google Scholar]
- 128.Carlsson I.B., Scott J.E., Visser J.A., Ritvos O., Themmen A.P.N., Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum. Reprod. 2006;21:2223–2237. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- 129.Durlinger A.L.L., Gruijters M.J.G., Kramer P., Karels B., Ingraham H.A., Nachtigal M.W., Uilenbroek J.J., Grootegoed J.A., Themmen A.P.N. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 130.Durlinger A.L., Kramer P., Karels B., de Jong F.H., Uilenbroek J.T., Grootegoed J.A., Themmen A.P. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 131.Druker B.J., Tamura S., Buchdunger E., Ohno S., Segal G.M., Fanning S., Zimmermann J., Lydon N.B. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 132.Demetri G.D., von Mehren M., Blanke C.D., van den Abbeele A.D., Eisenberg B., Roberts P.J., Heinrich M.C., Tuveson D.A., Singer S., Janicek M., et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 133.Jaeckle K.A., Anderson S.K., Twohy E.L., Dixon J.G., Giannini C., Jenkins R., Egorin M.J., Sarkaria J.N., Brown P.D., Flynn P.J., et al. Phase I-II trial of imatinib mesylate (Gleevec; STI571) in treatment of recurrent oligodendroglioma and mixed oligoastrocytoma. North central cancer treatment group study N0272 (ALLIANCE/NCCTG) J. Neurooncol. 2019;143:573–581. doi: 10.1007/s11060-019-03194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gonfloni S., di Tella L., Caldarola S., Cannata S.M., Klinger F.G., di Bartolomeo C., Mattei M., Candi E., de Felici M., Melino G., et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 135.Kharbanda S., Pandey P., Morris P.L., Whang Y., Xu Y., Sawant S., Zhu L.J., Kumar N., Yuan Z.M., Weichselbaum R., et al. Functional role for the c-Abl tyrosine kinase in meiosis I. Oncogene. 1998;16:1773–1777. doi: 10.1038/sj.onc.1201934. [DOI] [PubMed] [Google Scholar]
- 136.Kharbanda S., Yuan Z.M., Weichselbaum R., Kufe D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene. 1998;17:3309–3318. doi: 10.1038/sj.onc.1202571. [DOI] [PubMed] [Google Scholar]
- 137.Kerr J.B., Hutt K.J., Cook M., Speed T.P., Strasser A., Findlay J.K., Scott C.L. Cisplatin-induced primordial follicle oocyte killing and loss of fertility are not prevented by imatinib. Nat. Med. 2012;18:1170–1172. doi: 10.1038/nm.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim S.-Y., Cordeiro M.H., Serna V.A., Ebbert K., Butler L.M., Sinha S., Mills A.A., Woodruff T.K., Kurita T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013;20:987–997. doi: 10.1038/cdd.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morgan S., Lopes F., Gourley C., Anderson R.A., Spears N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS ONE. 2013;8:e70117. doi: 10.1371/journal.pone.0070117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morita Y., Perez G.I., Paris F., Miranda S.R., Ehleiter D., Haimovitz-Friedman A., Fuks Z., Xie Z., Reed J.C., Schuchman E.H., et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat. Med. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- 141.Li F., Turan V., Lierman S., Cuvelier C., de Sutter P., Affiliations K.O. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum. Reprod. 2014;29:107–113. doi: 10.1093/humrep/det391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hancke K., Strauch O., Kissel C., Göbel H., Schäfer W., Denschlag D. Sphingosine 1-phosphate protects ovaries from chemotherapy-induced damage in vivo. Fertil. Steril. 2007;87:172–177. doi: 10.1016/j.fertnstert.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 143.Kaya H., Desdicioglu R., Sezik M., Ulukaya E., Ozkaya O., Yilmaztepe A., Demirci M. Does sphingosine-1-phosphate have a protective effect on cyclophosphamide- and irradiation-induced ovarian damage in the rat model? Fertil. Steril. 2008;89:732–735. doi: 10.1016/j.fertnstert.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 144.Wikiel M.E.S., McGuire M.M., Sukhwani M., Donohue J., Chu T., Krivak T.C., Rajkovic A., Orwig K.E. Granulocyte colony-stimulating factor with or without stem cell factor extends time to premature ovarian insufficiency in female mice treated with alkylating chemotherapy. Fertil. Steril. 2013;99:2045–2054.e3. doi: 10.1016/j.fertnstert.2013.01.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Solaroglu I., Cahill J., Jadhav V., Zhang J.H. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 2006;37:1123–1128. doi: 10.1161/01.STR.0000208205.26253.96. [DOI] [PubMed] [Google Scholar]
- 146.Rigsby C.K., Siegel M.J. CT appearance of pediatric ovaries and uterus. J. Comput. Assist. Tomogr. 1994;18:72–76. doi: 10.1097/00004728-199401000-00016. [DOI] [PubMed] [Google Scholar]
- 147.Nicholson R., Coucher J., Thornton A., Connor F. Effect of a full and empty bladder on radiation dose to the uterus, ovaries and bladder from lumbar spine CT and X-ray examinations. Br. J. Radiol. 2000;73:1290–1296. doi: 10.1259/bjr.73.876.11205673. [DOI] [PubMed] [Google Scholar]
- 148.Kim S., Lee S., Hong J.H., Park Y.J., Song J.Y., Lee J.K., Lee N.W. Comparing efficacy of high-dose rate brachytherapy versus helical tomotherapy in the treatment of cervical cancer. J. Gynecol. Oncol. 2020;31:e42. doi: 10.3802/jgo.2020.31.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hamre M.R., Robison L.L., Nesbit M.E., Sather H.N., Meadows A.T., Ortega J.A., D’Angio G.J., Hammond G.D. Effects of radiation on ovarian function in long-term survivors of childhood acute lymphoblastic leukemia: A report from the Childrens Cancer Study Group. J. Clin. Oncol. 1987;5:759–765. doi: 10.1200/JCO.1987.5.11.1759. [DOI] [PubMed] [Google Scholar]
- 150.Livesey E.A., Brook C.G. Gonadal dysfunction after treatment of intracranial tumours. Arch. Dis. Child. 1988;63:495–500. doi: 10.1136/adc.63.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harden S.V., Twyman N., Lomas D.J., Williams D., Burnet N.G., Williams M.V. A method for reducing ovarian doses in whole neuro-axis irradiation for medulloblastoma. Radiother. Oncol. 2003;69:183–188. doi: 10.1016/j.radonc.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 152.Lee C.T., Bilton S.D., Famiglietti R.M., Riley B.A., Mahajan A., Chang E.L., Maor M.H., Woo S.Y., Cox J.D., Smith A.R. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: How do protons compare with other conformal techniques? Int. J. Radiat. Oncol. Biol. Phys. 2005;63:362–372. doi: 10.1016/j.ijrobp.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 153.Miralbell R., Lomax A., Bortfeld T., Rouzaud M., Carrie C. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuroectodermal tumors: Reduction of the supratentorial target volume. Int. J. Radiat. Oncol. Biol. Phys. 1997;38:477–484. doi: 10.1016/S0360-3016(97)00004-7. [DOI] [PubMed] [Google Scholar]
- 154.Yuh G.E., Loredo L.N., Yonemoto L.T., Bush D.A., Shahnazi K., Preston W., Slater J.M., Slater J.D. Reducing toxicity from craniospinal irradiation: Using proton beams to treat medulloblastoma in young children. Cancer J. 2004;10:386–390. doi: 10.1097/00130404-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 155.Hadar H., Loven D., Herskovitz P., Bairey O., Yagoda A., Levavi H. An evaluation of lateral and medial transposition of the ovaries out of radiation fields. Cancer. 1994;74:774–779. doi: 10.1002/1097-0142(19940715)74:2<774::AID-CNCR2820740234>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 156.Le Floch O., Donaldson S.S., Kaplan H.S. Pregnancy following oophoropexy and total nodal irradiation in women with Hodgkin’s disease. Cancer. 1976;38:2263–2268. doi: 10.1002/1097-0142(197612)38:6<2263::AID-CNCR2820380612>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 157.Thibaud E., Ramirez M., Brauner R., Flamant F., Zucker J.M., Fékété C., Rappaport R. Preservation of ovarian function by ovarian transposition performed before pelvic irradiation during childhood. J. Pediatr. 1992;121:880–884. doi: 10.1016/S0022-3476(05)80332-4. [DOI] [PubMed] [Google Scholar]
- 158.Loren A.W., Mangu P.B., Beck L.N., Brennan L., Magdalinski A.J., Partridge A.H., Quinn G., Wallace W.H., Oktay K., American Society of Clinical Oncology Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morice P., Ba R.T., Castaigne D., Haie-Meder C., Gerbaulet A., Pautier P., Duvillard P., Michel G. Fertility results after ovarian transposition for pelvic malignancies treated by external irradiation or brachytherapy. Hum. Reprod. 1998;13:660–663. doi: 10.1093/humrep/13.3.660. [DOI] [PubMed] [Google Scholar]
- 160.Al-Asari S., Abduljabbar A. Laparoscopic ovarian transposition before pelvic radiation in rectal cancer patient: Safety and feasibility. Ann. Surg. Innov. Res. 2012;6:9. doi: 10.1186/1750-1164-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dursun P., Ayhan A., Yanik F.B., Kuşçu E. Ovarian transposition for the preservation of ovarian function in young patients with cervical carcinoma. Eur. J. Gynaecol. Oncol. 2009;30:13–15. [PubMed] [Google Scholar]
- 162.Morice P., Juncker L., Rey A., El-Hassan J., Haie-Meder C., Castaigne D. Ovarian transposition for patients with cervical carcinoma treated by radiosurgical combination. Fertil. Steril. 2000;74:743–748. doi: 10.1016/S0015-0282(00)01500-4. [DOI] [PubMed] [Google Scholar]
- 163.Han S.S., Kim Y.H., Lee S.H., Kim G.J., Kim H.J., Kim J.W., Park N.H., Song Y.S., Kang S.B. Underuse of ovarian transposition in reproductive-aged cancer patients treated by primary or adjuvant pelvic irradiation. J. Obstet. Gynaecol. Res. 2011;37:825–829. doi: 10.1111/j.1447-0756.2010.01443.x. [DOI] [PubMed] [Google Scholar]
- 164.Scott S.M., Schlaff W. Laparoscopic medial oophoropexy prior to radiation therapy in an adolescent with Hodgkin’s disease. J. Pediatr. Adolesc. Gynecol. 2005;18:355–357. doi: 10.1016/j.jpag.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 165.Williams R.S., Littell R.D., Mendenhall N.P. Laparoscopic oophoropexy and ovarian function in the treatment of Hodgkin disease. Cancer. 1999;86:2138–2142. doi: 10.1002/(SICI)1097-0142(19991115)86:10<2138::AID-CNCR36>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 166.Bisharah M., Tulandi T. Laparoscopic preservation of ovarian function: An underused procedure. Am. J. Obstet. Gynecol. 2003;188:367–370. doi: 10.1067/mob.2003.38. [DOI] [PubMed] [Google Scholar]
- 167.Wallace W.H., Anderson R.A., Irvine D.S. Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol. 2005;6:209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 168.Gook D.A., Edgar D.H. Human oocyte cryopreservation. Hum. Reprod. Update. 2007;13:591–605. doi: 10.1093/humupd/dmm028. [DOI] [PubMed] [Google Scholar]
- 169.de Vos M., Smitz J., Woodruff T.K. Fertility preservation in women with cancer. Clin. Exp. Reprod. Med. 2012;39:46–51. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cakmak H., Rosen M.P. Ovarian stimulation in cancer patients. Fertil. Steril. 2013;99:1476–1484. doi: 10.1016/j.fertnstert.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 171.Devroey P., Polyzos N.P., Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum. Reprod. 2011;26:2593–2597. doi: 10.1093/humrep/der251. [DOI] [PubMed] [Google Scholar]
- 172.Roque M., Lattes K., Serra S., Solà I., Geber S., Carreras R., Checa M.A. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: A systematic review and meta-analysis. Fertil. Steril. 2013;99:156–162. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 173.Rienzi L., Gracia C., Maggiulli R., LaBarbera A.R., Kaser D.J., Ubaldi F.M., Vanderpoel S., Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update. 2017;23:139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.AbdelHafez F.F., Desai N., Setta A.M.A., Falcone T., Goldfarb J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: A systematic review and meta-analysis. Reprod. Biomed. Online. 2010;20:209–222. doi: 10.1016/j.rbmo.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 175.Debrock S., Peeraer K., Gallardo E.F., de Neubourg D., Spiessens C., D’Hooghe T.M. Vitrification of cleavage stage day 3 embryos results in higher live birth rates than conventional slow freezing: A RCT. Hum. Reprod. 2015;30:1820–1830. doi: 10.1093/humrep/dev134. [DOI] [PubMed] [Google Scholar]
- 176.Lee S., Oktay K. Does higher starting dose of FSH stimulation with letrozole improve fertility preservation outcomes in women with breast cancer? Fertil. Steril. 2012;98:961–964.e1. doi: 10.1016/j.fertnstert.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chung K., Donnez J., Ginsburg E., Meirow D. Emergency IVF versus ovarian tissue cryopreservation: Decision making in fertility preservation for female cancer patients. Fertil. Steril. 2013;99:1534–1542. doi: 10.1016/j.fertnstert.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 178.Courbiere B., Decanter C., Deutsch S.B., Rives N., Mirallié S., Pech J.C., de Ziegler D., Pigeon F.C., Panloup P.M., Sifer C., et al. Emergency IVF for embryo freezing to preserve female fertility: A French multicentre cohort study. Hum. Reprod. 2013;28:2381–2388. doi: 10.1093/humrep/det268. [DOI] [PubMed] [Google Scholar]
- 179.Cakmak H., Katz A., Cedars M.I., Rosen M.P. Effective method for emergency fertility preservation: Random-start controlled ovarian stimulation. Fertil. Steril. 2013;100:1673–1680. doi: 10.1016/j.fertnstert.2013.07.1992. [DOI] [PubMed] [Google Scholar]
- 180.Oktay K., Cil A.P., Bang H. Efficiency of oocyte cryopreservation: A meta-analysis. Fertil. Steril. 2006;86:70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 181.Dolmans M.M., de Ouderaen S.H., Demylle D., Pirard C. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J. Assist. Reprod. Genet. 2015;32:1233–1237. doi: 10.1007/s10815-015-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mayeur A., Puy V., Windal V., Hesters L., Gallot V., Benoit A., Grynberg M., Sonigo C., Frydman N. Live birth rate after use of cryopreserved oocytes or embryos at the time of cancer diagnosis in female survivors: A retrospective study of ten years of experience. J. Assist. Reprod. Genet. 2021 doi: 10.1007/s10815-021-02168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim S., Lee Y., Lee S., Kim T. Ovarian tissue cryopreservation and transplantation in patients with cancer. Obstet. Gynecol. Sci. 2018;61:431–442. doi: 10.5468/ogs.2018.61.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cobo A., Velasco J.A.G., Domingo J., Remohí J., Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil. Steril. 2013;99:1485–1495. doi: 10.1016/j.fertnstert.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 185.Cobo A., Diaz C. Clinical application of oocyte vitrification: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2011;96:277–285. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 186.Parmegiani L., Cognigni G.E., Bernardi S., Cuomo S., Ciampaglia W., Infante F.E., de Fatis C.T., Arnone A., Maccarini A.M., Filicori M. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reprod. Biomed. Online. 2011;23:505–512. doi: 10.1016/j.rbmo.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 187.Cobo A., Kuwayama M., Pérez S., Ruiz A., Pellicer A., Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil. Steril. 2008;89:1657–1664. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 188.Cobo A., Meseguer M., Remohí J., Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: A prospective, randomized, controlled, clinical trial. Hum. Reprod. 2010;25:2239–2246. doi: 10.1093/humrep/deq146. [DOI] [PubMed] [Google Scholar]
- 189.Rienzi L., Romano S., Albricci L., Maggiulli R., Capalbo A., Baroni E., Colamaria S., Sapienza F., Ubaldi F. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: A prospective randomized sibling-oocyte study. Hum. Reprod. 2010;25:66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ubaldi F., Anniballo R., Romano S., Baroni E., Albricci L., Colamaria S., Capalbo A., Sapienza F., Vajta G., Rienzi L. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum. Reprod. 2010;25:1199–1205. doi: 10.1093/humrep/deq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.National Collaborating Centre for Women’s and Children’s Health . Fertility: Assessment and Treatment for People with Fertility Problems. Royal College of Obstetricians & Gynaecologists; London, UK: 2013. National Collaborating Centre for Women’s and Children’s Health. National Institute for Health and Clinical Excellence: Guidance. [PubMed] [Google Scholar]
- 192.Practice Committees of the American Society for Reproductive Medicine. The Society for Assisted Reproductive Technology Intracytoplasmic sperm injection (ICSI) for non-male factor indications: A committee opinion. Fertil. Steril. 2020;114:239–245. doi: 10.1016/j.fertnstert.2020.05.032. [DOI] [PubMed] [Google Scholar]
- 193.Dittrich R., Lotz L., Mueller A., Hoffmann I., Wachter D.L., Amann K.U., Beckmann M.W., Hildebrandt T. Oncofertility: Combination of ovarian stimulation with subsequent ovarian tissue extraction on the day of oocyte retrieval. Reprod. Biol. Endocrinol. 2013;11:19. doi: 10.1186/1477-7827-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sánchez M., Maestre E.N., Teruel J., Ortiz E., Pellicer A. The Valencia Programme for Fertility Preservation. Clin. Transl. Oncol. 2008;10:433–438. doi: 10.1007/s12094-008-0227-4. [DOI] [PubMed] [Google Scholar]
- 195.Donnez J., Dolmans M.M., Pellicer A., Garcia C.D., Serrano M.S., Schmidt K.T., Ernst E., Luyckx V., Andersen C.Y. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: A review of 60 cases of reimplantation. Fertil. Steril. 2013;99:1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 196.Suzuki N. Ovarian tissue cryopreservation in young cancer patients for fertility preservation. Reprod. Med. Biol. 2015;14:1–4. doi: 10.1007/s12522-014-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Corkum K.S., Laronda M.M., Rowell E.E. A review of reported surgical techniques in fertility preservation for prepubertal and adolescent females facing a fertility threatening diagnosis or treatment. Am. J. Surg. 2017;214:695–700. doi: 10.1016/j.amjsurg.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee S., Ryu K.J., Kim B., Kang D., Kim Y.Y., Kim T. Comparison between Slow Freezing and Vitrification for Human Ovarian Tissue Cryopreservation and Xenotransplantation. Int. J. Mol. Sci. 2019;20:3346. doi: 10.3390/ijms20133346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Isachenko V., Isachenko E., Weiss J.M. Human ovarian tissue: Vitrification versus conventional freezing. Hum. Reprod. 2009;24:1767–1768. doi: 10.1093/humrep/dep094. [DOI] [PubMed] [Google Scholar]
- 200.Keros V., Xella S., Hultenby K., Pettersson K., Sheikhi M., Volpe A., Hreinsson J., Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum. Reprod. 2009;24:1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 201.Klocke S., Bündgen N., Köster F., Eichenlaub-Ritter U., Griesinger G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch. Gynecol. Obstet. 2015;291:419–426. doi: 10.1007/s00404-014-3390-6. [DOI] [PubMed] [Google Scholar]
- 202.Dolmans M.M., Luyckx V., Donnez J., Andersen C.Y., Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013;99:1514–1522. doi: 10.1016/j.fertnstert.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 203.Loren A.W., Senapati S. Fertility preservation in patients with hematologic malignancies and recipients of hematopoietic cell transplants. Blood. 2019;134:746–760. doi: 10.1182/blood.2018846790. [DOI] [PubMed] [Google Scholar]
- 204.Rosendahl M., Andersen M.T., Ralfkiær E., Kjeldsen L., Andersen M.K., Andersen C.Y. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil. Steril. 2010;94:2186–2190. doi: 10.1016/j.fertnstert.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 205.Kim B., Ryu K.J., Lee S., Kim T. Changes in telomere length and senescence markers during human ovarian tissue cryopreservation. Sci. Rep. 2021;11:2238. doi: 10.1038/s41598-021-81973-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bedaiwy M.A., Falcone T. Ovarian tissue banking for cancer patients: Reduction of post-transplantation ischaemic injury: Intact ovary freezing and transplantation. Hum. Reprod. 2004;19:1242–1244. doi: 10.1093/humrep/deh262. [DOI] [PubMed] [Google Scholar]
- 207.Madrid B.M., Dolmans M.M., van Langendonckt A., Defrère S., Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil. Steril. 2004;82:1390–1394. doi: 10.1016/j.fertnstert.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 208.Yin H., Wang X., Kim S.S., Chen H., Tan S.L., Gosden R.G. Transplantation of intact rat gonads using vascular anastomosis: Effects of cryopreservation, ischaemia and genotype. Hum. Reprod. 2003;18:1165–1172. doi: 10.1093/humrep/deg236. [DOI] [PubMed] [Google Scholar]
- 209.Zhang J.M., Sheng Y., Cao Y.Z., Wang H.Y., Chen Z.J. Cryopreservation of whole ovaries with vascular pedicles: Vitrification or conventional freezing? J. Assist. Reprod. Genet. 2011;28:445–452. doi: 10.1007/s10815-011-9539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang S., Yao H., Liu Y., Ren L., Xiang D., Wang Y. Hypothermic machine perfusion after static cold storage improves ovarian function in rat ovarian tissue transplantation. J. Assist. Reprod. Genet. 2020;37:1745–1753. doi: 10.1007/s10815-020-01797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hossay C., Donnez J., Dolmans M.M. Whole Ovary Cryopreservation and Transplantation: A Systematic Review of Challenges and Research Developments in Animal Experiments and Humans. J. Clin. Med. 2020;9:3196. doi: 10.3390/jcm9103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Soleimani R., Heytens E., van den Broecke R., Rottiers I., Dhont M., Cuvelier C.A., de Sutter P. Xenotransplantation of cryopreserved human ovarian tissue into murine back muscle. Hum. Reprod. 2010;25:1458–1470. doi: 10.1093/humrep/deq055. [DOI] [PubMed] [Google Scholar]
- 213.Gook D.A., Edgar D.H., Borg J., Archer J., Lutjen P.J., McBain J.C. Oocyte maturation, follicle rupture and luteinization in human cryopreserved ovarian tissue following xenografting. Hum. Reprod. 2003;18:1772–1781. doi: 10.1093/humrep/deg365. [DOI] [PubMed] [Google Scholar]
- 214.Gook D.A., Edgar D.H., Borg J., Archer J., McBain J.C. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum. Reprod. 2005;20:72–78. doi: 10.1093/humrep/deh550. [DOI] [PubMed] [Google Scholar]
- 215.Lotz L., Schneider H., Hackl J., Wachter D., Hoffmann I., Jurgons R., Beckmann M.W., Dittrich R. Does stimulation with human gonadotropins and gonadotropin-releasing hormone agonist enhance and accelerate the developmental capacity of oocytes in human ovarian tissue xenografted into severe combined immunodeficient mice? Fertil. Steril. 2014;101:1477–1484. doi: 10.1016/j.fertnstert.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 216.Kim S.S., Kang H.G., Kim N.H., Lee H.C., Lee H.H. Assessment of the integrity of human oocytes retrieved from cryopreserved ovarian tissue after xenotransplantation. Hum. Reprod. 2005;20:2502–2508. doi: 10.1093/humrep/dei099. [DOI] [PubMed] [Google Scholar]
- 217.Ayuandari S., Crepaz K.W., Paulitsch M., Wagner C., Zavadil C., Manzl C., Ziehr S.C., Wildt L., Tollinger S.H. Follicular growth after xenotransplantation of cryopreserved/thawed human ovarian tissue in SCID mice: Dynamics and molecular aspects. J. Assist. Reprod. Genet. 2016;33:1585–1593. doi: 10.1007/s10815-016-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Terada Y., Sato Y.T., Yoshimoto T.K., Hasegawa H., Ugajin T., Koyanagi Y., Ito M., Murakami T., Sasano H., Yaegashi N., et al. Development of human Graafian follicles following transplantation of human ovarian tissue into NOD/SCID/gammac null mice. Am. J. Reprod. Immunol. 2008;60:534–540. doi: 10.1111/j.1600-0897.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 219.Dolmans M.M., Yuan W.Y., Camboni A., Torre A., van Langendonckt A., Madrid B.M., Donnez J. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod. Biomed. Online. 2008;16:705–711. doi: 10.1016/S1472-6483(10)60485-3. [DOI] [PubMed] [Google Scholar]
- 220.Oktay K., Newton H., Mullan J., Gosden R.G. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum. Reprod. 1998;13:1133–1138. doi: 10.1093/humrep/13.5.1133. [DOI] [PubMed] [Google Scholar]
- 221.David A., Dolmans M.M., van Langendonckt A., Donnez J., Amorim C.A. Immunohistochemical localization of growth factors after cryopreservation and 3 weeks’ xenotransplantation of human ovarian tissue. Fertil. Steril. 2011;95:1241–1246. doi: 10.1016/j.fertnstert.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 222.Weissman A., Gotlieb L., Colgan T., Jurisicova A., Greenblatt E.M., Casper R.F. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol. Reprod. 1999;60:1462–1467. doi: 10.1095/biolreprod60.6.1462. [DOI] [PubMed] [Google Scholar]
- 223.Dath C., van Eyck A.S., Dolmans M.M., Romeu L., Vigne L.D., Donnez J., van Langendonckt A. Xenotransplantation of human ovarian tissue to nude mice: Comparison between four grafting sites. Hum. Reprod. 2010;25:1734–1743. doi: 10.1093/humrep/deq131. [DOI] [PubMed] [Google Scholar]
- 224.Paulini F., Vilela J.M.V., Chiti M.C., Donnez J., Jadoul P., Dolmans M.M., Amorim C.A. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod. Biomed. Online. 2016;33:425–432. doi: 10.1016/j.rbmo.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 225.Amorim C.A., David A., Dolmans M.M., Camboni A., Donnez J., van Langendonckt A. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J. Assist. Reprod. Genet. 2011;28:1157–1165. doi: 10.1007/s10815-011-9672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jafarabadi M., Abdollahi M., Salehnia M. Assessment of vitrification outcome by xenotransplantation of ovarian cortex pieces in gamma-irradiated mice: Morphological and molecular analyses of apoptosis. J. Assist. Reprod. Genet. 2015;32:195–205. doi: 10.1007/s10815-014-0382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Campos P.H.A., Jr., Alves T.J.M., Dias M.T., Assunçao C.M., Munk M., Mattos M.S., Kraemer L.R., Almeida B.G., Russo R.C., Barcelos L., et al. Ovarian Grafts 10 Days after Xenotransplantation: Folliculogenesis and Recovery of Viable Oocytes. PLoS ONE. 2016;11:e0158109. doi: 10.1371/journal.pone.0158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.van Eyck A.S., Bouzin C., Feron O., Romeu L., van Langendonckt A., Donnez J., Dolmans M.M. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil. Steril. 2010;93:1676–1685. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 229.Hormozi M., Talebi S., Zarnani A.H., Tehrani M.J., Gohari L.H., Soltanghoraei H., Jafarabadi M., Akhondi M.M. 5’-(N-ethylcarboxamido) adenosine improves angiogenesis in transplanted human ovarian tissue. Fertil. Steril. 2011;95:2560–2563.e1–5. doi: 10.1016/j.fertnstert.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 230.Fonseca H.H., Bosch P., Sirisathien S., Wininger J.D., Massey J.B., Brackett B.G. Effect of site of transplantation on follicular development of human ovarian tissue transplanted into intact or castrated immunodeficient mice. Fertil. Steril. 2004;81(Suppl. 1):888–892. doi: 10.1016/j.fertnstert.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 231.Maltaris T., Koelbl H., Fischl F., Seufert R., Schmidt M., Kohl J., Beckmann M.W., Binder H., Hoffmann I., Mueller A., et al. Xenotransplantation of human ovarian tissue pieces in gonadotropin-stimulated SCID mice: The effect of ovariectomy. Anticancer Res. 2006;26:4171–4176. [PubMed] [Google Scholar]
- 232.Friedman O., Orvieto R., Fisch B., Felz C., Freud E., Haroush A.B., Abir R. Possible improvements in human ovarian grafting by various host and graft treatments. Hum. Reprod. 2012;27:474–482. doi: 10.1093/humrep/der385. [DOI] [PubMed] [Google Scholar]
- 233.Fu S., Wang J., Sun W., Xu Y., Zhou X., Cheng W. Preclinical humanized mouse model with ectopic ovarian tissues. Exp. Ther. Med. 2014;8:742–746. doi: 10.3892/etm.2014.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.van Langendonckt A., Romeu L., Ambroise J., Amorim C., Bearzatto B., Gala J.L., Donnez J., Dolmans M.M. Gene expression in human ovarian tissue after xenografting. Mol. Hum. Reprod. 2014;20:514–525. doi: 10.1093/molehr/gau015. [DOI] [PubMed] [Google Scholar]
- 235.Wang L., Ying Y.F., Ouyang Y.L., Wang J.F., Xu J. VEGF and bFGF increase survival of xenografted human ovarian tissue in an experimental rabbit model. J. Assist. Reprod. Genet. 2013;30:1301–1311. doi: 10.1007/s10815-013-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ruan X., Cui Y., Du J., Jin J., Gu M., Chen S., Mueck A.O. Randomized study to prove the quality of human ovarian tissue cryopreservation by xenotransplantation into mice. J. Ovarian Res. 2019;12:46. doi: 10.1186/s13048-019-0521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Luyckx V., Durant J.F., Camboni A., Gilliaux S., Amorim C.A., van Langendonckt A., Irenge L.M., Gala J.L., Donnez J., Dolmans M.M. Is transplantation of cryopreserved ovarian tissue from patients with advanced-stage breast cancer safe? A pilot study. J. Assist. Reprod. Genet. 2013;30:1289–1299. doi: 10.1007/s10815-013-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kim S.S., Radford J., Harris M., Varley J., Rutherford A.J., Lieberman B., Shalet S., Gosden R. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum. Reprod. 2001;16:2056–2060. doi: 10.1093/humrep/16.10.2056. [DOI] [PubMed] [Google Scholar]
- 239.Greve T., Wielenga V.T., Grauslund M., Sørensen N., Christiansen D.B., Rosendahl M., Andersen C.Y. Ovarian tissue cryopreserved for fertility preservation from patients with Ewing or other sarcomas appear to have no tumour cell contamination. Eur. J. Cancer. 2013;49:1932–1938. doi: 10.1016/j.ejca.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 240.Lotz L., Montag M., van der Ven H., von Wolff M., Mueller A., Hoffmann I., Wachter D., Beckmann M.W., Dittrich R. Xenotransplantation of cryopreserved ovarian tissue from patients with ovarian tumors into SCID mice--no evidence of malignant cell contamination. Fertil. Steril. 2011;95:2612–2614.e1. doi: 10.1016/j.fertnstert.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 241.Dolmans M.M., Marinescu C., Saussoy P., van Langendonckt A., Amorim C., Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116:2908–2914. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 242.Lee J.A., Barritt J., Moschini R.M., Slifkin R.E., Copperman A.B. Optimizing human oocyte cryopreservation for fertility preservation patients: Should we mature then freeze or freeze then mature? Fertil. Steril. 2013;99:1356–1362. doi: 10.1016/j.fertnstert.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 243.Son W.Y., Henderson S., Cohen Y., Dahan M., Buckett W. Immature Oocyte for Fertility Preservation. Front. Endocrinol. 2019;10:464. doi: 10.3389/fendo.2019.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Prasath E.B., Chan M.L.H., Wong W.H.W., Lim C.J.W., Tharmalingam M.D., Hendricks M., Loh S.F., Chia Y.N. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum. Reprod. 2014;29:276–278. doi: 10.1093/humrep/det420. [DOI] [PubMed] [Google Scholar]
- 245.Uzelac P.S., Delaney A.A., Christensen G.L., Bohler H.C.L., Nakajima S.T. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil. Steril. 2015;104:1258–1260. doi: 10.1016/j.fertnstert.2015.07.1148. [DOI] [PubMed] [Google Scholar]
- 246.Tavana S., Azarnia M., Valojerdi M.R., Shahverdi A. Hyaluronic acid-based hydrogel scaffold without angiogenic growth factors enhances ovarian tissue function after autotransplantation in rats. Biomed. Mater. 2016;11:055006. doi: 10.1088/1748-6041/11/5/055006. [DOI] [PubMed] [Google Scholar]
- 247.Tavana S., Valojerdi M.R., Azarnia M., Shahverdi A. Restoration of ovarian tissue function and estrous cycle in rat after autotransplantation using hyaluronic acid hydrogel scaffold containing VEGF and bFGF. Growth Factors. 2016;34:97–106. doi: 10.1080/08977194.2016.1194835. [DOI] [PubMed] [Google Scholar]
- 248.Manavella D.D., Cacciottola L., Pommé S., Desmet C.M., Jordan B.F., Donnez J., Amorim C.A., Dolmans M.M. Two-step transplantation with adipose tissue-derived stem cells increases follicle survival by enhancing vascularization in xenografted frozen-thawed human ovarian tissue. Hum. Reprod. 2018;33:1107–1116. doi: 10.1093/humrep/dey080. [DOI] [PubMed] [Google Scholar]
- 249.Damous L.L., Nakamuta J.S., de Carvalho A.E.T.S., Carvalho K.C., José M.S., Jr., Simões M.D., Krieger J.E., Baracat E.C. Scaffold-based delivery of adipose tissue-derived stem cells in rat frozen-thawed ovarian autografts: Preliminary studies in a rat model. J. Assist. Reprod. Genet. 2015;32:1285–1294. doi: 10.1007/s10815-015-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Manavella D.D., Cacciottola L., Payen V.L., Amorim C.A., Donnez J., Dolmans M.M. Adipose tissue-derived stem cells boost vascularization in grafted ovarian tissue by growth factor secretion and differentiation into endothelial cell lineages. Mol. Hum. Reprod. 2019;25:184–193. doi: 10.1093/molehr/gaz008. [DOI] [PubMed] [Google Scholar]
- 251.Cho E., Kim Y.Y., Noh K., Ku S.Y. A new possibility in fertility preservation: The artificial ovary. J. Tissue Eng. Regen. Med. 2019;13:1294–1315. doi: 10.1002/term.2870. [DOI] [PubMed] [Google Scholar]
- 252.Luyckx V., Dolmans M.M., Vanacker J., Legat C., Moya C.F., Donnez J., Amorim C.A. A new step toward the artificial ovary: Survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 2014;101:1149–1156. doi: 10.1016/j.fertnstert.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 253.Vanacker J., Luyckx V., Dolmans M.M., Rieux A.D., Jaeger J., van Langendonckt A., Donnez J., Amorim C.A. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: First step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33:6079–6085. doi: 10.1016/j.biomaterials.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 254.Johnson J., Canning J., Kaneko T., Pru J.K., Tilly J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 255.Tilly J.L., Telfer E.E. Purification of germline stem cells from adult mammalian ovaries: A step closer towards control of the female biological clock? Mol. Hum. Reprod. 2009;15:393–398. doi: 10.1093/molehr/gap036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hutt K.J., Albertini D.F. Clinical applications and limitations of current ovarian stem cell research: A review. J. Exp. Clin. Assist. Reprod. 2006;3:6. doi: 10.1186/1743-1050-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Himelstein-Braw R., Peters H., Faber M. Morphological study of the ovaries of leukaemic children. Br. J. Cancer. 1978;38:82–87. doi: 10.1038/bjc.1978.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Familiari G., Caggiati A., Nottola S.A., Ermini M., di Benedetto M.R., Motta P.M. Ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin’s disease. Hum. Reprod. 1993;8:2080–2087. doi: 10.1093/oxfordjournals.humrep.a137985. [DOI] [PubMed] [Google Scholar]