Abstract
Multisystem inflammatory syndrome in children (MIS-C) is a post-infectious immune-mediated condition, seen 3–5 weeks after COVID-19. Maternal SARS-CoV-2 may potentially cause a similar hyperinflammatory syndrome in neonates due to transplacental transfer of antibodies. We reviewed the perinatal history, clinical features, and outcomes of 20 neonates with features consistent with MIS-C related to maternal SARS-CoV-2 in Kolhapur, India, from 1 September 2020 to 30 April 2021. Anti-SARS-CoV-2 IgG and IgM antibodies were tested in all neonates. Fifteen singletons and five twins born to eighteen mothers with a history of COVID-19 disease or exposure during pregnancy presented with features consistent with MIS-C during the first 5 days after birth. Nineteen were positive for anti-SARS-CoV-2 IgG and all were negative for IgM antibodies. All mothers were asymptomatic and therefore not tested by RTPCR-SARS-CoV-2 at delivery. Eighteen neonates (90%) had cardiac involvement with prolonged QTc, 2:1 AV block, cardiogenic shock, or coronary dilatation. Other findings included respiratory failure (40%), fever (10%), feeding intolerance (30%), melena (10%), and renal failure (5%). All infants had elevated inflammatory biomarkers and received steroids and IVIG. Two infants died. We speculate that maternal SARS-CoV-2 and transplacental antibodies cause multisystem inflammatory syndrome in neonates (MIS-N). Immunomodulation may be beneficial in some cases, but further studies are needed.
Keywords: neonate, multisystem inflammatory syndrome in children (MIS-C), anti SARS-CoV-2 antibodies, COVID-19
1. Introduction
COVID-19, caused by SARS-CoV-2, is a global public health crisis with a large recent surge in India. As of 24 June 2021, 179 million individuals were infected worldwide, with India contributing to half of all new daily cases in April–May 2021 [1]. Initial studies showed that children were spared of severe COVID-19 [2,3,4]. However, recently case reports of children experiencing a potentially life threatening pediatric inflammatory multisystem syndrome (PIMS)—also called multisystem inflammatory syndrome in children (MIS-C)—have been described [5,6,7].
MIS-C is a new disease in children, the exact mechanism of which is still unclear. It is thought to be due to immune dysregulation following exposure to SARS CoV-2 [8]. It usually presents as fever and multiorgan involvement, with blood investigations showing increased inflammatory markers weeks after exposure to SARS-CoV-2 [5,6,8]. MIS-C has clinical and serological similarities with Kawasaki disease and the severe COVID-19 cytokine storm seen in adults [9]. However, its pathophysiology and immunological response is different, and may be mediated by autoantibodies [10]. More than 80% of children with MIS-C have specific IgM and IgG antibodies against SARS-CoV-2, but only about one-third are positive for SARS-CoV-2 by RTPCR [5,11,12].
Unlike MIS-C, where SARS-CoV-2 infection and multisystem inflammation occur in the same subject, a few case reports suggest neonatal multisystem inflammation [13] occurs secondary to maternal SARS-CoV-2 infection [14,15,16,17]. A few weeks after the first wave of COVID-19 in Kolhapur, India, we found an increase in the number of neonates with structurally normal hearts who presented with conduction abnormalities and were born to mothers with a past history of COVID-19. Specifically, these neonates presented with prolonged QTc with 2:1 Atrioventricular (AV) block or thrombosis similar to older children with MIS-C within the first week after birth [18]. We present a case series of 20 neonates with multisystem involvement, hyperinflammatory syndrome and positive anti SARS-CoV-2 IgG antibodies, temporally related to maternal antenatal SARs-CoV-2 exposure. To our knowledge, this is the largest series of MIS-C presenting in the early neonatal period.
2. Materials and Methods
Access to chart reviews and publication was approved by the Institutional Ethics Committee (IEC) of the Dr D Y Patil Medical College Hospital and Research Institute, at Dr D Y Patil University, Kolhapur, India. Informed consent was obtained from parents/guardians for using clinical data and photographs. Neonates who met the criteria in Table 1 (with four exceptions, as explained below) and that were admitted to seven NICUs in Kolhapur between 1 September 2020 and 30 April 2021 were included. These criteria were modified from CDC criteria for MIS-C and interim guidance from AAP to accommodate lack of fever in neonates and source of primary infection (mother, instead of the child) [19,20]. Neonates with signs consistent with MIS-C, maternal history of COVID-19, and positive for anti-SARS CoV-2 antibodies were included. However, infants with these symptoms and culture positive sepsis, or proven infective pathology in other organ systems (e.g., meningitis, urinary tract infection, etc.) were excluded. Infants with low Apgar scores (≤3 at 5 min) and evidence of birth asphyxia were excluded. Preterm infants with findings attributable to early gestation (such as respiratory distress presenting immediately after birth and transient hypotension) were excluded. IgG and IgM against SARS CoV-2 were detected using VIDAS® SARS-COV-2 kits (BioMerieux SA, Marcy-I’Etioile, France), with MINIVIDAS using ELFA: enzyme linked fluorescent assay. Data are presented as median (range) or number (%).
Table 1.
Proposed inclusion criteria for neonatal multisystem inflammatory syndrome (MIS-N) secondary to maternal SARS CoV-2 exposure or infection.
|
We differentiated neonates presenting with multisystem inflammatory syndrome in the first week after birth secondary to possible maternal COVID-19 infection (labeled in this article as MIS-N), from neonates who had early onset neonatal COVID-19 or late-onset neonatal COVID-19 and subsequently present with multisystem inflammation during 2–4 weeks after birth (labeled in this article as MIS-C) (Figure 1). In patients with MIS-C, multisystem inflammation was secondary to prior COVID-19 in the same subject. However, in MIS-N, multisystem inflammation in the neonate was secondary to COVID-19 in the mother with passive transmission of antibodies.
Figure 1.
Various presentations of SARS-CoV-2 infection and its sequences in the neonatal period. Red colored subjects with a ‘+’ sign indicate COVID-19 positive patients. Pregnant mother A has COVID-19 and her baby is negative at birth but contracts late-onset COVID-19 due to transmission from the mother. Pregnant mother B has no COVID-19 but her neonate develops late-onset neonatal infection due to exposure to a family member 2–4 weeks after birth. Pregnant mother C is COVID-19 positive during the perinatal period and transmits the virus to her offspring during birth leading to early-onset infection in the neonate. This baby can potentially develop MIS-C 2–4 weeks later (a rare occurrence) [16]. Pregnant mother D has COVID-19 during pregnancy but the neonate remains healthy. Pregnant mother E has COVID-19 disease or exposure to SARS-COV-2 during pregnancy and the baby develops multisystem inflammation secondary to passive transfer of antibodies leading to MIS-N (multisystem inflammatory syndrome in neonates) [14].
3. Results
Clinical characteristics of 20 neonates are shown in Table 2. Individual patient characteristics are shown in Table 3. Three infants (# 17, 18 and 19 in Table 3) had IgG anti SARS-CoV-2 levels below the cut-off but were included because of maternal history and typical presentation (AV block or dilated coronaries). Case # 20 only had a cardiac thrombus without other organ involvement but was included due to maternal history, high IgG levels, elevated inflammatory markers, and lack of other explanation for the thrombus.
Table 2.
Characteristics of patients with suspected MIS-N.
| Characteristic | Median (Range) or Number (%) | Comments |
|---|---|---|
| Characteristic | Median (Range) or Number (%) | Comments |
| Maternal age | 26.5 years (20–34 years) | |
| Maternal symptoms (n = 18): | ||
| Asymptomatic Symptomatic Trimester when positive or had exposure |
11 (61.2%) 7 (38.8) First–1 (5.5%), Second–2 (11.1%), Third–15 (83.3%) |
|
| Mode of delivery–Cesarean | 7 (38.8%) | |
| Gestational age at birth (n = 20) | 34 weeks (27–38 weeks) | Term (≥37 weeks)–3 (15%) Late preterm (34–36 weeks)–13 (65%) <33 weeks–4 (20%) |
| Birth weight (kg) | 2.15 (1–4) | |
| Sex | Male (10), Female (10) | |
| Multiplicity | Singleton–15 Twins–5 |
|
| Neonate–age at presentation | Day 2 (day 1 to day 5) | Day 1 (<24 h of birth)–7 (35%) |
| Organ system involvement | ||
| • Cardiac • Hematologic/thrombosis • Respiratory • Gastrointestinal • Neurological • Cutaneous • Renal • Fever/temperature instability |
18 (90%) 2 (10%)-thrombosis; 2 (10%)-GI bleed 11 (55%)-requiring ventilator/CPAP 6 (30%) 2 (10%) 1 (5%) 1 (5%) 2 (10%) |
|
| Investigations: | ||
| • CRP • Procalcitonin • D-dimer • LDH • NT-Pro BNP |
24 (9–62) 2.05 (1.3–51) 5932 (2820–12,000) 1315 (793–6424) 24,300 (7361-> 30,000) |
Normal values 0-6 mg/L <0.5 ng/mL <2700 ng/mL 290-775 U/L <11,987 pg/mL for 0-2 days, |
| <5918 pg/mL for 3-11 days | ||
| • Blood culture | No growth in all infants | No growth |
| Cardiac findings | ||
| • Arrhythmia • Dilated coronaries • Intracardiac thrombus • Shock/ cardiac dysfunction |
11 (44%) 2 (12%) 2 (8%) 5 (20%) |
One thrombus in LPA, one in RA |
| Infant’s serology | ||
| • IgM SARS CoV-2 • IgG SARS CoV-2 |
0.0 (0–0) COI 3.49 (0.07–74.39) COI |
Cut-off-Index (COI) ≥ 1 is positive, for both IgG and IgM |
| Therapy | ||
| • Steroids • IVIG • LMWH • Inotropes |
20 (100%) 20 (100%) 14 (70%) 12 (60%) |
IV Methylprednisolone 2 mg/kg/day 1–2 g/kg 1.5 mg/kg/dose, twice a day IV Milrinone, Adrenaline, Dobutamine, Dopamine |
| Outcome | ||
| • Mortality | 2 (10%) | One due to necrotizing enterocolitis One due to Multiorgan dysfunction |
Table 3.
Clinical features, treatments, and outcomes of suspected patients with Neonatal Inflammatory Multisystem Syndrome (MIS-C) associated with SARS-CoV-2 infection.
| Subject Number | Age at Presentation/Sex/Weight/Gestation | Maternal COVID-19 Status | Neonatal Serology (All Were IgM-ve) | Lab Studies (Values) | Clinical Features | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1 * | Day 1/F 4 kg 38 weeks |
asymptomatic, RTPCR +ve 3 weeks before delivery | IgG +ve on day 1 | Elevated CRP (14), PCT (1.3), Ferritin (1500), D Dimer (5088). | Fever on day 1, hypotension; echo-LV dysfunction | Inotropes Steroids IVIG |
Discharged on day 13 |
| 2 * | Day 1/M 2.02 kg 35 weeks |
asymptomatic, COVID-19 contact 8 weeks before delivery, | IgG +ve on day 2 | Elevated CRP (9), D-Dimer (5100), Ferritin (393), LDH (1183), NT Pro BNP (>30,000) | Antenatal scan showing fetoplacental compromise; shock on day 1, Echo—mild LV dysfunction and bilateral pleural effusions | LMWH Steroids IVIG |
Discharged on day 14 |
| 3 * | Day 4/F 2 kg 33 weeks |
asymptomatic COVID-19 contact 6 weeks before delivery, IgG +ve | IgG +ve on day 6 | Elevated CRP (10), d-dimer (3020), Ferritin (407) | RDS, severe bradycardia with prolonged QTc and 2:1 AVB from day 4 of life (Figure 2A,B) | Surfactant MV, Steroids IVIG |
Sinus rhythm at discharge on day 16 (Figure 2C) |
| 4 * | Day 1/M 2 kg 36 weeks |
asymptomatic COVID-19 contact 6 weeks before delivery, | IgG +ve on day 1 | Elevated CRP (12), D Dimer (6848), NT Pro BNP (>25,000), LDH (1158) | Antenatal scan showing dilated RA/RV, pericardial and pleural effusions and ascites; Respiratory distress, PPV at birth, shock; echo–dilated hypertrophied RV with dysfunction, moderate TR, large thrombus at LPA origin on day 3 (Figure 3D,E) | LMWH, Alteplase, Inotropes, MV, IVIG, Steroids |
Discharged on day 19; LMWH and Aspirin x 6 weeks, complete resolution of thrombus at 8 weeks echo |
| 5 * | Day 3/M 3.5 kg 38 weeks |
Febrile illness at 7 months of gestation | IgG +ve on day 5 | Elevated CRP (60.2), PCT (2.1), D Dimer (6483), Ferritin (878), LDH (793), leucocytosis (18,600). | Grunting, tachypnea, and lethargy, feeding intolerance, intermittent bradycardia, hypotension | Inotropes, MV, Steroids, IVIG |
Discharged on day 13 |
| 6 * | Day 2/M 2.3 kg 34 weeks |
Febrile illness 2 weeks before delivery | IgG + ve on day 12 | Elevated CRP (24), d dimer (4200), thrombocytopenia (39 × 109/L) | feeding intolerance, decreased activity from day 2, brown gastric aspirates on day 4, treated like NEC, bleeding continued with rash, pedal edema, oral and skin lesions, skin peeling (Figure 3G,H) | CPAP, Inotropes, LMWH, Steroids, IVIG |
Discharged on day 38 |
| 7 * | Day 3/F 1.4 kg 34 weeks |
Asymptomatic RTPCR +ve, 5th month of gestation | IgG +ve on day 5 | Elevated CRP (50), D Dimer (5100), normal coagulation profile | Antenatal scan showing fetoplacental compromise; LBW. Brownish gastric aspirates from day 3, frank malena (Figure 3I) from day 6, episodes of SVT from day 8; Echo- bilateral pleural and pericardial effusion. | Beta- blockers, Steroids, IVIG |
Discharged on day 20 |
|
8 *
2nd of twins † |
Day 2/M 1.9 kg 32 weeks |
Asymptomatic RTPCR positive at 3rd month of gestation | IgG +ve on day 1 | Elevated CRP (43), IL-6 (116), D Dimer (6600) | distress at birth, bradycardia with prolonged QTc and 2:1 AVB on day 2 of life | MV and CPAP, inotropes, Steroids, IVIG |
Sinus rhythm at discharge on day 23 |
|
9 *
Twin 1 |
Day2/F 1.9 kg 33 weeks |
Asymptomatic COVID-19 contact 8 weeks before delivery | IgG +ve on day 4 | Elevated CRP (35), D Dimer (10,000) | Antenatal scan showing fetoplacental compromise, bradycardia with prolonged QTc and 2:1 AVB from day 2 | IVIG, Steroids, LMWH, | Sinus rhythm at discharge on day 18 |
|
10 *
Twin 2 |
Day 2/M 1.6 kg 33 weeks |
Asymptomatic COVID-19 contact 8 weeks before delivery | IgG +ve on day 5 | Elevated D Dimer (10,000), LDH (977) | Antenatal scan showing feto-maternal compromise, feeding intolerance, bradycardia with prolonged QTc and 2:1 AVB from day 2, | IVIG, steroids, LMWH | Sinus rhythm at discharge on day 18 |
|
11 *
Twin 1 |
Day 4/F 2.05kg 34 weeks |
Febrile illness 3 weeks before delivery–IgG level below cutoff | IgG +ve on day 4 | Elevated PCT (1.8), D Dimer (4840), NT Pro BNP (> 25,000) | bradycardia with prolonged QTc and 2:1 AVB on day 4 (Figure 2D,E) | IVIG, steroids, LMWH, inotropes, CPAP | Sinus rhythm at discharge on day 11 (Figure 2F) |
|
12 *
Twin 2 |
Day 4/M 2.1 kg 34 weeks |
Febrile illness 3 weeks before delivery–IgG level below cutoff | IgG +ve on day 4 | Elevated PCT (1.4), D Dimer (5932) | bradycardia with prolonged QTc and 2:1 AVB on day 4 (Figure 2G,H) | IVIG, steroids, LMWH, inotropes, CPAP | Sinus rhythm at discharge on day 11 (Figure 2I) |
| 13 * | Day 3/F 1 kg 27 weeks |
Asymptomatic COVID-19 contact 8 weeks before delivery | IgG +ve on day | Elevated PCT (51), D Dimer (10,000), LDH (6424), NT Pro BNP (25,000) | Extreme PT, Extreme LBW, bradycardia with prolonged QTc and 2:1 AVB with 2:1 AV block on day 4 (Figure 2J,K); sinus rhythm on day 7 (Figure 2L) | IVIG, Steroids, LMWH, MV | day 9 abdominal distension, NEC → death on day 11 |
| 14 * | Day 2/M 2.4 kg 36 weeks |
Asymptomatic COVID-19 contact 10 weeks before delivery, IgG +ve | IgG +ve on day 6 | Elevated CRP (11), D Dimer (4700), LDH (2143) | not accepting feeds on day2, Cardiomegaly on X-ray chest, cardiogenic shock on day 5, echo (Figure 3A)-dilated coronaries # (LMCA Z score = + 4.2, RCA Z score = +4.9) severe TR, mild MR, ASD, PDA, Severe PAH, | IVIG, steroids, LMWH, Inotropes, PPV, Aspirin |
Discharged on day 14; Coronaries normalized at discharge, Tab Aspirin x 6 weeks |
| 15 * | Day 4/M 2 kg 36 weeks |
Asymptomatic COVID-19 contact 4 weeks before delivery | IgG +ve on day 6 | Elevated CRP (18), BUN (99.2), serum Creatinine (1.9), NT Pro BNP (14,500), Potassium (6.9 mEq/L) |
Admitted on day 6, Seizures, shock, bradycardia, acute renal failure, hyperkalemia, Echo-small ASD, dilated all four chambers, mild LV dysfunction |
IVIG, Steroids, MV, Inotropes, Peritoneal dialysis, |
Death on day 8-Multi-organ dysfunction |
| 16 * | Day 1/F 2 kg 36 weeks |
Asymptomatic COVID-19 contact 4 weeks before delivery, IgG +ve | IgG +ve on day 1 | Elevated CRP (62), PCT (2.4), D Dimer (9734), NT Pro BNP (7361). | Fever on day 1, feeding intolerance, vomiting, tachypnea, desaturation on day 2 | IVIG, Steroids | Discharged on day 10 |
| 17 | Day 2/F 1.5 kg 32 weeks |
Asymptomatic COVID-19 contact 10 weeks before delivery | IgG -ve | Elevated CRP (18), D Dimer (12,000) | RDS, bradycardia with prolonged QTc and 2:1 AVB on day 3 | IVIG, steroids, inotropes, CPAP | Sinus rhythm at discharge on day 13 |
| 18 | Day 2/F 1.5 kg 32 weeks |
Febrile illness 8 weeks before delivery | IgG below cut-off level | Elevated CRP (25), D Dimer (10,000), NT Pro BNP (23,700) | bradycardia with prolonged QTc and 2:1 AVB on day 2 (Figure 2M,N) | IVIG, steroids | Sinus rhythm at discharge on day 14 (Figure 2O) |
| 19 | Day 1/M 1.9 kg 34 weeks |
Febrile illness 6 weeks before delivery; IgG below cutoff levels, IgM -ve | IgG below cutoff levels | Elevated D Dimer (2820), LDH (2661), NT Pro BNP (>25,000), thrombocytopenia (93 × 109/L) | Antenatal scan showing pleural, pericardial effusions, and ascites; not cried after birth; LBW, pitting edema over chest wall, hepatomegaly, tachypnea, crepitations; Echo (Figure 3C)—dilated coronaries #, (LMCA Z score = +2.7, LAD Z score = +2.7, RCA Z score = +2), large PDA, mild TR and MR, normal function (on inotropes), bilateral moderate pleural effusion; | IVIG, Steroids, LMWH, Inotropes, Lasix, MV, Aspirin | Discharged on day 15; Coronaries normal, Aspirin x 6 weeks. |
| 20 | Day 1/ F 2.7 kg 38 weeks |
Febrile illness 6 weeks before delivery, IgG +ve | IgG +ve on day 2 | Elevated CRP (53), D Dimer (3942), LDH (804), NT Pro BNP (17,018) | Not cried after birth, mottling and poor peripheral pulsations, hypotension; Echo (Figure 2F)–day 4-intracardiac thrombus in RA, normal LV function | PPV, surfactant, Inotropes, IVIG, Steroids, LMWH, MV |
Discharged on day 24; LMWH x 6 weeks, thrombus decreased in size at 4 weeks echo |
Normal ranges and units for lab values: CRP 0-6 mg/L; D-Dimer < 2740 ng/mL (0-3 days of life); NT Pro-BNP < 11,987 pg/mL for 0–2 days, <5918 pg/mL for 3–11 days; procalcitonin <0.5 ng/mL; LDH 290-775 U/L (for 0-4 days of life); IL-6 < 7 pg/mL; Ferritin = 25–200 ng/mL; BUN 2–19 mg/dL; serum creatinine 0.3–1 mg/dL; patients with (*) met the inclusion criteria mentioned in Table 1. Patients 17–19 did not have a positive IgG SARS CoV-2 level above the laboratory cut-off–however, patients had EKG consistent with AV block; patient 20 had delayed cry and might have had perinatal depression but had Apgar scores > 3 by 5 min but an unexplained intracardiac thrombus in the right atrium. † Twin A was positive for IgG SARS CoV-2 but other clinical features were consistent with prematurity. # Z scores for coronary diameter were calculated based on Kobayashi et al. [22]. Abbreviations: -ve = negative; +ve = positive; M = male, F = female; ASD = atrial septal defect; AVB = atrioventricular block; BUN = blood urea nitrogen; CKMB = creatinine kinase myocardial band; CNS = central nervous system; COVID-19 = corona virus disease 2019; CPAP = continuous positive airway pressure; CRP = C-reactive protein; IgG = immunoglobulin G, IgM = immunoglobulin M, IL-6 = interleukin-6; IVIG = intravenous immunoglobulin; LAD = left anterior descending coronary artery; LBW = low birth weight, LDH = lactate dehydrogenase; LMCA = left main coronary artery; LMWH = low molecular weight heparin; LPA = left pulmonary artery; LV = left ventricle; MR = mitral regurgitation; MV = mechanical ventilation; NEC = necrotizing enterocolitis; NT Pro BNP = N-terminal pro–B-type natriuretic peptide; PAH = pulmonary artery hypertension; PCT = procalcitonin; PDA = patent ductus arteriosus; PPHN = persistent pulmonary hypertension of the newborn; PPV = positive pressure ventilation; PT = preterm; QTc = corrected QT interval; RA and RV = right atrium and ventricle; RCA = right coronary artery; RT- PCR = reverse transcription-polymerase chain reaction; SVT = supraventricular tachycardia; TR = tricuspid regurgitation.
3.1. Maternal Features
Of the 18 mothers (three with twin pregnancy), seven (38.8%) were symptomatic for COVID-19 during pregnancy, three (16.6%) were asymptomatic but RT-PCR positive for COVID-19, and eleven (61.1%) were asymptomatic but had history of close contact with COVID-19 cases (usually a confirmed case in the family). Fifteen mothers (83.3%) were symptomatic or had contact during the last trimester of pregnancy, (five (27.7%) within the last 4 weeks before delivery), two (11.1%) during second trimester and one (5.5%) in the first trimester of pregnancy. None of them had symptomatic COVID-19 or febrile illness during admission for delivery, and none were tested for COVID -19 RT-PCR during the admission for delivery. Five mothers (27.7%) had an antenatal ultrasound scan showing fetoplacental compromise (reduced flow in uterine artery or umbilical artery and/or diastolic notch, diastolic flow reversal, fetal ascites, pericardial and pleural effusion). Mothers whose infants presented with cardiac conduction abnormalities were tested for lupus antibodies and were negative.
3.2. Resuscitation at Birth and Post-Resuscitation Period
Two neonates did not cry immediately after birth and two had significant respiratory distress in the delivery room. These four (20%) neonates required positive pressure ventilation (PPV) and subsequently required conventional mechanical ventilation on the day of birth. Sixteen (80%) neonates did not require any PPV in the delivery room. However, three of these infants required respiratory support (invasive mechanical ventilation or CPAP) on the day of birth in the NICU.
3.3. Clinical Presentation
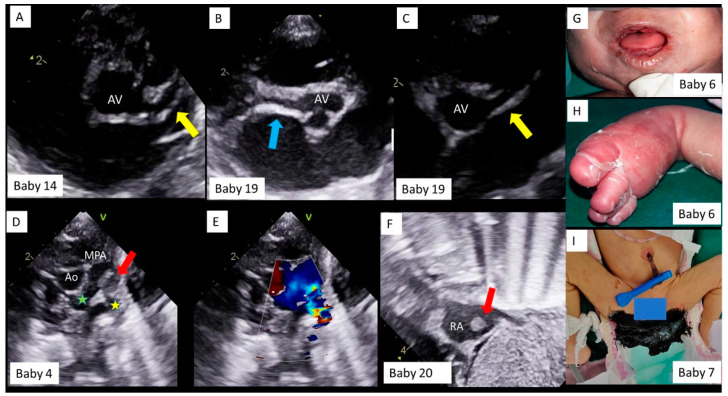
The most common presentation involved the cardiovascular system (Table 3). Eleven had rhythm disorders, of which nine presented with prolonged QTc interval with 2:1 AV block (Figure 2A,D,G,J,M). With immunomodulatory therapy with methylprednisolone and intravenous immunoglobulin (IVIG), 2:1 AV block disappeared first (Figure 2B,E,H,K,N), followed by normalization of QTc (C, F, I, L, O), in all of the nine neonates. One neonate had an episode of supraventricular tachycardia (SVT), requiring a short course of beta blockers, and one infant had bradycardia with tall, peaked T waves and broad QRS due to hyperkalemia secondary to acute renal failure. Shock with or without cardiac dysfunction on echocardiography was seen in five neonates. Two neonates had significant coronary dilatation on day one of life (Figure 3A–C). One neonate had a thrombus almost completely occluding the left pulmonary artery (LPA) (Figure 3D), requiring systemic thrombolysis with Alteplase (t-PA, 3 doses), and low molecular weight heparin (LMWH) for six weeks. One neonate had an intracardiac thrombus at the inferior vena cava–right atrial junction (Figure 3E), which partly resolved at discharge, after LMWH therapy.
Figure 2.
Representative EKGs of neonates presenting with bradycardia. Baby number sequence is the same as in Table 3. The first column showing EKGs at presentation (A,D,G,J,M), with sinus rhythm, prolonged QT interval and atrio-ventricular block. Middle column showing sinus rhythm and prolonged QT interval (B,E,H,K,N). The last column showing sinus rhythm with normal QT interval (C,F,I,L,O). Black arrows = atrial beats; horizontal square bracket = QT interval; QTc = corrected QT interval; ms = milliseconds. QTc values in the figure are derived by the formula QTc = QT/√RR.
Figure 3.
Echocardiography and clinical findings in neonates with MIS-C. Baby number sequence is the same as in Table 3. Transthoracic echocardiography, parasternal short axis view in Baby #14 (A) and Baby #19 (B,C). The left main and left anterior descending coronary artery (yellow arrow) and the right coronary artery (blue arrow) are significantly dilated. AV = aortic valve. Transthoracic echocardiography and color doppler, parasternal short axis view in Baby #4 (D), showing aorta (Ao) and main pulmonary artery (MPA) bifurcation, with a large thrombus (red arrow) obstructing the left pulmonary artery (yellow star) origin and causing flow turbulence on color doppler (E), but normal flows across right pulmonary artery (green star). Transthoracic echocardiography subcostal bi-caval view in Baby # 20 (F), showing a thrombus (red arrow) in right atrium (RA). Baby #6, showing oral and muco-cutaneous lesions (G) and, pedal edema and skin peeling (H) and Baby #7 with black, tarry stools (melena, I).
Eleven neonates required either mechanical ventilation (n = 8) or CPAP (n = 3), for respiratory distress syndrome associated with prematurity, shock, or respiratory depression. Two neonates presented with fever on day one of life. Two neonates did not cry immediately after birth but had Apgar scores >3 by 5 min of age. One infant presented with convulsions on day 4 and was admitted on day 6 with multiorgan failure leading to death.
Feeding intolerance and gastric aspirates were seen in 6 neonates, of which two had brownish gastric aspirates. Two had lower gastrointestinal bleeding, of which one had tarry stools (melena) (Figure 3I) and one had blood in stools on day 8 of life (with a normal coagulation profile).
Anti-SARS-CoV-2 IgM antibodies were negative in all the neonates, and IgG antibodies (cut-off-index (COI) ≥1 considered reactive) were positive (COI value > 1) in 17 (85%) neonates. Two (10%) had levels below positive cut-off, and one (5%) had no detectable levels. RTPCR for SARS-CoV-2 was not done in any of the neonates as the Indian Academy of Pediatrics Guidelines recommend this test after birth if mothers are symptomatic, or tested positive within 14 days before birth, or if there is history of contact with COVID-19 positive persons in the postnatal period. [21]
To summarize, we present a case series of 20 neonates born to mothers with a history of SARS-CoV-2 infection or exposure to COVID-19 patients. The majority of infants were late preterm, with equal sex distribution and presented with cardiac (90%), respiratory (55%) or gastrointestinal (30%) signs with elevated inflammatory markers and positive IgG SARS-CoV-2 titers. These infants were managed with supportive therapy, methylprednisolone, IVIG and was associated with a 10% mortality. Our protocol for diagnosis and management of MIS-N is shown in Table 4.
Table 4.
Protocol for laboratory investigations and management of MIS-N.
Laboratory investigations need to be titrated based on clinical presentation. [9]
|
Management of neonates with MIS-N is predominantly supportive.
|
Specific therapy for MIS-C includes the use of anticoagulants, steroids, IVIG and anti-inflammatory agents. As shown in the case reports in Table 2 and Table 3, neonates have received treatment with immunomodulatory therapies (IVIG, methylprednisolone, anti-platelet agents (aspirin), and anticoagulants (unfractionated heparin or low molecular weight heparin). Further studies are required to evaluate the benefits and risks of these therapies in MIS-C in neonates. Pending further studies, we recommend the following approach to MIS-C in neonates.
|
| Note: During the neonatal period, MIS-N is relatively rare. More common causes for cardiac dysfunction and elevated Troponin or BNP such as perinatal asphyxia should be considered. The use of glucocorticoids and IVIG should be limited to indications outlined above. |
Abbreviations are same as in Table 3.
4. Discussion
We present a case series of neonates born to mothers with a history of SARS-CoV-2 infection or exposure to a COVID-19 patient during pregnancy and presenting with features that cannot be explained by other causes. Whether these findings are unrelated to maternal COVID-19 or due to an inflammatory process induced by the transplacental passage of antibodies directed against autoantigens is not clear. However, the unusually high frequency of findings such as atrioventricular conduction abnormalities, resembling cardiac findings in older children with MIS-C [18], and response to immunomodulatory therapy with intravenous immunoglobulin (IVIG) and steroids suggests that “multisystem inflammatory syndrome in the neonate (MIS-N)” deserves further study [26]. We present this case series to increase awareness of this possibility amongst all care providers, especially obstetricians, pediatricians, pediatric cardiologists, and neonatologists.
We speculate that maternal infection with SARS CoV-2 results in development of protective IgG antibodies against spike protein of the virus (similar to a response following vaccination) [27]. These antibodies cross the placenta (with IgA versions in breastmilk) to provide passive immunity to the newborn [27]. In some genetically susceptible children, autoantibodies triggered by SARS CoV-2 infection may bind to receptors in neutrophils and macrophages causing activation and secretion of pro-inflammatory cytokines that results in development of MIS-C [9,28]. Children with MIS-C have higher SARS-CoV-2 IgG titers than those with severe COVID-19 [29], however, this trend is transient in MIS-C [30]. We speculate that the spike protein IgG antibodies are protective innocent bystanders, are a marker of prior infection and do not have a pathogenic role in MIS-C. On the other hand, autoantibodies against endothelial, gastrointestinal, and immune cells are also produced and may potentially play a role in MIS-C [31]. Patients with MIS-C have high levels of certain antibodies against autoantigens (anti-SSB, anti-Jo-1), lending credence to the hypothesis that MIS-C is mediated by a persistent autoimmune response to the original infection [31]. As such, and analogous to neonatal lupus, where anti-SSA and anti-SSB antibodies cross the placenta to cause manifestations such as rash and congenital heart block in newborns, it is plausible that similar antibodies against autoantigens crossed the placenta after a SARS CoV-2 infection and initiated MIS-N disease in these neonates. In our case series, atrioventricular conduction abnormalities were common (Figure 2) potentially secondary to transplacental transfer of similar antibodies.
We would like to differentiate MIS-C in the neonatal period due to early-onset SARS-CoV-2 infection in the neonate from “MIS-N” where the infection occurs in the mother and the neonates present early as shown in this case series (Figure 1). We acknowledge that the CDC has not labelled or described this condition and nomenclature may change in the future.
That maternal antibodies pass transplacentally is a known fact, and maternal infection with SARS-CoV-2 is no different. Multiple studies have reported the transplacental transfer of anti-SARS-CoV-2 IgG antibodies to neonates [32,33,34]. The majority (87%) infants born to seropositive mothers had detectable IgG antibody at birth, transfer ratios were more than 1.0, and there was a positive correlation between maternal and infant antibody titers, regardless of the presence of symptoms in the mother or the severity of disease [33].
None of the mothers in our case series had received vaccination against COVID-19 (vaccines were only administered to >45 years age strata in India during the study period). Although COVID-19 vaccines were not tested in pregnant mothers, many pregnant health care workers have received the Pfizer and Moderna vaccines in the US [35]. These mothers have a robust IgG and IgA response in their sera and breast milk respectively [27]. Umbilical cord sera were positive for IgG antibodies. We speculate that these vaccine induced antibodies against SARS CoV-2 spike protein are protective and do not pose a risk of MIS-C in babies because they are not directed towards autoantigens. Approximately 4500 pregnant mothers have registered in the V-safe COVID-19 vaccine pregnancy registry. Limited data from this registry have not reported any neonatal deaths to date [9,36].
The majority of infants in our case series were delivered at late preterm gestation. The NICHD Maternal Fetal Medicine Units (MFMU) network has reported a higher incidence of preterm labor and delivery in symptomatic pregnant mothers with COVID-19 (severe symptoms–42%; mild to moderate symptoms—15%; asymptomatic—12%) [37]. Therapy for MIS-N is mainly supportive. All patients in our case series received immunomodulatory therapies (intravenous immunoglobulin-IVIG and steroids), anti-platelet agents (aspirin), and anticoagulants (unfractionated heparin or LMWH). Further studies are required to evaluate the benefits and risks of these therapies in MIS-N [23,25]. While some cases, especially those with cardiac conduction abnormalities responded well to IVIG and steroid therapy, we need randomized trials to evaluate efficacy of these therapies in MIS-C. Overuse of these agents should be avoided. We admit that there was probably overtreatment with steroids, LMWH and IVIG among our patients and many of these patients might have improved without these therapies. More targeted therapy with these agents based on further research is prudent as IVIG use among neonates carries the potential risk of necrotizing enterocolitis [24].
5. Conclusions
We conclude that maternal history of SARS-CoV-2 infection or exposure to COVID-19 may potentially be associated with multisystem inflammation, thrombosis, and AV conduction abnormalities in the early neonatal period. However, neonatal MIS-C and MIS-N are relatively rare. More common causes for cardiac dysfunction and elevated troponin or BNP such as perinatal asphyxia and sepsis should be considered. Based on our case series, we recommend that among neonatal patients born to mothers with a history of COVID-19, neonatal MIS-C or MIS-N be considered in the differential diagnosis to explain unusual signs of multisystem inflammation, after excluding common causes.
Acknowledgments
We thank Rupali Patil, Amar Naik, and all post graduate residents in Pediatrics from all the included NICUs for their contribution in patient management. Also, thanks to R. S. Patil and Akshata Pawar (Pathologists), for laboratory tests and their interpretations in neonates. Special thanks to Shimpa Sharma, Pro-Vice Chancellor, D. Y. Patil Education Society (Institution deemed to be University), Kolhapur, R. K. Sharma, Dean and Anil Kurane, Head of Department of Pediatrics, Dr. D. Y. Patil Medical College Hospital and Research Institute, Kolhapur, for their guidance. We thank Ananya Nrusimha for her critical editing of the manuscript.
Author Contributions
Conceptualization, R.P.; methodology, R.P., V.G., N.P., V.M., A.G., S.L. (Sanjog Loya) and R.S.; validation, R.P., V.G., A.C., V.T., U.P. and N.N.; formal analysis, R.P. and S.L. (Satyan Lakshminrusimha); investigation, V.G., V.M., A.G., S.L. (Sanjog Loya) and A.C.; data curation, R.P., V.G., U.P. and N.P.; writing—original draft preparation, R.P.; writing—review and editing, R.P., N.N. and S.L. (Satyan Lakshminrusimha) All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of D. Y. Patil Medical College Kolhapur (438/2021/IEC, on 15 May 2021).
Informed Consent Statement
Informed consent was obtained from parents/guardians of all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO COVID-19 Dashboard. [(accessed on 24 June 2021)]; Available online: https://covid19.who.int/
- 2.Gupta S., Malhotra N., Gupta N., Agrawal S., Ish P. The curious case of coronavirus disease 2019 (COVID-19) in children. J. Pediatr. 2020;222:258–259. doi: 10.1016/j.jpeds.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodin P. Why is COVID-19 so mild in children? Acta Paediatr. 2020;109:1082–1083. doi: 10.1111/apa.15271. [DOI] [PubMed] [Google Scholar]
- 4.Rawat M., Chandrasekharan P., Hicar M.D., Lakshminrusimha S. COVID-19 in Newborns and Infants-Low Risk of Severe Disease: Silver Lining or Dark Cloud? Am. J. Perinatol. 2020;37:845–849. doi: 10.1055/s-0040-1710512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., Ramnarayan P., Fraisse A., Miller O., Davies P., et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA J. Am. Med. Assoc. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhadjer Z., Meot M., Bajolle F., Khraiche D., Legendre A., Abakka S., Auriau J., Grimaud M., Oualha M., Beghetti M., et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 8.Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., Johnson M., Griffiths B., du Pre P., Mohammad Z., et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: A multicentre observational study. Lancet Child Adolesc. Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children. 2020;7:69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., Tan Z., Zicari S., Ruggiero A., Pascucci G.R., et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell. 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godfred-Cato S., Tsang C.A., Giovanni J., Abrams J., Oster M.E., Lee E.H., Lash M.K., Le Marchand C., Liu C.Y., Newhouse C.N. Multisystem Inflammatory Syndrome in Infants<12 months of Age, United States, May 2020–January 2021. Pediatric Infect. Dis. J. 2021;40:601–605. doi: 10.1097/INF.0000000000003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godfred-Cato S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J., Roguski K., Wallace B., Prezzato E., Koumans E.H., et al. COVID-19-Associated Multisystem Inflammatory Syndrome in Children—United States, March–July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarty K.L., Tucker M., Lee G., Pandey V. Fetal Inflammatory Response Syndrome Associated With Maternal SARS-CoV-2 Infection. Pediatrics. 2020 doi: 10.1542/peds.2020-010132. [DOI] [PubMed] [Google Scholar]
- 14.Divekar A.A., Patamasucon P., Benjamin J.S. Presumptive Neonatal Multisystem Inflammatory Syndrome in Children Associated with Coronavirus Disease 2019. Am. J. Perinatol. 2021 doi: 10.1055/s-0041-1726318. [DOI] [PubMed] [Google Scholar]
- 15.Khaund Borkotoky R., Banerjee Barua P., Paul S.P., Heaton P.A. COVID-19-Related Potential Multisystem Inflammatory Syndrome in Childhood in a Neonate Presenting as Persistent Pulmonary Hypertension of the Newborn. Pediatric Infect. Dis. J. 2021;40:e162–e164. doi: 10.1097/INF.0000000000003054. [DOI] [PubMed] [Google Scholar]
- 16.Kappanayil M., Balan S., Alawani S., Mohanty S., Leeladharan S.P., Gangadharan S., Jayashankar J.P., Jagadeesan S., Kumar A., Gupta A., et al. Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS-CoV-2: A case report. Lancet Child Adolesc. Health. 2021;5:304–308. doi: 10.1016/S2352-4642(21)00055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaiba L.A., Hadid A., Altirkawi K.A., Bakheet H.M., Alherz A.M., Hussain S.A., Sobaih B.H., Alnemri A.M., Almaghrabi R., Ahmed M., et al. Case Report: Neonatal Multi-System Inflammatory Syndrome Associated With SARS-CoV-2 Exposure in Two Cases From Saudi Arabia. Front. Pediatr. 2021;9:652857. doi: 10.3389/fped.2021.652857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark B.C., Sanchez-de-Toledo J., Bautista-Rodriguez C., Choueiter N., Lara D., Kang H., Mohsin S., Fraisse A., Cesar S., Sattar Shaikh A., et al. Cardiac Abnormalities Seen in Pediatric Patients During the SARS-CoV2 Pandemic: An International Experience. J. Am. Heart Assoc. 2020;9:e018007. doi: 10.1161/JAHA.120.018007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C) [(accessed on 25 June 2021)]; Available online: https://www.cdc.gov/mis-c/hcp/
- 20.AAP Multisystem Inflammatory Syndrome in Children (MIS-C) Interim Guidance. [(accessed on 25 June 2021)]; Available online: https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance/
- 21.Chawla D., Chirla D., Dalwai S., Deorari A.K., Ganatra A., Gandhi A., Kabra N.S., Kumar P., Mittal P., Parekh B.J., et al. Perinatal-Neonatal Management of COVID-19 Infection—Guidelines of the Federation of Obstetric and Gynaecological Societies of India (FOGSI), National Neonatology Forum of India (NNF), and Indian Academy of Pediatrics (IAP) Indian Pediatr. 2020;57:536–548. doi: 10.1007/s13312-020-1852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi T., Fuse S., Sakamoto N., Mikami M., Ogawa S., Hamaoka K., Arakaki Y., Nakamura T., Nagasawa H., Kato T., et al. A New Z Score Curve of the Coronary Arterial Internal Diameter Using the Lambda-Mu-Sigma Method in a Pediatric Population. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016;29:794–801.e29. doi: 10.1016/j.echo.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Alsaleem M. Intravenous Immune Globulin Uses in the Fetus and Neonate: A Review. Antibodies. 2020;9:60. doi: 10.3390/antib9040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro M., Negre S., Matoses M.L., Golombek S.G., Vento M. Necrotizing enterocolitis following the use of intravenous immunoglobulin for haemolytic disease of the newborn. Acta Paediatr. 2009;98:1214–1217. doi: 10.1111/j.1651-2227.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 25.Whitworth H.B., Sartain S.E., Kumar R., Armstrong K., Ballester L., Betensky M., Cohen C., Diaz R., Diorio C., Goldenberg N.A., et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021 doi: 10.1182/blood.2020010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakshminrusimha S., Hudak M., Dimitriades V., Higgins R.D. Multisystem Inflammatory Syndrome (MIS-C) in Neonates (MIS-N) Following Maternal SARS CoV-2 COVID-19 Infection. Am. J. Perinatol. 2021 doi: 10.1055/a-1682-3075. (editorial under review) [DOI] [PubMed] [Google Scholar]
- 27.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., Medina Baez A., Shook L.L., Cvrk D., James K., et al. COVID-19 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabeerdoss J., Pilania R.K., Karkhele R., Kumar T.S., Danda D., Singh S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: Immunological mechanisms, clinical manifestations and management. Rheumatol. Int. 2021;41:19–32. doi: 10.1007/s00296-020-04749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson E.M., Diorio C., Goodwin E.C., McNerney K.O., Weirick M.E., Gouma S., Bolton M.J., Arevalo C.P., Chase J., Hicks P., et al. SARS-CoV-2 antibody responses in children with MIS-C and mild and severe COVID-19. J. Pediatric. Infect. Dis. Soc. 2020 doi: 10.1093/jpids/piaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vella L.A., Giles J.R., Baxter A.E., Oldridge D.A., Diorio C., Kuri-Cervantes L., Alanio C., Pampena M.B., Wu J.E., Chen Z., et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci. Immunol. 2021;6 doi: 10.1126/sciimmunol.abf7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruber C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., Wilson K.M., Onel K., Geanon D., Tuballes K., et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C) Cell. 2020;183:982–995.14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. JAMA J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flannery D.D., Gouma S., Dhudasia M.B., Mukhopadhyay S., Pfeifer M.R., Woodford E.C., Triebwasser J.E., Gerber J.S., Morris J.S., Weirick M.E., et al. Assessment of Maternal and Neonatal Cord Blood SARS-CoV-2 Antibodies and Placental Transfer Ratios. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atyeo C., Pullen K.M., Bordt E.A., Fischinger S., Burke J., Michell A., Slein M.D., Loos C., Shook L.L., Boatin A.A., et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–642.e10. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumberg D., Sridhar A., Lakshminrusimha S., Higgins R.D., Saade G. COVID-19 Vaccine Considerations during Pregnancy and Lactation. Am. J. Perinatol. 2021;38:523–528. doi: 10.1055/s-0041-1726390. [DOI] [PubMed] [Google Scholar]
- 36.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., Marquez P.L., Olson C.K., Liu R., Chang K.T., et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metz T.D., Clifton R.G., Hughes B.L., Sandoval G., Saade G.R., Grobman W.A., Manuck T.A., Miodovnik M., Sowles A., Clark K., et al. Disease Severity and Perinatal Outcomes of Pregnant Patients With Coronavirus Disease 2019 (COVID-19) Obstet. Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]