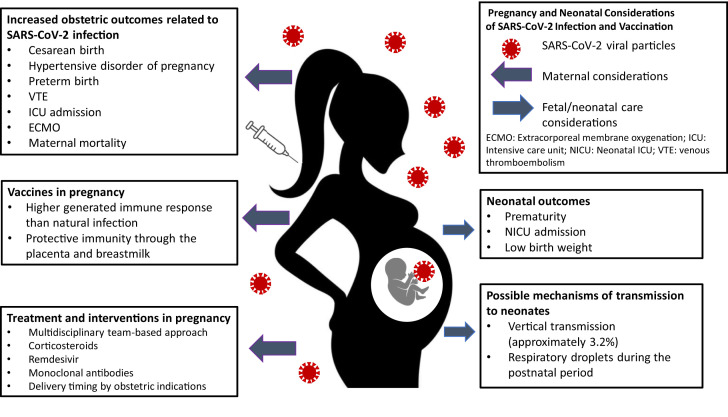
Over the past 18 months, the world has seen the largest pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of June 28, 2021, the Centers for Disease Control and Prevention (CDC) reported 98,948 cases of coronavirus disease 2019 (COVID-19) infection in pregnancy and 109 related maternal deaths in the United States alone.1 As the pandemic continues to evolve, the rapid and overwhelming increase in available evidence on the impact in pregnancy has resulted in studies of varying degrees of bias and quality. In this brief review, we seek to fill some of the knowledge gaps regarding maternal care considerations and answer some key questions about vaccination safety in pregnancy and evidence of protective immunity (Fig 1 ).
Fig 1.
Pregnancy and neonatal considerations of SARS-CoV-2 infection and vaccination. Depiction of risks associated with SARS-CoV-2 infection in pregnancy, treatment and intervention, and benefits of vaccine in pregnancy. Fetal/neonatal outcomes associated with SARS-CoV-2 infection in pregnancy and possible modes of transmission.
Effects of COVID-19 in pregnancy
Clinical findings: Symptoms, labs, imaging
Maternal COVID-19 varies widely, but clinical course, laboratory findings, and radiological patterns found in pregnancy (Table I ) are similar to those in the nonpregnant population.3 Although some patients may be asymptomatic, presence of any COVID-19 symptoms was found to be associated with increased maternal morbidity and mortality.1
Table I.
Clinical findings, laboratory parameters, and radiologic findings of frequency in pregnancies affected by COVID-19
| Sign and symptoms | Range of frequency in pregnancy |
|---|---|
| Clinical findings | |
| Fever | 32.8%-78%2 |
| Cough | 34%-70%2 |
| Dyspnea | 7.3%-35.6%2 |
| Asymptomatic | 8%-32.6%2 |
| Myalgia | 6%-24.4%2 |
| Sore throat | 3.4%-22.2%2 |
| Fatigue | 9.5%-18.5%2 |
| Diarrhea | 4%-10.4%2 |
| Laboratory parameters | |
| Elevated C-reactive protein | 40.8%-70.3%2 |
| Lymphopenia | 29%-68.2%2 |
| Leukocytosis | 13%-45.8%2 |
| Leukopenia | <45.3%2 |
| Abnormal liver function test result | 8%-27.3%2 |
| Thrombocytopenia | 2.7%-8.4%2 |
| Radiologic findings | |
| Ground-glass opacities | 41.5%-81.6%2 |
Maternal and neonatal outcomes
Maternal COVID-19 is associated with consistent and substantial increases in morbidity and mortality when infected pregnant versus nonpregnant individuals are compared.1 A large study conducted by the Maternal-Fetal Medicine Unit Network including 1219 patients reported that mothers with severe or critical COVID-19 and their neonates are at increased risk for a number of perinatal complications, including cesarean birth, hypertensive disorders of pregnancy, preterm birth, venous thromboembolism, neonatal intensive care unit admission, and lower birth weight, compared with asymptomatic mothers.4 Pregnancy is also independently associated with an increased risk for intensive care unit admission, needing extracorporeal membrane oxygenation, and maternal death among patients with symptomatic COVID-19 infection. Moreover, comorbidities (body mass index higher than 35 kg/m2, diabetes, and cardiovascular disorders) and advanced maternal age appear to have an independent risk for adverse maternal outcomes.
Vertical transmission of SARS-CoV-2, maternal immunity, and protection of the neonate
Vertical transmission is defined as evidence of transmission of the SARS-CoV-2 virus from the mother to the fetus or newborn. Studies have evaluated SARS-CoV-2 viral concentrations in umbilical cord blood and placenta. Real-time RT-PCR has been used to evaluate amniotic fluid, newborn blood, urine, nasopharyngeal, fecal, and rectal swabs. Positive samples have been rare, and significant neonatal respiratory disease, even in the presence of SARS-CoV-2 positivity, is even more infrequent.5 The CDC reported that transmission of SARS-CoV-2 virus to neonates occurred primarily through respiratory droplets during the postnatal period when neonates are exposed to mothers or other caregivers with COVID-19. Overall, the risk of vertical transmission of the SARS-CoV-2 virus is approximately 3.2%.6
In utero fetal production of IgG and IgM antibodies starts in the 20th week of gestation; therefore, most neonatal IgG is of maternal origin. IgG positivity cannot support or refute vertical transmission. IgM antibodies do not cross the placenta, and therefore IgM presence in the fetus or neonate is thought to represent fetal or neonatal production in response to an infection. However, in case reports describing identification of COVID-19 IgM antibodies in the neonate, infants have been asymptomatic and tested negative for SARS-CoV-2 viral RNA at birth. Although plausible that the presence of these IgM antibodies represents crossover from maternal to fetal circulation, the presence of IgM antibodies in these infants could provide evidence for intrauterine vertical transmission. There are some case reports demonstrating evidence of transplacental transmission; however, these reports remain scare. Overall, there is limited evidence of the timing for the production of IgM and IgG during COVID-19 infection or the timeline for development of long-term immunity, and more data are needed regarding the potential and appropriate testing to determine the risk of vertical transmission.
Vaccine safety in pregnancy
There are currently 3 approved vaccines for use in the United States (Table II ). Although not specifically included in the initial phase III vaccine trials, pregnant patients were not excluded as part of the Food and Drug Administration–issued Emergency Use Authorization. Given the increased risk of disease severity in pregnancy, professional organizations advocate for availability of vaccine for pregnant and lactating persons. Early data from developmental and reproductive toxicity studies for both the Pfizer and Moderna vaccine did not demonstrate direct or indirect harmful effects with respect to pregnancy, fetal development, delivery, or postnatal complications. In addition, developmental and reproductive toxicity data on the Johnson & Johnson vaccine have not demonstrated adverse outcomes.
Table II.
COVID-19 vaccines
| Vaccine | Pfizer-BioNTech BNT162b2 | Moderna mRNA 1273 | Janssen Biotech Ad26.COV2.S | AstraZeneca SKBio AZD1222 |
| Type | mRNA vaccines | mRNA vaccines | Adenoviral-vector vaccine | Adenoviral-vector vaccine |
| FDA issued EUA in the United States | Yes | Yes | Yes | No |
| Age eligibility | ≥12 y | ≥12 y | ≥18 y | ≥18 y |
| No. of pregnancies in placebo vs treatment arms | 11 in the placebo arm vs 12 in the treatment arm | 7 in the placebo arm vs 6 in the treatment arm | NA | NA |
| No. of doses and frequencies | 2 doses, 21 d apart | 2 doses, 28 d apart | 1 dose | 2 doses, 4-12 wk apart |
| Efficacy | 95.0% (95% CI, 90.3%-97.6%) after the second dose | 94.1% (95% CI, 89.3%- 96.8%) after the second dose | 72% moderate; 85% severe; 100% COVID-related hospitalization and death | 60% (per EMA) to 63.09% (per WHO) after the second dose |
| Contain live virus? | No | No | No | No |
| Mechanism of action | Contain mRNA, a genetic material that encodes the SARS-CoV-2 S protein | Contain mRNA, a genetic material that encodes the SARS-CoV-2 S protein | Uses an adenovirus to carry the gene for the coronavirus S protein, which is produced by the host cell and expressed on the cell membrane, where it is detected by the host immune system to mimic components of the pathogen without causing disease | Uses an adenovirus to carry the gene for the coronavirus S protein, which is produced by the host cell and expressed on the cell membrane, where it is detected by the host immune system to mimic components of the pathogen without causing disease |
| Enter cell nucleus/ integrated into the host DNA? | No/No | No/No | Yes/No | Yes/No |
| Other similar vaccines | None | None | Ebola, HIV, and RSV adenoviral vaccine | Ebola, HIV, and RSV adenoviral vaccine |
| Pregnancy test recommended before vaccination? | No | No | No | No |
| Vaccine contraindication | Acute illness | Acute illness | Acute illness | Acute illness |
| Risk of TTS in pregnancy | Not increased in pregnancy | Not increased in pregnancy | Not increased in pregnancy (occurred in 8.9 in 1 million doses in nonpregnant women age 18-49 y)7 | Not increased in pregnancy (occurred in 6.5 in 1 million doses in nonpregnant women age <60 y, reported by EMA) |
| Safety data in pregnancy | Evidence from 827 completed pregnancies7 | Evidence from 827 completed pregnancies7 | NA | NA |
EMA, European Medicines Agency; EUA, Emergency Use Authorization; FDA, Food and Drug Administration; NA, not applicable/available; RSV, respiratory syncytial virus; S, spike; SARS, severe acute respiratory syndrome; TTS, thrombosis with thrombocytopenia syndrome; WHO, World Health Organization.
Since the Emergency Use Authorization for the Pfizer and Moderna vaccines, more than 128,306 pregnant patients have received the vaccine and registered with the CDC V-safe program. Recent data from the CDC V-safe program found that side effects from the vaccine were similar between pregnant and nonpregnant women. In addition, when evaluating 827 completed pregnancies, there was no increased risk in adverse pregnancy outcomes including miscarriage, preterm birth, small for gestational age, and neonatal death when compared with data before the COVID-19 pandemic.7
A recent study of 131 patients found the mRNA COVID-19 vaccines to be highly effective in producing vaccine-induced antibody titers in pregnant (median, 5.74; interquartile range, 5.06-6.22) and lactating women (median, 5.62; interquartile range, 4.77-5.98), who had titers similar to those of nonpregnant women (median, 5.59; interquartile range, 4.68-5.89).8 All vaccine-generated titers were higher than those generated by SARS-CoV-2 infection during pregnancy. Furthermore, vaccine-generated antibodies were found in all umbilical cord and breast milk samples. This study provided reassuring data that pregnant individuals have a similar response to the vaccine as nonpregnant individuals. Another cohort study from Israel comparing vaccinated pregnant patients, PCR-confirmed SARS-CoV-2–infected pregnant patients, and unvaccinated noninfected pregnant controls looked at the effect of the mRNA vaccine (Pfizer-BioNTech) versus native infection on maternal humoral and transplacentally acquired fetal immune response. They found a robust maternal-induced humoral response to the vaccine that effectively transfers to the fetus, also supporting the role of vaccination during pregnancy.9
Regarding timing of vaccine and antibody production, Prabhu et al10 evaluated 122 pregnant patients who received an mRNA COVID-19 vaccine and found that 44% of cord blood samples were positive for IgG antibodies following 1 dose of the vaccine, compared with 99% of the samples following both doses of the vaccine. All patients and cord blood samples had detectable antibodies when delivered at least 4 weeks following the first dose of vaccine. They also found that the earliest antibody detection in maternal samples was 5 days following vaccine and 16 days following vaccine for cord blood samples.10
COVID-19–related maternal morbidity and mortality is lower than that which occurred during previous coronavirus-related epidemics, although greater than that observed in the nonpregnant population. Nevertheless, vertical transmission is rare. The novel Food and Drug Administration–approved mRNA and adenovirus vaccines have the ability to reduce the risk of severe maternal morbidity and mortality and induce an immunologic protection for neonates through antibody transfer in utero and during lactation. The benefits of these vaccines may outweigh the risks of COVID-19 in pregnancy and in the postpartum period. Ongoing research is needed on the effects of COVID-19 infection during pregnancy spanning all times in gestation as well as long-term studies related to the effect of COVID vaccine in pregnancy.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study [published online ahead of print April 22, 2021]. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed]
- 2.Papapanou M., Papaioannou M., Petta A., Routsi E., Farmaki M., Vlahos N. Maternal and neonatal characteristics and outcomes of COVID-19 in pregnancy: an overview of systematic reviews. Int J Environ Res Public Health. 2021;18:596. doi: 10.3390/ijerph18020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molteni E., Astley C.M., Ma W., Sudre C.H., Magee L.A., Murray B. SARS-CoV-2 (COVID-19) infection in pregnant women: characterization of symptoms and syndromes predictive of disease and severity through real-time, remote participatory epidemiology. medRxiv. 2020 2020.08.17.20161760. [Google Scholar]
- 4.Metz T.D., Clifton R.G., Hughes B.L., Sandoval G., Saade G.R., Grobman W.A. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaral W.N.D., Moraes C.L., Rodrigues A., Noll M., Arruda J.T., Mendonca C.R. Maternal coronavirus infections and neonates born to mothers with SARS-CoV-2: a systematic review. Healthcare (Basel) 2020;8:511. doi: 10.3390/healthcare8040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotlyar A.M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:35–53.e3. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study [published online ahead of print March 2, 2021]. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed]
- 9.Beharier O., Plitman Mayo R., Raz T., Nahum Sacks K., Schreiber L., Suissa-Cohen Y. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131:e150319. doi: 10.1172/JCI154834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prabhu M., Murphy E.A., Sukhu A.C., Yee J., Singh S., Eng D. Antibody response to coronavirus disease 2019 (COVID-19) messenger RNA vaccination in pregnant women and transplacental passage into cord blood. Obstet Gynecol. 2021;138:278–280. doi: 10.1097/AOG.0000000000004438. [DOI] [PMC free article] [PubMed] [Google Scholar]