Abstract
Background and Objective: Non-motor symptoms (NMS) progress in different ways between Parkinson’s disease (PD) patients. The aim of the present study was to (1) analyze the change in global NMS burden in a PD cohort after a 2-year follow-up, (2) to compare the changes with a control group, and (3) to identify predictors of global NMS burden progression in the PD group. Material and Methods: PD patients and controls, recruited from 35 centers of Spain from the COPPADIS cohort from January 2016 to November 2017, were followed-up with after 2 years. The Non-Motor Symptoms Scale (NMSS) was administered at baseline (V0) and at 24 months ± 1 month (V2). Linear regression models were used for determining predictive factors of global NMS burden progression (NMSS total score change from V0 to V2 as dependent variable). Results: After the 2-year follow-up, the mean NMS burden (NMSS total score) significantly increased in PD patients by 18.8% (from 45.08 ± 37.62 to 53.55 ± 42.28; p < 0.0001; N = 501; 60.2% males, mean age 62.59 ± 8.91) compared to no change observed in controls (from 14.74 ± 18.72 to 14.65 ± 21.82; p = 0.428; N = 122; 49.5% males, mean age 60.99 ± 8.32) (p < 0.0001). NMSS total score at baseline (β = −0.52), change from V0 to V2 in PDSS (Parkinson’s Disease Sleep Scale) (β = −0.34), and change from V0 to V2 in NPI (Neuropsychiatric Inventory) (β = 0.25) provided the highest contributions to the model (adjusted R-squared 0.41; Durbin-Watson test = 1.865). Conclusions: Global NMS burden demonstrates short-term progression in PD patients but not in controls and identifies worsening sleep problems and neuropsychiatric symptoms as significant independent predictors of this NMS progression.
Keywords: mood, non-motor symptoms, Parkinson’s disease, progression, quality of life
1. Introduction
Parkinson’s disease (PD), the second most common neurodegenerative disease after Alzheimer’s disease, is a progressive neurodegenerative disorder causing motor and non-motor symptoms (NMS) that result in disability, loss of patient autonomy, and caregiver burden [1]. Some NMS (e.g., olfactory disorders, constipation, or sleep disturbances) may precede motor symptoms and could be useful as prodromal/preclinical markers of PD [2]. Other symptoms, such as dementia and psychosis, more frequently develop during the late stages of the disease and are sometimes difficult to manage [3]. Early identification and proper management of NMS is important because NMS are common and negatively impact a patient’s quality of life (QoL) [4]. Many cross-sectional studies have analyzed the impact of NMS burden on QoL in PD patients [5,6,7,8]. However, there is a lack of knowledge about how NMS burden progresses over time and how its progression impacts on QoL [9,10,11,12]. Thus, there are limited data from large, controlled, prospective studies on the evolution of NMS in PD [12]. Very recently, we observed that NMS progression is an important factor which impacts the change in a patient’s QoL in PD patients from the COPPADIS cohort after a 2-year follow-up [13]. However, not all PD patients progress in the same way with regard to NMS. Identification of predictive factors for global NMS burden progression is necessary to be able to propose specific interventions (e.g., medication, other therapy, preventive action, etc.). In other words, avoiding or at least reducing the progression of NMS perceived by the patient could help avoid a deterioration in his/her QoL perception.
The aim of the present study was to (1) analyze the change in global NMS burden, and in some NMS in particular, in PD patients from the COPPADIS cohort after a 2-year follow-up, (2) to compare the changes with a control group, and (3) to identify predictors of global NMS burden progression in the PD group.
2. Methods
PD patients and controls, who were recruited from January 2016 to November 2017 from 35 centers of Spain from the COPPADIS cohort [14] and were evaluated again at 2-year follow-up, were included in the study. Methodology about COPPADIS-2015 study can be consulted at https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-016-0548-9 (25 February 2016) [15]. This is a multi-center, observational, longitudinal-prospective, 5-year follow-up study designed to analyze disease progression in a Spanish population of PD patients. All patients included were diagnosed according to UK PD Brain Bank criteria. Exclusion criteria were: atypical parkinsonism, dementia (Mini Mental State Examination (MMSE) <26), age <18 or >75 years, inability to read or understand the questionnaires, to receive any advanced therapy (continuous infusion of levodopa or apomorphine, and/or with deep brain stimulation at baseline), and the presence of comorbidity, sequelae, or any disorder that could interfere with the assessment.
Information on sociodemographic aspects, factors related to PD, comorbidity, and treatment was collected. V0 (baseline visit) and V2 (2 years ± 1 month) evaluations included motor assessment (Hoenh & Yahr [H&Y], Unified Parkinson’s Disease Rating Scale (UPDRS) part III and part IV, Freezing of Gait Questionnaire (FOGQ)), non-motor symptoms (Non-Motor Symptoms Scale (NMSS), Parkinson’s Disease Sleep Scale (PDSS), Visual Analog Scale-Pain (VAS-Pain), Visual Analog Fatigue Scale (VAFS)), cognition (MMSE, Parkinson’s Disease Cognitive Rating Scale (PD-CRS), completing a simple 16-piece puzzle), mood and neuropsychiatric symptoms (Beck Depression Inventory-II (BDI-II), Neuropsychiatric Inventory (NPI), Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale (QUIP-RS)), disability (Schwab & England Activities of Daily Living Scale (ADLS)), and QoL (the 39-item Parkinson’s disease Questionnaire (PDQ-39), PQ-10, the EUROHIS-QOL 8-item index (EUROHIS-QOL8)) [15]. In patients with motor fluctuations, the motor assessment was made during the OFF state (without medication in the last 12 h) and during the ON state. On the other hand, the assessment was only performed without medication in patients without motor fluctuations. The same evaluation as for the patients, except for the motor assessment, was performed in control subjects at V0 and at V2 (2 years ± 1 month). Furthermore, motor and non-motor assessment (NMSS and ADLS) was conducted in PD patients at 1 year ± 1 month [15].
The NMSS [16] was used for assessing the NMS and defining the global NMS burden at V0 and at V2 in both groups, PD patients and controls. It was applied in PD patients at V1 as well. The NMSS includes 30 items, each with a different non-motor symptom. The symptoms refer to the 4 weeks prior to assessment. The total score for each item is the result of multiplying the frequency (0, never; 1, rarely; 2, often; 3, frequent; 4, very often) x severity (1, mild; 2, moderate; 3, severe) and will vary from 0 to 12 points. The scale score ranges from 0 to 360 points (NMSS total score). The items are grouped into nine different domains: (1) Cardiovascular (items 1 and 2; score, 0 to 24); (2) Sleep/fatigue (items 3, 4, 5, and 6; score, 0 to 48); (3) Depression/apathy (items 7, 8, 9, 10, 11, and 12; score, 0 to 72); (4) Perceptual problems/hallucinations (items 13, 14, and 15; score, 0 to 36); (5) Attention/memory (items 16, 17, and 18; score, 0 to 36); (6) Gastrointestinal tract (items 19, 20, and 21; score 0 to 36); (7) Urinary symptoms (items 22, 23, and 24; score, 0 to 36); (8) Sexual dysfunction (items 25 and 26; score 0 to 24); and (9) Miscellaneous (items 27, 28, 29, and 30; score, 0 to 48). Each domain of the NMSS was expressed as a percentage: (score/total score) × 100. The global NMS burden was defined as the NMS total score and it was categorized in four groups: mild (NMSS 1–20); moderate (NMSS 21–40); severe (NMSS 41–70); very severe (NMSS >70) [17].
2.1. Data Analysis
Data were processed using SPSS 20.0 (IBM, Armonk, NY, USA) for Windows. For comparisons between groups, the Student’s t-test, Mann–Whitney U test, Chi-square test, or Fisher test was used as appropriate (distribution for variables was verified by one-sample Kolmogorov–Smirnov test).
General linear model (GLM) repeated measure was used to test whether the mean differences of the NMSS total score and the NMSS domain scores between the two visits (V0 and V2) were significant in both PD patients and controls. In PD patients, it was analyzed the change between V1 and V0, V2 and V1 and between all groups as well. Age, gender and LEDD (levodopa equivalent daily dose) at V0 and at V2 were included as covariates. This test was also applied for testing the difference between V2 and V0 in other variables. Cohen’s d formula was applied for measuring the effect size (in PD patients). It was considered: small effect = 0.2; medium effect = 0.5; large effect = 0.8. The Bonferroni method was used was used as a post-hoc test after ANOVA. Spearman’s or Pearson’s correlation coefficient, as appropriate, was used for analyzing the relationship between the change from V0 to V2 in continuous variables. Correlations were considered weak for coefficient values ≤0.29, moderate for values between 0.30 and 0.59, and strong for values ≥0.60. The p-value was considered significant when it was <0.05.
Linear regression models were used for determining predictive factors of global NMS burden progression (NMSS total score change from V0 to V2 as dependent variable). Any variable with univariate associations with p-values <0.20 were included in a multivariable model, and a backwards selection process was used to remove variables individually until all remaining variables were significant at the 0.10 level [12]. The variables considered for the analysis were: (1) at baseline (V0): age, gender, disease duration, total number of non-antiparkinsonian drugs, LEDD, daily dose of levodopa, daily dose of dopamine agonist, motor phenotype [18], UPDRS-III (OFF), UPDRS-IV, FOGQ, PD-CRS, NMSS, PDSS, NPI, QUIP-RS, VAS-PAIN, VASF-Physical, and VASF-Mental; (2) at 2-year follow-up (V2): to be receiving L-dopa, a MAO-B (monoamine oxidase type B) inhibitor, a COMT (catechol-o-methyl transferase) inhibitor, a dopamine agonist, and to practice regular exercise; (3) at the end of the follow-up (V2): change from V0 to V2 in total number of non-antiparkinsonian drugs, LEDD, daily dose of levodopa, daily dose of dopamine agonist, UPDRS-III, UPDRS-IV, FOGQ, PD-CRS, PDSS, NPI, QUIP-RS, VAS-PAIN, VASF-Physical, and VASF-Mental. Tolerance and variance inflation factor (VIF) were used to detect multicollinearity (multicollinearity was considered problematic when tolerance was less than 0.2 and, simultaneously, the value of VIF 10 and above). Finally, according to the change in the NMSS total score, patients were classified as “NMS—improver” (NMSS total score at V2 < NMSS total score at V0) or “non NMS improver” (NMSS total score at V2 ≥ NMSS total score at V0) and predictors of NMS improvement were identified. A level of p = 0.01 was considered for determining significant variables in the model.
2.2. Standard Protocol Approvals, Registrations, and Patient Consents
For this study, we received approval from the Comité de Ética de la Investigación Clínica de Galicia from Spain (2014/534; 2/12/2014). Written informed consent from all participants in this study were obtained before the start of the study. COPPADIS-2015 was classified by the AEMPS (Agencia Española del Medicamento y Productos Sanitarios) as a Post-authorization Prospective Follow-up study with the code COH-PAK-2014-01.
3. Results
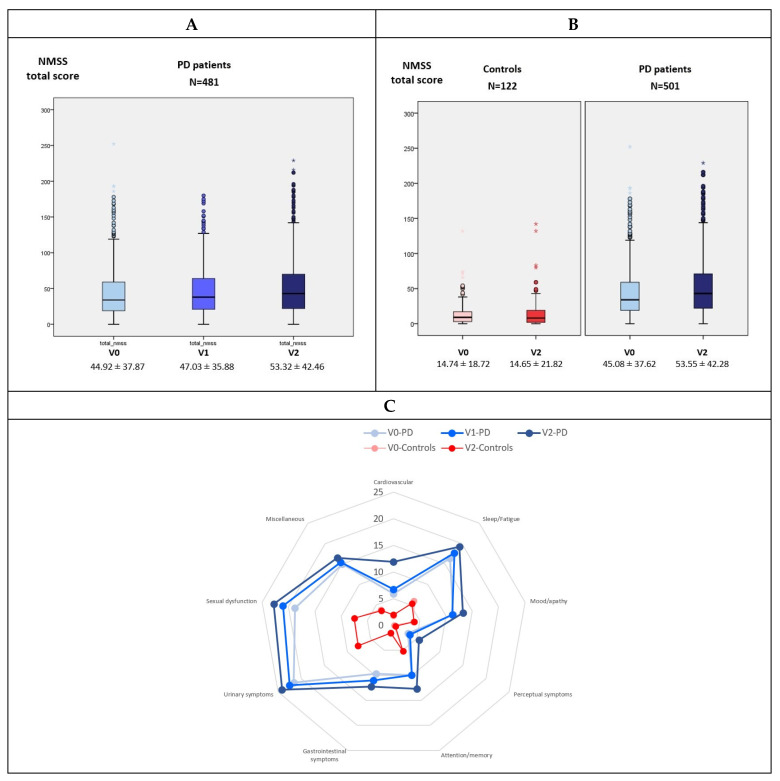
After the 2-year follow-up, the mean global NMS burden (NMSS total score) significantly increased in PD patients by 18.8% (from 45.08 ± 37.62 to 53.55 ± 42.28; p < 0.0001; N = 501; 60.2% males, mean age 62.59 ± 8.91; mean disease duration 5.5 ± 4.37 years) compared to no change observed in controls (from 14.74 ± 18.72 to 14.65 ± 21.82; p = 0.944; N = 122; 49.5% males, mean age 60.99 ± 8.32) (difference between groups, p = 0.002) (Table 1 and Figure 1B). Moreover, the increase in the mean NMSS total score from V0 to V1 and from V1 to V2 was significant as well (p < 0.0001; Figure 1A). By domains, the score of all the NMSS domains at V2 was significantly higher than at V0 in PD patients except domain 9 (Miscellaneous) but none in the control group (Table 1). In PD patients, the highest increase from V0 to V2 was for domain 1 (cardiovascular symptoms) with a 117.7% (Cohen’s effect size = 0.644), and the lowest was for domain 9 (miscellaneous) with a 10.7% (Figure 1C). With regard to other NMS, a significant change in the score of the PD-CRS (from 92 ± 15.65 to 90.26 ± 18.07; p < 0.0001; N = 500), VAS-Pain (from 2.61 ± 2.92 to 2.96 ± 2.88; p = 0.037; N = 501), and VAFS-Physical (from 2.86 ± 2.67 to 3.17 ± 2.8; p = 0.025; N = 501) was also observed in the PD group but not in controls (Table 1).
Table 1.
Changes in motor and non-motor symptoms, disability, and quality of life in PD patients and/or controls from V0 (baseline) to V2 (2 years ± 1 month).
| PD Patients V0 |
PD Patients V2 |
Cohen’s Test | p a | Controls V0 |
Controls V2 |
p b | p c | p d | |
|---|---|---|---|---|---|---|---|---|---|
| Hoehn & Yahr (OFF) (%) | 0.422 | <0.0001 | N.A. | N.A. | N.A. | N.A. | N.A. | ||
| Stage 1 | 22.7 | 13.3 | |||||||
| Stage 2 | 68 | 77 | |||||||
| Stage 3–5 | 9.3 | 9.7 | |||||||
| UPDRS-III (OFF) | 21.92 ± 10.53 | 25.26 ± 12.19 | 0.452 | <0.0001 | N.A. | N.A. | N.A. | N.A. | N.A. |
| UPDRS-IV | 1.99 ± 2.41 | 2.65 ± 2.75 | 0.371 | <0.0001 | N.A. | N.A. | N.A. | N.A. | N.A. |
| FOGQ | 3.76 ± 4.69 | 4.94 ± 5.18 | 0.4 | <0.0001 | N.A. | N.A. | N.A. | N.A. | N.A. |
| Daily dose L-dopa (mg) | 577.48 ± 412.09 | 767.56 ± 307.1 | 0.812 | <0.0001 | N.A. | N.A. | N.A. | N.A. | N.A. |
| Number of non-antipark. drugs | 2.35 ± 2.38 | 3.08 ± 2.65 | 0.507 | <0.0001 | 2.04 ± 2.16 | 2.76 ± 2.35 | 0.005 | ||
| PD-CRS | 92 ± 15.65 | 90.26 ± 18.07 | −0.207 | <0.0001 | 99.65 ± 13.56 | 99.68 ± 13.73 | 0.368 | 0.517 | 0.018 |
| NMSS | 45.08 ± 37.62 | 53.55 ± 42.28 | 0.343 | <0.0001 | 14.74 ± 18.72 | 14.65 ± 21.82 | 0.944 | 0.784 | 0.002 |
| Cardiovascular | 5.45 ± 9.79 | 11.87 ± 14.21 | 0.644 | <0.0001 | 1.78 ± 3.6 | 1.99 ± 3.91 | 0.929 | 0.151 | 0.004 |
| Sleep/fatigue | 16.28 ± 15.91 | 19.2 ± 17.52 | 0.241 | <0.0001 | 5.91 ± 7.98 | 5.32 ± 9.87 | 0.813 | 0.243 | 0.018 |
| Mood/apathy | 11.32 ± 16.07 | 13.21 ± 17.98 | 0.173 | 0.007 | 3.8 ± 8.85 | 3.93 ± 11.53 | 0.219 | 0.438 | 0.249 |
| Perceptual symptoms | 3.24 ± 9.24 | 5.51 ± 12.71 | 0.286 | <0.0001 | 0.13 ± 1.12 | 0.39 ± 2.41 | 0.352 | 0.643 | 0.316 |
| Attention/memory | 10.01 ± 14.5 | 12.69 ± 17.08 | 0.249 | <0.0001 | 5.13 ± 10.85 | 5.18 ± 11.25 | 0.567 | 0.747 | 0.321 |
| Gastrointestinal symptoms | 9.59 ± 13.1 | 12.28 ± 14.35 | 0.3 | <0.0001 | 1.58 ± 5.23 | 1.53 ± 4.39 | 0.385 | 0.414 | 0.001 |
| Urinary symptos | 21.47 ± 22.21 | 24.09 ± 23.14 | 0.181 | 0.013 | 7.63 ±12.57 | 7.71 ±11.74 | 0.851 | 0.982 | 0.012 |
| Sexual dysfunction | 18.83 ± 25.53 | 22.67 ± 27.84 | 1.182 | 0.005 | 7.43 ± 16.54 | 7.5 ± 14.68 | 0.903 | 0.714 | 0.002 |
| Miscellaneous | 14.98 ± 15.41 | 16.59 ± 15.69 | 0.149 | 0.054 | 3.73 ± 8.75 | 3.54 ± 8.31 | 0.633 | 0.698 | 0.004 |
| BDI-II | 8.28 ± 6.9 | 8.54 ± 7.48 | 0.048 | 0.369 | 4.56 ± 5.46 | 4.31 ± 5.5 | 0.484 | 0.541 | 0.043 |
| PDSS | 117.13 ± 24.48 | 117.85 ± 24.98 | 0.038 | 0.452 | 131.26 ± 17.41 | 126.67 ± 26.46 | 0.796 | 0.737 | 0.677 |
| QUIP-RS | 4.6 ± 8.8 | 4.66 ± 9.22 | 0.007 | 0.798 | 1.51 ± 3.73 | 1.32 ± 3.37 | 0.284 | 0.334 | 0.222 |
| NPI | 5.82 ± 7.88 | 6.17 ± 9.39 | 0.056 | 0.491 | 3.31 ± 7.15 | 2.64 ± 7.67 | 0.786 | 0.714 | 0.486 |
| VAS-PAIN | 2.61 ± 2.92 | 2.96 ± 2.88 | 0.147 | 0.037 | 1.49 ± 2.41 | 1.70 ± 2.32 | 0.272 | 0.661 | 0.177 |
| VASF-physical | 2.86 ± 2.67 | 3.17 ± 2.8 | 0.147 | 0.025 | 1.52 ± 2.35 | 1.29 ± 2.12 | 0.78 | 0.488 | 0.198 |
| VASF-mental | 2.09 ± 2.51 | 2.20 ± 2.61 | 0.056 | 0.545 | 1.29 ± 2.09 | 1.03 ± 1.97 | 0.699 | 0.96 | 0.254 |
| ADLSL | 88.58 ± 10.19 | 84.26 ± 13.38 | −0.519 | <0.0001 | 98.87 ± 6.65 | 99.52 ± 2.15 | 0.227 | 0.069 | <0.0001 |
| PDQ-39SI | 16.72 ± 13.02 | 20.3 ± 16.41 | 0.413 | <0.0001 | N.A. | N.A. | N.A. | N.A. | N.A. |
| PQ-10 | 3.77 ± 0.54 | 3.75 ± 0.58 | −0.114 | 0.063 | 8.07 ± 1.22 | 7.86 ± 1.65 | 0.06 | 0.486 | 0.831 |
| EUROHIS-QOL8 | 3.8 ± 0.7 | 3.68 ± 0.67 | 0.048 | 0.4 | 4.18 ± 0.5 | 4.12 ± 0.51 | 0.066 | 0.852 | 0.074 |
p values were computed using general linear models (GLM) repeated measures. The results represent mean ± SD or %; p a, change over time (V2 vs. V0) in PD patients; p b, change over time (V2 vs. V0) in controls. Age, gender and LEDD (levodopa equivalent daily dose) (except for assessing changes in this variable) at V0 and at V2 were included as covariates; p c, group visit interaction; p d, PD vs. controls. PD vs. controls is not applicable if test of interaction was significant (a significant test of interaction means the rates of changes over time are different between the two groups). All patients with the data at V0 and V2 were included for each comparative analysis. For NMSS and its domains, N = 501 in PD patients and N = 122 in the control group. ADLS, Schwab & England Activities of Daily Living Scale; BDI-II, Beck Depression Inventory-II; FOGQ, Freezing Of Gait Questionnaire; NMSS, Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDSS, Parkinson’s Disease Sleep Scale; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; VAFS, Visual Analog Fatigue Scale; VAS-Pain, Visual Analog Scale-Pain.
Figure 1.
(A) Mean NMSS total score at V0 (baseline), V1 (1 year follow-up ± 1 month), and V2 (2-year follow-up ± 1 month) in PD patients (N = 481). V1 vs. V0, p < 0.0001; V2 vs. V1, p < 0.0001; V2 vs. V0, p < 0.0001; All groups; p < 0.0001. (B) Change from V0 to V2 in the mean NMSS total score in PD patients (N = 501) and controls (N = 122). V2 vs. V0 in controls, p = 0.944; V2 vs. V0 in PD patients, p < 0.0001; difference in the change from V0 to V2 between PD patients and controls, p = 0.002. (C) Mean score on each domain of the NMSS scale at V0, V1, and V2 in PD patients (blue) and controls (red). Data (A and B) are presented as box plots, with the box representing the median and the two middle quartiles (25–75%). P values were computed using general linear models (GLM) repeated measures. Mild outliers (O) are data points that are more extreme than Q1 − 1.5 × IQR or Q3 + 1.5 × IQR. NMSS, Non-Motor Symptoms Scale.
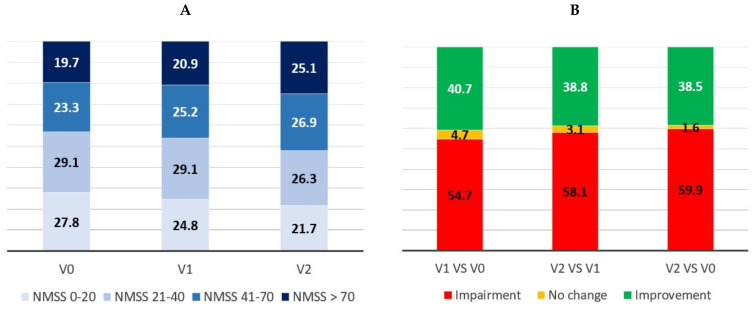
In 201/501 patients (40.1%) a score at V2 equal to or less than the baseline NMSS total score was observed compared to in 73/122 controls (59.8%) (p < 0.0001). Specifically, 38.5%, 1.6%, and 59.9% of the patients presented improvement (NMSS at V2 < NMSS at V0), no change (NMSS at V2 = NMSS at V0), and impairment (NMSS at V2 > NMSS at V0), respectively, with regard to the change in the NMSB from V0 to V2 (Figure 2B). A significant change in the percentage of patients with mild, moderate, severe, or very severe burden from V0 to V1 (p = 0.016; N = 600) and from V1 to V2 (p = 0.007; N = 482) was observed in the PD group (when all groups were considered, p < 0.0001 [N = 482]) (Figure 2A).
Figure 2.
(A) Percentage of PD patients with mild (NMSS 1–20), moderate (NMSS 21–40), severe (NMSS 41–70), and very severe (NMSS >70) NMS burden at V0 (baseline), V1 (1 year follow-up ± 1 month), and V2 (2-year follow-up ± 1 month). V1 vs. V0, p < 0.016 (N = 600); V2 vs. V1, p = 0.007 (N = 482), All groups; p < 0.0001 (N = 481). (B) Percentage of patients with a greater NMS burden (NMS burden impairment; in red), no change (No changes in the NMSS total score), and a lower NMS burden (NMS burden improvement; in green) at V1 compared to V0, V2 compared to V1, and V2 compared to V0. NMSS, Non-Motor Symptoms Scale.
A moderate correlation was observed between the change in the NMSS total score from V0 to V2 and the change in the BDI-II (r = 0.35; p < 0.0001), PDSS (r = −0.34; p < 0.0001), NPI (r = 0.31; p < 0.0001), and PDQ-39SI (r = 0.42; p < 0.0001) scores over the same period (Table 2). When the change in the score of the domains of the NMSS was considered, moderate correlations were observed between the changes from V0 to V2 in domain 1 (cardiovascular symptoms) and ADLS (r = −0.35; p < 0.0001), domain 2 and PDSS (r = −0.34; p < 0.0001) and PDQ-39SI (r = 0.33; p < 0.0001), and domain 3 and BDI-II (r = 0.33; p < 0.0001), VAS-PAIN (r = 0.41; p < 0.0001), and PDQ-39SI (r = 0.35; p < 0.0001) (Table 2). Correlation between the change from V0 to V2 in NMS (NMSS total score and score domains) and LEDD, daily dose of levodopa, and daily dose of dopamine agonist was negligible.
Table 2.
Correlations between the changes in non-motor symptoms (NMSS total score and NMSS domains) and other disease-related variables in PD patients from V0 (baseline) to V2 (2 years ± 1 month).
| NMSS TS |
NMSS D1 |
NMSS D2 |
NMSS D3 |
NMSS D4 |
NMSS D5 |
NMSS D6 |
NMSS D7 |
NMSS D8 |
NMSS D9 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Age at baseline | −0.06 | 0.12 *** | −0.03 | −0.01 | 0.04 | 0.04 | −0.04 | −0.08 | −0.03 | −0.04 |
| Disease duration (at V0) | 0.10 *** | 0.11 *** | 0.02 | 0.07 | 0.06 | 0.04 | 0.02 | 0.06 | 0.12 *** | 0.05 |
| N. of non-antipark. drugs (at V0) | −0.05 | 0.12 *** | −0.03 | −0.03 | 0 | −0.02 | −0.03 | −0.06 | −0.02 | −0.07 |
| Change at V2 (from V0 to V2) | ||||||||||
| LEDD | 0.02 | −0.02 | 0.03 | 0.02 | 0.02 | 0.01 | 0.08 | 0.04 | −0.01 | 0 |
| Daily dose L-dopa (mg) | 0.01 | −0.05 | 0.01 | 0.03 | −0.02 | 0.02 | 0.05 | 0.01 | −0.03 | 0.04 |
| Equivalent daily dose of DA (mg) | −0.04 | 0.02 | 0.02 | −0.1 | 0.02 | −0.09 *** | 0.05 | −0.04 | −0.06 | −0.05 |
| Number of non-antipark. drugs | 0.06 | 0.07 | −0.02 | 0 | 0.09 *** | 0.08 | 0.03 | 0.07 | 0 | 0.04 |
| UPDRS-III (OFF) | 0.16 * | 0.07 | 0.12 *** | 0.16 ** | 0.07 | 0.11 *** | 0.07 | 0.14 *** | 0 | 0.15 *** |
| UPDRS-IV | 0.10 *** | 0.02 | 0.12 *** | 0.09 | 0.06 | 0 | 0.06 | 0.11 *** | 0 | 0.04 |
| FOGQ | 0.21 * | 0.11 *** | 0.19 * | 0.13 ** | 0.09 *** | 0.13 *** | 0.12 *** | 0.14 *** | 0.10 *** | 0.11 *** |
| PD-CRS | 0.01 | −0.05 | −0.04 | −0.01 | −0.05 | −0.11 *** | 0.02 | −0.03 | 0.15 | 0.03 |
| BDI-II | 0.35 * | 0.17 * | 0.29 * | 0.33 * | 0.19 * | 0.22 * | 0.09 *** | 0.15 ** | 0.13 *** | 0.14 *** |
| PDSS | −0.34 * | −0.04 | −0.34 * | −0.23 | −014 ** | −0.15 ** | −0.17* | −0.24 * | −0.12 *** | −0.17 * |
| QUIP-RS | 0.10 *** | 0 | 0.06 | −0.03 | 0.17 * | 0.05 | −0.02 | 0.11 *** | 0.03 | 0.05 |
| NPI | 0.31 * | 0.12 *** | 0.19 * | −0.03 | 0.21 * | 0.18 * | 0.12 *** | 0.12 *** | 0.14 *** | 0.06 |
| VAS-PAIN | 0.12 *** | 0.09 *** | 0.09 *** | 0.41 * | 0.06 | 0.01 | 0.07 | 0.02 | 0.11 *** | 0.12 *** |
| VASF-physical | 0.21 * | 0.01 | 0.10 *** | 0.11 *** | 0.07 | 0.08 | 0.13 *** | 0.08 | 0.09 | 0.14 *** |
| VASF-mental | 0.21 * | 0 | 0.15 * | 0.18 * | 0.07 | 0.11 | 0.18 * | 0.09 | 0.07 | 0.10 *** |
| PDQ-39SI | 0.42 * | 0.09 *** | 0.33 * | 0.35 * | 0.15 ** | 0.23 * | 0.20 * | 0.19 *** | 0.12 *** | 0.17 * |
| PQ-10 | −0.17 * | −0.06 | −0.15 ** | −0.17 * | −0.11 *** | −0.11 *** | −0.07 | −0.07 | −0.01 | −0.08 |
| EUROHIS-QOL8 | −0.20 * | −0.03 | −0.25 * | −0.09 *** | −0.11 *** | −0.12 *** | −0.05 | −013 *** | −0.04 | −0.09 |
| ADLS | −0.24 * | −0.35 * | −0.19 * | −0.19 | −0.15 ** | −0.18 * | −0.17 | −0.15 ** | −0.17 * | −0.10 *** |
Spearman correlation test was applied. *, p < 0.0001; **, p < 0.001; ***, p < 0.05. In bold are expressed significant values. NMSS: TS, total score. D1, Cardiovascular (items 1 and 2; score, 0 to 24); D2, Sleep/fatigue (items 3, 4, 5, and 6; score, 0 to 48); D3, Depression/apathy (items 7, 8, 9, 10, 11, and 12; score, 0 to 72); D4, Perceptual problems/hallucinations (items 13, 14, and 15; score, 0 to 36); D5, Attention/memory (items 16, 17, and 18; score, 0 to 36); D6, Gastrointestinal tract (items 19, 20, and 21; score 0 to 36); D7, Urinary symptoms (items 22, 23, and 24; score, 0 to 36); D8, Sexual dysfunction (items 25 and 26; score 0 to 24); D9, Miscellaneous (items 27, 28, 29, and 30; score, 0 to 48). ADLS, Schwab & England Activities of Daily Living Scale; BDI-II, Beck Depression Inventory-II; DA, dopamine agonist; FOGQ, Freezing Of Gait Questionnaire; LEDD, levodopa equivalent dauly dose; N, number; NMSS, Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDSS, Parkinson’s Disease Sleep Scale; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; TS, total score; UPDRS, Unified Parkinson’s Disease Rating Scale; VAFS, Visual Analog Fatigue Scale; VAS-Pain, Visual Analog Scale-Pain.
In the multivariate analysis, a greater increase in the NMSS total score from V0 to V2 was associated with longer disease duration (p = 0.006), a higher NPI total score (indicative of more severe neuropsychiatric symptoms) (p < 0.0001) and a lower total score on the NMSS (p < 0.0001) and PDSS (indicative of a worse sleep quality) (p = 0.001) at baseline, a greater decrease in the PDSS total score from V0 to V2 (indicative of a greater sleep quality impairment) (p < 0.0001), a greater increase in the FOGQ from V0 to V2 (indicative of a more worsening in gait problems) (p = 0.001) and in the NPI total score from V0 to V2 (indicative of a more impairment in neuropsychiatric symptoms) (p < 0.0001), and to be taking an antipsychotic agent at V2 (p = 0.003) (Table 3). NMSS total score at baseline (β = −0.52), change from V0 to V2 in PDSS (β = −0.34), and change from V0 to V2 in NPI (β = 0.25) provided the highest contributions to the model (adjusted R-squared 0.41; Durbin-Watson test = 1.865) (Table 3). In the final model, tolerance was from 0.61 to 0.94 and VIF from 1.05 to 1.72.
Table 3.
Linear regression model about factors associated with global burden progression after 2-year follow-up (change in the NMSS total score from V0 to V2).
| β a | β b | 95% IC a | 95% IC b | p a | p b | |
|---|---|---|---|---|---|---|
| At V0 (baseline) | ||||||
| Age at baseline | −0.036 | N.A. | −0.501–0.211 | N.A. | 0.423 | N.A. |
| Gender | −0.045 | N.A. | −9.419–3.024 | N.A. | 0.313 | N.A. |
| Disease duration | 0.092 | 0.118 | 0.019–1.499 | 0.261–1.591 | 0.044 | 0.006 |
| Number of non-antipark. drugs | −0.041 | N.A. | −1.862–0.683 | N.A. | 0.363 | N.A. |
| LEDD | 0.062 | N.A. | −0.002–0.012 | N.A. | 0.17 | N.A. |
| Daily dose L-dopa (mg) | 0.077 | 0.152 | −0.001–0.019 | N.A. | 0.085 | N.A. |
| Equivalent Daily dose of DA (mg) | 0.08 | N.A. | −0.002–0.038 | N.A. | 0.075 | N.A. |
| No-tremoric motor phenotype | 0.062 | 0.094 | −1.788–10.373 | 0.675–12.195 | 0.166 | 0.029 |
| UPDRS-III | 0.026 | N.A. | −0.217–0.387 | N.A. | 0.58 | N.A. |
| UPDRS-IV | 0.015 | N.A. | −1.047–1.473 | N.A. | 0.74 | N.A. |
| FOGQ | −0.023 | N.A. | −0.823–0.482 | N.A. | 0.608 | N.A. |
| PD-CRS | −0.03 | N.A. | −0.262–0.129 | N.A. | 0.504 | N.A. |
| NMSS | −0.317 | −0.52 | −0.369–0.215 | −0.557–−0.370 | <0.0001 | <0.0001 |
| BDI-II | −0.174 | N.A. | −0.872 | N.A. | <0.0001 | N.A. |
| PDSS | 0.077 | −0.186 | −0.015–0.234 | −0.397–−0.106 | 0.085 | 0.001 |
| NPI | −0.12 | 0.208 | −0.912–0.107 | 0.441–1.353 | 0.013 | <0.0001 |
| QUIP-RS | −0.005 | N.A. | −0.310–0.271 | N.A. | 0.896 | N.A. |
| VAS-PAIN | 0.032 | N.A. | −0.62–0.148 | N.A. | 0.032 | N.A. |
| VASF-Physical | −0.021 | N.A. | −1.181– -0.684 | N.A. | 0.601 | N.A. |
| VASF-Mental | −0.015 | N.A. | −1.206–0.832 | N.A. | 0.719 | N.A. |
| Change at V2 (from V0 to V2) | ||||||
| Number of non-antipark. drugs | 0.087 | N.A. | −0.040–3.925 | N.A. | 0.055 | N.A. |
| LEDD | 0.072 | N.A. | −0.002–0.017 | N.A. | 0.115 | N.A. |
| Daily dose L-dopa (mg) | 0.044 | N.A. | −0.007–0.019 | N.A. | 0.339 | N.A. |
| Equivalent daily dose of DA (mg) | 0.009 | N.A. | −0.013–0.016 | N.A. | 0.843 | N.A. |
| UPDRS-III (OFF) | 0.178 | N.A. | 0.296–0.938 | N.A. | <0.0001 | N.A. |
| UPDRS-IV | 0.086 | N.A. | −0.041–2.434 | N.A. | 0.058 | N.A. |
| FOGQ | 0.284 | 0.149 | 1.658–3.074 | 0.522–1.922 | <0.0001 | 0.001 |
| PD-CRS | −0.026 | N.A | −0.339–0.186 | N.A. | 0.567 | N.A. |
| BDI-II | 0.334 | N.A. | 1.120–1.862 | N.A. | <0.0001 | N.A. |
| PDSS | −0.303 | −0.339 | −0.222 | −0.543–−0.292 | <0.0001 | <0.0001 |
| NPI | 0.307 | 0.249 | 0.798–1.521 | 0.595–1.296 | <0.0001 | <0.0001 |
| QUIP-RS | 0.095 | N.A. | 0.038–0.612 | N.A. | 0.027 | N.A. |
| VAS-PAIN | 0.156 | N.A. | 0.761–2.302 | N.A. | <0.0001 | N.A. |
| VASF-Physical | 0.192 | 0.101 | 1.258–2.973 | 0.179–2.061 | <0.0001 | 0.02 |
| VASF-Mental | 0.194 | N.A. | 1.282–3.010 | N.A. | <0.0001 | N.A. |
| At V2 | ||||||
| To be taking L-dopa | 0.025 | N.A. | −7.129–12.723 | N.A. | 0.58 | N.A. |
| To be taking a MAO-B inhibitor | 0.03 | N.A. | −4.722–9.684 | N.A. | 0.499 | N.A. |
| To be taking a COMT inhibitor | 0.003 | N.A. | −6.564–6.987 | N.A. | 0.951 | N.A. |
| To be receiving a DA | 0.067 | N.A. | −1.651–12.057 | N.A. | 0.136 | N.A. |
| To be taking an analgecic | −0.074 | N.A. | −11.116–0.388 | N.A. | 0.068 | N.A. |
| To be taking a benzodiazepin | 0.025 | N.A. | −4.350–8.277 | N.A. | 0.542 | N.A. |
| To be taking an antidepressive agent | 0.051 | N.A. | −2.049–9.514 | N.A. | 0.205 | N.A. |
| To be taking an antipsychotic agent | 0.067 | 0.129 | 6.067–29.381 | −3.084–22.227 | 0.138 | 0.003 |
| To practice regular exercise | 0.011 | N.A. | −6.668–8.440 | N.A. | 0.818 | N.A. |
| Cognitive stimulation therapy | −0.017 | N.A. | −7.935–5.396 | N.A. | 0.708 | N.A. |
| Physiotherapy | 0.016 | N.A. | −5.661–8.179 | N.A. | 0.721 | N.A. |
| Speech therapy | −0.008 | N.A. | −10.296–8.548 | N.A. | 0.885 | N.A. |
Dependent variable: change from V0 to V2 in the NMSS total score. β standardized coefficient and 95% IC are shown. a, univariate analysis; b, multivariate analysis (Durbin-Watson test = 1.865; R2 = 0.41). BDI-II, Beck Depression Inventory-II; COMT, catechol-o-methyl transferase; DA, dopamine agonist; FOG, freezing of gait; FOGQ, Freezing Of Gait Questionnaire; LEDD, levodopa equivalent daily dose (mg); MAO-B, monoamine oxidase type B; N.A., not applicable; NMS, non-motor symptoms; NMSS, Non-Motor Symptoms Scale; NPI, Neuropsychiatric Inventory; PD, Parkinson’s disease; PD-CRS, Parkinson’s Disease Cognitive Rating Scale; PDQ-39SI, 39-item Parkinson’s Disease Quality of Life Questionnaire Summary Index; PDSS, Parkinson’s Disease Sleep Scale; QoL, Quality of life; QUIP-RS, Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating Scale; UPDRS, Unified Parkinson’s Disease Rating Scale; VAFS, Visual Analog Fatigue Scale; VAS-Pain, Visual Analog Scale-Pain.
When change from V0 to V2 in the NMSS total score was considered as a binary variable (to be an NMS—improver as dependent variable), a lower dopamine agonist equivalent dose (OR = 0.998; 95%CI, 0.996–0.999; p = 0.013) and NPI total score (OR = 0.926; 95%CI, 0.884–0.970; p = 0.001) at V0, a higher NMSS total score (OR = 1.028; 95%CI, 1.018–1.039; p < 0.0001) at V0, and a greater decrease in the NPI total score (OR = 0.902; 95%CI, 0.864–0.943; p < 0.0001) and VASF-Physical (OR = 0.901; 95%CI, 0.831–0.977; p < 0.0001) from V0 to V2 were independent predictors of NMS improvement at 2-year follow-up (adjusted R-squared 0.27; Hosmer–Lemeshow test = 0.160). In NMS—improvers, the mean NPI and VASF-Physical total scores decreased from V0 to V2 in 2.34 ± 8.55 and of 0.39 ± 3.03 points, respectively, compared to an increase in 2.17 ± 8.57 and 0.74 ± 2.88 in non NMS improvers, respectively (p < 0.0001 for both analysis). The motor phenotype was not associated with NMS outcome, being NMS—improvers 104 out of 262 (39.7%) PD patients with a tremor-dominant motor phenotype vs. 89 out of 230 (37.2%) patients with a PIGD/indeterminate motor phenotype (p = 0.573).
4. Discussion
The present study observes that global NMS burden (defined as NMSS total score) demonstrates short-term progression in PD patients and identifies some factors associated with this progression. Specifically, worsening gait problems (FOGQ), sleep symptoms (PDSS), and neuropsychiatric symptoms (NPI) was associated with an increase in global NMS burden at 2-year follow-up after adjustment to baseline status. On the contrary, a progression in NMS burden in the control group was not observed. Awareness of the progression of these symptoms in clinical practice, with the aim to introduce interventions to reduce NMS burden progression when possible, could be important. As example, bilateral subthalamic stimulation (STN-DBS) improved significantly the global SNMS burden and QoL in advanced PD patients after 36 months but not in patients who received the standard-of-care medical therapy [19]. Since global NMS burden impacts the patient’s QoL [8] and its progression worsens QoL [13], understanding the role global NMS plays in PD is very relevant.
Although NMS are common and their importance has been increasing during the last years in PD [20], very little is known about the progression of NMS in PD patients, partially because there is a lack of longitudinal studies [12]. To our knowledge, our study represents the largest observational study about the longitudinal evolution of NMS in PD patients compared with non-PD controls in which a very extensive assessment and analysis have been conducted. Aligning with previous studies, we observed a significant increase in NMS burden over 2 years in PD patients compared with controls [9,10,11,12]. Erro et al. reported 2-year and 4-year longitudinal data about the baseline prevalence and longitudinal evolution of NMS in a cohort of PD patients, but the sample was small (N = 91 for 2-year and N = 61 for 4-year follow-up), a control group was not included, and a qualitative scale (NMSQ (Non-Motor Symptoms Questionnaire)) was used instead of a quantitative one [9,10]. As we observed, they found no association between NMS progression and motor disability as measured by the UPDRS-III and either drug class (i.e., L-dopa, DA, etc.) or total LEDD. In our study, the only motor feature related with NMS burden progression was gait problems. Previous studies have reported that gait problems including freezing of gait (FOG) are associated with higher NMSS total score and some NMS in particular such as cognitive impairment or anxiety [21,22,23]. Further, discrete gait impairment progresses in PD over time [24], as we noted here. In practice, it is important to keep in mind that patients with PD who develop an impairment in gait could progress more in their global NMS burden. Using gait impairment as a simple clinical biomarker of NMS progression could be helpful.
More recently, Simuni et al. [12], identified clinical and biological variables associated with NMS progression in 380 PD patients from the PPMI (Parkinson’s Progression Markers Initiative) and compared them with a control group (174 health control subjects) [12]. Although they used the MDS-UPDRS (Movement Disorder Society-Unified Parkinson’s Disease Rating Scale) Part I [25], a strong convergent validity was shown with the NMSS [26]. They observed a significant worsening in global cognition, depression, autonomic dysfunction, and impulse control disorders in PD subjects, while controls worsened only in cognition and trait anxiety. In our cohort, the mean score of all domains of the NMSS except domain 9 (miscellaneous) increased significantly in patients but not in controls, although a significant change from baseline to 2-year assessment was not observed in the rest of scales except for the PD-CRS and VAS for pain and physical fatigue. In general, these results align with a majority of previous reports [27,28,29] but contrary to others [30]. Antonini et al. [27] observed in 707 PD patients that sleep, gastrointestinal, attention/memory, and skin disturbances became more prevalent during a 24-month follow-up while psychiatric, cardiovascular, and respiratory disorders became less prevalent. In another study, Vu et al. [28] reported that anxiety and depression were significantly less frequent in 795 de novo PD patients, while pain, sexual difficulties, and weight change were more frequent at 2-year follow-up period. In another study, NMS severity in 117 PD patients increased after a mean follow-up visit of 21.6 ± 5.6 months [29]. Unlike the above, Prakash et al. [30] noted in 147 PD patients a small but significant median reduction of total NMS burden over a 12–18-month study period, postulating that the improvement in total NMS burden with various therapies may be attributed to the early stages of PD in a majority (more than 80%) of their study patients. The early stages of PD are known to have the best therapeutic window response to symptomatic therapy for motor symptoms and it could be the best window for NMS as well [30,31]. However, in the COPPADIS cohort more than 90% of the patients included at baseline were in stage 1 or 2 of H&Y and a progression of some symptoms impacting on NMS burden progression was still observed even after considering different treatments in the model (dopaminergic and non-dopaminergic therapies). In spite of a lack of longitudinal studies about cognitive changes in the short-term in PD patients, there seems to be a slow and heterogenic progression of cognitive function in PD [32]. A very recent study observed that PD patients had significantly more fatigue than the control group from baseline and throughout 9 years of follow-up [33]. Although there is no clear evidence about the progression of pain in PD and again, more longitudinal studies are needed, some reports suggest a higher frequency of pain after long-term progression [34]. Another study demonstrated that autonomic dysfunction is not only common in early-stage PD, but it increases in severity with increasing disease stage [35]. In addition, orthostatic hypotension has been associated with impaired daily living activities even in the asymptomatic stage [36]. In the COPPADIS cohort, cardiovascular symptoms was the domain of the NMSS with the greatest impairment, and a moderate correlation between the changes in cardiovascular symptoms and the changes in the ADLS at 2-year follow-up was observed. All these findings suggest that, despite symptomatic treatment, there is a lot of variability between patients with different non-motor phenotypes proposed [37] and that, globally, there is a slow progression of motor symptoms and NMS in PD. Results about the effect of size in this cohort suggest that the progression of NMS could be slower than motor symptoms.
Simuni et al. [12] identified only two predictors of the longitudinal change of NMS, age at the time of analysis and lower CSF Aβ1-42. In our analysis, as in some other reports [29,38,39] but unlike some cross-sectional and case–control studies [40,41], age was not related with the severity evolution of NMS in PD. The large heterogeneity of PD patients in terms of treatments and age (in our study patients older than 75 years old were not included) may account for this discrepancy; therefore, longer periods of follow-up studies are needed. On the contrary, a longer disease duration was related to a greater global NMS burden impairment, aligning with the observation that NMS become more prevalent and severe in advanced PD patients throughout the course of the disease [42]. We did not introduce MRI and CSF biomarkers. Mollenhauer et al., did it and did not identify an association of NMS with the CSF measures [43]. Unlike Simuni et al., information about the variance and β regression coefficients were provided in our analysis. A novel aspect to be highlighted of the present study is the fact that greater sleep symptoms (PDSS) and neuropsychiatric symptoms (NPI) impairment predicted a greater global NMS burden increase. The NMSS includes questions about these symptoms, but it has not been previously reported and this relationship was not observed for example with mood, a factor impacting very importantly in the perception of the patients about NMS [44]. As neuropsychiatric symptoms impact on QoL and several of these symptoms have a presumed effective treatment [45,46], doctors should be encouraged to assess the presence of these symptoms for the purpose of applying the proper treatment to improve the patients’ health-related QoL [46,47]. Remarkably, a reduction in the NPI score in this study from baseline visit to 2-year follow-up visit was a predictor of being an NMS—improver (i.e., to have a lower global NMS burden after 2 years of follow-up). With regard to sleep problems, there is a progressive increase in the frequency of sleep disturbances in PD, with the number of subjects reporting multiple sleep disturbances increasing over time [48]. Recently, we observed in the baseline analysis of the COPPADIS cohort that NMS burden was associated with sleep problems after adjustment for age, gender, disease duration, LEDD, H&Y, UPDRS-III, UPDRS-IV, PD-CRS, BDI-II, NPI, VAS-Pain, VAFS, FOGQ, and total number of non-antiparkinsonian treatments [49]. Here, after a 2-year follow-up we observed that changes in sleep predict changes in the global NMS burden. A large number of patients are not treated for their sleep disturbances [48] and these findings call for an increased awareness of sleep problems in PD patients. Finally, the most significant predictor of global NMS burden progression was the NMSS total score at baseline. The risk is higher when the score is lower, signifying that even in early PD patients it is necessary to be alert when the patient has a low global NMS burden because it is liable to increase. Again, it is logical to consider and on the contrary, a greater improvement in NMS after an intervention has been observed in patients with a greater NMS burden [50], so changes are related to the score at baseline.
The present study has some limitations. First, the information about NMS burden follow-up was recorded in 501 patients of 677 (74%). Thirty-eight patients dropped out of the study (1 death; 2 with change in diagnosis; 35 other reasons) at the 2-year follow-up and 132 were not evaluated. In six patients, NMSS was not assessed. However, this is a limitation observed in other prospective studies. In the study of Antonini et al. [27], 707 PD patients from 1142 initially included (61.9%) were evaluable at 24 months. The percentage in other studies was 89.8% (380/423) [12], 83.6% (117/140) [29], and 73.9% (147/199) [30]. Second, our sample was not fully representative of the PD population due to inclusion and exclusion criteria (i.e., age limit, no dementia, no severe comorbidities, no second line therapies, etc.) [15], which leads to an early PD bias in this cohort. Third, for some variables, the information was not collected in all cases. Fourth, a specific tool for assessing comorbidity, like Charlson Index or others, has not been used. However, the total number of non-antiparkinsonian medications has been suggested as a useful marker of comorbidity in PD [8]. Fifth, the smaller sample size of the control group. On the contrary, the strengths of our study include the large sample size of the PD group, a very thorough assessment, a prospective longitudinal follow-up design, the fact that this analysis was “a priori” planned as one objective of the multicenter COPPADIS project [15], and the extensive clinical and demographic information recorded.
In conclusion, the present study observes that global NMS burden demonstrates short-term progression in PD patients but not in controls and identifies worsening sleep problems and neuropsychiatric symptoms as significant independent predictors of this NMS progression. Strategies designed to act over these symptoms and to analyze the short-term and long-term progression of NMS burden could be of interest.
Acknowledgments
We would like to thank all patients and their caregivers who collaborated in this study. Many thanks also to Fundación Española de Ayuda a la Investigación en Parkinson y otras Enfermedades Neurodegenerativas (Curemos el Parkinson; www.curemoselparkinson.org), Alpha Bioresearch (www.alphabioresearch.com), and other institutions helping us.
Appendix A
COPPADIS STUDY GROUP
Adarmes AD, Almeria M, Alonso Losada MG, Alonso Cánovas A, Alonso Frech F, Alonso Redondo R, Álvarez I, Álvarez Sauco M, Aneiros Díaz A, Arnáiz S, Arribas S, Ascunce Vidondo A, Aguilar M, Ávila MA, Bernardo Lambrich N, Bejr-Kasem H, Blázquez Estrada M, Botí M, Borrue C, Buongiorno MT, Cabello González C, Cabo López I, Caballol N, Cámara Lorenzo A, Canfield Medina H, Carrillo F, Carrillo Padilla FJ, Casas E, Catalán MJ, Clavero P, Cortina Fernández A, Cosgaya M, Cots Foraster A, Crespo Cuevas A, Cubo E, de Deus Fonticoba T, de Fábregues-Boixar O, Díez-Fairen M, Dotor García-Soto J, Erro E, Escalante S, Estelrich Peyret E, Fernández Guillán N, Gámez P, Gallego M, García Caldentey J, García Campos C, García Moreno JM, Gastón I, Gómez Garre MP, Gómez Mayordomo V, González Aloy J, González-Aramburu I, González Ardura J, González García B, González Palmás MJ, González Toledo GR, Golpe Díaz A, Grau Solá M, Guardia G, Hernández Vara J, Horta-Barba A, Idoate Calderón D, Infante J, Jesús S, Kulisevsky J, Kurtis M, Labandeira C, Labrador MA, Lacruz F, Lage Castro M, Lastres Gómez S, Legarda I, López Ariztegui N, López Díaz LM, López Manzanares L, López Seoane B, Lucas del Pozo S, Macías Y, Mata M, Martí Andres G, Martí MJ, Martínez Castrillo JC, Martinez-Martin P, McAfee D, Meitín MT, Menéndez González M, Méndez del Barrio C, Mir P, Miranda Santiago J, Morales Casado MI, Moreno Diéguez A, Nogueira V, Novo Amado A, Novo Ponte S, Ordás C, Pagonabarraga J, Pareés I, Pascual-Sedano B, Pastor P, Pérez Fuertes A, Pérez Noguera R, Planas-Ballvé A, Planellas L, Prats MA, Prieto Jurczynska C, Puente V, Pueyo Morlans M, Redondo Rafales N, Rodríguez Méndez L, Rodríguez Pérez AB, Roldán F, Ruíz De Arcos M, Ruíz Martínez J, Sánchez Alonso P, Sánchez-Carpintero M, Sánchez Díez G, Sánchez Rodríguez A, Santacruz P, Santos García D, Segundo Rodríguez JC, Seijo M, Sierra Peña M, Solano Vila B, Suárez Castro E, Tartari JP, Valero C, Vargas L, Vela L, Villanueva C, Vives B, Villar MD.
| Name (Last Name, First Name) | Location | Role | Contribution |
| Astrid Adarmes, Daniela | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Almeria, Marta | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Alonso Losada, Maria Gema | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Alonso Cánovas, Araceli | Hospital Universitario Ramón y Cajal, Madrid, Spain |
Site investigator | Evaluation of participants and/or data management |
| Alonso Frech, Fernando | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Alonso Redondo, Ruben | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Aneiros Díaz, Ángel | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Álvarez, Ignacio | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Álvarez Sauco, María | Hospital General Universitario de Elche, Elche, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Arnáiz, Sandra | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Arribas, Sonia | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Ascunce Vidondo, Arancha | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Aguilar, Miquel | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Ávila Rivera, Maria Asunción | Consorci Sanitari Integral, Hospital General de L’Hospitalet, L’Hospitalet de Llobregat, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Bernardo Lambrich, Noemí | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator | Evaluation of participants and/or data management |
| Bejr-Kasem, Helena | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Blázquez Estrada, Marta | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator | Evaluation of participants and/or data management |
| Botí González, Maria Ángeles | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Borrué, Carmen | Hospital Infanta Sofía, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Buongiorno, Maria Teresa | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Cabello González, Carolina | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Scheduling of evaluations |
| Cabo López, Iria | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Caballol, Nuria | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain. | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Cámara Lorenzo, Ana | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Canfield Medina, Héctor | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Evaluation of participants and/or data management |
| Carrillo, Fátima | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Carrillo Padilla, Francisco José | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Casas, Elena | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Catalán, Maria José | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Clavero, Pedro | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cortina Fernández, A | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Coordination of blood extractions |
| Cosgaya, Marina | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cots Foraster, Anna | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Crespo Cuevas, Ane | Hospital del Mar, Barcelona, Spain. | Site investigator | Evaluation of participants and/or data management |
| Cubo, Esther | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| De Deus Fonticoba, Teresa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Nurse study coordinator Evaluation of participants and/or data management |
| De Fábregues-Boixar, Oriol | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Díez Fairen, M | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Dotor García-Soto, Julio | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator/PI | Evaluation of participants and/or data management |
| Erro, Elena | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Escalante, Sonia | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Estelrich Peyret, Elena | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Fernández Guillán, Noelia | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Gámez, Pedro | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Gallego, Mercedes | Hospital La Princesa, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| García Caldentey, Juan | Centro Neurológico Oms 42, Palma de Mallorca, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| García Campos, Cristina | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| García Moreno, Jose Manuel | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator/PI (until MAR/21) | Coordination at the center Evaluation of participants and/or data management |
| Gastón, Itziar | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Gómez Garre, María del Pilar | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Genetic studies coordination |
| Gómez Mayordomo, Víctor | Hospital Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aloy, Javier | Institut d’Assistència Sanitària (IAS) - Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aramburu, Isabel | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| González Ardura, Jessica | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator/PI (until FEB/21) | Evaluation of participants and/or data management |
| González García, Beatriz | Hospital La Princesa, Madrid, Spain | Site investigator | Nurse study coordinator |
| González Palmás, Maria Josefa | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| González Toledo, Gabriel Ricardo | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Golpe Díaz, Ana | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Laboratory analysis coordination |
| Grau Solá, Mireia | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Guardia, Gemma | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Hernández Vara, Jorge | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Horta Barba, Andrea | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Idoate Calderón, Daniel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigaor | Neuropsychologist; evaluation of participants |
| Infante, Jon | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Jesús, Silvia | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Kulisevsky, Jaime | Hospital de Sant Pau, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Kurtis, Mónica | Hospital Ruber Internacional, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Labandeira, Carmen | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator | Evaluation of participants and/or data management |
| Labrador Espinosa, Miguel Ángel | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging data analysis |
| Lacruz, Francisco | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Lage Castro, Melva | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| Lastres Gómez, Sonia | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Legarda, Inés | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| López Ariztegui, Nuria | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator/PI | Evaluation of participants and/or data management |
| López Díaz, Luis Manuel | Hospital Da Costa de Burela, Lugo, Spain | Site investigator/PI (until SEP/16) | Evaluation of participants and/or data management |
| López Manzanares, Lydia | Hospital La Princesa, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| López Seoane, Balbino | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Lucas del Pozo, Sara | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Macías, Yolanda | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Mata, Marina | Hospital Infanta Sofía, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí Andres, Gloria | Hospital Universitario Vall d’Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí, Maria José | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Martínez Castrillo, Juan Carlos | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Martinez-Martin, Pablo | Centro Nacional de Epidemiología y CIBERNED, Instituto de Salud Carlos III. Madrid | Collaborator in statistical and methods analysis | Methods and statistical reviewer |
| McAfee, Darrian | University of Pennsylvania, Philadelphia | Collaborator in english style | English style reviewer |
| Meitín, Maria Teresa | Hospital Da Costa de Burela, Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| Menéndez González, Manuel | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Méndez del Barrio, Carlota | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Mir, Pablo | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Miranda Santiago, Javier | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Morales Casado, Maria Isabel | Complejo Hospitalario de Toledo, Toledo, Spain. | Site investigator | Evaluation of participants and/or data management |
| Moreno Diéguez, Antonio | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Nogueira, Víctor | Hospital Da Costa de Burela, Lugo, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Novo Amado, Alba | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Novo Ponte, Sabela | Hospital Universitario Puerta de Hierro, Madrid, Spain. | Site investigator | Evaluation of participants and/or data management |
| Ordás, Carlos | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain. | Site Investigator | Evaluation of participants and/or data management |
| Pagonabarraga, Javier | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Isabel Pareés | Hospital Ruber Internacional, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Pascual-Sedano, Berta | Hospital de Sant Pau, Barcelona, Spain | Site Investigator | Evaluation of participants and/or data management |
| Pastor, Pau | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Pérez Fuertes, Aída | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Pérez Noguera, Rafael | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Planas-Ballvé, Ana | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Planellas, Lluís | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Prats, Marian Ángeles | Institut d’Assistència Sanitària (IAS)—Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Prieto Jurczynska, Cristina | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Puente, Víctor | Hospital del Mar, Barcelona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Pueyo Morlans, Mercedes | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Redondo, Nuria | Hospital La Princesa, Madrid, Spain | Site Investigator | Evaluation of participants and/or data management |
| Rodríguez Méndez, Luisa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Rodríguez Pérez, Amparo Belén | Hospital General Universitario de Elche, Elche, Spain | Site investigator | Evaluation of participants and/or data management |
| Roldán, Florinda | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging studies |
| Ruíz de Arcos, María | Hospital Universitario Virgen Macarena, Sevilla, Spain. | Site investigator | Evaluation of participants and/or data management |
| Ruíz Martínez, Javier | Hospital Universitario Donostia, San Sebastián, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Alonso, Pilar | Hospital Universitario Puerta de Hierro, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez-Carpintero, Macarena | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Sánchez Díez, Gema | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Rodríguez, Antonio | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Santacruz, Pilar | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Santos García, Diego | CHUAC, Complejo Hospitalario Universitario de A Coruña | Coordinator of the Project | Coordination of the COPPADIS-2015 |
| Segundo Rodríguez, José Clemente | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator | Evaluation of participants and/or data management |
| Seijo, Manuel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Sierra, María | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Solano, Berta | Institut d’Assistència Sanitària (IAS)—Instituí Cátala de la Salud. Girona, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Suárez Castro, Ester | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Evaluation of participants and/or data management |
| Tartari, Juan Pablo | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Valero, Caridad | Hospital Arnau de Vilanova, Valencia, Spain | Site investigator | Evaluation of participants and/or data management |
| Vargas, Laura | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Vela, Lydia | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator/PI | Coordination at the center Evaluation of participants and/or data management |
| Villanueva, Clara | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Vives, Bárbara | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator | Evaluation of participants and/or data management |
| Villar, Maria Dolores | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
Author Contributions
Conception, D.S.-G.; Organization, D.S.-G.; Execution of the project, D.S.-G.; statistical analysis, D.S.-G.; writing of the first draft of the manuscript, D.S.-G.; Recruitment, D.S.-G., T.d.D., E.S., S.J., M.A., P.P., L.P., M.C., J.G.C., N.C., I.L., J.H.-V., I.C., L.L.M., I.G.A., M.A.Á.R., M.J.C., V.N., V.P., J.D., C.B., B.S., M.Á.S., L.V., S.E., E.C., F.C., J.C.M.C., P.S.A., G.A., N.L.A., I.G., J.K., M.B., M.S., J.R.M., C.V., M.K., O.d.F., J.A., R.A., C.O., L.M.L.D. and P.M.; Review and critique: T.d.D., C.C., L.V.A., H.C., J.M.P.G., C.M.M., E.S., S.J., M.A., P.P., L.P., M.C., J.G.C., N.C., I.L., J.H.-V., I.C., L.L.M., I.G.A., M.A.Á.R., M.J.C., V.N., V.P., J.D., C.B., B.S., M.Á.S., L.V., S.E., E.C., F.C., J.C.M.C., P.S.A., G.A., N.L.A., I.G., J.K., M.B., M.S., J.R.M., C.V., M.K., O.d.F., J.A., R.A., C.O., L.M.L.D., D.M., P.M.-M. and P.M.; Evaluation of participants: D.S.-G., T.d.D., E.S., S.J., M.A., P.P., L.P., M.C., J.G.C., N.C., I.L., J.H.-V., I.C., L.L.M., I.G.A., M.A.Á.R., M.J.C., V.N., V.P., J.D., C.B., B.S., M.Á.S., L.V., S.E., E.C., F.C., J.C.M.C., P.S.A., G.A., N.L.A., I.G., J.K., M.B., M.S., J.R.M., C.V., M.K., O.d.F., J.A., R.A., C.O., L.M.L.D. and P.M.; Collaboration in the preparation of the manuscript: C.C. and L.V.A.; Review of english style: D.M.; Supervision: P.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundación Española de Ayuda a la Investigación en Parkinson y otras Enfermedades Neuro-degenerativas (Curemos el Parkinson; www.curemoselparkinson.org).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and ap-proved by Comité de Ética de la Investigación Clínica de Galicia from Spain (2014/534; 2/DEC/2014).
Informed Consent Statement
Written informed consent from all participants in this study were obtained before the start of the study.
Data Availability Statement
The protocol and the statistical analysis plan are available on request. Deidentified participant data are not available for legal and ethical reasons.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armstrong M.J., Okun M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 2.Tolosa E., Gaig C., Santamaría J., Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology. 2009;72(Suppl. S7):S12–S20. doi: 10.1212/WNL.0b013e318198db11. [DOI] [PubMed] [Google Scholar]
- 3.Poewe W., Mahlknecht P. The clinical progression of Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15(Suppl. S4):S28–S32. doi: 10.1016/S1353-8020(09)70831-4. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri K.R., Healy D.G., Schapira A.H. National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Martin P. What is quality of life and how do we measure it? Relevance to Parkinson’s disease and movement disorders. Mov. Disord. 2017;32:382–392. doi: 10.1002/mds.26885. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Martin P., Kurtis M.M. Health-related quality of life as an outcome variable in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2012;5:105–117. doi: 10.1177/1756285611431974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soh S.E., Morris M.E., McGinley J.L. Determinants of health-related quality of life in Parkinson’s disease: A systematic review. Parkinsonism Relat. Disord. 2011;17:1–9. doi: 10.1016/j.parkreldis.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Santos García D., de Deus Fonticoba T., Suárez Castro E., Borrué C., Mata M., Solano Vila B., Cots Foraster A., Álvarez Sauco M., Rodríguez Pérez A.B., Vela L., et al. Non-motor symptoms burden, mood, and gait problems are the most significant factors contributing to a poor quality of life in non-demented Parkinson’s disease patients: Results from the COPPADIS Study Cohort. Parkinsonism Relat. Disord. 2019;66:151–157. doi: 10.1016/j.parkreldis.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 9.Erro R., Picillo M., Vitale C., Amboni M., Moccia M., Longo K., Cozzolino A., Giordano F., De Rosa A., De Michele G., et al. Non-motor symptoms in early Parkinson’s disease: A 2-year follow-up study on previously untreated patients. J. Neurol. Neurosurg. Psychiatry. 2013;84:14–17. doi: 10.1136/jnnp-2012-303419. [DOI] [PubMed] [Google Scholar]
- 10.Erro R., Picillo M., Vitale C., Amboni M., Moccia M., Santangelo G., Pellecchia M.T., Barone P. The non-motor side of the honeymoon period of Parkinson’s disease and its relationship with quality of life: A 4-year longitudinal study. Eur. J. Neurol. 2016;23:1673–1679. doi: 10.1111/ene.13106. [DOI] [PubMed] [Google Scholar]
- 11.Prakash K.M., Nadkarni N.V., Lye W.K., Yong M.H., Tan E.K. The impact of non-motor symptoms on the quality of life of Parkinson’s disease patients: A longitudinal study. Eur. J. Neurol. 2016;23:854–860. doi: 10.1111/ene.12950. [DOI] [PubMed] [Google Scholar]
- 12.Simuni T., Caspell-Garcia C., Coffey C.S., Weintraub D., Mollenhauer B., Lasch S., Tanner C.M., Jennings D., Kieburtz K., Chahine L.M., et al. Baseline prevalence and longitudinal evolution of non-motor symptoms in early Parkinson’s disease: The PPMI cohort. J. Neurol. Neurosurg. Psychiatry. 2018;89:78–88. doi: 10.1136/jnnp-2017-316213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos García D., de Deus Fonticoba T., Cores C. Predictors of Clinically Significant Quality of Life Impairment in Parkinson’s Disease. Results from the COPPADIS Cohort at 2-Year Follow-up. 2021. Unpublished work.
- 14.Santos García D., Jesús S., Aguilar M., Planellas L.L., García Caldentey J., Caballol N., Legarda I., Hernández Vara J., Cabo I., López Manzanares L., et al. COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015): An ongoing global Parkinson’s disease project about disease progression with more than 1000 subjects included. Results from the baseline evaluation. Eur. J. Neurol. 2019;26:1399–1407. doi: 10.1111/ene.14008. [DOI] [PubMed] [Google Scholar]
- 15.Santos-García D., Mir P., Cubo E., Vela L., Rodríguez-Oroz M.C., Martí M.J., Arbelo J.M., Infante J., Kulisevsky J., Martínez-Martín P., et al. COPPADIS-2015 (COhort of Patients with PArkinson’s DIsease in Spain, 2015), a global-clinical evaluations, serum biomarkers, genetic studies and neuroimaging-prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurol. 2016;16:26. doi: 10.1186/s12883-016-0548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhuri K.R., Martinez-Martin P., Brown R.G., Sethi K., Stocchi F., Odin P., Ondo W., Abe K., MacPhee G., MacMahon D., et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov. Disord. 2007;22:1901–1911. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- 17.Ray Chaudhuri K., Rojo J.M., Schapira A.H., Brooks D.J., Stocchi F., Odin P., Antonini A., Brown R.J., Martinez-Martin P. A proposal for a comprehensive grading of Parkinson’s disease severity combining motor and non-motor assessments: Meeting an unmet need. PLoS ONE. 2013;8:e57221. doi: 10.1371/journal.pone.0057221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stebbins G.T., Goetz C.G., Burn D.J., Jankovic J., Khoo T.K., Tilley B.C. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 2013;28:668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 19.Jost S.T., Visser-Vandewalle V., Rizos A., Loehrer P.A., Silverdale M., Evans J., Samuel M., Petry-Schmelzer J.N., Sauerbier A., Gronostay A., et al. EUROPAR and the International Parkinson and Movement Disorders Society Non-Motor Parkinson’s Disease Study Group. Non-motor predictors of 36-month quality of life after subthalamic stimulation in Parkinson disease. NPJ Parkinsons Dis. 2021;7:48. doi: 10.1038/s41531-021-00174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermanowicz N., Jones S.A., Hauser R.A. Impact of non-motor symptoms in Parkinson’s disease: A PMDAlliance survey. Neuropsychiatr. Dis. Treat. 2019;15:2205–2212. doi: 10.2147/NDT.S213917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montero-Odasso M., Speechley M., Muir-Hunter S.W., Pieruccini-Faria F., Sarquis-Adamson Y., Hachinski V., Bherer L., Borrie M., Wells J., Garg A.X., et al. Canadian Gait and Cognition Network. Dual decline in gait speed and cognition is associated with future dementia: Evidence for a phenotype. Age Ageing. 2020;49:995–1002. doi: 10.1093/ageing/afaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghielen I., Koene P., Twisk J.W., Kwakkel G., van den Heuvel O.A., van Wegen E.E. The association between freezing of gait, fear of falling and anxiety in Parkinson’s disease: A longitudinal analysis. Neurodegener Dis. Manag. 2020;10:159–168. doi: 10.2217/nmt-2019-0028. [DOI] [PubMed] [Google Scholar]
- 23.Choi S.M., Jung H.J., Yoon G.J., Kim B.C. Factors associated with freezing of gait in patients with Parkinson’s disease. Neurol. Sci. 2019;40:293–298. doi: 10.1007/s10072-018-3625-6. [DOI] [PubMed] [Google Scholar]
- 24.Wilson J., Alcock L., Yarnall A.J., Lord S., Lawson R.A., Morris R., Taylor J.-P., Burn D.J., Rochester L., Galna B. Gait Progression Over 6 Years in Parkinson’s Disease: Effects of Age, Medication, and Pathology. Front Aging Neurosci. 2020;12:577435. doi: 10.3389/fnagi.2020.577435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goetz C.G. Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): A new scale for the evaluation of Parkinson’s disease. Rev. Neurol. 2010;166:1–4. doi: 10.1016/j.neurol.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Martin P., Chaudhuri K.R., Rojo-Abuin J.M., Rodriguez-Blazquez C., Alvarez-Sanchez M., Arakaki T., Bergareche-Yarza A., Chade A., Garretto N., Gershanik O., et al. Assessing the non-motor symptoms of Parkinson’s disease: MDS-UPDRS and NMS Scale. Eur. J. Neurol. 2015;22:37–43. doi: 10.1111/ene.12165. [DOI] [PubMed] [Google Scholar]
- 27.Antonini A., Barone P., Marconi R., Morgante L., Zappulla S., Pontieri F.E., Ramat S., Ceravolo M.G., Meco G., Cicarelli G., et al. The progression of non-motor symptoms in Parkinson’s disease and their contribution to motor disability and quality of life. J. Neurol. 2012;259:2621–2631. doi: 10.1007/s00415-012-6557-8. [DOI] [PubMed] [Google Scholar]
- 28.Vu T.C., Nutt J.G., Holford N.H. Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br. J. Clin. Pharmacol. 2012;74:267–283. doi: 10.1111/j.1365-2125.2012.04192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou R., Yang J., Cao B., Wei Q., Chen K., Chen X., Zhao B., Wu Y., Song W., Shang H. Progression of non-motor symptoms in Parkinson’s disease among different age populations: A two-year follow-up study. J. Neurol. Sci. 2016;360:72–77. doi: 10.1016/j.jns.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 30.Prakash K.M., Nadkarni N.V., Lye W.K., Yong M.H., Chew L.M., Tan E.K. A longitudinal study of non-motor symptom burden in Parkinson’s disease after a transition to expert care. Parkinsonism Relat. Disord. 2015;21:843–847. doi: 10.1016/j.parkreldis.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Bloem B.R., Okun M.S., Klein C. Parkinson’s disease. Lancet. 2021 doi: 10.1016/S0140-6736(21)00218-X. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Roheger M., Kalbe E., Liepelt-Scarfone I. Progression of Cognitive Decline in Parkinson’s Disease. J. Parkinsons Dis. 2018;8:183–193. doi: 10.3233/JPD-181306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ongre S.O., Dalen I., Tysnes O.B., Alves G., Herlofson K. Progression of fatigue in Parkinson’s disease-a 9-year follow-up. Eur. J. Neurol. 2021;28:108–116. doi: 10.1111/ene.14520. [DOI] [PubMed] [Google Scholar]
- 34.Tai Y., Lin C. An overview of pain in Parkinson’s disease. Clin. Parkinsonism Relat. Disord. 2020;2:1–8. doi: 10.1016/j.prdoa.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.B., Kim B.J., Koh S.B., Park K.W. Autonomic dysfunction according to disease progression in Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20:303–307. doi: 10.1016/j.parkreldis.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z., Li G., Liu J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020;134:104700. doi: 10.1016/j.nbd.2019.104700. [DOI] [PubMed] [Google Scholar]
- 37.Marras C., Chaudhuri K.R. Nonmotor features of Parkinson’s disease subtypes. Mov. Disord. 2016;31:1095–1102. doi: 10.1002/mds.26510. [DOI] [PubMed] [Google Scholar]
- 38.Brockmann K., Srulijes K., Pflederer S., Hauser A.-K., Schulte C., Maetzler W., Gasser T., Berg D. GBA-associated Parkinson’s disease: Reduced survival and more rapid progression in a prospective longitudinal study. Mov. Disord. 2015;30:407–411. doi: 10.1002/mds.26071. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan S., Sarma G., Sarma S., Kishore A. Do nonmotor symptoms in Parkinson’s disease differ from normal aging? Mov. Disord. 2011;26:2110–2113. doi: 10.1002/mds.23826. [DOI] [PubMed] [Google Scholar]
- 40.Guo X., Song W., Chen K., Chen X., Zheng Z., Cao B., Huang R., Zhao B., Wu Y., Shang H. Gender and onset age-related features of non-motor symptoms of patients with Parkinson’s disease—A study from Southwest China. Parkinsonism Relat. Disord. 2013;19:961–965. doi: 10.1016/j.parkreldis.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Szewczyk-Krolikowski K., Tomlinson P., Nithi K., Wade-Martins R., Talbot K., Ben-Shlomo Y., Hu M.T.M. The influence of age and gender on motor and non-motor features of early Parkinson’s disease: Initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat. Disord. 2014;20:99–105. doi: 10.1016/j.parkreldis.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Coelho M., Ferreira J.J. Late-stage Parkinson disease. Nat. Rev. Neurol. 2012;8:435–442. doi: 10.1038/nrneurol.2012.126. [DOI] [PubMed] [Google Scholar]
- 43.Mollenhauer B., Zimmermann J., Sixel-Döring F., Focke N.K., Wicke T., Ebentheuer J., Schaumburg M., Lang E., Trautmann E., Zetterberg H., et al. Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology. 2016;87:168–177. doi: 10.1212/WNL.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos-García D., de la Fuente-Fernández R. Impact of non-motor symptoms on health-related and perceived quality of life in Parkinson’s disease. J. Neurol. Sci. 2013;332:136–140. doi: 10.1016/j.jns.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Alvarado-Bolaños A., Cervantes-Arriaga A., Rodríguez-Violante M., Rodrigo L.-A., Humberto C.-F., Roxanna M.-C., Roberto L.-O., Ingrid E.-B., Carlos Z.-R. Impact of Neuropsychiatric Symptoms on the Quality of Life of Subjects with Parkinson’s Disease. J. Parkinsons Dis. 2015;5:541–548. doi: 10.3233/JPD-150597. [DOI] [PubMed] [Google Scholar]
- 46.Balestrino R., Martinez-Martin P. Neuropsychiatric symptoms, behavioural disorders, and quality of life in Parkinson’s disease. J. Neurol. Sci. 2017;373:173–178. doi: 10.1016/j.jns.2016.12.060. [DOI] [PubMed] [Google Scholar]
- 47.Antonini A., Tesei S., Zecchinelli A., Barone P., De Gaspari D., Canesi M., Sacilotto G., Meucci N., Mariani C., Pezzoli G. Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson’s disease and depression: Effect on quality of life. Mov. Disord. 2006;21:1119–1122. doi: 10.1002/mds.20895. [DOI] [PubMed] [Google Scholar]
- 48.Xu Z., Anderson K.N., Saffari S.E., Lawson R.A., Chaudhuri K.R., Brooks D., Pavese N. Progression of sleep disturbances in Parkinson’s disease: A 5-year longitudinal study. J. Neurol. 2021;268:312–320. doi: 10.1007/s00415-020-10140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos-García D., Castro E.S., de Deus Fonticoba T., Feal Panceiras M.J., Muñoz Enriquez J.G., Paz González J.M., Cores Bartolomé C., Planellas L.L., García Caldentey J., Caballol N., et al. Sleep Problems Are Related to a Worse Quality of Life and a Greater Non-Motor Symptoms Burden in Parkinson’s Disease. J. Geriatr. Psychiatry Neurol. 2020 doi: 10.1177/0891988720964250. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Santos García D., Labandeira Guerra C., Yáñez Baña R., Hernando M.I.C., López I.C., Gonález J.M.P., Losada M.G.A., Palmás M.J.G., Miró C.M. Safinamide Improves Non-Motor Symptoms Burden in Parkinson’s Disease: An Open-Label Prospective Study. Brain Sci. 2021;11:316. doi: 10.3390/brainsci11030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The protocol and the statistical analysis plan are available on request. Deidentified participant data are not available for legal and ethical reasons.