Abstract
Background and Objectives: Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a chronic condition distinguished by disabling fatigue associated with post-exertional malaise, as well as changes to sleep, autonomic functioning, and cognition. Mind-body interventions (MBIs) utilize the ongoing interaction between the mind and body to improve health and wellbeing. Purpose: To systematically review studies using MBIs for the treatment of ME/CFS symptoms. Materials and Methods: MEDLINE, EMBASE, CINAHL, PsycINFO, and Cochrane CENTRAL were searched (inception to September 2020). Interventional studies on adults diagnosed with ME/CFS, using one of the MBIs in comparison with any placebo, standard of care treatment or waitlist control, and measuring outcomes relevant to the signs and symptoms of ME/CFS and quality of life were assessed for inclusion. Characteristics and findings of the included studies were summarized using a descriptive approach. Results: 12 out of 382 retrieved references were included. Seven studies were randomized controlled trials (RCTs) with one including three reports (1 RCT, 2 single-arms); others were single-arm trials. Interventions included mindfulness-based stress reduction, mindfulness-based cognitive therapy, relaxation, Qigong, cognitive-behavioral stress management, acceptance and commitment therapy and isometric yoga. The outcomes measured most often were fatigue severity, anxiety/depression, and quality of life. Fatigue severity and symptoms of anxiety/depression were improved in nine and eight studies respectively, and three studies found that MBIs improved quality of life. Conclusions: Fatigue severity, anxiety/depression and physical and mental functioning were shown to be improved in patients receiving MBIs. However, small sample sizes, heterogeneous diagnostic criteria, and a high risk of bias may challenge this result. Further research using standardized outcomes would help advance the field.
Keywords: myalgic encephalomyelitis/chronic fatigue syndrome, mind-body interventions, systematic review, adults
1. Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a chronic condition distinguished by disabling fatigue associated with multiple symptoms including post-exertional malaise, orthostatic intolerance, pain, sleep problems, and impaired cognitive and immune functions [1]. While the true prevalence is unknown, Johnston et al., estimated the pooled prevalence of ME/CFS to be 3.28% and 0.76% according to self-reporting and clinical assessment, respectively [2]. In Canada, 1.4% of people older than 12 years old [3] suffer from ME/CFS. Patients report post-exertional malaise (69–100%), muscle pain (63–95%), impaired memory or concentration (88%), non-restorative sleep (87%), joint pain (55–85%), and sore throat (62%) [1,4]. Health-related quality of life in ME/CFS patients is consistently reported as significantly lower than otherwise healthy populations with regards to physical and mental health, self-care, and ability to perform usual activities [5,6]. Not surprisingly, ME/CFS reduces patients’ abilities to carry out normal working activities leading to higher unemployment rates [7]. It is estimated that annual household and labor force productivity of ME/CFS patients are decreased by 37% and 54%, respectively, costing an approximate annual loss of $9.1 billion in the United States (US) [8]. ME/CFS patients, their families and employers endure a high financial burden estimated to be between $18 to $51 billion annually in the US [9].
Despite extensive research, the etiology and pathophysiology of ME/CFS have not yet been fully understood. Disruptions in the autonomic nervous system, hypothalamic-pituitary-adrenocortical (HPA) axis, and immune system were shown in several studies [10,11]. Metabolic and mitochondrial dysfunction and abnormal gut microbiota were also shown to be interconnected with the above dysregulation [11]. A recent systematic review of neuroimaging studies showed inconsistent but widespread abnormalities in white matter, functional connectivity, and morphological changes of the autonomic nervous system [12].
With no specific etiology, there is no gold standard method to diagnose ME/CFS to date. A recent systematic review of diagnostic methods by Haney et al., identified nine case definitions [13]. Due to the lack of a biomarker, most of the case definitions require other competing diagnoses to be ruled out [14,15]. In the literature, the term myalgic encephalomyelitis (ME) [16] was used earlier than the term chronic fatigue syndrome (CFS) [17]. The Canadian case definition published in 2003 required post-exertional malaise as an essential symptom in these patients and recommended the umbrella term ME/CFS [18], used in this systematic review.
There is no cure for ME/CFS nor any FDA or Health Canada approved medication to treat it [14,19], therefore the focus tends to be on managing and minimizing the symptoms and improving quality of life. A variety of conventional and complementary therapies have been used to mitigate the symptoms of ME/CFS. As in other chronic conditions, long-term pharmacological interventions may have significant impacts on patients and their families in terms of adverse effects and financial burden [20,21]. Non-pharmacological options are of interest to patients as they may be less expensive and have fewer associated adverse effects.
Systematic reviews have shown low strength of evidence for the effectiveness of different complementary therapies [19], cognitive-behavioral therapy (CBT), counseling and behavioral therapies [14,22], and graded exercise therapy [23] for improvement of fatigue, physical functioning, sleep, and quality of life in patients with ME/CFS.
Mind-body approaches utilize the interactions between the brain, mind, and body, and behavior to improve health and wellbeing [24]. Using these interconnections strengthens self-awareness and self-care and helps to improve mood, quality of life, and increase one’s ability to cope. Examples of mind-body therapy interventions (MBIs) include progressive muscle relaxation, guided imagery, hypnosis, meditation, mindfulness, Tai chi, yoga, and biofeedback. Newer approaches are using the brain’s ability to change (i.e., neuroplasticity) associated with repeated, purposeful thoughts, feelings or behaviors [25]. The science behind how mind-body therapies work is expanding. It has been shown that the brain and body communicate in multiple directions using neurotransmitters/neuropeptides, hormones, and cytokines and MBIs may be influencing physical health by affecting these interactions [24,26].
Considering the complex nature of ME/CFS and the involvement of psycho-neuroendocrine and immune systems, these patients are an ideal population for evaluating MBIs. Furthermore, by enhancing self-knowledge and patients’ abilities to work through their problems and reduce stress, MBIs may improve their quality of life and wellbeing [27].
Several MBIs such as mindfulness-based stress reduction (MBSR), mindfulness-based cognitive therapy (MBCT), yoga, and Qigong have been studied in ME/CFS patients, but to our knowledge, have not yet been included in any systematic review or meta-analysis. There are some promising results to improve anxiety, fatigue, depression, quality of life, and physical functioning [28,29,30,31,32] in ME/CFS. In this systematic review, we evaluated the effectiveness and safety of MBIs that were studied in individuals diagnosed with ME/CFS. The results of this review will inform the design and methodology of future randomized controlled trials.
Objectives
The objectives of this study were to systematically review studies of MBIs for the treatment of ME/CFS symptoms and to report any adverse events reported for these approaches in ME/CFS patients.
2. Materials and Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [33]. The protocol of this systematic review was registered at PROSPERO (CRD42018085981).
2.1. Population, Intervention, Control, Outcome- Study Design (PICO-S)
The population of interest was adults (≥18 years old) diagnosed or symptom-matched with one of the ME/CFS case definitions (Appendix A, Table A1). Patients with any other conditions were included in this review, as long as they were diagnosed with ME/CFS. Interventions of interest included any of the MBIs listed in Table 1 and any placebo, the standard of care treatment or waiting list as a control group. To be eligible for inclusion, multiple-arm interventional studies were also required to have at least one of the control groups mentioned above.
Table 1.
Criteria for selecting studies.
| Population | Patients with a diagnosis of CFS, ME, and ME/CFS including: Patients who were previously treated Patients who are previously untreated Adults (≥18 years) |
| Interventions | Mind-body interventions (alone or in combination) including: Art Therapy Autogenic training Biofeedback/neurofeedback Breathing exercise Cognitive restructuring Dynamic Neural Retraining System Emotional Freedom Techniques (EFT) Eye movement desensitization and reprocessing (EMDR) Guided imagery Hypnotherapy/self-hypnosis Meditation (mindfulness, mantra, guided, transcendental) Mindfulness-based cognitive therapy (MBCT) Mindfulness-based Stress Reduction (MBSR) Music therapy Neurolinguistic programming Prayer/spirituality Psychological flexibility Qigong Relaxation therapy (relaxation response, progressive muscle relaxation) Tai Chi Visualization Yoga |
| Comparators | One or more of the following control conditions including: Placebo Standard of care treatments Waitlist |
| Outcomes | Any single or combination of, but not limited to, the following outcomes: Fatigue (energy, motivation) Refreshing sleep Pain Anxiety (stress, nervousness, etc.,) Depression (mood, hopefulness, helplessness) Quality of life Performance (physical, mental, emotional) Work-related outcome (employment, income, etc.) Changes in physical health such as sore throat, tender lymph nodes, and muscle weakness |
| Study Design | Parallel/Cross-over randomized controlled trials (RCTs) Controlled clinical trials (CCTs) Controlled before and after studies Single-arm interventional studies (within subject control group) Cohort |
| Other | English language |
All outcomes relevant to the signs and symptoms of ME/CFS and quality of life were considered. The outcomes included fatigue, sleep refreshment, pain, anxiety (stress, nervousness, etc.), depression (mood, hopefulness, and helplessness), quality of life, performance (physical, mental, emotional), work-related outcomes (employment, income, etc.), and physical health symptoms such as sore throat, tender lymph nodes, and muscle weakness (Table 1).
Study designs eligible for inclusion were parallel/cross-over/N-of-1 randomized controlled trials (RCTs), controlled clinical trials (CCTs), single-arm experimental (within subject control group), controlled before and after studies, or cohort studies.
2.2. Search Methods
Five electronic databases (MEDLINE, EMBASE, CINAHL, PsycINFO, and Cochrane Register of Controlled Trials (CENTRAL)) were searched from inception to September 2020. Search terms were based on those presented in Table 1; an example is found in Appendix B. No limitation was implemented in terms of publication dates. English language restriction was applied. The reference lists of included studies, and systematic reviews, were reviewed to identify additional studies.
2.3. Selection of Studies
Two review authors (MK, DJ) independently screened all the titles and abstracts retrieved from the search in order to identify those that may meet the inclusion criteria. They classified studies as being relevant, possibly relevant and irrelevant. Three reviewers (MK, DJ, SKA) independently assessed the full texts of all relevant and possibly relevant studies to assess inclusion. Discrepancies were resolved by referring to a senior review author (ES, SV).
2.4. Data Collection
Standardized data extraction forms were used to extract data from full-text articles. Extracted data included general characteristics of the study (first author, publication year, country, settings, design), sample size, age and sex distribution in groups, diagnosis methods, type of MBI and other relevant data including frequency and duration, control (active or passive), primary outcome, secondary outcomes, primary and secondary measurement tools, length of study, follow up period, statistically significant outcomes, and adverse events reported. Data extraction was completed by one reviewer (DJ) and independently verified by a second reviewer (SKA). Disagreements between the authors were resolved by discussion until consensus was reached; if consensus could not be reached, a senior reviewer’s opinion was sought.
2.5. Data Analysis
This systematic review was conducted to determine which outcomes and outcome measures were used in the studies of MBIs for the treatment of ME/CFS patients and whether the interventions were effective. General information of the included studies along with the statistically significant and insignificant outcomes were described. We present the findings of studies using different diagnostic criteria (e.g., Oxford criteria, CDC criteria) separately. We also report whether studies assessed adverse events, their absence or presence, and frequencies. A meta-analysis was not performed due to heterogeneous interventions and outcomes used in the included studies. Cochrane risk of bias assessment tool was used by two independent review authors (SKA, SP) to assess sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias [34] in RCTs. Other study designs including single-arm experimental studies were also appraised by two independent reviewers (SKA, SP) for risk of bias using Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies of Intervention (ACROBAT_NRSI) which was recently renamed ROBINS-I [35]. Domains for assessing the risk of bias in these studies include bias due to confounding, selection of participants, measurement of interventions, a departure from the intended intervention, missing data, measurement of outcomes, and selection of the reported result.
2.6. Patient Involvement
Patient engagement in health research can improve the quality, relevance and impact of the research [36,37]. To recruit patient research partners in this study, a “call for patient representative” letter was developed and distributed among patients, caregivers and advocates. Three patient partners were selected based on their educational background, personal experience, and health status to participate in the study team. They did not receive any financial compensation. They participated regularly in teleconference calls and skype meetings. They also provided feedback and participated in team discussions via email. They contributed to the protocol design, development of the literature search strategy, the condition/diagnosis definitions, and outcome selection.
3. Results
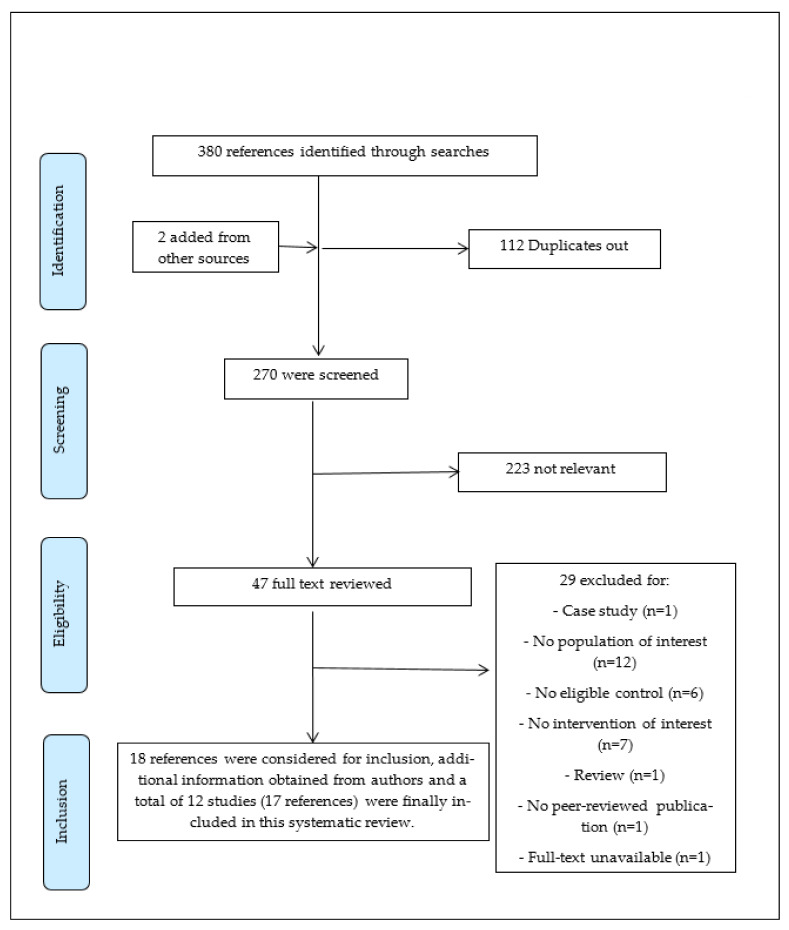
Our search results yielded 382 references. After removing duplicates, 270 were screened using title and abstracts, and 47 references were considered relevant for full-text screening. Considering the a priori inclusion criteria and obtaining additional clarifying information from authors of some of the references, twelve studies (17 reports) were ultimately included [10,28,29,30,38,39,40,41,42,43,44,45]. The flow of studies through the screening process of the review is shown in Figure 1. The excluded studies and the reasons for exclusion are shown in Table A2.
Figure 1.
Adapted version of PRISMA flow diagram of study selection for the ME/CFS systematic review.
3.1. Characteristics of the Included Studies
Table 2 shows the characteristics of all the included studies.
Table 2.
General characteristics of the included studies.
| First Author, Year, Country | Setting | Design, Sample Size (Enrolled/Completed/Analyzed), Treatment Duration | Study Population (Diagnosis, Age, Gender) | Mind-Body Intervention, Frequency, Duration, Self-Practice |
Control Group | Outcome, Measurement Tool and Validity |
|---|---|---|---|---|---|---|
| Surawy, Ch., 2005, UK [29] | Not reported | A series of exploratory studies: Study 1 Design: RCT, Sample size: Intervention: 9/9/9, Control: 9/8/8 Treatment duration: 8 weeks Study 2 Design: single-arm trial, Sample size: 12/9/9,Treatment duration: 8 weeks Study 3 Design: single-arm trial, Sample size: 11/9/9, Treatment duration: 8 weeks and a follow-up period of 3 months |
Patients diagnosed with Oxford criteria [46] Study 1 Age range: 18–65 y/o, 56% female Study 2 Age range: 18–65 y/o, 75% female Study 3 Age range: 18–65 y/o, 64% female |
MBSR/MBCT Frequency: Once a week, Duration: Not reported, Self-practice: Not reported |
Study 1: Wait list Study 2: No control group Study 3: No control group |
Study 1, 2, and 3 Anxiety and Depression: Hospital Anxiety and Depression Scale (HADS) [47] Fatigue Severity: Chalder’s Fatigue Scale [48] Quality of Life: SF36 physical functioning [49] Study 2 and 3 Effect of fatigue on quality of life: Fatigue impact scale [50] |
| Thomas, M., 2006 and 2008, UK [39,51] | Outpatient clinics | Design: RCT, Sample size: Intervention (relaxation group): 14/14/14, Control: 9/9/9 Treatment duration: 10 weeks Follow-up: 6 months |
Patients diagnosed with CFS by CDC diagnostic criteria for CFS [52] Age (mean ± SD): Intervention (relaxation): 45.7 ± 12.5, Control: 46.2 ± 11.04, Intervention (relaxation): 71.4% female, Control: 66.7% female |
Relaxation therapy Frequency: Once a week, Duration: 1 h, Self-practice: Not reported |
Standard medical care | Report 1 Illness history: Beck Depression Inventory [53] Centre for Epidemiological Studies-Depression Scale [54], Chalder Fatigue Scale [48], Cognitive Failures Questionnaire [55], Cohen–Hoberman Index of Physical Symptoms [56], Current State of Health [57], Fatigue Problem Rating Scale [58], Hospital Anxiety and Depression Scale [47], MOS SF-36 [49] Perceived Stress [56] Positive and Negative Affect [59], Profile of Fatigue Related Symptoms [60], Sleep Questionnaire [57], Symptom Check List [57] Mood testing: Alertness, hedonic tone and anxiety: measured using 18 computerized visual analogue mood scales Performance testing: Word recall, reaction time, vigilance tasks using a Viglen Dossier laptop computer connected to a simple 3-button response box [57] Report 2 Primary outcome: Functional performance: Karnofsky performance scale [61] Secondary outcome: Global measures of illness and satisfaction with treatment (including improvement and changes in fatigue and disability) |
| Bogaerts, K., 2007, Belgium [38] | University hospital clinic | Design: Single-arm trial, Sample size: 30/30/30 Treatment duration: Single time imagery trial |
Patients diagnosed with CFS by CDC diagnostic criteria for CFS [52] | Relaxation imagery Frequency: onceDuration: less than 5 min Self-practice: NA |
No control group | Ventilatory measures: Pet CO2 Subjective measures: Degree of fatigue, imagery vividness, concentration ability on the scripts and similarity of evoked feelings with daily life feelings: 9-point rating scale Positive and negative affectivity: Positive and Negative Affect Schedule (PANAS) [62] Hyperventilation complaints: Symptom checklist [63] Chronic fatigue acceptance: Acceptance Chronic Fatigue Test (ACFT) Tendency to worry: Penn-State Worry Questionnaire (PSWQ) [64] Valence, arousal and dominance: Self-assessment Manikin [65] |
| Lopez, C., 2011. USA [45] | Physician referrals, community | Design: RCT Sample size: 69/58/58 Treatment duration: 12 weeks |
Patients diagnosed with CFS by CDC diagnostic criteria for CFS [52], Age (mean ±SD): 45.9 ± 9.3 88.4% female |
Cognitive-behavioral stress management Frequency: Weekly Duration: Two hours Self-practice: Workbook and relaxation tapes |
Psychoeducation (half-day seminar) | Distress: Perceived Stress Scale (PSS) [66], Profile of Mood States (POMS) [67] Quality of life: Quality of Life Inventory (QOLI) [68] CFS symptoms: CDC Symptom Inventory for Chronic Fatigue Syndrome [69] |
| Chan, J., 2013, Hong Kong [41] (characteristics of Ho et al., 2012 [70] as the preliminary study and Li et al., 2015 [71] as the study conducted on a subset of participants suffering from bereavement are reported here as well) | Community | Design: RCT, Sample size: Ho, R., 2012 report: Intervention: 35 * /27/33 Control group: 35 ** /25/31 Chan, J., 2013 (main report): Intervention: 77/53/72 ^ Control: 77/58/65 ^^ Li, J., 2015, report: Intervention: 22/22/22 Control: 24/24/24 Treatment duration: 5 consecutive weeks training sessions + 12 weeks home-based qigong exercise (4 months in total) Li et al., however, reported their findings after three months of intervention. |
Patients diagnosed with CFS by CDC diagnostic criteria for CFS [52] Age (mean ± SD), % female: Ho, R., 2012 report: Intervention: 42.1 ± 7.3, Control: 42.5 ± 5.5, Intervention: 75.8% female, Control: 83.9% female Chan, J., 2013 (main report): Intervention: 42.4 ± 6.7, Control: 42.5 ± 6.4, Intervention: 72.2% female, Control: 81.5% female Li, J., 2015, report: Patient with CFS had been bereaved within the previous 2 years. Age (median, range): Intervention: 46 (23–52), Control: 45 (32–51), Intervention: 86.4% female, Control: 87.5% female |
Qigong exercise training (Wu Xing Ping Heng Gong), Frequency: twice a week, Duration: 2 h, Self-practice: 30 min, every day at home |
Waitlist | Chan, J., 2013 (main report): Primary outcome: Fatigue severity: Chalder’s Fatigue Scale [48,72] Secondary outcomes: Anxiety and Depression: Hospital Anxiety and Depression Scale (HADS) [47,73] Ho, R., 2012 report: In addition to fatigue severity, they measured Physical functioning and mental functioning: the Chinese version of the Medical Outcomes Study 12- Item Short-Form Health Survey [74,75] as their primary outcome and Telomerase Activity as their secondary outcome. Li, J., 2015, report: In addition to fatigue severity and anxiety and depression, they measured quality of life: Short form health survey (SF-12) [74,75] and Spiritual well-being: the “spirituality” subscale of the Body-Mind-Spirit Well-being Inventory (BMSWBI-S) [76] |
| Rimes, K., 2013, U.K. [30] | A specialist National Health Service CFS Unit | Design: RCT, Sample size: Intervention group: 18/16/16 Control group: 19/19/19 Treatment duration: Introductory session + 8 weeks Follow-up: at 2 months, and at 6 months for MBCT group only |
Patients diagnosed with CFS by Fukuda et al. [52] criteria or Oxford criteria [46] Age (mean ± SD): Intervention: 41.4 ± 10.9, Control: 45.2 ± 9.4, Intervention:75% female, Control: 89.5% female |
MBCT, Frequency: Once a week, Duration: 2.25 h, Self-practice: Home practice with the support of CDs. |
Waitlist | Primary outcome: Fatigue: Chalder Fatigue Scale [48] Secondary outcomes: Impairment: The Work and Social Adjustment Scale [77] Physical Functioning: Physical Functioning (PF-10) scale) [78,79] Beliefs about Emotions: Beliefs about Emotions Scale [80] Self-Compassion: Self-Compassion Scale [81] Mindfulness: Five-Facet Mindfulness Questionnaire [82] Anxiety and Depression: Hospital Anxiety and Depression Scale (HADS) [47] All-or-Nothing Behaviour and Catastrophic Thinking about Fatigue: five-item subscale of the Cognitive and Behavior Responses to Symptoms Questionnaire (Moss-Morris and Chalder, in preparation; King’s College London, UK) Acceptability and Engagement: Record of class attendance and amount of home practice undertaken |
| Chan, J., 2014 and 2017, Hong Kong [40,83] | Community | Design: RCT, Sample size: Report 1: Intervention: 75/57/75 Control: 75/58/75 Report 2: Intervention:46 Control: 62 Treatment duration: 9 consecutive weeks Follow-up: 3-month post-intervention. |
Patients diagnosed with CFS by CDC diagnostic criteria for CFS [52] Report 1: Age (mean ± SD): Intervention: 39.1 ± 7.8, Control: 38.9 ± 8.1, Intervention: 61.3% female, Control: 82.7% female Report 2 Age (mean ± SD): 39 ± 7.9 All females |
Qigong exercise: Baduanjin Qigong Frequency: 16 sessions, Duration: 1.5 h, Self-practice: 30 min, every day |
Waitlist | Report 1 Primary outcomes: Sleep Quality: Pittsburgh Sleep Quality Index (PSQI) [84,85,86] Fatigue severity: Chalder Fatigue Scale (ChFS) [48,72] Anxiety and Depression: Hospital Anxiety and Depression Scale (HADS) [47,73] Secondary outcome: Dose-response relationship between Qigong exercise and improvements. Global Assessment, Satisfaction Report 2 Anxiety and Depression: Hospital Anxiety and Depression Scale (HADS) [47,73] Plasma Adiponectin Levels |
| Oka, T., 2014, Japan [28] | Outpatients with CFS who visited the Department of Psychosomatic Medicine of KyushuUniversity Hospital | Design: RCT, Sample size: Intervention: 15/15/15 Control: 15/15/15 Treatment duration: Two months |
Patients diagnosed with CFS by CDC diagnostic criteria for CFS [52] Age (mean ±SD): Intervention:38.0 ±11.1, Control:39.1 ± 14.2, Intervention: 80% female, Control: 80% female |
Isometric yoga Frequency: every two to three weeks, at least 4 times during the intervention period, Duration: 20 min, Self-practice: With the aid of a digital videodisk and booklet |
Waitlist | Acute effects of isometric yoga on fatigue: The fatigue and vigor score of the Profile of Mood States (POMS) questionnaire [67] immediately after the final 20-min yoga session Chronic effects of isometric yoga on fatigue: Chalder’s Fatigue Scale [48] Quality of Life: Medical Outcomes Study Short Form 8, standard version (SF-8) [87] |
| Sollie, K., 2017. Norway [43] | Community | Design: Single-arm trial Sample size: 10 Treatment duration: Eight weeks with three months follow-up |
Patients diagnosed with CFS by Canada criteria [88] Age (mean ± SD):43.5 ± 9.9, 80% female |
MBCT, Frequency: Weekly Duration: Two hours Self-practice: Homework with the aid of workbook and CD |
No control group | Fatigue: Chalder Fatigue Scale [48] Symptom burden: Likert scale Anxiety and depression: Hospital Anxiety and Depression Scale (HADS) [47] Tendency to ruminate: Ruminative Response Scale [89] Dispositional mindfulness: Five Facet Mindfulness questionnaire [82] Quality of life: Satisfaction with Life Scale (SWLS) [90] |
| Oka, T., 2018 and 2019, Japan [42,91] | Outpatients with CFS who visited the Department of Psychosomatic Medicine of KyushuUniversity Hospital | Design: Single-arm trial Sample size: 15 Treatment duration: Eight weeks |
Patients diagnosed with CFS by CDC diagnostic criteria for CFS [52], the 2011 international consensus criteria for myalgic encephalomyelitis [92] and the 2015 diagnostic criteria for systemic exertion intolerance disease [1] Age (mean ± SD): 38.0 ± 11.1 80% female |
Sitting isometric yoga Frequency: Biweekly with a yoga instructor Duration: 20 min Self-practice: Daily in-home session |
No control group | Report 1: Fatigue and vigor: The fatigue and vigor score of the Profile of Mood States (POMS) questionnaire [67] Autonomic nervous system (ANS) functions: Heart rate and Heart rate variability (HRV) Blood biomarkers: Serum cortisol, DHEA-S, TNF-α, IL-6, IFN-α, IFN-γ, PRL, total carnitine, free carnitine, and acylcarnitine, and plasma TGF-β1, BDNF, MHPG, and HVA Report 2: Fatigue severity: Chalder fatigue scale (FS) score [48] Levels of the blood biomarkers: Cortisol, DHEA-S, TNF-α, IL-6, prolactin, carnitine, TGF-β1, BDNF, MHPG, HVA, and α-MSH The autonomic nervous functions: Heart rate (HR) and HR variability Alexithymia: The 20-item Toronto Alexithymia Scale (TAS-20) [93] Anxiety and depression: Japanese version of the Hospital Anxiety and Depression Scale (HADS) [47] |
| Jonsjo, M., 2019, Sweden [44] | Tertiary specialist clinic | Design: Single-arm trial Sample size: 40/32/32 Treatment duration: 13 sessions with three- and six-month follow-up |
Patients diagnosed with CFS according to CDC [52] and 2003 Canadian criteria for ME/CFS [88] Age (mean ± SD): 49.02 ± 10.78 76.7% female |
Acceptance and commitment therapy Frequency: Weekly to biweekly depending on illness severity (13 sessions) Duration: 45 min Self-practice: Home assignments |
No control group | Primary outcomes: Disability: The pain disability index [94] Psychological inflexibility: The Psychological Inflexibility in Fatigue Scale (PIFS) [95] Secondary outcomes: ME/CFS symptoms and severity: 5-point scale Fatigue: The Multidimensional Fatigue Inventory (MFI-20) [96] Anxiety and depression: The Hospital Anxiety and Depression Scale (HADS) [47] Dimensions of mental and physical health: SF-36 Health Survey [79] Health-related quality of life: EQ-5D-3L [97] |
| Takakura, S., 2019, Japan [10] | Outpatients with CFS who visited the Department of Psychosomatic Medicine of Kyushu University Hospital | Design: Single-arm trial Sample size: 9 Treatment duration: Three months |
Patients diagnosed with CFS according to the 1994 Fukuda case definition of CFS [52], the 2011 International Consensus Criteria for ME [92], and the 2015 diagnostic criteria for systemic exertion intolerance disease [1] Age (mean ± SD): 37.2 ± 9.9 All female |
Recumbent isometric yoga Frequency: Every two to four weeks Duration: 20–30 min depending on patient’s preference Self-practice: In-home daily sessions |
No control group | Fatigue: Japanese version of 11 item Chalder Fatigue Scale [98,99] Human microRNA |
* 2 dropped out before the intervention ** 4 dropped out before the intervention ^ 5 dropped out before the intervention ^^ 12 dropped out before the intervention.
3.2. Design
Six studies were prospective RCTs with at least one eligible control group [28,30,39,40,41,45]. One manuscript presented a brief report of three studies in which one was a prospective RCT and the other two were single-arm experimental studies [29]. Five additional publications were also single-arm experimental studies [10,38,42,43,44].
3.3. Population
Participants were all adults diagnosed with ME/CFS (n = 564 total; sample size range n = 9–150). Six studies used the Center for Disease Control and Prevention (CDC) criteria for the diagnosis of their patients [28,39,40,41,45,51]. One study used the 2003 Canadian criteria [43]. One study used Oxford criteria [29], one study used both CDC and Oxford criteria [30] and three studies used a combination of CDC criteria with 2003 or 2005 versions of Canadian criteria and 2011 international consensus criteria [10,42,44].
The healthcare settings included outpatient settings [28,39], community [40,41,43,45], a university hospital clinic [38], department of psychosomatic medicine [10,42] and a specialist ME/CFS unit [30,44]. One study did not report the setting from which their patients were recruited [29].
Three studies were conducted in the United Kingdom [29,30,39], three in Japan [10,28,42], two were conducted in Hong Kong, China [40,41], and one each in Belgium [38], Norway, Sweden, and USA [43,44,45].
3.4. Intervention
A variety of different interventions were implemented in the included studies comprising mindfulness-based stress reduction/mindfulness-based cognitive therapy (MBSR/MBCT) [29], MBCT [30,43], relaxation therapy [39], relaxation imagery [38], Qigong exercise training [41], Baduanjin Qigong [40], and isometric yoga [28], seated isometric yoga [42], recumbent isometric yoga [10], acceptance and commitment therapy [44] and cognitive-behavioral stress management [45]. Treatment duration ranged between 5–12 weeks.
3.5. Comparison
Participants assigned to the control group were either placed on the waiting list [28,29,30,40,41] or received standard medical care [39]. They were advised to keep their usual lifestyle activities including seeking general medical care but not to participate in any activities similar to the intervention of interest.
3.6. Outcomes
Many different outcomes and outcome measures were reported in the included studies. Four studies clearly stated their primary and secondary outcomes/objectives [30,39,40,41]. Fatigue severity was measured by seven studies using Chalder fatigue scale [10,28,29,30,40,41,43]. One study (published as two reports), listed Chalder fatigue scale in one of the reports as the administered questionnaire [51]. In the other report, however, they measured fatigue using patient-rated Likert-type scales [39]. Other studies used either profile of mood state (POMS) [42,45] or multidimensional fatigue inventory (MFI-20) [44].
Eight studies measured anxiety and depression using the Hospital Anxiety and Depression Scale (HADS) [29,30,40,41,43,44,51,91]. Six studies measured quality of life or physical and/or mental functioning using different quality of life outcome measures [28,29,30,41,45,51]. Seven studies measured objective outcomes including ventilatory parameters [38], performance testing by computer programs [51], telomerase activity [70], autonomic nervous system functions, blood biomarkers [42,91], adiponectin levels [83], and microRNA changes [10]. Table 2 describes the details of these outcome measures and the other outcomes measured in the included studies.
3.7. Effects of Interventions
Due to heterogeneous interventions and outcome measures used in the included studies, a meta-analysis was not performed. The statistically significant outcomes reported by these studies are presented in Table 3 and Table 4. Table A3 and Table A4 show the statistically insignificant outcomes.
Table 3.
Significant outcomes in the included studies using CDC, Canadian and international consensus criteria for diagnosing CFS.
| Intervention Type | First Author, Year | Outcome (Assessed by) | Comparison Groups | Comparison Time Point | p-Value | |
|---|---|---|---|---|---|---|
| Relaxation-based | Thomas, M., 2006 and 2008 [39,51] | Report 1 (2006) | Alertness (as part of a subjective mood scale) | Relaxation group (pre-post) | Post follow-up (6 months) | <0.027 |
| Anxiety (as part of a subjective mood scale) | Relaxation group (pre-post) | Post follow-up (6 months) | <0.002 | |||
| Current state of health (self-reporting scale) | Relaxation group (pre-post) | Post-treatment (10 weeks) | Reported significant (value not reported) | |||
| Report 2 (2008) | Performance score-10% improvement (Karnofsky scale) | Relaxation group (pre-post) | Post-treatment (10 weeks) | Reported significant (value not reported) | ||
| Global measures of health: overall condition (Likert-type scale) | Relaxation group (pre-post) | Post-treatment (10 weeks) | Reported significant (value not reported) | |||
| Global measures of health: Fatigue levels (Likert-type scale) | Relaxation group (pre-post) | Post-treatment (10 weeks) | Reported significant (value not reported) | |||
| Global measures of health: Fatigue levels (Likert-type scale) | Relaxation group (pre-post) | Post follow-up (6 months) | Reported significant (value not reported) | |||
| Global measures of health: reduction in disability (Likert-type scale) | Relaxation group (pre-post) | Post-treatment (10 weeks) | Reported significant (value not reported) | |||
| Global measures of health: reduction in disability (Likert-type scale) | Relaxation group (pre-post) | Post follow-up (6 months) | Reported significant (value not reported) | |||
| Bogaerts K., 2007 [38] | PetCO2 | Relaxation imagery (pre-post) | Post intervention | <0.01 | ||
| Cognitive-based | Lopez C., 2011 [45] (Cognitive restructuring) |
Distress (Perceived stress scale) | CBSM compared to control | Time X group ** | 0.03 | |
| Total mood disturbance (Profile of Mood States (POMS) | CBSM compared to control | Time X group ** | 0.05 | |||
| Quality of life (QOLI Category) | CBSM compared to control | Time X group ** | 0.002 | |||
| Quality of life (QOLI Raw score) | CBSM compared to control | Time X group ** | 0.05 | |||
| Quality of life (QOLI Total score) | CBSM compared to control | Time X group ** | 0.05 | |||
| CFS symptoms (Total CDC symptom severity) | CBSM compared to control | Time X group ** | 0.04 | |||
| Sollie K., 2017 [43] (Mindfulness-based cognitive therapy) | Fatigue (Chalder Fatigue Scale) | MBCT (pre-post) | Post intervention (8 weeks) | p value not reported, medium effect size was reported (d = 0.56) | ||
| Anxiety (HADS) | MBCT (pre-post) | Post intervention (8 weeks) | p value not reported, medium to large effect size was reported (d = 0.68) | |||
| Anxiety (HADS) | MBCT (pre-post) | Post follow-up (3 months) | p value not reported, medium effect size was reported (d = 0.48) | |||
| Dispositional mindfulness (Five Facet Mindfulness questionnaire) | MBCT (pre-post) | Post follow-up (3 months) | p value not reported, large effect size was reported (d = 0.77) | |||
| Jonsjo, M., 2019 [44] (Psychological flexibility) | Disability (Pain disability index) | ACT (pre-post) | Post intervention (after 13 sessions) | 0.000 | ||
| Psychological flexibility (Psychological inflexibility fatigue scale) | ACT (pre-post) | Post intervention (after 13 sessions) | 0.000 | |||
| CFS symptoms | ACT (pre-post) | Post intervention (after 13 sessions) | 0.017 | |||
| Anxiety (HADS) | ACT (pre-post) | Post intervention (after 13 sessions) | 0.001 | |||
| General fatigue (MFI-20) | ACT (pre-post) | Post intervention (after 13 sessions) | 0.024 | |||
| General fatigue (MFI-20) | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.049 | |||
| Physical fatigue (MFI-20) | ACT (pre-post) | Post intervention (after 13 sessions) | 0.046 | |||
| Mental fatigue (MFI-20) | ACT (pre-post) | Post intervention (after 13 sessions) | 0.004 | |||
| Reduced activity (MFI-20) | ACT (pre-post) | Post intervention (after 13 sessions) | 0.041 | |||
| Reduced motivation (MFI-20) | ACT (pre-post) | Post intervention (after 13 sessions) | 0.043 | |||
| SF-36 physical | ACT (pre-post) | Post intervention (after 13 sessions) | 0.009 | |||
| Movement-based | Chan J., 2013 [41] | Ho, R., 2012 (Preliminary report) | Quality of life: Mental functioning score (MOS SF-12) | Qigong (pre-post) | Post-training (5 weeks) | <0.01 |
| Quality of life: Mental functioning score (MOS SF-12) | Qigong (pre-post) | Post intervention (4 months) | <0.01 | |||
| Quality of life: Mental functioning score (MOS SF-12) | Qigong compared to control | Time X group ** | 0.001 | |||
| Telomerase activity * (Telomerase PCR ELISA) | Qigong (pre-post) | Post intervention (4 months) | <0.05 | |||
| Telomerase activity * (Telomerase PCR ELISA) | Qigong compared to control | Time X group ** | 0.029 | |||
| Chan J., 2013 (main report) |
Total fatigue score (ChFS) | Qigong (pre-post) | Post intervention (4 months) | <0.001 | ||
| Total fatigue score (ChFS) | Qigong compared to control | Time X group ** | 0.000 | |||
| Physical fatigue score (ChFS) | Qigong (pre-post) | Post intervention (4 months) | <0.001 | |||
| Physical fatigue score (ChFS) | Qigong compared to control | Time X group ** | 0.000 | |||
| Mental fatigue score (ChFS) | Qigong (pre-post) | Post intervention (4 months) | <0.001 | |||
| Mental fatigue score (ChFS) | Qigong compared to control | Time X group ** | 0.050 | |||
| Anxiety score (HADS) | Qigong (pre-post) | Post intervention (4 months) | <0.001 | |||
| Depression score (HADS) | Qigong (pre-post) | Post intervention (4 months) | <0.001 | |||
| Depression score (HADS) | Qigong compared to control | Time X group ** | 0.002 | |||
| Li J., 2015 (Subset study report) | Spirituality (the spirituality subscale of BMSWBI-S) | Qigong compared to control | Post intervention (3 months) | 0.013 | ||
| Quality of life: mental component summary (MOS SF-12) | Qigong compared to control | Post intervention (3 months) | 0.002 | |||
| Quality of life: mental component summary (MOS-SF 12) | Qigong compared to control | Post intervention (change score from baseline to 3 months) | 0.002 | |||
| Chan, J. 2014 [40] and Chan, J. 2017 [83] | Report 1 (2014) | Sleep quality: total score (PSQI) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.05 | |
| Subjective sleep quality (PSQI) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.01 | |||
| Subjective sleep quality (PSQI) | Baduanjin Qigong compared to waitlist | Post follow-up (change score from baseline to 3 months) | <0.01 | |||
| Subjective sleep quality (PSQI) | Baduanjin Qigong compared to wait list | Time X group ** | 0.002 | |||
| Sleep latency (PSQI) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.05 | |||
| Sleep latency (PSQI) | Baduanjin Qigong compared to waitlist | Time X group ** | 0.044 | |||
| Sleep duration (PSQI) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.05 | |||
| Total fatigue score (ChFS) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.001 | |||
| Total fatigue score (ChFS) | Baduanjin Qigong compared to waitlist | Post follow-up (change score from baseline to 3 months) | <0.001 | |||
| Total fatigue score (ChFS) | Baduanjin Qigong compared to waitlist | Time X group ** | <0.001 | |||
| Physical fatigue score (ChFS) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.001 | |||
| Physical fatigue score (ChFS) | Baduanjin Qigong compared to waitlist | Post follow-up (change score from baseline to 3 months) | <0.001 | |||
| Physical fatigue score (ChFS) | Baduanjin Qigong compared to waitlist | Time X group ** | <0.001 | |||
| Mental fatigue score (ChFS) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.001 | |||
| Mental fatigue score (ChFS) | Baduanjin Qigong compared to waitlist | Post follow-up (change score from baseline to 3 months) | <0.01 | |||
| Mental fatigue score (ChFS) | Baduanjin Qigong compared to waitlist | Time X group ** | <0.001 | |||
| Anxiety (HADS) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.01 | |||
| Anxiety (HADS) | Baduanjin Qigong compared to waitlist | Post follow-up (change score from baseline to 3 months) | <0.05 | |||
| Anxiety (HADS) | Baduanjin Qigong compared to waitlist | Time X group ** | 0.016 | |||
| Depression (HADS) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.001 | |||
| Depression (HADS) | Baduanjin Qigong compared to waitlist | Time X group ** | <0.001 | |||
| Report 2 (2017) | Increase in adiponectin levels | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.05 | ||
| Depression (HADS) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | <0.001 | |||
| Anxiety (HADS) | Baduanjin Qigong compared to waitlist | Post intervention (9 weeks) | <0.05 | |||
| Oka T, 2014 [28] | Fatigue score -acute effect (POMS) | Isometric yoga (pre-post) | Before to after the final 20-min session | <0.001 | ||
| Vigor score- acute effect (POMS) | Isometric yoga (pre-post) | Before to after the final 20-min session | <0.01 | |||
| Physical fatigue score (ChFS) | Isometric yoga (pre-post) | Post intervention (2 months) | 0.004 | |||
| Physical fatigue score (ChFS) | Isometric yoga compared to control | Time X group ** | 0.009 | |||
| Mental fatigue score (ChFS) | Isometric yoga (pre-post) | Post intervention (2 months) | 0.004 | |||
| Mental fatigue score (ChFS) | Isometric yoga compared to control | Time X group ** | 0.007 | |||
| Total fatigue score (ChFS) | Isometric yoga (pre-post) | Post intervention (2 months) | 0.002 | |||
| Total fatigue score (ChFS) | Isometric yoga compared to control | Time X group ** | 0.003 | |||
| Quality of life: bodily pain (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | 0.0001 | |||
| Quality of life: general health perception (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | 0.0021 | |||
| Quality of life: Physical component summary (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | 0.024 | |||
| Oka, T., 2018 and Oka, T., 2019 [42,91] | Report 1 (2018) | Fatigue (POMS) | Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | 0.001 | |
| Vigor (POMS) | Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | 0.002 | |||
| Decreased heart rate | Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | 0.047 | |||
| Increased high-frequency power of HR variability | Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | 0.028 | |||
| Increased serum levels of DHEA-S | Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | 0.012 | |||
| Decreased levels of cortisol | Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | 0.016 | |||
| Decreased level of TNF-α | Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | 0.035 | |||
| Report 2 (2019) | Fatigue (POMS) | Longitudinal effects of sitting isometric yoga (pre-post) | Post intervention (2 months) | 0.002 | ||
| Depression (HADS) | Longitudinal effects of sitting isometric yoga (pre-post) | Post intervention (2 months) | 0.02 | |||
| Takakura, S., 2019 [10] | Fatigue (11 score Chalder’s fatigue scale) | Recumbent isometric yoga (pre-post) | Post intervention (3 months) | <0.0001 | ||
| Changes in miRNA expression | Recumbent isometric yoga (pre-post) | Post intervention (3 months) | <0.05 (Four miRNAs significantly upregulated and 42 were significantly downregulated) | |||
BMSWBI-S: Body-Mind-Spirit Well-being Inventory, ChFS: Chalder’s Fatigue Scale, HADS: Hospital Anxiety and Depression Scale, MOS SF-12: Medical Outcomes Study 12- Item Short-Form Health Survey, POMS: Profile of Mood States, PSQI: Pittsburgh Sleep Quality Index, SF-8: Medical Outcomes Study Short Form 8, QOLI: Quality of life inventory, MFI-20: Multidimensional fatigue inventory-20, ACT: Acceptance and commitment therapy, MBCT: Mindfulness-based cognitive therapy, CBSM: Cognitive-based stress management. * measure of stress-related damage at a cellular level. ** to test the interaction effect of time and group.
Table 4.
Significant outcomes in the included studies using Oxford criteria for diagnosing CFS.
| Intervention Type | First Author, Year | Outcome (Assessed by) | Comparison Groups | Comparison Time Point | p-Value | |
|---|---|---|---|---|---|---|
| Mindfulness and cognitive-based | Surawy, Ch., 2005 [29] | Study 1 (RCT) | Anxiety (HADS) | MBSR/MBCT compared to controls | Post treatment (8 weeks) | 0.010 |
| Study 2 (single-arm experimental study) | Anxiety (HADS) | MBCT/MBSR (pre-post) | Post treatment (8 weeks) | 0.000 | ||
| Fatigue impact: total score (FIS) | MBCT/MBSR (pre-post) | Post treatment (8 weeks) | 0.010 | |||
| Study 3 (single-arm experimental study) | Anxiety (HADS) | MBCT/MBSR (pre-post) | Post treatment (8 weeks) | 0.010 | ||
| Anxiety (HADS) | MBCT/MBSR (pre-post) | Post follow-up (3 months) | 0.010 | |||
| Depression (HADS) | MBCT/MBSR (pre-post) | Post-treatment (8 weeks) | 0.010 | |||
| Depression (HADS) | MBCT/MBSR (pre-post) | Post follow-up (3 months) | 0.050 | |||
| Fatigue score (ChFS) | MBCT/MBSR (pre-post) | Post treatment (8 weeks) | 0.010 | |||
| Fatigue score (ChFS) | MBCT/MBSR (pre-post) | Post follow-up (3 months) | 0.000 | |||
| Quality of life: physical functioning (SF-36) | MBCT/MBSR (pre-post) | Post treatment (8 weeks) | 0.010 | |||
| Quality of life: physical functioning (SF-36) | MBCT/MBSR (pre-post) | Post follow-up (3 months) | 0.000 | |||
| Fatigue impact: total score (FIS) | MBCT/MBSR (pre-post) | Post treatment (8 weeks) | 0.020 | |||
| Fatigue impact: total score (FIS) | MBCT/MBSR (pre-post) | Post follow-up (3 months) | 0.050 | |||
| Rimes, K., 2013 [30] | Fatigue (ChFS) | MBCT compared to waitlist | Post treatment (8 weeks) | 0.014 | ||
| Fatigue (ChFS) | MBCT compared to waitlist | Post follow-up (2 months) | 0.033 | |||
| Fatigue (ChFS) | MBCT (pre-post) | Post follow-up (6 months) | 0.010 | |||
| Impairment (The work and social adjustment scale) | MBCT compared to wait list | Post-treatment (8 weeks) | 0.04 | |||
| Impairment (The work and social adjustment scale) | MBCT compared to waitlist | Post follow-up (2 months) | 0.054 | |||
| Impairment (The work and social adjustment scale) | MBCT (pre-post) | Post follow-up (6 months) | 0.004 | |||
| Impairment (The work and social adjustment scale) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.004 | |||
| Beliefs about Emotions (Self-reporting scale) | MBCT compared to waitlist | Post treatment (8 weeks) | 0.01 | |||
| Beliefs about Emotions (Self-reporting scale) | MBCT compared to waitlist | Post follow-up (2 months) | 0.012 | |||
| Beliefs about Emotions (Self-reporting scale) | MBCT (pre-post) | Post follow-up (6 months) | 0.004 | |||
| Self-Compassion (Self-reporting scale) | MBCT compared to waitlist | Post treatment (8 weeks) | 0.007 | |||
| Self-Compassion (Self-reporting scale) | MBCT compared to waitlist | Post follow-up (2 months) | 0.006 | |||
| Self-Compassion (Self-reporting scale) | MBCT (pre-post) | Post follow-up (6 months) | 0.003 | |||
| Mindfulness (5 facet mindfulness questionnaire) | MBCT compared to waitlist | Post follow-up (2 months) | 0.035 | |||
| Mindfulness (5 facet mindfulness questionnaire) | MBCT (pre-post) | Post follow-up (6 months) | 0.006 | |||
| Mindfulness (5 facet mindfulness questionnaire) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.017 | |||
| Catastrophizing (Self-reporting scale) | MBCT compared to waitlist | Post treatment (8 weeks) | 0.004 | |||
| Catastrophizing (Self-reporting scale) | MBCT (pre-post) | Post follow-up (6 months) | 0.012 | |||
| All-or-nothing behavior (Self-reporting scale) | MBCT compared to waitlist | Post treatment (8 weeks) | 0.005 | |||
| All-or-nothing behavior (Self-reporting scale) | MBCT (pre-post) | Post follow-up (6 months) | 0.017 | |||
| Depression (HADS) | MBCT compared to waitlist | Post-treatment (8 weeks) | 0.038 | |||
ChFS: Chalder’s Fatigue Scale, HADS: Hospital Anxiety and Depression Scale, MBSR: Mindfulness-based stress reduction, MBCT: Mindfulness-based cognitive therapy, SF-36: 36- Item Short-Form Health Survey.
In comparison to the control group, both mental and physical fatigue scores improved significantly in four included studies using MBCT [30], isometric yoga [28], Qigong exercise [41] and Baduanjin Qigong [40]. Two studies showed within-group fatigue improvement in participants receiving an 8-week mindfulness therapy [29] and in participants receiving a 10-week relaxation program [39] (Table 3 and Table 4)
Anxiety and depression were improved in participants receiving Baduanjin Qigong compared to the controls after 16 sessions (9 weeks) of therapy [40]. Depression was improved in participants after 4 months of Qigong exercise [41] and 8 weeks of MBCT [30] compared to the control groups. Surawy et al. [29] also showed improvement of anxiety after 8 weeks of MBSR/MBCT intervention compared to the control group.
In comparison to the control group, quality of life improved in participants receiving Qigong exercise [41,70,71] and cognitive-behavioral stress management [45].
Table 3 and Table 4 show the details of all the significant outcomes of the included studies according to the diagnosis of ME/CFS (Oxford or CDC criteria).
3.8. Adverse Events
Seven studies assessed adverse events: Four did not identify any adverse events [30,39,41,43]; and three studies recorded adverse events such as deterioration of their symptoms, muscle ache, palpitation, dizziness, knee pain, backache, fatigue, and nervousness [28,40,44]. Five studies did not report if they assessed adverse events [10,29,38,42,45]
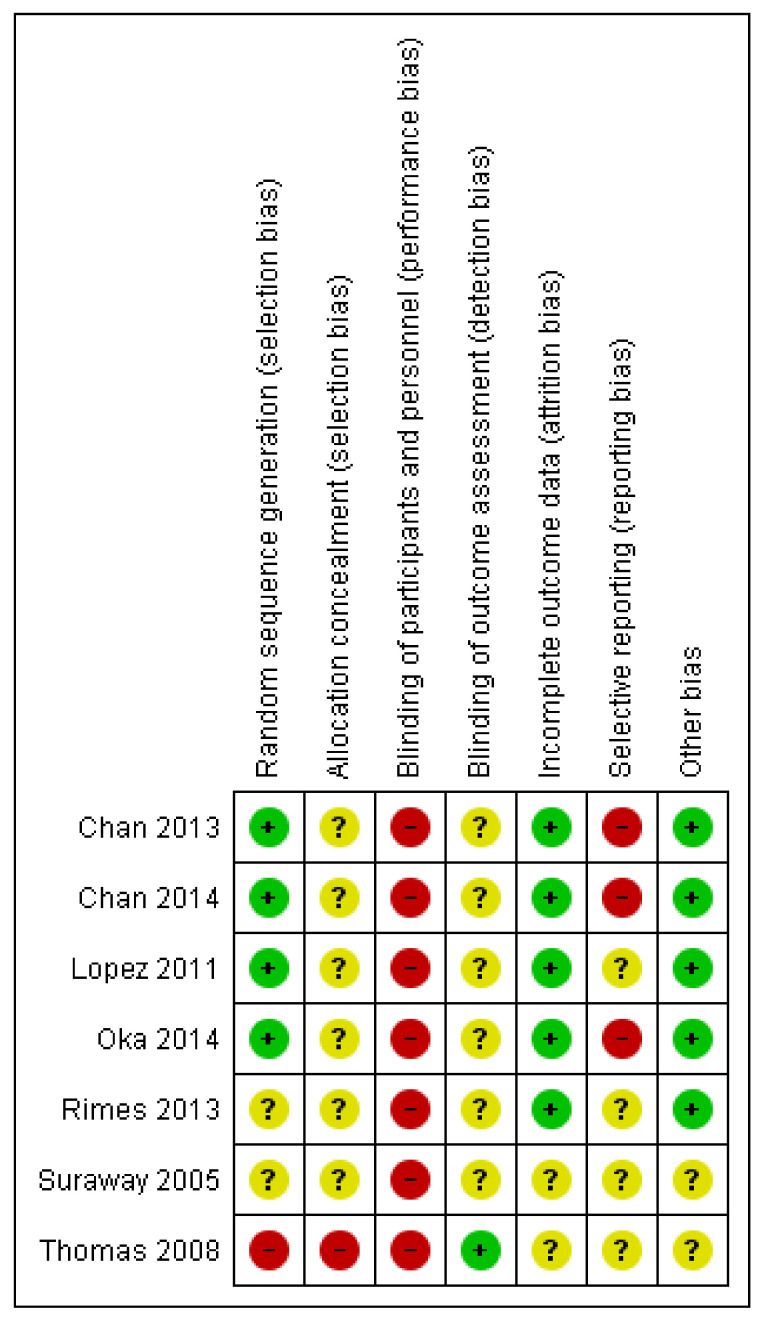
3.9. Risk of Bias in the Included Studies
All the included RCT studies were assessed at a high risk of bias in relation to the lack of blinding of participants and personnel. We were not able to assess the risk of bias in many areas as most of the studies were poorly reported (Figure 2). The risk of bias assessment for the single-arm experimental studies using the ROBINS-I assessment tool is shown in Table A5.
Figure 2.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
4. Discussion
This is the first systematic review of studies using MBIs in patients with ME/CFS. The MBIs used in these studies were mindfulness-based stress reduction and mindfulness-based cognitive therapy, relaxation, Qigong, and yoga.
The etiology and pathogenesis of ME/CFS are still unknown [1]. Researchers have shown changes in some biological markers [100,101,102,103]. Other studies highlight changes in the hypothalamic-pituitary-adrenal (HPA) axis in these patients [104].
It was also suggested that ME/CFS may be a neurophysiological disorder in the brain caused by repeated incidental or unnecessary stimuli in the limbic system, which is known as the threat response/protection center. These stimuli can be emotional, psychological, chemical, and/or physiological and they can keep the threat response center on a continuous high alert [105]. Connections between the amygdala and sympathetic, hypothalamic and other limbic brain systems can initiate a series of stimulations and uncontrolled reactions throughout the whole body, which could be considered as the root cause of CFS symptoms [105].
With increasing knowledge based on neuroplasticity and the impact of limbic function on somatic symptoms, the potential mechanisms of MBIs might be explained. There is growing interest in using MBIs and many programs are being offered directly to the public to assist with mental and physical health. One of these programs developed specifically for ME/CFS (25) has shown modest success in functional ability in a clinical audit. Because patients are accessing MBI programs, there is an urgent need for evidence as to whether these programs are having an impact on the core symptoms of ME/CFS or mainly address the secondary dissatisfaction that comes with having a chronic, poorly understood disease for which there is no cure. In this review, the MBIs used in the included studies were quite heterogenous. Two studies used relaxation techniques, five studies used movement-based therapies including different forms of yoga and Qigong and the remaining ones used various forms of mindfulness and cognitive-based approaches. Table A6 describes these interventions briefly.
In this systematic review, we found the most commonly measured outcomes were fatigue severity, anxiety and depression, and quality of life or its components (e.g., physical and mental functioning). When compared to the control group, fatigue severity, mental functioning and anxiety/depression mostly improved in patients receiving MBIs. However, poor reporting, small sample sizes, different diagnostic criteria, and a high risk of bias may challenge this result. It is also worth noting that these symptoms are not specific and can be found not only in some individuals with ME/CFS but also in individuals with many other physical and mental health conditions.
According to the 2015 Institute of Medicine report [1], impaired function, post-exertional malaise and unrefreshing sleep are the core symptoms in ME/CFS patients. None of our included studies, however, measured post-exertional malaise. One study measured sleep using a self-reporting scale which improved after 9 weeks of Qigong exercise [40]. Physical or mental functioning and functional performance were mostly measured using self-report scales and only one study measured performance using objective measures [51].
In contrast, anxiety and depression and some cognitive constructs were commonly measured in the included studies. While these symptoms are important, they are secondary and not the key features of ME/CFS. Reporting secondary outcomes while omitting measurement of the core symptoms of a disease may lead to inaccurate conclusions about treatment effectiveness.
Previous studies have used a variety of definitions for the diagnosis of ME/CFS. Lack of consensus and competing definitions act as a barrier for research in this field. Most of the studies in this systematic review used the 1994 CDC criteria for the diagnosis of ME/CFS and two studies used Oxford criteria.
The Oxford criteria were developed at a consensus meeting [46]. They do not require the presence of any symptom other than disabling fatigue. The presence of other symptoms such as immune, autonomic and mood symptoms differentiate ME/CFS from other common medical and psychiatric conditions including major depression. It has long been suspected that the Oxford criteria may therefore fail to exclude individuals with other fatiguing conditions [14,19].
To address this concern, the Agency for Healthy Research and Quality (AHRQ) in the United States conducted a sensitivity analysis in which the outcomes of treatment studies using the Oxford criteria were compared with studies using other criteria (mostly the 1994 CDC Criteria) [14]. They found that whereas most studies using the Oxford criteria showed some benefits for CBT, studies using the CDC criteria were mixed with no overall benefit. With regards to graded exercise therapy, exclusion of the trials using the Oxford case definition left insufficient evidence about the effectiveness of graded exercise therapy on any outcome. Studies of other therapies were not affected as primary studies had small sample sizes and a high risk of bias. These findings confirm that the choice of inclusion criteria impacts study outcomes. The AHRQ concluded that future research should retire the use of the Oxford case definition. The National Institutes of Health held a consensus workshop to guide the future of ME/CFS research [19]. For similar reasons as the AHRQ, they also recommended that the Oxford Criteria should be retired.
The 1994 CDC criteria also have significant drawbacks. They require four out of eight criteria but none are mandatory. This means two subjects identified with these criteria may have no symptoms in common with each other—one might have four and the other, another four. Moreover, minor symptoms may overlap with the symptoms of psychiatric disorders including major depression [14].
The Institute of Medicine [1] has proposed diagnostic criteria which are very similar to the Canadian Consensus Criteria [88]. They require patients to have moderate, substantial or severe disabling fatigue, post-exertional malaise and unrefreshing sleep for at least half of the time and one of the cognitive impairments or orthostatic intolerance symptoms. Conclusions about the effectiveness of interventions will be possible once studies use the same diagnostic criteria and measure core outcomes using standardized measures.
4.1. Strengths and Limitations
Assessment of a broad range of mind-body approaches and outcomes in a systematic fashion was one of the main strengths of this systematic review. Engaging patients in the process of designing the review protocol and in reviewing the findings increase the applicability and relevance of the findings of this study.
As we found a diverse range of interventions and outcomes across the included studies; we were not able to perform a meta-analysis. We also may have missed some relevant information by including only studies published in the English language.
4.2. Research Implications
As recommended by the Institute of Medicine report, using objective measures is a priority in studies of ME/CFS. There are several symptoms such as post-exertional malaise, cognitive dysfunction, orthostatic intolerance, and changes including impaired immune function and abnormal brain functions that could be measured objectively.
Future RCTs will benefit from larger sample sizes. Investigators must use an appropriate randomization method and ensure outcome assessors are blinded to the group identity of the participants. They should measure and report the outcomes specified in their protocol in order to avoid selective reporting.
5. Conclusions
In this systematic review, we described the current literature on MBIs for the treatment of ME/CFS. Future clinical trials will benefit from the findings of this study in terms of what outcomes and outcome measures are mostly used in previous studies. We showed that the included studies did not report measuring post-exertional malaise as a core outcome of ME/CFS. On the other hand, fatigue severity, anxiety/depression and mental functioning were shown to be improved in the patients receiving MBIs. However, poor reporting, small sample sizes, different diagnostic criteria, and a high risk of bias may challenge this result. We highlight the need for further research to use objective and standardized outcomes and outcome measures for making definitive conclusions.
Acknowledgments
The authors thank Susanne King-Jones for her help in the search strategy and contributions to the project.
Appendix A
Table A1.
Case definitions for the diagnosis of ME/CFS over time.
| Advisor Group, Year | Identifier | Case Definition and Required Symptom(s) |
|---|---|---|
| For Adults | ||
| Holmes et al., 1988 (CDC) [17] | CFS | Major criteria New onset of persistent or relapsing, debilitating fatigue or easy fatigability in a person who has no previous history of similar symptoms, that does not resolve with bedrest, and that is severe enough to reduce or impair average daily activity below 50% of the patient’s premorbid activity level for a period of at least 6 months Minor criteria Mild fever Sore throat Painful lymph node in the anterior or posterior cervical or axillary distribution Unexplained generalized muscle weakness Muscle discomfort or myalgia Prolonged generalized fatigue (≥24 h) after normal level of exercise Migratory arthralgia without joint swelling or redness Neurological complains one or more of: photophobia, transient visual scotomata, forgetfulness, excessive irritability, confusion, difficulty thinking, inability to concentrate, depression Sleep disturbances |
| Sharp et al., 1991 (Oxford) [46] | CFS | Fatigue as the principal symptom A definite onset that is not lifelong The fatigue is severe, disabling, and affects physical and mental functioning The fatigue should have been present for a minimum of 6 months during which it was present for more than 50% of the time Other symptoms may be present, particularly myalgia, mood and sleep disturbance. |
| Fukuda et al., 1994, (CDC) [52] | CFS | Clinically evaluated, “unexplained”, persistent or relapsing fatigue for ≥6 months. Not the result of ongoing exertion Not substantially alleviated by rest Resulting in a substantial reduction in previous activity level. Four or more of the following concurrently present for ≥ 6 months: impaired memory or concentration sore throat tender cervical or axillary lymph nodes muscle pain multi-joint pain new headaches unrefreshing sleep post-exertion malaise |
| London criteria-V2, (Dowsett et al., 1994) [106] | These three criteria must all be present for a diagnosis of M.E./PVFS Exercise-induced fatigue precipitated by trivially small exertion -physical or mental -relative to the patient’s previous exercise tolerance Impairment of short-term memory and loss of powers of concentration, usually coupled with other neurological and psychological disturbances such as emotional lability, nominal dysphasia, disturbed sleep patterns, disequilibrium or tinnitus Fluctuation of symptoms, usually precipitated by either physical or mental exercise |
|
| Canadian Consensus Criteria, (Carruthers et al., 2003) [88] |
ME/PVFS | For a diagnosis of CFS/ME, a patient must meet the following criteria 1–6 and adhere to item 7: Fatigue Post-exertional malaise and/or fatigue Sleep dysfunction Pain Two or more of the following neurological/cognitive manifestations: Confusion Impairment of concentration and short-term memory consolidation Disorientation difficulty with information processing categorizing and word retrieval perceptual and sensory disturbances One or more symptoms from two of the following categories: Autonomic manifestation (e.g., orthostatic intolerance, postural orthostatic tachycardia syndrome, …) Neuroendocrine manifestation (e.g., loss of thermostatic stability, sweating episode, …) Immune manifestation (e.g., tender lymph nodes, recurrent sore throat, …) Illness lasting ≥6 months |
| Revised Canadian Consensus Criteria, (Jason et al., 2010) [107] |
ME/CFS | Definition of Research CFS/ME criteria: Over the past 6 months, persistent or recurring chronic fatigue that is not lifelong and results in substantial reductions in previous levels of occupational, educational, social and personal activities Post-exertional malaise and/or fatigue Unrefreshing sleep or disturbance of sleep quantity or rhythm disturbance Pain (or discomfort) that is often widespread and migratory in nature. At least one symptom from any of the following: Myofascial and/or joint pain (e.g., deep pain, abdomen/stomach pain, or achy and sore muscles. Pain, stiffness, or tenderness may occur in any joint but must be present in more than one joint and lacking edema or other signs of inflammation) Abdominal and/or head pain (e.g., stomach pain or chest pain). Headaches often described as localized behind the eyes or in the back of the head (includes headaches localized elsewhere, including migraines; headaches would need to be more frequent than they were before, which would indicate a new pattern of a new type as compared to headaches previously experienced (i.e., location of pain has changed, nature of pain has changed), or different in severity type as compared to headaches previously experienced by the patient) Two or more of the following neurological/cognitive manifestations: Impaired memory (self-reported or observable disturbance in the ability to recall information or events on a short-term basis) Difficulty focusing vision and attention (disturbed concentration may impair the ability to remain on task, to screen out extraneous/excessive stimuli) Loss of depth perception Difficulty finding the right word Frequently forget what wanted to say Absent-mindedness Slowness of thought Difficulty recalling information Need to focus on one thing at a time Trouble expressing thought Difficulty comprehending information Frequently lose train of thought Sensitivity to bright lights or noise Muscle weakness/muscle twitches At least one symptoms from two of the following categories: Autonomic manifestation: Neurally mediated hypotension, postural orthostatic tachycardia, delayed postural hypotension, palpitations with or without cardiac arrhythmias, dizziness or fainting, feeling unsteady on the feet--disturbed balance, shortness of breath, nausea, bladder dysfunction, or irritable bowel syndrome Neuroendocrine manifestation: Recurrent feelings of feverishness and cold extremities, subnormal body temperature and marked diurnal fluctuations, sweating episodes, intolerance of extremes of heat and cold, marked weight change-loss of appetite or abnormal appetite Immune manifestation: Recurrent flu-like symptoms, non-exudative sore or scratchy throat, repeated fevers and sweats, lymph nodes tender to palpitation--generally minimal swelling observed, new sensitivities to food, odors, or chemicals |
| International Consensus Criteria, (Carruthers et al., 2011) [90] |
ME | Myalgic encephalomyelitis is an acquired neurological disease with complex global dysfunctions. Pathological dysregulation of the nervous, immune and endocrine systems, with impaired cellular energy metabolism and ion transport, are prominent features. Although signs and symptoms are dynamically interactive and causally connected, the criteria are grouped by regions of pathophysiology to provide general focus. A patient will meet the following criteria A. Post-exertional neuro-immune exhaustion (PENEpen’-e): Compulsory This cardinal feature is a pathological inability to produce sufficient energy on demand with prominent symptoms primarily in the neuro-immune regions. Characteristics are as follows:
|
Table A2.
Excluded studies.
| Primary Author, Publication Year | Reason for Exclusion |
|---|---|
| Aaron L. 2003 | Review study |
| Arroll M. 2012 | Not intervention of interest |
| Arroll MA. 2014 | Not eligible control group |
| Benor D. 2017 | Not population of interest |
| Bentler S. 2005 | Not population of interest |
| Craske N. 2009 | Not population of interest |
| Crawley E. 2017 | Not population of interest |
| Deale A. 1997 | Not intervention of interest |
| Deale A. 2001 | Not eligible control group |
| Densham S. 2016 | Not population of interest |
| Fjorback LO. 2012 | Not population of interest |
| Fjorback LO. 2013 | Not population of interest |
| Fjorback LO. 2013 | Not population of interest |
| Guthlin C. 2012 | Not intervention of interest |
| Hlavaty LE. 2011 | Not intervention of interest |
| Hall DL. 2017 | Not eligible control group |
| Jacobson HB. 2017 | Not population of interest |
| James, L. 1996 | Case study |
| Jason L. 2007 | Not intervention of interest |
| Kos D. 2015 | Not eligible control group |
| Lee J. 2015 | Not population of interest |
| Nijs J. 2008 | Not intervention of interest |
| Oka T. 2017 | Not eligible control group |
| Pauzano-Slamm N. 2005 | Not peer-reviewed publication |
| Ryan M. 2004 | Not population of interest |
| Sampalli T. 2009 | Not population of interest |
| Stevens MW. 1999 | Full-text not available |
| Toussaint L. 2012 | Not population of interest |
| Walach H. 2008 | Not intervention of interest |
| Windthorst P. 2017 | Not eligible control group |
Table A3.
Statistically insignificant outcomes in the included studies using CDC, Canadian and international consensus criteria for diagnosing CFS.
| Intervention Type | First Author, Year | Outcome (Assessed by) | Comparison Groups | Comparison Time Point | p-Value | |
|---|---|---|---|---|---|---|
| Relaxation-based | Thomas, M., 2006 and 2008 [39,51] | Report 1 (2006) | Anxiety (as part of a self-report subjective mood scale) | Relaxation group (pre-post) | Post treatment (10 weeks) | Non-significant |
| Performance (word recall, reaction time and vigilance tasks) | Relaxation group (pre-post) | Post follow-up (6 months) | Non-significant | |||
| Report 2 (2008) | Performance score-10% improvement or 80% attainment (Karnofsky scale) | Relaxation group compared to MCT and control groups | Post treatment (10 weeks) | Non-significant | ||
| Cognitive-based | Lopez C., 2011 [45] (Cognitive restructuring) |
Fatigue (Profile of Mood States (POMS) | CBSM compared to control | Time X group ** | 0.06 | |
| Sollie K., 2017 [43] (Mindfulness-based cognitive therapy) | Fatigue (Chalder Fatigue Scale) | MBCT (pre-post) | Post follow-up (3 months) | p value not reported, small effect size was reported (d = 0.26) | ||
| Depression (HADS) | MBCT (pre-post) | Post intervention (8 weeks) | p value not reported, small effect size was reported (d = 0.32) | |||
| Depression (HADS) | MBCT (pre-post) | Post follow-up (3 months) | p value not reported, small effect size was reported (d = 0.33) | |||
| Dispositional mindfulness (Five Facet Mindfulness questionnaire) | MBCT (pre-post) | Post intervention (8 weeks) | p value not reported, small effect size was reported (d = 0.11) | |||
| Rumination (Ruminative Response Scale) | MBCT (pre-post) | Post intervention (8 weeks) | p value not reported, small effect size was reported (d = 0.26) | |||
| Rumination (Ruminative Response Scale) | MBCT (pre-post) | Post follow-up (3 months) | p value not reported, small effect size was reported (d = 0.32) | |||
| CFS symptom burden | MBCT (pre-post) | Post intervention (8 weeks) | p value not reported, small effect size was reported (d = 0.07) | |||
| CFS symptom burden | MBCT (pre-post) | Post follow-up (3 months) | p value not reported, small effect size was reported (d = 0.04) | |||
| Satisfaction with life (Satisfaction With Life Scale) | MBCT (pre-post) | Post intervention (8 weeks) | p value not reported, small effect size was reported (d = −0.09) | |||
| Satisfaction with life (Satisfaction With Life Scale) | MBCT (pre-post) | Post follow-up (3 months) | p value not reported, small effect size was reported (d = 0.09) | |||
| Jonsjo, M., 2019 [44] (Psychological flexibility) | Disability (Pain disability index) | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.608 | ||
| Psychological flexibility (Psychological inflexibility fatigue scale) | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.775 | |||
| CFS symptoms | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.652 | |||
| Anxiety (HADS) | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.922 | |||
| Depression (HADS) | ACT (pre-post) | Post intervention (after 13 sessions) | 0.574 | |||
| Depression (HADS) | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.066 | |||
| Physical fatigue (MFI-20) | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.352 | |||
| Mental fatigue (MFI-20) | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.943 | |||
| Reduced activity (MFI-20) | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.449 | |||
| Reduced motivation (MFI-20) | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.918 | |||
| SF-36 physical | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.325 | |||
| SF-36 mental | ACT (pre-post) | Post intervention (after 13 sessions) | 0.520 | |||
| SF-36 mental | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.301 | |||
| EQ. 5D-Index | ACT (pre-post) | Post intervention (after 13 sessions) | 0.065 | |||
| EQ. 5D-Index | ACT (pre-post) | Post intervention to post follow-up (3 months) | 0.524 | |||
| Movement-based | Chan J., 2013 [41] | Ho, R., 2012 (Preliminary report) | Quality of life: physical functioning score (MOS SF-12) | Qigong (pre-post) | Post training (5 weeks) | Non-significant |
| Quality of life: physical functioning score (MOS SF-12) | Qigong (pre-post) | Post intervention (4 months) | Non-significant | |||
| Quality of life: physical functioning score (MOS SF-12) | Qigong compared to control | Time X group ** | 0.484 | |||
| Quality of life: physical functioning score (MOS SF-12) | Control (pre-post) | Post training (5 weeks) | Non- significant | |||
| Quality of life: physical functioning score (MOS SF-12) | Control (pre-post) | Post intervention (4 months) | Non- significant | |||
| Quality of life: mental functioning score (MOS SF-12) | Control (pre-post) | Post training (5 weeks) | Non-significant | |||
| Quality of life: mental functioning score (MOS SF-12) | Control (pre-post) | Post intervention (4 months) | Non-significant | |||
| Telomerase activity* (Telomerase PCR ELISA) | Control (pre-post) | Post intervention (4 months) | Non-significant | |||
| Chan J., 2013 (main report) |
Anxiety score (HADS) | Qigong (pre-post) | Time X group ** | 0.584 | ||
| Depression score (HADS) | Control (pre-post) | Post-intervention (4 months) | 0.365 | |||
| Li J., 2015 (Subset study report) | Quality of life: physical component summary (MOS SF-12) | Qigong compared to control | Post intervention (change score from baseline to 3 months) | 0.451 | ||
| Chan, J. 2014 [40] and Chan, J. 2017 [83] | Report 1 (2014) | Sleep quality: total score (PSQI) | Baduanjin Qigong compared to waitlist | Time X group ** | 0.064 | |
| Sleep duration (PSQI) | Baduanjin Qigong compared to waitlist | Time X group ** | 0.151 | |||
| Sleep efficacy (PSQI) | Baduanjin Qigong compared to wait list | Time X group ** | 0.522 | |||
| Sleep disturbance (PSQI) | Baduanjin Qigong compared to waitlist | Time X group ** | 0.062 | |||
| Use of sleep medication (PSQI) | Baduanjin Qigong compared to waitlist | Time X group ** | 0.803 | |||
| Daytime dysfunction (PSQI) | Baduanjin Qigong compared to waitlist | Time X group ** | 0.253 | |||
| Report 2 (2017) | Adiponectin levels | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 3-month) | Non-significant | ||
| Depression (HADS) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 3-month) | Non-significant | |||
| Anxiety (HADS) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 9 weeks) | Non-significant | |||
| Anxiety (HADS) | Baduanjin Qigong compared to waitlist | Post intervention (change score from baseline to 3-month) | Non-significant | |||
| Oka T, 2014 [28] | Quality of life: vitality (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | Non-significant | ||
| Quality of life: role emotional (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | Non-significant | |||
| Quality of life: mental health (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | Non-significant | |||
| Quality of life: physical functioning (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | Non-significant | |||
| Quality of life: mental component summary (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | Non-significant | |||
| Quality of life: role physical (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | Non-significant | |||
| Quality of life: social functioning (SF-8) | Isometric yoga (pre-post) | Post intervention (2 months) | Non-significant | |||
| Oka, T., 2018 and Oka, T., 2019 [42,91] | Report 1 (2018) | Autonomic function indices (low-frequency power of HR variability, CVR-R: Coefficient of variation of R-R intervals) | Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | Non-significant | |
| Serum biomarkers (IL-6, prolactin, Free carnitine, total carnitine, acylcarnitine) | Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | Non-significant | |||
| Plasma biomarkers (Transforming growth factor-beta1; Brain-derived Neurotrophic factor, Homovanillic acid, 3-methoxy-4-hydroxyphenylglycol) |
Acute effects of sitting isometric yoga (pre-post) | Before to after the final 20-min session | Non-significant | |||
| Report 2 (2019) | Autonomic function tests, serum, and blood biomarkers | Longitudinal effects of sitting isometric yoga (pre-post) | Post intervention (2 months) | Non-significant | ||
| Anxiety (HADS) | Seated isometric yoga compared to controls | Time X group ** | 0.786 | |||
| Depression (HADS) | Seated isometric yoga compared to controls | Time X group ** | 0.008 | |||
| Alexithymia (TAS-20) | Seated isometric yoga compared to controls | Time X group ** | 0.950 | |||
BMSWBI-S: Body-Mind-Spirit Well-being Inventory, ChFS: Chalder’s Fatigue Scale, HADS: Hospital Anxiety and Depression Scale, MCT: Multi-convergent therapy, MOS SF-12: Medical Outcomes Study 12- Item Short-Form Health Survey, POMS: Profile of Mood States, PSQI: Pittsburgh Sleep Quality Index, SF-8: Medical Outcomes Study Short Form 8, QOLI: Quality of life inventory, MFI-20: Multidimensional fatigue inventory-20, ACT: Acceptance and commitment therapy, MBCT: Mindfulness-based cognitive therapy, CBSM: Cognitive-based stress management, TAS: 20-item Toronto alexithymia scale. ** to test the interaction effect of time and group.
Table A4.
Statistically insignificant outcomes in the included studies using Oxford criteria for diagnosing CFS.
| Intervention Type | First Author, Year | Outcome (Assessed by) | Comparison Groups | Comparison Time Point | p-Value | |
|---|---|---|---|---|---|---|
| Mindfulness and cognitive-based | Surawy, Ch., 2005 [29] | Study 1 (RCT) | Depression (HADS) | MBSR/MBCT compared to controls | Post treatment (8 weeks) | 0.28 |
| Fatigue score (ChFS) | MBSR/MBCT compared to controls | Post treatment (8 weeks) | 0.08 | |||
| Study 2 (single-arm experimental study) | Depression (HADS) | MBCT/MBSR (pre-post) | Post treatment (8 weeks) | 0.16 | ||
| Fatigue score (ChFS) | MBCT/MBSR (pre-post) | Post treatment (8 weeks) | 0.06 | |||
| Quality of life: physical functioning (SF-36) | MBCT/MBSR (pre-post) | Post treatment (8 weeks) | 0.69 | |||
| Rimes, K., 2013 [30] | Mindfulness (5 facet mindfulness questionnaire) | MBCT compared to waitlist | Post treatment (8 weeks) | 0.067 | ||
| Catastrophizing (Self-reporting scale) | MBCT compared to waitlist | Post follow-up (2 months) | 0.152 | |||
| All-or-nothing behavior (Self-reporting scale) | MBCT compared to waitlist | Post follow-up (2 months) | 0.089 | |||
| Depression (HADS) | MBCT compared to waitlist | Post follow-up (2 months) | 0.153 | |||
| Anxiety (HADS) | MBCT compared to waitlist | Post treatment (8 weeks) | 0.173 | |||
| Anxiety (HADS) | MBCT compared to waitlist | Post follow-up (2 months) | 0.296 | |||
| Quality of life: physical functioning (SF-36) | MBCT compared to waitlist | Post treatment (8 weeks) | 0.124 | |||
| Quality of life: physical functioning (SF-36) | MBCT compared to waitlist | Post follow-up (2 months) | 0.345 | |||
| Impairment (The work and social adjustment scale) | MBCT compared to waitlist | Post follow-up (2 months) | 0.054 | |||
| Fatigue (ChFS) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.089 | |||
| Depression (HADS) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.069 | |||
| Catastrophizing (Self-reporting scale) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.063 | |||
| All-or-nothing behavior (Self-reporting scale) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.082 | |||
| Self-Compassion (Self-reporting scale) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.110 | |||
| Anxiety (HADS) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.211 | |||
| Quality of life: physical functioning (SF-36) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.164 | |||
| Beliefs about Emotions (Self-reporting scale) | MBCT (pre-post) | Between 2- and 6-month follow-up | 0.84 | |||
| Quality of life: physical functioning (SF-36) | MBCT (pre-post) | Post follow-up (6 months) | 0.051 | |||
| Depression (HADS) | MBCT (pre-post) | Post follow-up (6 months) | 0.051 | |||
| Anxiety (HADS) | MBCT (pre-post) | Post follow-up (6 months) | 0.206 | |||
ChFS: Chalder’s Fatigue Scale, HADS: Hospital Anxiety and Depression Scale, MBSR: Mindfulness-based stress reduction, MBCT: Mindfulness-based cognitive therapy, SF-36: 36- Item Short-Form Health Survey.
Table A5.
Risk of Bias (ROBINS-I).
| Domains | Bogaerts 2007 | Surawy 2005 Study 2 | Surawy 2005 Study 3 | Sollie 2017 | Oka 2018 and 2019 | Jonsjo 2019 | Takakura 2019 |
|---|---|---|---|---|---|---|---|
| Confounding | No information | No information | No information | Low | Low | Low | Low |
| Selection bias | Low | Serious | Serious | Low | Moderate | Low | Low |
| Measurement of intervention | Low | Low | Low | Low | Low | Low | Low |
| Deviation from the intended intervention | Low | Low | Low | Low | Low | Low | Low |
| Missing data | Low | Low | Low | Low | Low | Low | Low |
| Measurement of outcomes | Moderate | Serious | Serious | Moderate | Moderate | Moderate | Moderate |
| Reported results | No information | No information | No information | Low | Low | Low | Low |
| Overall | Moderate | Serious | Serious | Moderate | Moderate | Moderate | Moderate |
Low risk of bias (the study is comparable to a well-performed randomized trial with regard to this domain), Moderate risk of bias (the study is sound for a nonrandomized study with regard to this domain but cannot be considered comparable to a well-performed randomized trial), Serious risk of bias (the study has some important problems).
Table A6.
Brief descriptions of mind-body interventions used in the included studies.
| MBIs | Definition |
|---|---|
| Relaxation therapies |
https://www.nccih.nih.gov/health/relaxation-techniques-for-health (accessed on 13 June 2021). “Relaxation techniques include a number of practices such as progressive relaxation, guided imagery, biofeedback, self-hypnosis, and deep breathing exercises. The goal is similar in all: to produce the body’s natural relaxation response, characterized by slower breathing, lower blood pressure, and a feeling of increased well-being”. |
| Movement-based interventions |
https://www.nccih.nih.gov/health/yoga-what-you-need-to-know (accessed on 13 June 2021). “Although classical yoga also includes other elements, yoga as practiced in the United States typically emphasizes physical postures (asanas), breathing techniques (pranayama), and meditation (dyana). There are many different yoga styles, ranging from gentle practices to physically demanding ones. Differences in the types of yoga used in research studies may affect study results. This makes it challenging to evaluate research on the health effects of yoga. Yoga and two practices of Chinese origin—tai chi and qi gong—are sometimes called “meditative movement” practices. All three practices include both meditative elements and physical ones”. |
|
https://www.nccih.nih.gov/health/tai-chi-and-qi-gong-in-depth (accessed on 13 June 2021). “Tai chi and qi gong are centuries-old practices that involve certain postures and gentle movements with mental focus, breathing, and relaxation. The movements can be adapted or practiced while walking, standing, or sitting. In contrast to qi gong, tai chi movements, if practiced quickly, can be a form of combat or self-defense”. | |
| Mindfulness and cognitive-based | Mindfulness-based stress reduction (MBSR): “The program is conducted as an 8- to 10-week course for groups of up to 30 participants who meet weekly for 2—2.5 hr for instruction and practice in mindfulness meditation skills, together with a discussion of stress, coping, and homework assignments”. 1 |
| Mindfulness-based cognitive therapy (MBCT): “MBCT incorporates elements of cognitive therapy that facilitate a detached or de-centered view of one’s thoughts, including statements such as “thoughts are not facts” and “I am not my thoughts.” This decentered approach also is applied to emotions and bodily sensations”. 1 | |
| Cognitive-behavioral stress management (CBSM) is based on cognitive restructuring: “CBSM interventions reduce distress by teaching relaxation techniques; modifying patients’ outlook, cognitive appraisals, and coping strategies; and when performed in a group format may also improve their perceptions of social support”. 2 | |
| Acceptance commitment therapy (ACT) is based on psychological flexibility. “This is defined as the ability to act in line with important long-term goals or values in life, even in the presence of negative experiences (e.g., non-acute somatic symptoms or psychological distress). Psychological flexibility is a complex overarching behavioral construct that includes several behavioral processes such as acceptance/non-acceptance and cognitive fusion/diffusion”. 3 |
1 Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clinical psychology: Science and practice. June 2003, 10,125–143. 2 Lopez C, Antoni M, Penedo F, Weiss D, Cruess S, Segotas M-C, et al. A pilot study of cognitive-behavioral stress management effects on stress, quality of life, and symptoms in persons with chronic fatigue syndrome. Journal of psychosomatic research. 2011, 70, 328–334. 3 Jonsjö MA, Wicksell RK, Holmström L, Andreasson A, Olsson GL. Acceptance and commitment therapy for ME/CFS (Chronic Fatigue Syndrome)–a feasibility study. Journal of Contextual Behavioral Science. 2019, 12, 89–97.
Appendix B
Medline Search Strategy
Fatigue Syndrome, Chronic/
Myalgic Encephalomyelitis.mp.
exp Encephalomyelitis/
Fatigue/
3 and 4
1 or 2 or 5
(chronic$ adj3 fatig$ adj3 syndrom$).mp.
(myalg$ adj3 encephal$).mp.
6 or 7 or 8
exp Mind-Body Therapies/
exp Biofeedback, Psychology/
exp Neurofeedback/
exp “Imagery (Psychotherapy)”/
exp Hypnosis/
exp Relaxation Therapy/
exp Mindfulness/
exp Meditation/
exp Yoga/
exp Tai Ji/
(Mindfulness-based adj2 cognitive adj2 therapy).mp.
self-hypnosis.mp.
Guided imagery.mp.
exp Art Therapy/
mindfulness-based stress reduction.mp.
guided meditation.mp.
exp Autogenic Training/
(progressive adj2 muscle adj2 relaxation).mp.
exp Breathing Exercises/
Chi Gong.mp.
exp Qigong/
Psychological flexibility.mp.
Relaxation Response.mp.
exp Spirituality/
Mindful meditation.mp.
Mantra.mp.
Transcendental Meditation.ti,ab.
Mindfulness-based cognitive therapy.ti,ab.
Prayer.ti,ab.
Visualization.ti,ab.
Neurolinguistic programming.ti,ab.
Cognitive restructuring.ti,ab.
exp Music Therapy/
(Eye movement desensitization and reprocessing).ti,ab.
Emotional Freedom Techniques.ti,ab.
Dynamic Neural Retraining System.ti,ab.
or/10–45
9 and 46
limit 47 to English language
Author Contributions
Conceptualization, S.V., E.S., and K.O; methodology, S.K.A., M.K., E.S., S.P., D.R.J., T.K., M.P., L.S., K.O. and S.V.; validation, S.K.A., M.K., E.S., D.R.J., S.P. and S.V.; formal analysis, S.K.A.; investigation, S.K.A., M.K., D.R.J. and E.S.; resources, S.V.; data curation, S.K.A.; writing—original draft preparation, S.K.A., M.K. and E.S.; writing—review and editing, S.P., E.S. and S.V.; visualization, S.K.A.; supervision, S.V.; project administration, S.P.; funding acquisition, S.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Calgary.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Institute of Medicine . Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness, Committe on the Diagnosis for ME/CFS. The National Academies Pres; Washington, DC, USA: 2015. [PubMed] [Google Scholar]
- 2.Johnston S., Brenu E.W., Staines D., Marshall-Gradisnik S. The prevalence of chronic fatigue syndrome/myalgic encephalomyelitis: A meta-analysis. Clin. Epidemiol. 2013;5:105–110. doi: 10.2147/CLEP.S39876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rusu C., Gee M.E., Lagacé C., Parlor M. Chronic fatigue syndrome and fibromyalgia in Canada: Prevalence and associations with six health status indicators. Chronic Dis. Inj. Can. 2015;35:1678508400. doi: 10.24095/hpcdp.35.1.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jason L.A., Richman J.A., Rademaker A.W., Jordan K.M., Plioplys A.V., Taylor R.R., McCready W., Huang C.F., Plioplys S. A community-based study of chronic fatigue syndrome. Arch. Intern. Med. 1999;159:2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 5.Hvidberg M.F., Brinth L.S., Olesen A.V., Petersen K.D., Ehlers L. The Health-Related Quality of Life for Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) PLoS ONE. 2015;10:e0132421. doi: 10.1371/journal.pone.0132421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nacul L.C., Lacerda E.M., Campion P., Pheby D., Drachler M.d.L., Leite J.C., Poland F., Howe A., Fayyaz S., Molokhia M. The functional status and well being of people with myalgic encephalomyelitis/chronic fatigue syndrome and their carers. BMC Public Health. 2011;11:402. doi: 10.1186/1471-2458-11-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bombardier C.H., Buchwald D. Chronic fatigue, chronic fatigue syndrome, and fibromyalgia. Disability and health-care use. Med. Care. 1996;34:924–930. doi: 10.1097/00005650-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds K.J., Vernon S.D., Bouchery E., Reeves W.C. The economic impact of chronic fatigue syndrome. Cost Eff. Resour. Alloc. 2004;2:1–4. doi: 10.1186/1478-7547-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Diseases Control and Prevention Chronic Fatigue Syndrome Awareness Day: CDC. [(accessed on 21 August 2018)];2018 Available online: http://www.cdc.gov/Features/cfsawarenessday/
- 10.Takakura S., Oka T., Sudo N. Changes in circulating microRNA after recumbent isometric yoga practice by patients with myalgic encephalomyelitis/chronic fatigue syndrome: An explorative pilot study. Bio. Psycho. Soc. Med. 2019;13:1–10. doi: 10.1186/s13030-019-0171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Missailidis D., Annesley S.J., Fisher P.R. Pathological mechanisms underlying myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics. 2019;9:80. doi: 10.3390/diagnostics9030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maksoud R., du Preez S., Eaton-Fitch N., Thapaliya K., Barnden L., Cabanas H., Staines D., Marshall-Gradisnik S. A systematic review of neurological impairments in myalgic encephalomyelitis/chronic fatigue syndrome using neuroimaging techniques. PLoS ONE. 2020;15:e0232475. doi: 10.1371/journal.pone.0232475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haney E., Smith M.E.B., McDonagh M., Pappas M., Daeges M., Wasson N., Nelson H.D. Diagnostic Methods for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for a National Institutes of Health Pathways to Prevention WorkshopDiagnostic Methods for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Ann. Intern. Med. 2015;162:834–840. doi: 10.7326/M15-0443. [DOI] [PubMed] [Google Scholar]
- 14.Smith M., Nelson H.D., Haney E., Pappas M., Daeges M., Wasson N., McDonagh M. Diagnosis and Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Agency for Healthcare Research and Quality Evidence Report/Technology Assessment No 219 (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No 290-2012-00014-I) Rockville, MD: AHRQ Publication No. 15-E001-EF. [(accessed on 20 June 2021)]; Available online: www.effectivehealthcare.ahrq.gov/reports/final.cfm.
- 15.Holgate S.T., Komaroff A.L., Mangan D., Wessely S. Chronic fatigue syndrome: Understanding a complex illness. Nat. Rev. Neurosci. 2011;12:539–544. doi: 10.1038/nrn3087. [DOI] [PubMed] [Google Scholar]
- 16.Acheson E.D. The clinical syndrome variously called benign myalgic encephalomyelitis, Iceland disease and epidemic neuromyasthenia. Am. J. Med. 1959;26:569–595. doi: 10.1016/0002-9343(59)90280-3. [DOI] [PubMed] [Google Scholar]
- 17.Holmes G.P., Kaplan J.E., Gantz N.M., Komaroff A.L., Schonberger L.B., Straus S.E., Jones J.F., Dubois R.E., Cunningham-Rundles C., Pahwa S., et al. Chronic Fatigue Syndrome: A Working Case Definition. Ann. Intern. Med. 1988;108:387–389. doi: 10.7326/0003-4819-108-3-387. [DOI] [PubMed] [Google Scholar]
- 18.Jason L.A., Brown A., Evans M., Sunnquist M., Newton J.L. Contrasting Chronic Fatigue Syndrome versus Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Fatigue: Biomedicine, health & behavior. Ann. Intern. Med. 2013;1:168–183. doi: 10.1080/21641846.2013.774556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith M.E.B., Haney E., McDonagh M., Pappas M., Daeges M., Wasson N., Fu R., Nelson H.D. Treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for a National Institutes of Health Pathways to Prevention WorkshopTreatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Ann. Intern. Med. 2015;162:841–850. doi: 10.7326/M15-0114. [DOI] [PubMed] [Google Scholar]
- 20.Toward Optimal Practice . Identification and Symptom Management of ME/CFS. Clinical Practice Guideline; Calgary, AB, Canada: 2016. [Google Scholar]
- 21.McCrone P., Darbishire L., Ridsdale L., Seed P. The economic cost of chronic fatigue and chronic fatigue syndrome in UK primary care. Psychol. Med. 2003;33:253–261. doi: 10.1017/S0033291702006980. [DOI] [PubMed] [Google Scholar]
- 22.Price J.R., Mitchell E., Tidy E., Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst. Rev. 2008 doi: 10.1002/14651858.CD001027.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larun L., Brurberg K.G., Odgaard-Jensen J., Price J.R. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD003200.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Wahbeh H., Elsas S.-M., Oken B.S. Mind–body interventions: Applications in neurology. Neurology. 2008;70:2321–2328. doi: 10.1212/01.wnl.0000314667.16386.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A. Can amygdala retraining techniques improve the wellbeing of patients with chronic fatigue syndrome. J. Holist. Healthc. 2010;7:12–15. [Google Scholar]
- 26.Vitetta L., Anton B., Cortizo F., Sali A. Mind-body medicine: Stress and its impact on overall health and longevity. Ann. N. Y. Acad. Sci. 2005;1057:492–505. doi: 10.1196/annals.1322.038. [DOI] [PubMed] [Google Scholar]
- 27.Theadom A., Cropley M., Smith H.E., Feigin V.L., McPherson K. Mind and body therapy for fibromyalgia. Cochrane Database Syst. Rev. 2015;1057:492–505. doi: 10.1002/14651858.CD001980.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oka T., Tanahashi T., Chijiwa T., Lkhagvasuren B., Sudo N., Oka K. Isometric yoga improves the fatigue and pain of patients with chronic fatigue syndrome who are resistant to conventional therapy: A randomized, controlled trial. BioPsychoSocial Med. 2014;8:1–9. doi: 10.1186/s13030-014-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surawy C., Roberts J., Silver A. The Effect of Mindfulness Training on Mood and Measures of Fatigue, Activity, and Quality of Life in Patients with Chronic Fatigue Syndrome on a Hospital Waiting List: A Series of Exploratory Studies. Behav. Cogn. Psychother. 2005;33:103–109. doi: 10.1017/S135246580400181X. [DOI] [Google Scholar]
- 30.Rimes K.A., Wingrove J. Mindfulness-Based Cognitive Therapy for People with Chronic Fatigue Syndrome Still Experiencing Excessive Fatigue after Cognitive Behaviour Therapy: A Pilot Randomized Study. Clin. Psychol. Psychother. 2013;20:107–117. doi: 10.1002/cpp.793. [DOI] [PubMed] [Google Scholar]
- 31.Collinge W., Yarnold P.R., Raskin E. Use of mind/body selfhealing practice predicts positive health transition in chronic fatigue syndrome: A controlled study. Subtle Energ. Energy Med. J. Arch. 1998;9:107–117. [Google Scholar]
- 32.Dybwad M., Frøslie K., Stanghelle J. Work Capacity, Fatigue and Health Related Quality of Life in Patients with Myalgic Encephalopathy or Chronic Fatigue Syndrome, before and after Qigong Therapy, a Randomized Controlled Study. Sunnaas Rehabilitation Hospital; Nesoddtangen, Norway: 2007. [Google Scholar]
- 33.Moher D., Hopewell S., Schulz K.F., Montori V., Gotzsche P.C., Devereaux P.J., Elbourne D., Egger M., Altman D.G., Consolidated Standards of Reporting Trials Group CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 2010;63:e1-37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domecq J.P., Prutsky G., Elraiyah T., Wang Z., Nabhan M., Shippee N., Brito J.P., Boehmer K., Hasan R., Firwana B., et al. Patient engagement in research: A systematic review. BMC Health Serv. Res. 2014;14:89. doi: 10.1186/1472-6963-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pollock A., Campbell P., Struthers C., Synnot A., Nunn J., Hill S., Goodare H., Morris J., Watts C., Morley R. Stakeholder involvement in systematic reviews: A scoping review. Syst. Rev. 2018;7:208. doi: 10.1186/s13643-018-0852-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogaerts K., Hubin M., Van Diest I., De Peuter S., Van Houdenhove B., Van Wambeke P., Crombez G., Van den Bergh O. Hyperventilation in patients with chronic fatigue syndrome: The role of coping strategies. Behav. Res. Ther. 2007;45:2679–2690. doi: 10.1016/j.brat.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Thomas M.A., Sadlier M.J., Smith A.P. A multiconvergent approach to the rehabilitation of patients with chronic fatigue syndrome: A comparative study. Physiotherap. 2008;94:35–42. doi: 10.1016/j.physio.2007.04.013. [DOI] [Google Scholar]
- 40.Chan J.S., Ho R.T., Chung K.-F., Wang C.-W., Yao T.-J., Ng S.-M., Chan C.L. Qigong exercise alleviates fatigue, anxiety, and depressive symptoms, improves sleep quality, and shortens sleep latency in persons with chronic fatigue syndrome-like illness. Evid. Based Complementary Altern. Med. 2014;2014:106048. doi: 10.1155/2014/106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan J.S., Ho R.T., Wang C.-W., Yuen L.P., Sham J.S., Chan C.L. Effects of qigong exercise on fatigue, anxiety, and depressive symptoms of patients with chronic fatigue syndrome-like illness: A randomized controlled trial. Evid. Based Complementary Altern. Med. 2013;2013:1–8. doi: 10.1155/2013/485341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oka T., Tanahashi T., Sudo N., Lkhagvasuren B., Yamada Y. Changes in fatigue, autonomic functions, and blood biomarkers due to sitting isometric yoga in patients with chronic fatigue syndrome. BioPsychoSocial Med. 2018;12:3. doi: 10.1186/s13030-018-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sollie K., Næss E.T., Solhaug I., Thimm J. Mindfulness training for chronic fatigue syndrome: A pilot study. Health Psychol. Rep. 2017;3:240–250. doi: 10.5114/hpr.2017.65469. [DOI] [Google Scholar]
- 44.Jonsjö M.A., Wicksell R.K., Holmström L., Andreasson A., Olsson G.L. Acceptance & commitment therapy for ME/CFS (Chronic Fatigue Syndrome)–a feasibility study. J. Contextual Behav. Sci. 2019;12:89–97. [Google Scholar]
- 45.Lopez C., Antoni M., Penedo F., Weiss D., Cruess S., Segotas M.-C., Helder L., Siegel S., Klimas N., Fletcher M.A. A pilot study of cognitive behavioral stress management effects on stress, quality of life, and symptoms in persons with chronic fatigue syndrome. J. Psychosom. Res. 2011;70:328–334. doi: 10.1016/j.jpsychores.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharpe M.C., Archard L.C., Banatvala J.E., Borysiewicz L.K., Clare A.W., David A., Edwards R.H., Hawton K.E., Lambert H.P., Lane R.J. A report—Chronic fatigue syndrome: Guidelines for research. J. R. Soc. Med. 1991;84:118–121. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 48.Chalder T., Berelowitz G., Pawlikowska T., Watts L., Wessely S., Wright D., Wallace E.P. Development of a fatigue scale. J. Psychosom. Res. 1993;37 doi: 10.1016/0022-3999(93)90081-P. [DOI] [PubMed] [Google Scholar]
- 49.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care. 1992;30:473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Fisk J.D., Ritvo P.G., Ross L., Haase D.A., Marrie T.J., Schlech W.F. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin. Infect. Dis. 1994;18(Suppl. 1):S79–S83. doi: 10.1093/clinids/18.Supplement_1.S79. [DOI] [PubMed] [Google Scholar]
- 51.Thomas M., Sadlier M., Smith A. The effect of Multi Convergent Therapy on the psychopathology, mood and performance of Chronic Fatigue Syndrome patients: A preliminary study. Couns. Psychother. Res. 2006;6:91–99. doi: 10.1080/14733140600711955. [DOI] [Google Scholar]
- 52.Fukuda K., Straus S.E., Hickie I., Sharpe M.C., Dobbins J.G., Komaroff A. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann. Intern. Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 53.Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 54.Radloff L. The center for epidemiologic studies depression index. Appl. Psychol. Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 55.Broadbent D.E., Cooper P.F., FitzGerald P., Parkes K.R. The cognitive failures questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 56.Cohen S., Hoberman H.M. Positive events and social supports as buffers of life change stress1. J. Appl. Soc. Psychol. 1983;13:99–125. doi: 10.1111/j.1559-1816.1983.tb02325.x. [DOI] [Google Scholar]
- 57.Smith A., Pollock J., Thomas M., Llewelyn M., Borysiewicz L. The relationship between subjective ratings of sleep and mental functioning in healthy subjects and patients with chronic fatigue syndrome. Hum. Psychopharmacol. Clin. Exp. 1996;11:161–167. doi: 10.1002/(SICI)1099-1077(199605)11:3<161::AID-HUP784>3.0.CO;2-5. [DOI] [Google Scholar]
- 58.Marks I.M. Behavioural Psychotherapy: Maudsley Pocket Book of Clinical Management. Wright/IOP Publishing; Bristol, UK: 1986. [Google Scholar]
- 59.Zevon M.A., Tellegen A. The structure of mood change: An idiographic/nomothetic analysis. J. Personal. Soc. Psychol. 1982;43:111. doi: 10.1037/0022-3514.43.1.111. [DOI] [Google Scholar]
- 60.Ray C., Weir W., Stewart D., Miller P., Hyde G. Ways of coping with chronic fatigue syndrome: Development of an illness management questionnaire. Soc. Sci. Med. 1993;37:385–391. doi: 10.1016/0277-9536(93)90268-9. [DOI] [PubMed] [Google Scholar]
- 61.Karnofsky D., Abelmann W., Craver L. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer. 1948;1:634–656. doi: 10.1002/1097-0142(194811)1:4<634::AID-CNCR2820010410>3.0.CO;2-L. [DOI] [Google Scholar]
- 62.Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Personal. Soc. Psychol. 1988;54:1063. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 63.Wientjes C.J., Grossman P. Overreactivity of the psyche or the soma? Interindividual associations between psychosomatic symptoms, anxiety, heart rate, and end-tidal partial carbon dioxide pressure. Psychosom. Med. 1994;56:533–540. doi: 10.1097/00006842-199411000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Meyer T.J., Miller M.L., Metzger R.L., Borkovec T.D. Development and validation of the penn state worry questionnaire. Behav. Res. Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 65.Bradley M.M., Lang P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 66.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 67.McNair D., Lorr M., DroppLemn L. Manual for the Profile of Mood States (POMS) Educational and Industrial Testing Service; San Diego, CA, USA: 1971. [Google Scholar]
- 68.Frisch M.B. Quality of Life Inventory (QOLI) National Computer Systems; Minneapolis, MN, USA: 1994. [Google Scholar]
- 69.Wagner D., Nisenbaum R., Heim C., Jones J.F., Unger E.R., Reeves W.C. Psychometric properties of the CDC symptom inventory for assessment of Chronic Fatigue Syndrome. Popul. Health Metr. 2005;3 doi: 10.1186/1478-7954-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho R.T., Chan J.S., Wang C.-W., Lau B.W., So K.F., Yuen L.P., Sham J.S., Chan C.L. A randomized controlled trial of qigong exercise on fatigue symptoms, functioning, and telomerase activity in persons with chronic fatigue or chronic fatigue syndrome. Ann. Behav. Med. 2012;44:160–170. doi: 10.1007/s12160-012-9381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J., Chan J.S., Chow A.Y., Yuen L.P., Chan C.L. From body to mind and spirit: Qigong exercise for bereaved persons with chronic fatigue syndrome-like illness. Evid. Based Complementary Altern. Med. 2015;2015:1–7. doi: 10.1155/2015/631410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong W.S., Fielding R. Construct validity of the Chinese version of the Chalder Fatigue Scale in a Chinese community sample. J. Psychosom. Res. 2010;68:89–93. doi: 10.1016/j.jpsychores.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Leung C., Wing Y., Kwong P., Shum A.L.K. Validation of the Chinese-Cantonese version of the Hospital Anxiety and Depression Scale and comparison with the Hamilton Rating Scale of Depression. Acta Psychiatr. Scand. 1999;100:456–461. doi: 10.1111/j.1600-0447.1999.tb10897.x. [DOI] [PubMed] [Google Scholar]
- 74.Ware J.E., Jr., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Lam C.L., Eileen Y., Gandek B. Is the standard SF-12 health survey valid and equivalent for a Chinese population? Qual. Life Res. 2005;14:539–547. doi: 10.1007/s11136-004-0704-3. [DOI] [PubMed] [Google Scholar]
- 76.Ng S., Yau J.K., Chan C.L., Chan C.H., Ho D.Y. The measurement of body-mind-spirit well-being: Toward multidimensionality and transcultural applicability. Soc. Work Health Care. 2005;41:33–52. doi: 10.1300/J010v41n01_03. [DOI] [PubMed] [Google Scholar]
- 77.Mundt J.C., Marks I.M., Shear M.K., Greist J.M. The Work and Social Adjustment Scale: A simple measure of impairment in functioning. Br. J. Psychiatry. 2002;180:461–464. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- 78.Stewart A.L., Hays R.D., Ware J.E. The MOS short-form general health survey: Reliability and validity in a patient population. Med. Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 79.McHorney C.A., Ware J.E., Jr., Lu J.R., Sherbourne C.D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med. Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 80.Rimes K.A., Chalder T. The Beliefs about Emotions Scale: Validity, reliability and sensitivity to change. J. Psychosom. Res. 2010;68:285–292. doi: 10.1016/j.jpsychores.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 81.Neff K.D. The development and validation of a scale to measure self-compassion. Self Identity. 2003;2:223–250. doi: 10.1080/15298860309027. [DOI] [Google Scholar]
- 82.Baer R.A., Smith G.T., Hopkins J., Krietemeyer J., Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- 83.Chan J.S., Li A., Ng S.-M., Ho R.T., Xu A., Yao T.-J., Wang X.M., So K.F., Chan C.L. Adiponectin potentially contributes to the antidepressive effects of Baduanjin Qigong exercise in women with chronic fatigue syndrome-like illness. Cell Transpl. 2017;26:493–501. doi: 10.3727/096368916X694238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buysse D.J., Reynolds C.F., III, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 85.Chong A.M., Cheung C.-K. Factor structure of a Cantonese-version pittsburgh sleep quality index. Sleep Biol. Rhythms. 2012;10:118–125. doi: 10.1111/j.1479-8425.2011.00532.x. [DOI] [Google Scholar]
- 86.Ho R.T., Fong T.C. Factor structure of the Chinese version of the Pittsburgh Sleep Quality Index in breast cancer patients. Sleep Med. 2014;15:565–569. doi: 10.1016/j.sleep.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 87.Fukuhara S., Suzukamo Y. Manual of the SF-8 Japanese version. Kyoto: Institute for Health Outcomes & Process Evaluation Research. Environ. Health Prev. Med. 2011;16:97–105. [Google Scholar]
- 88.Carruthers B.M., Jain A.K., De Meirleir K.L., Peterson D.L., Klimas N.G., Lerner A.M., Bested A.C., Flor-Henry P., Joshi P., Powles A.P., et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003;11:7–115. doi: 10.1300/J092v11n01_02. [DOI] [Google Scholar]
- 89.Nolen-Hoeksema S., Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta Earthquake. J. Personal. Soc. Psychol. 1991;61:115. doi: 10.1037/0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- 90.Diener E., Emmons R.A., Larsen R.J., Griffin S. The life satisfaction scale. J. Personal. Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 91.Oka T., Tanahashi T., Lkhagvasuren B., Yamada Y. The longitudinal effects of seated isometric yoga on blood biomarkers, autonomic functions, and psychological parameters of patients with chronic fatigue syndrome: A pilot study. BioPsychoSocial Med. 2019;13:1–13. doi: 10.1186/s13030-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carruthers B.M., van de Sande M.I., De Meirleir K.L., Klimas N.G., Broderick G., Mitchell T., Staines D., Powles A.P., Speight N., Vallings R., et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Komaki G. The reliability and factorial validity of the Japanese version of the 20-item Toronto Alexithymia Scale. J. Psychosom. Res. 2003;55:143. doi: 10.1016/S0022-3999(03)00360-X. [DOI] [PubMed] [Google Scholar]
- 94.Tait R.C., Pollard C.A., Margolis R.B., Duckro P.N., Krause S.J. The Pain Disability Index: Psychometric and validity data. Arch. Phys. Med. Rehabil. 1987;68:438–441. [PubMed] [Google Scholar]
- 95.Wicksell R.K., Renöfält J., Olsson G.L., Bond F.W., Melin L. Avoidance and cognitive fusion–central components in pain related disability? Development and preliminary validation of the Psychological Inflexibility in Pain Scale (PIPS) Eur. J. Pain. 2008;12:491–500. doi: 10.1016/j.ejpain.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 96.Smets E., Garssen B., Bonke B., De Haes J. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-O. [DOI] [PubMed] [Google Scholar]
- 97.Rabin R., Charro F.D. EQ-SD: A measure of health status from the EuroQol Group. Ann. Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 98.Tanaka M., Fukuda S., Mizuno K., Imai-Matsumura K., Jodoi T., Kawatani J., Takano M., Miike T., Tomoda A., Watanabe Y. Reliability and validity of the Japanese version of the Chalder Fatigue Scale among youth in Japan. Psychol. Rep. 2008;103:682–690. doi: 10.2466/pr0.103.3.682-690. [DOI] [PubMed] [Google Scholar]
- 99.Jackson C. The Chalder fatigue scale (CFQ 11) Occup. Med. 2015;65:86. doi: 10.1093/occmed/kqu168. [DOI] [PubMed] [Google Scholar]
- 100.Hornig M., Montoya J.G., Klimas N.G., Levine S., Felsenstein D., Bateman L., Peterson D.L., Gottschalk C.G., Schultz A.F., Che X., et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Landi A., Broadhurst D., Vernon S.D., Tyrrell D.L.J., Houghton M. Reductions in circulating levels of IL-16, IL-7 and VEGF-A in myalgic encephalomyelitis/chronic fatigue syndrome. Cytokine. 2016;78:27–36. doi: 10.1016/j.cyto.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 102.Maes M., Mihaylova I., Kubera M., Leunis J.C., Twisk F.N.M., Geffard M. IgM-mediated autoimmune responses directed against anchorage epitopes are greater in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) than in major depression. Metab. Brain Dis. 2012;27:415–423. doi: 10.1007/s11011-012-9316-8. [DOI] [PubMed] [Google Scholar]
- 103.Strayer D.R., Victoria S., William C. Low NK Cell Activity in Chronic Fatigue Syndrome (CFS) and Relationship to Symptom Severity. J. Clin. Cell. Immunol. 2015;1 doi: 10.4172/2155-9899.1000348. [DOI] [Google Scholar]
- 104.Papadopoulos A.S., Cleare A.J. Hypothalamic–pituitary–adrenal axis dysfunction in chronic fatigue syndrome. Nat. Rev. Endocrinol. 2011;8 doi: 10.1038/nrendo.2011.153. [DOI] [PubMed] [Google Scholar]
- 105.Gupta A. Unconscious amygdalar fear conditioning in a subset of chronic fatigue syndrome patients. Med. Hypotheses. 2002;59:727–735. doi: 10.1016/S0306-9877(02)00321-3. [DOI] [PubMed] [Google Scholar]
- 106.Dowsett E., Goudsmit E., Macintyre A., Shepherd C. Report from the National Task Force on Chronic Fatigue Syndrome (CFS), Post Viral Fatigue Syndrome (PVFS), Myalgic Encephalo-myelitis (ME); An Initiative of the Registered Charity Westcare. Department of Health and with Financial Assistance from the Wellcome Trust; Westcare, UK: 1994. London criteria for ME; pp. 96–98. [Google Scholar]
- 107.Jason L., Evans M., Porter N., Brown M., Brown A., Hunnell J., Biotechnol A.J. The development of a revised Canadian myalgic encephalomyelitis chronic fatigue syndrome case definition. Am. J. Biochem. Biotechnol. 2010;6:120–135. doi: 10.3844/ajbbsp.2010.120.135. [DOI] [Google Scholar]