Several scientific societies, notably the European Union Against Rheumatism (EULAR) and the French Society of Rheumatology, have published recommendations regarding the prescription of biologic agents during the COVID-19 epidemic [1], [2] Here, we report on the evolution of biologic therapy prescription in outpatient care during the first lockdown in France in 2020.
This was a retrospective observational study that used a non-medicalized source of anonymized dispensing data for biologic medications prescribed in outpatient care (i.e., LRx). LRx includes data for a panel of nearly 10,000 French retail pharmacies (historical data since 2012, nearly 40 million patients/year), representing 45% of the French retail pharmacies in terms of geographical spread in continental France and age of population coverage [3]. This coverage allows for the extrapolation of data to the overall French population.
The following therapeutic classes were studied: anti-tumor necrosis factor (TNF; adalimumab, certolizumab pegol, etanercept, golimumab), anti-interleukin 6 (IL-6; tocilizumab, sarilumab), anti-IL-17 (ixekizumab, secukinumab, brodalumab), anti-IL-12/23 (ustekinumab), anti-IL-23 (guselkumab, rizankizumab, tildrakizumab), Janus kinase (JAK) inhibitors (baricitinib, tofacitinib), abatacept, and other drugs such as apremilast, hydroxychloroquine (HCQ), and methotrexate (MTX).
We studied the impact of COVID-19 on both the initiation and renewal of biologic prescriptions in 2020 by using:
-
•
year 2019 as the reference and;
-
•
dispensing delivery data from pharmacies.
We studied weeks 2 to week 25 with a focus on the lockdown weeks (weeks 12–19). Data for 49,807 and 55,858 patients with at least 1 delivery of biologic agents in 2019 and 2020, respectively, were analyzed. Treatment initiation was defined as a treatment not delivered in the previous 12 months and the converse for treatment renewal.
The impact of prescriptions was described at a national and regional level. Three French regions were considered: Grand-Est and Île-De-France, with a large number of infected patients, compared to Pays-de-la-Loire, with a markedly lower prevalence of COVID-19.
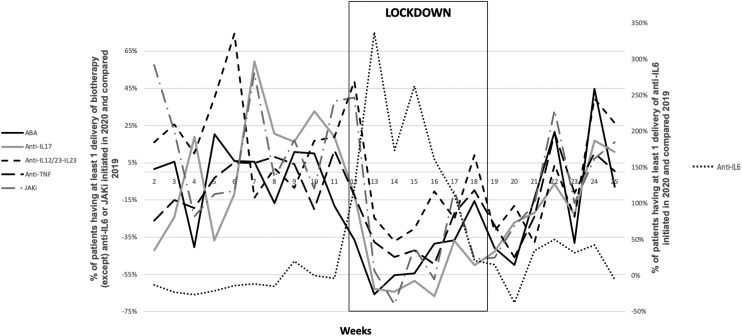
Fig. 1 shows the initiation of biologic therapy prescription in 2020 compared to those in 2019. During the lockdown, prescription initiation was greatly reduced for abatacept (405 patients in 2019 vs. 227 patients in 2020; −44%, P < 0.0001), anti-TNF agents (1,156 vs. 1,058; −31%, P < 0.001), anti-IL17 (415 vs. 206; −50%, P < 0.0001) and JAK inhibitors (289 vs. 174; −39%, P< 0.01) but was greatly increased for anti-IL6 mainly due to tocilizumab (117 vs. 445; +152%, P <0.05), with a non-significant decrease for p19 (IL-23) and p40 (IL-12/23) inhibitors.
Fig. 1.
Evolution of the proportion of patients with at least 1 delivery of biologic therapy or Janus kinase inhibitors initiated in 2020 and 2019. ABA: abatacept; JAKi: JAK inhibitor.
Overall, these reductions in prescription initiation were mainly observed in the area where the epidemic was more pronounced (i.e., East of France; P < 0.01).
We also looked at disease-modifying anti-rheumatic drugs and found a marked increase in HCQ prescription initiation (+173%; P < 0.05, occurring mainly during the first 4 weeks of the lockdown: +492%, +646%, +127%, and +49% at weeks 12, 13, 14 and 15, respectively) but a significant decrease in prescription initiation of apremilast (−44%; P < 0.001) and MTX (−30%; P < 0.001).
In contrast, we found no change in renewal of treatments, whatever the therapeutic class (data not shown).
In conclusion, during the COVID-19 epidemic peak in France, we found a sharp increase in new prescriptions of both HCQ and tocilizumab, two drugs suspected to improve COVID-19 outcomes [4]. By contrast, physicians less frequently prescribed other biologic agents, MTX and apremilast, as previously reported [5], [6].
The delay in the initiation of biologic therapy prescription might have affected the quality of care and might lead to worse outcomes. This point remains to be addressed by further studies.
Funding declaration
None.
Disclosure of interest
PR received fees from Abbvie, Pfizer, UCB, Roche, Fresenius, Amgen, Janssen, Lilly, Novartis, IQVIA.
MA: received fees from Amgen, Biogen, Bms/Celgene, Celltrion, Ferring, Janssen, Novartis, Pfizer, Roche/Genentech, Takeda and IQVIA.
LP, SP and MM are employed by IQVIA.
The other authors declare that they have no competing interest.
References
- 1.Landewe R.B., Machado P.M., Kroon F., et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79:851–858. doi: 10.1136/annrheumdis-2020-217877. [DOI] [PubMed] [Google Scholar]
- 2.Richez C., Flipo R.M., Berenbaum F., et al. Managing patients with rheumatic diseases during the COVID-19 pandemic: The French Society of Rheumatology answers to most frequently asked questions up to May 2020. Joint Bone Spine. 2020;87:431–437. doi: 10.1016/j.jbspin.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilcu A.M., Blanchon T., Sabatte L., et al. Cross-validation of an algorithm detecting acute gastroenteritis episodes from prescribed drug dispensing data in France: comparison with clinical data reported in a primary care surveillance system, winter seasons 2014/15 to 2016/17. BMC Med Res Methodol. 2019;19:110. doi: 10.1186/s12874-019-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benucci M., Damiani A., Infantino M., et al. Old and new antirheumatic drugs for the treatment of COVID-19. Joint Bone Spine. 2020;87:195–197. doi: 10.1016/j.jbspin.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quere B., Saraux A., Marhadour T., et al. Impact of the COVID-19 pandemic on therapeutic management of rheumatoid arthritis in Brittany (France) Joint Bone Spine. 2021;88:105179. doi: 10.1016/j.jbspin.2021.105179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costantino F., Bahier L., Tarancon L.C., et al. COVID-19 in French patients with chronic inflammatory rheumatic diseases: Clinical features, risk factors and treatment adherence. Joint Bone Spine. 2021;88:105095. doi: 10.1016/j.jbspin.2020.105095. [DOI] [PMC free article] [PubMed] [Google Scholar]